Funding: this work was supported by grants from the Italian Association for Cancer Research (AIRC) and Beneficientia Foundation (both to GZ), and by FIRB-MIUR Projects (to PS and to GZ).
Manuscript received on January 12, 2012. Revised version arrived on May 2, 2012. Manuscript accepted on May 11, 2012. Correspondence:
Giorgio Zauli, Institute for Maternal and Child Health, IRCCS “Burlo Garofolo”, via dell’Istria 65/1, 34137 Trieste, Italy. Phone: international +39.040.3785478. Fax: international +39.0532.207351. E-mail: [email protected] The online version of this article has a Supplementary Appendix.
Background
Both the multi-kinase inhibitor sorafenib and the small molecule inhibitor of the MDM2/p53 interaction, nutlin-3, used alone, have shown promising anti-leukemic activity in acute myeloid leukemia cells. Thus, in this study we investigated the effect of the combination of sorafenib plus nutlin-3 in acute myeloid leukemia.
Design and Methods
Primary acute myeloid leukemia blasts (n=13) and FLT3wild-type/p53wild-type(OCI-AML3), FLT3 mutat-ed/p53wild-type (MOLM), FLT3mutated/p53mutated (MV4-11), FLT3wild-type/p53deleted (HL60) or FLT3 wild-type/p53mutated(NB4) acute myeloid cell lines were exposed to sorafenib, used alone or in associa-tion with nutlin-3 at a 1:1 ratio, in a range of clinically achievable concentraassocia-tions (1-10 mM). Induction of apoptosis and autophagy was evaluated by transmission electron microscopy and by specific flow cytometry analyses. The levels of Mcl-1, p53 and Bak proteins were analyzed by western blotting. Knock-down of Bax and Bak gene expression was performed in transfec-tion experiments with specific short interfering RNA.
Results
The sorafenib+nutlin-3 drug combination exhibits synergistic cytotoxicity in primary acute myeloid leukemia blasts and in acute myeloid leukemia cell lines with maximal cytotoxicity in FLT3mutated MV4-11 and MOLM, followed by the FLT3wild-type OCI-AML3, HL60 and NB4 cell lines. The cytotoxic activity of sorafenib+nutlin-3 was characterized by an increase of both apoptosis and autophagy. Moreover, Bax and Bak showed prominent roles in mediating the decrease of cell viability in response to the drug combination in p53wild-type OCI-AML3 and p53deletedHL-60 cells, respectively, as demonstrated in transfection experiments performed with specific short interfering RNA.
Conclusions
Our data demonstrate that acute myeloid leukemia cells show a variable but overall good sus-ceptibility to the innovative therapeutic combination of sorafenib+nutlin-3, which differential-ly involves the pro-apoptotic Bcl-2 famidifferential-ly members Bax and Bak in p53wild-typeand p53deletedcells. Key words: sorafenib, nutlin-3, acute myeloid leukemia, p53, apoptosis, autophagy.
Citation: Zauli G, Celeghini C, Melloni E, Voltan R, Ongari M, Tiribelli M, di Iasio MG, Lanza F, and Secchiero P. The sorafenib plus nutlin-3 combination promotes synergistic cytotoxicity in acute myeloid leukemic cells irrespectively of FLT3 and p53 status. Haematologica 2012;97(11):1722-1730. doi:10.3324/haematol.2012.062083
©2012 Ferrata Storti Foundation. This is an open-access paper.
The sorafenib plus nutlin-3 combination promotes synergistic cytotoxicity
in acute myeloid leukemic cells irrespectively of FLT3 and p53 status
Giorgio Zauli,1Claudio Celeghini,2Elisabetta Melloni,3Rebecca Voltan,3Manuele Ongari,4Mario Tiribelli,5
Maria Grazia di Iasio,3Francesco Lanza,4and Paola Secchiero3
1Institute for Maternal and Child Health, IRCCS “Burlo Garofolo”, Trieste; 2Department of Life Sciences, University of Trieste, Trieste;
3Department of Morphology and Embryology and LTTA Center, University of Ferrara, Ferrara; 4Hematology Section and Bone
Marrow Transplantation Unit, Hospital of Cremona, Cremona; 5Department of Medical and Morphological Research, Division of
Hematology and Bone Marrow Transplantation, University Hospital, Udine, Italy
Introduction
The second-generation protein kinase inhibitors consti-tute a new class of pharmacological compounds with the potential to target acute myeloid leukemia (AML) cells.1 Sorafenib (Nexavar) was found to be particularly active in six of seven FMS-like tyrosine kinase-3-internal tandem duplication (FLT3)-ITD-positive patients included in a phase 1 clinical trial performed on 16 AML patients,2as well as in FLT3-ITD positive cell lines.3In addition, com-plete molecular remission has been reported in a subset of AML patients treated with sorafenib,4while a long-term response was observed in refractory and relapsed disease before and after allogeneic stem cell transplantation.5 At the molecular level, sorafenib shows the ability to inhibit several critical tyrosine kinases, besides FLT3-ITD, such as vascular endothelial growth factor receptor (VEGFR-2), c-Kit, Ret, as well as the serine threonine kinase RAF1, an upstream activator of the ERK1/ERK2 pathway.2-5
In spite of this promising clinical evidence, recent stud-ies suggest that after a favorable initial outcome, subse-quent non-responsiveness occurs.6It is, therefore, unlikely that sorafenib will display maximal efficacy as monother-apy and combined therapies have recently been pro-posed.7In this context, we sought to investigate the anti-leukemic activity of this multi-kinase inhibitor in combi-nation with nutlin-3, a small molecule inhibitor of the MDM2/p53 interaction.8 The rationale for testing this association relies on solid data demonstrating that muta-tions and/or delemuta-tions of p53 in hematologic malignancies are present in less than 20% of patients at diagnosis.9In addition, recent studies have demonstrated that nutlin-3, used alone or in combination with chemotherapeutic drugs, effectively increases the degree of cytotoxicity in AML.10-12 Finally, a reciprocal interplay between MDM2 and FLT3 in AML cells has been proposed.13
Thus, we investigated the potential therapeutic efficacy of sorafenib in association with nutlin-3 in primary AML blasts as well as in a panel of myeloid leukemic cells with different FLT3 (FLT3wild-typeand FLT3mutated) and p53 (p53 wild-type, p53deletedor p53mutated) status.
