Erika Carla Pierattini
Effects of Xenobiotics
in Poplar
Academic Year 2016-2017 PhD course in Agrobiosciences
Effects of Xenobiotics on Poplar
PhD Candidate:
Erika Carla Pierattini
Supervisor:
Index
Abstract ... 1
Introduction ... 3
Pharmaceutical and Personal Care Products... 3
Caffeine ... 4
Erythromycin ... 8
Diclofenac... 10
Sodium Dodecyl Sulfate ... 12
Plant uptake and metabolism of organic xenobiotics ... 14
Heavy metals ... 17
Zinc... 19
Phytoremediation ... 21
Poplar ... 25
Aims ... 29
Results and Discussion ... 30
Poplar and Caffeine ... 30
Poplar and Erythromycin... 32
Poplar, SDS and Zn... 35
Poplar and Diclofenac ... 38
Conclusions and further perspectives ... 43
Acknowledgements ... 45
References ... 46
Publications and conference papers ... 58
Abstract
Environmental pollution from pharmaceutical and personal care products has become in recent years issue of increasing concern, since the widespread use and continuous release of these substances into the aquatic environment, together with the scarce removal efficiency of traditional water treatment plants. Plant uptake has been demonstrated to be effective for the removal of heavy metals as well as organic contaminants, since plants have the ability to absorb, translocate, and eventually metabolize organic xenobiotic compounds.
The potential of trees in the remediation of some xenobiotics was explored using Populus alba L. Villafranca clone as model plant species. Different contaminants belonging to the class of pharmaceutical and personal care products (caffeine, erythromycin, sodium dodecyl sulfate, and diclofenac), and heavy metals (zinc), were tested in order to investigate poplar tolerance to these pollutants, and its capability to take up and eventually metabolize pharmaceutical and personal care products. Villafranca clone maintained a healthy phenotype during the treatments with all the tested pollutants, excluding sodium dodecyl sulfate, where severe foliar necrosis occurred, caused by the release of sodium from the molecule.
With the exception of caffeine, that has been found to be rapidly translocated in leaves, the other pollutants (i.e. erythromycin, dodecyl sulfate, and diclofenac) were preferentially retained at higher concentrations in roots.
The ability of Villafranca clone to metabolize the target xenobiotics has been demonstrated. In particular, degradation of caffeine-(trimethyl-13C)
in theobromine-(dimethyl-13C) and theophylline-(dimethyl-13C) was
observed.
Additional peaks were detected in the mass chromatograms of erythromycin-treated plant extracts, and they are thought to be epimers of erythromycin.
Moreover, in plants treated with diclofenac, the hydroxylated form was detected, as well as the conjugated forms of both native diclofenac and OH-diclofenac.
This work puts the basis for a further investigation of tree tolerance to heavy metals and pharmaceutical and personal care products pollutants, and for studies on tree metabolism of these xenobiotics.
Introduction
Pharmaceutical and Personal Care Products
Pharmaceutical and personal care products (PPCPs) have become issues of increasing concern in the last decades due to their widespread use and continuous release into the aquatic environment, both via domestic and industrial wastewaters (Zhang et al., 2014). Due to their persistence in the environment, these molecules are found as microcontaminants in soil and surface waters (Li, 2014). Conventional waste water treatment plants (WWTPs) cannot eliminate efficiently many pharmaceutical compounds (Fig. 1); removal rate of pharmaceuticals varies greatly amongst different WWTPs technologies, such as coagulation-flocculation, activated carbon adsorption, ozonation and other oxidation processes, membrane processes and biofiltration (Matamoros et al., 2012; Rivera-Utrilla et al., 2013; Luo et al., 2014). Contamination from pharmaceutical active compounds may cause serious risks both for the environment, disrupting micro-fauna and microbial communities (Lawrence et al., 2012), and also for human health, since reclaimed waters are used for irrigation, and vegetables uptake of PPCPs have been widely demonstrated (Fatta-Kassinos et al., 2011; Herklotz et al., 2010; Wu et al., 2013). Contamination from PPCPs has been detected also in bottled mineral water, reaching up to 15 ng L-1 for
nicotine (González Alonso et al., 2012). Although according to the World Health Organization (2012), pharmaceutical compounds found in drinking water did not constitute a serious risk for human health, since their concentration is far below the therapeutic threshold, to date no monitoring routine programs nor threshold concentrations for PPCPs in
drinking water and wastewater treatment plants effluent have been instituted (World Health Organization, 2012).
Fig. 1. Average concentrations (on logarithmic Y axis) of micropollutants in WWTPs influents and effluents. Data are representative of surveys from European Union, United Kingdom, East Europe, China, Korea, USA. Modified from Luo et al. (2014).
Caffeine
Among PPCPs, caffeine is one of the most widely consumed drugs in the world and has been considerably detected in WWTPs effluents, surface water, and in groundwater (Loos et al., 2013). Its average concentration in groundwaters used as drinking water sources in Europe was found to be 3 ng L-1 (Loos et al., 2010), while concentrations below 15 ng L-1 were observed in spring waters used for bottled mineral water in Spain (González Alonso et al., 2012). Due to the relatively high concentration
and worldwide distribution of caffeine, this compound is commonly considered an anthropogenic marker (Buerge et al., 2003).
Zhang and coworkers (2013a) demonstrated in mesocosms trials that caffeine does not undergo photodegradation and that microbial populations have a minor role in its removal from water. Therefore, this molecule is persistent in the aquatic environment and it has been demonstrated to damage microbial biofilm communities and micro-fauna (Lawrence et al., 2012).
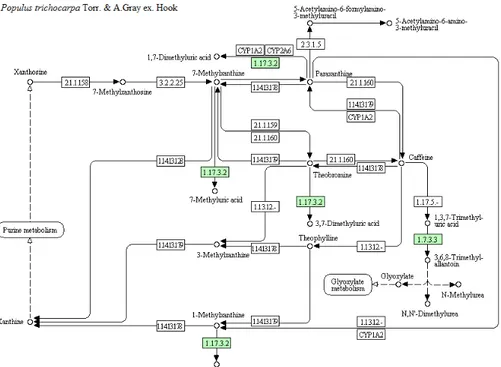
Caffeine is a plant secondary metabolite mainly studied in coffee, tea, and cocoa species (Ashihara and Crozier, 2001): caffeine plant metabolism, and in particular its degradation pathway is well known in coffee plants (Ashihara and Crozier, 2001; Ashihara et al., 2008). The main metabolites derived from caffeine catabolism in plants are theophylline and theobromine, resulting from demethylation of positions 1 and 7 of the purinic ring of the caffeine molecule, respectively (Fig. 2) (Ashihara et al., 2008). A model pathway for caffeine metabolism has been predicted in
silico in Populus trichocarpa (KEGG pathway pop00232); in particular,
proteins belonging to the xanthine dehydrogenase family (POPTR_0009s05940g) and a uricase (POPTR_0010s24880g) were identified (Fig. 3).
Fig. 2. Caffeine catabolic pathway. Solid lines represent main metabolic pathways, dotted lines represent alternative metabolic pathways. Modified from Ashihara et al., 2008.
Fig. 3. Caffeine metabolic pathway. Green boxes represent the genes identified in Populus trichocarpa (KEGG pathway pop00232); xanthine dehydrogenases (1.17.3.2; POPTR_0009s05940g) and uricase (1.7.3.3; POPTR_0010s24880g).
Erythromycin
Erythromycin is an antibiotic belonging to the class of macrolides, widely used in human and veterinary medicine (Manzetti and Ghisi, 2014); The mean daily European erythromycin consumption is estimated to be 1.336 mg/capita (Johnson et al., 2015). Macrolides are large-spectrum antibiotics with bacteriostatic activity, and inhibit protein synthesis: in particular, erythromycin binds to the 50S ribosomal subunit of bacteria, promoting the detachment of the peptidyl-tRNA (Menninger and Otto, 1982).
Erythromycin has been detected in various aquatic environments in several countries, including USA (180–1200 ng L-1) (Karthikeyan and Meyer, 2006), United Kingdom (71–141 ng L-1), (Roberts and Thomas,
2006), Germany (6000 ng L-1) (Hirsch et al., 1999), and Italy (median
concentration of 47 ng L-1) (Zuccato et al., 2005).
