IDENTIFICATION AND FUNCTIONAL CHARACTERIZATION
OF MASTER REGULATORS OF THE ONSET OF BERRY
OF MASTER REGULATORS OF THE ONSET OF BERRY RIPENING
IN GRAPEVINE (Vitis vinifera L.)
DEPARTMENT OF BIOTECHNOLOGY
Graduate School of Natural Sciences and Engineering
Doctoral program in Biotechnology
CYCLE XXXI
PhD THESIS
IDENTIFICATION AND FUNCTIONAL
CHARACTERIZATION OF MASTER REGULATORS OF
THE ONSET OF BERRY RIPENING IN GRAPEVINE
(Vitis vinifera L.)
S.S.D. AGR/03
Coordinator: Prof. Matteo Ballottari
Tutor: Prof. Giovanni Battista Tornielli
Co-tutor: Prof.ssa Sara Zenoni
Grapevine is one of the most important and cultivated fruit crops in the world. Its economic importance is especially related to winemaking and the production of high-quality grape is one of the major concerns of the viticulturists. In the last years, continuous temperatures increasing are altering the maturation process; in particular, higher temperatures have caused an anticipation of the onset of berry ripening, called veraison, with reducing grape color and increasing volatilization of aroma compounds. This change could modify the physiological characteristics of grape, its final quality and consequently wine quality. In this contest of climate changes, the development of strategies to prevent these negative effects is indispensable. Many agronomic practices have been tested, but they are very complex and expensive and their application on large scale could be economically unsustainable. Accordingly, the identification of alternative approaches seems to be essential. The interpretation of the molecular mechanisms controlling the onset of berry ripening could provide allow the development of more specific and targeted intervention strategies. To this aim, many molecular studies have been performed. One of the most important is represented by the generation of the grapevine gene expression atlas (Fasoli et al., 2012); this study showed a transcriptomic reprogramming during the vegetative-to-mature transition, suggesting the existence of key regulator genes. Further studies (Palumbo et al., 2012; Massonnet et al., 2017) showed that this phase transition seem to be regulated by specific genes, defined switch genes: they are mainly transcription factors and they could be master regulators of the ripening process in grapevine. Furthermore, some of these transcription factors are characterized by a strong induction during the first phase of veraison, confirming their specific role in the regulation of the onset of berry ripening (Fasoli et al., 2018). The identification of the functions of these transcription factors could provide important details about the molecular mechanism controlling the maturation process in grapevine. Among these transcription factors, five of them, VviNAC33, VviNAC60, VviAGL15,
VviWRKY19 and VvibHLH75, have been selected for functional characterization. They are five
switch genes and, excluding VviAGL15, they are markers of the first transition during veraison. Furthermore, they belong to 4 of the most important transcription factors families in plants. Their functional analysis in grapevine has been performed using stable genetic transformation and transient gene expression approaches. The application, improvement and development of these approaches has supported the functional characterization of the five selected genes.
different cultivars (Shiraz, Garganega and Sangiovese), using GFP as reporter gene, have been tested. Different parameters, including the type of embryogenic tissue, different Agrobacterium OD600 and media, have been analyzed. The results showed that the regeneration of transgenic
somatic embryos and plants occurred only in Shiraz and Garganega cultivars using embryogenic calli as transformation material, indicating that this complex process is cultivar-dependent. Furthermore, there weren’t remarkable differences in terms of regenerated plants between the protocol tested. Stable genetic transformation was used for the functional analysis of both
VviNAC33 and VviNAC60. In a previous work (D’Incà, 2017), both NAC genes have been
overexpressed in grapevine plants; the overexpression of VviNAC33 has altered the chlorophyll metabolism, while the overexpression of VviNAC60 has caused stunted growth and anthocyanins leaf accumulation, indicating that both genes are involved in the regulation of vegetative-to-mature transition. Furthermore, their overexpression showed an upregulation of many genes involved in the maturation process. In this PhD project, both NAC gene have been fused with EAR motif, the strongest transcriptional repression domain in plants, and stably expressed in Garganega and Shiraz plants. The results showed that some putative target genes of both NAC transcription factors are less expressed than WT plants, indicating that EAR motif represents a good approach to study the function of a transcription factor and to identify their target genes. Regarding transient gene expression, this method was used for the functional analysis of VviAGL15, VviWRKY19 and VvibHLH75. Leaf agroinfiltration, the historic and the most used assay of this method, was optimized using YFP as reporter gene and tested in different cultivars by a vacuum system. The analysis of YFP transient expression showed that the fluorescence signal is especially localized in the first and second leaf from apex and the day post infiltration of maximum YFP expression is cultivar dependent. Moreover, agroinfiltration was tested using grapevine berry, a more complex tissue than leaf, obtained from fruiting cuttings. This approach was performed using again YFP as reporter gene and two different agroinfiltration methods: syringe with needle and vacuum system. The YFP transient expression analysis showed that the efficiency of this approach is not very high, but the visualization of fluorescence signal only in the inner part of vacuum agroinfiltrated berries, indicates that this approach can be further improved and subsequently used for gene functional analysis directly in berry. However, VviAGL15, VviWRKY19 and VvibHLH75 have been functionally characterized
transcription factor was co-expressed with YFP gene: the visualization of its expression has allowed to select only agroinfiltrated leaves. Next microarray analysis of overexpressing leaves showed that many upregulated genes are involved in processes associate with ripening, and an exhaustive molecular interpretation of these preliminary results seem to indicate that
VviAGL15, VviWRKY19 and VvibHLH75 are master regulators of the onset of berry ripening,
controlling many aspects of the maturation programs. Finally, the last topic of this thesis is the regeneration of grapevine plants from embryogenic calli-derived protoplasts. This approach was tested in two different grapevine cultivars, Garganega and Sangiovese. The results showed that plant regeneration occurred in both cultivars, but the efficiency was higher in Garganega. Furthermore, protoplast transfection with a vector harboring a cassette for YFP overexpression showed a high and uniform YFP expression until 72 hours post transfection. The successful of these results indicate that protoplast technology can be used for functional studies, including genome editing, an innovative and emergent system to genetic modifications and improvement of plants.
Chapter 1 ...………..………1
Introduction
Chapter 2 .………..………17
Identification of master regulators of the onset of berry ripening and candidates’ selection for functional characterization
Chapter 3 ...………49
Application, improvement and development of gene transfer technology in grapevine (Vitis Vinifera L.)
Chapter 4 ...………96
Roles of VviNAC33 and VviNAC60 in grapevine development
Chapter 5 ...………116
Functional analysis of VviAGL15a, VviWRKY19 and VvibHLH75
Chapter 6 ...………187
Plant regeneration from protoplasts: a new perspective for genome editing application in grapevine
Chapter 7 ...199
1
Chapter 1
INTRODUCTION
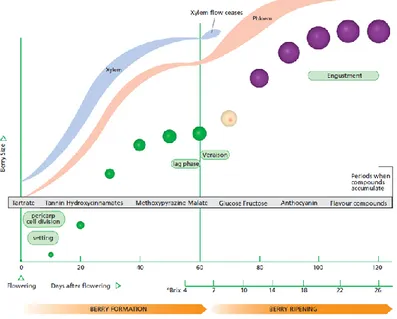
Grapevine development and berry ripeningGrapevine (Vitis vinifera L.) is a woody perennial plant from the family Vitaceae. It is one of the most important fruit crops in the world, and viticulture and enology play an important role in the economy of many developed and emerging countries (Martínez-Esteso et al, 2013). Grapevine has a biennal reproductive cycle: buds formed in the first year give rise to shoots bearing fruit in the second year. Its annual growth cycle is represented by a period of active growth from spring to fall, followed by a rest period in the winter. During the dormant season, the organs undergo an acclimation process to survive freezing temperatures. In the spring, following the increase of day length and temperatures, the dormancy is released. After that, the budburst takes place and the first shoots start to grow. Early shoot growth is relatively slow, but soon it enters a phase of rapid growth which typically continues until just after fruit set to a halt by about the time the fruit begins to ripen. As the shoot grows, the flower cluster development takes place, rapidly also forming individual flower. Following the flowering, during which processes of pollination and fertilization take place, the next phases are the fruit set and the berry development. This latter phase and the ripening are the most important processes of the annual growth cycle of grapevine. Grape berry development and ripening are represented by double sigmoid growth pattern (Conde et al., 2007; Kennedy, 2002; Figure 1). The first growth phase is characterized by not only rapid cell division, which increases the number of cells, but also by an expansion of existing cells; during this phase, the berry is formed, the seed embryos are produced, and several solutes are accumulated. The most prevalent compounds are tartaric and malic acids, followed by hydroxycinnamic acids, tannins, amino acids, micronutrients and aroma compounds. The first phase is followed by a lag phase with little or no growth. The second growth phase coincides with the onset of ripening, called veraison, a French word used to describe the change in berry skin colour; it is characterized by important biochemical and physiological changes such as softening, coloring and engustment of berry. As grape berries develop, they change in size and composition: in fact, during this phase, berry
2
approximately doubles in size between veraison and harvest and most of compounds accumulated in berries during the first growth phase (malic acid, tannins and aroma compounds) are significantly reduced while others, especially fructose and glucose sugars and anthocyanins, are considerably increased.
