Absorption and pharmacokinetics of green tea catechins in beagles
Maria de Lourdes Mata-Bilbao
1, Cristina Andre´s-Lacueva
1, Elena Roura
1, Olga Ja´uregui
2, Elvira Escribano
3,
Celina Torre
4and Rosa M. Lamuela-Ravento´s
1*
1Department of Nutrition and Food Science, XARTA, INSA, Faculty of Pharmacy, University of Barcelona, Av. Joan XXIII s/n, 08028 Barcelona, Spain
2Scientific and Technical Services, University of Barcelona, Llu´is, Sole i Sabaris 1-3, E-08028 Barcelona, Spain 3Biopharmaceutics and Pharmacokinetics Unit, Faculty of Pharmacy, University of Barcelona, Av. Joan XXIII s/n, 08028 Barcelona, Spain
4Affinity Pet-care, 08174 St Cugat Barcelona, Spain
(Received 7 August 2007 – Revised 26 November 2007 – Accepted 28 November 2007 – First published online 21 January 2008)
The present study evaluates for the first time in dogs, the kinetics of green tea catechins and their metabolic forms in plasma and urine. Ten beagles were administered 173 mg (12·35 mg/kg body weight) of catechins as a green tea extract, in capsules. Blood samples were collected during 24 h after intake and urine samples were collected during the following periods of time: 0 – 2, 2 – 6, 6 – 8 and 8 – 24 h. Two catechins with a galloyl moiety and
three conjugated metabolites were detected in plasma. Most of the detected forms in plasma reached their maximum plasma concentration (Cmax) at
around 1 h. Median Cmaxfor (2 )-epigallocatechin-3-gallate (EGCG), (2 )-epicatechin-3-gallate (ECG), (2 )-epigallocatechin glucuronide
(EGC-glucuronide), (2 )-epicatechin glucuronide (EC-(EGC-glucuronide), (2 )-epicatechin sulphate (EC-sulphate) were 0·3 (range 0·1 – 1·9), 0·1 (range 0 – 0·4), 0·8 (range 0·2 – 3·9), 0·2 (range 0·1 – 1·7) and 1 (range 0·3 – 3·4) mmol/l, respectively. The areas under the plasma concentration v. time
curves (AUC0!24) were 427 (range 102 – 1185) mmol/l £ min for EGC-glucuronide, 112 (range 53 – 919) mmol/l £ min for EC-sulphate, 71
(range 26 – 306) mmol/l £ min for EGCG, 40 (range 12 – 258) mmol/l £ min for EC-glucuronide and 14 (range 0·1 – 124) mmol/l £ min for ECG.
The values of mean residence time (MRT0!24) were 5 (range 2 – 16), 2 (range 1 – 11), 10 (range 2 – 13), 3 (range 2 – 16) and 2·4 (range 1 – 18) h
for EGCG, ECG, EGC-glucuronide, EC-glucuronide and EC-sulphate, respectively. In urine, catechins were present as conjugated forms, suggesting bile excretion of EGCG and ECG. Green tea catechins are absorbed following an oral administration and EGC-glucuronide is the metabolic form that remains in the organism for a longer period of time, suggesting that this compound could suffer an enterohepatic cycle.
Flavanols: Metabolites: Pharmacokinetic: Extract: Plasma: Dogs: Urine
Green tea is the most consumed beverage in the world aside from water, and has recently attracted significant attention, both in the scientific and in consumer communities for its health benefits for a variety of disorders, protection against cancer and promotion of weight loss. The beneficial effects of green tea are attributed to the polyphenolic compounds present in this beverage. Green tea polyphenols have been extensively studied as CVD and cancer chemopreventive agents(1 – 4). The natural product (2 )-epigallocatechin-3-gallate (EGCG) is the major polyphenolic constituent found in green tea (dried fresh leaves of the plant Camellia sinensis)(5). Several other fla-vanols are also found in lower abundance in this beverage, including (2 )-epicatechin-3-gallate (ECG), (2 )-epigallocate-chin (EGC), (2 )-epicate)-epigallocate-chin (EC) and (þ )-cate)-epigallocate-chin (Fig. 1). More than 50 % of the mass of this catechin combination is composed of EGCG which has proven beneficial effects in studies of Parkinson’s disease(6), Alzheimer’s disease(7,8),
stroke(9,10), obesity(11), diabetes(12,13), chemoprevention(14 – 16) and high antioxidant activity(17). It has also been demonstrated to be a potential anti-inflammatory molecule(18,19).
