Facoltà di Medicina e Chirurgia
Scuola di Specializzazione in Oncologia Medica Direttore: Professor Alfredo Falcone
FOLFOXIRI plus bevacizumab as initial treatment for
metastatic colorectal cancer patients: results of the phase III
TRIBE trial
Dott.ssa Chiara Cremolini Prof. Alfredo Falcone
Anno accademico: 2013-2014
INDEX
1. BACKGROUND ... 2
2. METHODS ... 4
2.1. STUDY DESIGN AND OVERSIGHT ... 4
2.2. PATIENTS ... 4
2.3. TREATMENT ... 5
2.4. ASSESSMENTS ... 5
2.5. KRAS AND BRAF MUTATIONAL STATUS ... 6
2.6. STATISTICAL ANALYSIS ... 6
2.7. POOLED ANALYSIS OF FOLFOXIRI AND FOLFOXIRI PLUS BEV ... 6
3. RESULTS ... 8
3.1. FOLFOXIRI PLUS BEVACIZUMAB VS FOLFIRI PLUS BEVACIZUMAB: RESULTS FROM THE PHASE III TRIBE TRIAL ... 8
3.1.1. STUDY POPULATION ... 8
3.1.2. SAFETY ... 11
3.1.3.PRIMARY ENDPOINT: PROGRESSION FREE SURVIVAL ... 12
3.1.4. SECONDARY ENDPOINTS: RESPONSE RATE AND RESECTION RATE ... 16
3.1.5. SECONDARY ENDPOINTS: EARLY TUMOR SHRINKAGE AND DEEPNESS OF RESPONSE ... 17
3.1.6. SECONDARY ENDPOINTS: OVERALL SURVIVAL ... 20
3.1.7. SUBSEQUENT LINES TREATMENTS ... 22
3.1.8. RAS AND BRAF MUTATIONAL ANALYSES ... 23
3.2. FOLFOXIRI WITH OR WITHOUT BEVACIZUMAB: A POOLED ANALYSIS OF TWO CONSECUTIVE CLINICAL TRIALS ... 26 3.2.1. STUDY POPULATIONS ... 26 3.2.2. SURVIVAL RESULTS ... 26 3.2.2. ACTIVITY RESULTS ... 27 4. DISCUSSION ... 29 5. FUTURE PERSPECTIVES ... 33 6. REFERENCES ... 36
1. BACKGROUND
Upfront treatment with 2-‐drugs combinations of fluorouracil based chemotherapy with irinotecan or oxaliplatin plus bevacizumab are a widely adopted approach for metastatic colorectal cancer.1,2 Initial treatment strategies adopting irinotecan containing doublets compared with oxaliplatin led to similar results,3 therefore the choice of the upfront combination is up to each single clinician and is commonly based on patient's preferences, regional differences and having or not already received an adjuvant oxaliplatin-‐containing treatment.4
In the pivotal randomized phase 3 study AVF 2107 g,1 the addition of bevacizumab to irinotecan and bolus fluorouracil led to an improvement of objective response rate, progression-‐free survival and overall survival. Bevacizumab was added to the irinotecan and infusional fluorouracil in a phase 4 trial producing similar results.5
A triple combination of fluorouracil, oxaliplatin and irinotecan proved to be feasible and highly active in phase 2 studies.6,7 In a phase 3 study of the Gruppo Oncologico Nord Ovest (GONO), 12 cycles of treatment with FOLFOXIRI demonstrated superior response rate, progression-‐free survival and overall survival compared to 12 cycles of FOLFIRI.8
Activity and safety of FOLFOXIRI plus bevacizumab were previously tested in a phase II study named FOIB. A response rate of 77% and a disease control rate of 100% were reported. Median progression-‐free survival was 13.1 months and median overall survival was 30.9 months. Toxicity results were consistent with the previous experience.9
On the basis of such promising results, we conducted TRIBE trial, a phase III randomized study of FOLFOXIRI plus bevacizumab as compared with FOLFIRI plus bevacizumab in patients with previously untreated metastatic colorectal cancer.
In the meanwhile, a growing amount of data have demonstrated the importance of an extensive molecular characterization of metastatic colorectal cancer, beyond the assessment of KRAS exon 2 mutational status. On the basis of the post-‐hoc analysis of phase III randomized PRIME trial of first-‐line FOLFOX plus or minus the anti-‐EGFR monoclonal antibody panitumumab,10 the use of both cetuximab and panitumumab is now restricted in Europe to patients bearing RAS wt tumors. Moreover, the negative prognostic impact of BRAF V600E mutation was clearly evidenced. BRAF mutant tumors actually share specific clinical and molecular characteristics, affecting their extremely aggressive behaviour.11-‐13 The retrospective analysis of phase II FOIB trial suggested that an intensive first-‐line regimen such
as the triplet plus bevacizumab may be able to counteract the biologic and clinical aggressiveness of such a poor prognosis disease.9 That finding was prospectively validated in an independent cohort of 15 BRAF mutant metastatic colorectal cancer patients, where promising results in terms of both PFS (median PFS: 9.2 months) and OS (24.1 months) were reported.14 Therefore, tissue samples from patients randomized in TRIBE trial were collected and RAS and BRAF mutational status were centrally assessed in order to investigate their prognostic and/or predictive impact.
2. METHODS
2.1. STUDY DESIGN AND OVERSIGHT
The TRIplet plus BEvacizumab (TRIBE) study was a randomized, open-‐label, multicenter phase 3 trial conducted in 34 italian centers involving patients with unresectable metastatic colorectal cancer who had not received chemotherapy or biologic therapy for their metastatic disease. The study was conducted in accordance with the Declaration of Helsinki, and adhered to Good Clinical Practice Guidelines. Approval for the protocol was obtained from the local ethics committee for each participating site. All patients provided written informed consent, including a separate, specific signature consenting to blood sampling and specimen donation for translational analyses.
Patients were assigned (in a 1:1 ratio) to receive up to 12 cycles of FOLFOXIRI plus bevacizumab or FOLFIRI plus bevacizumab. A maintenance treatment with fluorouracil plus bevacizumab until tumor progression was then administered in both arms. Stratification criteria included: ECOG performance status (0 vs. 1-‐2), center, prior adjuvant treatment (yes vs. no).
The primary end point was progression-‐free survival, defined as the time from randomization to disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0, 15 or death from any cause. Tumor assessment was centrally reviewed. Secondary endpoints included response rate, overall survival, resection rate of metastases and safety. Adverse events were graded according to NCI Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.16
2.2. PATIENTS
Main inclusion criteria were: age between 18 and 75 years, ECOG performance status of 2 or less (patients aged above 70 years were eligible if their ECOG performance status was 0), histologically confirmed adenocarcinoma of the colon or rectum, first occurrence of metastatic disease deemed unresectable with curative intent, measurable disease according to RECIST version 1.0, adequate bone marrow, liver and renal function. Main exclusion criteria were adjuvant oxaliplatin completed less than 12 months before relapse, peripheral neuropathy of grade 1 or more according the NCI CTCAE version 3.0
,
evidence of bleedingevents within 6 months before study entry, serious cardiac events requiring medication, New York Heart Association grade 2 or more heart failure, need for full dose anticoagulation.