Figure 1: diagram showing the two phases of grape berry development and ripening (Kennedy, 2002). Berry ripening is a complex developmental process affected by many endogenous and exogenous factors. Hormonal signaling but also many environmental influences, such as sunlight, temperature, inorganic nutrients and water, are the main factors involved in the regulation of berry ripening (Jackson, 2014; Kuhn et al., 2014). Grape is a non-climacteric fruit and the respiratory burst and rise in ethylene production are absent at the onset of ripening. However, ethylene and other two hormones, abscisic acid (ABA) and brassinosteroids, have been suggested to promote ripening through complex interactions (Fortes et al., 2015; Conde et al., 2007), while auxins delay ripening associated processes, such us berry size, sugar accumulation and anthocyanin content (Kuhn et al., 2014). The levels of ABA increase after
veraison in berry tissues where it plays a role in seed maturation, acquisition of seed dormancy
and resistance to water stress deficit. ABA is also specifically involved in maturation control, regulating positively sugar and phenolics accumulation in grape (Conde et al., 2007). Despite
3
ethylene levels are always very low in ripening grape berries, itmay influence berry acidity and the development of grape flavor and aroma (Conde et al., 2007; Fortes et al., 2015). Finally, brassinosteroids are hormones involved in plant growth and development, but they dramatically increase at the onset of berry ripening suggesting they may play a primary role in the regulation of this process (Kuhn et al., 2014).
Berry ripening is also strongly affected by environmental factors: in particular, light exposure regulates the flavonoid pathway and promotes flavonol and anthocyanin synthesis that results in a a deeper berry skin coloration in red cultivars (Jackson, 2014; Kuhn et al., 2014). Temperatures is another important parameter: low temperatures are necessary to increase total soluble solid and anthocyanin content and to decrease total acidity, while high temperatures have negative effects on berry ripening, causing a reduction in berry weight, total soluble solid, anthocyanins and flavonol contents (Kuhn et al., 2014). Water and inorganic nutrients supply also affect berry ripening: a moderate water deficit after veraison can be beneficial to grape quality, because it enhances anthocyanin synthesis and limits berry enlargement, but it also increases stilbenoids and sugar contents (Jackson, 2014). Regarding inorganic nutrients, low soil nitrogen and phosphorus deficiencies are known to increase anthocyanin content while high potassium levels can increase berry juice pH and, thereby, lower wine color (Jackson, 2014).
Finally, the very detailed transcriptomic maps produced in the last years evidenced that berry development and ripening are characterized by a fine genetic regulation (Fasoli et al., 2012; Massonnet et al., 2017; Fasoli et al., 2018); however, excluding some processes, such as for example the regulation of anthocyanin synthesis(Walker et al., 2007; Kobayashi et al., 2004), and the identification of the gene responsible of the flb (fleshless berry) mutant phenotype (Fernandez et al., 2006), the precise function and contribution of the huge amount of genes modulated during berry development and ripening remain unknown.
Climate changes, grape quality and intervention strategies
Control of the ripening timing, berry size and coloration, acidity and the relative assortment of volatile and non-volatile aroma and flavor compounds in wine grape cultivars are major concerns to viticulturists (Conde et al., 2007). Continued and specific study of the key control points in grape ripening are crucial to improve grape and wine quality. One of the most
4
important factors affecting these parameters is represented by weather and climate change projections for the 21st century is expected to have important impacts on viticulture. In fact, grapevine physiology and fruit metabolism/composition are highly influence by the mean temperature along the growing season and extreme heat or heat weaves may also permanently affect vine physiology and yield attributes (Fraga et al., 2012). Winemaking regions under extremely hot temperatures may lead to a significant increase in the risk of organoleptic degradation and wine spoilage. In particular, higher temperatures may inhibit the formation of anthocyanin thus reducing grape color and increasing volatilization of aroma compounds. Under a future warmer climate, springtime warming may lead to earlier budburst and continue increases in temperature cause a trend towards earlier flowering, veraison and harvest. The timing of veraison may be of importance, because earlier veraison implies that the critical ripening period shifts towards the hotter part of the season (Keller, 2010). This change of the timing of grape ripening and harvest date may affect grape quality and yield and consequently wine quality. Altogether, the profound climate changes, the modification of grape quality and therefore the production of high-quality wine, could have substantial consequences for the global economy of wine industry.
To reduce the negative effects of climate changes, many adaptation strategies, represented especially by agricultural practices, have been performed. Among them, there are the late winter pruning, late irrigation, late defoliation, the use of sunscreens for leaf protection, the use of specific products affecting the maturation phases (especially auxin and cytokinin) and the increase of buds to produce more grapes and to slow down ripening (Palliotti et al., 2012). All these short-term management practices are finalized to regulate/delay the maturation, avoiding the alteration of grape quality caused by temperatures increase. The positive results obtained by their application indicate that these agricultural practices are efficient; however most of them are based on principles related to traditional viticulture. The interpretation of the molecular mechanisms involved in the regulation of berry ripening could provide important information about these processes and allow the development of more effective intervention strategies.
5
Molecular studies of berry development and ripening
The recent grapevine genome sequencing (Jaillon et., 2007) has allowed to perform many molecular studies related to grapevine development and berry ripening. One of the most important is represented by the generation of grapevine gene expression atlas (Fasoli et al., 2012, see Chapter 2 for a more detailed description). This complex work has showed a deep transcriptomic shift during the immature-to-mature shift in all grapevine organs, suggesting the existence of key regulators genes involved in the regulation of this complex phase transition. These specific genes, named switch genes (Palumbo et al., 2014), are expressed at low level in immature organs but their expression increase considerably in mature organs, indicating that they are a specific role in the regulation of transcriptomic changes during ripening process in grapevine. To obtain more information about the regulation of berry ripening process, the specific switch genes involved in the immature-to-mature transition in both red and white berry have been identified (Palumbo et al., 2014; Massonnet et al., 2017, see Chapter 2 for a more detailed description). The comparison of switch genes of grapevine expression atlas and switch genes berry specific shows that many genes are common while others are specific of only one transcriptomic dataset. Switch genes of both expression atlas and berry transcriptome include genes belong to many functional categories, from carbohydrate metabolic process and cell wall metabolism to response to hormone stimulus and secondary metabolic process, but the functional category overrepresented in both datasets is transcription factor activity. These results suggest a fundamental role of transcription factors in the regulation of immature-to-mature in grapevine organs, including berry.
