ContentslistsavailableatScienceDirect
Applied
Surface
Science
j o ur na l ho me pa g e :w w w . e l s e v i e r . c o m / l o c a t e / a p s u s c
Full
length
article
In
situ
reduction
of
antibacterial
silver
ions
to
metallic
silver
nanoparticles
on
bioactive
glasses
functionalized
with
polyphenols
S.
Ferraris
a,∗,
M.
Miola
a,b,
A.
Cochis
b,
B.
Azzimonti
b,
L.
Rimondini
b,1,
E.
Prenesti
c,1,
E.
Vernè
a,1aDepartmentofAppliedScienceandTechnology,PolitecnicodiTorino,C.soDucadegliAbruzzi24,10129,Torino,Italy
bDepartmentofHealthSciences,UniversitàdelPiemonteOrientaleUPO,ViaSolaroli17,28100,Novara,Italy
cDepartmentofChemistry,UniversitàdegliStudidiTorino,ViaPietroGiuria7,Torino,10125,Italy
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received28June2016
Receivedinrevisedform24October2016
Accepted27October2016
Availableonline3November2016
Keywords: Bioactiveglasses Polyphenols Silvernanoparticles Insitureduction Antibacterialactivity
a
b
s
t
r
a
c
t
Therealizationofsurfaceswithantibacterialpropertiesduetosilvernanoparticlesloadedthrougha greenapproachisapromisingresearchchallengeofthebiomaterialfield.
Inthisresearchwork,twobioactiveglasseshavebeendoublysurfacefunctionalizedwith polyphe-nols(gallicacidornaturalpolyphenolsextractedfromredgrapeskinsandgreentealeaves)andsilver nanoparticlesdepositedbyinsitureductionfromasilvernitrateaqueoussolution.Thepresenceof biomolecules–showingreducingabilitytodirectlyobtaininsitumetallicsilver–andsilver nanopar-ticleswasinvestigatedbymeansofUV–visspectroscopy,X-RayPhotoelectronSpectroscopy(XPS)and FieldEmissionScanningElectronMicroscopy(FESEM).Theantibacterialactivityofthemodifiedsurfaces wastestedagainstamultidrugresistantStaphylococcusaureusbacterialstrain.
©2016ElsevierB.V.Allrightsreserved.
1. Introduction
Silverisknownfromancienttimesforitsbroadspectrumof
antibacterialactivityandiswidelyinvestigatedasmulti-purpose
antibacterialagent.Theraisingdiffusionofbacterialresistanceto
commonantibiotics,recentlydefinedasglobalthreat[1],increases
theinterestinalternativeantibacterialsubstancesandinparticular
inorganicones,suchassilver.Thedevelopmentof
nanotechnolo-giesfocuses the attention onsilver nanoparticles as promising
antibacterial agents [2,3] and numerous silver
nanoparticles-loadedproductscomeintothemarketinvariousapplicationfields
(e.g. drugs, personal care/cosmetics, textiles/shoes, electronics,
householdproducts, filtration/sanitization, medical devices)[4].
Despitethewidediffusionofsilvernanoparticles,itspotential
tox-icityforhealthandenvironmentisnotcompletelyknownupto
now[4].Theantibacterialmechanismofsilvernanoparticlesisnot
yetfullyunderstood,howeveramultipleactionwasproposed:i)
interactionwiththebacterialcellwallanditsconsequentdamage,
ii)inductionofoxidativestressbyReactiveOxygenSpecies(ROS)
productionandiii)sustainedreleaseofsilverions[5,6].Thanksto
∗ Correspondingauthor.
E-mailaddress:[email protected](S.Ferraris).
1 Co-sharedauthorship.
thehighsurfacetovolumeratioandtheprobablemultiplemodeof
action,silvernanoparticlespresentsuperiorantibacterialactivity
comparedtobulkmetallicsilver.
Silvernanoparticlescanbeproducedbytopdownapproaches,
whichforeseethedimensionalreductionoflargerstructures(e.g.
mechanical, ball milling, chemical etching, thermal/laser
abla-tion,sputtering)orbottomupapproaches,basedonaggregation
processes(suchaschemical/electrochemicalprecipitation,vapor
deposition,atomic/molecularcondensation,sol-gel,spray
pyroly-sis,laserpyrolysisoraerosolpyrolysis)[7,8].Themaindrawbacksof
commonsynthesisroutesforsilvernanoparticlesarethe
employ-ment of toxic chemicals and high temperature, pressure and
energy,sothatnewgreenandenvironmentallyfriendlystrategies
areneeded.Variousgreensynthesisapproacheshavebeen
investi-gatedsuchasthoseusingmicroorganisms(bacteria,fungi,yeasts)
ratherthanalgaeorpolysaccharidesand plantsextracts[8–11].
Amongthem,theuseofnaturalsubstancessuchasplantextracts
seemsmorepromisingfortheindustrialapplicationbecauseitis
easilyscaledupanddoesnotrequirecomplexsystemsfor
microor-ganisms’ cultureand related biohazardconcerns [8]. Moreover,
reducing agents canbeobtainedfromvegetal productscoming
fromthewastesoffoodandwineproductionchains,openingthe
opportunityforasustainableuseofresourcesobtainedfrom
vege-talresiduesthatwouldbecomewastes.
http://dx.doi.org/10.1016/j.apsusc.2016.10.177
Table1
Glass composition (molar percentages), melting temperatures and annealing
conditions.
Glasscomposition(mol%) Tmelt[◦C] Annealing SiO2 Na2O CaO Al2O3
SCNA 57.0 6.0 34.0 3.0 1550 10h@600◦C
SCN1 57.0 9.0 34.0 0.0 1500 12h@550◦C
Numerousexperimentalstrategieshavebeenreportedinthe
recentscientificliteratureforthegreensynthesisofcolloidal
sil-vernanoparticles[8–21]althoughfewpapersconsiderspecifically
insitureductionofsilvernanoparticlesonsubstrates[22–24].