2.3. TREATMENT
Patients in the control arm received up to 12 cycles of FOLFIRI plus bevacizumab, consisting of a 30-‐minute infusion of bevacizumab at a dose of 5 mg per kilogram; a 60-‐minute infusion of irinotecan at a dose of 180 mg per square meter of body-‐surface area; a 120-‐minute infusion of l-‐leucovorin at a dose of 200 mg per square meter; a bolus of fluorouracil at a dose of 400 mg per square meter followed by a 46-‐hour flat continuous infusion of fluorouracil at a dose of 2400 mg per square meter. Cycles were repeated every 14 days. Patients in the experimental arm received up to 12 cycles of FOLFOXIRI plus bevacizumab, consisting of a 30-‐minute infusion of bevacizumab at a dose of 5 mg per kilogram; a 60-‐minute infusion of irinotecan at a dose of 165 mg per square meter of body-‐surface area; a 120-‐minute infusion of oxaliplatin at a dose of 85 mg per square meter and a concomitant 120-‐minute infusion of l-‐ leucovorin at a dose of 200 mg per square meter, followed by a 48-‐hour flat continuous infusion of fluorouracil at a dose of 3200 mg per square meter. Cycles were repeated every 14 days
Thereafter, in both arms, maintenance treatment with bevacizumab, fluorouracil and l-‐ leucovorin was continued until disease progression, unacceptable toxicity or consent withdrawal. In case of prespecified adverse events treatment modifications were permitted according to study protocol.
2.4. ASSESSMENTS
Tumor assessment by means of computed tomography was performed every 8 weeks until the evidence of disease progression.
Tumor response was evaluated according to RECIST 1.0 criteria. Early tumor shrinkage was defined as the relative change in the sum of the longest diameters of RECIST target lesions at week 8 compared to baseline. Patients achieving a ETS > 20% were defined as early responders. Deepness of response was defined as the relative change in the sum of the longest diameters of RECIST target lesions at the nadir in the absence of new lesions or progression of non-‐target lesions, as compared to baseline At the start of every cycle patients' medical history, ECOG performance status, physical examination and adverse events were recorded.
2.5. KRAS AND BRAF MUTATIONAL STATUS
DNA was extracted from archival tissue specimens from the primary tumor or metastasis.
KRAS and NRAS codons 12, 13 and 61 and BRAF codon 600 were centrally analysed by means
of pyrosequencing as previously reported.14
2.6. STATISTICAL ANALYSIS
The trial was planned as a phase 3 randomized study. We planned to enroll 450 patients in order to observe 379 events of disease progression or death from any cause; with that number of events, it was estimated that the study would have 80% power to detect a hazard ratio for progression-‐free survival of 0.75 at a two-‐sided significance level of 5%. All efficacy analyses were performed on an intention-‐to-‐treat basis. Median period of follow-‐up was calculated for the entire study cohort according to the reverse Kaplan-‐Meier method. Distributions of time-‐to-‐event variables were estimated with the use of the Kaplan–Meier product-‐limit method. The stratified log-‐rank test was used as the primary analysis for comparison of treatment groups. Cox proportional-‐hazards model was also performed as supportive analyses. Subgroup analyses of progression-‐free survival were performed by means of interaction test to determine the consistency of the treatment effect according to key baseline characteristics. Overall survival was analyzed with the same methods as used for the analysis of progression-‐free survival. Objective response rate, the resection rate for metastases and the incidence of adverse events in the two groups were compared with the use of the Chi-‐square test for heterogeneity, or the Fisher's exact test when approriate. All statistical tests were two sided, and P values of 0.05 or less were considered to be statistically significant. Odds and hazard ratios and 95% CIs were estimated with a logistic regression model and a Cox proportional hazard model, respectively. No adjustment for multiple comparisons was made.
2.7. POOLED ANALYSIS OF FOLFOXIRI AND FOLFOXIRI PLUS BEV
From May 2001 to April 2005 122 mCRC patients received first-‐line FOLFOXIRI in the phase III trial by the GONO8 (FOLFOXIRI group) and from July 2008 to May 2011 252 patients received first-‐line FOLFOXIRI plus bev in the TRIBE trial (FOLFOXIRI plus bev group). Estimates of the effect of adding bev to FOLFOXIRI were obtained with the use of the Cox's proportional hazard and logistic regression models and the ANOVA. Group differences have
liver-‐only disease, timing of metastases, previous surgery and adjuvant CT and Kohne score.
3. RESULTS
3.1. FOLFOXIRI PLUS BEVACIZUMAB VS FOLFIRI PLUS BEVACIZUMAB: RESULTS FROM THE PHASE III
TRIBE TRIAL
3.1.1. STUDY POPULATION
From July 2008 through May 2011, a total of 508 patients from 34 Italian centers were enrolled in the study; 256 and 252 patients were randomly assigned to FOLFIRI plus bevacizumab (control group) and FOLFOXIRI plus bevacizumab, respectively, and were included in the intention-‐to-‐treat population. Two patients per arm did not receive any cycle of treatment according to the randomization arm and therefore were not included in the safety population (Fig. 1). The cutoff date for collection of follow-‐up data was April 26, 2013. Demographic and baseline characteristics of the patients were similar in the two groups (Table 1), but a higher percentage of patients had a right-‐sided primary tumor in the FOLFOXIRI plus bevacizumab group than in the FOLFIRI plus bevacizumab group (34.9% vs 23.8%). Altogether, the 89.8% of the study population had an ECOG performance status of 0, the 79.5% presented with synchronous metastases, the 32.7% had the primary tumor in site and the 12.6% had previously received an adjuvant treatment. The 72.6% of enrolled patients had multiple sites of metastases and the 20.7% had a liver-‐limited disease.
The median number of cycles administered per patient as induction treatment was 12 (range 1-‐25) in the control group and 11 (range 1-‐21) in the FOLFOXIRI plus bevacizumab group. Only 23 patients in the control group and 12 patients in the FOLFOXIRI plus bevacizumab group received more than the 12 planned cycles per investigator's choice, resulting in a protocol violation. In the FOLFOXIRI plus bevacizumab group a higher number of cycles was delayed (16.4% vs 6.1%, P<0.001) or administered with reduced dose (21.4% vs 8.2%, P<0.001). Dose reductions were not permitted for bevacizumab. In the control group the average relative dose intensities of fluorouracil and irinotecan were 83% and 84%, respectively. In the FOLFOXIRI plus bevacizumab arm the average relative dose intensities of fluorouracil, irinotecan and oxaliplatin were 73%, 74% and 75%, respectively. More patients in the control group discontinued treatment because of disease progression (20.1% vs 12.8%, P=0.028).
