Sapienza University of Rome
Department of Internal Medicine and Medical Specialties
PhD program in Immunological, Haematological and Rheumatological Sciences
XXXII cycle
PhD program director: Prof. Angela Santoni
Rheumatology research program coordinator: Prof. Guido Valesini
PhD project supervisor: Prof. Fabrizio Conti
“Expression and function of KLRG1 and IL1R8 in Systemic Lupus
Erythematosus”
PhD candidate
Lucia Novelli, MD
INDEX
ABSTRACT ... 5
Chapter 1 ... 9
1.1 Introduction ... 9
1.2 Systemic Lupus Erythematosus ...10
1.2.1 Epidemiology... 10 1.2.2 Etiology ...11 1.2.2.1 Genetic factors ... 11 1.2.2.3 Environmental factors ... 12 1.2.2.4 Immunological factors ... 14 1.2.2.4.1 Innate immunity ... 14 1.2.2.4.2 Adaptive Immunity ... 18 1.2.3 Clinical manifestations ...22 1.2.3.1 General symptoms ... 22 1.2.3.2 Muco-cutaneous involvement ... 22 1.2.3.3 Musculoskeletal involvement ... 23 1.2.3.4 Respiratory involvement ... 23 1.2.3.5 Cardiovascular involvement ... 24 1.2.3.6 Gastro-intestinal involvement ... 24 1.2.3.7 Lupus nephritis... 25 1.2.3.8 Neuropsychiatric Lupus ... 28 1.2.3.9 Hematological involvement ... 28 1.2.4 Classification criteria ...29 1.2.5 Clinimetric indices ...30
1.2.6 Quality of life indices...31
1.2.7 Therapy ...31
1.3 KLRG1 ... 33
1.4 KLRG1 and SLE ... 35
1.5 IL1R8 ... 35
1.6 IL1R8 and SLE ... 37
Chapter 2 ... 39
2.1 AIM ... 39
Chapter 3 ... 39
3.1 Materials and methods ... 39
3.1.1 Patients and controls ... 39
3.1.2 PBMCs purification ... 40
3.1.3 Assessment of KLRG1 and IL1R8 by flow cytometry ... 40
3.1.4 NK cells assay: CD107a mobilization and intracellular IFN-g production ... 41
3.1.5 PBMCs in vitro treatment by hydroxychloroquine ... 42
3.1.6 Statistical analysis ... 43
4.1.4 KLRG1 expression on NK cell subsets, NKT cells and CD56+CD3bright cells ... 45
4.1.5 Distribution of CD4+ and CD8+ T cell subpopulations ... 45
4.1.6 KLRG1 expression on CD4+ and CD8+ T cell subpopulations... 46
4.1.7 Expression of IL1R8 ... 46
4.1.8 KLRG1 expression associates with SLE patients’ clinical features ... 46
4.1.9 KLRG1 expression on NK cells associates with Hydroxychloroquine ... 47
4.1.10 Hydroxychloroquine increases KLRG1 expression on NK cells ... 47
4.1.11 E-cadherin reduces IFNg and CD107a production in KLRG1+ NK cells... 47
5.1 Discussion ... 48
Chapter 6 ... 52
6.1 Conclusions ... 52
Tables and Figures ... 53
ABSTRACT
Background:
Systemic Lupus Erythematosus (SLE) is a multifactorial autoimmune disease, characterized by several immunological alterations and different clinical phenotypes1. KLRG1 is a transmembrane protein expressed in humans on NK cells and on CD4+ and CD8 + T cells. KLRG1 inhibits the cytotoxic activity of NK cells against tissues expressing its principal ligands: E-cadherin, N-cadherin and R-cadherin. Moreover, it has been shown that KLRG1 has an inhibitory effect on CD8+ T cells proliferative capacity and also their effector functions2. Thus, KLRG1-mediated signal might have a role in preventing autoimmunity, increasing the activation threshold of NK cells and T cells. Recently, KLRG1 gene emerged as a disease susceptibility gene for Systemic Lupus Erythematosus in four different ethnic groups3. Interleukin -1R like receptors (ILRs) and Toll Like Receptors (TLRs) are key receptors of innate immunity, inflammation and also adaptive immune responses. IL1R8, also known as SIGIRR and TIR8, is a member of ILRs family, it is ubiquitously expressed and it is a regulatory molecule with an inhibitory function towards other ILRs and TLRs receptors4. Uncontrolled or deregulated activation of ILRs- or TLRs- dependent inflammatory and immune responses can cause tissue damage and acute or chronic inflammatory disorders. SLE mice models IL1R8 knock-out develop massive lymphoproliferations, serositis, glomerulonephritis, and increased autoantibodies production. In humans there are only a few data, showing a relationship between IL1R8 and SLE pathogenesis.