These works have provided important information about the transcriptional changes between green and mature berries, but the specific molecular mechanism controlling the onset of berry ripening remain unknown. To further improve these molecular knowledges related to berry ripening and to better identify the key genes involved in the regulation of this process, transcriptomic analysis of berry at different developmental stages (from fruit set to full maturity) has been performed (Fasoli et al., 2018, see Chapter 2 for a more detailed description), confirming again the transcriptional shift during immature-to-mature transition and suggesting the existence of specific key regulators genes. Furthermore, the transcriptomic analysis of berry around veraison (early veraison, mid-veraison and late veraison), showed that the onset of berry ripening could be represented by two molecular transitions starting from 14
6
days before veraison (Figure 2). Positive biomarkers of transition 1 were characterized by a stronger induction 14 days before veraison than positive biomarkers of transition 2, indicating the first set of genes trigger the expression of the second set, which in turn mediates the different processes that characterize ripening. As previously described for switch genes, the positive biomarkers of both transitions are represented by genes involved in many processes, such as response to hormone stimulus, cell wall metabolism and secondary metabolic processes, but one of the most represented functional categories are transcription factors. Furthermore, many of them were already identified as switch gene of expression atlas and of both red and white berry transcriptome. This data indicates that transcription factors are key genes during the immature-to-mature transition and they have a very important role in the regulation of berry development and ripening.
Figure 2: the averaged expression profile of transition-specific putative biomarker genes shown over the whole of development (left plot) and during pre-veraison phase (right plot).
Gene transfer technologies in grapevine
After the identification of a specific gene of interest, the next phase is represented by its functional characterization. In fact, the genetic improvement of grapevine can be performed only after the complete characterization of the gene of interest. Gene transfer technologies are very useful for this purpose. In grapevine, the most important are stable genetic transformation and the transient gene expression (Jelly et al., 2014). Stable transformation allows the study of stable gene expression at the whole plant level, in different tissues and at different developmental stages; it is based on Agrobacterium tumefaciens-mediated transformation of embryogenic culture derived by explants of stamens and pistils or leaves. It is a complex, long and random process, with a very low efficiency; it is characterized by numerous limitations, include poor embryogenic potential of genotypes, wide variations among varieties in their
7
response to genetic transformation, Agrobacterium-induced post-cocultivation necrosis of embryogenic cultures, and poor plant recovery from transformed somatic embryos. However, the grapevine stable transformation and the regeneration of transformed plants has been reported in some works (Iocco et al., 2001; Li et al., 2006, 2008, 2015; Dhekney et al., 2009; Kandel et al., 2016). On the other hand, transient expression provides the most efficient way to study many genes in a very short time (Jelly et al., 2014). It is based on temporary, high-level transcription of DNA sequences that do not necessarily integrate into the plant genome. Methods for transient gene expression in plants mainly involve Agrobacterium tumefaciens-mediated transformation: during a short period immediately following the cultivation with
Agrobacterium tumefaciens, many copies of the transgene are actively transcribed in the plant
cells, allowing a high expression of gene of interest. Leaf agro-infiltration represents a major historic breakthrough in transient expression assays. It is easy and rapid, and it is based on the forced infiltration of Agrobacterium tumefaciens into the intercellular spaces of the leaf parenchyma, using a needleless syringe or a vacuum pump. Many reports (Zottini et al., 2008; Bertazzon et al., 2012; Santos-Rosa et al., 2008; Ben-Amar et al., 2013) have stated the success of grapevine leaf agroinfiltration using both methods above mentioned.
Both stable genetic transformation and transient gene expression approaches cane be used for the functional analysis of gene of interest. However, despite its simplicity and rapidity, transient expression represents a good strategy for a rapid and preliminary study of gene function but a complete characterization of gene of interest can be performed by the stable transformation. Furthermore, the generation of genetically modified grapevine plants harboring important traits is fundamental for the genetic improvement of grapevine (Vidal et al., 2010).
Genetic improvement of grapevine
In the last years, the economic importance of grapevine has considerably increased the studies related with its genetic modification. The historic approach used for grapevine genetic improvement is represented by conventional breeding. This method was largely used during 19th century to generate grapevine plants resistant to fungal diseases, especially against
phylloxera (Riaz et al., 2007). Breeding requires a cross between parent plants characterized by genomes can interact between them, with the formation of progeny that combine both the positive and negative traits from each parent. Based on searched trait, the progeny
8
performance is evaluated during the growth, keeping the plants with desired trait and discarding the others. The presence of undesirable traits, the long time before the fruit is produced and the difficulty to obtain a specific set of genes to confer improved properties, make the use of this system in grapevine very complex and time-consuming. An alternative, more prominent approach of genetic improvement is represented by genetic engineering; this method has been further implemented after the complete sequencing of grapevine genome (Jaillon et al, 2007). As previously described, stable transformation, the most representative technology of genetic engineering, can be used for the functional analysis of gene of interest, but also for the generation of genetically modified plants, obtained by transferring of single gene coding for specific traits, with the minimum alteration of the original genome. Transgenic plants could show many advantages than non-transformed wild type plants, such us biotic or abiotic stress resistance or higher capacity of production, but the introduction of exogenous DNA sequences, the use of a transformation agent, Agrobacterium tumefaciens, the incomplete understanding of the genetic mechanisms underlying the trait of interest and the substantial scepticism among the general public, hinders or limits the complete use of these type of plants (Holme et al., 2013). An alternative to transgenic technology is represented by cisgenesis: the definition of this approach is: “Full CDS including introns of a gene originating from the sexually compatible gene pool of the recipient plant” (Schouten et al., 2006). This method avoids the use of exogenous DNA, but the use of Agrobacterium as transformation agent to obtain cisgenic plants and the scepticism related to transformed plants could still limit the application of this technology. A recent and innovative approach of genetic modification of plants, including grapevine, is represented by genome editing. This method enables targeted genome modification using sequence-specific nucleases, including zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALEN) and the clustered regularly interspaced short palindromic repeats/CRISPR-associated protein (CRISPR/Cas) system (Yin et al., 2017). These sequence-specific nucleases generate double-stranded breaks (DSBs) at targeted genome sites, which are generally repaired by either non-homologous end joining (NHEJ) or homologous recombination (HR), which lead to gene knockout or gene replacement, respectively. In particular, the CRISPR/Cas system can be apply in transformable plants for functional characterization of gene of interest and to improve delivered traits; to this aim, it is possible to use both Agrobacterium-based traditional transformations systems, to deliver in
9
plant plasmids containing specific CRISPS-Cas expression cassette, and DNA and Agrobacterium-free systems, based on use of ribonucleoparticles (RNPs), a mixture of Cas protein and RNA guide. The last approach is very prominent for the genetic improvement of grapevine, because the absence of Agrobacterium and of sequences of exogenous DNA and the minimal modification of the genome, would allow the generation of plants indistinguishable from those obtained by conventional breeding, exempting them from the current genetic modified organisms (GMOs) regulations.
10
Outline of the thesis
The great goal of this PhD project was the identification and functional characterization of putative master regulators of the onset of berry ripening. This main topic was flanked by the application, development and improvement of technologies for functional studies and for genetic improvement of grapevine. The genes selected are the transcription factors VviNAC33,
VviNAC60, VviAGL15a, VviWRKY19, VvibHLH75: they are five switch genes of red and white
berry transcriptomes (Palumbo et al., 2014; Massonnet et al., 2017) and, excluding VviAGL15a, they were identified as markers of first transition of veraison (Fasoli et al., 2018). The functional characterization of genes selected was performed using stable genetic transformation and transient gene expression approaches. The phenotypic and molecular analysis of transgenic plants allowed to obtain important information about their roles in the regulation of the onset of berry ripening.
Chapter 2 describes the analysis of different transcriptomic dataset of grapevine and berry development and ripening, the selection of the best candidates for functional characterization in grapevine and the analysis of their expression profile in different grapevine organs and developmental stages and correlated genes.
Chapter 3 reports the application of gene transfer technology in grapevine. To identify a standard and defined protocol, stable genetic transformation was tested using different methods in different cultivars and gfp as reporter gene. Regarding the transient gene expression, the use of yfp as reporter gene allowed to improve the leaf agroinfiltration, identifying the best expressing leaves at the day post infiltration of maximum expression, and to develop the berry agroinfiltration.