One-‐hundred-‐thirty-‐nine patients in the control group and 142 patients in the FOLFOXIRI plus bevacizumab group were candidate to continue fluorouracil, l-‐leucovorin and bevacizumab as maintenance after the induction phase (Fig. 1). One-‐hundred-‐fourteen
patients in the control group (82%) and 130 patients in the FOLFOXIRI plus bevacizumab group (91.5%) actually received maintenance.
* ECOG denotes Eastern Cooperative Oncology Group; FOLFIRI fluorouracil, L-‐lederfolin, irinotecan; FOLFOXIRI fluorouracil, L-‐lederfolin, oxaliplatin, irinotecan. ** A significantly higher percentage of patients in the FOLFOXIRI plus bevacizumab arm had a right-‐sided primary tumor (p=0.022).
Table 1. Demographic and Baseline Characteristics of Patients in the Intention-‐to-‐Treat
Population*
Characteristic FOLFIRI plus Bevacizumab (N= 256) FOLFOXIRI plus Bevacizumab (N= 252)
Age -‐ yr
Median 60.0 60.5
Range 29-‐75 29-‐75
Sex – no. (%) Male 156 (60.9) 150 (59.5)
Female 100 (39.1) 102 (40.5)
ECOG Performance Status – no. (%) 0 229 (89.5) 227 (90.1)
1-‐2 27 (10.5) 25 (9.9)
Primary tumor site – no. (%) Right colon 61 (23.8) 88 (34.9)**
Left colon or rectum 179 (70.0) 152 (60.3)
Missing data 16 (6.2) 12 (4.8)
Prior adjuvant – no. (%) Yes 32 (12.5) 32 (12.7)
No 224 (87.5) 220 (87.3)
Time to metastases – no. (%) Synchronous 207 (80.9) 197 (78.2)
Metachronous 49 (19.1) 55 (21.8)
Liver-‐only disease – no. (%) Yes 46 (18.0) 59 (23.4)
No 210 (82.0) 193 (76.6)
Surgery on primary – no. (%) Yes 167 (65.2) 175 (69.4)
No 89 (34.8) 77 (30.6)
Kohne Score – no. (%) High 29 (11.3) 18 (7.1)
Intermediate 113 (44.2) 111 (44.0)
Low 105 (41.0) 108 (42.9)
FIGURE 1. CONSORT DIAGRAM
3.1.2. SAFETY
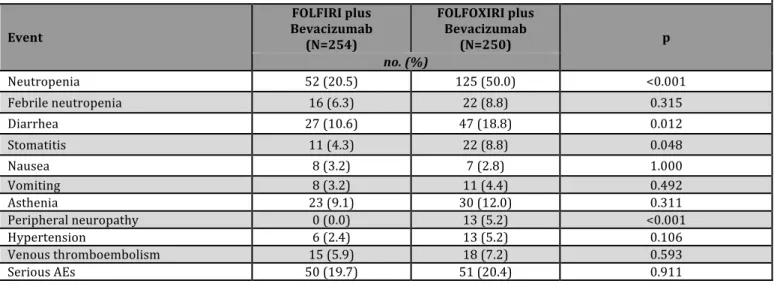
Treatment-‐related grade 3 or 4 adverse events occurring in at least 3% of patients are summarized in Table 2. Incidences of grade 3 or 4 neutropenia, diarrhea, stomatitis and neurotoxicity were significantly higher in the FOLFOXIRI plus bevacizumab than in the FOLFIRI plus bevacizumab group. No differences in bevacizumab-‐related adverse events were observed between groups. The incidence of serious adverse events was similar in the two groups (19.7% in the control arm and 20.4% in the experimental arm, p=0.912).
In the safety population, most of the deaths were attributed to disease progression: 142 of the deaths in the control group (55.5%) and 121 of the deaths in the FOLFOXIRI plus bevacizumab group (48.0%). A similar number of patients died as a result of adverse events (4 [1.6%] in the FOLFIRI plus bevacizumab group and 6 [2.4%] in the FOLFOXIRI plus bevacizumab group).
** Events listed are those that occurred in at least the 3% of patients in either treatment group
Table 2. Most Common Grade 3 or 4 Adverse Events Occurring in At Least 3% of Patients in the
Safety Population** Event FOLFIRI plus Bevacizumab (N=254) FOLFOXIRI plus Bevacizumab (N=250) p no. (%) Neutropenia 52 (20.5) 125 (50.0) <0.001 Febrile neutropenia 16 (6.3) 22 (8.8) 0.315 Diarrhea 27 (10.6) 47 (18.8) 0.012 Stomatitis 11 (4.3) 22 (8.8) 0.048 Nausea 8 (3.2) 7 (2.8) 1.000 Vomiting 8 (3.2) 11 (4.4) 0.492 Asthenia 23 (9.1) 30 (12.0) 0.311 Peripheral neuropathy 0 (0.0) 13 (5.2) <0.001 Hypertension 6 (2.4) 13 (5.2) 0.106 Venous thromboembolism 15 (5.9) 18 (7.2) 0.593 Serious AEs 50 (19.7) 51 (20.4) 0.911
3.1.3.PRIMARY ENDPOINT: PROGRESSION FREE SURVIVAL
Median follow-‐up duration was 32.2 months (range 24.7-‐40.6). The progression-‐free survival analysis was based on 439 events among the 508 patients (86.4%). More events occurred in the control group than in the FOLFOXIRI plus bevacizumab group (226 [88.3%] vs. 213 [84.5%]). The median progression-‐free survival times were 12.1 months with FOLFOXIRI plus bevacizumab and 9.7 months with FOLFIRI plus bevacizumab. FOLFOXIRI plus bevacizumab reduced the risk of progression by 25% as compared with FOLFIRI plus bevacizumab (hazard ratio, 0.75: 95% confidence interval [CI], 0.62 to 0.90; P=0.003) (Fig. 2).
FIGURE 2. KAPLAN–MEIER ESTIMATES OF PROGRESSION-‐FREE SURVIVAL ACCORDING TO TREATMENT GROUP.