In the present paper, for the first time, silver nanoparticles
(Ag-NPs)have been obtainedby in situ reduction onbioactive
glassesfunctionalized withgallic acid(usedas a simple model
moleculeto represent thepolyphenols reactivity) or with
nat-ural polyphenols extracted from red grape skin and green tea
leaves.Theeffectofglasssurfacereactivityontheabilitytograft
biomolecules and, subsequently, to induce in situ reduction of
silverionswasinvestigatedbymeansofUV–visphotometry,
X-RayPhotoelectronSpectroscopy(XPS)andFieldEmissionScanning
ElectronMicroscopy(FESEM).TheantibacterialactivityofAg-NPs
dopedbioactiveglasseswasevaluatedagainstamultidrug
resis-tantstrainofStaphylococcusaureusbymeansofbioactivecoating
derivedmetabolicreductionevaluation.
Thisresearchworkpresentsapromisingstrategytoobtain
inno-vativesmartpolyfunctionalbiomaterialscombiningthepeculiar
featuresofi)bioactiveglasses(bioactivity,ionreleaseability),ii)
polyphenols(antioxidant,antibacterial,vascularprotective,bone
stimulatingactivities)andiii)silver(antibacterial).Inthisroute,
Ag-NPsresultsembeddedontheglasssurfacereducingrisk
con-cernsrelatedtothefreemetallicnanoparticles.
2. Materialsandmethods
2.1. Glasssynthesis
Twobioactive glasses,designed in theauthors’laboratories,
wereconsidered:SCNAandSCN1.Themolarcompositionand
melt-ing/annealingconditionsarereportedinTable1.Thereactivityof
bioactiveglassesstronglydependsontheircomposition.In
par-ticular,itwasevidencedthatthesilicacontent(formeltderived
bioactiveglasses)shouldnotexceed60%(mol),inordertoobtain
abioactivebehavior[25]andthattheadditionofaluminainthe
glasscompositionreducetheglassbioactivitybecauseitinhibits
theionexchangeimprovingthematerialstability[25,26].
More-oversodiumisinvolvedintheionexchangeprocess,whichisthe
firststepinthebioactivitymechanism[25–27].Thetwobioactive
glassesconsideredinthisresearchworkpresentthesamesilica
contentanddiffersforthepresence/absenceofaluminaandforthe
sodiumcontent.SCNAisahighlystableglassduetothepresence
ofaluminaamongitsconstituentoxides.SCN1presentsalarger
reactivitybecauseofahigherNacontentandtheabsenceof
alu-mina.Theseglasseswerechosenfortheirsimplecompositionsand
controllablereactivityinordertoinvestigatetheirabilitytograft
polyphenolsandinduceinsitureductionofsilvernanoparticles.
2.2. Surfacefunctionalizationwithpolyphenols
Gallicacid(GA),usedassimplemodelmolecule,orpolyphenols
extractedfromredgrapeskin(GPH)andgreentealeaves(TPH)
wereconsideredfortheorganicsurfacefunctionalization.
GallicacidwaspurchasedfromSigmaAldrich(GA97.5–102.5%
titration,G7384,SigmaAldrich,Milan,Italy)whilenatural
polyphe-Table2
Samplesnames,treatmentsandsurfacefeatures.
Samplename Treatment Surfacefeature
SCNA-wash Acetoneandwater
washing
OHgroups
SCNA+GA Acetoneandwater
washing+GAgrafting
GAmolecules
SCNA+GPH Acetoneandwater
washing+GPHgrafting
GPHmolecules
SCNA+TPH Acetoneandwater
washing+TPHgrafting
TPHmolecules
SCN1-wash Acetoneandwater
washing
OHgroups
SCN1+GA Acetoneandwater
washing+GAgrafting
GAmolecules
SCN1+GPH Acetoneandwater
washing+GPHgrafting
GPHmolecules
SCN1+TPH Acetoneandwater
washing+TPHgrafting
TPHmolecules SCNA-wash+Ag Acetoneandwater
washing+insitu reductionAgnps
OHgroups/AgNPs
SCNA+GA+Ag Acetoneandwater
wash-ing+GAgrafting+in situreductionAgNPs
GAmolecules/AgNPs
SCNA+GPH+Ag Acetoneandwater
wash-ing+GPHgrafting+in situreductionAgNPs
GPHmolecules/AgNPs
SCNA+TPH+Ag Acetoneandwater
wash-ing+TPHgrafting+in situreductionAgNPs
TPHmolecules/AgNPs
SCN1-wash+Ag Acetoneandwater washing+insitu reductionAgnps
OHgroups/Agnps
SCN1+GA+Ag Acetoneandwater
wash-ing+GAgrafting+in situreductionAgNPs
GAmolecules/AgNPs
SCN1+GPH+Ag Acetoneandwater
wash-ing+GPHgrafting+in situreductionAgNPs
GPHmolecules/AgNPs
SCN1+TPH+Ag Acetoneandwater
wash-ing+TPHgrafting+in situreductionAgNPs
TPHmolecules/AgNPs
nolswereextractedbyconventionalsolventextractionmethod,as
previouslydescribedbytheauthorsin[28,29].
Theabovecitedbiomoleculesweredirectlygraftedonthe
sur-faceof SCNA and SCN1 afterhydroxylsexposition ontheglass
surface.ReactiveOHgroupswereexposedbymeansofacetoneand
waterwashingsinultrasonicbath,asdescribedin[28–32].Washed
sampleswerenamedSCNA-washandSCN1-wash(Table2).The
graftingofbiomoleculeswasperformedbysoakingwashedglasses
inasolutionofpolyphenols(1mg/mlforGAandTPHand5mg/ml
forGPH)for3hat37◦C[29,33].Attheendofthesoakingperiod
samplesweregentlywashedtwotimesinultrapurewaterandlet
dryunderalaminarflowcabinet(FASTERCYTOSAFE)indark
condi-tions.FunctionalizedsampleswerenamedSCNA+GA,SCNA+GPH,
SCNA+TPH,SCN1+GA,SCN1+GPHandSCN1+TPH(Table2).