Objective:
effector memory and effector) from SLE patients compared to healthy subjects (HS). Expression of these receptors have been investigated through flow cytometry analysis after isolation of PBMCs from peripheral blood. Possible correlations of these results with clinical data, including SLE disease activity score (SLEDAI-2K), were also investigated. As secondary aims, functional studies about KLRG1-mediated signaling on NK cells in SLE have been performed.
Results and methods:
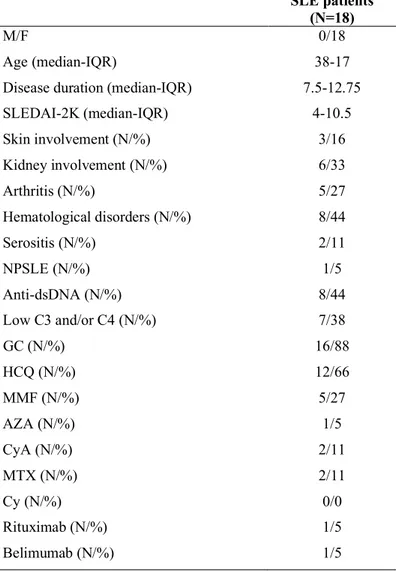
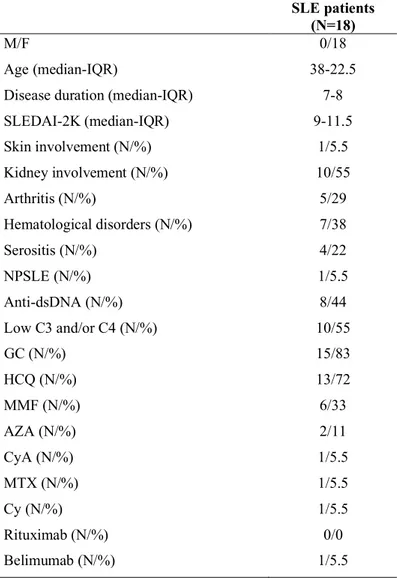
Blood samples were obtained from SLE patients and HS. Peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation (Lympholyte-H; Cedarlane Laboratories, Hornby, Ontario, Canada), and phenotypic characterization was performed. All experiments were conducted by flow cytometry. For the statistical analysis Mann-Whitney U test and Spearman’s rank correlation test were used appropriately. Eighteen patients (18F, median age 38 years IQR 17, median disease duration 7.5 years IQR 12.75) affected by SLE according to the 1997 ACR criteria, and twelve healthy subjects (12F, median age 34 years, IQR 18.5) were enrolled for the evaluation of KLRG1 expression on NK cells. Eighteen SLE patients (18F, median age 38 years IQR 22.5, median disease duration 7 years IQR 8) and fourteen healthy subjects (14F, median age 31.5 years, IQR 23) were enrolled for the characterization of IL1R8. Two lupus patients with active disease (SLEDAI-2K=6 and SLEDAI-2K=14) were enrolled for the in vitro study about hydroxychloroquine (HCQ) influence on KLRG1 expression. Preliminary in vitro functional studies about KLRG1-mediated signaling on NK cells have been also performed. All patients were enrolled from Sapienza Lupus Cohort. Disease activity was measured by SLEDAI-2K.