The metabolic fate of polyphenols in the organism after their ingestion as food or beverages should be thoroughly studied, since these are the active forms that may generate the biological effect of these compounds. Green tea catechin in vivo activity is limited by effective concentrations at target sites, since it is assumed that plasma concentration of catechins and their metabolites are dynamically equilibrated with their concentration at successfully reached tissues. The changes observed in plasma concentrations reflect transform-ations the metabolites undergo in the tissues. It is, therefore, of special interest to study in depth the behaviour of catechins in the organism, that is, to evaluate the process of absorption, distribution, metabolism and excretion through plasma and urine levels of their metabolites. The use of enzymatic
* Corresponding author: Dr Rosa M. Lamuela-Ravento´s, fax þ34 93 4035931, email [email protected]
Abbreviations:AUC, area under the plasma concentration v. time curve; Cmax, maximum plasma concentration; EC, (2 )-epicatechin; ECG, (2
)-epicatechin-3-gallate; EC-glucuronide, (2 )-epicatechin glucuronide; EGC, (2 )-epigallocatechin; EGCG, (2 )-epigallocatechin-3-)-epicatechin-3-gallate; EGC-glucuronide, (2 )-epigallocatechin glucuronide; MRT, mean residence time; Tmax, time needed to reach Cmax.
qThe Authors 2008
British
Journal
of
hydrolysis for the determination of tea catechins in plasma has been extensively applied in previous studies(20 – 26). However, information about individual metabolites is not available in these studies, nor have they carried out a pharmacokinetic evaluation of the specific active molecules that could reach target cells.
The use of dogs as a model has been shown to be useful in evaluating the absorption of polyphenols from different food sources, allowing identification of specific forms present in the systemic circulation. In a previous study we evaluated in plasma, the kinetics of flavanones and their metabolites in beagle dogs after oral ingestion of a grapefruit fruit extract(27). Our earlier results demonstrated the absorption of grapefruit flavanone via the presence of its metabolites in plasma. Swezey et al.(28)studied in beagles the absorption, tissue dis-tribution and elimination of 4-[3H]epigallocatechin-3-gallate after intravenous and oral administration of radiolabelled EGCG. Their results indicate that, after oral administration, EGCG is rapidly and widely distributed to tissues where it can exert its biological effects.
Therefore, the purpose of the current study is to assess, for the first time in beagles, the major flavanol forms in plasma and urine after oral administration of a green tea extract. We also evaluate the pharmacokinetics of these metabolic forms in both biological matrices by considering their biotransformation, thus providing a general model that can be used for studies on flavonoid bioavailability.
Materials and methods Standards and reagents
EGCG (95 %), EGC and ECG (98 %) from green tea, (2 )-epi-catechin and blank dog plasma were purchased from
Sigma-Aldrich (St Louis, MO, USA). Ethylgallate (internal standard) was purchased from Extrasynthese (Genay, France). Methanol, acetonitrile and n-n-dimethylformamide (HPLC grade) and formic acid were purchased from Scharlau Chemie SA (Barcelona, Spain); o-phosphoric acid (85 %) was purchased from Panreac Quimica SA (Barcelona, Spain). Ultrapure water (Milli-Q) was obtained from a Millipore System (Bedford, MA, USA). Green tea capsules contained 124 mg EGCG, 9 mg EGC, 21 mg EC, 19 mg ECG, 4·38 mg (þ )-catequin and talc as an excipient. The capsules were stored at room temperature and protected from extreme environmental conditions.
Subjects and study design
Twelve healthy adult (14 (SD 3) kg) female beagle dogs were randomly chosen and were deprived of food overnight before the experiment. The dogs were orally administered with two capsules containing 200 mg green tea extract; two dogs were chosen as a control and were given an excipient containing talc. Blood samples (5 ml) were drawn before capsule admin-istration and at the following times: 30, 45, 60, 75, 90 min, 2, 4, 8 and 24 h and were collected in vacutainer tubes containing EDTA as anticoagulant (Becton Dickinson, Franklin Lakes, NJ, USA). Plasma was obtained after blood centrifugation at 1000 g for 10 min and stored in Eppendorf tubes at 2 808C until analysis. Urine samples were taken before and at the foll-owing intervals: 0 – 2, 2 – 6, 6 – 8 and 8 – 24 h, after oral administration of green tea extract. Urine was acidified with 200 mM-HCl and stored at 2 808C until analysis.
The study was carried out at the University of Zaragoza (Spain) in accordance with the Guide for the Care and Use of Laboratory Animals(29). The study protocol was approved by the Ethics Committee of the University of Zaragoza. Fig. 1. Chemical structures of green tea catechins.