2.3. Insitureductionofsilvernanoparticles
Functionalized biomaterials with the mentioned organic
molecules were soaked1h at 37◦C in a 0.005MAgNO3
aque-ous solution in order to obtain the in situ reduction of silver
nanoparticles(AgNPs)ontheglasssurface,exploitingthereducing
actionofpreviouslygraftedpolyphenols.Attheendofthe
dryunderalaminarflowcabinetindarkconditions.Polyphenols
graftedandsilvermodifiedsampleswerenamedSCNA+GA+Ag,
SCNA+GPH+Ag,SCNA+TPH+Ag,SCN1+GA+Ag,SCN1+GPH+Ag
and SCN1+TPH+Ag(Table2).Washedsamples weresubjected
tothesametreatmentforcomparisonpurposesandwerenamed
SCNA-wash+AgandSCN1-wash+Ag(Table2).
2.4. Physico-chemicalcharacterization
PhotometricmeasurementsintheUV–visspectralregion(CARY
500Varianspectrophotometer)wereperformedtoquantifythe
amountofactivepolyphenols ontheglass surfacebymeansof
theFolin&Ciocalteumethod[34],aspreviouslydescribedbythe
authors[29,33].Astandardcalibrationcurvewasobtainedwith
GAsolutionsofknownconcentrationandusedforGA
quantifica-tiononthesamples[30,28,29,33].Asfarasnaturalpolyphenols
(acomplexblendofvariousmono-andpolyciclictypeof
phenol-basedmolecules)areconcerned,theirconcentrationwascalculated
ingallicacidequivalentsunits,employingtothesamecalibration
curve[28,29].
Surfacechemicalcompositionandchemicalstateofelements
wereanalyzedbymeansofX-rayPhotoelectronSpectroscopy(XPS,
PHI5000VERSAPROBE,PHYSICALELECTRONICS)inorderto
deter-mine the presence of biomolecules after functionalization and
silvernanoparticlesafterinsitureduction.
Field Emission Scanning Electron Microscopy (FESEM-EDS
SUPRATM40,Zeissand MerlinGeminiZeiss)wasemployedfor
theinvestigationofsilvernanoparticlesprecipitationonsamples
surface.SamplesweresputtercoatedwithathinCrlayer(<5nm)
beforeanalyses.
2.5. Antibacterialactivityevaluation
SCNA and SCN1 samples functionalized with polyphenols
extractedfromgreentealeavesandthesameafterinsitureduction
ofAgNPswereconsideredfortheantibacterialtests,becausethey
showedthebestresultsintermsoffunctionalizationamongthe
moleculesofnaturalorigin.Justwashedsampleswerealsotested
forcontrolpurposes.
2.5.1. Bacterialstrainsandgrowthconditions
The exponentially-growing biofilm pathogen Staphylococcus
aureus(clinicalisolatefromtheHospitalMaggioreofNovara)strain
wasusedtoevaluatetheantibacterialactivityofsamples.Bacteria
werecultivatedonblood-agarplates(SintakS.r.l.,Corsico,Milan,
Italy) at 37◦C in aerobicconditions for 48huntil roundsingle
colonieswereobtained.Plateswerethenstoredat4◦Cuntiluse.
2.5.2. Biofilmformation
Specimenswereplacedintothewellsofa12multiwellplate
(NuncDelta, Nunclone). Then,500mLof freshbacterialculture
were prepared by inoculating about 4–5 single colonies into
LuriaBertanibroth(LB,Sigma-Aldrich,Milan,Italy);cultureswere
incubatedat 37◦C in a Gallenkamp orbitalshaker incubator at
200rpm for 16h. Exponentially-growing bacterial suspensions
werethen diluted in freshLB medium ata final concentration
of 1×107cellsmL−1 according toMcFarland standard 1.0 [35].
1mLofthebrothculturewascollectedandusedtocontaminate
specimens;platewasincubatedat37◦Cinrotation(90rpm)for
90min(adhesionphase).Thesupernatant containingplanktonic
cellswasthenremoved,whilebiofilmformercells,attachedtothe
specimens’surfaces,wererinsedwith1mLof freshLBmedium
(separationphase)[36,37].Platewasincubated24hat37◦Cina
humidatmospheretoallowmaturebiofilmgrowth.
Table3
Polyphenolsamountonthesurfaceofglasssamples.
Glass Polyphenolcontent–GA-equivalents[mg/ml](mean±stdev)
GA GPH TPH
SCNA 0.0000±7.2832·10−8 0.0000±7.071·10−6 0.0000±7.071·10−6 SCN1 0.0004±0.0002 0.0000±0.0005 0.0025±0.0006
2.5.3. Bacterialcellviability
To assess the growth capacity of the bacterial after 24h
of direct contact to specimens’ surface, compared to that
of untreated controls, bacterial viability was evaluated by
the validated quantitative colorimetric metabolic 2,3-bis
(2-methoxy-4-nitro-5-sulphophenyl)-5-[(phenyl amino)
carbonyl]-2H-tetrazoliumhydroxide assay(XTT,Sigma). Briefly, 100Lof
XTTsolution(3mgmL-1inacetonecontaining0.1Mmenadione)
wereaddedtoeachwellandplateswereincubatedat37◦Cfor
5hinthedark;100Lwerethencollectedfromeachwell,
cen-trifugedfor2minat1200rpmtoremoveanydebris,andtheoptical
density(o.d.)wasevaluatedusingaspectrophotometer
(Spectra-Count,IBM,NY,USA)at490nm[38].Experimentswereperformed
intriplicate.
2.6. Statisticalanalysis
Alltestswereperformedintriplicate.TheKruskal–Wallis
anal-ysisfollowedbyConovertestaspost-hocwereusedtodetermine
significancethatwassetatp<0.05
3. Resultsanddiscussion
3.1. Photometricdeterminationofthepolyphenolcontentonthe
glasssurface
The resultsof theFolin&Ciocalteu test onglass samplesare
reportedinTable3.Itwasimpossibletodetectasignificantamount
ofpolyphenols(GA,GPHandTPH)onSCNAsurfacebythis
tech-nique,whileacertainamountofGAandTPHwasregisteredon
SCN1 one. These resultsare in accordance with previous ones
obtainedby the authorsonthe same glass[30,28] and can be
attributedtothelow reactivityof SCNAand themoderate
sur-faceareaofglassslices.Theincreaseinreactivityobtainedwith
SCN1compositionallowsamoreeffectivegraftingofbiomolecules,
exceptforGPH.
3.2. XPSanalyses
Atomic percentages of elements from survey spectra are
reportedinTable4.