Design and Methods
Primary samples from patients with acute myeloid
leukemia and leukemic cell lines
For experiments with primary cells, peripheral blood samples from 13 AML patients, diagnosed according to French-American-British (FAB) criteria, were collected into heparin-coated tubes fol-lowing informed consent, in accordance with the Declaration of Helsinki and in agreement with institutional guidelines after Ethics Committee approval (Udine University-Hospital and Cremona Hospital). The main clinical parameters of the patients were abstracted from clinical records and are reported in Online
Supplementary Table S1.
The p53wild-type(OCI-AML3 and MOLM), p53mutated(NB4 and
MV4-11) and p53deleted(HL60) leukemic cell lines were purchased from the
American Type Culture Collection (ATCC, Manassas, VA, USA) or the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ; Braunschweig, Germany).
Further details on primary AML samples and leukemic cell lines cultures are described in the Online Supplementary Design and
Methods.
Culture treatments and flow cytometric assessment
of cell cycle, apoptosis and autophagy
Leukemic cells were treated with sorafenib (range 1-10 mM, Custom Selleck Chemicals, Houston, TX, USA) and nutlin-3 (range 1-10 mM, Cayman Chemicals, Ann Arbor, MI, USA), used alone or in combination. At different time points after treatment, cell viability was examined by trypan blue dye exclusion, as pre-viously described.14,15 Details of the cytometric analysis of cell cycle,9,11 apoptosis and autophagy are provided in the Online
Supplementary Design and Methods.
Transmission electron microscopy
For transmission electron microscopy (TEM), cells were cen-trifuged at 2000 rpm for 5 min and the pellet fixed with 2.5% glu-taraldehyde/0.1 M phosphate-buffered saline (pH 7.3) overnight at 4°C. After several rinses with phosphate-buffered saline, the cells were post-fixed in 1% osmium tetroxide at 4°C for 3 h and then rinsed thoroughly with distilled water, dehydrated by graded ethanol, and freeze-dried. The specimens were sputter-coated with platinum and examined with a TEM H-800 (Hitachi) for the presence of putative autophagic features. The apoptotic and autophagic cells were quantified by counting a total of 500 cells, scoring several fields (magnification 1000X) for each culture con-dition.
Antibody arrays, western blot and immunoprecipitation
analyses
Details on antibody arrays, western blot16,17and immunoprecip-itation analyses18are provided in the Online Supplementary Design
and Methods.
Transfection experiments
Leukemic cells (1.2x106cells/0.1 mL) were mixed with either 1
mg of control enhanced green fluorescence protein (EGFP) plasmid or 2 mg of small interfering RNA (siRNA) cocktails, transferred to a 2.0-mm electroporation cuvette, and nucleofected with the nucleofector kit V (Amaxa, Cologne, Germany) using a nucleofec-tor device (Amaxa Nucleofecnucleofec-tor II apparatus). After electropora-tion, cells were immediately transferred to a complete medium supplemented with 10% fetal calf serum and cultured at 37°C until analysis. Transfection efficiency was monitored in each experiment by scoring the percentage of EGFP-positive cells by flow cytometry analysis. For specific Bax and Bak gene knock-down, siRNA were designed and manufactured by Ambion Inc. (Woodward, Austin, TX, USA) according to the current guidelines for effective gene knock-down by this method. A cocktail of three different negative control siRNA, each comprising a 19 bp-scram-bled sequence with 3’ dT overhangs (Ambion’s Silencer negative control siRNA), was used to demonstrate that the transfection did not induce non-specific effects on gene expression. The Ambion’s Silencer negative control siRNA sequences have no significant homology to any known gene sequences from humans and they have been previously tested to ensure that they do not have non-specific effects on gene expression (Ambion).
RNA analyses
Details on RNA analyses are provided in the Online
Supplementary Design and Methods.
Statistical analysis and assessment of the effect
of combination treatment
Results were evaluated by using analysis of variance with sub-sequent comparisons by Student’s t-test and with the Mann-Whitney rank-sum test. Statistical significance was defined as
combination, leukemic cells were treated with serial doses (range 1-10 mM) of sorafenib or nutlin-3, individually or in combination using a constant 1:1 ratio (sorafenib:nutlin-3). Results were ana-lyzed with the method of Chou and Talalay19 to determine whether combined treatment yielded greater effects than expect-ed from summation alone: a combination index (CI) of 1 indicates an additive effect, while a CI below 1 indicates synergism. For this purpose cell viability data were analyzed with CalcuSyn software and reported either as dose-effect curves drawn directly by the CalcuSyn software (Online Supplementary Table S1A) or as CI val-ues (Online Supplementary Table S2).
Results
The sorafenib+nutlin-3 combination exhibits synergistic
cytotoxicity in both primary acute myeloid leukemia
blasts and myeloid cell lines with different FLT3
and p53 status
In the first group of experiments, we investigated the cytotoxic effect of the combination of sorafenib+nutlin-3. Both primary AML cells from patients (Online
Supplementary Table S1) and AML cell lines were exposed
to serial concentrations (1-3-10 mM) of sorafenib and nut-lin-3, used either alone or in combination at a constant 1:1 sorafenib:nutlin-3 ratio, and analyzed for cell viability by trypan blue dye exclusion 24 and 48 h after treatment. Synergy (average CI<1) of the sorafenib+nutlin-3 combi-nation was documented in all primary AML samples from patients, including those cells bearing FLT3-ITD (Online
Supplementary Table S2 and Online Supplementary Figure S1A,B). Since the percentage of blasts was around 40% in
several patients, we cannot exclude that the toxicity of the drug combination also affected non-leukemic peripheral blood mononuclear cells. However, it is noteworthy that sorafenib+nutlin-3 promoted synergistic cytotoxicity in all AML leukemic cell lines investigated (Online Supplementary
Table S2), with FLT3mutated/p53mutated(MV4-11) and FLT3
mutat-ed/p53wild-type(MOLM) leukemic cells being the most sensi-tive to the drug combination, followed by FLT3 wild-type/p53wild-type (OCI-AML3), FLT3wild-type/p53deleted (HL60) and FLT3wild-type/p53mutated (NB4) (Figure 1). In this respect, it is noteworthy that treatment for 48 h with a low concentra-tion of sorafenib+nutlin-3 (1 mM each) killed all MV4-11 cells and reduced the viability of MOLM cells by about 80% (Figure 1).