In the Chapter 4, the functional analysis of VviNAC33 and VviNAC60 by stable genetic transformation was performed. In a previous work (D’Incà, 2017), their overexpression showed an upregulation of many genes involved in the maturation process, suggesting their involvement in the regulation of grapevine development. Here, their conversion into transcriptional repressors confirmed previous results, showing a downregulation of some target of both NAC genes, and allowed to complete the characterization of these two transcription factors.
In the Chapter 5, VviAGL15a, VviWRKY19 and VvibHLH75 were functionally characterized by transient overexpression, using the improved leaf agroinfiltration approach based on YFP
11
expression. The exhaustive molecular analysis of overexpressing leaves allowed to identify their target genes and to better define their specific roles in the transcriptional regulatory network controlling the onset of berry ripening.
Finally, the Chapter 6 describes the application of a protocol to isolate grapevine protoplast from embryogenic calli and to regenerate plants by somatic embryogenesis. Plant regeneration and the positive results obtained after protoplast transfection using a plasmid carrying the yfp reporter gene, indicate that this technology can be used for many studies, including the application of genome editing by CRISP-Cas system.
12
REFERENCES
Ben-Amar A., Cobanov P., Buchholz G., Mliki A., Reustle G.. In planta agro-infiltration system for transient gene expression in grapevine (Vitis spp.). Acta Physiol Plant. 2013. Vol 35:3147– 3156.
Bertazzon N., Raiola A., Castiglioni C., Gardiman M., Angelini E., Borgo M. and Ferrari S.. Transient silencing of the grapevine gene VvPGIP1 by agroinfiltration with a construct for RNA interference. Plant Cell Rep. 2012. Vol 31: 133–143.
Conde C., Silva P., Fontes N., Dias A. C. P., Tavares R.M., Sousa M. J., Agasse A., Delrot S., Gerós H.. Biochemical Changes throughout Grape Berry Development and Fruit and Wine Quality.
Food. 2007. Vol 1 (1): 1-22.
Dhekney S. A., Li Z. T., Zimmerman T. W. and Gray D. J.. Factors Influencing Genetic Transformation and Plant Regeneration of Vitis. American journal of Enology and Viticulture. 2009. Vol 60 (3): 285-292.
D’Incà E. Master regulators of the vegetative-to-mature organ transition in grapevine: the role of NAC transcription factors. PhD thesis. 2017. Verona University.
Fasoli M., Dal Santo S., Zenoni S., Tornielli G. B., Farina L., Zamboni A., Porceddu A., Venturini L., Bicego M., Murino V., Ferrarini A., Delledonne M. and Pezzotti M.. The Grapevine Expression Atlas Reveals a Deep Transcriptome Shift Driving the Entire Plant into a Maturation Program.
The Plant Cell. 2012. Vol. 24: 3489–3505.
Fasoli M., Richter C. L., Zenoni S., Bertini E., Vitulo N., Dal Santo S., Dokoozlian N., Pezzotti M., Tornielli G.B.. The timing and order of the molecular events that mark the onset of berry ripening in grapevine. Plant Physiology. 2018. Vol. 178: 1187-1206.
Fernandez L., Romieu C., Moing A., Bouquet A., Maucourt M., Thomas M. R. and Torregrosa L.. The Grapevine fleshless berry Mutation. A Unique Genotype to Investigate Differences between Fleshy and Nonfleshy Fruit. Plant Physiology. 2006. Vol. 140: 537-547.
Fortes A. M., Teixeira R. T. and Agudelo-Romero P.. Complex Interplay of Hormonal Signals during Grape Berry Ripening. Molecules. 2015. Vol. 20: 9326-9343.
Fraga H., Malheiro A.C., Moutinho-Pereira J. and Santos J. A.. An overview of climate change impacts on European viticulture. Food and Energy Security. 2012. Vol 1(2): 94–110.
13
Holme I. B., Wendt T. and Holm P. B.. Intragenesis and cisgenesis as alternatives to transgenic crop development. Plant Biotechnology Journal. 2013. Vol 11: 395–407.
Iocco P., Franks T., Thomas M.R. Genetic transformation of major wine grape cultivars of Vitis vinifera L. Transgenic Research. 2001. Vol. 10: 105–112.
Jackson R. S.. Grapevine Structure and Function. In: Wine Science - Principles and Applications. Chapter 3, 4th edn. Elsevier Inc. 2014.
Jaillon O., Aury J.M., No€el B., Policriti A., Clepet C., Casagrande A., Choisne N., Aubourg S., Vitulo N., Jubin C., Vezzi A., Legeai F., Hugueney P., Dasilva C., Horner D., Mica E., Jublot D., Poulain J., Bruyere C., Billault A., Segurens B., Gouyvenoux M., Ugarte E., Cattonaro F., Anthouard V., Vico V., Del Fabbro C., Alaux M., Di Gaspero G., Dumas V., Felice N., Paillard S., Juman I., Moroldo M., Scalabrin S., Canaguier A., Le Clainche I., Malacrida G., Durand E., Pesole G., Laucou V., Chatelet P., Merdinoglu D., Delledonne M., Pezzotti M., Lecharny A., Scarpelli C., Artiguenave F., Pe M.E., Valle G., Morgante M., Caboche M., Adam-Blondon A.F., Weissenbach J., Qu_etier F. and Wincker P. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007. Vol. 449: 463–467.
Jelly N.S., Valat L., Walter B. and Maillot P. Transient expression assays in grapevine: a step towards genetic improvement. Plant Biotechnology Journal. 2014. Vol 12, pp. 1231–1245.
Kandel R., Bergey D. R., Dutt M., Sitther V., Li Z. T., Gray D. J. and Dhekney S. A.. Evaluation of a grapevine-derived reporter gene system for precision breeding of Vitis. Plant Cell Tiss Organ
Cult. 2016. Vol 124: 599–609.
Keller M.. Managing grapevines to optimise fruit development in a challenging environment: a climate change primer for viticulturists. Australian Journal of Grape and Wine Research. 2010. Vol 16: 56–69.
Kennedy J.. Understanding grape berry development. Pract Winer Vineyard. 2002.
Kobayashi S., Goto-Yamamoto N. and Hirochika H.. Retrotransposon-induced mutations in grape skin color. Science. 2004. 304
(
5673): 982.Kuhn N., Guan L, Wu Dai Z., Wu B. H., Lauvergeat V., Gomès E., Li S. H., Godoy F., Arce-Johnson P. and Serge Delrot S.. Berry ripening: recently heard through the grapevine. Journal of
14
Li Z. T., Dhekney S., Dutt M., Van Aman M., Tattersall J., Kelley K. T. and Gray D. J.. Optimizing Agrobacterium-mediated transformation of grapevine. In Vitro Cell. Dev. Biol. – Plant. 2006 Vol. 42: 220–227.
Li Zhijian T., Dhekney S. A., Dutt M., Gray D.J. An improved protocol for Agrobacterium-mediated transformation of grapevine (Vitis vinifera L.). Plant Cell Tiss Organ Cult. 2008. Vol 93: 311–321.
Li Zhijian T., Hopkins Donald L., Gray Dennis J. Overexpression of antimicrobial lytic peptides protects grapevine from Pierce’s disease under greenhouse but not field conditions.
Transgenic Res. 2015. Vol 24: 821–836.
Martínez-Esteso M.J., Vilella-Antón M.T., Pedreño M.A., Valero M.L. and Bru-Martínez R.. iTRAQ-based protein profiling provides insights into the central metabolism changes driving grape berry development and ripening. BMC Plant Biology. 2013. Vol 13, 167.
Massonnet M, Fasoli M, Tornielli G.B., Altieri M, Sandri M, Zuccolotto P, Paci P, Gardiman M, Zenoni S. and Pezzotti M.. Ripening Transcriptomic Program in Red and White Grapevine Varieties Correlates with Berry Skin Anthocyanin Accumulation. Plant Physiology. 2017. Vol. 174: 2376–2396.
Palliotti A., Silvestroni O., Leoni F. and Poni S. Maturazione dell’uva e gestione della chioma in Vitis vinifera: processi e tecniche da riconsiderare in funzione del cambiamento del clima e delle nuove esigenze di mercato. Italus Hortus. 2012. Vol. 19 (20): 1-15.
