POLITECNICO DI MILANO
Scuola di Ingegneria Industriale e dell’Informazione
Corso di Laurea Magistrale in Ingegneria Biomedica
Analysis of a hospital clinical
engineering service and development of
e-Learning tools for electrical safety of
medical devices
Relatore: Prof.ssa Veronica Cimolin Correlatore: Prof. Leandro Pecchia
Tesi di Laurea di: Edoardo von Morgen Matr. 905580
3
Ringraziamenti
Desidero ringraziare la mia famiglia. Il loro incessabile sostegno durante tutti questi anni è stato essenziale.
Grazie ai miei Professori. Il loro aiuto è stato indispensabile per approfondire e comprendere nel profondo le potenzialità e le responsabilità dell’Ingegnere.
Il professor Leandro Pecchia mi ha trasmesso la sua grande esperienza e la sua profonda passione. La professoressa Veronica Cimolin è stata fondamentale con la sua disponibilità, il suo supporto, e i suoi consigli. Ringrazio anche la professoressa Anna Maria Bianchi per l'opportunità offertami e per avermi guidato durante questi anni. Entrare a far parte del dipartimento di Ingegneria Clinica al Nottingham University Hospital mi ha permesso di conoscere molte persone instaurando rapporti professionali, di scambio concreto di conoscenze ed esperienze, e di amicizia.
Infine, voglio ringraziare in miei compagni di studio, i miei coinquilini, e i miei amici, con i quali ho condiviso gioie e dolori della mia esperienza universitaria.
Acknowledgements
I want to thank my family. Their ever-lasting support during all these years has been essential.
Thanks to my Professors. Their help has been indispensable to deepen and fully understand the potentialities and the responsibilities of an Engineer.
Professor Leandro Pecchia has shown me his great experience and his deep passion. Professor Veronica Cimolin has been fundamental with her kindness, support, and advices. Thanks also to Professor Anna Maria Bianchi for giving me this opportunity and guiding me during these years.
Joining the Department of Clinical Engineering at Nottingham University Hospital allowed me to meet many people, establishing professional relationships, with concrete exchange of knowledge and experience, and friendship.
Finally, I would like to thank in my fellow students, my roommates, and my friends with whom I shared the joy and pain of my university experience.
5
Sommario
Un ospedale è caratterizzato da una complessa struttura organizzativa. Tutte le figure coinvolte svolgono ruoli fondamentali nell’utilizzare le risorse disponibili (economiche, tecnologiche, informative etc.) per fornire servizi medici ai pazienti. All’interno della struttura organizzativa, esiste una particolare sezione di professionisti che costituisce il servizio di Ingegneria Clinica. Il loro obiettivo principale non è quello di fornire direttamente l’assistenza in termini di terapie, cure o diagnostica, ma consiste nel sostenere il personale clinico con essenziali servizi accessori di gestione delle tecnologie sanitarie. In ospedale, una delle più importanti cause di rischio per i pazienti è l'elettroshock, che può avvenire quando il corpo umano entra in contatto con un'apparecchiatura medicale difettosa. Durante il contatto, diretto o indiretto, una corrente elettrica fluisce attraverso il corpo umano causando potenzialmente gravi danni come interruzione della respirazione, fibrillazione ventricolare, ustioni tissutali. Per prevenirlo, il protocollo di gestione del rischio, eseguito dagli ingegneri clinici richiede di eseguire test di sicurezza elettrica sui dispositivi che utilizzano e conducono energia elettrica in normali condizioni di lavoro.
Uno dei due obiettivi di questa tesi è lo sviluppo di un modello di servizio di Ingegneria Clinica in cui identificare gli attori e le unità coinvolte nelle specifiche attività necessarie per la gestione delle tecnologie sanitarie. L'altro obiettivo è la produzione di e-Learning tools per test di sicurezza elettrica di dispositivi medici.
Per raggiungere il primo obiettivo, è possibile creare un modello utilizzando un metodo induttivo ed esaminando l'intera struttura organizzativa del dipartimento, facendo l’analisi e la valutazione dei compiti e delle interazioni tra le unità di lavoro. Un’iniziale analisi della letteratura di fonti accademiche e istituzionali aiuta a contestualizzare e definire il processo di gestione delle tecnologie mediche eseguito dal servizio di ingegneria clinica all'interno dell'ospedale. Un'indagine sul campo durante l’affiancamento dei professionisti del dipartimento, con incontri e interviste non strutturate, ha permesso una comprensione più profonda delle dinamiche, dei compiti, e dei ruoli di ciascuno all'interno del reparto. Queste interazioni, insieme alle informazioni primarie provenienti da
6
documenti interni e forniti dall’ospedale, permettono la raccolta di informazioni soggettive, più complete e dettagliate. La generalizzazione e schematizzazione delle informazioni raccolte ha permesso la creazione del modello teorico della struttura organizzativa di un complesso dipartimento di ingegneria clinica. Invece, per raggiungere il secondo obiettivo sono stati studiati standard internazionali, normative italiane, linee guida inglesi, e manuali del tester di sicurezza elettrica. In seguito, sono stati seguiti e implementati protocolli per la sicurezza elettrica, l'ispezione e i test sulle apparecchiature mediche, come elettrocardiogramma, monitor di segnali vitali, concentratore di ossigeno. I materiali didattici test di sicurezza elettrica per dispositivi medici sono stati prodotti come strumenti di e-Learning e strutturati come una combinazione di video, realizzati presso il laboratorio dell'Università, e di un manuale. Questi, sono stati poi esaminati da alcuni dei professionisti del dipartimento di ingegneria clinica del Nottingham University Hospital.
Il metodo induttivo e la soggettività di interviste qualitative utilizzati non sono garanzia del rigore e nella generalizzazione del modello, ma proprio grazie a questa soggettività è stato anche possibile ottenere una visione più accurata e una migliore comprensione delle dinamiche dell’organizzazione del dipartimento. Gli strumenti di e-Learning prodotti potranno essere proposti come supporto per alcuni professionisti di tali dipartimenti, in particolare, potrebbe essere utile a supportare la progettazione, la pianificazione, l'installazione e la messa in servizio di dispositivi medici. Un'applicazione più importante potrebbe essere nel campo della gestione del rischio di sicurezza elettrica dei dispositivi medici. In questo processo, al fine di garantire la gestione delle attrezzature in armonia con l'ottimizzazione delle cure per i pazienti, è necessario un dialogo continuo tra ingegneri clinici e diverse altre figure come medici, infermieri, tecnici specializzati e aziende fornitrici esterne. Un'altra applicazione degli e-Learning tools è come riferimento e supporto durante la manutenzione quotidiana dei dispositivi, da usare per la formazione e l’aggiornamento dei tecnici biomedici. Anche studenti e ricercatori possono utilizzare gli strumenti di e-Learning per comprendere e migliorare la sicurezza su una questione così complessa e pericolosa, come la sicurezza elettrica, senza dover studiare gli standard immediatamente, mantenendoli però come riferimento per un'illustrazione più dettagliata.
Parole chiave: ingegneria clinica, struttura organizzativa, sicurezza elettrica, e-Learning tools
8
Abstract
The Hospital is characterized by a complex organisation structure. All the actors involved play fundamental roles processing the resources available (e.g. economical, technological, information) in order to utilise these to provide healthcare services for patients. Within the hospital, there is a particular team of professionals, constituting the department of Clinical Engineering, whose main goal is not to directly provide the Profile of care in terms of therapies, cures, or diagnostics, but it consists in supporting clinical staff with ancillary services of management of health technologies. Inside an hospital, one of the most important sources of harm for patients is the electro-shock. It can happen when the human body gets in contact with a medical equipment. When a direct or indirect hazardous contact happens, an electrical current flow through the human body, potentially causing serious hazards such as interruption of respiration, ventricular fibrillation, tissue burns. In order to prevent electro-shock, risk management process requires to perform electrical safety testing on the devices that utilise and conduct electrical power under normal working conditions.