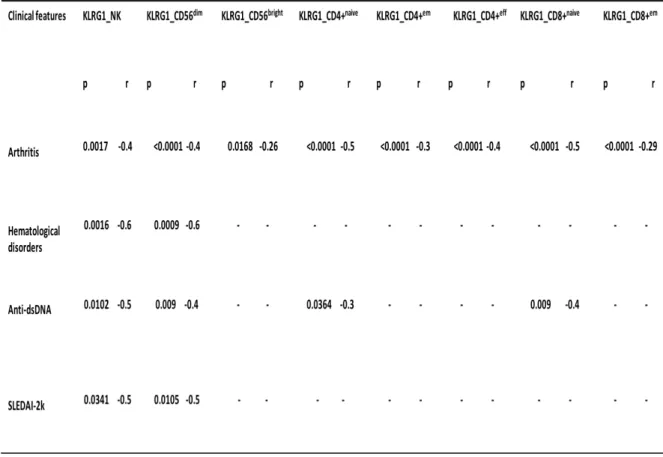
patients compared to HS (p=0.0017 and p=0.0079, respectively). KLRG1 was also expressed in a lower amount on NKT cells and CD56+ CD3bright cells from SLE patients compared to HS (p=0.0048 and p=0.0016). Examination of CD4+ and CD8+ T cell subsets showed less quantity of KLRG1 in SLE patients compared to HS on CD4+ naïve (p=0.0009), effector memory (p=0.04), effector (p=0.029) and also on CD8+ naïve (p=0.0027) and effector memory T cells (p=0.0185). The lower levels of KLRG1 on NK cells inversely correlated with the SLEDAI-2K (p=0.034, r=-0.5) and were inversely associated with arthritis (p=0.0017, 0.4), hematological disorders (p=0.0016, 0.6) and anti-dsDNA (p=0.01, r=-0.5). A direct association between KLRG1 expression on NK cells and the use of HCQ was found (p=0.0002). Analysis of the two main subpopulations of NK cells showed an inverse association between KLRG1 on CD56dim cells and arthritis (p<0.0001, r=-0.4), hematological disorders (p=0.0009, r=-0.6), the SLEDAI-2K (p=0.01, r=-0.5), and the presence of anti-dsDNA (p=0.009, r=-0.4). KLRG1 on CD56dim was also directly associated with the use of HCQ (p=0.0005). KLRG1 on CD56bright cells was also inversely associated with arthritis (p=0.01, r=-0.26) and directly associated with the use of HCQ (p=0.0064).Moreover, inverse associations were also found between KLRG1 on CD4+ naïve, effector memory, effector T cells and arthritis (p<0.0001 r=-0.5, p<0.0001 r=-0.3 and p<0.0001 r=-0.4 respectively) and between CD8+ naïve T cells and arthritis (p>0.0001, 0.5) and anti-dsDNA (p=0.009, r=-0.4) and between CD8+effector memory T cells and arthritis (P<0.0001, r=-0.29). There was no statistically significant difference about IL1R8 expression on NK cells subpopulations, on NKT cells and on CD56+CD3bright cells and T cells subpopulations between SLE patients and HS, despite in this last group there was a tendency to a higher expression in SLE patients compared to HS. PBMCs of three lupus patients with active disease, who were not taking
reaching a statistically significant difference. Preliminary in vitro functional studies in two HS and one SLE patient, confirmed inhibition of NK cells IFNg and CD107a production on KLRG1+ cells when treated with E-cadherin, in the two healthy subjects. In the lupus patient compared to the two healthy subjects, E-cadherin does not seem to be able to interfere with CD107a mobilization but it can reduce IFNg production. More experiments, extended to a larger population, are needed to evaluate if there is a difference about the grade of this inhibition between SLE patients and HS.