British
Journal
of
Sample extraction procedure for green tea catechins and their metabolites
Green tea catechins in plasma and urine were obtained by solid-phase extraction. Dog plasma samples were treated as follows: 280 ml internal standard (2525 nmol/l) were added to 1 ml plasma and then mixed with 370 ml antioxidant solution (containing 0·2 g/ml ascorbic acid and 1 mg/ml EDTA) and with 20 ml o-phosphoric acid. After 2 min of vortex mixing, samples were diluted with 3 ml water. A solid-phase extraction (30 mg) with a Waters Oasisw HLB 30 mm ninety-six-well plate (Milford, MA, USA) was applied to the mixture. Cartridge activation was achieved by adding 1 ml: methanol, water, 70 % (v/v) n-n-dimethylformamide containing 0·1 % (v/v) formic acid and water, respectively. The cartridges were washed with 2 ml water and 1 ml 30 % (v/v) methanol. Tea catechin metabolites were then eluted with 0·7 ml 70 % (v/v) n-n-dimethylformamide containing 0·1 % (v/v) formic acid. After filtration with a 4 mm, 0·45 mm polytetrafluoroethane filter (Waters Corporation, USA), 20 ml of the resulting filtrate was injected into the LC – MS/MS system.
Preparation of standards and sample treatments were per-formed in a darkened room with a red safety light to avoid oxidation of the analytes.
Instrumentation
LC – MS/MS. Plasma and urine green tea catechin metabo-lites were identified and quantified by LC – MS/MS analysis. LC analysis was performed using a Perkin-Elmer series 200 (Norwalk, CT, USA) equipped with a quaternary pump and a refrigerated auto-sampler. A Luna C18 column (50 £ 2·0 mm internal diameter, 5 mm) from Phenomenex (Torrance, CA, USA) was used at room temperature, and the injected volume was 20 ml. Gradient elution was carried out with water (0·1 % formic acid) and acetonitrile (0·1 % formic acid) at a constant flow-rate of 600 ml/min. A gradient profile with the following proportions (v/v) of acetonitrile (0·1 % formic acid) was applied: 0 min, 0·5 %; 3 min, 15 %; 6 min, 100 %; 9 min, 100 %. A triple quadrupole mass spectrometer (API 3000; Applied Biosystems, PE Sciex, Concord, Ontario, Canada), equipped with a Turbo IonSpray source was used to obtain the MS and MS/MS data. Prior to its use, the instru-ment was checked to meet the acceptance specifications defined by the manufacturer. The triple quadrupole mass spec-trometer was calibrated with the Turbo IonSpray using a test mixture solution of poly (propyleneglycol) obtained from Applied Biosystems. The mass spectrometer was calibrated so that mass accuracy specifications and sensitivity were achieved over the entire mass range. Turbo Ionspray source settings were as follows: capillary voltage, 3500 V; nebulizer gas (N2), 10 (arbitrary units); curtain gas (N2), 12 (arbitrary units); collision gas (N2), 4 (arbitrary units); focusing poten-tial, 200 V; entrance potenpoten-tial, 10 V; drying gas (N2) heated to 4008C and introduced at a flow rate of 8000 cm3/min. The declustering potential and the collision energy were optimized for each compound in infusion experiments: individual stan-dard solutions (21·8 – 34·5 mmol/l) dissolved in 80:20 mobile phase were infused at a constant flow rate of 5 ml/min into the mass spectrometer using a model syringe pump (Harvard Apparatus, Holliston, MA, USA). Full scan data were acquired
by scanning from m/z 100 to 800 in profile mode, using a cycle time of 2 s with a step size of 0·1 unit. For MS/MS, a product ion scan utilizing a cycle time of 2 s was used. MS/MS product ions were produced by collision-activated dissociation of selected precursor ions in the collision cell of the triple – quad-rupole mass spectrometer and mass analysed using the second analyser of the instrument. The multiple reaction monitoring, the method of choice owing to its highest selectivity and sen-sitivity in quantitative LC – MS/MS, has monitored several transitions for each analysis. Both quadrupoles (Q1 and Q3) were operated at unit resolution. The criteria for identification of green tea catechin metabolites such as retention time, mul-tiple reaction monitoring transition as mentioned earlier and transitions 481 ! 305 ((2 )-epigallocatechin-glucuronide), 385 ! 305 ((2 )-epigallocatechin-sulphate), 305 ! 125 ((2 )-epigallocatechin), 465 ! 289 ((2 )-epicatechin-glucuro-nide), 369 ! 289 ((2 )-epicatechin-sulphate (m/z 369/289), 289 ! 245 ((2 )-epicatechin) (at a higher declustering poten-tial value) were chosen as confirmation of the multiple reac-tion monitoring trace for each metabolite in collisionally induced dissociation MS/MS experiments(30,31).