One main goal of this thesis is the development of a model of a Clinical Engineering Service, in which it is possible to identify the actors and units involved in all the specific activities necessary in the Health Care Management. The other objective is the production of e-Learning tools on the Electrical Safety Testing for medical devices.
In order to obtain the first objective, an organisation case study is performed. Examining the whole structure of the department organisation and evaluating the fundamental tasks and interactions of its working units, is possible to create a model using an inductive method. An initial literature analysis of academic and institutional sources helps to contextualise and define the process of Health Technology Management performed by the clinical engineers within the hospital setting. An investigation on field through shadowing, meetings, and non-structured interviews with professionals of the department enables a deeper understanding of dynamics, tasks, and roles within the department. These interactions, alongside primary information from internal documents,
9
provides the research of subjective, but more complete and detailed information. Generalising and schematising all the information gathered, permits the creation of the theoretical model of the organisational structure of a complex clinical engineering service. In order to reach the second goal, international standards, Italian regulations, English guidance notes, and user manuals of the electrical safety analyser were studied. Then, protocols for electrical safety, inspection, and testing are followed and implemented on medical equipment, such as electrocardiograph, patient monitor, oxygen concentrator. Didactic materials on the medical devices Electrical Safety Testing are produced as e-Learning tools and structured as a combination of videos, taken at the University laboratory, and a booklet. The e-Learning tools are reviewed by professionals of the Clinical Engineering department at Nottingham University Hospital.
Through an inductive methodology, in which the subjectivity of qualitative interviews determined the poorer rigorousness and generalisability of the model, it is possible to achieve a deeper insight and better conceptualization of the dynamics of the Health Technology Management organisation. The e-Learning tools could be proposed as support for some professionals of such organisation. In particular, they can be useful in the design, the planning, the installation, and the commissioning of medical devices. However, one major application would be in the field of risk management and governance of medical devices electrical safety. In this case, in order to ensure the management of equipment in harmony with the optimisation of patients’ outcomes, a fluid dialogue is needed between clinical engineers and several other clinical figures such as doctors, nurses, specialized technicians, and external biomedical companies. Another important application of the e-Learning tools is the support as reference during the daily servicing of devices, the training, and the teaching of biomedical technicians. Students and researchers can use the tools for understanding and being more confident on such complex and hazardous issue, without having to immediately study on the standards, while keeping them as reference for a more detailed illustration.
Keywords: clinical engineering, organisation structure, electrical safety, e-Learning tools
10
Index
RINGRAZIAMENTI ... 3 SOMMARIO ... 5 ABSTRACT ... 8 INDEX ... 10 INDEX OF FIGURES ... 12 INDEX OF TABLES ... 15 1 INTRODUCTION... 162 OBJECTIVES OF THE THESIS ... 19
3 CLASSIFICATION MEDICAL DEVICES ... 22
3.1CLINICAL ENGINEERING AND HEALTH TECHNOLOGY MANAGEMENT ... 22
3.2HEALTH TECHNOLOGIES,MEDICAL DEVICES, AND MEDICAL EQUIPMENT ... 22
3.3MEDICAL DEVICES DEFINITION MDR2017/745 ... 23
3.4MEDICAL DEVICES RISK CLASSIFICATION ... 26
3.5MEDICAL EQUIPMENT ... 29
3.6APPLIANCE CLASSES AND TYPES OF MEDICAL DEVICES ... 30
3.7TYPES OF MEDICAL DEVICES ... 31
3.8CLASSES BASED ON ELECTRICAL INSULATION ... 34
4 CLINICAL ENGINEERING MODEL ... 37
4.1LIFECYCLE OF MEDICAL DEVICES ... 37
4.2HOSPITAL AS A PROCESS ... 39
4.3PROFILE AND PROCESS OF CARE ... 41
4.4INPUTS AND OUTPUTS OF CLINICAL ENGINEERING ... 43
4.5HEALTH TECHNOLOGY MANAGEMENT CYCLE ... 44
4.6CLINICAL ENGINEERING DEPARTMENT ORGANISATION ... 50
4.7INTEGRATED ACTIVITIES OF HEALTH TECHNOLOGY MANAGEMENT CYCLE AND CLINICAL ENGINEERING DEPARTMENT ORGANISATION ... 55
11
5.1PATIENT SAFETY ... 61
5.2THE PATIENT ENVIRONMENT ... 63
5.3THE ELECTRICAL SUPPLY SYSTEM ... 64
5.4EFFECTS OF ELECTRICAL CURRENT ON THE BODY ... 67
5.5ELECTRICAL BODY MODEL ... 72
5.6ELECTRICAL SAFETY STANDARDS ... 74
5.7SINGLE FAULT CONDITION ... 75
5.8RECOMMENDATIONS ... 76
6 E-LEARNING TOOLS ...78
6.1ELECTRICAL SAFETY TESTS INDEX ... 78
6.2TESTER EQUIPMENT ... 79
6.3VISUAL INSPECTION ... 80
6.4PROTECTIVE EARTH CONTINUITY TEST ... 81
6.5INSULATION RESISTANCE TEST (CLASS I) ... 84
6.6INSULATION RESISTANCE TEST (CLASS II) ... 88
6.7LEAKAGE CURRENT TESTS ... 90
6.8EARTH LEAKAGE CURRENT TEST ... 95
6.9ENCLOSURE LEAKAGE CURRENT TEST ... 97
6.10PATIENT LEAKAGE CURRENT TEST ... 98
6.11MAINS ON APPLIED PARTS TEST ... 100
6.12PATIENT AUXILIARY CURRENT TEST ... 102
6.13POINT-TO-POINT MEASUREMENTS ... 103
6.14SIMULATING ECGWAVEFORMS ... 104
6.15ELECTRICAL SAFETY TESTS IEC62353 ... 104
6.16DIRECT EQUIPMENT LEAKAGE TEST ... 106
6.17ALTERNATIVE EQUIPMENT LEAKAGE TEST ... 107
6.18DIFFERENTIAL LEAKAGE CURRENT TEST ... 108
6.19DIRECT APPLIED PART LEAKAGE TEST ... 109
6.20ALTERNATIVE APPLIED PART LEAKAGE TEST ... 110
6.21ACCESSIBLE LEAKAGE CURRENT TEST ... 111