The sorafenib+nutlin-3 combination promotes both
apoptosis and autophagy in p53
wild-typeand p53
deleted/mutatedleukemic cells
In order to appreciate the morphological and molecular aspects of the synergistic cytotoxicity of sorafenib+nutlin-3 better, we performed most of the following experiments on FLT3wild-typeOCI-AML3 and HL60 cells, considering that in these cell lines the toxicity of the single agents (sorafenib or nutlin-3), as well as of the sorafenib+nutlin-3 combination, was not as substantial as observed in the FLT3mutated MOLM and MV4-11 cell lines (Figure 1). The concentrations (3-10 mM) of nutlin-3 and sorafenib used in the experiments performed on OCI-AML3 and HL60 cells were chosen on the basis of previous clinical studies demonstrating that when given twice daily at 400 mg, the maximum plasma concentrations of sorafenib reach 9.9 mM after 6 h,209.7 mM after 6 days21and 8.5 mM after 28
days.22 When used at maximal concentration (10 μM) sorafenib almost completely abrogated the phosphoryla-tion levels of ERK1/2 but not of other kinases, such as JNK and Akt (Online Supplementary Figure S2A,B). In addition, sorafenib variably down-regulated the phosphorylation levels of STAT transcription factor family members, while nutlin-3 had minor effects on these pathways and para-doxically increased the phosphorylation levels of ERK1/2 (Online Supplementary Figure S2A). Of note, the effect of sorafenib+nutlin-3 on the potential signaling mediators analyzed was not significantly different from the effects of sorafenib alone and even the suppressive effects of sorafenib on ERK1/2 phosphorylation prevailed over the induction by nutlin-3 (Online Supplementary Figure S2A,B). With respect to the drug concentrations used, it is also noteworthy that while our study was under considera-tion, another study showed a synergistic cytotoxic effect of sorafenib+nutlin-3 in renal cell carcinoma cells, using high concentrations of both sorafenib (up to 50 mM) and nutlin-3 (up to 20 mM).23
Before characterizing the aspects of cell death induced by the sorafenib+nutlin-3 combination, we investigated the potential anti-proliferative effects. As shown in Online
Supplementary Figure S3, while nutlin-3 alone induces a
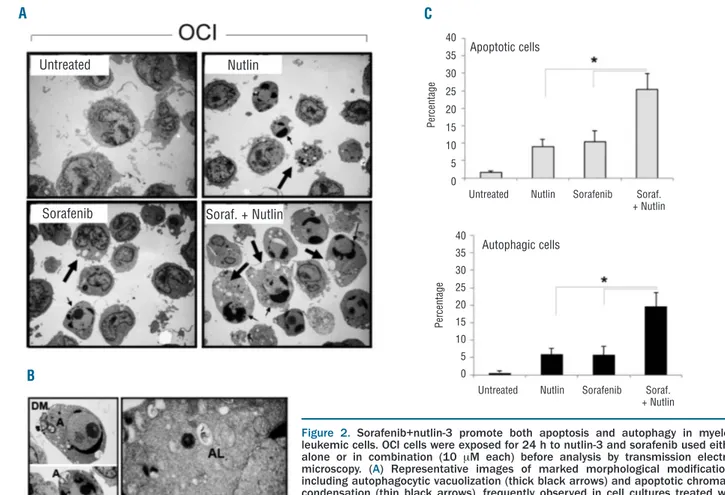
prevalent accumulation in G1and G2/M phases of the cell cycle in p53wild-type OCI-AML3 and p53deleted HL60 cells, respectively, as previously demonstrated by us and by other groups,10-12 sorafenib had minor effects on the leukemic cell cycle, whether used alone or in combination with nutlin-3. In the next experiments, the morphological aspects of cell death were characterized by TEM (Figure 2) on cell cultures harvested after 24 h of drug treatment, to avoid the excessive cell death observed at 48 h (Figure 1). After treatment with sorafenib and nutlin-3, leukemic cells showed mixed aspects of apoptosis, such as nuclear shrinkage, chromatin condensation, and membrane bleb-bing, and of autophagy, such as membrane-bound vesicles occupying the major cytoplasmic space, which frequently contained electron-dense material of cytoplasmic frag-ments and organelles (Figure 2A,B). Higher magnification of images clearly revealed multiple or double membrane-bound autophagosomes with fragmented cellular organelles and debris inside (Figure 2B). By scoring inde-pendent fields, the most evident morphological aspect characterizing the dying leukemic cells treated with sorafenib+nutlin-3 with respect to cells treated with single drugs was the appearance of a significantly higher (P<0.05) percentage of cells showing features of apoptosis and autophagy (Figure 2C).