Safety considerations
Dog plasma samples were considered as potentially infectious. We have respected general guidelines regarding working with biological fluids, organic solvents and acids. Universal precau-tions regarding handling of chemicals and fluids were applied.
Pharmacokinetic analysis
Pharmacokinetic parameters were determined by means of a non-compartmental analysis using the WinNonlinwProfessional software version 3.3 (Pharsight Corporation, USA). The linear trapezoidal method was used to calculate the area under the plasma concentration curve (AUC0 – t) from time 0 until the last detectable concentration. The maximum plasma concentration (Cmax) and the time needed to reach Cmax(Tmax) were determined by visual inspection of the experimental data. MRT was esti-mated by means of the AUMC/AUC ratio, where AUMC is from the first measurement of the plasma concentration v. time curve. The maximum accumulated amount of catechins excreted in urine was also determined.
Statistical analysis
The pharmacokinetic parameters of green tea catechins and its metabolites were compared by one-way ANOVA on ranks followed by a Scheffe´’s multiple comparison test. P, 0·05 was considered significant. The statistical analysis was per-formed using SPSS software version 11.5 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Green tea catechins present in plasma and urine samples Two catechins with a galloyl moiety were present in plasma (EGCG and ECG) and three conjugated forms were also detected, however, only the conjugated forms of EC and EGC were present in urine. Regarding conjugated catechins found
British
Journal
of
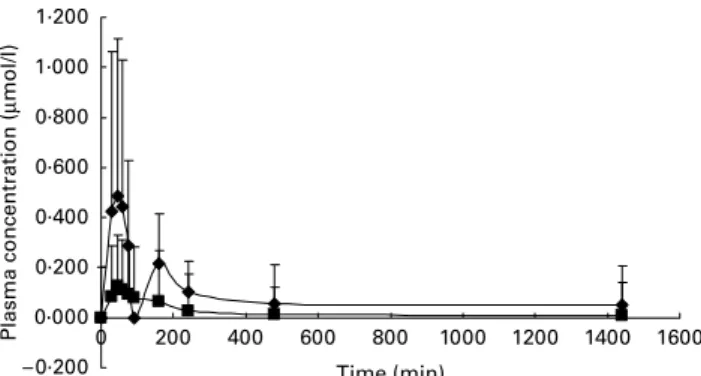
in plasma, EGC was mainly present in the glucuronide form while EC was both in the glucuronide and sulphate form. Figs. 2 and 3 show plasma concentration v. time curves corre-sponding to the catechins and catechin metabolites detected in plasma. In urine tea catechins EGC and EC were mostly present in the conjugated forms, as glucuronides and sulphates. Pharmacokinetics of green tea catechins after oral intake of green tea extract
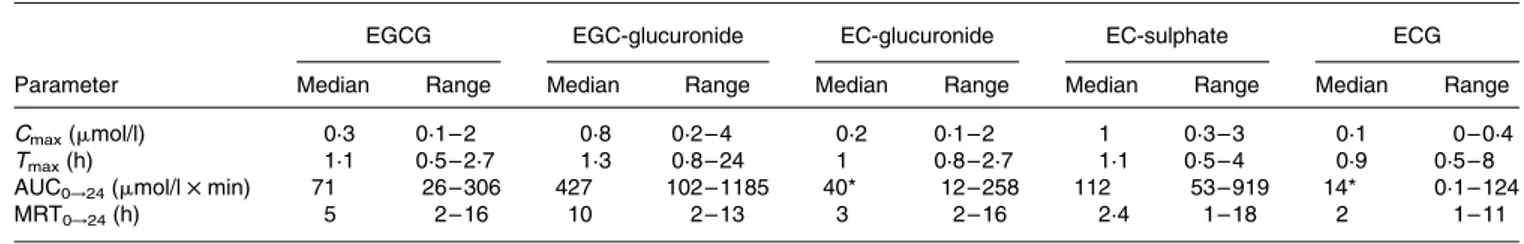
The following pharmacokinetic parameters corresponding to each of the catechins and catechin metabolites (Cmax, AUC0!24, MRT0!24, Tmax) are summarized in Table 1. Values are expressed as median and range, together with the results of the statistical analysis carried out. No significant statistical differences were found between most of the pharma-cokinetic parameters corresponding to both catechins and catechin conjugates. This was not the case, however, with AUC0!24, whose values showed significant differences between (2 )-epigallocatechin glucuronide (EGC-glucuronide) and (2 )-epicatechin glucuronide (EC-glucuronide), and EGC-glucuronide and ECG.