Conclusions:
This study explored for the first time a possible involvement of KLRG1 receptor in Systemic Lupus Erythematosus pathogenesis. SLE patients appear to express less amount of this receptor compared to healthy subjects on their lymphocytes, especially on NK cells population. KLRG1 expression on NK cells inversely associates with the SLEDAI-2K and directly associates with the use of HCQ. Moreover, after in vitro treatment with HCQ, KLRG1 levels on NK cells tends to increase. In healthy subjects, KLRG1 binding to its ligand E-cadherin inhibits NK cells activity. This inhibition seems to be altered in SLE, especially for the NK cells degranulation capacity. These results suggest a possible role of KLRG1 in the pathogenesis of this disease. However, more experiments, extended to larger cohorts of patients, are necessary to better understand its function. IL1R8 is a well-known receptor for its role as a negative regulator of immune responses and inflammation. In this study no differences emerged about its expression between SLE patients and healthy subjects. More studies, including larger populations are necessary to better understand if this receptor might actually have a role in this disease.
Chapter 1
1.1 Introduction
Systemic Lupus Erythematosus (SLE) is a multifactorial autoimmune disease, characterized by several immunological alterations and different clinical phenotypes1. KLRG1 is a transmembrane protein expressed in humans on NK cells, mostly CD56dim, cells with high cytotoxic activity (50-80%) and on CD4+ and CD8 + T cells, especially with an effector or effector memory phenotype. KLRG1 inhibits the cytotoxic activity of NK cells against tissues expressing its principal ligands: E-cadherin, N-cadherin and R-cadherin. In particular, KLRG1-E-cadherin interaction might be involved in cancer immuno-surveillance like other NK inhibitor receptors binding MHC class I molecules. Recent studies also demonstrated a role for KLRG1 as an inhibitory receptor in T cells, but only when there is also a co-engagement with the CD3/TCR mediated signaling and MHC/antigen and KLRG1 ligands are expressed on the same target cells. Moreover, it has been shown that KLRG1 has an inhibitory effect on CD8+ T cells proliferative capacity and also their effector functions2. Thus, KLRG1-mediated signal might have a role in preventing autoimmunity, increasing the activation threshold of NK and T cells. Recently, KLRG1 gene emerged as a disease susceptibility gene for Systemic Lupus Erythematosus3. Its expression on NK and T cells might be altered in SLE contributing to its pathogenesis, however, this receptor has never been investigated in this disease.
Interleukin -1R like receptors (ILRs) and Toll Like Receptors (TLRs) are key receptors of innate immunity, inflammation and also adaptive immune responses. IL1R8, also known as SIGIRR and TIR8, is a member of ILRs family, it is ubiquitously expressed and it is a regulatory molecule with an inhibitory function towards other ILRs and TLRs receptors4.
immune responses can cause tissue damage and acute or chronic inflammatory disorders. Several evidences in both mice models and humans have demonstrated that IL1R8 expression is reduced in inflammatory conditions, describing IL1R8 as a fundamental modulator of autoimmunity. IL1R8 role in SLE has been mostly studied in murine models; SLE mice models IL1R8 knock-out develop massive lymphoproliferation, serositis, glomerulonephritis, and increased autoantibodies production. In humans there are only a few data, mostly from genetic studies, showing a relationship between IL1R8 and SLE pathogenesis. For this reason, further studies are necessary to better understand IL1R8 role in this autoimmune disease. Modulation of IL1R8 might reduce autoimmune and inflammatory responses, becoming a possible future therapeutic target for autoimmune diseases.
1.2 Systemic Lupus Erythematosus
The word “lupus” was used for the first time in the Middle Ages to describe a skin lesion similar to a wolf bite. In 1846 an Austrian physician named Hebra compared the shape of the typical malar rash of this disease to a butterfly and named this disease “lupus erythematosus”. Later, Dr. Osler in Baltimore and Dr. Jadassohn in Wien, described the existence of a systemic form of the disease.
