ContentslistsavailableatScienceDirect
Annals
of
Anatomy
j o ur na l h o me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / a a n a t
Probiotic
supplementation
affects
the
glycan
composition
of
mucins
secreted
by
Brunner’s
glands
of
the
pig
duodenum
夽
Gianluca
Accogli
a,
Alberto
Maria
Crovace
b,
Maria
Mastrodonato
c,
Giacomo
Rossi
d,
Edda
G.
Francioso
a,
Salvatore
Desantis
a,∗aSectionofVeterinaryClinicsandAnimalProductions,DepartmentofEmergencyandOrganTransplantation(DETO),UniversityofBariAldoMoro,S.P.
CasamassimaKm3,70010Valenzano,Bari,Italy
bDottoratodiRicercainSanitàeScienzeSperimentaliVeterinarie,UniversityofPerugia,Perugia,Italy cDepartmentofBiology,UniversityofBari“AldoMoro”,ViaE.Orabona4,70124Bari,Italy
dSchoolofBiosciencesandVeterinaryMedicine,UniversityofCamerino,ViaCirconvallazione93/95,62024Matelica,MC,Italy
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:Received24October2017
Receivedinrevisedform21March2018 Accepted29March2018 Keywords: Mucins Glycohistochemistry Lectins Probiotics Diet Swine
a
b
s
t
r
a
c
t
Theeffectofadietaryprobioticblendonthecarbohydratecompositionofmucinssecretedbythe Brun-ner’sglandsintheduodenumofgrowing-finishingpigswasinvestigatedbymeansofconventional (periodicacid-Schiff,AlcianBluepH2.5,highirondiaminestaining)andlectin(15lectins) histochem-istry.Pigswereassignedtotwodietarytreatments:acontrolbasaldietwithouttheprobioticblend (No-Pro)andatestdietthatincludedtheprobioticblend(Pro).Duodenaltissuefragmentswerefixedin 4%phosphate-buffered-saline-bufferedparaformaldehyde,dehydratedthroughagradedalcoholseries, andembeddedinparaffinwax.ThesecretorycellsoftheBrunner’sglandsfromNo-Propigsprimarily producedneutralglycoproteinsandasmallamountofacidicnon-sulphatedmucins.Thisglycan pat-ternwasoppositethatoftheBrunner’sglandsfromProanimals.Acomparisonoflectin-bindingprofiles ofthesecretorycellsofBrunner’sglandsinthesetwogroupsshowedthatinPropigs,therewas(i)a decreaseinN-linkedglycanscontaining␣1,2-linkedfucose(ConA,UEAI);(ii)alossofcomplextypesof N-glycans(PHA-L,PHA-E)terminatingwithlactosamine(RCA120),␣1,6-and␣1,3-linkedfucose(LTA),and
␣-galactose(GSAI-B4),aswellasofO-glycanswithterminalGal1,3GalNAc(PNA);and(iii)anincrease
inO-glycanscontainingGalNAcHPA.No-ProandProsamplesshowednochangeintheexpressionof␣2,6 sialoglycansandterminalGlcNAcresiduesandnoaffinityforMALII,DBA,andSBA.Theseresults indi-catethatprobioticsupplementationaffectstheglycancompositionofmucinsproducedintheBrunner’s glandsofgrowing-finishingpigs.Thesechangescouldeffectivelyactonthegastrointestinalfunctionand healthstatusoftheseanimalsbecausetheprobioticblendinducedhighergrowthperformanceandmeat qualityinthetestprobioticgroupthanitdidinthecontrolbasaldietgroup(Tufarellietal.,2017).
©2018ElsevierGmbH.Allrightsreserved.
1. Introduction
Brunner’s glands, or duodenal glands, are specific to
mam-mals and are located in the submucosa of the duodenalwall,
althoughtheycanbefound,evenifdiscontinuously,inthejejunum
ofbigherbivores and pigs (Krause, 2000).Brunner’s glands are
mucin-secretingtubulo-alveolarglands.Mucinsconstitutea
fam-ilyofdenselyglycosylatedproteins,thehighglycosylationgiving
themgel-likepropertiesandtheabilitytoresistproteolysisand
holdwater(Perez-VilarandHill,1999).Thehighlyviscousmucus
夽 ThispaperbelongstothespecialissueAnimalAnatomyIII. ∗ Correspondingauthor.
E-mailaddress:[email protected](S.Desantis).
secretedbyBrunner’sglandsprotectstheunderlyingmucosafrom
mechanicalinsults,neutralizestheacidityofgastricjuice(Florey
andHarding,1933),modulatesabsorptionofingesta,inhibitsattack
bypathogens,andmaintainsbacterialmicroflora(Krause,2000;
FlemstromandIsenberg,2001).
Theimportanceoftheintestinalmicrobiotaforgastrointestinal
functionandhealthhasbeenshowninmanystudies(Heinritzetal.,
2013;BüsingandZeyner,2015;Huetal.,2015;Liuetal.,2017).In
particular,probioticshaveastimulatingeffectonthedigestive
pro-cessesandimmunityofanimals(Fuller,2006;Mengetal.,2010).