Fig. 4 represents the curves of accumulated excretion in urine for each of the conjugated catechins. Values are expressed as means and standard deviations for ten dogs. Discussion
During the past 10 years, there have been several pharmacoki-netics studies(22 – 26,32 – 36)with green tea catechins where only unchanged catechins were evaluated, suggesting that the cal-culated bioavailability in these studies were probably overes-timated. Thus, additional studies, where catechin metabolites are determined, are necessary. Once the polyphenol has been ingested it may suffer a number of changes during absorption and when it reaches the systemic circulation it may be present in its native or in its metabolic form. Thus, in order to under-stand the mechanism by which polyphenols exert their beneficial effects, extensive data from metabolic and distri-bution studies are required. Previous studies with green tea
catechins have applied different forms of administration, including green tea as a beverage, green tea extracts, pure cate-chins (EGCG) or polyphenolic preparations (Polyphenon E), demonstrating that the form of administration has a pronounced effect on the bioavailability of these compounds.
In the present study, we have administered green tea cate-chins as a green tea extract contained in capsules. The pre-sence of conjugated EGCG forms in dog plasma was not detected, only the free forms of EGCG and ECG were present. The present results were in accordance with Chow et al.(23), who indicated that after ingestion of EGCG and Polyphenon E (a tea polyphenol preparation) by human volunteers, plasma EGCG was mainly present in the free (unconjugated) form and may be eliminated in faeces by biliar excretion. The presence of unconjugated forms of EGCG and ECG in dog plasma could be explained by the fact that EGCG and ECG have a galloyl group on the C ring and EGCG has a hydroxyl group in the 50 position (Fig. 1). Possibly this group could have decreased access to the active site of uridine diphosphate glucuronosyl transferase, since EGCG (galloyl group and hydroxyl in 50 position) has shown an even lower glucuronidation rate than that of ECG(37). Another expla-nation, as has been reported before, may be the fact that green tea catechins can be strong inhibitors of phase II enzymes (caffeic o-methyltransferases, uridine diphosphate glucuronosyl transferases and sulfotransferases). It has also been found that methylation of EGC can be strongly inhibited by ECG and EGCG(38), justifying the absence of methylated conjugate.
As can be observed in Table 1, EGC-glucuronide showed the highest AUC0!24 values and also the highest MRT0!24, indicating that this specific metabolite remains within the organism for approximately 10 h. Considering that in the orally administered green tea extract, EGC is in the lowest proportion (4·7 %) compared to the other catechins, these high AUC and MRT values may be due to an enterohepatic cycle. The glucuronide formation could take place during EGC absorption and/or in the liver by the action of uridine diphosphate glucuronosyl transferase. The resulting conju-gated product (EGC-glucuronide) could be again excreted to the intestinal lumen through the bile. This conjugate could be hydrolysed by action of the intestinal enzyme b-glucuroni-dase generating EGC that could once again be absorbed, gen-erating the enterohepatic cycle, and remaining in the organism Fig. 2. Time v. plasma concentration curves for the following catechin
metab-olites: (2 )-epigallocatechin glucuronide (V), (2)-epicatechin glucuronide (B) and (2 )-epicatechin sulphate (O) for ten beagles receiving 173 mg green tea catechins. Values are means with their standard deviations depicted by verti-cal bars.
Fig. 3. Time v. plasma concentration curves for the following catechins: (2 )-epi-gallocatechin-3-gallate (V) and (2)-epicatechin-3-gallate (B) for ten beagles receiving 173 mg green tea catechins. Values are means with their standard deviations depicted by vertical bars.
British
Journal
of
for a longer period of time. As has been reported before, the health benefits of flavonoids can be due to different properties of their major phase II metabolites, such as antioxidants, pro-tein inhibitors of human Multidrug Resistance Propro-teins 1 and 2, and monocyte – endothelial cell interaction(39 – 41). The high MRT value is very interesting because EGC-glucuronide could be a potentially active molecule that remains in the organism long enough to exert its beneficial actions.
Tmaxand AUC values for EGCG compared well with data generated in previous studies with human subjects(21,24,34,42). However, Cmaxvalues differ, suggesting that the bioavailabil-ity of green tea catechins is variable and could be due to differences in species, quantity and form of catechin adminis-tration. It should be considered that the presence or absence and the time-dependent appearance and disappearance of specific catechin metabolites in plasma can vary significantly among subjects and species, suggesting that interindividual variation is an important factor that must always be taken into consideration when performing dietary assessment studies.