6.22TABLES OF ELECTRICAL SAFETY TESTS LIMITS ... 112
6.23VIDEOS OF THE ELECTRICAL SAFETY TESTING ... 117
7 CONCLUSIONS AND FUTURE DEVELOPMENTS ... 120
12
Index of figures
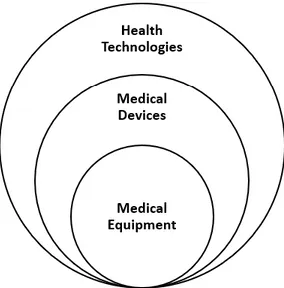
FIGURE 1:SUBDIVISION OF HEALTH TECHNOLOGIES,MEDICAL DEVICES, AND MEDICAL EQUIPMENT. ... 23
FIGURE 2: IDENTIFICATION OF MEDICAL EQUIPMENT, APPLIED PARTS, AND PATIENT CONNECTIONS IN A MULTIFUNCTION PATIENT MONITOR WITH INVASIVE PRESSURE MONITORING FACILITIES ... 31
FIGURE 3:LIFECYCLE OF MEDICAL DEVICES.FROM THE DEVELOPMENT, THROUGH THE VALIDATION, TO THE USE, AND THE OBSOLESCENCE. ... 38
FIGURE 4:THE HOSPITAL PROCESSING INPUTS INTO OUTCOMES. ... 39
FIGURE 5:THE PROFILE OF CARE AND THE PROCESS OF CARE AS FUNDAMENTAL ACTIONS IN THE HEALTHCARE SERVICE. ... 41
FIGURE 6: THE HEALTH TECHNOLOGY MANAGEMENT CYCLE PERFORMED BY THE CLINICAL ENGINEERING DEPARTMENT. ... 45
FIGURE 7:THE ORGANISATION CHART OF THE CLINICAL ENGINEERING DEPARTMENT OF THE NOTTINGHAM UNIVERSITY HOSPITAL. ... 51
FIGURE 8:THE ANTHONY MODEL OF THE ORGANISATIONAL STRUCTURE OF THE CLINICAL ENGINEERING DEPARTMENT. ... 55
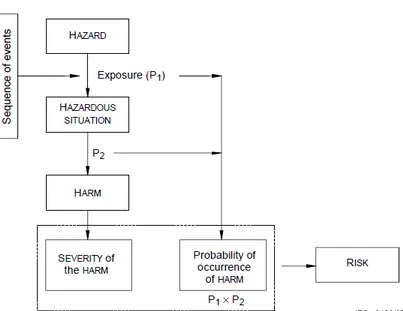
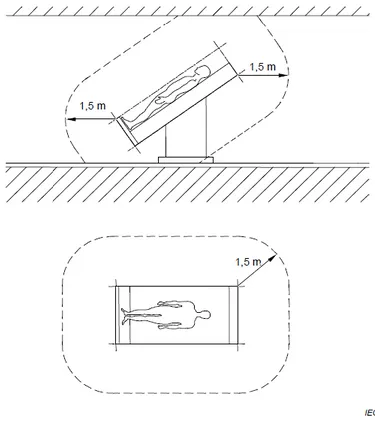
FIGURE 9:THE RELATIONSHIP BETWEEN, SEQUENCE OF EVENTS, HAZARDOUS SITUATION, HARM AND RISK. 63 FIGURE 10:AN EXAMPLE OF PATENT ENVIRONMENT.THE DIMENSIONS IN THE FIGURE SHOW ITS MINIMUM EXTENT IN A FREE SURROUNDING. ... 64
FIGURE 11:ARRANGEMENT OF THE MAINS SUPPLY IN A 3-PHASE STAR CONFIGURATION... 65
FIGURE 12:THE DIFFERENT DYNAMICS OF MACROSHOCK AND MICROSHOCK. ... 68
FIGURE 13:THRESHOLD OF SENSATION AS A FUNCTION OF FREQUENCY FOR AN ELECTRIC CURRENT APPLIED BETWEEN 5 MM WIDE BAND ELECTRODES ENCIRCLING THE BASE OF TWO ADJACENT FINGERS OF A NORMAL SUBJECT. ... 69
FIGURE 14:PERCENTAGE OF ADULT MALES WHO CAN "LET GO" AS A FUNCTION OF FREQUENCY AND INTENSITY OF THE ELECTRICAL CURRENT. ... 70
FIGURE 15:IMPACT OF CURRENT ON THE HUMAN BODY ... 71
FIGURE 16:PROBABILITY OF VENTRICULAR FIBRILLATION IN CARDIAC PATHOLOGICAL PATIENTS. ... 72
FIGURE 17:THE ELECTRONIC CIRCUIT FOR AN ELECTRICAL SAFETY ANALYSER OF MEDICAL DEVICES. ... 74
FIGURE 18: A CLASS I MEDICAL EQUIPMENT WITH A BASIC INSULATION PLUS THE PROTECTIVE EARTH CONDUCTOR. ... 81
13
FIGURE 20: THE TEST CONFIGURATION TO MEASURE THE INSULATION RESISTANCE BETWEEN THE MAINS
VOLTAGE AND THE PROTECTIVE EARTH. ... 87
FIGURE 21:THE TEST CONFIGURATION TO MEASURE THE INSULATION RESISTANCE BETWEEN THE PROTECTIVE EARTH AND THE APPLIED PARTS. ... 88
FIGURE 22: THE TEST CONFIGURATION TO MEASURE THE INSULATION RESISTANCE BETWEEN THE MAINS VOLTAGE AND THE APPLIED PARTS. ... 88
FIGURE 23:THE TEST CONFIGURATION TO MEASURE THE INSULATION RESISTANCE BETWEEN THE APPLIED PARTS AND THE EXPOSED CONDUCTIVE PARTS. ... 89
FIGURE 24: THE TEST CONFIGURATION TO MEASURE THE INSULATION RESISTANCE BETWEEN THE MAINS VOLTAGE AND THE EXPOSED CONDUCTIVE PARTS. ... 90
FIGURE 25: THE TEST CONFIGURATION TO MEASURE THE INSULATION RESISTANCE BETWEEN THE MAINS VOLTAGE AND THE APPLIED PARTS. ... 90
FIGURE 26:SCHEMATIC OF A LEAKAGE CURRENT FLOWING THROUGH THE HUMAN BODY... 91
FIGURE 27:SCHEMATIC OF THE STRAY CAPACITANCES IN A CLASS I MEDICAL EQUIPMENT WITHOUT APPLIED PARTS. ... 91
FIGURE 28:SCHEMATIC OF THE STRAY CAPACITANCES IN A CLASS II MEDICAL EQUIPMENT WITHOUT APPLIED PARTS. ... 92
FIGURE 29:SCHEMATIC OF THE STRAY CAPACITANCES IN A CLASS I MEDICAL EQUIPMENT WITH APPLIED PARTS. ... 93
FIGURE 30: SCHEMATIC EXPLANATION OF THE EARTH LEAKAGE TESTING ... 96
FIGURE 31:SCHEMATIC EXPLANATION OF THE ENCLOSURE LEAKAGE TESTING ... 98
FIGURE 32:SCHEMATIC OF THE PATIENT LEAKAGE MEASUREMENT INCLUDING THE SWITCHES OPERATING THE SINGLE FAULT CONDITIONS. ... 100
FIGURE 33: SCHEMATIC EXPLANATION OF THE F-TYPE LEAKAGE MEASUREMENT INCLUDING THE SWITCHES OPERATING THE SINGLE FAULT CONDITIONS. ... 101
FIGURE 34:SCHEMATIC OF THE PATIENT AUXILIARY LEAKAGE MEASUREMENT INCLUDING THE SWITCHES OPERATING THE SINGLE FAULT CONDITIONS. ... 103
FIGURE 35:SCHEMATIC OF THE DIRECT EQUIPMENT LEAKAGE TEST. ... 107
FIGURE 36:SCHEMATIC OF THE ALTERNATIVE EQUIPMENT LEAKAGE TEST. ... 108
FIGURE 37:SCHEMATIC OF THE DIFFERENTIAL EQUIPMENT LEAKAGE TEST. ... 109
FIGURE 38: SCHEMATIC OF THE DIRECT APPLIED PART LEAKAGE TEST... 110
FIGURE 39: SCHEMATIC OF THE ALTERNATIVE APPLIED PART LEAKAGE TEST. ... 111
FIGURE 40: AN ELECTROCARDIOGRAM UNDER TEST WITH THE ELECTRICAL SAFETY ANALYSER IN THE BACKGROUND... 117
FIGURE 41:MAINS ON APPLIED PARTS LEAKAGE TEST.IEC60601-1 ... 117
FIGURE 42:DIRECT EQUIPMENT LEAKAGE TEST.IEC62353... 118
14
FIGURE 44:PROTECTIVE EARTH RESISTANCE TEST.IEC60601-1 ... 119 FIGURE 45:APPLIED PARTS LEAKAGE TEST.IEC60601-1... 119
15
Index of tables
TABLE 1DIFFERENT CLASSIFICATIONS FOR MEDICAL DEVICES ... 30
TABLE 2:SYMBOLS THAT CAN BE PRINTED ON THE MEDICAL DEVICE RATING PLATE ... 34
TABLE 3:UNITS OF THE CLINICAL ENGINEERING SERVICE AND THE TASK TO WHICH THEY CONTRIBUTE. ... 60
TABLE 4:PROTECTIVE EARTH RESISTANCE TEST LIMITS OF IEC60601-1 AND IEC62353 ... 112
TABLE 5:INSULATION TEST LIMITS FOR IEC62353. ... 113
TABLE 6:LEAKAGE TEST LIMITS OF IEC60601-1 ... 115
16
1 Introduction
This thesis was born in the context of the internship done, between August 2019 and March 2020, at the University of Warwick and at the Nottingham University Hospital NHS Trust. The Clinical Engineering Laboratory of the Applied Biomedical Signal Processing and Intelligent eHealth Laboratory at University of Warwick had been set up for teaching and research purposes on Clinical Engineering. The Medical Physics and Clinical Engineering Department of the Nottingham University Hospital NHS Trust is one of the biggest public hospitals in the United Kingdom.