Persistence of antibiotics in the environment constitute a serious risk for the rising and spreading, through horizontal gene transfer, of antibiotics-resistance mechanisms into bacterial communities (Dantas et al., 2008; Guo et al., 2015). Moreover, erythromycin concentrations up to 1 µg L-1 could be effective on wildlife (Johnson et al., 2015), as proved for aquatic organisms such as fishes, where muscles bioaccumulation of erythromycin occurs (Liu et al., 2014). Several negative effects in terms of growth reduction, oxidative stress, and impairment of photosynthesis have been observed in algae (Pomati et al., 2004; Liu et al., 2011; Deng et al., 2014; Wan et al., 2015). On the other hand, erythromycin presence in surface waters has also been related with algal bloom, since concentrations below 0.1 µg L-1 stimulated Microcystis flos-aquae growth
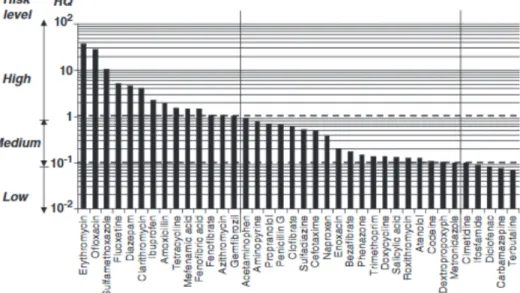
with an hormesis-like trend (Wan et al., 2015). The environmental risk quotient of this pollutant, defined as the average concentration in WWTPs effluents on the predicted no-effect concentration, is calculated to be really high (Verlicchi et al., 2012) (Fig. 4).
Fig. 4. Risk quotient of some of the most detected pharmaceuticals in WWTPs. Modified from Verlicchi et al. (2012).
Sandmann and Boger (2008), as well as Pan and coworkers (2009) observed that activities of PS I and PS II seem to be the target of several antibiotics, including erythromycin; in particular a strong effect of this molecule on photosynthetic efficiency was observed in cyanobacteria (Deng et al., 2014; Wan et al., 2015).
Diclofenac
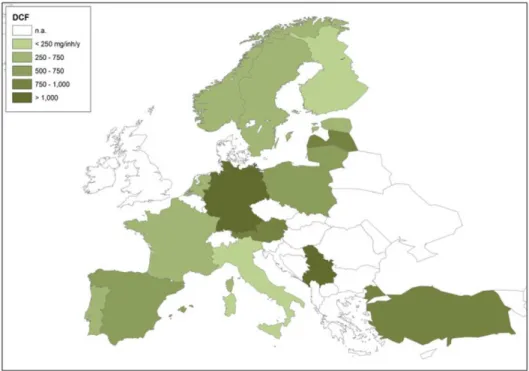
Diclofenac is a non-steroidal anti-inflammatory drug widely used as painkiller across Europe, reaching consumption doses up to 1033 mg per inhabitant per year in Germany (Fig. 5) (Schröder et al., 2016). In European WWTP effluents, the average diclofenac concentration was reported to be 49.5 ng L−1, with the highest being 174 ng L−1 (Loos et al. 2013). Its removal from wastewater has been reported to be extremely variable; Luo and coworkers reported a removal efficiency between 0 and 81%, with an average removal of 35.8%. Diclofenac has also been described as sensitive to photodegradation, showing an half-life of 3 to 10 days (Zhang et al., 2011; Zhang et al., 2012; Matamoros et al., 2012). Photodegradation products originate from UV-induced dechlorination and decarboxilation of the diclofenac molecule (Musa and Eriksson, 2009; Yan and Song, 2014).
Ecological concern about diclofenac arouse in the early 2000’s, when Oaks and coworkers (2004) established that the decline of vulture population in Asia was associated to the widespread use of diclofenac for cattle treatments. Negative effects of this pharmaceutical compound were observed also on aquatic organisms by several authors; in particular, loss of cell membrane integrity was observed in duckweed (Kummerová et al., 2016), while tissue-specific oxidative stress was induced by diclofenac in mussels (Gonzalez-Rey and Bebianno, 2014). Accumulation of diclofenac and its metabolites has been reported in fishes, together with enhanced activity of enzymatic antioxidant systems (Islas-Flores et al., 2013; Guiloski et al., 2015). Regarding terrestrial species, Chen and colleagues (2015) observed reduction of survival and fertility in soil arthropods.
Fig. 5. European diclofenac consumption in mg per inhabitant per year. Modified from Schröder et al. (2016).
Uptake and metabolism of diclofenac has been described in mesocosms studies using herbaceous species such as Scirpus (Zhang et al., 2012), and Typha (Bartha et al., 2014), as well as in alfalfa (Christou et al., 2016). Scirpus validus has been noticed to tolerate concentrations up to 2 mg L-1 (Zhang et al., 2012), while oxidative stress response was observed in Typha latifolia (Bartha et al., 2014), and Medicago sativa, where an increase of lipid peroxidation was observed (Christou et al., 2016). After entering the plant cells, the diclofenac molecule is modified by plant metabolism (Sandermann, 1994); the main metabolites were found to be the hydroxylated forms 4-OH-diclofenac and 5-OH-diclofenac, as well as the conjugated forms O-glucopyranosyl-oxydiclofenac and 4-OH-glutathionyl-diclofenac (Huber et al., 2012; Bartha et al., 2014).
Moreover, formation of the intermediate compound diclofenac-2,5-iminoquinone, due to plant peroxidases activity, has been demonstrated (Huber et al., 2016).
Sodium Dodecyl Sulfate
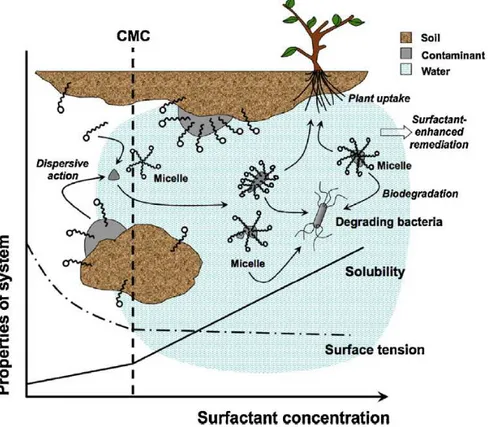
Surfactants are a class of compounds with numerous industrial applications worldwide (Cserháti et al., 2002), including the remediation of contaminated environments. In particular, they are used for their ability to meliorate the solubility of petroleum hydrocarbons and heavy metals in polluted soils, hence increasing their removal from the polluted site, both in soil flushing (Torres et al., 2002) and in phytoremediation trials with herbaceous species (Fig. 6) (Liu et al., 2008; Almeida et al., 2009; Liu et al.,2009; Mao et al., 2015).
Nevertheless, surfactants are molecules with a high environmental impact (Cserháti et al., 2002; Kumar et al., 2014), and their ecotoxicity is a matter of ever-increasing interest since their continuous introduction in the aquatic environment via domestic and industrial wastewaters. Anionic surfactants such as sodium dodecyl sulfate (SDS) are amphipatic compounds, that interact readily with polar and apolar macromolecules, leading to membrane damages (Anderberg and Artursson, 1993) and oxidative stress (Wang et al., 2016).
Biological activity of anionic surfactants have been widely reported (Cserháti et al., 2002). In particular, SDS has been shown to have toxic effects on duckweed, where it affects phenols content and stress enzymes activity (Dirilgen and Ince, 1995; Forni et al., 2012) and in mussel, where
reactive oxygen species production was enhanced (Messina et al., 2014). It has been described to alter carbohydrates and proteins distribution in tissues of the fish Scophthalmus maximus (Rosety-Rodríguez et al., 2002). Mariani and coworkers (2006) performed an acute toxicity study including various trophic levels (bacteria, algae, crustacea, echinodermata, pisces), showing that, for the tested species, the half maximal effective concentration (EC50) of SDS ranged from 2.36 mg L-1 (bacterium Vibrio
fischeri) to 7.42 mg L-1(crustacean Tigriopus fulvus).
Fig. 6. Schematic mechanism of surfactant-enhanced phytoremediation. CMC= critical micellar concentration. From Mao et al. (2015).
Although SDS is generally considered as safe in remediation trials, because it can be degraded by some strains of Pseudomonas species that are able to use this molecule as carbon source (Chaturvedi and Kumar, 2011; Paulo et al., 2013), and by photoelectrochemical processes, that can reduce up to 90% of SDS-related total organic carbon (Nguyen et al., 2016), the direct effect of this surfactant on plants has not been thoroughly investigated.