It is assumed that plasma concentrations of catechins and their metabolites are equilibrated with their concentrations at successfully reached tissues, Swezey et al.(28) dem-onstrated that radioactive EGCG was distributed to a variety of epithelial tissues in beagles; the highest concentrations being observed in the liver and gastrointestinal tract
tissues. However, additional investigation is needed on tissue distribution, specifically with the metabolites present in plasma after oral intake, since these compounds, such as EGC-glucuronide, are the ones that remain in the organism for a longer period of time.
The maximum amount of metabolites excreted in urine is directly related to the quantity of the absorbed compound(43). In Fig. 4, the accumulative amount excreted per sampling interval is referred to the mid-point of the collection period, for example, for the 0 – 2 h period, the mid-point would be 1 h; for the interval 2 – 6 h, the mid-point would be 4 h. The values of the 7 h mid-point correspond to the interval 6 – 8 h. Unfortunately not all the subjects could urinate during these hours, obtaining less sample and consequently, lower values. This tendency is only observed in the graphics with mean values; individual graphics do not show this tendency. As pre-sented in Fig. 4, EC conjugates are the metabolites with the highest excreted amounts, sulphates being the major ones; (2 )-epicatechin sulphate is the metabolite with the highest maximum amount excreted. The cumulated urine excretion during 24 h after ingestion of the green tea extract accounted for 1·12 and 0·58 % for EC and EGC, respectively, suggesting that a considerable amount of these metabolites could have bile excretion as an elimination pathway as well. On the other hand, the absence of unconjugated EGCG and ECG in urine may possibly be due to most of the absorbed unconju-gated EGCG being excreted through the bile(28,44).
To our knowledge, we have evaluated, for the first time in beagles, the pharmacokinetics of green tea catechins and their metabolites in plasma and urine after oral administration of a green tea extract. We have demonstrated that catechins are absorbed from the intestine as their intact form or as conju-gates following oral administration, and that the conjugated catechins remain in the organism for a longer period of time probably due to an enterohepatic cycle. The present finding is very interesting because if the conjugated metabolites of green tea catechins are indeed active molecules, their MRT values are high enough to exert potentially beneficial effects. These data also provide a general model for future pharmaco-kinetics studies.
Acknowledgements
The authors would like to thank the R&D Department, Affi-nity Pet-care Barcelona, without whose support this project Table 1. Pharmacokinetic parameters of green catechins and their metabolites in beagles, after oral intake of green tea extract
(Median values and ranges for ten beagles)
EGCG EGC-glucuronide EC-glucuronide EC-sulphate ECG
Parameter Median Range Median Range Median Range Median Range Median Range
Cmax(mmol/l) 0·3 0·1 – 2 0·8 0·2 – 4 0·2 0·1 – 2 1 0·3 – 3 0·1 0 – 0·4
Tmax(h) 1·1 0·5 – 2·7 1·3 0·8 – 24 1 0·8 – 2·7 1·1 0·5 – 4 0·9 0·5 – 8
AUC0!24(mmol/l £ min) 71 26 – 306 427 102 – 1185 40* 12 – 258 112 53 – 919 14* 0·1 – 124
MRT0!24(h) 5 2 – 16 10 2 – 13 3 2 – 16 2·4 1 – 18 2 1 – 11
AUC, area under the plasma concentration v. time curve; Cmax, maximum plasma concentration; ECG, (2 )-epicatechin-3-gallate; EC-glucuronide, (2 )-epicatechin glucuronide;
EC-sulphate, (2 )-epicatechin sulphate; EGCG, (2 )-epigallocatechin-3-gallate; EGC-glucuronide, (2 )-epigallocatechin glucuronide; MRT, mean residence time; Tmax, time
needed to reach Cmax.
Median values were significantly different from those of the EGC-glucuronide group: *P, 0·05.
Fig. 4. Accumulated excreted quantities (mmol) of catechin conjugates in urine during 24 h, after capsule administration for ten beagles. Values are means with their standard deviations depicted by vertical bars. V, (2)-Epica-techin glucuronide; O, (2)-epica(2)-Epica-techin sulphate; X, (2)-epigalloca(2)-Epica-techin glu-curonide; B, (2)-epigallocatechin sulphate.
British
Journal
of
would not have been possible. M. L. M.-B. is also grateful to the Danone Institute for its partial contribution to the study through her pre-doctoral fellowship. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of analyses. All authors were involved in the conception and design of the study, the collection and assembly of data, the analysis and interpretation of the data, and provided administrative, techni-cal and material support. Crititechni-cal revision of the manuscript for important intellectual content was completed by all the authors. M. L. M.-B. and E. E. performed the statistical ana-lyses. R. M. L.-R., C. A.-L. and C. T. obtained funding.