Palumbo M. C., Zenoni S., Fasoli M., Massonnet M., Farina L., Castiglione F., Pezzotti M. and Paci P.. Integrated Network Analysis Identifies Fight-Club Nodes as a Class of Hubs Encompassing Key Putative Switch Genes That Induce Major Transcriptome Reprogramming during Grapevine Development. The Plant Cell. 2014. Vol. 26: 4617–4635.
Riaz S., Doligez A., Henry R. J. and Walker M. A.. Grape. In: C. Kole (Ed.), Fruits and Nuts, Chapter 2. Genome Mapping and Molecular Breeding in Plants, Volume 4. Springer-Verlag Berlin Heidelberg. 2007.
Santos-Rosa M., Poutaraud A., Merdinoglu D. and Mestre P.. Development of a transient expression system in grapevine via agro-infiltration. Plant Cell Rep. 2008. Vol 27: 1053–1063.
Schouten H. J., Krens F. A. and Jacobsen, E.. Cisgenic plants are similar to traditionally bred plants. 2006. EMBO Rep. Vol 7: 750–753.
15
Vidal J. R., Gomez C., Cutanda M. C., Shrestha B. R., Bouquet A., Thomas M. R. and Torregrosa L.. Use of gene transfer technology for functional studies in grapevine. Australian Journal of
Grape and Wine Research. 2010. Vol 16: 138–151.
Walker A. R., Lee E., Bogs J., McDavid D. A. J., Thomas M. R. and Robinson S.P.. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant Journal. 2007. Vol. 49: 772-785.
Yin K., Gao C. and Qiu J.L.. Progress and prospects in plant genome editing. Nature Plants. 2017. Vol 3. Article number 17107.
Zottini M., Barizza E., Costa A., Formentin E., Ruberti C., Carimi F. and Lo Schiavo F.. Agroinfiltration of grapevine leaves for fast transient assays of gene expression and for long-term production of stable transformed cells. Plant Cell Rep. 2008. Vol 27: 845–853.
17
IDENTIFICATION OF MASTER REGULATORS OF THE ONSET
OF BERRY RIPENING AND CANDIDATES’ SELECTION FOR
FUNCTIONAL CHARACTERIZATION
ABSTRACT
Grapevine (Vitis vinifera L.) is one of the most economically important fruit crops in the world. Qualitative characteristics of grape are acquired during berry development and ripening phases and they are widely affected by both agronomic practicals and environmental factors. In particular, the negative effects related to ripening anticipation, caused by high temperature season, are well known. The onset of ripening (veraison) is a complex developmental process, influenced by many exogenous and endogenous factors, whose molecular bases are only partially known. In the last years, several molecular studies showed that veraison is characterized by a profound transcriptomic reprogramming and some genes, which are promptly induced during this process, could be key master regulators of berry ripening. The identification of their functions could allow to control the timing of ripening initiation. Among these genes, the functional category overrepresented are transcription factors and some of them have been selected to functionally characterized. They are VviNAC33, VviNAC60,
VviAGL15a, VviWRKY19 and VvibHLH75, belonging to four of the most important transcription
factors families in plants. The analysis of their expression profile in different grapevine organs at different developmental stages and the coexpression analysis showed that they are characterized by a high expression level only at veraison and in the mature berry and that their highly correlated putative target genes are involved in processes associated with ripening. These results suggest their specific role in the regulation of this process.
18
1. INTRODUCTION
Grapevine (Vitis vinifera L.) is one of the major fruit crops in the world. The economic importance of grapevine is closely correlated to fruit quality; grape is highly valued for its multiple uses as a fresh fruit and processed food product such as jelly, juice, raisins, and especially wine. The grape quality characteristics are acquired during berry formation, growth and ripening phases and they are widely affected by agronomic and environmental factors. In a context of profound climate changes, these specific characteristics could be highly altered, with dramatic consequences for wineries basing the identification of their products on grape varieties cultivated in determinate geographical areas. In particular, the high temperatures cause a ripening anticipation, with deep changes in the biochemical and physiological characteristics of grape and consequently the final qualities of grape. In some cases, these changes can be modulated by adopting specific agronomical practices that may not be economically sustainable. The interpretation of the molecular mechanisms controlling the maturation process in grapevine could provide better strategies to control and manipulate grape ripening preserving/enhancing specific quality characteristics.
Veraison is a key event during the berry ripening and it coincides with the onset of ripening.
During veraison the main events involved in the shift from immature to mature berry take place. This complex transition is marked by the colouring and softening of berries, related to important biochemical and physiological changemost of which are well known. On the other hand, the molecular mechanisms involved in the regulation of this process are only partially known and in the last years, many studies have been performed to discover the transcriptional programs associated to veraison and the ripening process.
One of them is represented by the generation of grapevine global genes expression atlas (Fasoli et al., 2012). This analysis was carried out in 54 different samples of Vitis vinifera cv Corvina representing green and mature organ and tissue at different development stages using a comprehensive grapevine genome microarray. This research has revealed a clear distinction between the green/vegetative and woody/mature sample transcriptomes, suggesting a fundamental shift in global gene expression as the plant switches from the immature to the mature developmental program. These results indicate the existence of specific regulatory genes that promote the vegetative-to-mature transcriptomic transition. The identification of the key genes involved in deep transcriptome shift that occurs in grapevine was carry out using
19
a gene network analysis (Palumbo et al, 2014). Using the differential expressed genes among woody/mature organs and vegetative/green organs, a co-expression network has been generated. The specific topological properties of the co-expression network were analyzed and a subset of 113 genes was classified as switch genes. These switch genes of the global gene expression atlas were expressed at a low level in vegetative/green tissues but at significantly higher levels in mature/woody organs, suggesting they participate in the regulation of the transition from immature to mature development. Among these, the functional categories overrepresented are secondary metabolic process, carbohydrate metabolic process and transcription factors activity. Regarding the transcription factors, two LATERAL ORGAN
BOUNDARIES DOMAIN, two NAC DOMAIN-CONTAINING PROTEINS and many ZINC FINGER have
been identified.
To elucidate the immature-to-mature transition in berry, the most important organ in grapevine, and to identify the key genes of this process, the same approach was used (Palumbo et al, 2014; Massonnet et al., 2017). Berries from 5 red and 5 white varieties were sampled at different phenological stages and the transcriptomic profiles were obtained by RNA-Seq. After the identification of differential expressed genes, the gene co-expression network has revealed the existence of 190 switch genes for red varieties and 212 switch genes. They are likely to be involved in the regulation of the grape berry development transition and they are expressed at low level during the immature phase and were significantly induced at veraison. Among the switch genes of both red and white varieties, the functional categories overrepresented are carbohydrate metabolic process, cell wall metabolism, secondary metabolic process and transcription factor activity. Among the transcription factors of both sets of switch genes, 1
MADS-box gene, 3 LATERAL ORGAN BOUNDARIES DOMAIN, 4 NAC DOMAIN-CONTAINING PROTEINS, 3 MYBA genes, 3 WRKY genes, 3 bHLH genes and many ZINC FINGER have been
identified. Among the switch genes, the identification of a high number of transcription factors could indicate the existence of a specific transcriptional regulatory controlling the onset of berry ripening.