Consistently with the morphological data, analyses of leukemic cell cultures by flow cytometry confirmed the induction of both apoptosis and autophagy upon treat-ment with sorafenib+nutlin-3. In particular, the staining of leukemic cells with annexin V/7-amino-actinomycin D revealed a significant (P<0.05) increase in the percentage of apoptotic cells following treatment with the sorafenib+nutlin-3 combination, with respect to following treatment with either sorafenib or nutlin-3 used alone, in both p53wild-typeOCI-AML3 and p53deletedHL60 leukemic cul-tures (Figure 3A). On the other hand, evidence of autophagy was first documented by analysis of the phys-ical parameters FSC-H/SSC-H by flow cytometry (Figure 3B). Autophagic cells were represented by a distinct pop-ulation of cells that were smaller and denser with respect to viable cells and that were observed in a significantly
higher percentages after treatment with sorafenib+nutlin-3 than after treatment with the single compounds (Figure 3B). The occurrence of autophagy was confirmed by flow cytometry analysis after MDC staining of both p53wild-type OCI-AML3 and p53deletedHL60 cells (Figure 3C), as well as by western blot analysis of autophagosome-associated generation of endogenous LC3-II (Figure 3D). It is note-worthy that similar results were confirmed by exposing FTL-3mutatedMV4-11 leukemic cells to low concentrations of sorafenib+nutlin-3 (1 mM each), as shown in Online
Supplementary Figure S4A-D. In addition, cell viability of
OCI-AML3 and HL60 cultures was analyzed after pre-treatment with a specific autophagy inhibitor24 (3MA) before adding the sorafenib+nutlin-3 combination. As shown in Online Supplementary Figure S5, in the presence of 3MA a small increase of cell viability was observed upon drug treatment. Of note, pre-treatment with 3MA down-modulated autophagy and induced a concomitant increase of apoptosis. Taken together, these data indicate that both apoptosis and autophagy, which are intimately intertwined processes, are involved in the cytotoxic response of leukemic cells to sorafenib+nutlin-3.
Molecular interplay between sorafenib and nutlin-3
in p53
wild-typeleukemic cells
It has been previously shown that Mcl-1 degradation is an early event not only following induction of apoptosis, but also under conditions in which Mcl-1 levels regulate
activation of autophagy.25In particular, we have recently shown that Mcl-1 shows a marked decline in myeloid leukemic cells as early as 2 h after treatment with sorafenib.26 Thus, in order to investigate the mechanism underlying the synergistic cytotoxicity of sorafenib+nut-lin-3 in AML cells, we next investigated changes in the lev-els of Mcl-1 and p53, two known targets of sorafenib and nutlin-3, respectively. Consistently with previous studies in both leukemic cells26 and solid tumor models,27 sorafenib alone showed the ability to down-regulate Mcl-1 in lysates obtained from primary AML blasts as well as OCI-AML3 and HL60 cells (Figure 4A). Although nutlin-3 alone showed either no effects or a paradoxical induction of Mcl-1 (Figure 4A), in keeping with its ability to induce ERK1/2 phosphorylation (Online Supplementary Figure
S2A,B), the sorafenib+nutlin-3 combination promoted
potent down-regulation or a complete shut-off of Mcl-1 protein (Figure 4A), indicating that the suppressive activity of sorafenib was prominent with respect to the induction of Mcl-1 by nutlin-3. On the other hand, nutlin-3 alone selectively induced p53 accumulation in p53wild-type OCI-AML3 but not in p53deleted HL60 cells (Figure 4B), while sorafenib alone had no effects on p53. Of note, the associ-ation of sorafenib+nutlin-3 did not interfere with the accu-mulation of p53 protein induced by nutlin-3 in either pri-mary AML blasts or p53wild-typeOCI-AML3 cells (Figure 4B). With respect to the p53 transcriptional activity, however, it is noteworthy that sorafenib+nutlin-3 induced
signifi-Figure 1. Synergistic cytotoxicity by the sorafenib+nutlin-3 combi-nation in myeloid leukemic cell lines. Leukemic cell lines were exposed to the indicated concentrations of nutlin-3 or sorafenib used either alone or in combination, at a fixed 1:1 ratio. Cell via-bility was analyzed at 24 and 48 h and is reported as percentage of untreated cultures set to 100% (hatched lines). Data are reported as means±SD of at least three independent experi-ments. 24 hours 48 hours MV4-11 120 100 80 60 40 20 0 120 100 80 60 40 20 0 120 100 80 60 40 20 0 120 100 80 60 40 20 0 120 100 80 60 40 20 0
24 hours 48 hours MOLM
24 hours 48 hours HL60
24 hours 48 hours OCI
1 mM (each) 3 mM (each) 10 mM (each) 1 mM (each) 3 mM (each) 10 mM (each)
1 mM (each) 3 mM (each) 10 mM (each) Nutlin Soraf. Soraf.
+ Nutlin
Nutlin Soraf. Soraf. + Nutlin
Nutlin Soraf. Soraf. + Nutlin
Nutlin Soraf. Soraf. + Nutlin
Nutlin Soraf. Soraf. + Nutlin
Nutlin Soraf. Soraf. + Nutlin Nutlin Soraf. Soraf.
+ Nutlin
Nutlin Soraf. Soraf. + Nutlin
Nutlin Soraf. Soraf. + Nutlin Nutlin Soraf. Soraf.
+ Nutlin
Nutlin Soraf. Soraf. + Nutlin
Nutlin Soraf. Soraf. + Nutlin
Nutlin Soraf. Soraf. + Nutlin Nutlin Soraf. Soraf.
+ Nutlin Nutlin Soraf. Soraf.
+ Nutlin
1 mM (each) 3 mM (each) 10 mM (each)
24 hours 48 hours NB4 C e ll v ia b il it y ( % ) C e ll v ia b il it y ( % ) C e ll v ia b il it y ( % )
cantly (P<0.05) higher levels of Bax and MDM2 than did nutlin-3 alone in OCI-AML3 cells but not in HL60 cells (Figure 4C). In order to start to address the molecular mechanism mediating the cytotoxic activity of sorafenib+nutlin-3, it is worth noting that a previous study showed that the autophagic response is converted to apoptosis by the concomitant activation of Bax.25Thus, in subsequent experiments OCI-AML3 and HL60 cells were transfected with Bax siRNA and then treated with sorafenib+nutlin-3 (Figure 4D). Down-regulation of Bax efficiently (P<0.05) counteracted the cytotoxicity of sorafenib+nutlin-3 in OCI-AML3 but not in HL60 cells (Figure 4D), suggesting that Bax plays a prominent role in mediating the sorafenib+nutlin-3 cytotoxicity in p53wild-type cells.