Plant uptake and metabolism of organic xenobiotics
Organic pollutants uptake and translocation to the aerial parts of the plant is known to be related to the octanol-water partition coefficient (log Kow)
of the molecule (Miller et al., 2016). Historically, compounds with low polarity (log Kow > 1) are considered more likely to enter the plant via
passive diffusion through root membranes (Briggs et al., 1982; Burken and Schnoor, 1998; Dettenmaier et al., 2009), although they are not likely transported to the aerial part by the transpiration stream (Briggs et al., 1982). However, plants have been demonstrated also to absorb polar compounds such as caffeine (log Kow = - 0.07; Zhang et al., 2013a). A better
estimation of lipophilicity than Kow for ionizable organic xenobiotics is
represented by Dow, calculated as Dow = αneutral·Kow,neutral (Miller et al., 2016).
This formula include correction for the pH-dependent speciation of compounds, as αneutral is the fraction of the compound present as neutral species and Kow,neutral
is the octanol-water partition coefficient of the neutral species.
Anionic PPCPs have been observed to have a different accumulation pattern in comparison with cationic and neutral species, showing higher accumulation in
roots, while cationic and neutral PPCPs can be more easily translocated to the aerial part of the plant (Dodgen et al., 2015).
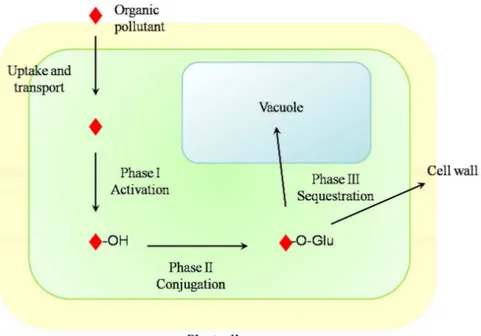
Once organic xenobiotics such as PPCPs enter the plant cells, they could be modified by plant enzymes according to what has been described as the “green liver” model (Sandermann, 1994) (Fig. 7).
Metabolic modifications of organic xenobiotics could be grouped into three phases. The first phase of plant metabolism (Phase I) consists in the hydrolysis, hydroxylation and/or oxidation of the molecule, involving enzymes belonging to cytochrome P-450 group (Coleman et al., 1997). Phase I acts as “activation” of the xenobiotic in order to enhance its response to Phase II reactions, i.e. conjugation with endogenous molecules such as glutathione and glucose (Coleman et al., 1997; Schröder and Collins, 2002): Phase I reactions are often necessary in order to permit the formation of conjugates (Huber et al., 2012). Subsequently (Phase III), organic xenobiotics could be sequestered in cell walls and vacuoles, where they can be further degraded by peroxidases activity (Coleman et al., 1997; Passardi et al., 2007) (Fig. 7).
Products of the “green liver” metabolism are usually more polar and less harmful than the original xenobiotic compounds (Sandermann, 1994). However, plant transformation of PPCPs may also lead to the formation of molecules that are more toxic than the parent compounds (Plewa and Wagner, 1993).
Pollutants such as polychlorinated biphenyls (PCBs) have been largely demonstrated to undergo the above mentioned metabolic processes (Van Aken, 2008; Zhai et al., 2011).
In addition, organic xenobiotics such as PPCPs could be metabolized by activity of endophytic bacteria, as it has been demonstrated for carbamazepine (Sauvêtres and Schröder, 2015).
Fig. 7. Schematic representation of the “green liver” concept: Phase I results in the activation of the organic pollutant (e.g. via hydroxylation); Phase II determines the conjugation of the activated pollutant with endogenous molecules (e.g. glucose); Phase III consists in the sequestration of the molecule in vacuole or cell wall. Modified from Van Aken (2008).
Plants ability to absorb, translocate, sequester and eventually detoxify pollutants could be improved thanks to genetic engineering. In fact, several transgenic lines of both herbaceous species (e.g. Arabidopsis, tobacco, Indian mustard) and trees (e.g. poplar) have been made in order to enhance plant uptake, tolerance, and metabolism of both elemental and organic pollutants (Cherian and Oliveira, 2005; Eapen et al., 2007; Van Aken, 2008; Abhilash et al., 2009).
Heavy metals
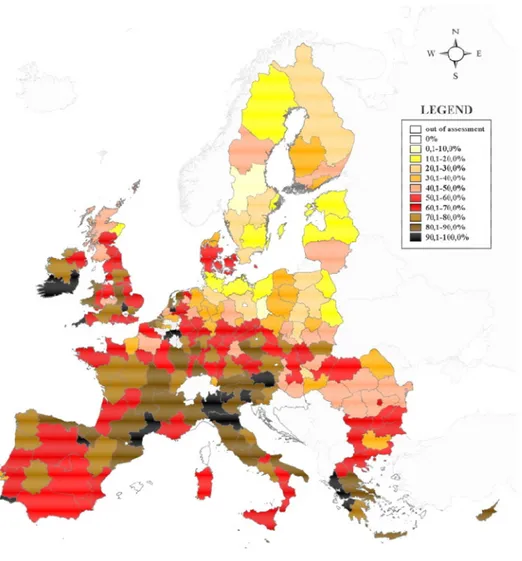
Heavy metals are known to be one of the major class of soil and water contaminants, representing more than 30% of total contamination agents in European groundwaters (Panagos et al., 2013). Industrial and mining activities are considered the main sources of heavy metal pollution; according to the European Environmental Agency, 37% of the soil contaminating activities in the European Union lead to heavy metals contamination (EEA, 2007). According to recent surveys (Tóth et al., 2016), an estimated 6.24% of agricultural land in the European Union, corresponding to 137,000 km2, needs remedial actions against heavy metal pollution (Fig. 8).
Environmental problems consequent to contamination of soils from heavy metals are related mainly to reduction of soil microbial biodiversity, decrease of soil fertility, and reduction of crop yield (McGrath et al., 1995). Moreover, increase of heavy metal contamination in plants could create potential toxic effects at higher trophic levels, since biomagnification phenomena may occur (David et al., 2012).
Fig. 8. For each European region, percentage of samples from agricultural land that were found to have any heavy metal concentration above the threshold value. For each region, at least 5 sampling sites from agricultural land were considered. From Tóth et al. (2016).
Zinc
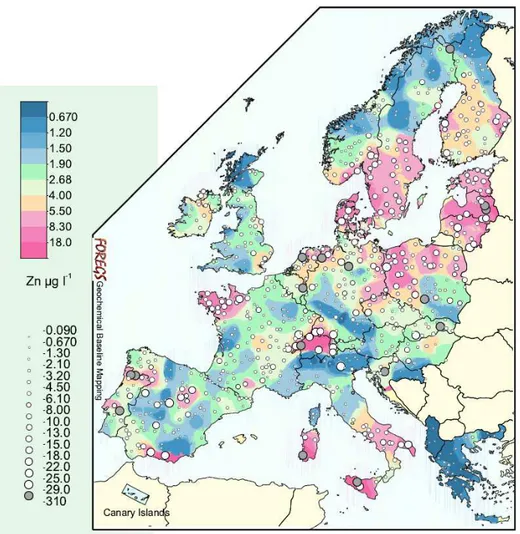
Zinc (Zn) is a heavy metal whose main inputs in soil and waters are human activities such as mining operations, coal and waste combustion, smelting and steel processing, as well as sewage sludge application to land (Fig. 9).
Zn is a plant micronutrient; due to the formation of tetrahedral complexes with N- , O- and S-donor ligands (Marschner, 1995), in its Zn+2 form is enrolled as structural cofactor to ensure appropriate protein folding (e.g. alcohol dehydrogenases, protein kinases, Zn finger domain-containing proteins), or it can be involved in the catalytic function of enzymes (e.g. carbonic anhydrases) (Broadley et al., 2007). A structural, catalytic, and regulatory action of Zn can also be observed in superoxide dismutases (Broadley et al., 2007).
Enzymatic reactions of photosynthesis are also dependent on Zn, since this metal determine the availability of HCO3-, by regulating both
carbonic anhydrase activity and stomatal opening (Marschner, 1995; Tsonev and Cebola Lidon, 2012). Moreover, Zn was observed also to have a protective role against damage caused by reactive oxygen species, as well as in assuring ion channels functionality (Cakmak, 2000).