To further improve the molecular knowledge related to berry ripening and to better identify the key genes involved in the regulation of this process, transcriptomic analysis by RNA-Seq of berry of two different cultivars, Cabernet Sauvignon and Pinot Noir, at different developmental stages (from fruit set to full maturity) has been performed (Fasoli et al., 2018). The results
20
showed that during the progress of berry development and maturation, transcripts are divided into four classes: genes expressed during pre-veraison (Class 1), during veraison/mid-ripening (Classes 2 and 3) and during later ripening (class 4). The expression of class 1 transcripts rapidly decreases during berry development, transcripts of classes 2 and 3 show a peak at veraison and subsequently declining while genes of class 4 are expressed during late-ripening stages with increasing expression throughout development. These data confirming the transcriptional shift during immature-to-mature transition and suggesting again the existence of specific key regulators genes. To obtain more information about the molecular mechanisms that regulate the transition from lag phase to ripening, the berry transcriptome around veraison (early
veraison, mid-veraison and late veraison) have been analyzed. The results showed that veraison
could be resolved into two back-to-back molecular transitions starting from 14 days before veraison. Each transition contains positive and negative molecular biomarkers; negative biomarkers of transition 1 showed a very low expression level while negative biomarkers of transition 2 showed a more complex trend with a small initial increase of expression until
veraison followed by a very slight decline thereafter. Indeed, the positive biomarkers of
transitions 1 and 2 showed similar upward expression, but they differed during the pre-veraison phase. Positive biomarkers of transition 1 started at very low expression levels, but they were characterized by a strong induction 14 days before veraison and their average expression value doubled in less than 1 week. In contrast, positive biomarkers of transition 2 started with a higher level of expression but their upregulation 14 days before veraison occurs at a slower rate than the transition-1 positive biomarkers. This result could indicate that the first set of genes trigger the expression of the second set, which in turn mediates the different processes that characterize ripening. Furthermore, as previously described for switch genes, among the positive biomarkers of both transitions, there are genes involved in many processes, such as response to hormone stimulus, cell wall metabolism and secondary metabolic processes, but one of the most represented functional categories are transcription factors. Furthemore, many of these transcription factors have been identified as switch genes of grapevine expression atlas and of both red and white berry transcriptomes. This data indicates that these genes have a very important roles in the regulation of berry development and ripening.
Altogether, these results have provided important and detailed information about the specific set of genes, represented especially by transcription factors, putatively controlling the
21
development and ripening of grape berry. At this point, an in-depth functional characterization of specific transcription factors is necessary for a complete interpretation of the molecular mechanism involved in the regulation of the onset of berry ripening.
In this chapter, the close inspection of the expression and co-expression behavior of switch genes has allowed to select specific transcription factors representing candidates to be functionally characterized to define their putative role of master regulators of grapevine berry ripening.
2. MATERIALS AND METHODS
2.1 Gene selection criteriaThe selection of candidate genes for functional analysis was performed using four criteria: 1) their belonging to the functional category of transcription factors, 2) their role as switch genes in both grapevine expression atlas (Fasoli et al., 2012) and in the transcriptomic dataset of red and white berries (Massonnet et al., 2017), 3) their identification as marker of first and/or second transition during the onset of berry ripening (Fasoli et al., 2018) and 4) the specific biological role of the gene family they belong to.
2.2 Expression analysis of selected genes in grapevine
The expression profiles of VviNAC33, VviNAC60, VviAGL15a, VviWRKY19 and VvibHLH75 were analyzed in the Vitis vinifera cultivar Corvina (clone 48) gene expression atlas of different organs at various developmental stages (Fasoli et al., 2012). Microarray data were obtained from Gene Expression Omnibus website (https://www.ncbi.nlm.nih.gov/geo/) searching for the GSE36128 entry.
The expression profiles of selected genes were also analyzed in a berry specific expression map. Transcriptomic data were obtained by RNA-Seq performed on whole berry samples collected from 10 different grapevine varieties (Sangiovese, Barbera, Negroamaro, Refosco, Primitivo, Vermentino, Garganega, Glera, Moscato, Passerina) at four different developmental stages (Massonnet et al., 2017).
Finally, the expression analysis of VviNAC33, VviNAC60, VviAGL15a, VviWRKY19 and VvibHLH75 was performed using a berry specific expression map of both Cabernet Sauvignon and Pinot
22
Noir cultivars (Fasoli et al., 2018). Transcriptomic data were obtained by RNA-Seq using berry samples collected every 7 to 10 days, from fruit set to full maturity.
2.3 Co-expression analysis
The gene co-expression analyses of VviNAC33, VviNAC60, VviAGL15a, VviWRKY19 and
VvibHLH75 was performed using the global gene expression dataset of V. vinifera cv. Corvina
obtained by microarray approach (Fasoli et al., 2012) by means of the CorTo software (http://www.usadellab.org/cms/index.php?page=corto), setting Pearson’s coefficient as correlation metric.
3. RESULTS
3.1 Candidate selection
The candidate genes selected for functional characterization belong to the functional category of transcription factors, one of the most represented among the list of switch genes (Palumbo et al., 2014; Massonnet et al., 2017). The selection has been performed by analyzing and combining the list of transcription factors identified as switch genes in the grapevine expression atlas and in the transcriptomic dataset from red and white berries (Table 1). Another level of selection comes from the information obtained by Fasoli et al. (2018) that indicates some of these transcription factors as marker of first and/or second transition during the onset of berry ripening. Finally, the transcription factors to be functionally analyzed have been selected based on specific role of the gene family they belong to.
Table 1: Transcription factors identified as switch genes of grapevine expression atlas (A), of red (R) and white (W) berry transcriptome and marker of the first and/or second transition of the onset of ripening.
VIT FUNCTIONAL ANNOTATION A R W
Marker of the 1° transition Marker of the 2° transition
VIT_17S0000G00430 basic helix-loop-helix (bHLH) family
(VvibHLH75) * * *
VIT_11S0037G01230 basic helix-loop-helix (bHLH) family * VIT_05S0077G00750 basic helix-loop-helix (bHLH) family * VIT_18S0122G01340 BTB/POZ domain-containing protein * VIT_15S0046G00150 DOF affecting germination 1 * *
23
VIT_06S0004G07790 Lateral organ boundaries Domain 15 * * * * VIT_03S0091G00670 Lateral organ boundaries protein 38 * *
VIT_15S0048G00830
LOB domain-containing 18 (Asymmetric leaves 2-like protein
20)
* *
VIT_12S0028G00980 myb family *
VIT_07S0031G01930 myb TKI1 (TSL-KINASE INTERACTING
PROTEIN 1) * *
VIT_02S0033G00380 Myb VvMYBA1 * *
VIT_14S0108G01070 NAC domain-containing protein
(VviNAC11) * *
VIT_02S0012G01040 NAC domain-containing protein
(VviNAC13) * *
VIT_19S0027G00230 NAC domain-containing protein
(VviNAC33) * * * *
VIT_08S0007G07670 NAC domain-containing protein
(VviNAC60) * * * *
VIT_13S0158G00100 putative MADS-box Agamous-like
15a (VviAGL15a) * *
VIT_02S0033G00410 VvMybA1 * *
VIT_02S0033G00390 VvMybA2 * * *
VIT_02S0033G00450 VvMybA3 * *
VIT_17S0000G01280 WRKY Transcription Factor
(VviWRKY75) *
VIT_07S0005G01710 WRKY Transcription Factor
(VviWRKY19) * * * *
VIT_12S0059G00880 WRKY Transcription Factor
(VviWRKY37) *
VIT_13S0064G01210 Zf A20 and AN1 domain-containing
stress-associated protein 2 * VIT_13S0047G01130 Zfwd2 protein (ZFWD2) * VIT_10S0071G00580 Zfwd2 protein (ZFWD2) * VIT_12S0059G02510 Zinc finger (B-box type) * VIT_00S0347G00030 Zinc finger (B-box type) * * VIT_00S0203G00210 Zinc finger (B-box type) * * VIT_06S0061G00760 Zinc finger (C2H2 type) family * VIT_06S0004G04180 Zinc finger (C2H2 type) protein
(ZAT11) *
VIT_14S0219G00040 Zinc finger (C3HC4-type ring finger) * * *
VIT_05S0020G04730 Zinc finger (C3HC4-type ring finger) * * * VIT_08S0040G01950 Zinc finger (C3HC4-type ring finger) * * * *
24
VIT_12S0028G03860 Zinc finger (C3HC4-type ring finger)
protein (RMA1) * *
VIT_03S0091G00260 Zinc finger protein 4 * * *
Based on the criteria previously described, five transcription factors have been selected for functional characterization. The first two genes are represented by VviNAC33 (VIT_19S0027G00230) and VviNAC60 (VIT_08S0007G07670). They are two switch genes of grapevine expression atlas and of both red and white berry transcriptome; furthermore, they are two markers of the first transition of the onset of ripening. NAC transcription factors family is one of the most important transcription factors families in plants; their involvement in plant growth regulation and response to abiotic and biotic stress emerged after many studies in
Arabidopsis thaliana and rice. Regarding grapevine, a comprehensive analysis of NAC
transcription factors has been performed (Wang et al., 2013), but the specific biological function of each of them remains unknown. The functional analysis of these two transcription factors has been initiated in a previous project (D’Incà, 2017) that highlighted an effective role of these two genes in the regulation of vegetative-to-mature transition. A more detailed description of these preliminary results and other information about NAC transcription factors are reported in the Chapter 4.