NosignificantdifferencesinthesurfaceamountofSiandCacan
behighlightedbetweenSCNAandSCN-1, inaccordancetotheir
theoreticalbulkcomposition.Thelowamountofsodiumrecorded
onSCN-1anditsabsenceonSCNA(comparedtothetheoretical
bulkcomposition)canbeascribedtobothionreleaseinthe
wash-ingsolutionandalsotoasortofhidingduetocarbonpresence
atthesurface.XPSissensitivetotheoutermostsurfacelayer(few
nanometers)andasignificantamountofcarbonhasbeen
regis-teredalsoonwashedsamplesduetohydrocarbonabsorptionfrom
theatmosphere,aswidelydocumentedintheliteraturefor
reac-tivesurfaces[39–42].EDSanalyses(notreported),thatinteresta
higherpenetrationdepth(around1m)detectasodiumamount
closertothetheoreticalone.ThisphenomenonmakesXPSsurvey
analysesnotcompletelysuitableandexhaustiveforthe
Table4
AtomicpercentagesofelementsfromXPSsurveyspectra.
SCNA
Element wash wash+Ag GA GA+Ag GPH GPH+Ag TPH TPH+Ag
O 56.8 55.1 59.6 55.6 57.0 49.9 53.6 54.6 C 21.7 18.0 15.1 15.2 20.6 26.3 22.3 20.5 Si 15.4 20.1 22.9 23.4 21.3 19.3 19.6 19.2 Ca 3.5 0.8 0.3 1.3 0.5 Al 2.6 2.6 1.6 1.2 2.7 N 0.4 Ag 4.2 5.9 4.0 5.2 Cl 0.2 0.1 SCN1 O 53.4 48.7 53.0 49.3 52.6 50.7 50.0 50.9 C 25.3 29.3 27.1 29.5 30.0 27.4 31.2 26.6 Si 17.1 16.8 17.9 16.4 14.3 16.4 17.5 16.4 Ca 2.8 2.1 1.1 0.2 1.9 0.3 1.0 1.1 Na 1.3 1.0 1.0 0.3 0.9 N 1.0 0.9 0.5 0.2 0.1 0.1 Ag 1.1 4.1 5.0 0.2 3.9
spectraoftheoxygenregion(notreported)hasbeenrecordedand
confirmedhydroxylsexpositiononbothglassesafterwashing.
Nosignificanttrendscanberegisteredforcarbonandoxygen
ascharacteristicelementsof graftedbiomolecules.The detailed
analysisofcarbonregionisnecessaryinordertoinvestigatethe
effectivenessof the surfacefunctionalizationprocedure, as
dis-cussedinthefollowing.
Ontheotherhand,asignificantamountofsilvercanbedetected
onAg-treatedsamples,confirmingthesurface abilitytoinduce
thereductionof Ag(I)andthesubsequentuptake. Ahigher
sil-vercontentcanbeobservedonpolyphenolgraftedSCN1,whileno
significantdifferencesbetweenfunctionalizedandwashed
sam-plescanbenotedonSCNAsamples.Thenatureofdepositedsilver
(metallic/ionic)was investigatedby the detailed analysisof Ag
regioninthefollowing.Asmallamountofsilverhasbeendetected
onSCN1+TPH,theamountcanbeconsiderednegligiblebecauseit
fallsintheinstrumentalerror(0.1–0.2%at.)andcanbeattributed
tocontamination.
Thedetailedanalysisofcarbonregionforwashedand
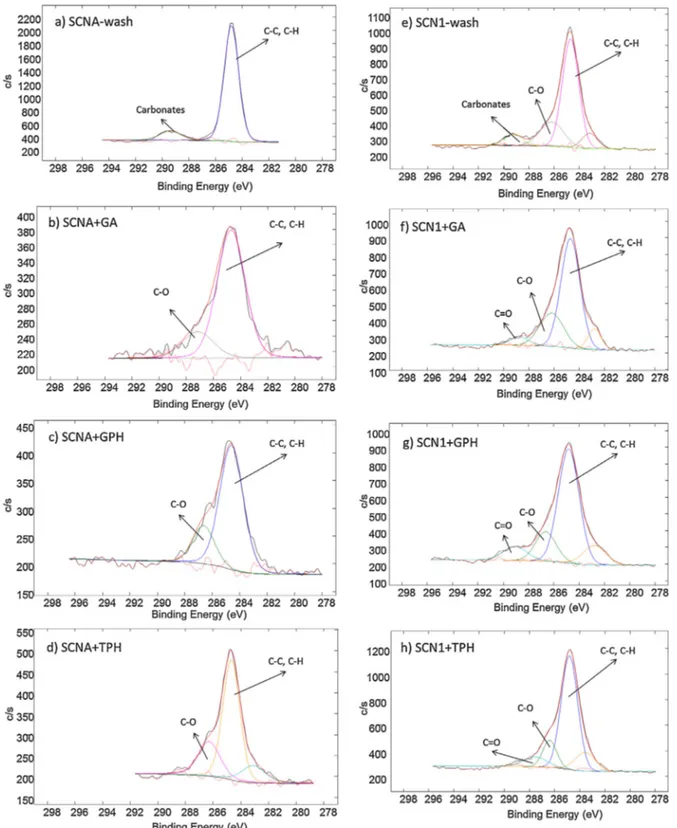
function-alizedsamplesisreportedinFig.1.
On thewashed samples (Fig. 1a and e) two main
contribu-tions,atabout284.8eVand289eV,canbedetectedandattributed
to C C/C H from hydrocarbon contaminants and carbonates
respectively [39–42]. Surface contaminations by atmospheric
hydrocarbonsarealwayspresentontoreactivesurfaces[39–41],
asalreadyobserved,togetherwithcarbonates,onbioactiveglasses
bytheauthors[28–30,43].Asmallsignalatabout287eVcanbealso
detectedonSCN1-wash;C Obondscancorrespondtothisenergy,
butitcanalsocomefromcontaminantsonthissample.