Fig. 9. Zinc distribution in European surface waters (µg L-1). Dot size scale represents
logarithmic percentile distribution between minimum and maximum of Zn concentrations (µg L-1). Modified from GTK FOREGS Geochemical Atlas of Europe
(http://weppi.gtk.fi/publ/foregsatlas/index.php).
Nevertheless, when exposed to supra optimal concentrations of Zn, plants show toxicity symptoms such as reduced yield, leaves chlorosis and necrosis, impairment of photosynthesis and imbalance of nutrients uptake (Chaney, 1993; Di Baccio et al., 2009; Romeo et al., 2014b). Moreover, restriction of stomatal conductance as well as modification of size and
number of stomata cells, and of the mesophyll tissues have been observed under Zn excess (Di Baccio et al., 2005; 2009).
The threshold of Zn toxicity greatly differs among plant species (Tsonev and Cebola Lidon, 2012). Hyper-accumulator plants can sequestrate and mobilize in the aerial parts high amounts of heavy metals such as Zn, Cd and Pb in comparison with other plants (Baker, 1981). Hyper-accumulator species belong to the family of Brassicaceae, and unfortunately their application in phytoremediation of soils is scarce, because of their slow growth rate and poor biomass production (Baker et al., 1991).
Poplar has been demonstrated to be a good candidate for the phytoremediation of heavy metals, and in particular Zn (Di Baccio et al., 2003; 2009; 2011; Sebastiani et al., 2004; Romeo et al., 2014a; 2014b). As a consequence of Zn excess, it has been observed that development of apoplasmic barriers in poplar roots as well as lignification of xylem vessels occur closer to the root apex than in control conditions (Stoláriková et al., 2012).
However, insights in phytoremediation of Zn and other heavy metals in presence of organic compounds, and in particular of pharmaceutical and personal care products, are needed (Guittonny-Philippe et al., 2015).
Phytoremediation
In the last decades, the increasing need for new technologies aimed at the removal of pollutants from the environment caused a growing interest in phytoremediation, an evolving field in technology and science that use
plants for cleaning polluted soils, waters, and air (Morikawa and Erkin, 2003; Schröder et al., 2007). Phytoremediation offers an effective, low-cost, and environmentally friendly alternative to traditional remediation techniques such as acid leaching, excavation and landfilling, thermal desorption, and soil washing (Cunningham et al., 1995). Plants could be used for the remediation of both elemental and organic pollutants (Meagher, 2000; Mench et al., 2009). Elemental pollutants include heavy metals (such as cadmium, Zn, lead, mercury, arsenic) and radionuclides (such as uranium, cesium, strontium and tritium): these class of pollutants cannot be degraded by plant metabolism and are mainly stored inside vacuoles and cell walls (Coleman et al., 1997). On the other hand, organic pollutants such as PCBs, polycyclic aromatic hydrocarbons (PAHs), herbicides, trinitrotoluene (TNT), trichloroethylene (TCE), and pharmaceutical active compounds, could additionally enter the plant metabolism and being converted in harmless molecules (Salt et al., 1998). Plant capability to reduce the harm of pollutants or to remove them from the environment, and in particular from soils and waters, is to be ascribed mainly to the following mechanisms (Pilon-Smits, 2005) (Fig. 10):
- Phytoextraction: pollutants are taken up from the roots and translocated to the aerial parts of the plant, where they are stored mainly in vacuoles and cell walls. The aerial parts can subsequently be harvested and incinerated as hazardous waste. Together with phytostabilization, phytoextraction is the main mean of remediation of heavy metals and radionuclides polluted areas (Garbisu and Alkorta, 2001).
- Phytostabilization: pollutants are stored in root compartments and are not translocated into the aerial parts. This mechanism prevents pollutants leaching into ground waters but does not remove the contaminants from the polluted area (Salt et al., 1998).
- Phytodegradation: pollutants enter the roots and are modified by plant enzymes in order to be degraded or to form less harmful compounds. Metabolism of pollutants could occur in roots or these molecules could be translocated in the aerial parts where they will be further metabolized. Phytodegradation is the main fate of organic pollutants (Newman and Reynolds, 2004).
- Phytovolatilization: pollutants are taken up by roots and are further released in the atmosphere in volatile form. The pollutants can be released unaltered (volatile organic compounds, such as TCE), or can be modified by plant metabolism in order to make them volatile (e.g. selenium) (Limmer and Burken, 2016).
- Rhizodegradation: pollutants are metabolized either by root-released plant enzymes or by plant-associated bacteria in the rhizosphere (Bano et al., 2015).
The above mentioned mechanisms are not mutually exclusive; in fact, a same pollutant could undergo various phytoremediation pathways at once (Pilon-Smits, 2005).
Fig. 10. Schematic representation of pollutants fate in phytoremediation. Modified from Pilon-Smits, 2005.
As concerns removal of PPCPs from the environment, in the last decades several types of WWTPs were tested in order to determine their efficiency in PPCPs elimination from wastewaters (Rivera-Utrilla et al., 2013; Luo et al., 2014). Phytoremediation may be an effective and low-cost technology for the removal of these compounds (Schröder et al., 2007). Most of the studies regarding phytoremediation of PPCPs are based on herbaceous species such as Scirpus (Zhang et al., 2012; 2013a,b),
Phragmites (Liu et al., 2013), and Thypa (Bartha et al., 2014), that have
caffeine (Zhang et al., 2013a), diclofenac (Zhang et al., 2012; Bartha et al., 2014), and various antibiotics (Liu et al., 2013).
Very few studies investigate the removal of these compounds by tree species, namely poplar and willow: in particular, Iori and coworkers established that these species are very tolerant to high concentrations (>10 mg L-1) of ibuprofen, and are able to enhance the removal of this PPCP from nutrient medium (Iori et al., 2012, 2013). Accumulation of sulfonamide antibiotics in willow roots was investigated by Michelini and colleagues (2012, 2014), highlighting adverse effects on root growth at environmentally relevant soil concentrations (10 mg kg−1 sulfadiazine).
Poplar
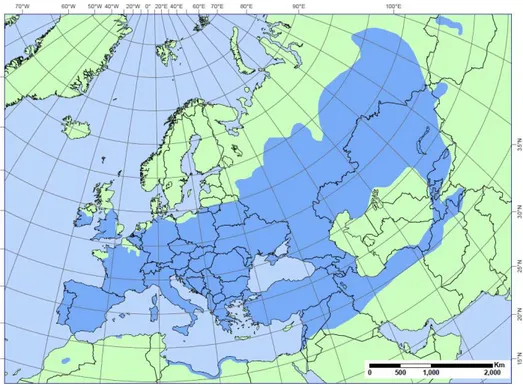
Poplars trees (genus Populus) belong to the Salicaceae family, whose species are well known for their phytoremediation potential of both heavy metals and organic pollutants (Taylor, 2002; Sebastiani et al., 2004; Giachetti and Sebastiani, 2006; Marmiroli et al., 2011; Zacchini et al., 2011; Romeo et al., 2014b; Pilipović et al., 2015). Poplars distribution spreads in a wide range of different climatic areas and latitudes (Fig. 11). The wide geographical distribution of this genus indicate that poplars are able to face many different environmental conditions, circumstance that makes these species good candidates in stress tolerance experiments (Cronk, 2005). Moreover, they could be easily propagated both as woody cuttings and via in vitro micropropagation, guaranteeing highly homogeneous plant material (Ahuja, 1987). The complete sequencing of
Populus trichocarpa genome (Tuskan et al., 2006) paved the way to in-depth exploration of -omics traits concerning poplar tolerance to abiotic stress, such as variation of transcriptome profiles under Zn stress (Di Baccio et al., 2011), proteomic response to heavy metals such as cadmium and Zn (Kieffer et al., 2009; Romeo et al., 2014a), and variation of epigenetic traits, that are main factors in determining phenotypic plasticity and adaptation to unfavorable environments (Ariani et al., 2016). Poplars great ability to remove contaminants as heavy metals and organic xenobiotics from soils and waters is due to the high levels of transpiration, the deep root system, and the high growth rate of these species (Capuana, 2011; Pilipović et al., 2015). In particular, they have been demonstrated to be excellent candidates for the phytoremediation of organic compounds (Burken and Schnoor, 1998); in fact, these species have been demonstrated to absorb and metabolize herbicides such as atrazine (Burken and Schnoor, 1997), solvents such as TCE (Newman et al., 1997; Shang and Gordon, 2002), and other toxic compounds such as PBC (Zhai et al., 2010; 2011). Moreover, poplar species are of interest for the remediation of pharmaceutical active compounds from surface waters, since they have been demonstrated to absorb ibuprofen, a widespread used painkiller (Iori et al., 2012).