Therefore,theiruseissuggestedasanalternativetoantibioticsor
anti-inflammatorydrugs.However,themodeofactionofprobiotics
ispoorlyunderstoodandthereportedmechanismsofactionare
oftentheresultsofinvitroexperiments.Theseresultsshould
there-https://doi.org/10.1016/j.aanat.2018.03.008 0940-9602/©2018ElsevierGmbH.Allrightsreserved.
forebeconfirmedbyinvivostudies(Oelschlaeger, 2010).More
recently,theuseofaprobioticcomplex(SivoyTM,SLAB51)hasbeen
showntoenhancethegrowthperformanceandmeatqualityof
growing-finishingpigsandtoreducepollutionfromanimalexcreta
(Tufarellietal.,2017).
Despitethephysiologicalimportanceofthemucinssecretedby
Brunner’sglands, todate nothingis knownabouttheeffects of
microbiotaonthecompositionofthemucinssecretedbythese
glands. Previous studies have, however, demonstrated that the
compositionofmucinsfromBrunner’sglandscouldbeaffectedby
dietarychange(seeKrause,2000,forreference).
Theaimofthepresentstudywastoexaminetheinsitueffect
of a dietary probiotic complex (SivoyTM, SLAB51) on the
gly-cancompositionofmucinsproducedin theBrunner’s glandsof
growing-finishingpigs.Weusedbothconventionalandlectin
his-tochemistry.The conventional technicalapproach discriminates
neutral and acidic classes of glycoconjugates, whereas lectins
allowanalysisofthecarbohydratecompositionofcomplexglycans
(Spicer and Schulte, 1992; Sharon and Lis, 2004). The
inves-tigation was carried out in pigs. Because of their anatomical,
physiological,andgeneticcomparabilitytohumans,they
repre-sentapromisinganimalmodeltodeterminequestionsofbasic,
applied,andtranslationalbiomedicalresearch(Aigneretal.,2010;
Stramandinoli-Zanicottietal.,2014),includingstudiesofhuman
nutritionandhealthproblems(Guilloteauetal.,2010;Vermaetal.,
2011;Pratheretal.,2013;Gonzalezetal.,2015).
2. Materialsandmethods
2.1. Probioticsources
The probiotic preparation used in the present trial was
obtainedfromacommercialcompany(SLAB51,MendesSA,Lugano,
Switzerland).TheprobioticSLAB51iscomposedofablendofthe
followingstrains:StreptococcusthermophilesDSM32245,amixture
ofthetwostrainsBifidobacteriumanimalisssp.lactisDSM32246and
DSM32247,LactobacillusacidophilusDSM32241,Lactobacillus
hel-veticusDSM32242,LactobacillusparacaseiDSM32243,Lactobacillus
plantarumDSM32244,andLactobacillusbrevisDSM27961.
2.2. Animals
ThetrialreceivedethicalapprovalfromtheItalianMinistryof
Health(n.597/2015-PRdel23/06/2015)andwasconductedinstrict
accordancewiththerecommendationsoftheGuidefortheCare
andUseofLaboratoryAnimalsoftheNationalInstitutesofHealth
(Art.18D.L.4March2014,no.26).
Twentypigs[(Landrace×Yorkshire)×Talent]withanaverage
initialbodyweight(BW)of22.80±0.95kg(SE)wereusedina
12-weekexperiment.Pigswereassignedtotwodietarytreatments:
thecontrolbasaldietwithouttheprobioticblend(No-Pro)andthe
experimentaldietthatincludedtheprobioticblend(Pro).The
pro-bioticmixturewasusedasadietarysupplementforthepigsduring
theentirefeedingperiodatadoseof100mg/kgofBW.Thebasal
dietwasformulatedtomeetorexceedthenutrientrequirements
ofpigsaccordingtotheNRC(1998).Pigswerehousedinan
envi-ronmentallycontrolledroomwithaconcretefloorandwerefedad
libitum.
2.3. Samplingandhistologyprocessing
Attheendofthetrial,pigswereslaughteredandspecimensof
duodenaltissuewereimmediatelyremovedfrom5cmofthecaudal
partofthepyloricregionandfixedin4%(v/v)
phosphate-buffered-saline-buffered paraformaldehydefor 24hat 4◦C. Thesamples
werethendehydratedthroughagradedalcoholseriesand
embed-ded inparaffinwax.Serialsections(4-mthick) werecutand,
afterbeingde-waxedwithxyleneandhydratedinanethanolseries
ofdescendingconcentrations,stainedwithhematoxylin-eosinfor
morphologicalandmorphometricstudiesandbyconventional
his-tochemicalproceduresorlectinhistochemistryforglycoconjugate
characterization.
2.4. Conventionalhistochemistry
Sectionsweretreatedwith(1)periodicacid-Schiff(PAS)
reac-tionforneutralglycoconjugates(McManus,1948);(2)AlcianBlue
pH2.5(AB2.5)forsulphateestersandcarboxylgroupsin
glycocon-jugates(Pearse,1968);and(3)combinedhighirondiamine-Alcian
BluepH2.5(HID-AB2.5)forsimultaneousstainingofsulphated
(brown-black) and non-sulphated (blue) acidic glycans (Spicer,
1965).Torevealcellularcombinationsofbothacidicandneutral
glycoconjugates,weperformedAB2.5/PASandHID/AB2.5staining
sequences.