At University of Warwick, the work was on the set-up of the Clinical Engineering Laboratory under the coordination of Professor Leandro Pecchia, the supervisor for this thesis. International protocols for electrical safety, inspection, and testing of electro-medical devices such as electrocardiograph, patient monitor, oxygen concentrator) were studied and implemented. Also protocols and standards for the set-up and the safety of surgical theatres were studied. Then, collaborating with a PhD student of the University, didactic e-Learning tools on the Electrical Safety Testing for medical devices were produced. These tools were requested by Professor Pecchia in order to be used for teaching, studying and research purposes by his students and researchers of University of Warwick.
Afterwards, during the internship at the Nottingham University Hospital, there was the possibility of, what in gergo is called, “shadowing” several members of the Clinical Engineering Service understanding their roles and tasks within it. It helped gaining a deeper understanding of the whole working system of such complex department and its different sections with their tasks. At Nottingham University Hospital NHS values and required behaviours, confidentiality issues, risk assessment, and health and safety measures in the hospital were also reviewed.
A consistent part of my time in the Nottingham University Hospital was spent with biomedical technicians of the workshop. Their tasks are:
17
• the application of standards and inspection and testing protocols I worked on in the laboratory at University of Warwick.
• The analysis of medical equipment and hospital areas for which Medical Equipment Service Units (MESU) are responsible for.
• The How safety and equipment are ensured for use through Annual Preventive Maintenance, Performance verification, Maintenance, Repair, Electrical Safety Testing, Equipment Manuals and Work Instructions.
• These tasks were performed on medical equipment such as infusion and syringe pump, life support ventilator, anesthetic machine, infrared thermometer, defibrillator, electrocardiograph, invasive (and non-invasive) blood pressure sensor, pulse oximeter, Electrosurgical system generator, vascular doppler system. Several tasks helpful to the Clinical Engineering service were performed, working on an audit of flowmeters, updating medical devices data, developing template for software-automatized defibrillator testing, and creating a decommissioning guideline for delivering medical equipment to the Clinical Engineering Laboratory at University of Warwick.
There was also the possibility to review and investigate the e-Learning tools already produced during the first part of the internship at the University of Warwick. Different actors of the clinical engineering service (such as, the technicians, the engineers assigned to the safety governance, and the engineers working on the internal development and production of medical equipment) reviewed and gave feedbacks to the work done.
This thesis aims to illustrate a model of a clinical engineering service, or department, which has been produced as contextualisation of a complex and efficacy hospital environment. The second part of the work analyses electrical safety, its standards and testing on medical devices, with the development of E-Learning tools for teaching and training purposes.
The thesis is articulated in seven chapters:
• The first introduces the context where the work has been done between University of Warwick and Nottingham University Hospital.
• The second chapter explains the methods and the objectives of the thesis.
• The third chapter introduces medical devices, their regulations, and classifications. • The fourth chapter describes the model of a Clinical Engineering service.
18
• The fifth chapter presents the hospital environment as source of risk, focusing on the electrical risks associated to medical devices.
• The sixth chapter shows the E-Learning tools developed.
• The seventh and last chapter is dedicated to the discussion and application of the model and the eLearning tool, and to conclusions.
19
2 Objectives of the Thesis
In this work, the aim was pointed at two different aspects of the Clinical Engineering that were considered fundamental. On one side, the complex organisation of a department for the Health Technology Management, on the other side, the electrical safety of medical devices.
The focus on electrical safety has started with the study of international standards [12][13] [14][15][16], and English guidelines [26][27]. In particular, considering the standards analysed referred to the electrical safety of medical devices, in the United Kingdom there are no regulations on the health care setting that pose proper requirements, while there are recommended guidelines. These guidelines are only recommended and can be not used if a proper risk assessment process is performed taking into consideration alternative adequate requirements. Also protocols and standards required for the electrical set-up and safety of medical locations were analysed [2][7][8]. Instead, in Italy, there are regulations that are introduced by State government and applied by the regional administrations. The latter can apply additional and stricter requirements on the regulations in order to adapt it to specific needs or precautions. However, these modifications cannot soften the State law. In any case, all the laws and directives regarding the healthcare service, must be respected by public and private health care services providers, whose actions must be verified by the local agency of ASL.
After the study of this documentation, the next step was studying the manual of the tester for electrical safety available in the Clinical Engineering Laboratory at University of Warwick. The model of the tester is the Electrical Safety Analyser ESA620 produced by the company Fluke. Then, protocols for electrical safety, inspection and testing of electro-medical devices were performed on the devices available at the moment in the laboratory: electrocardiograph, patient monitor, oxygen concentrator. Collaborating with a PhD student of the University, didactic materials on medical devices Electrical Safety Testing were produced as e-Learning tools. The e-Learning tools have been structured as a combination of videos and booklets following a literature research on the e-Learning
20
[1][17][18][30], corresponding to each fundamental test required in the electrical safety testing. The videos were taken in the laboratory of the university with a smartphone on a tripod. The booklet has been written as a guideline for technicians, engineers, and students involved in testing medical electrical equipment and cannot be considered to replace the standards mentioned within it, but a support tool that can ease the understanding of the theory and the performance of the tests. All reasonable care has been taken to ensure accuracy of the information. Reference figures and data have been taken from the latest versions of various standards, guidance notes, user manual [6], and recognised “best practices” to establish the recommended testing requirements. During the time spent at Nottingham University Hospital, there has been the possibility for reviewing the e-Learning tools together with professionals such as clinical safety governance engineers, biomedical technicians, engineers, developers of medical devices.
In the other part of the work, the aim has been to analyse the functioning of the Clinical Engineering department of Nottingham University Hospital and creating a model of its complex organisation. In order to create the model, a qualitative case study methodology was performed [24]. This method can be called organisation-exploratory case study, since its aim is to investigate a clinical engineering department, considered as a whole organisation, in order to individuate and describe the structure and the most important actions and interactions present within the department. Results were expected to be used to create a functioning model of a complex clinical engineering service. To do that, an inductive method was used. In this method, at the beginning literature sources and empirical analysis from on field work are considered and studied. These help to formulate related concepts on the same topics. Generalising these topics is possible to create the theoretical model that can describe a complex clinical engineering department organisation. The starting point of the process was the analysis of documents related to the Health Technology Management or Clinical Engineering such as a guide of the World Health Organisation [10], the Italian AIIC [28], or research books [3][4][9]. These sources of information have been considered as external because they were not produced by the hospital or clinical engineering department themselves. They were fundamental for giving a first introduction and explanation of such complex system that was going to be analysed. The second step of the analysis was the investigation on field, where several meetings with
21
professionals of the department took place. This type of research, performed during non-structured interviews, considers also the subjective aspects emerging spontaneously from the meeting and exchange of reflections between interviewer and interviewed. It leads to the collection of information and primary data in a non-numeric form, but through a series of labels, descriptions, and classifications. Moreover, some internal documents given by clinical engineers were analysed providing the research of other subjective but more comprehensive points of view. Since the main purpose of this qualitative research is to provide a complete and detailed model of the empirical findings.