Fig. 11. In blue, natural European distribution area of Populus nigra L. Modified from EUFORGEN 2015 (www.euforgen.org).
Concerning tolerance and uptake of heavy metals such as cadmium, chromium, copper, and Zn, numerous studies have been performed on several poplar species and clones (Di Baccio et al., 2003; 2009; 2014; Sebastiani et al., 2004; Tognetti et al., 2004; Giachetti and Sebastiani, 2006; Borghi et al., 2007, 2008; Gaudet et al., 2011; Romeo et al., 2014b; Romè et al., 2016). It has been observed that different poplar clones have different responses when exposed to heavy metals, regarding biomass allocation, heavy metal uptake and translocation, and tolerance to stress. In particular, Populus alba L. Villafranca clone has been found to accumulate heavy metals such as nickel (Katanić et al., 2015), Zn (Romeo et al., 2014b), cadmium (Romè et al., 2016), copper (Borghi et al., 2008) and arsenic (Di Lonardo et al., 2011), showing less toxicity symptoms
when compared to other species and clones (Di Lonardo et al., 2011; Romeo et al., 2014b; Katanić et al., 2015). Villafranca clone has been also used as standard genotype to compare lead accumulation in white poplars under in vitro conditions (Kovačević et al., 2013).
Aims
The aim of the present thesis was to determine the fate of some of the pharmaceutical and personal care products (PPCPs) more widely detected in wastewater treatment plants effluents and surface waters in phytoremediation trials using poplar, a model tree species. The target pollutants were investigated alone (as for caffeine, see Annex I; erythromycin, see Annex II; diclofenac, see Annex IV), or in association with heavy metals (SDS and Zn, see Annex III).
In particular uptake, translocation, and plant metabolism of the target compounds, as well as the physiological response of poplar in terms of growth, photosynthetic efficiency, and photosynthetic pigments content were investigated.
The objectives were reached conducting the experiments in hydroponic systems, using Populus alba L. Villafranca clone derived from in vitro culture. Experiments started only after an acclimation period from in vitro to in vivo conditions, which was protracted until abscission of the leaves developed under the microenvironment and formation of new in vivo leaves. Liquid chromatography coupled with tandem mass spectrometry was chosen for the detection and quantification of PPCPs and their metabolites in poplar organs, being the leader methodology for the analyses of these compounds.
Results and Discussion
Poplar and Caffeine
(Annex I)
Since endogenous caffeine metabolism had been predicted in silico in
Populus trichocarpa (KEGG pathway pop00232), uptake of
caffeine-(trimethyl-13C) and its degradation in theobromine-(dimethyl-13C) and theophylline-(dimethyl-13C) in roots, stem, and leaves of P. alba Villafranca clone was investigated. The use of caffeine-(trimethyl-13C) allowed us to discriminate the exogenous caffeine and its metabolites from possible endogenous ones, thanks to tandem mass spectrometric measurements in combination with liquid chromatography (LC-MS/MS). Poplar plants were grown in hydroponics, and irrigated with either 2 mg L-1 caffeine-(trimethyl-13C) or control Hoagland nutritive solution. Caffeine-(trimethyl-13C) concentration was chosen according to literature (Zhang et al., 2013a). Plants were harvested after 7 and 28 days of exposure.
Results showed that caffeine enhanced poplar growth both after 7 days of exposure (+ 43 % in 2 mg L-1 caffeine-(trimethyl-13C)-treated plants in
comparison to control ones), and after 28 days of exposure (+ 31 %). Our findings in terms of physiological parameters confirmed the results from Vitória and Mazzafera (1997), describing that caffeine can induce internodes elongation and fresh weight allocation.
Endogenous caffeine, theobromine, and theophylline were detected in all plant organs: in particular, theophylline was the most abundant endogenous compound both in control and treated plants, having the
highest concentration in roots of control plants after 7 days of treatment (57±29.5 µg g-1 fresh weight, FW). A general variation in concentration of endogenous caffeine, theobromine, and theophylline in caffeine-(trimethyl-13C)-treated plants was detected in comparison with control ones. In roots, we measured a highly significant (P≤0.001) decrease of all three endogenous metabolites in plants treated with
caffeine-(trimethyl-13C): all three compounds showed a decrease in a range of 99±0.05 %.
The difference in concentrations observed as a consequence of caffeine-(trimethyl-13C) treatment could be due to the activation of metabolic pathways aimed at maintaining caffeine homeostasis into the plant.
After 28 days of caffeine-(trimethyl-13C) exposure, we observed a reduction of all compound concentrations, especially of endogenous theophylline and theobromine, both in control and treated plants. As suggested by Mazzafera (2004), synthesis and degradation of caffeine are related to the tissue developmental stage.
Xanthines such as theophylline and theobromine have been proved to have a role as natural pesticides (Nathanson, 1984). Moreover, occurrence of caffeine and theophylline have also been described in Citrus flowers, in which they were thought to have a cytokinin-like effect in addition to their defense role (Kretschmar and Baumann, 1999).
Concerning uptake and metabolism of exogenous compounds, caffeine-(trimethyl-13C) could be detected in all plant organs of Villafranca clone, as well its metabolites theobromine-(dimethyl-13C) and theophylline-(dimethyl-13C). The highest concentrations (2679±507.1 ng g-1 FW caffeine-(trimethyl-13C); 1067±70.1 ng g-1 FW
(theobromine-(dimethyl-13C); 55±65.4 ng g-1 FW theophylline-(dimethyl-13C) were found after 7
days of treatment in leaves. The main metabolite derived from the catabolism of caffeine-(trimethyl-13C) was theobromine-(dimethyl-13C),
while theophylline-(dimethyl-13C) was present only in a less extent at the
first sampling time.
These findings confirmed similar results obtained by Zhang et al. (2013a) on Scirpus validus exposed to caffeine in hydroponic conditions, and proved that P. alba Villafranca clone absorbs and degrades exogenous caffeine (log Kow = − 0.07), although historically compounds with high
polarity (log Kow < 1) were considered not to be significantly taken up by
plants (Briggs et al. 1982; Burken and Schnoor 1998).
Moreover, to our knowledge, this was the first time that presence of endogenous caffeine, theobromine, and theophylline was reported in poplar.
Poplar and Erythromycin
(Annex II)
Uptake of the antibiotic erythromycin as well as Villafranca clone physiological response were investigated in a phytoremediation trial with four erythromycin concentrations (0, 0.01, 0.1 and 1 mg L-1) and two sampling times (3 and 28 days). Moreover, a timeline of chlorophyll a fluorescence measurements was performed, in order to gain insights on the performance of photosystem II, since it has been observed to be strongly affected by erythromycin (Deng et al., 2014; Wan et al., 2015). The range of concentration was chosen to induce poplar physiological response and to gain evidence of drug uptake in different plant organs, starting from environmentally realistic erythromycin concentration (0.01 mg L-1).
Plants showed good health all over the period of treatment, for all the concentrations of erythromycin tested and no differences in growth was observed between control and treated plants, neither after 3 nor 28 days of exposure.
No differences between treatments were observed also concerning neoxanthin, violaxanthin, lutein, chlorophyll a, chlorophyll b, and β carotene concentrations. Moreover, we did not observe the presence of zeaxanthin or other pigments related to the activation of xanthophyll cycle in leaves of treated plants. These data diverge with previous literature, where a decrease in chlorophyll content was observed as a consequence of erythromycin treatment, both in Triticum aestivum (Opriş et al., 2013) and in the blue-green algae Microcystis flos-aquae (Wan et al., 2015).
Erythromycin could be found in all organs of P. alba Villafranca clone at both sampling times, although this antibiotic has been considered not likely to be absorbed through the cellular membranes and translocated into crops, since its relatively high molecular weight (Pan et al., 2014). For all treatments, erythromycin was found to be mainly concentrated in roots. Since plant uptake of PPCPs in mainly driven by their solubility, polarity and hydrophobicity, described by the octanol-water partition coefficient (Kow) (Schnoor et al., 1995; Dettenmaier et al., 2009), the
hydrophobic behavior of erythromycin (log Kow = 3.06) could explain the
higher concentration of erythromycin found in roots.