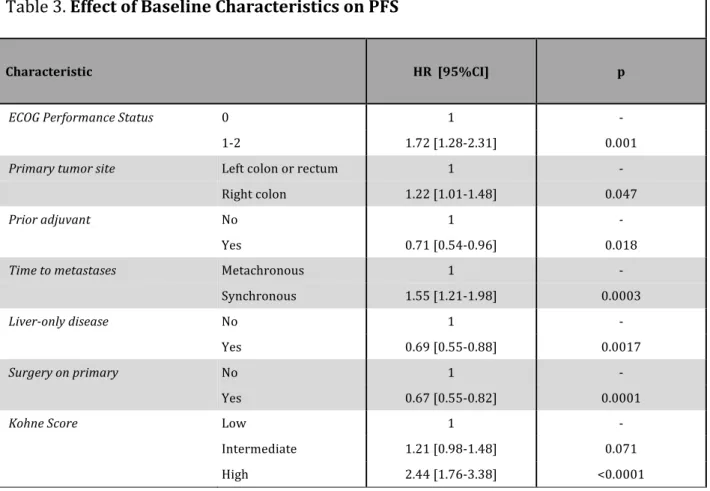
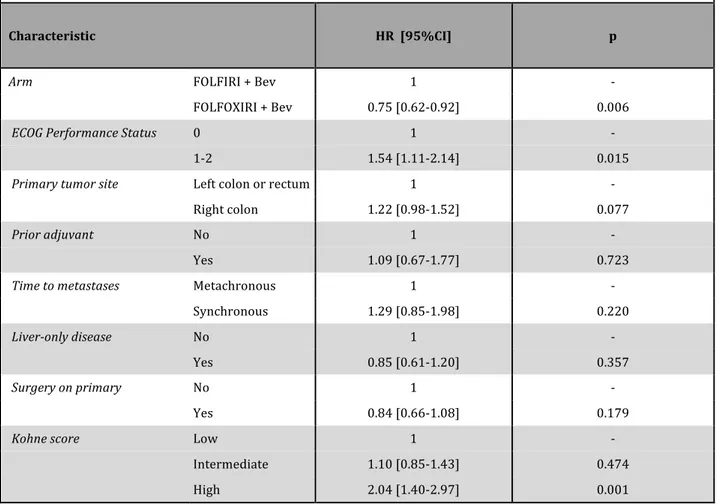
ECOG performance status of 1 or 2, right-‐sided primary tumor, synchronous metastases, disease not confined to the liver, unresected primary and high Kohne score were identified as adverse prognostic factors for progression-‐free and overall survival at the univariate model (Table 3). At an exploratory analysis adjusting for these variables, the hazard ratio for progression with FOLFOXIRI plus bevacizumab was 0.75 (95% CI, 0.62 to 0.92; P=0.006) (Table 4).
Table 3. Effect of Baseline Characteristics on PFS
Characteristic HR [95%CI] p
ECOG Performance Status 0 1 -‐
1-‐2 1.72 [1.28-‐2.31] 0.001
Primary tumor site Left colon or rectum 1 -‐
Right colon 1.22 [1.01-‐1.48] 0.047
Prior adjuvant No 1 -‐
Yes 0.71 [0.54-‐0.96] 0.018
Time to metastases Metachronous 1 -‐
Synchronous 1.55 [1.21-‐1.98] 0.0003
Liver-‐only disease No 1 -‐
Yes 0.69 [0.55-‐0.88] 0.0017
Surgery on primary No 1 -‐
Yes 0.67 [0.55-‐0.82] 0.0001
Kohne Score Low 1 -‐
Intermediate 1.21 [0.98-‐1.48] 0.071
Table 4. Multivariable model for PFS
Characteristic HR [95%CI] p
Arm FOLFIRI + Bev 1 -‐
FOLFOXIRI + Bev 0.75 [0.62-‐0.92] 0.006
ECOG Performance Status 0 1 -‐
1-‐2 1.54 [1.11-‐2.14] 0.015
Primary tumor site Left colon or rectum 1 -‐
Right colon 1.22 [0.98-‐1.52] 0.077
Prior adjuvant No 1 -‐
Yes 1.09 [0.67-‐1.77] 0.723
Time to metastases Metachronous 1 -‐
Synchronous 1.29 [0.85-‐1.98] 0.220
Liver-‐only disease No 1 -‐
Yes 0.85 [0.61-‐1.20] 0.357
Surgery on primary No 1 -‐
Yes 0.84 [0.66-‐1.08] 0.179
Kohne score Low 1 -‐
Intermediate 1.10 [0.85-‐1.43] 0.474
The benefit of FOLFOXIRI plus bevacizumab with respect to progression-‐free survival was homogenous in clinical and molecular subgroups, except for patients who had previously received an adjuvant treatment. A significant interaction of the exposure to a prior adjuvant treatment with progression-‐free survival was observed (p=0.039) (Fig. 3).
FIGURE 3. FORREST PLOT OF THE TREATMENT EFFECT ON PROGRESSION-‐FREE SURVIVAL IN CLINICAL SUBGROUP ANALYSES
3.1.4. SECONDARY ENDPOINTS: RESPONSE RATE AND RESECTION RATE
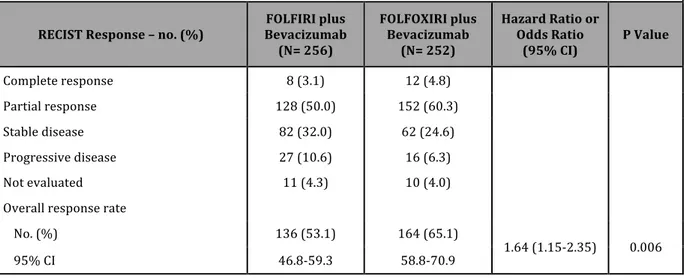
The response rate was 53.1% in the FOLFIRI plus bevacizumab group, as compared with 65.1% in the FOLFOXIRI plus bevacizumab group (odds ratio, 1.64: 95% CI, 1.15 to 2.35; P=0.006) (Table 5). The rate of R0 surgery for metastases was not significantly different in treatment groups (12% in the FOLFIRI plus bevacizumab group vs. 15% in the FOLFOXIRI plus bevacizumab group, P=0.327).