In the end, is possible to propose the inclusion of the e-Learning tools into the modelled functioning of the Clinical Engineering department, contextualising and providing a possible useful use not only at University of Warwick, but also on the actual field of clinical engineering.
22
3 Classification Medical Devices
3.1 Clinical Engineering and Health Technology Management
Healthcare is one of the most complex environments when it comes to management. Several aspects are vital at the same time, and the risks associated to the services provided are extremely high. In fact, managing human resources and physical assets has an effect on the quality, the efficiency and the economic sustainability of health services in whichever context is considered: both in an advanced and technologically sophisticated tertiary hospital, or in a simple healthcare facility where only simple equipment is used for patients’ diagnosis and treatment of patients. No matter the context, is always fundamental to have access to properly functioning, appropriate, and affordable technologies. They must also be used and applied correctly by competent staff, minimising risks to patients and themselves. Simple and effective tools, technical management, and clear policies are necessary for effective and efficient functioning of the single healthcare service and for the hospital considered as a system of technologies. Eventually, the final aim still is to have an impact on patients and population health issues with an adequate response capacity to health needs. So, Health Technology Management, or Clinical Engineering, is the field of management of medical technologies in real settings where organization and coordination of several technical activities are needed.
3.2 Health Technologies, Medical Devices, and Medical Equipment
As “health technology” can be considered any devices, drugs, medical and surgical procedures (and the knowledge associated) used in the prevention, diagnosis, and treatment of disease as well as in rehabilitation, and the organizational and supportive systems within which care is
23
provided [29]. Healthcare technologies can be classified in several ways, but a fundamental distinction must be done between medical devices, medical equipment, and the other medical technologies such as drugs, health care procedures. Medical devices are a subgroup of health (or medical) technologies. Medical equipment is a specific subgroup of medical devices (Figure 1).
3.3 Medical Devices Definition MDR 2017/745
Medical devices are probably the category, among health care technologies, on which clinical engineers’ profession is more focused on. Also, the domain of medical devices is broad and includes many objects used in hospitals and intended by the manufacturer to have an impact on human health (definition). The official definition of what is a medical device can be found in the new Medical Device Regulation MDR 2017/745 [25] [26] and is the following:
“medical device” means any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used alone or in combination for human beings for one or more of the following specific medical purposes:
24
• diagnosis, prevention, monitoring, prediction, prognosis, treatment, or alleviation of disease,
• diagnosis, monitoring, treatment, alleviation, or compensation for, an injury or disability,
• investigation, replacement, or modification of the anatomy or of a physiological or pathological process or state,
• providing information by means of in vitro examination of specimens derived from the human body including organ blood and tissue donations,
and which should not achieve its principal intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its function by such means.
The following products shall also be deemed to be medical devices: • devices for the control or support of conception,
• product specifically intended for the cleaning, disinfection or sterilisation of devices as referred to in article 1(4) and those products referred to in the first paragraph of this point.
Through a careful review of the definition, is possible to divide it in four parts and summarise in shorter sentences, in order to understand it more easily. The first part is related to specific categories of instruments used in medicine. A medical device, then, can be described as a product for human use has a medical purpose. The second part of the definition is related to the intended use of the medical device, which acts on disease, injuries, disabilities, anatomy, physiological, or pathological state, or provide information trough in vitro examination. The third part of the definition specifies that a medical device should not achieve its principal intended action by pharmacological, immunological, or metabolic means, but may contribute with those function. The fourth part includes a list of medical devices that are excluded by the previous part of the definition. Those products are, whether to control or support conception, or specifically intended for cleaning, disinfecting, or sterilising the following devices listed in annex XVI of MDR 2017/745:
25
• products intended to be totally or partially introduced into the human body through surgically invasive procedures for the purpose of modifying the anatomy or fixation of body parts with the exception of tattooing products and piercings, • substances, combinations of substances, or items intended to be used for facial or
other dermal or mucous membrane filling by subcutaneous, submucous or intradermal injection or other introduction, excluding those for tattooing,
• equipment intended to be used to reduce, remove, or destroy adipose tissue, such as equipment for liposuction, lipolysis or lipoplasty,
• high intensity electromagnetic radiation (e.g. infra-red, visible light and ultra-violet) emitting equipment intended for use on the human body, including coherent and non-coherent sources, monochromatic and broad spectrum, such as lasers and intense pulsed light equipment, for skin resurfacing, tattoo or hair removal or other skin treatment,
• equipment intended for brain stimulation that apply electrical currents or magnetic or electromagnetic fields that penetrate the cranium to modify neuronal activity in the brain.
To summarise a less accurate, but more practical definition can be:
A medical device is product for human use with a medical purpose. It acts on disease, injuries, disabilities, anatomy, physiological, or pathological state, or provide information by in vitro or live examination. It cannot be a drug, but can be a device for the control or support of conception, or a product specifically intended for the cleaning, disinfection, or sterilisation of:
• contact lenses or other items intended to be used into or onto the eye,
• a product intended to be totally or partially introduced into the human body to modify the anatomy,
• equipment emitting high intensity electromagnetic radiation for use on the human body,
• equipment intended for brain stimulation through electrical currents or magnetic or electromagnetic fields.
26
A fundamental focus must be done on the term “intended action” which means the main task, intended by the manufacturer, to be performed by the device. If a device is falling under the definition given by the MDR 2017/745 but its principal intended action is not the medical device action but for example pharmacological or cosmetic, then the product is not a medical device. There are some products that are called combination products or borderline products, where the intended action is fundamental to distinguish. An easy example can be a drug syringe: if considered itself, then can be seen as medical device because its intended mean is to pump the drug, but if it is considered the combination of the drug and the syringe, then its intended use is the pharmacological effect. It legally stops to be a medical device, instead must be considered a drug.
3.4 Medical Devices Risk Classification
Medical devices can be classified with three different criteria. The first, and most important classification is based on the level of risk associated to the device. A second classification is based on the type of applied parts of a medical device, and indirectly on the risk associated to its use. Another classification is used only for electromedical devices, comes from a classification of electronical devices. It divides electromedical devices based on their method of electric insulation.
Following the European Regulation MDR 2017/745, medical devices are classified in four classes ordered by incremental risk posed to the patient by utilising each device: I, IIa, IIb, and III. A manufacturing company, producing medical devices, has to follow the regulation assigning a class to the device models produced and set them to the specific requirements given by the MDR 2017/745.
At Class I, the medical device is supposedly not risky at all and if you are putting that is class III or it means that it is really a risky product.
Under Class I, there are four subclasses: I, Is, Im, and Ir. Class Is is a class I product delivered sterile. Class Im is a product with a measuring function. Class Ir is a New sub-class for products that are reprocessed. Class I comprehend medical devices of low risk
27
but without the previous mentioned characteristics. There are some parameters that are needed to analyse in order to proceed with the classification rules for a medical device.