In all organs we observed a significant increase in erythromycin concentration as a consequence of 1 mg L-1 treatment, in comparison with 0.01 and 0.1 mg L-1 erythromycin.
Mass chromatograms of leaves and roots samples of P. alba plants treated with erythromycin revealed the presence of three additional peaks
(retention times = 6.82, 7.23, 7.50 min), that differed from the erythromycin peak (retention time = 7.02 min). Although these peaks were partially observed also at the lowest erythromycin concentration, they were considerably over the signal-to-noise ratio only in the highest erythromycin treatment (1 mg L-1). These metabolites are characterized by the same parent ion-daughter ion transitions of erythromycin (734.7-158.1 m/z and 734.7-576.5 m/z). These additional peaks could be considered epimers of erythromycin, since they share the same parent ion-daughter ion transitions but have a different retention time. Epimers formation has been previously observed in Phragmites australis exposed to oxytetracycline and other antibiotics (Liu et al., 2013).
Concerning the efficiency of photosystem II, no negative effects could be observed as a consequence of erythromycin treatments, in contradiction with the studies on cyanobacteria, where a general reduction of photosystem II efficiency was observed (Deng et al., 2014; Wan et al., 2015). In new leaves (1 ≤ Leaf Plastochron Index ≤ 7) of treated plants a statistical significant increase in quantum efficiency of photosystem II (ΦPSII) at day 3 was observed, as well as an increase of electron transport rate (ETR) at 3, 7, 14, and 21 days of experiment. Old leaves (LPI > 7) showed an increase in ΦPSII, ETR, and photochemical quenching (qP) after 3 and 7 days of treatment along with a reduction of non-photochemical quenching (NPQ) and regulated energy dissipation (YNPQ) at day 7, as well as a decrease of non-regulated energy dissipation (YNO) after 3, 7, and 21 days of experiment. Differences observed between control and treated plants in terms of photosystem II efficiency were not depending on the dose of erythromycin given.
An increase in ETR was observed on maize plants under abiotic stress like low temperatures (Fryer et al., 1998), when re-directing of the
electron flux towards the creation of antioxidant systems was hypothesized. Otherwise, in Paulownia plants, the enhancement of ETR observed under salt stress (Stefanov et al., 2016) was associated with possible structural changes in the thylakoid membranes aimed at maintaining photosynthetic efficiency.
Principal component analysis of photosynthetic parameters such as maximum quantum efficiency of photosystem II photochemistry (Fv/Fm), ΦPSII, ETR, NPQ, qP, YNPQ, YNO, showed that distribution of the data is a consequence of different leaves age, while no differences were detected between erythromycin treatments, nor sampling times.
In conclusion, P. alba can tolerate moderate erythromycin pollution maintaining good health, as also confirmed by the time-course monitoring of photosystem II efficiency.
Poplar, SDS and Zn
(Annex III)
In order to asses plant toxicity of SDS
and
how this molecule influence Zn uptake, P. alba Villafranca clone was exposed to four treatments: 1 mM Zn(NO3)2; 0.5 mM SDS; 1 mM Zn(NO3)2 + 0.5 mM SDS; andcontrol. Plants were harvested after 21 days of treatment. Zn concentration is known to be sub-symptomatic for Villafranca clone, despite the high rate of metal accumulation of this clone (Romeo et al., 2014), while a concentration of 0.5 mM SDS can be found in literature in studies concerning enhancement of phytoremediation in heavy metals polluted soils (Liu et al., 2008).
Control and 1 mM Zn(NO3)2 treated plants maintained healthy traits for
the whole experimental period, as already described by Romeo and coworkers (2014b). On the contrary, 0.5 mM SDS treated plants showed wide necrosis areas on basal leaves already after 2 days of treatment, which expanded with an acropetal trend and eventually lead to the loss of the most damaged leaves (-13% leaves compared to control after 21 days of treatment). Surprisingly, the same symptoms appeared in the cross-stressed plants (1 mM Zn+0.5 mM SDS) only after 9 days of exposure, and their furtherance was faster (-25% leaves compared to control after 21 days of treatment). The extensive foliar necrosis starting from older leaves, and subsequent leaves abscission observed in poplar plants treated with 0.5 mM SDS and 1 mM Zn + 0.5 mM SDS is a clear symptom of sodium toxicity (Beritognolo et al., 2007). The SDS molecule in aqueous solution dissociates in Na(+) and dodecyl sulfate(-), inducing a salt stress-like phenotype. Populus alba L. response to salt stress varies significantly according to the genotype: a wide range of cases has been reported in literature (Beritognolo et al., 2007; Mao et al., 2008; Beritognolo et al., 2011). Beritognolo and coworkers (2007) in particular, reported that genotypes from coastal regions tolerate saline concentrations up to 200mM NaCl, while the same concentration induced cuttings’ death according to Mao and colleagues (2008).
HPLC determination of photosynthetic pigments in apical and median leaves revealed no differences in lutein, chlorophyll a and b, and β-carotene contents between control and treated plants. On the contrary, in basal leaves, a statistically significant increase of lutein (+52.9 %), chlorophyll a (+48.4 %) and β-carotene (+66.4 %) in 1 mM Zn treated plants was observed, compared to control. Increase of chlorophylls and
carotenoids content under Zn exposure was observed also in willow plants treated with up to 5 mM Zn (Borowiak et al., 2015).
Analysis of chlorophyll a fluorescence showed that PSII efficiency of basal and median leaves was the most affected by factor SDS, as expected from the expansion of wide necrosis areas in leaves of 0.5 mM SDS and 1 mM Zn + 0.5 mM SDS treated plants, indicating Na damage to the photosynthetic apparatus. In fact, several authors described impairment of photosynthetic rate and of stomatal conductance in various poplar species under salt stress (Ma et al., 1997; Abbruzzese et al., 2009). Moreover, decrease of ETR and increase of NPQ have been also observed in tomato plants as a consequence of Na exposure (Zribi et al., 2009).
Regarding Zn and Na organ distribution, Zn concentration was higher in roots both in 1 mM Zn and 1 mM Zn+ 0.5 mM SDS treated plants, confirming the root-accumulator phenotype of Villafranca clone (Romeo et al., 2014b). Both for Zn and Na uptake, no differences could be observed depending on leaves age and position; in fact, for each treatment, apical, median and basal leaves showed a similar accumulation trend of both elements. One-tailed t-test analysis between Zn concentration in 1 mM Zn and 1 mM Zn+ 0.5 mM SDS treated plant organs revealed a significant (P=0.03) increase in Zn concentration in basal leaves of the cross-stressed plants, while for the same treatment a decrease (P=0.05) of Zn concentration was observed in roots. Contrasting results are found in literature about effective increase of plant absorption of heavy metals as a consequence of co-exposure with surfactants; SDS enhanced uptake of Cd in Althaea rosea and Calendula officinalis (Liu et al., 2008; 2009), while no effects on Cu uptake was observed in Halimione portulacoides (Almeida et al., 2009). In our experiment, there was no statistical difference (P= 0.4564) between total Zn concentration of 1 mM Zn and 1
mM Zn + 0.5 mM SDS treated plants. However, since SDS caused serious damage to leaves and a consequent reduction of plant biomass, total Zn content in plants treated with 1 mM Zn + 0.5 mM SDS at the end of the experiment was lower than 1 mM Zn treated plants (-23%).
SDS has been found to be a major widespread contaminant; in fact, LC-MS/MS analyses revealed traces of dodecyl sulfate also in samples belonging to 1 mM Zn and control treatments. However, dodecyl sulfate accumulates in significantly larger amounts (P < 0.01) in all organs (apical, median, and basal leaves, stem and roots) of plants treated with 0.5 mM SDS and 1 mM Zn + 0.5 mM SDS. In particular, accumulation of dodecyl sulfate up to 4 mg g-1 fresh weight could be observed in roots of SDS treated plants. Plant uptake of detergents is usually not investigated in trials concerning surfactant-enhanced phytoremediation of heavy metals (Liu et al., 2008; 2009; Almeida et al., 2009). In trials concerning the ecotoxicity of SDS, it has been observed that this molecule is accumulated in fronds of Lemna minor, and that there is a positive correlation between the concentration of this detergent in the growth media and its accumulation in plant tissues (Dirilgen and Ince, 1995).