Molecular interplay between sorafenib and nutlin-3
in p53
deletedleukemic cells
The ability of sorafenib to potentiate the p53 transcrip-tional activity of Bax may account for the synergistic cyto-toxicity of sorafenib+nutlin-3 in p53wild-type cells, while it does not explain why this drug combination promotes cytotoxicity also in p53deletedHL60 cells. In this respect, it is noteworthy that the pro-apoptotic Bcl-2 family member Bak interacts with Mcl-1 under normal conditions and is released to be activated upon cell death stimuli.18 As
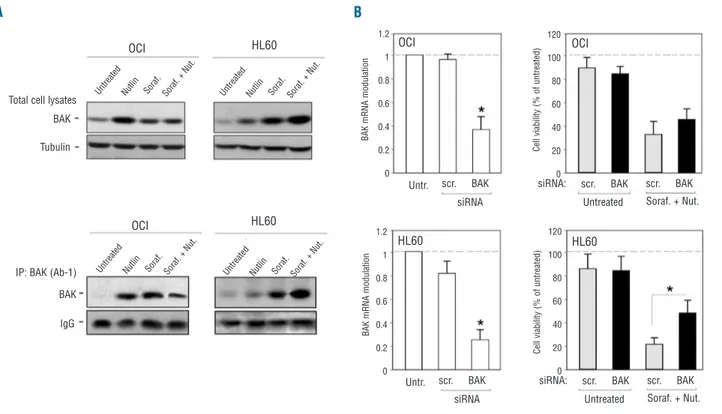
shown in Figure 5A, sorafenib+nutlin-3 selectively increased the levels of both total and the conformationally active form of Bak in p53deleted HL60 but not in p53wild-type OCI-AML3 cells. To elucidate the functional role of Bak activation in mediating the cytotoxic activity of sorafenib+nutlin-3, leukemic cells were transfected with Bak siRNA before exposure to sorafenib+nutlin-3 (Figure 5B). Down-regulation of Bak significantly (P<0.05) coun-teracted cytotoxicity of the drug combination in HL60 cells whereas it had little effect in OCI-AML3 cells (Figure 5B), suggesting that sorafenib+nutlin-3-induced cytotoxic-ity in p53deletedHL60 cells is Bak-dependent.
Discussion
We have demonstrated for the first time that the sorafenib+nutlin-3 combination exhibits synergistic cyto-toxicity in primary AML blasts as well as in a panel of myeloid cell lines. In all the cell lines and primary AML blasts investigated, we found that the cytotoxicity of sorafenib+nutlin-3 showed aspects of both autophagy and apoptosis, which are intimately intertwined processes. The greatest susceptibility to the drug combination was observed in the FLT3mutatedMV4-11 and MOLM cell lines, a finding particularly relevant since it has been shown that
Figure 2. Sorafenib+nutlin-3 promote both apoptosis and autophagy in myeloid leukemic cells. OCI cells were exposed for 24 h to nutlin-3 and sorafenib used either alone or in combination (10 mM each) before analysis by transmission electron microscopy. (A) Representative images of marked morphological modifications, including autophagocytic vacuolization (thick black arrows) and apoptotic chromatin condensation (thin black arrows), frequently observed in cell cultures treated with sorafenib+nutlin-3 (magnification, 2.500X). (B) High-magnification images of autophagosomes (A), an autophagolysosome (AL) and a double membrane vesicle (DM) (a: magnification, 5,000X; b: magnification, 30,000X). (C) Cells with morpholog-ical aspects of apoptosis and autophagy were quantified by counting a total of 500 cells (magnification 1,000X) for each culture condition. Data are expressed as means±SD of 11 independent fields. *P<0.05.
A C
B
Nutlin Untreated
Sorafenib Soraf. + Nutlin
40 35 30 25 20 15 10 5 0 40 35 30 25 20 15 10 5 0 P e rc e n ta g e Apoptotic cells Autophagic cells
Untreated Nutlin Sorafenib Soraf. + Nutlin
Untreated Nutlin Sorafenib Soraf. + Nutlin P e rc e n ta g e
Figure 3. Sorafenib+nutlin-3 synergisti-cally promote apoptosis and autophagy in both p53wild-typeand p53 delet-ed leukemic cells. p53wild-type (OCI) and
p53deleted (HL60) leukemic cells were
exposed for 24 h to nutlin-3 and sorafenib used either alone or in com-bination (10 mM each). In (A), the per-centage of apoptotic cells was deter-mined by flow-cytometry after annexin-V/7-ADD staining. Upper panels show representative experiments, while the lower panels report means±SD of three independent experiments. The asterisk indicates P<0.05. In (B), upper panels show representative flow-cytometry analysis for physical param-eters (FSC/SSC): large boxes indicate viable cells; small boxes indicate denser (autophagic) cells. Lower pan-els report means±SD of autophagic cells, evaluated in three independent experiments. In (C), quantification of MDC staining was assessed by flow cytometry. Data are expressed as MDC mean fluorescence intensity after sub-traction of background fluorescence from unstained cells. Results are reported as means±SD of three inde-pendent experiments. The asterisk indicates P<0.05. In (D), equal amounts of cell lysates were analyzed for LC3II cleavage by western blot. Tubulin staining is shown as a loading control. Representative examples of western blot results are shown. A D B C 40 35 30 25 20 15 10 5 0 7000 6000 5000 4000 3000 2000 1000 0 7000 6000 5000 4000 3000 2000 1000 0 45 40 35 30 25 20 15 10 5 0 102 103 104 105 105 104 103 102 105 104 103 102 105 104 103 102 105 104 103 102 105 104 103 102 105 104 103 102 105 104 103 102 105 104 103 102 40 35 30 25 20 15 10 5 0 A p o p to ti c c e ll s ( % ) 45 40 35 30 25 20 15 10 5 0 Untreated Nutlin Sorafenib Soraf.