Thesignalofcarbonatesdisappearsaftersurface
functionaliza-tion,aspreviouslyobservedbytheauthors[28–30].Asignificant
signalataround 286eVappearsonall thepolyphenols grafted
glasses(Fig.1b,c, d, f–h) and can beattributed toC Obonds
in polyphenols molecules [44–46]. This signal was previously
observedonpolyphenolsgraftedbioactiveglassesbytheauthors
[28–30].A moderate contributionin the 288eV around canbe
observedonSCN1samplesafterpolyphenolsgrafting. Itcanbe
attributed to the C O bonds [44–46] in carboxylic groups of
polyphenols or in hydroxyl groups of polyphenols oxidized to
quinones, as previously observed by the authors [28–30]. The
appearanceoftheC Osignalonlyonthemostreactiveglasscan
beassociatedwitha higherion release inthefunctionalization
medium withconsequent higher alkalinization and polyphenol
oxidationduringgraftingforthismaterial.
Thesignalat284.8eVpersistsafterthefunctionalizationbut
itsrelativeintensityislowerthanonthewashedsurfaces,
espe-ciallyfor SCNA. Itmust beunderlinedthattheC C/C Hsignal
onthewashedsamplecanbeattributedtosurfacehydrocarbon
contaminantsfromtheatmosphere.Inabsenceofsurface
function-alization,andforastableglassforwhichcarbonatationislow,itis
themostsignificantsignalinthecarbonregion.After
functionaliza-tionthesignalrelatedtothecharacteristicfunctionalgroupsofthe
biomoleculesbecomemorerelevantcomparedtothiscontribution
anditsrelativeintensitydecreases.
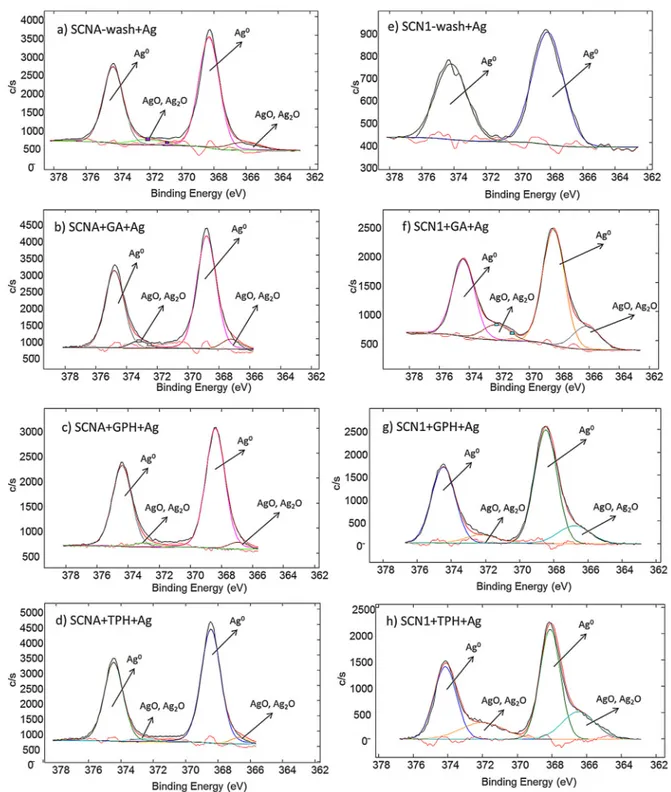
ThedetailedanalysisofthesilverregionforAg-treatedsamples
isreportedinFig.2.Forallthesamples,themaincontributionis
givenbythesignalsatabout368.2eVand374.3eV,attributableto
metallicsilver(Ag0)[47,48].Asecondcontributionatlowerbinding
energies(about367.7eVand373.1eV)canbeobserved,exceptfor
SCN1-wash+Agsample,andattributedtosilveroxides(AgO,Ag2O)
[47,48].ThiscontributionisalmostnegligibleforSCNAsamples,
whileit becomes significantforSCN1+GA+Ag,SCN1+GPH+Ag
andSCN1+TPH+Ag.
Thepresenceofmetallicsilveronthesurfacesconfirmstheir
abilitytoreducesilverionsfromtheAgNO3solutionofthe
treat-ment.Thisreducingabilitycanbeascribedtograftedpolyphenols
forfunctionalizedsurfaces.Inthecase ofwashedglassesa
cer-tainreducingabilitycancomefromthesurfaceexposedhydroxyl
groups.Thisresultisinaccordancewiththeantioxidantabilityof
bioactiveglassespreviouslyobservedbytheauthors[29].
Thepresenceofsilveroxidescanberelatedtotheabsorption
ofsilverionsinthesurfacereactionlayerduringthemodification
process.ThisphenomenonismoreevidentonfunctionalizedSCN1
glassbecauseofahigherreactivityandamorepronouncedreaction
layer,asconfirmedbyFESEMobservations(paragraph3.3).
3.3. FESEMobservations
FESEMimageshavebeenrecordedbothinsecondaryelectrons
andinback-scatteredonesmodesinordertoinvestigatesurface
morphologyanddiscriminatethepresenceofsilvernanoparticles.
InfactAgisheavierthantheglassconstituentsandsilver
nanopar-ticlescanbedetectedasbrightspotsonback-scatteredelectrons
images.
FESEM images of samples (secondary electrons and
back-scatteredones)arereportedinFig.3.
Numerousparticles,withabrightappearanceinback-scattered
images, can be observed on all the samples except of
SCN1-wash+Ag. Particles form aggregates of at about 200nm on
SCNA-wash+Ag while particles with dimensions lower than
100nmandsmallaggregatescanbeobservedonSCNA+GA+Ag,
Fig.1. XPShighresolutionspectraofcarbonregion.
dimensionscanbeevidencedonSCN1+GA+Ag,SCN1+GPH+Ag
andSCN1+TPH+Ag.Ontheotherhandonlyprecipitateswith
big-gerdimensions(fewm)andcaltrop shapecanbedetectedon
SCN1-wash+Ag(Fig.4).
AmoderatereactionlayercanbeobservedonSCNAsamples
afterthevarious modificationprocesses asan irregulartexture
(Fig.3).Ontheotherhandasignificantreactionlayer,moreevident
aftergraftingofbiomolecules,canbeobservedonSCN1samples,
confirmingthehigherreactivityofthisglass(Fig.3).Considering
bothFESEMandXPSresultsitcanbehypothesizedthatsilver
pre-cipitatesonlyasmetallicnanoparticlesonSCNAsubstratesandon
SCN1-washones,whileitisalsoadsorbedinionicforminthe
reac-tionlayeronSCN1+GA+Ag,SCN1+GPH+AgandSCN1+TPH+Ag.