Table 5. Activity in the Intention-‐to-‐Treat Population According to Treatment Arm
RECIST Response – no. (%) Bevacizumab FOLFIRI plus (N= 256)
FOLFOXIRI plus Bevacizumab
(N= 252)
Hazard Ratio or Odds Ratio (95% CI) P Value Complete response 8 (3.1) 12 (4.8) Partial response 128 (50.0) 152 (60.3) Stable disease 82 (32.0) 62 (24.6) Progressive disease 27 (10.6) 16 (6.3) Not evaluated 11 (4.3) 10 (4.0)
Overall response rate
No. (%) 136 (53.1) 164 (65.1)
1.64 (1.15-‐2.35) 0.006
3.1.5. SECONDARY ENDPOINTS: EARLY TUMOR SHRINKAGE AND DEEPNESS OF RESPONSE
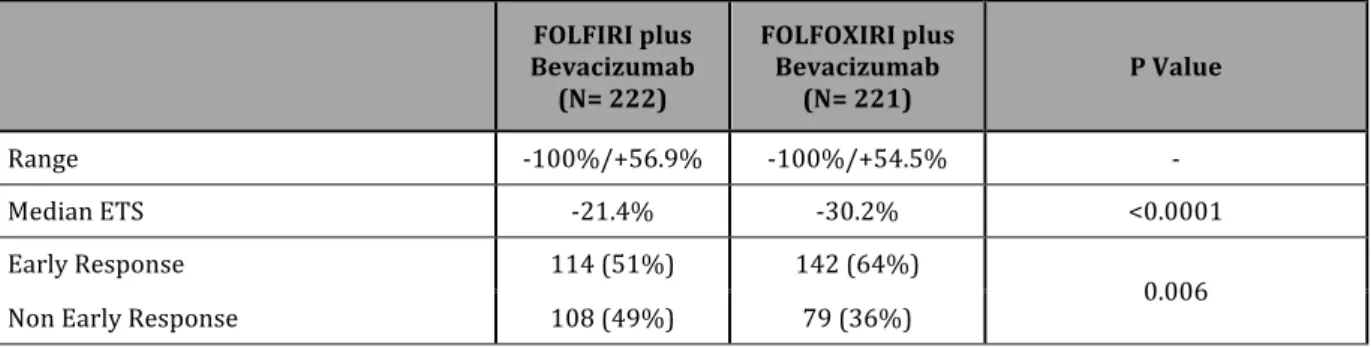
Early tumor shrinkage (ETS) and deepness of response (DoR) were evaluable in 443 (%) and 484 (%) patients, respectively.
142 (64%) out of 221 patients in the FOLFOXIRI plus bev arm achieved early response, compared to 114 (51%) out of 222 patients in the FOLFIRI plus bev arm (p=0.006). A mean ETS of 30.2% was reported in the experimental arm compared to 21.4% in the control arm (p=0.0001). (Table 6)
A mean DoR of 41.1% was reported in the FOLFOXIRI plus bev arm compared to 32.9% in the FOLFIRI plus bev arm (p=0.003). Median time to DoR was 4.3 and 3.9 months in the experimental and control arm, respectively. Adopting the median DoR as cut-‐off value, a significantly higher percentage of patients in the FOLFOXIRI plus bev arm achieved a DoR higher than the median value (58% vs 42%, p=0.0008). (Table 7)
Table 6. Early Tumor Shrinkage according to Treatment Arm
Bevacizumab FOLFIRI plus (N= 222) FOLFOXIRI plus Bevacizumab (N= 221) P Value Range -‐100%/+56.9% -‐100%/+54.5% -‐ Median ETS -‐21.4% -‐30.2% <0.0001 Early Response 114 (51%) 142 (64%) 0.006 Non Early Response 108 (49%) 79 (36%)
Table 7. Deepness of Response according to Treatment Arm
Bevacizumab FOLFIRI plus (N= 245) FOLFOXIRI plus Bevacizumab (N= 239) P Value Range -‐100%/+56.9% -‐100%/+54.5% -‐ Median DoR -‐33.8% -‐42.2% 0.0009 DoR > 38.9% 103(42%) 138 (58%) 0.0008 DoR < 38.9% 142 (58%) 101 (42%)
In the global population, early responders achieved significantly longer PFS (median PFS: 12.7 months vs 10.0 months, HR: 0.66 [0.52-‐0.79], p<0.0001), OS (median OS: 35.8 months vs 22.4 months, HR: 0.54 [0.39-‐0.67], p<0.0001) and Post-‐Progression Survival (median PPS: 17.1 months vs 10.7 months, HR: 0.64 [0.47-‐0.81], p=0.0005) (Figure 4). A significant correlation of ETS as a continuous variable with PFS (HR: 0.983 [0.978-‐0.987], p<0.0001), OS (HR: 0.979 [0.973-‐0.984], p<0.0001) and PPS (HR: 0.987 [0.982-‐0.993], p<0.0001) was also observed.
FIGURE 4. CORRELATION OF EARLY RESPONSE WITH SURVIVAL PARAMETERS
In the global population, a significant correlation of DpR as a continuous variable with PFS (HR: 0.983 [0.980-‐0.987], p<0.0001), OS (HR: 0.979 [0.975-‐0.983], p<0.0001) and PPS (HR: 0.987 [0.984-‐0.991], p<0.0001) was evidenced.
Patients achieving a DpR higher than the median value achieved significantly longer PFS (median PFS: 13.1 months vs 9.3 months, HR: 0.61 [0.49-‐0.73], p<0.0001), OS (median OS: 36.8 months vs 21.3 months, HR: 0.47 [0.35-‐0.58], p<0.0001) and PPS (median PPS: 18.4 months vs 10.5 months, HR: 0.58 [0.44-‐0.73], p<0.0001) (Figure 5).
FIGURE 5. CORRELATION OF DEEPNESS OF RESPONSE WITH SURVIVAL PARAMETERS
3.1.6. SECONDARY ENDPOINTS: OVERALL SURVIVAL
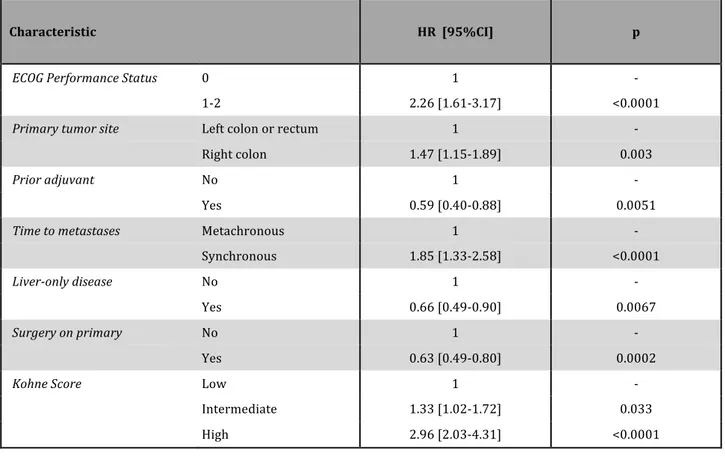
The overall survival analysis was based on 286 deaths among the 508 patients (56.3%). More deaths occurred in the control group than in the FOLFOXIRI plus bevacizumab group (155 [60.5%] vs. 131 [52.0%]). The median overall survival times were 31.0 months with FOLFOXIRI plus bevacizumab and 25.8 months with FOLFIRI plus bevacizumab, which corresponds to a hazard ratio for death of 0.79 (95% CI, 0.63 to 1.00; P=0.054) (Fig.6). At the exploratory analysis adjusting for prognostic variables, the hazard ratio for death with FOLFOXIRI plus bevacizumab, was 0.72 (95% CI, 0.56 to 0.94; P=0.014) (Tables 8 and 9).
FIGURE 6. KAPLAN–MEIER ESTIMATES OF OVERALL SURVIVAL ACCORDING TO TREATMENT GROUP.