2.5. Lectinhistochemistry
Thebindingof15lectinswastestedtoinvestigatethe
compo-sitionanddistributionofoligosaccharidicchainsintheBrunner’s
glandsofthepigs(Table1).AlllectinswerepurchasedfromVector
LaboratoriesInc.(Burlingame,CA,USA).
Tissue sectionsstained with fluorescent lectins were rinsed
in0.05MTris–HCl-bufferedsaline(TBS)pH7.4andincubatedin
appropriatedilutionsofeachlectindilutedinTBS(Table1)for1h
atRTinthedark.AfterthreerinsesinTBS,slidesweremountedin
Vectashieldmountingmedium(VectorLaboratories,Burlingame,
CA,USA).Tissuesectionsstainedwithbiotinylated MALIIwere
immersedin3%v/vsolutionofH2O2inmethanolfor10minto
sup-presstheendogenousperoxidaseactivity,rinsedinTBSpH7.4,and
incubatedinalectinsolution(25g/mlfor1hatRTinthedark).
AfterthreerinsesinTBS,thesectionsweretreatedwith
strepta-vidin/peroxidasecomplexfor30minandsubsequentlywith0.05%
(w/v)3,3-diaminobenzidine(VectorLaboratories,Burlingame,CA,
USA)plus 0.003%(v/v)H2O2 in0.05MTBS (pH7.6)for10min.
SectionsweredehydratedandmountedusingEukitt.
Eachexperimentwasrepeatedtwiceforeachsample.Controls
forlectinstainingincluded(1)substitutionofthesubstratemedium
withbufferwithoutlectinand(2)incubationwitheachlectinin
thepresenceofitshaptensugar(0.5MinTBS).Allcontrol
experi-mentsgavenegativereactions.Slideswereobservedwiththelight
photomicroscopeEclipseNi-U(Nikon,Japan)at20×magnification
and photographedwitha digital camera(DS-U3, Nikon,Japan).
Theimageswereanalyzedbytheimage-analyzing programNIS
ElementsBR(Version4.20)(Nikon,Japan).
2.6. Morphometryandstatisticalanalysis
ThediameterofBrunner’sglandadenomeresfrombothNo-Pro
and Prosampleswasmeasuredon15microphotographicfields
casuallydetectedandphotographedwithadigitalcamera(DS-U3,
Nikon,Japan)connectedtothelightphotomicroscopeEclipse
Ni-U(Nikon,Japan),usinga20×lens.Imageswereanalyzedbythe
image-analyzingprogramNISElementsBR(Vers.4.30)(Nikon,JP).
Eachfieldsurfacewas140,000m2.Wemeasuredthediameter
of450transversallycutadenomeresofBrunner’sglandfromthe
No-ProandProsamples.Valueswereexpressedasmeans±SD.The
resultswereevaluatedforstatisticalsignificancebyStudent’sttest
andthecompareddatawereconsideredstatisticallysignificantat
Table1
Lectinsused,theirsugarspecificities,andtheinhibitorysugarsusedincontrolexperiments.
Lectinabbreviation Sourceoflectin(g/ml) Sugarspecificity Inhibitorysugar
MALIIa Maackiaamurensis 25 NeuNAc˛2,3Gal1,3(±NeuNAc␣2,6)GalNAc NeuNAc
SNA Sambucusnigra 15 Neu5Ac˛2,6Gal/GalNAc NeuNAc
PNAb Arachishypogaea 25 Gal1,3GalNAc -D-Gal
RCA120 Ricinuscommunis 20 Gal1,4GlcNAc Gal
GSAI-B4 Griffoniasimplicifolia 20 ␣Gal Gal
DBA Dolichosbiflorus 25 GalNAc␣1,3(LFuc␣1,2)Gal1,3/4GlcNAc1 D-GalNAc
SBAb Glycinemax 20 ␣/GalNAc D-GalNAc
HPA Helixpomatia 20 ␣GalNAc D-GalNAc
ConA Canavaliaensiformis 15 ␣Man>␣Glc Man
PHA-E Phaseolusvulgaris 20 Gall,4GlcNAc1,2Man␣1,6 Man
PHA-L Phaseolusvulgaris 20 GlcNAcl,2Man,triantennarycomplexoligosaccharides Man
succWGAb Triticumvulgaris 15 GlcNAc D-GlcNAc
GSAII Griffoniasimplicifolia 20 D-GlcNAc D-GlcNAc
UEAI Ulexeuropaeus 20 L-Fuc␣1,2Gal1,4GlcNAc ␣-L-Fuc
LTA Lotus
tetragonolobus
25 L-Fuc␣1,6GlcNAc
L-Fuc␣1,2Gal1,4[L-Fuc1,3]GlcNAc1,6R ␣-L-Fuc Fuc:fucose,Gal:galactose,GalNAc:N-acetylgalactosamine,Glc:glucose,GlcNAc:N-acetylglucosamine,Man:mannose,NeuNAc:N-acetylneuraminic(sialic)acid,succ: succinylatedWGA.
aBiotin-labeledlectin.
b Rhodamine-labeledlectin.Non-markedlectinsarefluoresceinisothiocyanate-labeledlectins.