EDSanalysesconfirmthepresenceofsilverinalltheobserved
precipitates.
3.4. Antibacterialactivity
SinceTPHgraftedandTPHgrafted/Agtreatedsamplesshowed
Fig.2. XPShighresolutionspectraofthesilverregion.
ofnaturalorigin,theywerebothconsideredfortheantibacterial
teststogetherwithwashedcontrolsinordertoevaluatetheeffect
ofbothnaturalpolyphenolsandsilvernanoparticlesonbacterial
viability.Themainadvantagesintheuseofnaturalextractsinstead
ofsyntheticchemicalsarethepossibilityofrecoveryfromnatural
sources,witheconomicandenvironmentalbenefits,andreduced
toxicologicalconcerns.
TheresultsofantibacterialtestsarereportedinFig.5.
XTTanalysis(Fig.5a)revealed a statisticalsignificant
differ-encebetween washed control specimens (wash) and
polyphe-nols+silver doped (TPH+Ag) ones for both SCNA (p<0.05,
indicatedby*)andSCN-1(p<0.05,indicatedby#).Nosignificant
differenceswerenoticedbetweencontrolsandpolyphenols(TPH)
graftedspecimens(p>0.05forbothSCNAandSCN-1bioglasses).
Finally,bacteriaviabilityafter24hisreportedinFig.5bin
func-tionofcontrols.
No significant differences have been observed between the
behaviorofthetwoglassesdespiteofthedifferentreactivityand
the differentchemical stateof silver on theirsurfaces (mainly
metalliconSCNAand metallicandioniconfunctionalized
SCN-1).Theantibacterialactionofsilverionsandnanoparticlesiswidely
discussed in the literature and not completely understood yet
[49–51]aswellastheoptimaleffectivetherapeuticdosage[52].As
Fig.4.FESEMimage(secondaryandback-scatteredelectrons)ofSCN1-wash+Ag(5000xmagnification).
Fig.5.S.aureusviabilityonthetestedsamples.XTTassay(a)showedsignificantdifferencesbetweencontrol(washed)andTPH+Agspecimens(p<0.05,indicatedby*and
#forSCNAandSCN-1,respectively).In(b)the24hsbiofilmviabilitynormalizedtowardscontrolsarereported.Barsrepresentmeansandstandarddeviations.
themechanismoftheantibacterialactionshouldbeinvestigated
inmoredetailtogetherwiththesilverreleaseandthe
biocompat-ibility,butthisisoutofthescopeofthepresentpreliminarystudy
andwillbediscussedinafutureresearchpaper.
4. Conclusions
Gallicacidandnaturalpolyphenolsextractedfromredgrape
skinandgreentealeaveshavebeensuccessfullygraftedontwo
bioactiveglasseswithdifferentsurfacereactivity. Theabilityto
graft biomolecules increases with the glass surface reactivity.
Polyphenolicbiomolecules(asgallicacidornaturalonesextracted
byspecificplantsources)graftedonthesurfaceofbioactiveglasses
and,inacertainmeasure,alsothesurfacehydroxylgroupsofthe
glass,makeitpossiblethereductionofthesilverions(comingfrom
asilvernitrateaqueoussolution)tometallicsilvernanoparticleson
theglasssurface.Thespecificnoveltyofthispaperconsistsinthe
onthesurfaceofeach bioactiveglassunderexamination
previ-ouslygraftedwithnaturalorganicreducingmolecules(gallicacid
orblendsofpolyphenolsextractedfromplants)alsoabletoact
asprotective agents (antioxidant,antibacterial, vascular
protec-tive,bonestimulatingactivities).Bioactiveglassesfunctionalized
withteapolyphenolspresentastatisticallysignificant
antibacte-rialactivityagainstStaphylococcus aureusafterinsitu reduction
ofsilvernanoparticles.Finally,thestrategyofpreparationofthe
functionalizedbiomaterialsdevelopedopenstheopportunityfor
asustainableuseofresourcesobtainedfromvegetalresiduesthat
wouldbecomewastes.Thevegetablescrapsofdifferent
machin-ingoperationscanbecomevaluablesourcesofbiomoleculeswhose
activitiesareusefulinvarioussectorsofimpacttothehealthand
totheeconomy.
References
[1]P.Stephens,AntibioticResistanceNow‘globalThreat’,WHOWarns,2014,
http://www.bbc.com/news/health-27204988(Accessed20April2016). [2]M.Rai,A.Yadav,A.Gade,Silvernanoparticlesasanewgenerationof
antimicrobials,Biotechnol.Adv.27(2009)76–83.
[3]X.Chen,H.J.Schluesener,Nanosilver:ananoproductinmedicalapplication, Toxicol.Lett.176(2008)1–12.
[4]S.W.P.Wijnhoven,W.J.G.M.Peijnenburg,C.A.Herberts,W.I.Hagens,A.G. Oomen,E.H.W.Heugens,B.Roszek,J.Bisschops,I.Gosens,D.vandeMeent,S. Dekkers,W.H.DeJong,M.vanZijverden,A.J.A.M.Sips,R.E.Geertsma, Nano-silver–areviewofavailabledataandknowledgegapsinhumanand environmentalriskassessment,Nanotoxicology3(2009)109–138.
[5]J.T.Seil,T.J.Webster,Antimicrobialapplicationsofnanotechnology:methods andliterature,Int.J.Nanomed.7(2012)2767–2781.
[6]M.J.Hajipour,K.M.Fromm,A.A.Ashkarran,D.JimenezdeAberasturi,I.Ruizde Larramendi,T.Rojo,V.Serpooshan,W.J.Parak,M.Mahmoudi,Antibacterial propertiesofnanoparticles,TrendsBiotechnol.30(2012)499–511.
[7]M.Ocwieja,Z.Adamczyk,M.Morga,K.Kubiak,Silverparticlemonolayers– formationstability,applications,Adv.ColloidInterfaceSci.222(2015) 530–563.