The other three transcription factors selected are VviAGL15a (VIT_07S0005G01710),
VviWRKY19 (VIT_13S0158G00100) and VvibHLH75 (VIT_17S0000G00430). They are three
switch genes of both red and white berry transcriptomes; furthermore, VviWRKY19 and
VvibHLH75 are two markers of the first transition and VviWRKY19 is also a marker of the second
transition of the onset of ripening. These three transcription factors belong to three large families of transcription factors in plants: VviAGL15a belongs to the MADS-box transcription factors family, involved especially in process related to reproductive development (Gramzow and Theissen, 2010), VviWRKY19 belongs to the WRKY transcription factor family, whose main roles are biotic and abiotic stress responses (Schluttenhofer and Yuan, 2015) and VvibHLH75 belongs bHLH transcription factors family, involved in many processes, from hormone signaling and regulation of secondary metabolism to flower and fruit development (Carretero-Paulet et al., 2010). Each of these transcription factors families has been described in grapevine (Grimplet et al., 2016; Wang et., 2014; Wang et al., 2018). A more detailed description of these transcription factors and their gene families is reported in Chapter 5.
25
3.2 Expression profiles of the selected transcription factors
The expression analysis of VviNAC33, VviNAC60, VviAGL15a, VviWRKY19, VvibHLH75, was determined by inspecting the grapevine gene expression atlas (Fasoli et al., 2012), the transcriptome dataset of both red and white varieties (Massonnet et al, 2017) and the transcriptomic data related to berry ripening of Cabernet Sauvignon and Pinot Noir (Fasoli et al., 2018)
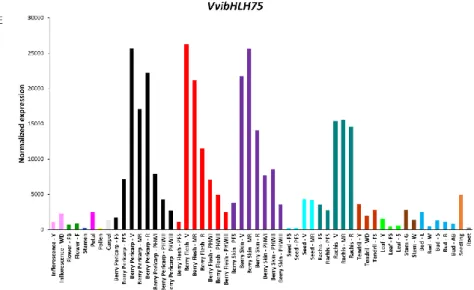
The expression profiles of each transcription factor in the gene expression atlas are shown in Figure 1. Regarding VviNAC33 (Figure 1A), it shows a very high expression during senescence phase in leaves, but it also expressed in berry pericarp, flesh and skin during post-veraison phases and in woody stem. The expression of VviNAC60 (Figure 1B) occurs especially in berry pericarp, flesh and skin during post-veraison phases but this gene is also expressed in woody stem, senescent leaf, rachis and seed during post-veraison phases. Regarding VviAGL15a (Figure 1C), its expression occurs mainly in berry pericarp, flesh and skin, starting from veraison phase and continuing until post-harvest phases; furthermore, VviAGL15 show a very high expression in stamen and pollen and while its expression in seed decrease from fruit set phase to veraison phase. The expression of VviWRKY19 (Figure 1D) is very high in berry pericarp, flesh and skin during all post-veraison phases, but it is very high also in rachis at post-veraison phases. Moreover, VviWRKY19 is expressed in seed at veraison and mid-ripening phases, in woody stem and bud and in roots. Finally, regarding VvibHLH75 (Figure 1E), this gene is preferentially expressed in berry pericarp, flesh and skin during all post-veraison phases and in rachis after
veraison. The high expression of VviNAC33 and VviNAC60 in each mature/woody organ/tissue
confirms their putative role of master regulators of the vegetative-to-mature transition of various organs while the preferential expression of VviAGL15a, VviWRKY19 and VvibHLH75 in berry during post-veraison phases confirms their role of putative master regulators of the onset of berry ripening.
28
Figure 1: VviNAC33 (A), VviNAC60 (B) VviAGL15a (C), VviWRKY19 (D) and VvibHLH75 (E) expression profiles in 54 grape organs at different developmental stages; transcriptomic data were obtained by a
global expression map of Vitis vinifera cv. Corvina by microarray (Fasoli et al., 2012). Description of ATLAS abbreviations:
Bud – L = latent bud; – W = winter bud; – S = bud swell; – B = bud burst; – AB = bud after-burst; Inflorescence
– Y = young inflorescence; – WD = well developed inflorescence; Flower – FB = flowering begins; – F = flowering; Stamen = pool of stamen from undisclosed flowers; Pollen = pollen from disclosed flowers;
Carpel = pool of carpels from undisclosed flowers; Petal = pool of petals from undisclosed flowers; Tendril
– Y = young tendril; – WD = well developed tendril; – FS = mature tendril; Leaf – Y = young leaf; – FS = mature leaf; – S = senescencing leaf; Berry Pericarp – FS = fruit set; – PFS = post-fruit set; – V = véraison; – MR = mid-ripening; – R = ripening; – PHWI = postharvest withering I; – PHWII = post-harvest withering II; – PHWIII = post-harvest withering III; Berry Skin – PFS = post-fruit set; – V = véraison; – MR = mid-ripening; – R = ripening; – PHWI = post-harvest withering I; – PHWII = post-harvest withering II; – PHWIII = post-harvest withering III; Berry Flesh – PFS = post-fruit set; – V = véraison; – MR = mid- ripening; – R = ripening; – PHWI = post-harvest withering I; –PHWII = post-harvest withering II; – PHWIII = post-harvest withering III; Seed – FS = fruit set; – PFS = fruit set; – V = véraison; – MR = mid-ripening; Rachis – FS = fruit set; – PFS = post-fruit set; – V = véraison; – MR = mid- ripening; – R = ripening; Stem – G = green stem; - W = woody stem;
Root = in-vitro cultivated roots; Seedling = pool of 3 developmental stages.
The expression profile of VviNAC33, VviNAC60, VviAGL15a, VviWRKY19 and VvibHLH75 retrieved from the berry transcriptomic survey of Massonnet et al., 2017 show that each transcription factors is mainly expressed in both red and white berries at end of veraison and harvest phases (Figure 2). The expression of VviNAC33 (Figure 2A) and VviNAC60 (Figure 2B) is slightly different among the varieties and it shows maximum level of expression at end of
29
veraison or harvest phase depending on the cultivars. In particular, VviNAC33 show a higher
expression during harvest phase in all varieties, excluding Refosco, Vermentino, Garganega and Passerina, while the expression of VviNAC60 is higher at end of veraison phase in all varieties, excluding Sangiovese, Refosco, Primitivo and Passerina. The expression of VviNAC33 and
VviNAC60 follow the same trend showed in Figures 1A and 1B, with high expression in berry
tissues during post-veraison phases. Regarding VviAGL15a (Figure 2C), its maximum expression value in both red and white varieties occurs during harvest phase, confirming the results previously described using the grapevine expression atlas (Figure 1C). VviWRKY19 (Figure 2D) and VvibHLH75 (Figure 2E) are preferentially expressed during end of veraison phase in each variety, confirming again the results previously described (Figure 1D, E). These results confirm again the high expression of the five transcription factors only in ripening berry and support their role of master regulators of the vegetative-to-mature transition in berry.