Characteristic HR [95%CI] p
ECOG Performance Status 0 1 -‐
1-‐2 2.26 [1.61-‐3.17] <0.0001
Primary tumor site Left colon or rectum 1 -‐
Right colon 1.47 [1.15-‐1.89] 0.003
Prior adjuvant No 1 -‐
Yes 0.59 [0.40-‐0.88] 0.0051
Time to metastases Metachronous 1 -‐
Synchronous 1.85 [1.33-‐2.58] <0.0001
Liver-‐only disease No 1 -‐
Yes 0.66 [0.49-‐0.90] 0.0067
Surgery on primary No 1 -‐
Yes 0.63 [0.49-‐0.80] 0.0002
Kohne Score Low 1 -‐
Intermediate 1.33 [1.02-‐1.72] 0.033
High 2.96 [2.03-‐4.31] <0.0001
Table 9. Multivariable model for OS
Characteristic HR [95%CI] p
Arm FOLFIRI + Bev 1 -‐
FOLFOXIRI + Bev 0.72 [0.56-‐0.94] 0.014
ECOG Performance Status 0 1 -‐
1-‐2 2.16 [1.48-‐3.17] 0.0003
Primary tumor site Left colon or rectum 1 -‐
Right colon 1.47 [1.13-‐1.92] 0.006
Prior adjuvant No 1 -‐
Yes 1.25 [0.66-‐2.35] 0.492
Time to metastases Metachronous 1 -‐
Synchronous 1.63 [0.94-‐2.80] 0.064
Liver-‐only disease No 1 -‐
Yes 1.00 [0.64-‐1.56] 0.996
Surgery on primary No 1 -‐
Yes 0.80 [0.59-‐1.08] 0.147
Kohne score Low 1 -‐
3.1.7. SUBSEQUENT LINES TREATMENTS
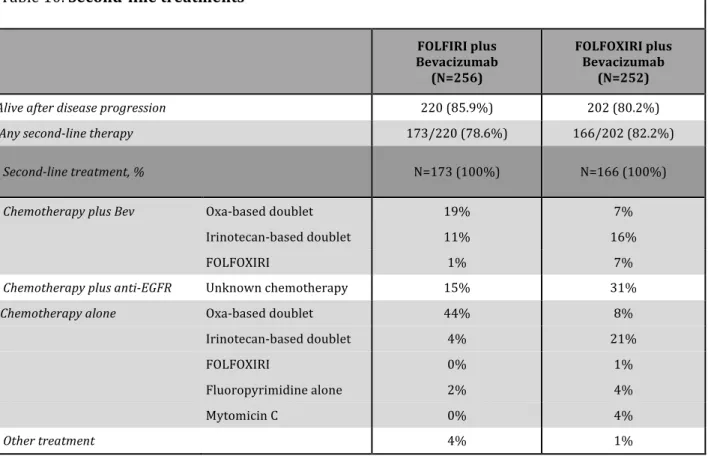
Second-‐line treatment was administered in 173 patients in the control group and in 166 patients in the FOLFOXIRI plus bevacizumab group. A detailed description of second-‐line regimens is provided in Table 10. Among patients receiving a second-‐line treatment a higher percentage of patients in the control group received an oxaliplatin containing regimen (64% vs 23%). In the control group another 14% of patients received oxaliplatin as part of the third or fourth line treatment. Similar percentages of patients continued bevacizumab beyond disease progression (31% in the control group vs 30% in the FOLFOXIRI plus bevacizumab group) and received an anti-‐EGFR monoclonal antibody as second-‐ or third-‐line treatment (29% in the control group and 33% in the FOLFOXIRI plus bevacizumab group).
Table 10. Second-‐line treatments
Bevacizumab FOLFIRI plus
(N=256)
FOLFOXIRI plus Bevacizumab
(N=252)
Alive after disease progression 220 (85.9%) 202 (80.2%)
Any second-‐line therapy 173/220 (78.6%) 166/202 (82.2%)
Second-‐line treatment, % N=173 (100%) N=166 (100%)
Chemotherapy plus Bev Oxa-‐based doublet 19% 7%
Irinotecan-‐based doublet 11% 16%
FOLFOXIRI 1% 7%
Chemotherapy plus anti-‐EGFR Unknown chemotherapy 15% 31%
Chemotherapy alone Oxa-‐based doublet 44% 8%
Irinotecan-‐based doublet 4% 21%
FOLFOXIRI 0% 1%
Fluoropyrimidine alone 2% 4%
Mytomicin C 0% 4%
3.1.8. RAS AND BRAF MUTATIONAL ANALYSES
Tissue samples from 407 (80.1%) out of 508 randomized patients were centrally collected and analyzed. Molecular analyses provided not conclusive results in 32 (7.9%) out of 407 cases. Therefore, 375 patients were included in the RAS & BRAF evaluable population. The ascertainment rate was 73.8%. RAS was found mutated in 218 (58.1%) cases and KRAS was more frequently affected (52.8%) than NRAS (5.3%). BRAF mutations occurred in 28 (7.5%) cases. “All-‐wt” patients, that is patients not bearing RAS or BRAF mutations, were 139 (34.4%).
The prognostic impact of RAS and BRAF mutations was assessed in the overall RAS & BRAF evaluable population, including 185 patients treated with FOLFIRI plus bevacizumab in the control arm and 190 patients treated with FOLFOXIRI plus bevacizumab in the experimental arm. A significant difference among RAS mutated, BRAF mutated and all-‐wt patients was evidenced in terms of PFS (median PFS: 11.0 vs 7.0 vs 12.2 months, log-‐rank test p=0.001). No difference between RAS mutated and all-‐wt patients was observed (HR: 1.15 [0.91-‐1.45], p=0.241) while BRAF mutated patients showed significantly shorter PFS as compared both to all-‐wt (HR: 2.78 [1.61-‐4.80], p<0.001) and RAS mutated patients (HR: 2.22 [1.33-‐3.70], p=0.002) (Figure 7).
FIGURE 7. KAPLAN–MEIER ESTIMATES OF PROGRESSION FREE SURVIVAL ACCORDING TO MOLECULAR SUBGROUPS.
A significant difference among RAS mutated, BRAF mutated and all-‐wt patients was evidenced in terms of OS (median PFS: 26.3 vs 13.4 vs 37.9 months, log-‐rank test p<0.0001). RAS mutated patients reported significantly shorter OS as compared to all-‐wt patients (HR: 1.44 [1.07-‐1.92], p=0.015) while BRAF mutated patients showed significantly shorter OS as compared both to all-‐wt (HR: 5.67 [2.88-‐11.18], p<0.001) and RAS mutated patients (HR: 2.86 [1.59-‐5.14], p<0.001) (Figure 8).
FIGURE 8. KAPLAN–MEIER ESTIMATES OF OVERALL SURVIVAL ACCORDING TO MOLECULAR SUBGROUPS.