+ Nutlin
Untreated Nutlin Sorafenib Soraf. + Nutlin
Untreated Nutlin Sorafenib Soraf. + Nutlin Untreated Nutlin Sorafenib Soraf.
+ Nutlin
Untreated Nutlin Sorafenib Soraf. + Nutlin
Untreated Nutlin Sorafenib Soraf. + Nutlin 102 103 104 105 102 103 104 105 102 103 104 105 102 103 104 105 102 103 104 105 102 103 104 105 102 103 104 105
Untreated Nutlin Sorafenib Soraf. + Nutlin
Annexin V HL60 HL60 HL60 OCI OCI HL60 autophagic cells 0 200 400 600 800 1000 FSC-Height 0 200 400 600 800 1000 FSC-Height 0 200 400 600 800 1000 FSC-Height 0 200 400 600 800 1000 FSC-Height 0 200 400 600 800 1000 0 200 400 600 800 1000 0 200 400 600 800 1000 0 200 400 600 800 1000 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 0 2 0 0 4 0 0 6 0 0 8 0 0 1 0 0 0 S S C -H e ig h t A u to p h a g ic c e ll s ( % ) M D C m e a n f lu o re s c e n c e i n te n s it y S S C -H e ig h t HL60 HL60 OCI
Untreated Nutlin Sorafenib Soraf. + Nutlin
OCI OCI OCI 7 -A A D Unt reat ed Nut lin Sor af. Sor af. + N ut. LC3 II Tubulin LC3 II Tubulin Unt reat ed Nut lin Sor af. Sor af. + N ut.
FLT3-ITD can up-regulate Mcl-1 and inactivate p53wild-type through hyper-phosphorylation to promote survival of stem cells in AML.28-30Although most of the mechanistic experiments were generated in FLT3wild-typeOCI-AML3 and HL60 cells, further investigation of mechanistic studies in FLT3mutatedAML cells are warranted.
A large body of data has shown that there are two main types of programmed cell death: type I, also called apop-tosis, refers specifically to a genetically controlled process involving transcription of specific proteins, such as the Bcl-2 pro-apoptotic family members and leading eventu-ally to a cell’s demise.31,32Type II programmed cell death, also called autophagy, is a non-selective process in which cytoplasm and organelles are (apparently) randomly assorted into the autophagosome, where they are degrad-ed.33-35 The levels of autophagy in cells treated with a combination of sorafenib+nutlin-3 rapidly increased, as judged by increased processing of LC3 to LC3II, morpho-logical tests and flow cytometry assays. Since sorafenib alone,36 similarly to other stress inducers,37,38 can induce either a protective form of autophagy or a toxic form of autophagy, which appears to be based on the stimulus and the tumor cell type being examined, the ability of the small molecule inhibitor 3MA to increase the number of apoptotic cells suggests that the sorafenib+nutlin-3 com-bination induces a protective form of autophagy in AML
cell models.
At the molecular level, it is noteworthy that while nut-lin-3 was unable to down-regulate Mcl-1 and rather pro-moted an increase of Mcl-1 levels in both p53wild-type OCI-AML3 and p53deletedHL60 cells, sorafenib potently down-regulated Mcl-1 both when used alone and when used in association with nutlin-3 in all leukemic cell lines investi-gated. In addition, the combined treatment selectively enhanced the transcriptional activation of the p53 target pro-apoptotic gene Bax in p53wild-typeAML leukemic cells with respect to nutlin-3 alone. This is an important find-ing since, in contrast to solid tumors, more than 80% of the AML cases at diagnosis comprise wild-type p53,5but, on the other hand, the p53 inhibitor MDM2 is usually strongly expressed in AML, contributing to blocking the effect of p53.6The role of Bax up-regulation in mediating the synergistic cytotoxicity of sorafenib+nutlin-3 in p53wild-type leukemic cells, but not in p53deleted cells, was underscored in knock-down experiments performed with siRNA for Bax.
Another important finding of our study was that the synergistic cytotoxicity of sorafenib+nutlin-3 was docu-mented also in p53deletedHL60 cells, clearly suggesting that nutlin-3 also exerts p53-independent effects. In this respect, we have demonstrated that sorafenib+nutlin-3 act synergistically in promoting the up-regulation of Bak in Figure 4. Role of Bax in mediating the anti-leukemic activity of
sorafenib+nutlin-3 in p53wild-type leukemic cells. Equal amounts of cell
lysates, obtained from leukemic cells treated for 24 h as indicated, were analyzed for Mcl-1 (A) and p53 (B) protein levels by western blot. Tubulin staining is shown as a loading control. In (A) and (B), blots representa-tive of at least three independent experiments yielding equivalent results, are shown. (C) Transcriptional activation of p53 target genes, MDM2, BAX and p21 was assessed by quantitative RT-PCR. RNA levels are expressed as folds of modulation, with respect to the control untreat-ed cultures set at 1. Results are reportuntreat-ed as means±SD of three inde-pendent experiments. Asterisks, P<0.05. (D) OCI-AML3 and HL60 cells were transfected with either control scrambled (scr.) siRNA or Bax siRNA before treatment with sorafenib+nutlin-3. After transfection, efficiency of Bax knock-down was documented by analyzing levels of Bax mRNA by quantitative RT-PCR. Results are expressed as fold of modulation with respect to the control cultures (set at 1, hatched line). Cultures transfect-ed with either control scrambltransfect-ed (scr.) siRNA or Bax siRNA were ana-lyzed for cell viability upon exposure to sorafenib+nutlin-3. Results are expressed as percentage of viable cells with respect to the control cul-tures (set at 100%, hatched line). Data are reported as means±SD of results from three independent experiments. Asterisks, P<0.05.