1.2.1 Epidemiology
SLE is more frequent among females compared to males with a ratio of 1:9 and a peak of incidence between 25 and 45 years (childbearing ages)5. To date, the prevalence of the disease is variable from 20 to 150 new cases every 100.000 inhabitants, with an annual incidence of 1-10 cases every 100.000 individuals1. Some ethnicities such as African and Asian have a higher risk of developing the disease which is also usually more severe6. Currently, the
10-compared to the past and to more efficacious therapies. Nowadays, death causes are probably a persistently active disease, an accelerated atherosclerosis, infections, thrombotic phenomena usually secondary to the presence of anti-phospholipid antibodies8.
1.2.2 Etiology
SLE etiology is still largely unknown. However, it has been demonstrated that SLE is a multifactorial disease, with the involvement of genetic, environmental, hormonal and immunological factors.
1.2.2.1 Genetic factors
Several studies suggest a genetic susceptibility for SLE. In monozygotic twins it has been demonstrated a higher prevalence of the disease, moreover, relatives of SLE patients have a major risk of developing SLE variable from 5% to 12%. SLE is also more frequent among some ethnic groups, such as Asians, African-Americans and Afro-Caribbeans. Generally, SLE is a result of mutations of several genes, however, sometimes SLE can be associated to a single gene mutation (for example, C1q, C2 and C4 complement factors deficiency)8. The first discovered genetic association with SLE is the HLA class II, specifically, HLA-DR2 and HLA-DR3. Several other genetic studies showed the importance of other no-HLA-related genes, and many of these genes are also involved in other autoimmune diseases (e.g. STAT4 and PTPN22 which are associated to diabetes and rheumatoid arthritis). Recent Genome Wide Association (GWA) studies on bigger cohorts of patients and controls have highlighted new
two are involved in T cells function9. Epigenetic also plays an important role in SLE pathogenesis; in fact, it has been shown that some susceptibility genes for SLE (CD70, CD40LG, PPP2CA e ITGAL) are hypomethylated in SLE patients.
1.2.2.2 Hormonal factors
Notably, SLE is more common among females in fertile age. Sex female hormones influence the activity of the immune system, predisposing to autoimmunity10. In particular, estrogens stimulate the humoral immune response, activate T cells and promote T-B cells interactions leading to antibodies production11. Moreover, estrogens receptors have been identified on B cells, CD8+ T cells, monocytes, neutrophils and NK cells12.
1.2.2.3 Environmental factors
Among the environmental factors, infections seem to play a major role in SLE pathogenesis. It has been hypothesized that infections might trigger an immune response because of molecular mimicry phenomena (cross-reactions between viral/microbial antigens and self-antigens)13.
According to another theory, some infective agents might cause apoptosis and expose antigens that may trigger the immune system14. This last hypothesis is consistent with the
Danger Model theory from the French-American immunologist Polly Matzinger. In 1994
Matzinger published an article “Tolerance, Danger and the Extended family” where she explained her theory, according to which, damaged cells send danger signals able to trigger the immune response15. This theory was an alternative thinking compared to the self-no-self
Charles Janeway, who believed that the immune system uses ancient recognition patterns which start the immune response after recognizing a pathogen from its evolutionary preserved features16.
Several viruses have been claimed to be involved in SLE development, such as Epstein Barr virus (EBV). It has been shown that SLE patients have a higher title of anti-EBV antibodies compared to controls17. EBV also cross-react with different autoantigens in SLE. In particular, similar sequences have been identified between the viral protein EBNA I and Sm and Ro antigens18. Besides, mice-immunization with EBV viral proteins induce a lupus-like syndrome19. Other viruses which seem to play a role in SLE because of molecular mimicry phenomena with SLE antigens are: Parvovirus B19, Coxsackie 2B and the Retroviruses HIV-1 and HTLV-HIV-120.