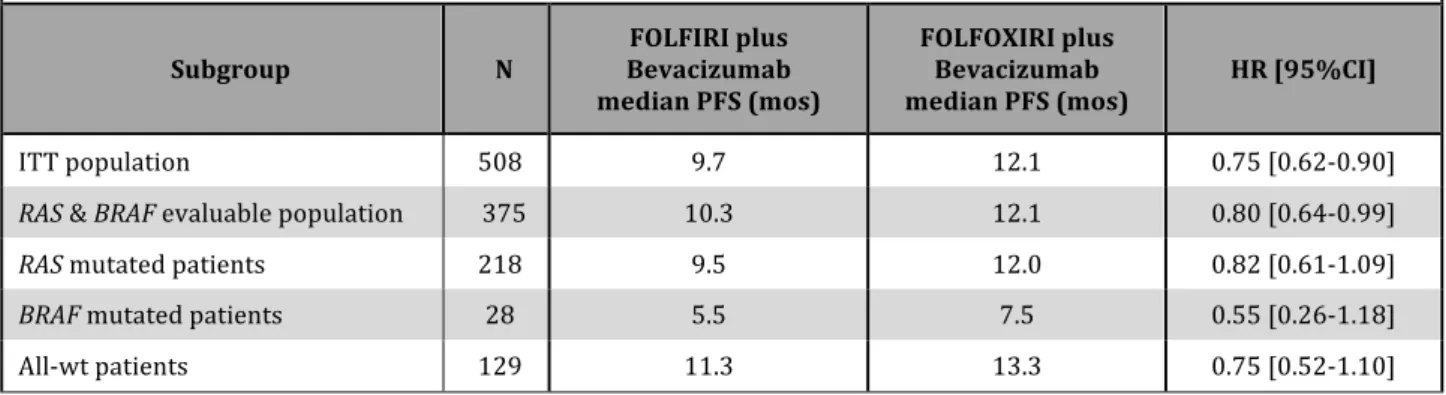
No significant interaction of RAS and BRAF mutational status with treatment effect was evidenced. As shown in Table 11 and 12 the relative benefit from the intensification of the upfront chemotherapy backbone was consistent across molecular subgroups in terms of both PFS and OS. Of note, a clinically relevant HR in favour of the triplet plus bevacizumab was observed in the small subgroup of BRAF mutant patients (HR for PFS: 0.55 [0.26-‐1.18]; HR for OS: 0.55 [0.24-‐1.23]).
Table 11. Subgroup analyses according to RAS and BRAF mutational status -‐ PFS
Subgroup N Bevacizumab FOLFIRI plus median PFS (mos)
FOLFOXIRI plus Bevacizumab
median PFS (mos) HR [95%CI]
ITT population 508 9.7 12.1 0.75 [0.62-‐0.90]
RAS & BRAF evaluable population 375 10.3 12.1 0.80 [0.64-‐0.99]
RAS mutated patients 218 9.5 12.0 0.82 [0.61-‐1.09]
BRAF mutated patients 28 5.5 7.5 0.55 [0.26-‐1.18]
All-‐wt patients 129 11.3 13.3 0.75 [0.52-‐1.10]
Table 12. Subgroup analyses according to RAS and BRAF mutational status -‐ OS
Subgroup N Bevacizumab FOLFIRI plus median PFS (mos)
FOLFOXIRI plus Bevacizumab
median PFS (mos) HR [95%CI]
ITT population 508 25.8 31.0 0.79 [0.63-‐1.00]
RAS & BRAF evaluable population 375 25.8 31.0 0.86 [0.65-‐1.12]
RAS mutated patients 218 23.1 30.8 0.86 [0.60-‐1.22]
BRAF mutated patients 28 10.8 19.1 0.55 [0.24-‐1.23]
3.2. FOLFOXIRI WITH OR WITHOUT BEVACIZUMAB: A POOLED ANALYSIS OF TWO CONSECUTIVE
CLINICAL TRIALS
3.2.1. STUDY POPULATIONS
As compared to the FOLFOXIRI group, in the FOLFOXIRI plus bev group more patients had ECOG PS 0 (p<0.001) and synchronous disease (p=0.031), less patients had received a prior adjuvant chemotherapy (p=0.012), had the primary tumor resected (p<0.001) and a high Kohne score (p<0.001).
3.2.2. SURVIVAL RESULTS
After adjusting for propensity score, the FOLFOXIRI plus bev group a significantly longer PFS (median PFS: 12.1 vs 9.8 months, HR: 0.75 [95%CI: 0.58-‐0.96], p=0.022) was reported, as well as a strong trend toward longer OS (median OS: 31.0 vs 23.4 months, HR: 0.76 [95%CI: 0.57-‐ 1.02], p=0.067).
FIGURE 9. KAPLAN–MEIER ESTIMATES OF PROGRESSION-‐FREE SURVIVAL ACCORDING TO TREATMENT GROUP
FIGURE 10. KAPLAN–MEIER ESTIMATES OF OVERALL SURVIVAL ACCORDING TO TREATMENT GROUP
3.2.2. ACTIVITY RESULTS
No significant differences in terms of RECIST RR (65% vs. 56%; Odds Ratio: 1.19 [95%CI: 0.73-‐1.95], p=0.494), early response rate (cut-‐off: 20%; 63% vs 58%; Odds Ratio: 1.19 [95%CI: 0.69-‐2.07], p=0.532) and deepness of response (42.2% vs 53.8%, p=0.486) were reported.
Table 13. Activity According to Treatment Group
RECIST Response – no. (%) FOLFOXIRI (N= 122) FOLFOXIRI plus Bevacizumab
(N= 252) Odds Ratio (95% CI) P Value
Complete response 10 (8.2) 12 (4.8)
Partial response 58 (47.5) 152 (60.3)
Stable disease 25 (20.5) 62 (24.6)
Progressive disease 10 (8.2) 16 (6.3)
Not evaluated 19 (15.6) 10 (4.0)
Overall response rate
No. (%)
Table 14. Early response and Deepness of Response According to Treatment Group
Early response – no. (%) FOLFOXIRI (N= 90) FOLFOXIRI plus Bevacizumab
(N= 221) Odds Ratio (95% CI) P Value
Early response 52 (57.8) 142 (64.2) 1.23 (0.75-‐2.03) 0.418 Non Early Response 38 (42.2) 79 (35.8) Adj OR: 1.19 (0.69-‐2.07) 0.532
Deepness of Response FOLFOXIRI (N= 98) FOLFOXIRI plus Bevacizumab
(N= 239) Odds Ratio (95% CI) P Value
Median -‐53.8% -‐42.2% 0.520
4. DISCUSSION
Phase III TRIBE study met its primary endpoint of improving first-‐line progression-‐free survival of metastatic colorectal cancer patients by adopting the combination of FOLFOXIRI plus bevacizumab as compared to FOLFIRI plus bevacizumab (hazard ratio for progression, 0.75: 95% CI, 0.62 to 0.90; P=0.003). The median progression-‐free survival was prolonged of 2.4 months, achieving 12.1 months in the experimental arm. Moreover, an absolute increase in response rate of 12% was reported and median overall survival was extended of 5.2 months, from 25.8 to 31.0. These results are clinically meaningful and represent one of the major achievements in the treatment of metastatic colorectal cancer in the last years.