There are no conflicts of interest.
References
1. Zaveri NT (2006) Green tea and its polyphenolic catechins:
medicinal uses in cancer and noncancer applications. Life Sci 78, 2073 – 2080.
2. Cooper R, Morre DJ & Morre DM (2005) Medicinal benefits of
green tea, I: review of noncancer health benefits. J Altern Comp-lement Med 11, 521 – 528.
3. Frei B & Higdon JV (2003) Antioxidant activity of tea
polyphe-nols in vivo: evidence from animal studies. J Nutr 133, 3275S – 3284S.
4. Bettuzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G &
Corti A (2006) Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res 66, 1234 – 1240.
5. Graham HN (1992) Green tea composition, consumption, and
polyphenol chemistry. Prev Med 21, 334 – 350.
6. Choi JY, Park CS, Kim DJ, Cho MH, Jin BK, Pie JE & Chung
WG (2002) Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin-3-gallate. Neurotoxi-cology 23, 367 – 374.
7. Ramassamy C (2006) Emerging role of polyphenolic compounds
in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol 545, 51 – 64.
8. Obregon DF, Rezai-Zadeh K, Bai Y, et al. (2006) ADAM10
activation is required for green tea-EGCG-induced alpha-secre-tase cleavage of amyloid precursor protein. J Biol Chem 281, 16419 – 16427.
9. Choi YB, Kim YL, Lee KS, Kim BS & Kim DJ (2004)
Protec-tive effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res 1019, 47 – 54.
10. Koh SH, Lee SM, Kim HY, et al. (2006) The effect of
epigal-locatechin gallate on suppressing disease progression of ALS model mice. Neuro Lett 395, 103 – 107.
11. Lin JK & Lin-Shiau SY (2006) Mechanisms of hypolipidemic
and anti-obesity effects of tea and tea polyphenols. Mol Nutr Food Res 50, 211 – 217.
12. Anderson RA & Polansky MM (2002) Tea enhances insulin
activity. J Agric Food Chem 50, 7182 – 7186.
13. Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T &
Kimura L (2004) Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. Pharmacology 4, 18.
14. Adhami VM, Malik A, Zaman N, Sarfaraz S, Siddiqui IA, Syed
DN, Afaq F, Pasha FS, Saleem M & Mukhtar H (2007) Com-bined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate
cancer cells both in vitro and in vivo. Clin Cancer Res 13, 1611 – 1619.
15. Kumar N, Shibata D, Helm J, Coppola D & Malafa M (2007)
Green tea polyphenols in the prevention of colon cancer. Front Biosci 12, 2309 – 2315.
16. Clark J & You M (2006) Chemoprevention of lung cancer by
tea. Mol Nutr Food Res 50, 144 – 151.
17. Guo Q, Zhao B, Shen S, Hoou J, Hu J & Xin W (1999) ESR
study on the structure – antioxidant activity relationship of tea catechins and their epimers. Biochim Biophys Acta 1427, 13 – 23.
18. Ahmed S, Pakozdi A & Koch AE (2006) Regulation of
interleu-kin-1beta-induced chemokine production and matrix metallopro-teinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 54, 2393 – 2401.
19. Tedeshi E, Suzuki H & Megazzi M (2002) Antiinflammatory
action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann N Y Acad Sci 973, 435 – 437.
20. Unno T, Sagesaka YM & Kakuda T (2005) Analysis of tea
cate-chins in human plasma by high-performance liquid chromatog-raphy with solid-phase extraction. J Agric Food Chem 53, 9885 – 9889.
21. Henning SM, Niu Y, Liu Y, Lee NH, Hara Y, Thames GD, Minutti
RR, Carpenter CL, Wang H & Heber D (2005) Bioavailability and antioxidant effect of epigallocatechin gallate administered in pur-ified form versus as green tea extract in healthy individuals. J Nutr Biochem 16, 610 – 616.
22. Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S,
Lambert G, Mohr S & Yang CS (2002) Pharmacokinetics of tea catechins after ingestion of green tea and (2 )-epigallocate-chin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev 11, 1025 – 1032.
23. Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F,
Cro-well JA, Yang CS & Hara Y (2001) Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev 10, 53 – 58.
24. Lee MJ, Wang ZY, Li H, Chen L, Sun Y, Gobbo S, Balentine D
& Yang CS (1995) Analysis of plasma and urinary tea polyphe-nols in human subjects. Cancer Epidemiol Biomarkers Prev 4, 393 – 399.