Among other environmental factors, UV light is relevant for the disease development and flare. Sun exposure in fact can trigger skin lesions inflammation and stimulate the systemic disease. UV light stimulate keratinocytes to produce pro-inflammatory cytokines such as IL-1, IL-6 and TNF. Besides, UV light induce B cells to produce autoantibodies such as anti-dsDNA, promote the formation of autoreactive T cell clones and modify apoptosis leading to self- antigens exposure21.
Finally, some drugs such as hydralazine, procainamide, penicillamine, isoniazid and α-methyldopa, can cause a particular kind of SLE, called drug-induced SLE, characterized by a good clinical outcome usually without nephritis, by the presence of anti-histone antibodies and the absence of anti-dsDNA22.
1.2.2.4 Immunological factors
1.2.2.4.1 Innate immunity
The primary mechanisms of the immune system represent one of the most innovative fields in the basic immunology research, especially for their role in the development of new and more effective therapeutic strategies from tumors to autoimmune diseases, such as SLE. The complement system is one of the components of the innate immunity, implicated in many immunological actions, including cell lysis, chemotaxis, immuno-adhesion, phagocytosis, neutralization of bacterial endotoxins, inactivation of viruses and anaphylaxis. It consists of a complex group of glycoproteins with peculiar biochemical characteristics, present in the serum in the inactive form but capable of activating according to precise sequences: the classical route, the alternative route, the lectin route23. SLE patients usually have hypocomplementemia, in particular, components of the classical pathway are reduced, and they also have a deposition of complement and immunoglobulins in the affected tissues such as kidneys and skin.
Complement genetic defects are rare, when present, they strongly predispose to the disease development. In physiological conditions, the complement system has the function to promote clearance of apoptotic or necrotic cells. Apoptotic or necrotic cells in fact, can activate the classical pathway through the binding to the fragment C1q24. These observations have been confirmed in a C1q knockout mouse model, where apoptotic cells deposit in glomeruli cause a proliferative glomerulonephritis25.
Cytokines play a fundamental role in the regulation of the immune system and in tissue damage in SLE. Generally, cytokines are distinguished in anti-inflammatory and pro-inflammatory26. However, it is not always possible to establish the exact role of all different
anti-inflammatory role in different animal models of the disease. In murine models administration of TNF ameliorated the disease and improved the survival. In the kidneys of patients with lupus nephritis high levels of TNF have been found and so this molecule seems to have a pro-inflammatory role. Moreover, the use of anti-TNF antibodies in other autoimmune diseases such as Rheumatoid Arthritis, can lead to the production of anti-dsDNA antibodies and sometimes can worsen lupus-like syndromes27.
High serum levels of (IL)-10, correlating with disease activity have been found in SLE patients. IL-10 stimulates B cell proliferation and autoantibodies production. A few studies demonstrated that IL-10 inhibition by a monoclonal antibody could improve skin involvement, arthritis and reduce disease activity in SLE patients28.
More recently, a major role as been attributed to the interferon (IFN) signature. Besides an association with different genetic variant of IFN, SLE patients have high serum levels of IFN -a. Moreover, patients who receive anti-IFN antibodies for other diseases, may develop autoantibodies and a lupus-like syndrome. Several data in scientific literature have underlined the importance of the interferon signature, which is overexpressed in SLE. IFN-α is a ligand of CD40, nucleosome and immunocomplexes; it is able to induce differentiation and
activation of dendritic cells and to stimulate cytokines production by these cells. Plasmacytoid dendritic cells (pDCs) secrete big amounts IFN-α after exposition to viruses because of toll-like-receptors activation (TLR). The number of pDCs is reduced in the peripheral blood in SLE but these cells can infiltrate skin and kidneys suggesting a possible pathogenetic role30. The B-lymphocyte stimulator (Blys), belonging to the TNF family is an important molecule that stimulates B cell proliferation and survival; several data suggest its involvement in SLE pathogenesis. High levels of Blys have been identified in the peripheral blood of SLE
Moreover, a drug that can inhibit Blys (Belimumab, Benlysta) is currently used for moderate disease without kidney involvement.