In line with previous experiences,8 the treatment with FOLFOXIRI plus bevacizumab was feasible in a multicentric setting. The intensification of the treatment was associated to a significant increase in the occurrence of grade 3 or 4 neurotoxicity, stomatitis, diarrhea and neutropenia. Notwithstanding, no differences in febrile neutropenia, serious adverse events or toxic deaths were observed. In our opinion, early recognition and active management of adverse events is crucial. The percentage of bevacizumab-‐related adverse events was in line with previous trials5 and no differences between arms were reported, thus showing that chemotherapy intensification does not influence the safety profile of the antiangiogenic. As a limitation, patients’ health-‐related quality-‐of-‐life was not assessed.
In order to exploit the potential benefit of a more intensive treatment without compromising its feasibility, specific selection criteria were adopted. Patients over 75 years old were excluded and for those aged between 70 and 75 ECOG performance status of 0 was required. At the same time, the clinical characteristics of patients enrolled in both arms reflect a rather unselected population. 79.5% of patients presented with synchronous metastases, only 20.7% had liver-‐limited disease and 32.7% had the primary tumor in site. These patients' characteristics should be taken into account when cross-‐comparing reported results with those from other trials. Subgroup analyses did not reveal any interaction between baseline characteristics and treatment effect, with the only exception of the prior exposure to an adjuvant chemotherapy. Indeed, patients previously treated with an adjuvant treatment, containing oxaliplatin in the 64% of cases, seem not to derive benefit from the intensification of the upfront treatment. Therefore, even if the choice of the triplet plus bevacizumab is not contraindicated in these patients, they may not be the optimal candidates to an intensified upfront chemotherapy.
No significant interaction of the extent of the metastatic disease (liver-‐only versus not liver-‐ only) and treatment effect was evidenced. In the present trial most patients had diffuse, extrahepatic disease and those with liver-‐limited metastases were not selected for a conversion approach, thus preventing from drawing conclusions about the impact of the triplet when secondary resectability is pursued. However, differently from TRIBE trial, where initially unresectable mCRC patients were included, independently from the intent of the treatment and the extent of the metastatic spreading, a phase II randomized trial (OLIVIA)17 was designed and conducted to assess the efficacy of the triplet plus bevacizumab, as compared to a doublet plus bevacizumab, in the specific setting of the liver-‐limited disease. In that trial, the triplet was able to significantly improve R0 resection rate, as well as PFS. The lack of a significant difference in terms of resection rate in the TRIBE trial, that was not designed to specifically address this question, does not weaken at all findings from the OLIVIA trial, so that the triplet plus bevacizumab may still represent a valid option in this setting. All the more so, looking at results of TRIBE trial in terms of activity, the triplet plus bevacizumab is able to significantly increase not only the RECIST response rate, but also the percentage of patients achieving an early response (after 8 weeks of treatment) and the deepness of response.
In recent years, the potential relevance of measures of treatments’ activity beyond RECIST has catched more and more attention. In particular, the analysis of phase III CRYSTAL and OPUS trial of first-‐line chemotherapy with or without cetuximab evidenced that the anti-‐EGFR is able to increase both the early response rate and the deepness of response and that both early tumor shrinkage and deepness of response are significantly associated to long-‐term outcome.18 Present results confirm the importance of achieving an early and deep response in order to improve long-‐term survival. A strong correlation of the early response with OS has been recently evidenced in the large ARCAD (Aide et Recherche en Cancérologie Digestive) data-‐set, including individual-‐patient data from 13.949 patients randomized in 15 first-‐line trials. 19 These results support the hypothesis that the advantage in terms of activity of an intensive upfront regimen may translate into a prolongation of survival independently of the opportunity to achieve secondary resections.
A hot issue in metastatic colorectal cancer is the choice of the best upfront regimen for RAS wt patients. Preliminary data on triplet plus cetuximab or panitumumab were promising and randomized studies are ongoing. A recent phase III trial20 randomized patients to receive
rate, primary endpoint, and progression-‐free survival in the RAS wt subgroup, while an improvement in overall survival was reported. In our trial, the treatment effect was independent of RAS and BRAF status. Consistently with previous retrospective and prospective observations,9,10 the subgroup of BRAF mutant patients seems to benefit relatively more from the triplet. This exploratory analysis is affected by the small size of the subgroup (N=28) and the significance for interaction was not reached. A large translational program is currently exploring other potential biomarkers of benefit from the intensive approach. All wild-‐type patients treated with FOLFOXIRI plus bevacizumab achieved impressive PFS (13.3 months) and OS (41.7 months) results.
Independently of treatment arm, RAS or BRAF mutant patients had shorter long-‐term outcome. With regard to the prognostic impact of RAS mutations, the wide adoption of anti-‐ EGFR monoclonal antibodies in later lines (up to 75% in RAS wild-‐type) makes it difficult to clarify their independent prognostic vs predictive impact.
From a clinical perspective, the upfront concomitant use of the three available cytotoxics may raise some issues about possible options in subsequent lines. Thus, it should be noticed that the 12.1 months median progression-‐free survival in the experimental arm was achieved with a 6 months induction phase of FOLFOXIRI plus bevacizumab, followed by a maintenance period with fluorouracil plus bevacizumab. This allows to reintroduce at progression the cytotoxic agents administered upfront, possibly modified according to clinical considerations. Actually, 78% of patients who progressed to the experimental regimen, then received again fluorouracil +/-‐ oxaliplatin +/-‐ irinotecan as part of their 2nd-‐line treatment. Results in terms of overall survival suggest that the use of a 4 drug regimen in first-‐line does not compromise the feasibility and the efficacy of these salvage treatments. The importance of a maintenance phase and the concept of continuum-‐of-‐care for metastatic colorectal cancer patients is supported by recent results and recommended by major guidelines.21-‐23
The TRIBE trial demonstrates that 6-‐months induction FOLFOXIRI plus bevacizumab followed by maintenance improved the activity and the efficacy of first-‐line therapy at the cost of manageable toxicities. Therefore, FOLFOXIRI plus bevacizumab stands as a new option for the upfront treatment of metastatic colorectal cancer patients with similar characteristics to those included in this study.
Since no direct comparison of FOLFOXIRI with or without bevacizumab is available (and such a trial would not be feasible nowadays), the actual impact of the addition of bevacizumab to