A C D B MDM2 BAX p21 OCI Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed R N A m o d u la ti o n ( fo ld /u n tr e a te d ) B A X m R N A m o d u la ti o n C e ll v ia b il it y ( % o f u n tr e a te d ) 40 35 30 25 20 15 10 5 0 40 35 30 25 20 15 10 5 0 5 4 3 2 1 0 1.2 1 0.8 0.6 0.4 0.2 0
Untr. scr. BAX scr. BAX
Untreated Soraf. + Nut. scr. BAX
scr. BAX
Untreated Soraf. + Nut. scr. BAX siRNA siRNA: siRNA: Untr. scr. BAX siRNA B A X m R N A m o d u la ti o n 1.2 1 0.8 0.6 0.4 0.2 0 120 100 80 60 40 20 0 C e ll v ia b il it y ( % o f u n tr e a te d ) 120 100 80 60 40 20 0 5 4 3 2 1 0 5 4 3 2 1 0 7 6 5 4 3 2 1 0 Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut. OCI OCI HL60 Mcl-1 Tubulin Tubulin p53 HL60 HL60
AML patient # 4 OCI HL60
HL60 cells and the role of Bak in mediating the cytotoxic-ity of sorafenib+nutlin-3 in this cell line was suggested by knock-down experiments performed with siRNA specific for Bak. The ability of nutlin-3 to up-regulate Bak in syn-ergism with sorafenib may be due to the existence of a molecular mimicry between MDM2 and the anti-apoptot-ic family members Bcl-2 and Bcl-Xl,39,40 which suggests that nutlin-3 could bind with comparable efficacy to MDM2 and to Bcl-2 and Bcl-Xl and free Bak from seques-tration.
In conclusion, we have established for the first time that the sorafenib+nutlin-3 combination is particularly active in FLT3mutatedleukemic cells but also in FLT3wild/type/p53wild-type
and in FLT3wild/type/p53deleted leukemic cells, opening new therapeutic perspectives for the combined use of sorafenib+nutlin-3 in the majority of AML cases.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Figure 5. Role of Bak in mediating the anti-leukemic activity of sorafenib+nutlin-3 in p53deletedleukemic cells. (A) Equal amounts of cell
lysates, obtained from OCI-AML3 and HL60 leukemic cells, treated for 24 h as indicated, were analyzed for Bak protein by western blot peformed on total cell lysates or after immunoprecipitation (IP) of conformationally active Bak. Blots representative of three independent experiments, yielding equivalent results, are shown. (B) OCI-AML3 and HL60 cells were transfected with either control scrambled (scr.) siRNA or Bak siRNA before treatment with sorafenib+nutlin-3. After transfection, the efficiency of Bak knockdown was documented by analyzing levels of Bak mRNA by quantitative RT-PCR. Results are expressed as fold of modulation with respect to the control cultures (set at 1, hatched line). Cultures transfected with either control scrambled (scr.) siRNA or Bak siRNA were analyzed for cell viability upon exposure to sorafenib+nutlin-3. Results are expressed as percentage of viable cells with respect to the control cultures (set at 100%, hatched line). Data are reported as means±SD of results from three independent experiments. Asterisks, P<0.05.
A B B A K m R N A m o d u la ti o n B A K m R N A m o d u la ti o n C e ll v ia b il it y ( % o f u n tr e a te d ) C e ll v ia b il it y ( % o f u n tr e a te d ) scr. BAK scr. BAK siRNA Untr. scr. BAK siRNA Untr. scr. BAK Untreated Soraf. + Nut. siRNA:
scr. BAK scr. BAK Untreated Soraf. + Nut. siRNA: 120 100 80 60 40 20 0 120 100 80 60 40 20 0 1.2 1 0.8 0.6 0.4 0.2 0 1.2 1 0.8 0.6 0.4 0.2 0 HL60 HL60 HL60 HL60 OCI OCI OCI OCI Tubulin BAK BAK IgG Total cell lysates
IP: BAK (Ab-1)
Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut. Unt reat ed Nut lin Sor af. Sor af. + N ut.
References
1. Appelbaum FR, Rosenblum D, Arceci RJ, Carroll WL, Breitfeld PP, Forman SJ, et al. End points to establish the efficacy of new agents in the treatment of acute leukemia. Blood. 2007;109(5):1810-6.
2. Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myeloge-nous leukemia. J Natl Cancer Inst. 2008;
100(3):184-98.
3. Lierman E, Lahortiga I, Van Miegroet H, Mentens N, Marynen P, Cools J. The ability of sorafenib to inhibit oncogenic PDGFRbeta and FLT3 mutants and over-come resistance to other small molecule inhibitors. Haematologica. 2007;92(1):27-34. 4. Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96(1):62-8.
5. Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, et al. Compassionate use of sorafenib in FLT3-ITD positive acute myeloid leukemia: sus-tained regression before and after allogene-ic stem cell transplantation. Blood. 2009; 113(15):6567-71.
6. Man CH, Fung TK, Ho C, Han HH, Chow HC, Ma AC, et al. Sorafenib treatment of FLT3-ITD+ acute myeloid leukemia: favor-able initial outcome and mechanisms of subsequent non-responsiveness associated
with a D835 mutation. Blood. 2012;119 (22):5133-43.
7. Rahmani M, Aust MM, Attkisson E, Williams DC Jr, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 anti-apoptotic mem-bers by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012;119(25): 6089-98.
8. Secchiero P, di Iasio MG, Gonelli A, Zauli G. The MDM2 inhibitors Nutlins as an innovative therapeutic tool for the treat-ment of hematological malignancies. Curr Pharmac Des. 2008;14(21):2100-10. 9. Mitani N, Niwa Y, Okamoto Y. Surveyor
nuclease-based detection of p53 gene muta-tions in haematological malignancy. Ann Clin Biochem. 2007;44(Pt6):557-9. 10. Kojima K, Konopleva M, Samudio IJ,
Shikami M, Cabreira-Hansen M, McQueen T, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106 (9):3150-9.