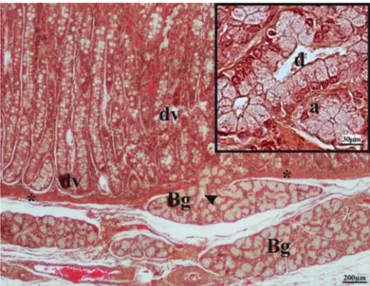
Fig.1. LightmicrographofpigduodenumshowingBrunner’sglands(Bg)and duodenalvilli(dv).Notetheduct(arrowhead)ofaBrunner’sglandpiercingthe muscularismucosae(asterisk)andenteringtheoverlyingduodenalmucosa.Inset displaysthedetailsofanacinousadenomere(a)andduct(d).Hematoxylin-eosin staining.
3. Results
3.1. Morphology
SwineBrunner’sglandsweretubulo-acinouswithamain
excre-toryduct opening at the base of the duodenal crypts (Fig. 1).
MorphologicalanalysisofBrunner’sglandsdidnotreveal
signif-icantdifferencesbetweentheNo-ProandProsamples.Therewas
alsonosignificantdifferenceinadenomerediameter,which
mea-sured42.66±7.38mintheNo-Proand44.3±6.32minthePro
specimens.
3.2. Glycohistochemistry
Theresultsofconventionalandlectinstainingpatternsof
Brun-ner’sglandsofbothNo-ProandPropigsaresummarizedinTable2.
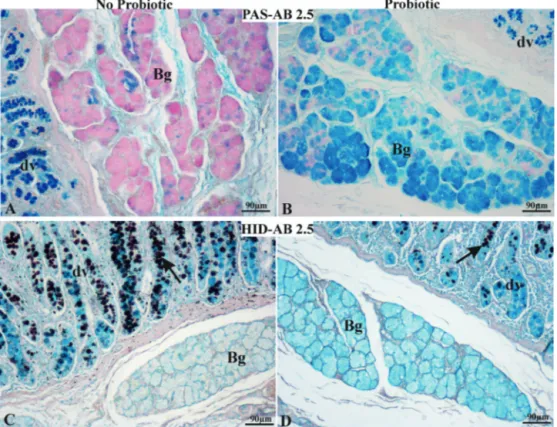
ThecombinationAB2.5/PASprocedurerevealedthewidespread
presenceofPAS-positive(magenta)cellsandafewAB2.5-positive
(blue)cellsintheBrunner’sglandsfromNo-Prosamples(Fig.2A).
Thisstaining pattern was reversed in the Pro group Brunner’s
Table2
ConventionalandlectinhistochemistrystainingpatternoftheduodenalBrunner’s glandsofno-probioticandprobiotic-fedpigs.
No-probiotic Probiotic PAS + * AlcianBluepH2.5 * ++ HID − − SNA +/++lc +/++lc PHA-L +/++lc −/++lc PHA-E ±/++lc −/±lc succinylWGA −/+lc −/+lc GSAII ++ ++ ConA ++ + UEAI + ± GSAI-B4 ++ − LTA + − PNA + − RCA120 ± − HPA + ++ MALII − − DBA − − SBA − −
Lc:luminalcontent,*:fewreactivecells,−:negativereaction,±:faintlyvisible reaction,+:weakpositivereaction,++:intensepositivereaction.
glands(Fig.2B).ThecombinationHID/AB2.5methodsrevealedthe
absenceofHIDreactivity(brown)inbothProandNo-ProBrunner’s
glands(Fig.2C,D).
Comparisonof lectin reactivity in No-Pro and Pro Brunner’s
glandsshowedseveraldifferentbindingpatterns.ExceptforMALII,
DBA,andSBA,whichwereunreactivewithBrunner’sglandsfrom
bothNo-ProandPropigs,theotherlectinsboundtheglandularcells
and/ortheluminalcontent(SNA,PHA-L,PHA-E,succinylWGA)or
onlytheadenomericcells(GSAII,ConA,UEAI,GSAI-B4,LTA,PNA,
RCA120,HPA).TheresultsaresummarizedinTable2.