Poplar and Diclofenac
(Annex IV)
Plant uptake and metabolism of diclofenac was investigated in a trial with P. alba Villafranca clone treated with 1 mg L-1 diclofenac Hoagland solution, and control. Physiological parameters, antioxidant enzymes activity, and diclofenac and its metabolites concentrations were
investigated after 1, 7, and 28 days of treatment. Diclofenac concentration was chosen as a good compromise between environmental realistic concentrations and the possibility of observing plant metabolites of this pharmaceutical active compound (Bartha et al., 2014).
Moreover, a parallel trial without plants was set up in order to investigate the eventual environmental degradation of diclofenac. Daily LC-MS/MS measurements revealed that after 7 days under greenhouse conditions only 5% of the initial diclofenac concentration (1 mg L-1) was still present in the nutritive solution without plants. Diclofenac susceptibility to photodegradation have been described by several authors (Musa and Eriksson, 2009; Zhang et al., 2011; Zhang et al., 2012). However, the environmental degradation of diclofenac observed in the trial without plants seems to be attributable to microbial degradation rather than photolysis, since correlation between diclofenac removal rate and solar radiation was found not significant (P=0.563). Matamoros and colleagues (2012) found that diclofenac in uncovered mesocosms has a half-life of 10 days; in our experimental conditions, diclofenac half-life in absence of plants was reduced to 3 days, as measured also by Zhang and coworkers (2012).
Despite the high environmental degradation rate of diclofenac, LC- MS/MS analyses of roots extracts revealed presence of diclofenac and of its metabolites at all sampling times. In particular, diclofenac was observed to rapidly undergo plant metabolism, as after 1 day of treatment 14.76±2.42 ng g-1 fresh weight of native diclofenac could be found in roots as well as its phase I metabolite hydroxydiclofenac (OH-diclofenac). Moreover, already after 1 day of treatment, conjugates of both diclofenac and of OH-diclofenac were observed. Relative amounts of the metabolites, calculated as the peak area of the metabolites on the peak area of
diclofenac, were 0.96 for OH-diclofenac, 1.06 for total diclofenac conjugates, and 0.29 for total OH-diclofenac conjugates. DCF represented the 35% on the totality of metabolites detected in roots. After 7 days of treatment was not possible to detect native diclofenac in poplar roots. However, OH-diclofenac, and both diclofenac and OH-diclofenac conjugates were present at much more higher concentrations (about 4, 19 and 23 times in comparison with day 1, respectively). Among detected metabolites, total diclofenac conjugates represent 65% of the total. Due to the renewal of the nutritive solution, native diclofenac was found in roots after 28 days of treatment (26±2.2 ng g-1 fresh weight). Accumulation of metabolites in long-term treated plant was observed: in particular, OH-diclofenac relative amount was 19.89 (43 times more in comparison with day 1), diclofenac conjugates relative amount was 98.4 (195 times more in comparison with day 1), and OH-diclofenac relative amount was 63.6 (483 times more in comparison with day 1). After 28 days of exposure, DCF accounted for less than 1% of total metabolites, being diclofenac conjugates the most detected (54%).
It has been reported in herbaceous species that diclofenac can be translocated in the aerial part of the plant, although the highest concentrations are found in roots (Zhang et al., 2012; Bartha et al., 2014). In the present analyses, presence of diclofenac and/or its related plant metabolites was not detected in stem and leaves of P. alba Villafranca clone at none of the sampling times. This may be due to the high limit of detection of the instrument (30 ng mL-1, corresponding to 120 ng g-1 FW). In fact, signals detection in root samples was improved thanks to a strong root matrix enhancement effect (+397.8%).
In order to perform an in-depth plant phenotyping, activities of stress-related enzymes such as glutathione-S-transferase (GST), glutathione
reductase (GR), ascorbate peroxidase (APX), and peroxidases (POX) were investigated in all plant organs. Two different substrates (fluorodifen and 1-Chloro-2,4-dinitrobenzene, CDNB) were applied in order to investigate the activity of different GST isoforms (Bartha et al., 2014). Stress enzymes activity showed a distinct pattern according to different plant organs and sampling times. In particular, in roots, were DCF and its plant metabolites were detected, GST activity was found to be increased after 28 days of treatment. GSTs could have a direct role in plant metabolism of PhACs, mediating the addition of the thiol group of glutathione to the electrophilic centers of the pollutants, thus increasing the hydrophilicity of the target compound (Christou et al., 2016). APOX activity increased in roots in the short-term exposure, indicating an enhancement of ascorbate-dependent scavenging of reactive oxygen species (Foyer and Noctor, 2011), together with an enhanced activity of GR at day 7. Enhancement of GR activity at low DCF concentrations (<100 µg L-1) was reported to occur in Lemna minor (Kummerová et al., 2016). On the contrary, in roots of Typha latifolia exposed to DCF no changes in GR activity were observed (Bartha et al., 2014).
Concerning the aerial parts of the plants, GST activity was found to be increased in stem after 28 days of treatment when CDNB was used as substrate, while no change in GST activity was recorded when RM containing fluorodifen was used: these findings highlight the different roles of GST isoforms in stress response (Schröder et al., 1997). APOX activity was found decreased in leaves and stem in the shot-term response, that corresponded to the enhanced activity of this enzyme in roots at the same sampling times. Regarding POX activity, it was observed to be diminished as a consequence of DCF treatment in short (1 day) and medium (7 days) term. Peroxidases have been demonstrated to have
multiple functions in plant metabolism (Passardi et al., 2005). Moreover, plant peroxidases are considered a class of enzymes with the capability to detoxify xenobiotics, as demonstrated for DCF in hairy root cultures of Armoracia rusticana (Huber et al., 2016), and for the toxic pesticide 2,4-dichlorophenol (2,4-DCP) in cell cultures of Brassica napus (Agostini et al., 2003). In literature, POX activity is usually found enhanced as a consequence of DCF exposure (Bartha et al., 2014). Anyway, some pollutants such as polycyclic aromatic hydrocarbons have been demonstrated to cause a decrease in POX activity (Dubrovskaya et al., 2017); these findings may be related with an inhibitory effect of the native pollutant or of its products of enzymatic oxidation.
Conclusions and further perspectives
Results highlighted the potential of Populus alba L. Villafranca clone for the remediation of compounds belonging to the class of PPCPs. All target compounds were successfully detected in poplar organs, thus indicating the capability of this clone to uptake and translocate this class of organic pollutants. Most importantly, Villafranca clone maintained good health when treated with all the tested pollutants, with the exception of SDS, where the release of sodium from the molecule caused severe foliar necrosis.
Moreover, plant metabolism of PPCPs was detected: in particular, degradation of caffeine-(trimethyl-13C) in theobromine-(dimethyl-13C) and theophylline-(dimethyl-13C) through N-demethylases of the
endogenous caffeine metabolism pathway was observed.
As for erythromycin, additional peaks in the mass chromatograms of plant extracts were detected, and considering the short shifts of retention times and the mass transitions, they are thought to be epimers of erythromycin. Further studies are needed in order to confirm the structures of these metabolites.
Identification of products of phase I and II of plant metabolism was possible in the diclofenac treatment, where the hydroxylated form of diclofenac was detected, as well as the conjugated forms of both native diclofenac and OH-diclofenac.
The present work puts the basis for further investigations of poplar tolerance mechanisms to organic pollutants such as PPCPs; studies on tree uptake of PPCPs mixtures, as well as studies on clonal and genotype-specific response to selected PPCPs, both in hydroponics and field
conditions, should be performed in order to assess the feasibility of PPCPs phytoremediation.
Moreover, the present thesis represents a starting point for studies on tree metabolism of pharmaceutical pollutants; future in vitro studies could have a great importance in characterizing PPCPs modifications due to plant metabolism, as well as in identifying the enzymes involved in the metabolism of these xenobiotics. In addition, studies on root exudates and studies considering the associations between roots, bacteria, and fungi, could provide further insights in the removal of PPCPs from the environment.
Acknowledgements
I would like to express sincere gratitude to Prof. Luca Sebastiani and Dr. Alessandra Francini for their high quality scientific guidance. Heartfelt thanks also to Cristina Ghelardi, Gaia Monteforti, and Antonio Minnocci for their scientific and human support through these years, and to all my colleagues at Biolabs, for their help and friendship.