One is the continuous duration of use, which does not consider temporary removal for cleaning or disinfection, or exchange of two different device of the same model. If the product is used less than 60 minutes then, it is transient use. If it is used between 60 minutes and 30 days, it is considered short term use. And if it is used more than 30 days then, it is classified as long-term use. The invasiveness of a medical device is also fundamental. A surgically invasive device penetrates inside the body through its surface, or through mucous membranes, or body orifices with the aid or in the context of a surgical operation. A body orifice is considered any natural, or permanent artificial opening in the body. A reusable surgical instrument is used in surgery procedures for cutting, drilling, sawing, scratching, scraping, clamping, retracting, clipping or similar procedures, without a connection to an active device. It is intended by the manufacturer to be reused after appropriate cleaning, disinfection and sterilisation have been carried out. An active device is used, alone or in combination with other devices, to perform a treatment or alleviation of an illness, injury, or disability or for detecting, diagnosing, monitoring, or treating physiological conditions, states of health, illnesses, or congenital deformities. The application of the classification rules must be governed by the intended purpose of the device analysed. If devices are intended to be used in combination, their classification must be applied to each one separately. Accessories and disposables for a medical device must be classified separately from the device. A software, which drives a device or influences the use of a device, must have the same class as the device. Otherwise, it shall be classified by its own. A device must be considered and classified on the basis of the most critical specified use. A direct diagnosis is considered so when it is provided by the device itself or when this provides decisive information for the diagnosis.
The classification rules are divided in four groups: Non-Invasive devices, Invasive devices, Active devices, and Special rules.
Rules applied to Non-Invasive devices are the first four.
1) All Non-invasive devices are unless other rules are applied.
2) Non-invasive devices intended for channelling or storing fluids (including cells), that might be introduced into the body.
28
3) Non-invasive devices that modify biological or chemical composition of blood, body liquids, other liquids, and cells.
4) Non-invasive devices in contact with injured skin or mucous membrane.
Rules applied to Invasive devices are from 5 to 8. 5) Devices invasive in body orifices.
6) Surgically invasive devices for transient use. 7) Surgically invasive devices for short term use.
8) Surgically invasive devices for long term use and implantable (including any device administering medicinal products, surgical mesh, or spinal disc).
Rules applied from 9 to 13 are for Active devices.
9) Active therapeutic devices intended to exchange or administer energy. 10) Active devices for diagnosis & monitoring, emit ionizing radiation.
11) Software intended to provide information which is used to take decisions with diagnosis or therapeutic purposes (from class I to class III).
12) Active devices intended to administer and/or remove medicinal products, body liquids or other substances.
13) All other active devices.
All other medical devices are classifies following special rules.
14) Devices incorporating a medicinal substance including human blood or plasma 15) Contraception or prevention of the transmission of sexually transmitted diseases 16) Specific disinfecting, cleaning, and rinsing devices
17) Devices specifically intended for recording of diagnostic images generated by X-ray radiation
18) Devices utilizing non-viable tissues or cells of human origin or tissues of animal or derivatives
19) Devices incorporating or consisting of nanomaterial
20) Invasive devices with respect to body orifices to administer medicinal products by inhalation
21) Substances or of combinations of substances that are intended to be introduced into the human body via a body orifice or applied to the skin and that are absorbed
29
22) Active therapeutic devices with an integrated or incorporated diagnostic function which significantly determines the patient management
Examples of medical device classified following the European MDR 2017 745 are: Class I: wheelchair, otoscope, stethoscope.
Class IIa: syringe for drugs, tooth implant, tracheotomy tube. Class IIb: blood bag, implantable plate, screws, condom.
Class III: drug coated stent, spinal disc cage, breast implants, pacemaker.
Considering the, just mentioned medical device classification, it is clear to imagine the different amount of time is spent on medical devices of different risk class. The risks for the patient, related to a medical device, are usually connected also to the complexity of the device and to the amount on time spent for performing maintenance of it.
A bigger amount of time is spent on more complex and risky medical devices, compared singularly, to the simpler ones.
However, the wide majority of the budget for medical devices is spent on class I medical devices because of their immensely higher number of units bought. Therefore, the majority of the effort goes back on managing, moving, cleaning, maintaining, changing spear parts of simple class I medical devices. Instead, maintenance of more complex devices, can be done only by experienced technicians, which could be in the hospital, or coming from an outsource company.
3.5 Medical Equipment
And within medical devices there is a narrower kind of devices called medical equipment, also called medical electrical equipment. They are medical devices that require calibration, maintenance, repair, user training. Medical equipment is used for the specific purposes of diagnosis and treatment of disease or rehabilitation following disease or injury; it can be used either alone or in combination with any accessory, consumable or other piece of medical equipment. It can be a defibrillator, an electrocardiograph, an X Ray
30
machine, a CT scanner, an electro-surgery generator, a pacemaker. It excludes implantable, disposable or single-use medical devices.
3.6 Appliance Classes and Types of Medical Devices
It is fundamental to know and understand the type and the class of a medical device because, on it depend which electrical safety requirements should be followed or respected. It is important to notice that the classification of electromedical devices can be done following two different categorizations. One is based on the level of electrical insulation of each device against electric current leakages and, so, electro-shocks. The other one is related to the use of the applied parts of medical devices and their level of risk. Electrical insulation is made using a basic insulation layer plus a protective earthing terminal, or a supplementary insulation. Based on the design and the construction of a device, the manufacturer must define to which class their device belongs to. Before the put-on sale, manufacturers have also to test their own products in order to determine the compliance of each device model to the assigned class. The next step is printing the specific symbol of the class on the rating plate of the device. Table 1 shows the different classes of medical devices categorised with different methodologies.
Risk Applied Parts Electric Insulation
Class I Type B Class I
Class IIa, IIb Type BF Class II
Class III Type CF Internally powered
31
3.7 Types of Medical Devices
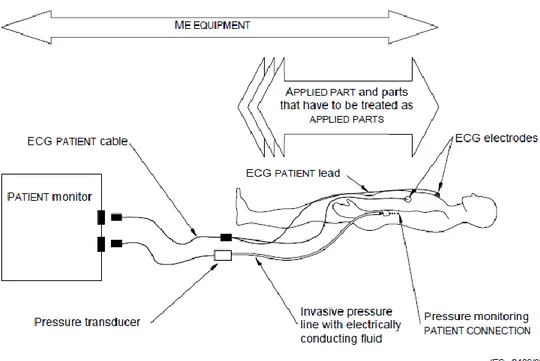
Electromedical devices can be divided also in three types of devices basing the classification on the use they are built for and on the risk related to it. The manufacturer, during the design and the production, must do a risk-assessment and choose the classification. Particular attention must be given to the usage of applied parts and patient connections of the device. Many types of medical equipment have parts connected to them that are intended to be in contact with the patient who is commonly vulnerable because of their illness or treatment. Such parts are called Applied Parts. They are defined [13] as “Parts of the medical equipment that in normal use necessarily have to come into physical contact with the patient in order for the equipment to perform its function”. Applied Parts can be non-electrically conductive, or they can have an electrical connection to the patient, called as Patient Connection (Figure 2). For example, an electrocardiogram electrode or defibrillator paddles are Patient Connection Applied Parts. Their design must provide a high degree of protection from electric shock because of their nature of intended electrical connection. Patient Connections intended to be in direct contact to the heart must be designed to provide the best degree of protection from microshock.
Figure 2: Identification of medical equipment, applied parts, and patient connections in a multifunction patient monitor with invasive pressure monitoring facilities
32
An applied part is a part of the device that must be in contact with the patient for the proper use of the device. While, a patient connection is the conductive part of the device that enters in contact with the body of the patient (for example, a couch can be an applied part, while the metallic electrodes of an ECG are the patient connections).
The three types of applied parts are defined by the degree of invasiveness and of protection for the patient, how it is separated from earth or how well separated from earth, so by its risk. The evaluation of risk depends also on the difference of contact between the patient and the normal user of the medical device. The user interface might have a different risk for the patient than for the medical staff. For some applied parts is possible to say, with a risk assessment, that there is no difference for the patient or the user. That can be called an accessible part. Is fundamental noticing, during the assessment of the device, that patients are a particular category of electronic devices users. Their body and health are probably not completely healthy. Moreover, their ability to react to eventual excessive stimulations, as leakage currents, are limited or even non-existent. In fact, often patients are unable to give a feedback of discomfort or pain to the medical staff. They can be unconscious, or insensitive in critical body regions, or unable to communicate the problem, or also unable to simply have physiological reflexes.