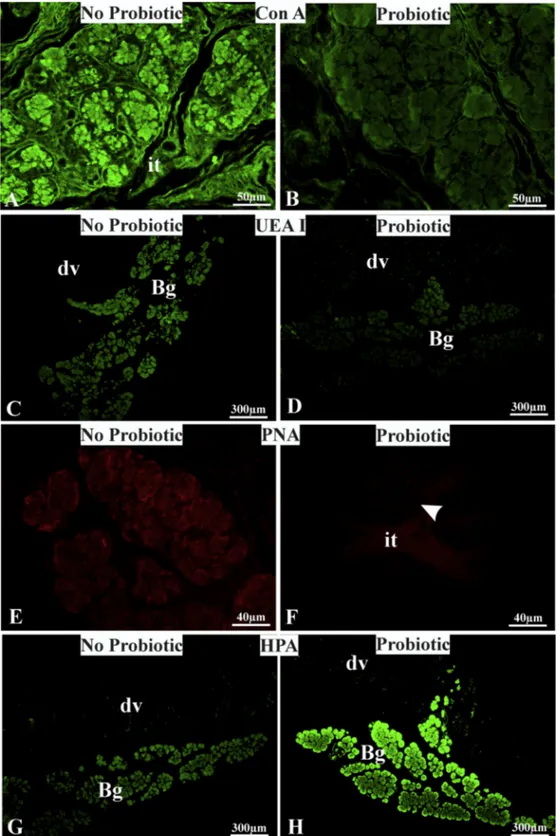
Inparticular,SNAdisplayednochangeinthestainingintensity
ofthesecretorycellsandtheadenomericluminalcontentof
Brun-ner’sglandsfrombothNo-ProandProanimals(Fig.3A,B);PHA-L
reactedwiththeadenomericcellsandtheluminalcontentof
No-ProBrunner’sglands(Fig.3C)and withtheadenomericluminal
contentoftreatedanimals(Fig.3D);PHA-Eboundweakly with
theadenomeresandstronglywiththeirluminalcontentinNo-Pro
specimens(Fig.3E),whereasitdidnotbindtheadenomeresbut
weaklystainedtheluminalcontentoftreatedpigs(Fig.3F);and
succinylWGAshowednoreactionwiththesecretorycells,whereas
itstainedtheluminalcontentinallinvestigatedBrunner’sglands
Fig.2.Duodenumfromno-probiotic(A,C)andprobiotic-fedpigs(B,D),stainedwithPAS/AlcianBlue(AB)2.5(A,B)andHID/AB2.5(C,D)procedures.(A)No-probiotic Brunner’sglandsexhibitbroadPASpositivity(magenta)andafewAB2.5-positive(blue)cells.(B)Brunner’sglandsofprobiotic-fedpigsshowbroadAB2.5positivityand somePAS-positivecells.(A)and(B)demonstrateAB2.5-positivegobletcellsinduodenalvilli(dv).(C,D)HID/AB2.5methodsrevealedtheabsenceofHID(brown)reactivity intheBrunner’sglandsofbothno-probioticandprobiotic-fedanimals.ThereliabilityofHIDstainingwasdemonstratedbytheHIDpositivityofthegobletcells(arrow)in duodenalvilli(dv).Bg,Brunner’sgland.(Forinterpretationofthereferencestocolorinthisfigurelegend,thereaderisreferredtothewebversionofthisarticle.)
Concerningthelectinsthatboundonlytheadenomericcells,
GSAIIexhibitednodifferenceinthestainingintensitybetween
No-ProandProsamples.ConA(Fig.4A,B)andUEAI(Fig.4C,D)showed
strongeraffinityintheNo-Pro samplesthanintheProsamples.
GSAI-B4,LTA,RCA120,andPNAboundtheadenomericcellsofthe
Brunner’sglandsfromNo-Propigs,whereasitdidnotreactwiththe
secretoryepitheliumofProBrunner’sglands(Fig.4E,F).HPAwas
thesolelectinshowinga decreaseinreactivityintheBrunner’s
glandsofNo-PropigscomparedwiththatintheBrunner’sglands
ofPropigs(Fig.4G,H).
4. Discussion
Thisis thefirststudy todemonstratethat feedingof a
pro-biotic complex affects the glycosylation pattern of the mucins
secretedbypigBrunner’sglands.Thisinvestigationfollowsarecent
reportdemonstratingthattheuseofadietaryprobioticcomplex
(SivoyTM,SLAB51)enhancesgrowthperformanceandmeatquality
ingrowing-finishingpigs(Tufarellietal.,2017).
ConventionalhistochemistryrevealedthatBrunner’sglandsof
no-probiotic-fedpigsproduceprimarilyneutralglycoproteins(PAS
reaction),asmallamountofacidiccarboxylatedmucins(AB2.5
positivity), and no sulfoglycans (HID negativity). These results
are consistentwith those of previousstudies onthe Brunner’s
glandsofseveralmammals(Krause,2000;Verdiglioneetal.,2002;
Schumacheretal.,2004;ScillitaniandMentino,2015),including
youngadultpigs(TakehanaandAbe,1986).
Using15differentlectins,wecharacterizedthecarbohydrate
compositionofthemucinsfromBrunner’sglandsmorethoroughly.
Thesecretory epithelium ofBrunner’s glands from No-Pro pigs
showedthepresenceofbothN-andO-linkedglycans.N-Glycans
werehighmannose(ConA)andcomplextypes(PHA-L,PHA-E).
O-GlycanscontainedtheterminaldisaccharideGal1,3GalNA(PNA)
andthesimplestmucinO-glycanmadebyasingleGalNAclinkedto
serineorthreonine(HPA).Moreover,weobservedglycans
termi-natingwithfucose(LTA,UEAI),GlcNAc(GSAII),galactose(GSA
I-B4), lactosamine (RCA120), and Neu5Ac␣2,6Gal/GalNAc (SNA).