IL-17 is mostly produced by activated TH17 cells and play an important role in the immune response against bacteria and fungi; it is a pro-inflammatory cytokine and besides its role in the host defense against extracellular microorganisms, contribute to the development and the pathogenesis of autoinflammatory and autoimmune diseases. IL-17A is a member of the cytokines family which includes IL-17B, -C, -D, -E and –F32. The main function of IL-17 is to coordinate local inflammation trough neutrophils recruitment and stimulation of other cytokines and chemokines in the injured tissues acting on fibroblasts, endothelial, epithelial and astrocytes cells33.
When TH17-mediated response is aberrant, it can lead to the development of inflammatory and autoimmune diseases such as, rheumatoid arthritis, multiple sclerosis, psoriasis and of course SLE34. In SLE patients in fact, it has been found an high percentage of CD4+ T cells and an increase in the number of double negative T cells which can produce IL-1735.
In SLE a reduced production of IL-2 can be observed; this reduction is due to an altered TCR-mediated intracellular signal and can cause a reduced cytotoxicity by T cells and so to a major risk of infections but also to a dysregulation of T cell activity and so to autoimmunity36. Cytokines are important because they can represent new biomarkers of disease activity, prognosis and new therapeutic targets.
In SLE pathogenesis a role of neutrophils emerged in the last few years; neutrophils genes in fact, are unusually expressed in SLE and specific neutrophils proteins can be found in the urines of SLE patients. Moreover, studies by Lande et al., showed that in SLE patients a certain substance based on chromatin can be found; this is the product of a specific type of
complexes are produced by activated neutrophils and create sort of “nets”; these nets are able to start the activation of innate immunity activating TLR 9 and pDCs and they may also function as autoantigens able to activate B cells. The key mediator seems to be the antimicrobic peptide LL-37 which can lead to immunogenicity against self-DNA. Members of TLRs have been considered involved in the pathogenesis of several autoimmune diseases including SLE. Circulating immunocomplexes can in fact activate TLR7 and TLR9 inducing production of IFN-alfa and other cytokines and chemokines. On the light of these data, activation of TLRs is fundamental to start the inflammation and for the production of the interferon signature38. Leucocytes isolated from SLE patients express higher levels of TLR2 mRNA compared to healthy subjects39. MRLlpr/lpr mice which spontaneously develop lupus disease have higher expression of TLR1, TLR2, TLR3 and TLR6 in kidneys during lupus nephritis40. TLR4 when is upregulated in mice induce a lupus-like syndrome41. On the contrary, TLR5 seems to have a protective role against SLE: Polymorphisms in TLR5 gene, in fact are associated to a reduced risk of the disease in caucasic patients42.TLR7 and TLR9 are necessary for anti-dsDNA and anti-RNA antibodies production. Role of TLR8 is unclear. To date, the literature has disregarded Natural Killer (NK) cells as relevant modulators in systemic lupus erythematosus pathogenesis, as these cells are few in number and show a dysfunctional phenotype in patients with active systemic lupus erythematosus43. NK cells are innate lymphoid cells with an important role in immune surveillance and immune response against infected and tumor cells through natural cytotoxicity or Ab-dependent cellular cytotoxicity (ADCC). NK cells are also a major source of chemokines and cytokines, such as IFN-g and TNF, which modulate adaptive immune responses upon activation44. Alteration of NK cell numbers and function leads to deregulation of the immune system and the
IFN-g, decreased ADCC, and altered natural cytotoxicity45,46. NK cells in the kidney and lungs from MRL/lpr also display an activated phenotype with increased natural cytotoxicity and IFN-g production but reduced ADCC47,48. Both are suggested contributors to tissue damage. The molecular alterations responsible for the SLE NK cell deregulation are largely unknown. Recently, Fueyo et al.,49 showed that in NK cells in SLE patients CD3z is downregulated. CD3z is a transmembrane molecule expressed in T cells and NKT cells where it associates with the TCR complex50 and in NK cells where it associates with CD16. Downregulation of CD3z confers a proinflammatory phenotype to SLE NK cells and contributes to their altered function in patients with SLE, increasing IFNg production and degranulation. The innate complexity of NK cell function and development, together with the multifactorial components that characterize SLE, have made it difficult for researchers to understand the role of NK cells in the pathogenesis of this disease. Whereas the importance of NK cells as factors in SLE has almost been disregarded, a closer look into their function and phenotype during active disease stages shows that they could have a larger role than originally thought.