11. Secchiero P, Zerbinati C, di Iasio MG, Melloni E, Tiribelli M, Grill V, et al. Synergistic cytotoxic activity of recombi-nant TRAIL plus the non-genotoxic activa-tor of the p53 pathway nutlin-3 in acute myeloid leukemia cells. Curr Drug Metab. 2007;8(4):395-403.
12. Long J, Parkin B, Ouillette P, Bixby D, Shedden K, Erba H, et al. Multiple distinct molecular mechanisms influence sensitivity and resistance to MDM2 inhibitors in adult acute myelogenous leukemia. Blood. 2010;116(1):71-80.
13. Wergeland L, Sjøholt G, Haaland I, Hovland R, Bruserud Ø, Gjertsen BT. Pre-apoptotic response to therapeutic DNA damage involves protein modulation of Mcl-1, Hdm2 and Flt3 in acute myeloid leukemia cells. Mol Cancer. 2007;6:33. 14. Campioni D, Corallini A, Zauli G, Possati
L, Altavilla G, Barbanti-Brodano G. HIV type 1 extracellular tat protein stimulates growth and protects cells of BK virus/tat transgenic mice from apoptosis. AIDS Res Human Retroviruses. 1995;11(9):1039-48. 15. Campioni D, Secchiero P, Corallini F,
Melloni E, Capitani S, Lanza F, et al. Evidence for a role of TNF-related apopto-sis-inducing ligand (TRAIL) in the anemia of myelodysplastic syndromes. Am J Pathol. 2005;166(2):557-63.
16. Zauli G, Visani G, Bassini A, Caramelli E, Ottaviani E, Bertolaso L, et al. Nuclear translocation of protein kinase C-alpha and -zeta isoforms in HL-60 cells induced to dif-ferentiate along the granulocytic lineage by all-trans retinoic acid. Br J Haematol. 1996;93(3):542-50.
17. Gibellini D, Bassini A, Pierpaoli S, Bertolaso L, Milani D, Capitani S, et al. Extracellular
HIV-1 Tat protein induces the rapid Ser133 phosphorylation and activation of CREB transcription factor in both Jurkat lym-phoblastoid T cells and primary peripheral blood mononuclear cells. J Immunol. 1998;160(8):3891-8.
18. Nguyen TK, Rahmani M, Harada H, Dent P, Grant S. MEK1/2 inhibitors sensitize Bcr/Abl+ human leukemia cells to the dual Abl/Src inhibitor BMS-354/825. Blood. 2007; 109(9):4006-15.
19. Chou T, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27-55.
20. Haouala A, Zanolari B, Rochat B, Montemurro M, Zaman K, Duchosal MA, et al. Therapeutic drug monitoring of the new targeted anticancer agents imatinib, nilotinib, dasatinib, sunitinib, sorafenib and lapatinib by LC tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(22):1982-96.
21. Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11(15):5472-80. 22. Strumberg D, Clark JW, Awada A, Moore
MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitu-mor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007; 12(4):426-37.
23. Vatsyayan R, Singhal J, Nagaprashantha LD, Awasthi S, Singhal SS. Nutlin-3 enhances sorafenib efficacy in renal cell car-cinoma. Mol Carcinog. 2011 Oct 17. doi: 10.1002/mc.20875. [Epub ahead of print] 24. Kondo Y, Kanzawa T, Sawaya R, Kondo S.
The role of autophagy in cancer develop-ment and response to therapy. Nat Rev Cancer. 2005;5(9):726–34.
25. Germain M, Slack RS. Mcl-1 regulates the balance between autophagy and apoptosis. Autophagy. 2011;7(5):549-51.
26. Secchiero P, Melloni E, Voltan R, Norcio A, Celeghini C, Zauli G. MCL1 down-regula-tion plays a critical role in mediating the higher anti-leukaemic activity of the multi-kinase inhibitor Sorafenib with respect to Dasatinib. Br J Haematol. 2012;157(4):510-4. 27. Inuzuka H, Fukushima H, Shaik S, Liu P, Lau AW, Wei W. Mcl-1 ubiquitination and destruction. Oncotarget. 2011;2(3):239-44. 28. van Stijn A, van der Pol MA, Kok A, Bontje
PM, Roemen GM, Beelen RH, et al. Differences between the CD34+ and CD34- blast compartments in apoptosis resistance in acute myeloid leukemia. Haematologica. 2003;88(5):497-508.
29. Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, et al. FLT3-ITD up-regulates MCL-1 to pro-mote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 acti-vation. Blood. 2009;114(24):5034-43. 30. Yokota S, Kiyoi H, Nakao M, Iwai T,
Misawa S, Okuda T, et al. Internal tandem duplication of the FLT3 gene is preferential-ly seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies: a study on a large series of patients and cell lines. Leukemia. 1997;11(10):1605-9.
31. Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cas-cade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7(12):1166-73.
32. Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17(14):3878-85.
33. Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3(4):295-9.
34. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132 (1):27-42.
35. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–8.
36. Park MA, Reinehr R, Hussinger D, Voelkel-Johnson C, Ogretmen B, Yacoub A, et al. Sorafenib activates CD95 and promotes autophagy and cell death via Src family kinases in gastrointestinal tumor cells. Mol Cancer Ther. 2010;9(8):2220-31.
37. Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther. 2009;8(21):2084-96.
38. Yacoub A, Park MA, Gupta P, Rahmani M, Zhang G, Hamed H, et al. Caspase-, cathepsin-, and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in pri-mary human glioma cells. Mol Cancer Ther. 2008;7(2):297-313.
39. Jin L, Tabe Y, Kojima K, Zhou Y, Pittalunga S, Konopleva M, et al. MDM2 antagonist Nutlin-3 enhances bortezomib-mediated mitochondrial apoptosis in TP53-mutated mantle cell lymphoma. Cancer Lett. 2010;299(2):161-70.
40. Ha JH, Won EY, Shin JS, Jang M, Ryu KS, Bae KH, et al. Molecular mimicry-based repositioning of nutlin-3 to anti-apoptotic Bcl-2 family proteins. J Am Chem Soc. 2011;133(5):1244-7.