[8]S.Ahmed,M.Ahmad,B.L.Swami,S.Ikram,Areviewonplantsextract mediatedsynthesisofsilvernanoparticlesforantimicrobialapplications:a greenexpertise,JARE7(2016)17–28.
[9]P.Rauwel,S.Kuunal,S.Ferdov,E.Rauwel,Areviewonthegreensynthesisof silvernanoparticlesandtheirmorphologiesstudiedviaTEM,Adv.Mater.Sci. Eng.2015(2015),ArticleID682749.
[10]V.K.Sharma,R.A.Yngard,Y.Lin,Silvernanoparticles:greensynthesisand theirantimicrobialactivities,Adv.ColloidInterfaceSci.145(2009) 83–96.
[11]M.Ramya,S.Subapriya,Greensynthesisofsilvernanoparticles,Int.J.Med. Biol.Sci.1(2012)54–61.
[12]G.A.Martinez-Castanon,N.Nino-Martinez,F.Martinez-Gutierrez,J.R. Martinez-Mendoza,F.Ruiz,Synthesisandantibacterialactivityofsilver nanoparticleswithdifferentsizes,J.Nanopart.Res.10(2008)1343–1348.
[13]S.Kheybari,N.Samadi,S.V.Hosseini,A.Fazeli,M.R.Fazeli,Synthesisand antimicrobialeffectsofsilvernanoparticlesproducedbychemicalreduction method,DARU18(2010)168–172.
[14]A.A.El-Kheshen,S.F.G.El-Rab,Effectofreducingandprotectingagentsonsize ofsilvernanoparticlesandtheirantibacterialactivity,DerPharmaChem.4 (2012)53–65.
[15]S.Panigrahi,S.Kundu,S.K.Ghosh,S.Nath,T.Pal,Generalmethodofsynthesis formetalnanoparticles,J.Nanopart.Res.6(2004)411–414.
[16]S.N.Barnaby,S.M.Yu,K.R.Fath,A.Tsiola,O.Khalpari,I.A.Banerjee,Ellagicacid promotedbiomimeticsynthesisofshape-controlledsilvernanochains, Nanotechnology22(2011)225605,10pp.
[17]J.A.Jacob,H.S.Mahal,N.Biswas,T.Mukherjee,S.Kapoor,Roleofphenol derivativesintheformationofsilvernanoparticles,Langmuir24(2008) 528–533.
[18]P.Dauthal,M.Mukhopadhyay,In-vitrofreeradicalscavengingactivityof biosynthesizedgoldandsilvernanoparticlesusingPrunusarmeniaca(apricot) fruitextract,J.Nanopart.Res.15(2013)1366,11pp.
[19]A.Panacek,L.Kvitek,R.Prucek,M.Kolar,R.Vecerova,N.Pizurova,V.K. Sharma,T.Nevecna,R.Zboril,Silvercolloidnanoparticles:synthesis, characterizationandtheirantibacterialactivity,J.Phys.Chem.B110(2006) 16248–16253.
[20]K.M.Kumar,M.Sinha,B.K.Mandal,A.R.Ghosh,K.S.Kumar,P.S.Reddy,Green synthesisofsilvernanoparticlesusingterminaliachebulsextractatroom temperatureandtheirantimicrobialstudies,Spectrochim.ActaPartA91 (2012)228–233.
[21]Q.Sun,X.Cai,J.Li,M.Zheng,Z.Chen,C.-P.Yu,Greensynthesisofsilver nanoparticlesusingtealeafextractandevaluationoftheirstabilityand antibacterialactivity,ColloidsSurf.A:Physiochem.Eng.Aspects444(2014) 226–231.
[22]R.Aladpoosh,M.Montazer,N.Samadi,Insitugreensynthesisofsilver nanoparticlesoncottonfabricusingSidlitziaRosmarinusashes,Cellulose21 (2014)3755–3766.
[23]Z.Liu,J.Yan,Y.-E.Miao,Y.Huang,T.Liu,Catalyticandantibacterialactivitiesof green-synthesizedsilvernanoparticlesonelectrospunpolystirenenanofiber membranesusingteapolyphenols,Compos.PartB79(2015)217–223.
[24]Z.Wang,C.Xu,X.Li,Z.Liu,InsitugreensynthesisofAgnanoparticlesontea polyphenols-modifiedgrapheneandtheircatalyticreductionactivityon 4-nitrophenol,ColloidsSurf.A485(2015)102–110.
[25]W.Cao,L.L.Hench,Bioactivematerials,Ceram.Int.22(1996)493–507.
[26]S.M.Rabiee,N.Nazparvar,M.Azizian,D.Vashaee,L.Tayebi,Effectofion substitutiononpropertiesofbioactiveglasses:areview,Ceram.Int.41(2015) 7241–7251.
[27]H.M.Kim,F.Miyaji,T.Kokubo,C.Ohtsuki,T.Nakamura,Bioactivityof Na2O-CaO-SiO2glasses,J.Am.Ceram.Soc.78(1995)2405–2411.
[28]X.Zhang,S.Ferraris,E.Prenesti,E.Vernè,Surfacefunctionalizationof bioactiveglasseswithnaturalmoleculesofbiologicalsignificance,partII: graftingofpolyphenolsextractedfromgrapeskin,Appl.Surf.Sci.287(2013) 341–348.
[29]M.Cazzola,I.Corazzari,E.Prenesti,E.Bertone,E.Vernè,S.Ferraris,Bioactive glasscouplingwithnaturalpolyphenols:surfacemodification,bioactivityand anti-oxidantability,Appl.Surf.Sci.367(2016)237–248.
[30]X.Zhang,S.Ferraris,E.Prenesti,E.Vernè,Surfacefunctionalizationof bioactiveglasseswithnaturalmoleculesofbiologicalsignificance,partI: gallicacidasmodelmolecule,Appl.Surf.Sci.287(2013)329–340.
[31]E.Vernè,C.Vitale-Brovarone,E.Bui,C.L.Bianchi,A.R.Boccaccini, Surfacefunctionalizationofbioactiveglasses,J.Biomed.Mater.Res.90A (2009)981–992.