AlthoughourresultsagreewithpreviousreportsonpigBrunner’s
glands(TakehanaandAbe,1986;Gelbergetal.,1992),ourstudy
providesamorein-depthcharacterizationoftheoligosaccharide
chainsofBrunner’sglandglycoproteinsbecauseSNA,PHA-L,
PHA-E,LTA,andHPAhavenotbeenusedpreviously.Theluminalcontent
accumulatedhighmannoseandcomplextypesofN-glycansaswell
asglycanswithinternalGlcNAc(succinylWGA)andterminal
␣2,6-linkedsialicacid(SNA).Theroleofsugarresiduesinthesecretory
glycoproteins ofBrunner’s glands is not wellknown. However,
oligosaccharidechainscontaininglactosamineinhigh
mannosy-latedN-glycanshavebeenobservedinthehumanmucinMUC6
(Toribaraetal.,1997).Notably,thegeneencodingthelattermucin
isalsoexpressedinhumanBrunner’sglands(Bartmanetal.,1998).
Concerningsialic acid,thenegativechargeof thismoleculehas
beenshowntohavearoleinthetransportofchloride,
bicarbon-ate,water,andprotonsinBrunner’sglands(Collacoetal.,2013).
Insecretedmucus,sialicacidcancontributetotheviscosityand
protectionoftheunderlyingepitheliumfromlysisbygastricjuice
andbacteria(Schauer,2004)andcanactasaligandforseveral
symbioticandpathogenicmicroorganisms(Lehmannetal.,2006).
GalNAcandGalresidualscaninhibitthecellbindingofthe
tropho-zoitesofEntamoebahistolytica,thecausativeagentofamoebiasis
(RalstonandPetri,2011).Regardingfucosylatedresiduals,theycan
beimportantinboththemaintenanceofthebacterialflorainvolved
inthedegradationoffucosefromfood(BeckerandLowe,2003)and
inincreasingtheviscosityofmucus(Liquorietal.,2012).O-Glycans
nat-Fig.3.LectinbindingpatternofBrunner’sglandsfromno-probioticandprobiotic-fedpigs.NotethedecreasedreactivityofPHA-LandPHA-EinBrunner’sglandadenomeres ofprobiotic-fedanimals.Bg,Brunner’sgland;dv,duodenalvilliwithpositivegobletcells;arrow,Brunner’sglandluminalcontent;arrowhead,basementmembrane.(A–F): FITC-conjugatedlectins;(G,H)rhodamine-labeledsuccinylatedWGA.
uralantibioticandasatumorsuppressorfordifferentiated-type
adenocarcinoma(Nakayama,2014).
Theprobioticblendinduceda greatchangeinthe
glycosyla-tionpathwayoftheepitheliumofBrunner’sglands.Conventional
histochemistryshowedadrasticreductioninneutralmucinsand
theprimarypresenceofcarboxylacidicglycoproteins.Inaddition,
lectinhistochemistryrevealedareductioninhighmannose(Con
A)and␣1,2-linkedfucosylatedglycans(UEAI)andthe
disappear-anceofcomplextypesofN-glycans(PHA-L,PHA-E)terminating
withlactosamine(RCA120)andfucosylatedoligomersbindingLTA,
aswellasO-linkedglycansterminatingwithGal1,3GalNAc(PNA)
and terminal galactose (GSA I-B4). Moreover, theluminal
con-tentofBrunner’sglandsexhibitedareducedamountofbisecting
GlcNAc-and Gal-bearingglycoproteins(PHA-E)whencompared
withthatfromprobiotic-fedpigs.However,thedietaryprobiotic
Fig.4. LectinbindingpatternofBrunner’sglandsfromno-probioticandprobiotic-fedpigs.NoteConA,UEAI,andPNA(B,D,F)decreasedreactivity,aswellasHPA(H)increased affinityintheBrunner’sglandsofprobioticfedpigs.Bg,Brunner’sgland;dv,duodenalvilli;it,interstitialtissue.(A–D,G,H)FITC-conjugatedlectins;(E,F)rhodamine-labeled PNA.
O-linkedglycansterminatingwithGalNAc(HPA).Thesefindings
areinlinewiththeevidencethatcomponentsofthedietandthe
gutmicrofloraareincontactwithintheintestinaltractandthat
dietarycomposition may influence thecarbohydrate structures
ofthemucosaland mucinglycoconjugateswithmarked
conse-quencesfortheadherenceofmicrofloraandforthegutitself(Kelly
etal.,1992).Somestudiesdemonstratedthatpre-treatmentwith
probioticsincreasedtheexpressionofMUC2,MUC5AC,andMUC6
in ratstomach(Caballero-Franco etal., 2007;Lam et al.,2007;
Gomietal.,2013).Moreover,experimentalevidencesupportsthe
hypothesisoftheadaptabilityofBrunner’sglandmucinstodietary
changes(Krause,2000;Desantisetal.,2011).
Inconclusion,this studydemonstratesthatprobiotic
supple-mentationaffectstheglycancompositionofthemucinsproduced
intheBrunner’sglandsofgrowing-finishingpigs.Sinceprobiotics
haveastimulatingeffectondigestiveprocessesandtheimmune
systemofpigs(Mengetal.,2010;Huetal.,2015;Liuetal.,2017)
meatqualitythandidthecontrolbasaldiet(Tufarellietal.,2017),
webelievethattheobservedchangesinthecompositionofthe
secretorymucinsfromBrunner’sglands couldacteffectivelyon
thegastrointestinalfunctionandhealthstatusofanimals.However,
furtherstudiesarenecessarytounderstandthemechanismof
pro-bioticactionontheglycosylationpathwayofthemucinssecreted
byBrunner’sglands.