31
Figure 2: VviNAC33 (A), VviNAC60 (B) VviAGL15a (C), VviWRKY19 (D) and VvibHLH75 (E), expression profiles in 10 different grapevine varieties at four developmental stages. Transcriptomic data were retrieved by a berry specific expression map obtained by RNAseq (Massonnet et al., 2017). Abbreviations:
32
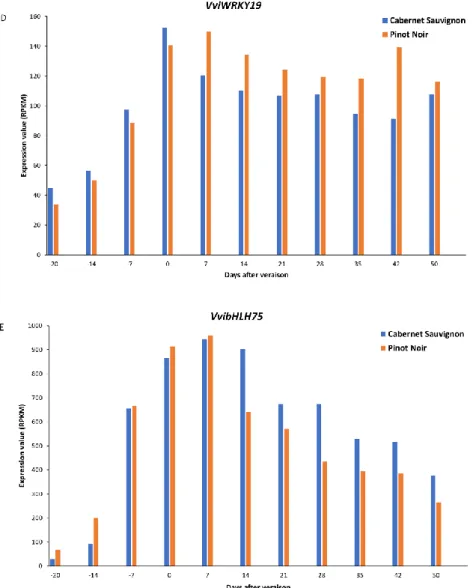
Finally, the expression analysis of VviNAC33, VviNAC60, VviAGL15a, VviWRKY19 and VvibHLH75 using the transcriptomic data related to berry ripening process of Cabernet Sauvignon and Pinot Noir (Fasoli et al., 2018) shows that each transcription factor, excluding VviAGL15a, is characterized by a sudden increase of expression just before veraison (Figure 3). These data are consistent with their classification as positive bio-markers of first transition of veraison and suggest that they act as master regulators during the onset of ripening. After veraison, the expression of VviNAC33 (Figure 3A), VviNAC60 (Figure 3B), VviWRKY19 (Figure 3D) and
VvibHLH75 (Figure 3E) can further increase or decrease, but their level of expression remain
higher than the pre-veraison stages, indicating that they may have a role during the whole ripening process. The steep decrease of expression of VvibHLH75 (Figure 3E), suggests that it plays a major role during the initial phases of ripening. Finally, concerning VviAGL15a, it is the only transcription factor characterized by the absence of a sudden expression before veraison, but its expression increases during the ripening process, suggesting its involvement in the regulation of specific processes associated with late ripening stages (Figure 3C).
34
Figure 3: VviNAC33 (A), VviNAC60 (B), VviAGL15a (C), VviWRKY19 (D) and VvibHLH75 (E) expression profiles related to berry ripening, from fruit set to full maturity, of Cabernet Sauvignon and Pinot Noir. Transcriptomic data were retrieved by a berry specific expression map obtained by RNAseq (Fasoli et al.,
35
3.3 Co-expression analysis
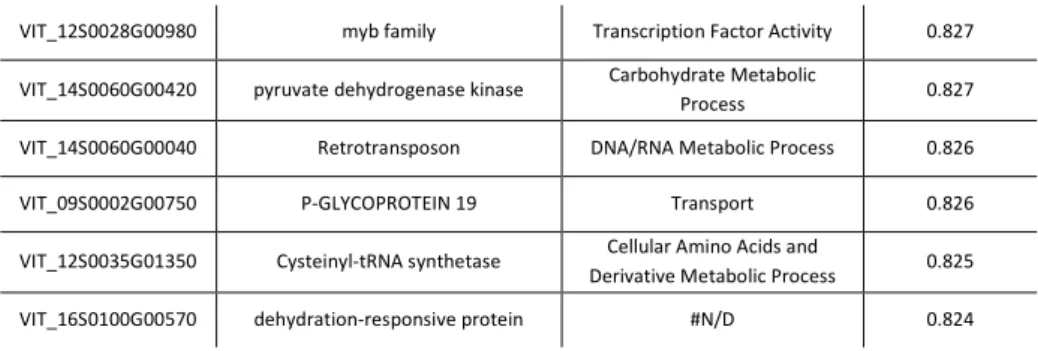
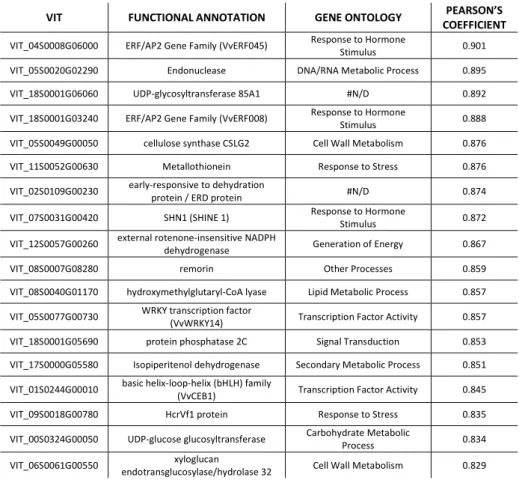
To obtain preliminary information about genes transcriptionally related to the selected transcription factors, that may include their targets, regulators or partners, a co-expression analysis by the CorTo software has been performed on the transcriptomic dataset of the grapevine gene expression atlas (Fasoli et al., 2012). For each transcription factors, only the first thirty co-expressed genes, excluding those with no similarity to known sequences or function (no hit/unknown protein), are reported.
Regarding VviNAC33, the first thirty co-expressed genes are indicated in table 2. Among them, there two RECEPTOR KINASE RK20-1 (VIT_00S2634G00010, VIT_00S0398G00030) and two
RECEPTOR SERINE/THREONINE KINASE (VIT_00S0409G00050, VIT_05S0049G01190), involved
in signal transduction, two proteins related to senescence, SENESCENCE-INDUCIBLE
CHLOROPLAST STAY-GREEN PROTEIN 1 (VIT_02S0025G04660) and senescence-related gene 1
(SRG1, VIT_13S0019G02010), two GALACTINOL SYNTHASE (VIT_05S0077G00430, VIT_05S0020G00330), related to sugar signaling, and one NAC transcription factor, VviNAC36 (VIT_12S0028G00860).
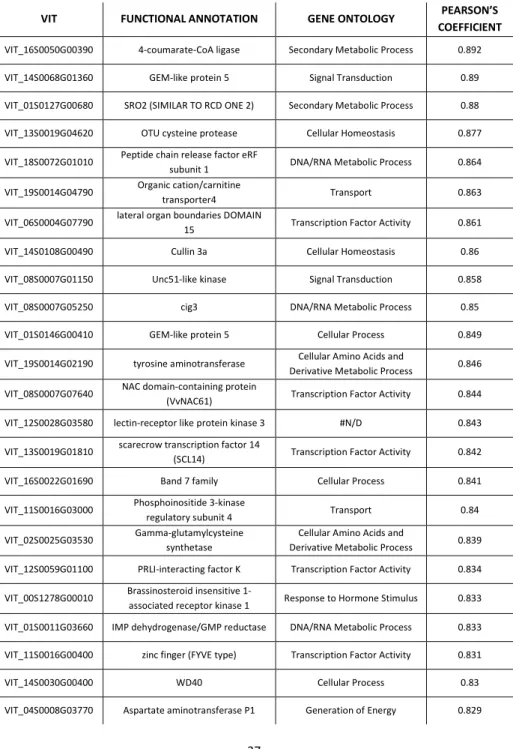
Table 2: the first thirty genes co-expressed with VviNAC33.
VIT FUNCTIONAL ANNOTATION GENE ONTOLOGY PEARSON’S COEFFICIENT
VIT_15S0048G01010
2-hydroxy isoflavone/dihydroflavonol
reductase
Secondary Metabolic Process 0.881
VIT_18S0001G08300 tubulin alpha-6 chain Cellular Process 0.83 VIT_00S2634G00010 receptor kinase RK20-1 Signal Transduction 0.821 VIT_14S0060G01530 DNA polymerase III subunit epsilon DNA/RNA Metabolic Process 0.812 VIT_00S0398G00030 receptor kinase RK20-1 Signal Transduction 0.807 VIT_14S0006G01610 PMI2 (plastid movement impaired
2) #N/D 0.802
VIT_18S0001G13780 Cytochrome P450, family 83,
subfamily B, polypeptide 1 Secondary Metabolic Process 0.801 VIT_04S0008G05400 serine hydrolase [Vitis vinifera] Cellular Amino Acids and
Derivative Metabolic Process 0.793 VIT_02S0025G04660 senescence-inducible chloroplast
stay-green protein 1 Developmental Process 0.78 VIT_00S0409G00050 Receptor-like serine-threonine