[32]E.Vernè,S.Ferraris,C.Vitale-Brovarone,S.Spriano,C.L.Bianchi,A.Naldoni,M. Morra,C.Cassinelli,Alkalinephosphatasegraftingonbioactiveglasses andglass–ceramics,ActaBiomater.6(2010)229–240.
[33]S.Ferraris,X.Zhang,E.Prenesti,I.Corazzari,F.Turci,M.Tomatis,E.Vernè, Gallicacidgraftingtoaferrimagneticbioactiveglass-ceramic,J.Non-Cryst. Solids432(2016)167–175.
[34]V.L.Singleton,J.A.Rossi,Colorimetryoftotalphenolicswithphosphomolybdic –phosphotungsticacidreagents,Am.J.Enol.Vitic.16(1965)144–158.
[35]A.Cochis,L.Fracchia,M.G.Martinotti,L.Rimondini,Biosurfactantsprevent invitroCandidaalbicansbiofilmformationonresinsandsiliconmaterialsfor prostheticdevices,OralSurg.OralMed.OralPathol.OralRadiol.113(2012) 755–761.
[36]A.Cochis,B.Azzimonti,C.DellaValle,R.Chiesa,C.R.Arciola,L.Rimondini, Biofilmformationontitaniumimplantscounteractedbygraftinggalliumand silverions,J.Biomed.Mater.Res.A103(2015)1176–1187.
[37]A.Cochis,B.Azzimonti,C.DellaValle,E.DeGiglio,N.Bloise,L.Visai,S. Cometa,L.Rimondini,R.Chiesa,Theeffectofsilverorgalliumdopedtitanium againstthemultidrugresistantAcinetobacterbaumannii,Biomaterials80 (2016)80–95.
[38]B.Azzimonti,A.Cochis,M.E.Beyrouthy,M.Iriti,F.Uberti,R.Sorrentino,M.M. Landini,L.Rimondini,E.M.Varoni,Essentialoilfromberriesoflebanese JuniperusexcelsaM.Biebdisplayssimilarantibacterialactivityto chlorhexidinebuthighercytocompatibilitywithhumanoralprimarycells, Molecules20(2015)9344–9357.
[39]M.Textor,C.Sittig,V.Frauchiger,S.Tosetti,D.M.Brunette,Propertiesand bio-logicalsignificanceofnaturaloxidefilmsontitaniumanditsalloys,in:M. Tengvall(Ed.),TitaniuminMedicine,Springler-Verlag,Berlin,Heidelberg, NewYork,NY,2001,pp.171–230.
[40]M.Morra,C.Cassinelli,G.Buzzone,A.Carpi,G.DiSanti,R.Giardino,M.Fini, Surfacechemistryeffectsoftopographicmodificationoftitanium
dentalimplantsurfaces:1.Surfaceanalysis,Int.J.OralMaxillofac.Implants18 (2003)40–45.
[41]M.P.Ferraz,F.J.Monteiro,J.D.Santos,CaO–P2O5glasshydroxyapatite doublelayerplasmaspraycoating:invitrobioactivityevaluation,J.Biomed. Mater.Res.45(1999)376–383.
[42]X-RayPhotoelectronSpectroscopyReferencepages,C1sCarbonates:http://
www.xpsfitting.com/2011/03/c-1s-carbonates.html,CopyrightM.C.Biesinger (http://www.xpsfitting.com/)2013(8May2013,11:14a.m.).
[43]E.Vernè,S.Ferraris,C.Cassinelli,A.R.Boccaccini,Surfacefunctionalizationof bioglass®withalkalinephosphatase,Surf.Coat.Technol.264(2015)132–139.
[44]A.V.Naumkin,A.Kraut-Vass,S.W.Gaarenstroom,C.J.Powell,NISTX-ray
photo-electronspectroscopydatabase,in:NISTStandardReferenceDatabase
20,Version4.1,2013,http://srdata.nist.gov/xps/selEnergyType.aspx(8May
2013,11:20a.m.),2012copyrightbytheU.S.SecretaryofCommerceon
behalfoftheUnitedStatesofAmerica.Allrightsreserved.
[45]J.F.Mowlder,W.F.Stickle,P.E.Sobol,K.D.Bomben,HandbookofX-ray photo-electronspectroscopy,in:AReferenceBookofStandardSpectrafor IdentificationandInterpretationofXPSData,PhysicalElectronics,USA,1995.
[46]G.Qiao,J.Su,M.He,Effectof(−)epigallocatechingallateon
electrochemicalbehaviorandsurfacefilmcompositionofCo–Cralloyusedin dentalrestora-tions,Dent.Mater.J.31(2012)564–574.
[47]M.Ferraris,S.Ferraris,M.Miola,S.Perero,C.Balagna,E.Verne,G.Gautier,C. Manfredotti,A.Battiato,E.Vittone,G.Speranza,I.Bogdanovic,Effectof thermaltreatmentsonsputteredsilvernanocluster/silicacompositecoatings onsoda-limeglasses:ionicexchangeandantibacterialactivity,J.Nanopart. Res.14(2012)1287.
[48]S.Ferraris,A.Venturello,M.Miola,A.Cochis,L.Rimondini,S.Spriano, Antibacterialandbioactivenanostructuredtitaniumsurfacesforbone integration,Appl.Surf.Sci.311(2014)279–291.
[49]B.Reidy,A.Haase,A.Luch,K.A.Dawson,I.Lynch,Mechanismsofsilver nanoparticlerelease,transformationandtoxicity:acriticalreviewofcurrent knowledgeandrecommendationsforfuturestudiesandapplications, Materials6(2013)2295–2350.
[50]G.A.Sotiriou,S.E.Pratsinis,Antibacterialactivityofnanosilverionsand particles,Environ.Sci.Technol.44(2010)5649–5654.
[51]S.Ferraris,S.Spriano,Antibacterialtitaniumsurfacesformedicalimplants, Mat.Sci.Eng.C61(2016)965–978.
[52]J.L.GravesJr.,M.Tajkarimi,Q.Cunningham,A.Campbell,H.Nonga,S.H. Harrison,J.E.Barrick,Rapidevolutionofsilvernanoparticleresistancein Escherichiacoli,Front.Genet.6(2015)42.