Patient connection applied parts shall not be earth referred. The connection between parts and the rest of the device must be isolated in order to ensure that potentials or currents flowing in the patient connection are safely isolated. However, due to effects of stray capacitance and imperfect insulation, leakage currents cannot be completely avoided. So, manufacturers must design their devices to keep Patient Leakage Currents within limits that are appropriate for the intended use of the device.
These patient circuits and their connections are called electrically floating, or F-Type, and not referenced to earth. They can be Body-Protected connections and be designated as Type BF, or Cardiac-Protected connections and be designated as Type CF. Considering also the non-floating patient connections applied parts, the three types of medical equipment applied parts classified depending on the risk are:
● Type B ● Type BF ● Type CF
33
A Type B applied part has no insulation from earth. It can be conductive, but also used without any conductive parts in contact with the patient. The parts in contact with the patient can immediately be separated from the patient, so they constitute a low risk for the patient. They can be of Class I, II, or III. Leakage currents are permitted under specific values and measured under the respective testing procedures.
Type BF devices use conductive parts that can be conductive and ordinarily will be conductive. These will be in contact with the patient, but not with the patient's heart. These applied parts, in touch with the patient, have a level of added insulation between them and all the earthed parts and the mains power. Leakage currents are permitted under specific values and measured under the respective testing procedures. If the equipment can be used combined with a defibrillator, another symbol has to be printed on the device rating plate.
Type CF devices use applied parts in direct contact with the patient's heart or at risk of passing a current through it. Even smaller currents could cause problems for direct connection to the
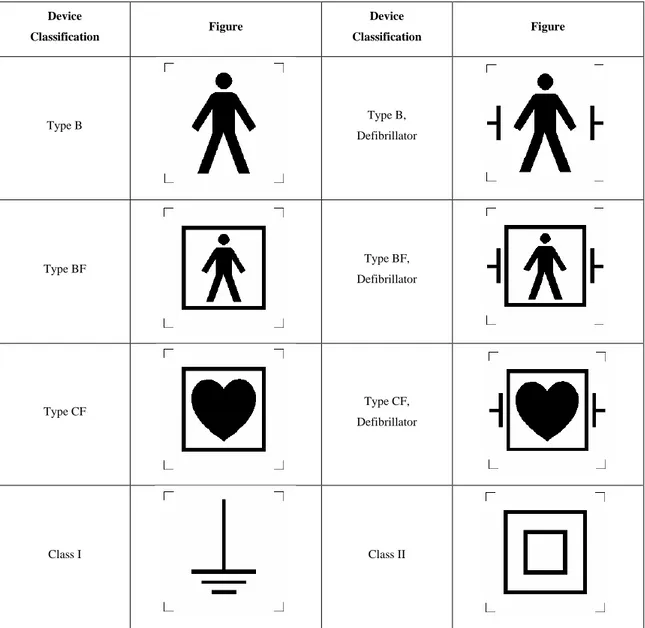
Heart. So, is important to isolate even better in order to be sure that any current flow at an intensity not enough strong to be harmful. These applied parts, in touch with the patient, are isolated from the earth and the power supply circuit. Leakage currents are permitted under specific values and measured under the respective testing procedures. Limits are much lower than the types B and BF. If the equipment can be used combined with a defibrillator, another symbol has to be printed on the device rating plate. Table 1 shows the different classes of medical devices categorised with different methodologies. Table 2 shows the symbols printed on the metallic rating plate of a medical device.
34 Device Classification Figure Device Classification Figure Type B Type B, Defibrillator Type BF Type BF, Defibrillator Type CF Type CF, Defibrillator Class I Class II
Table 2: Symbols that can be printed on the medical device rating plate
3.8 Classes based on Electrical Insulation
Classes of electromedical devices, can be created also depending on their method of electrical insulation for the users and for patients. The classes are three: I, II, and Internally Powered. Table 1 shows the different classes of medical devices categorised with different methodologies. In order to build an electrically safe equipment, it must have two Means of Operator Protection (MOOP). There must always be Basic Insulation plus one additional means of protection for the user, that could be a Protective Earth connection or a Secondary Insulation.
35
Class I electrical devices protect users, or patients, through the single Basic and a protective Earth terminal (PE). The PE terminal is connected to all the exposed conductive parts of the device, exposed and potentially leading electrical current to an external body who touches them. which permits the dispersion of the electric current to ground when a fault happens in the primary insulation. During a fault of the primary insulation barrier, an electrical current could flow from the PE terminal to the ground of the socket. The PE connection and cables have a lower impedance than the human body, so that the electrical current will naturally flow through those instead of human body. If the current is intense enough, the fuse inside the device or the plug burns and the power supply to the medical device gets interrupted. This PE cable is one of the three core mains inside the power cable of devices of Class I. The power plug, then, has three pins.
The symbol for the PE terminal is the earthing sign inside a circle. It must be put on the rating plate of the device. All medical equipment with this symbol should be considered as medical devices of Class I. Sometimes, several different medical devices of Class I are used at the same time. If so, their PE terminals must be connected to a common PE terminal in the room. This allows to put all those medical devices at the same voltage and permits to protect users and patients from earth leakage currents from different PE terminals. Is common, in Class I devices to have some exposed conductive parts constructed with Double or Reinforced Insulation. The requirement is to always have two MOOPs.
Like Class II electrical medical devices do not have Protective Earth terminal. Instead, devices of this class are equipped with both Basic and Supplementary Insulation or with a Reinforced Insulation that protects users or patients from live parts of the device. It is made of a basic insulation plus the supplementary insulation. The power cable of these devices contains only two core mains. The power plug, then, has only two pins. In some cases, a 3-pin cable can be used, with the earth cable working not as a PE but as functional earth. In this situation, the functional earth cable must not be connected to the exposed metallic parts of the device, while it should be used to discharge interference current, earth testing adapters, and common reference potentials of different electrical devices and equipment. It is important to pay attention to the orientation of the plug because, in many countries, mains supply Live and Neutral are not specified on the plug and the socket, so that the power plug can be inserted into the socket on the wall in both ways (which is unlikely to
36
constitute a problem). The symbol for Class II devices is the double square one inside the other and must be put on the rating plate of the device, located adjacent to the point of mains connection. Functional Earths often happens to be in Class II, these are earthed connections via another part of the system to provide protection against interference, but this is not for Protective Earth means. In medical equipment, a Functional Earth must be separated by two MOOPs from live parts or from any external conductive parts.
There is a third type of electrical medical devices, these devices are powered by an internal battery and do not have protective earth terminal. Their classification can be related to the previous one of devices with Safety Extra Low Voltage (SELV). SELV is a voltage low enough so that a person cannot get an electroshock even if touches the exposed live parts. The device uses an internal battery or an external power supply of less than 50 V in AC. There is no symbol for internally powered devices.
Even though its use is not specified for any class of electronic device, its use is an extra electric safety means of protection form electroshocks. This transformer is 1:1 (the voltage reached, on the input side of it, is the same that can be reached on the output side) but provides a galvanic isolation from the live potential to the ground. The output voltage is no more from live to ground, but from two live connectors. So, it can avoid easy electric dispersions to the ground. Then, the output socket has not a PE connection. The symbol for an isolation transformer is a double-crossed circle inside a shield. It must be put on the outside of external power supplies if they use it.
37
4 Clinical Engineering Model
As it has been said before, Health Technology Management, or Clinical Engineering, is the field of management of medical technologies in real contexts where organization and coordination of several technical activities are needed.