References
Aigner,B.,Renner,S.,Kessler,B.,Klymiuk,N.,Kurome,M.,Wünsch,A.,Wolf,E., 2010.Transgenicpigsasmodelsfortranslationalbiomedicalresearch.J.Mol. Med.Berl.Ger.88,653–664.
Bartman,A.E.,Buisine,M.P.,Aubert,J.P.,Niehans,G.A.,Toribara,N.W.,Kim,Y.S.,Kelly, E.J.,Crabtree,J.E.,Ho,S.B.,1998.TheMDC6secretorymucingeneisexpressed inawidevarietyofepithelialtissues.J.Pathol.186,398–405.
Becker,D.J.,Lowe,J.B.,2003.Fucose:biosynthesisandbiologicalfunctionin mam-mals.Glycobiology13,41R–53R.
Büsing,K.,Zeyner,A.,2015.EffectsoforalEnterococcusfaeciumstrainDSM10663 NCIMB10415ondiarrhoeapatternsandperformanceofsuckingpiglets.Benef. Microbes6,41–44.
Caballero-Franco,C.,Keller,K.,DeSimone,C.,Chadee,K.,2007.TheVSL#3probiotic formulainducesmucingeneexpressionandsecretionincolonicepithelialcells. Am.J.Physiol.Gastrointest.LiverPhysiol.292,G315–G322.
Collaco,A.M.,Jakab,R.L.,Hoekstra,N.E.,Mitchell,K.A.,Brooks,A.,Ameen,N.A.,2013. RegulatedtrafficofaniontransportersinmammalianBrunner’sglands:arole forwaterandfluidtransport.Am.J.Physiol.Gastrointest.LiverPhysiol.305, G258–G275.
Desantis,S.,Zizza,S.,Accogli,G.,Tufarelli,V.,Laudadio,V.,2011.Morphometric featuresandglycoconjugatepatternofrabbitintestineareaffectedbyparticle sizeofpelleteddiets.Anat.Rec.294,1875–1889.
Flemstrom,G.,Isenberg,J.I.,2001.Gastroduodenalmucosalalkalinesecretionand mucosalprotection.NewsPhysiol.Sci.16,23–28.
Florey,H.W.,Harding,H.E.,1933.ThefunctionofBrunner’sglandsandpyloricglands ofthestomach.J.Pathol.Bacteriol.37,431–453.
Fuller,R.,2006.Reasonsfortheapparentvariationintheprobioticresponse. Biolo-gia,Bratislava61,751–754.
Gelberg,H.,Whiteley,H.,Ballard,G.,Scott,J.,Kuhlenschmidt,M.,1992.Temporal lectinhistochemicalcharacterizationofporcinesmallintestine.Am.J.Vet.Res. 53,1873–1880.
Gomi,A.,Harima-Mizusawa,N.,Shibahara-Sone,H.,Kano,M.,Miyazaki,K.,Ishikawa, F.,2013.EffectofBifidobacteriumbifidumBF-1ongastricprotectionandmucin productioninanacutegastricinjuryratmodel.J.DairySci.96,832–837. Gonzalez,L.M.,Moeser,A.J.,Blikslager,A.T.,2015.Porcinemodelsofdigestive
dis-ease:thefutureoflargeanimaltranslationalresearch.Transl.Res.166,12–27. Guilloteau,P.,Zabielski,R.,Hammon,H.M.,Metges,C.C.,2010.Nutritional
program-mingofgastrointestinaltractdevelopment.Isthepigagoodmodelforman? Nutr.Res.Rev.23,4–22.
Heinritz,S.N.,Mosenthin,R.,Weiss,E.,2013.Useofpigsasapotentialmodelfor researchintodietarymodulationofthehumangutmicrobiota.Nutr.Res.Rev. 26,191–209.
Hu,Y.,Dun,Y.,Li,S.,Zhang,D.,Peng,N.,Zhao,S.,Liang,Y.,2015.DietaryEnterococcus faecalisLAB31improvesgrowthperformance,reducesdiarrhea,andincreases fecalLactobacillusnumberofweanedpiglets.PLoSOne10,e0116635. Kelly,D.,Begbie,R.,King,T.P.,1992.Postnatalintestinaldevelopment.In:Varley,
M.A.,Williams,P.E.V.,Lawrence,T.L.J.(Eds.),NeonatalSurvivalandGrowth. OccasionalPublicationNo.15,BritishSocietyofAnimalProduction,Edinburgh, UK,pp.63–79.
Krause,W.J.,2000.Brunner’sglands:astructural,histochemicalandpathological profile.Prog.Histochem.Cytochem.35,255–367.
Lam,E.K.,Tai,E.K.,Koo,M.W.,Wong,H.P.,Wu,W.K.,Yu,L.,So,W.H.,Woo,P.C.,Cho, C.H.,2007.EnhancementofgastricmucosalintegritybyLactobacillusrhamnosus GG.LifeSci.80,2128–2136.
Lehmann,F.,Tiralongo,E.,Tiralongo,J.,2006.Sialicacid-specificlectins:occurrence, specificityandfunction.Cell.Mol.LifeSci.63,1331–1354.