I would like also to thank Dr. Andrea Raffaelli, for sharing his expertise in mass spectrometry, and Prof. Peter Schröder, who gave me the opportunity to work in an international and stimulating research group at Helmholtz Zentrum in Munich, Germany.
46
References
Abbruzzese G, Beritognolo I, Muleo R, Piazzaia M, Sabatti M, Scarascia Mugnozza G, Kuzminsky E (2009) Leaf morphological plasticity and stomatal conductance in three Populus alba L. genotypes subjected to salt stress. Environmental and Experimental Botany 66: 381–388.
Abhilash PC, Jamil S, Singh N (2009) Transgenic plants for enhanced biodegradation and phytoremediation of organic xenobiotics. Biotechnology Advances 27: 474-488. Agostini E, Coniglio MS, Milrad SR, Tigier HA, Giulietti AM (2003) Phytoremediation of 2,4-dichlorophenol by Brassica napus hairy root cultures. Biotechnology and Applied Biochemistry 37: 139–144.
Ahuja MR (1987) In vitro propagation of poplar and aspen. In Cell and tissue culture in forestry - Case Histories: Gymnosperms, Angiosperms and Palms, pp 207-223, Springer Netherlands, Dordrecht. ISBN 978-94-017-0992-7.
Almeida CMR, Dias AC, Mucha AP, Bordalo AA, Vasconcelos MTSD (2009) Influence of surfactants on the Cu phytoremediation potential of a salt marsh plant. Chemosphere 75:135–140.
Anderberg EK, Artursson P (1993) Epithelial transport of drugs in cell culture. VIII: effects of sodium dodecyl sulfate on cell membrane and tight junction permeability in human intestinal epithelial (Caco-2) cells. Journal of Pharmaceutical Science 82: 392-46under salinity stress. Trees 21: 465–477.
Ariani A, Romeo S, Groover AT, Sebastiani L (2016) Comparative epigenomic and transcriptomic analysis of Populus roots under excess Zn. Environmental and Experimental Botany 132: 16–27.
Ashihara H, Crozier A (2001) Caffeine: a well known but little mentioned compound in plant science. Trends in Plant Science 6: 407-413.
Ashihara H, Sano H, Crozier A (2008) Caffeine and related purine alkaloids: biosynthesis, catabolism, function and genetic engineering. Phytochemistry 69: 841-856. Baker AJM (1981): Accumulators and excluders - strategies in the response of plants to heavy metals. Journal of Plant Nutrition 3: 643-654.
Baker AJM, Reeves RD, McGrath SP (1991) In situ decontamination of heavy metal polluted soils using crops of metal-accumulating plants-a feasibility study. In: Hinchee RE, Olfenbuttel RF, editors. In situ bioreclamation. Boston: Butterworth-Heinemann, pp. 600-605.
Bano A, Shahzad A, Siddiqui S (2015) Rhizodegradation of hydrocarbon from oily sludge. Journal of Bioremediation and Biodegradation 6:289-300.
Bartha B, Huber C, Schröder P (2014) Uptake and metabolism of diclofenac in Typha latifolia- how plants cope with human pharmaceutical pollution. Plant science 227:12-20.
Beritognolo I, Harfouche A, Brilli F, Prosperini G, Gaudet M, Brosche M, Salani F, Kuzminsky E, Auvinen P, Paulin L, Kangasjarvi J, Loreto F, Valentini R, Scarascia Mugnozza G, Sabatti M (2011) Comparative study of transcriptional and physiological responses to salinity stress in two contrasting Populus alba L. genotypes. Tree Physiology 31: 1335-1355.
Borghi M, Tognetti R, Monteforti G, Sebastiani L (2007) Responses of Populus × euramericana (P. deltoids × P. nigra) 40 clone Adda to increasing copper concentrations. Environmental and Experimental Botany 61: 66-73.
Borghi M, Tognetti R, Monteforti G, Sebastiani L (2008) Responses of two poplar species (Populus alba and Populus x canadensis) to high copper concentrations. Environmental and Experimental Botany 62: 290-299
Borowiak K, Gąsecka M., Mleczeck M, Dąbrowski J, Chadzinikolau T, Magdziak Z, Golin´ski P, Rutkowski P, Kozubik T (2015) Photosynthetic activity in relation to chlorophylls, carbohydrates, phenolics and growth of a hybrid Salix purpurea x triandra x viminalis 2 at various Zn concentrations. Acta Physiologiae Plantarum 37: 155-167. Briggs GG, Bromilow RH, Evans AA (1982) Relationships between lipophilicity and root uptake and translocation of non-ionized chemicals by barley. Pesticide Science 13: 495-504.
Broadley MR,White PJ, Hammond JP, Zelko I, Lux A (2007). Zinc in plants. New Phytologist 173: 677–702.
Buerge II, Poiger T, Müller MD, Buser HR (2003) Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environmental Science and Technology 37: 691-700.
Burken JG, Schnoor JL (1997) Uptake and metabolism of atrazine by poplar trees. Environmental Science and Technology 31: 1399-1406.
Burken JG, Schnoor JL (1998) Predictive relationships for uptake of organic contaminants by hybrid poplar trees. Environmental Science and Technology 32: 3379-3385.
Capuana M (2011) Heavy metals and woody plants biotechnologies for phytoremediation. iForest Biogeosciences and Forestry 4: 7-15.
Cakmak I (2000). Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytologist 146: 185–205.
Chaney RL (1993) Zinc phytotoxicity. In: Robson AD, ed. Zinc in soil and plants. Dordrecht, the Netherlands: Kluwer Academic Publishers 135-150.
Chaturvedi V, Kumar A (2011) Diversity of culturable sodium dodecyl sulfate (SDS) degrading bacteria isolated from detergent contaminated ponds situated in Varanasi city, India. International Biodeterioration and Biodegradation 65: 961–971.
Chen G, den Braver MW, van Gestel CAM, van Straalen NM, Roelofs D (2015) Ecotoxicogenomic assessment of diclofenac toxicity in soil. Environmental Pollution 199 : 253-260.
Cherian S, Oliveira MM (2005) Transgenic plants in phytoremediation: recent advances and new possibilities. Environmental Science and Technology 39: 9377-9390.
Christou A, Antoniou C, Christodoulou C, Hapeshi E, Stavrou I, Michael C, Fatta-Kassinos D, Fotopoulos V (2016) Stress-related phenomena and detoxification mechanisms induced by common pharmaceuticals in alfalfa (Medicago sativa L.) plants. Science of the Total Environment 557–558: 652–664
Coleman JOD, Blake-Kalff MMA, Davies TGE (1997) Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends in Plant Science 2: 144-151.
Cronk QCB (2005) Plant eco-devo: the potential of poplar as a model organism. New Phytologist 166: 39-48.
Cserháti T, Forgács E, Oros G (2002) Biological activity and environmental impact of anionic surfactants. Environment International 28: 337– 348.
Cunningham SD, Berti WR, Huang JW (1995) Phytoremediation of contaminated soils. Trends in Biotechnology 13: 393-397.
Dantas G, Sommers MOA, Oluwasegun RD, Church GM (2008) Bacteria subsisting on antibiotics. Science 320: 100-103.
David IG, Matache ML, Tudorache A, Chisamera G, Rozylowicz L, Radu GL (2012) Food chain biomagnification of heavy metals in samples from the lower Prut floodplain natural park. Environmental Engineering and Management Journal 11: 69-73.
Deng C-N, Zhang D-Y, Pan, X-L (2014) Toxic effects of erythromycin on photosystem I and II in Mycrocistis aeruginosa. Photosynthetica 52: 574-580.
Di Baccio D, Tognetti R, Sebastiani L Vitagliano C (2003) Responses of Populus deltoides × Populus nigra (Populus × euramericana) clone I-214 to high zinc concentrations. New Phytologist 159: 443-452.
Di Baccio D, Kopriva S, Sebastiani L, Rennenberg H (2005). Does glutathione metabolism have a role in the defence of poplar against zinc excess? New Phytologist 167: 73-80.
Di Baccio D, Tognetti R, Minnocci A, Sebastiani L (2009) Responses of the Populus × euramericana clone I-214 to excess zinc: Carbon assimilation, structural modifications, metal distribution and cellular localization. Environmental and Experimental Botany 67: 153-163