4.1 Lifecycle of Medical Devices
Clinical Engineering is a branch of Biomedical Engineering. It becomes a fundamental part only when we talk about the management of medical technologies to provide healthcare services. That comes at a specific time of the life cycle of medical technologies. Then, becomes important contextualising where it stays in the context of the whole field of biomedical within the life cycle of medical devices. Figure 3 explains clearly which is the life of all medical devices [9]. From the designing, passing through the testing and the use, and finishing at the decommissioning.
The beginning of the lifecycle starts with research or development. It is composed by all the processes necessary to ideate and design a new medical technology. Then, the manufacturing company (which is not always the same of the one which creates the idea) proceeds with the prototyping, a loop of manufacturing, tests, and evaluations of the prototype. A few units of device prototypes are produced and tested in loop in order to improve the research and design outcomes. Before putting the device on the market is needed to perform preclinical testing in order to know if this medical device is compliant with the latest requirements, among which, there are also electrical safety testing standards like such as the IEC 60601-1.
38
Then, there is the stage called “innovation” when the device must be tested in real world conditions on part of its functioning. Firstly, is done everything that can possibly be done in a lab on a device, or in silico, in vitro, in simulation. Right after, the device is tested on animals.
The last step is done moving to clinical studies on humans, building evidence in medicine. Right after that is possible to obtain regulatory approval by the competent authorities. If also this is successful then, it is possible to start with “in line” manufacturing of the device. But in order to do that, it is needed to prove that it is safe its effective is compliant with national or international regulations for manufacturing and safety. A huge number of medical devices are produced nowadays, and then put on the market for sell and clinical use. So far, actors involved in this part of the medical device’s lifecycle have been several, such as biomedical, mechanical, electronic engineers, clinicians, regulation technicians. It is now, that clinical engineers start to be engaged managing the medical device. The first step is the introduction of the device into the hospital or in the facility where it is supposed to operate. The initial analysis done on the device is called Health Technology Assessment (HTA) of the medical device. It defines the way in which the
39
device will be managed, and the amount of money needed to introduce it into the hospital. Then the device is commissioned, installed, used, and, more generically, managed in the hospital. It will remain available on the market until it will eventually become obsolescent because after some years, a time span that is rapidly becoming shorter, a novel technology, able to perform the same tasks with higher efficacy and/or efficiency, will be introduced into the market and will become the clinical benchmark or gold standard. When the old device will be decommissioned to introduce the new technology, usually, it will be dismantled and thrown in the bin.
4.2 Hospital as a process
Now, the focus is shifting to the part of the lifecycle of medical devices that is managed by clinical engineers: the use of medical device on the field of health care service, within the hospital. An interesting representation of the fundamental functioning of an hospital can be pictured imaging the hospital as a system made of inputs, transformation processing, outputs, and outcomes (Figure 4).
First of all, can be considered inputs the staff, the money, the devices, the facilities, and the knowledge. Medical and non-medical staff, as human resources, are the most important inputs, able to perform a wide range of tasks, and reason utilising their
40
knowledge. The budget for the hospital comes from different actors depending on the context of the healthcare service of each hospital. Technologies, comprehending devices and facilities, are fundamental to give the best service as possible, increasing efficacy of treatments. Also, policies, regulations, are fundamental for running healthcare processes with high efficacy and low risk. Usually national or international regulators provide standards and policies, which implementation is monitored by national authorities. All inputs, once entered the hospital, are processed and applied together and by other inputs, eventually becoming outputs and outcomes of the process. These two different results of the process are closely related, but there are significant differences. Outputs are numerical values such as medical locations created, number of imaging tests per day, number of surgical procedures per day. On the other hand, outcomes represent the so-called health service provision to the population which is the final goal of the healthcare system and direct effects of the outputs: the improvement of measures like lives saved, improved quality of life, increased life expectancy or quality adjusted life years.
The professional clinical engineers operate within the hospital during transformation processes of inputs in outputs and outcomes. They try to innovate and improve the ratio between outcomes and inputs in an effort to increase the efficiency of the healthcare service, improving the health or the wellbeing of the target population, or maintaining health of chronic patients. Before commission a medical technology, during the initial planning and assessment, also inputs, outputs, and outcomes related to it must be considered.
Is important to consider that, since the hospital is an extremely complex system, within the internal process there are smaller interconnected processes with their own inputs and outputs.
41
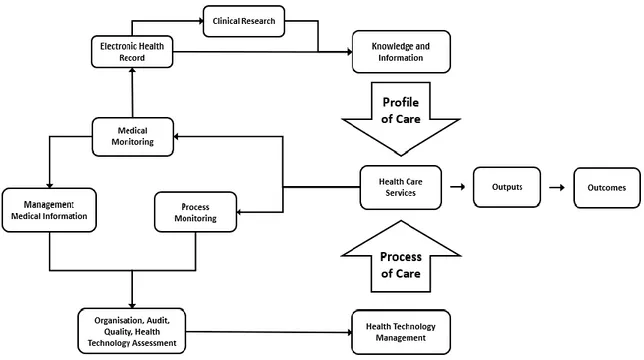
4.3 Profile and process of care
It is fundamental to underline that, within an hospital, internal processes are divided in two main categories: the profile of care and the process of care. The diagram of Figure 5 shows the two.
All the activities performed by clinicians, nurses, physiotherapists, surgeons, in general by all the medical staff, on and for the patients are called in gergo “profile of care”. The profile of care includes procedures of treatment, diagnosis, therapy, discharge, rehabilitation, diagnostic exams, service requests; all actions focused on improving the outcomes of the patient health. An important source of information for clinical staff is generated by patients’ biological signs, or by the medical services provided, or taken from the knowledge of medical sciences, which is also increased by their own on-field work on their current patients.
All these components of the profile of care are usually associated as the only ones performed in a hospital. Instead, in order to provide such a profile of care, there is an underlying process of care that enables medical staff to perform at best their job. Clinical
42
engineers work specifically on the management of the underlying infrastructure and ensemble of knowledge, techniques, procedures, methods, and equipment to maximise the efficacy of medical practice. During every healthcare service, data and information are generated. Data related to patients health statuses are relevant for the professionals working on their profile of care, while all the information, related to the process and the technologies involved, become extremely relevant for managing medical devices and procedures in the hospital according to the high quality standard required. An exemplificatory use of the knowledge related to medical technology can be related to a type of technology that should be changed because of its luck of efficiency. This could lead to the introduction of a new model, or maybe only the frequency of use of that specific device, or drugs, or disposables should change. Clearly, the information gathered on the clinical side of the profile of care, and relevant for the patient, and merged to the Electronic Patient Record (EPR) are also relevant to the management of the process of care. A clear example of the continuous generation of information can be done on the hospitalization. Several patients require technologies for monitoring vital signs and others to perform treatments. All of these produce data which go into the EPR’s. On the other side, the management the hospitality has an equivalent complex issue because of the requirements needed, performed, and registered in the hospitalisation ward. In fact, the hospitalisation requires management of the devices introduced, cleaning, food and water, disposables blankets and clothes for the patients, air changing system. More data are collected and analysed, and higher is the quality of the medical service. With this purpose, often technologies and tools of the hospital must undergo audits. Especially if the hospital is within the National Health Service it is more probable an audit is performed. During the internship I have done at Nottingham University Hospital, I worked on an audit of oxygen flowmeters, devices that control the flow of oxygen from the wall installation to the patient through a dial regulator. The hospital engineers noticed inaccuracies and irregularities between some flowmeters. This audit was useful because I could discover, investigate, and report those and more issues.
Before going on, it is important to explain, what in is difference between the National Health Service and the National Health System in the United Kingdom. The National Health Service is made of every service funded by public money. While, the Healthcare