Liquori,G.E.,Mastrodonato,M.,Mentino,D.,Scillitani,G.,Desantis,S.,Portincasa,P., Ferri,D.,2012.InsitucharacterizationofO-linkedglycansofMuc2inmouse colon.ActaHistochem.114,723–732.
Liu,C.,Zhu,Q.,Chang,J.,Yin,Q.,Song,A.,Li,Z.,Wang,E.,Lu,F.,2017.Effectsof LactobacilluscaseiandEnterococcusfaecalisongrowthperformance:immune functionandgutmicrobiotaofsucklingpiglets.Arch.Anim.Nutr.71,120–133. McManus,J.F.A.,1948.Histologicalandhistochemicalusesofperiodicacid.Stain
Technol.23,99–108.
Meng,Q.W.,Yan,L.,Ao,X.,Zhou,T.X.,Wang,J.P.,Lee,J.H.,Kim,I.H.,2010. Influ-enceofprobioticsindifferentenergyandnutrientdensitydietsongrowth performance,nutrientdigestibility,meatquality,andbloodcharacteristicsin growing-finishingpigs.J.Anim.Sci.88,3320–3326.
Nakayama,J.,2014.Dualrolesofgastricglandmucin-specificO-glycansin preven-tionofgastriccancer.ActaHistochem.Cytochem.47,1–9.
NRC,1998.NutrientRequirementsofSwine,10threv.ed.NationalAcademiesPress, Washington,DC,pp.110–142.
Oelschlaeger,T.A.,2010.Mechanismsofprobioticactions—areview.Int.J.Med. Microbiol.300,57–62.
Pearse,A.G.E.,1968.HistochemistryTheoreticalandApplied,vol.I.,3rded.Churchill, London.
Perez-Vilar,J.,Hill,R.L.,1999.Thestructureandassemblyofsecretedmucins.J.Biol. Chem.274,31751–31754.
Prather,R.S.,Lorson,M.,Ross,J.W.,Whyte,J.J.,Walters,E.,2013.Genetically engi-neeredpigmodelsforhumandiseases.Annu.Rev.Anim.Biosci.1,203–219. Ralston,K.S.,PetriJr.,W.A.,2011.TissuedestructionandinvasionbyEntamoeba
histolytica.TrendsParasitol.27,254–263.
Schauer,R.,2004.Sialicacids:fascinatingsugarsinhigheranimalsandman.Zoology 107,49–64.
Schumacher,U.,Duku,M.,Katoh,M.,Jorns,J.,Krause,W.J.,2004.Histochemical similaritiesofmucinsproducedbyBrunner’sglandsandpyloricglands:a com-parativestudy.Anat.Rec.A:Discov.Mol.Cell.Evol.Biol.278,540–550. Scillitani,G.,Mentino,D.,2015.Comparativeglycopatternanalysisofmucinsin
theBrunner’sglandsoftheguinea-pigandthehousemouse(Rodentia).Acta Histochem.117,612–623.
Sharon,N.,Lis,H.,2004.Historyoflectins:fromhemagglutininstobiological recog-nitionmolecules.Glycobiology14,53R–62R.
Spicer,S.S.,1965.Diaminemethodsfordifferentialingmucosubstances histochem-ically.J.Histochem.Cytochem.13,211–234.
Spicer,S.S.,Schulte,B.A.,1992.Diversityofcellglycoconjugatesshown histochem-ically:aperspective.J.Histochem.Cytochem.40,1–38.
Stramandinoli-Zanicotti,R.T.,Carvalho,A.L.,Rebelatto,C.L.K.,Sassi,L.M.,Torres,M.F., Senegaglia,A.C.,Boldrinileite,L.M.,Correa-Dominguez,A.,Kuligovsky,C., Brof-man,P.R.S.,2014.Brazilianminipigasalarge-animalmodelforbasicresearch andstemcell-basedtissueengineering:characterizationandinvitro differen-tiationofbonemarrow-derivedmesenchymalstemcells.J.Appl.Oral.Sci.Rev. F.O.B.22,218–227.
Takehana,K.,Abe,M.,1986.Histochemistryofcomplexcarbohydratesinthe duo-denalglandsinthepig.J.Coll.Dairy.11,371–380.
Toribara,N.W.,Ho,S.B.,Gum,E.,GumJr,J.R.,Lau,P.,Kim,Y.S.,1997.The carboxyl-terminalsequenceofthehumansecretorymucinMUC6.Analysisoftheprimary aminoacidsequence.J.Biol.Chem.272,16398–16403.
Tufarelli,V.,Crovace,A.M.,Rossi,G.,Laudadio,V.,2017.Effectofadietaryprobiotic blendonperformance,bloodcharacteristics,meatqualityandfaecalmicrobial sheddingingrowing-finishingpigs.S.Afr.J.Anim.Sci.47,875–882.
Verdiglione,R.,Mammola,C.L.,Filotto,U.,2002.Glycoconjugatehistochemistryof bovineBrunner’sglands.Ann.Anat.184,61–69.
Verma,N.,Rettenmeier,A.W.,Schmitz-Spanke,S.,2011.Recentadvancesinthe useofSusscrofa(pig)asamodelsystemforproteomicstudies.Proteomics11, 776–793.