25. Van Amelsvoort JMM, Hof KHV, Mathot JNJJ, Mulder TPJ,
Wiersma A & Tijburg LBM (2001) Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica 31, 891 – 901.
26. Yang CS, Chen L, Lee MJ, Balentine D, Chen Kuo M &
Schantz SP (1998) Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human vol-unteers. Cancer Epidemiol Biomarkers Prev 7, 351 – 354.
27. Mata ML, Lacueva CA, Roura E, Ja´uregui O, Escribano E,
Torre C & Lamuela-Ravento´s RM (2007) Absorption and pharmacokinetics of grapefruit flavanones in beagles. Br J Nutr 98, 86 – 92.
28. Swezey RR, Aldridge DE, LeValley SE, Crowell JA, Hara Y &
Green CE (2003) Absorption, tissue distribution and elimination of 4-[H-3]-epigallocatechin gallate in beagle dogs. Int J Toxicol 22, 187 – 193.
29. Committee on Care and Use of Laboratory Animals (1985)
Guide for the Care and Use of Laboratory Animals. Washing-ton, DC: Institute of Laboratory Animals Resources, National Research Council.
30. Roura E, Andres-Lacueva C, Jauregui O, Badia E, Estruch R,
Izquierdo-Pulido M & Lamuela-Raventos RM (2005) Rapid liquid chromatography tandem mass spectrometry assay to quantify plasma (2 )-epicatechin metabolites after ingestion of a standard portion of cocoa beverage in humans. J Agric Food Chem 53, 6190 – 6194.
British
Journal
of
31. Urpı´-Sarda` M, Ja´uregui O, Lamuela-Ravento´s RM, Jaeger W, Miksits M, Covas MI & Andre´s-Lacueva C (2005) Uptake of diet resveratrol into the human low-density lipoprotein. Identifi-cation and quantifiIdentifi-cation of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometry. Anal Chem 77, 3149 – 3155.
32. Meng X, Sang S, Zhu N, Lu H, Sheng S, Lee MJ, Ho CT &
Yang CS (2002) Identification and characterization of methyl-ated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem Res Toxicol 15, 1042 – 1050.
33. Unno T, Kondo K, Itakura H & Takeo T (1996) Analysis of
(2 )-epigallocatechin gallate in human serum obtained after ingesting green tea. Biosci Biotechnol Biochem 60, 2066 – 2068.
34. Kimura M, Umegaki K, Kasuya Y, Sugisawa A & Higuchi M
(2002) The relation between single/double or repeated tea cate-chin ingestions and plasma antioxidant activity in humans. Eur J Clin Nutr 56, 1186 – 1193.
35. Van het Hof KH, Kivits GA, Weststrate JA & Tijburg LB
(1998) Bioavailability of catechins from tea: the effect of milk. Eur J Clin Nutr 52, 356 – 359.
36. Pietta PG, Simonetti P, Gardana C, Brusamolino A &
Bombardelli E (1998) Catechin metabolites after intake of green tea infusions. Biofactors 8, 111 – 118.
37. Crespy V, Nancoz N, Oliveira M, Hau J, Courtet-compondu MC
& Williamson G (2004) Glucuronidation of the green tea
catechins, (2 )-epigallocatechin-3-gallate and (2 )-epicatechin-3-gallate, by rat hepatic and intestinal microsomes. Free Radic Res 38, 1025 – 1031.
38. Feng WY (2006) Metabolism of green tea catechins: an
over-view. Curr Drug Metab 7, 755 – 809.
39. Morand C, Crespy V, Manach C, Besson C, Demigne´ C &
Re´me´sy C (1998) Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol 275, 212 – 219.
40. Koga T & Meydani M (2001) Effect of plasma metabolites of
(þ )-catechin and quercetin on monocyte adhesion to human aortic endothelial cells. Am J Clin Nutr 73, 941 – 948.
41. Van Zanden JJ, Van der Woude H, Vaessen J, Usta M,
Wortel-boer HM, Cnubben N & Rietjens I (2007) The effect of querce-tin phase II metabolism on its MRP1 and MRP2 inhibiquerce-ting potential. Biochem Pharmacol 74, 345 – 351.
42. Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, Wang
H, Go VL & Heber D (2004) Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr 80, 1558 – 1564.
43. Shargel L & Yu A (1999) Applied Biopharmaceutics and
Phar-macokinetics, 4th ed. New York: Lange.
44. Chen L, Lee MJ, Li H & Yang CS (1997) Absorption,
distri-bution, and elimination of the tea polyphenols in rats. Drug Metab Dispos 25, 1045 – 1050.