1.2.2.4.2 Adaptive Immunity
B cells play a crucial role in SLE pathogenesis. In SLE patients with active disease a reduced number of naive B cells and an increased number of plasma cells in the periphery have been observed. B cells produce autoantibodies but they also work as antigen presenting cells51. A new population of B cells with regulatory functions has been recently identified52. This population can modulate the balance Th1/Th2 promoting a switch to T regulatory cells, producing anti-inflammatory cytokines and reducing dendritic cells activation. Blair et al.,
In SLE T cells also play a fundamental role. Their TCR-mediated intracellular signal is in fact amplified. CD3 molecule, part of the TCR complex, does not use regular chain z to mediate the signal but the common FCR-g chain in SLE patients54. Because of this change the intracellular signal is altered with the use of the kinase Syk instead of ZAP -70.
One of the crucial events in SLE pathogenesis is the polyclonal activation of B cells with increased production of autoantibodies55. Autoantibodies can be found in the peripheral circle but also in the interested tissues. In SLE anti-dsDNA antibodies are fundamental for the diagnosis, the prognosis and the disease outcome56. Besides anti-dsDNA antibodies, other autoantibodies can be present, usually associated to specific clinical features: anti-Ro/SSA, anti-La/SSB, anti-Sm, anti-RNP and anti-phospholipid antibodies (aPL). Anti-nuclear antibodies (ANA) appear much earlier compared to the other groups; these antibodies are present in the 95-99% of SLE patients, and are extremely important for the diagnosis, their absence in fact, lead to the exclusion of the disease. ANA can be against DNA, RNA, histones and other antigens. If they are present for a long time with a high serum levels they represent a sign of a pathogenic autoimmune process. Anti-dsDNA antibodies are frequently present in SLE57, they are the most important in this disease and can be found in the 50-90% of lupus patients. They are very specific and they are useful for the diagnosis and for the evaluation of disease activity. They also seem to have a pathogenic role especially in lupus nephritis58. To date it is still unknown the target antigen of these antibodies in vivo; since DNA per se is not immunogenic, probably complexes of altered self-DNA and protein of viral or microbic nature are the target59. Anti-histones antibodies are directed against proteins that organize DNA structure. They are usually found in drug-induced Lupus60. Among ANA there are antibodies anti-extractable nuclear antigens (ENA). This is a big group including anti-Sm,
associated to specific disease features, such us anti-SSA which associates with skin involvement and neonatal lupus61.
Anti-C1q antibodies can be found in 95% of SLE patients with kidney involvement and their levels correlate with renal disease activity.62 Anti-phospholipid antibodies (aPL) can be also present in SLE patients; they include lupus anticoagulant (LA), anti-cardiolipin (aCL) and anti-b2glycoprotein (anti-beta2GPI) antibodies. These antibodies associate with an increased risk of thrombosis, recurrent abortion and fetal death (aPL)63. In SLE there are also other antibodies directed against antigens on cell surface: erythrocytes, platelets, anti-lymphocytes antibodies. In SLE patients with neurological manifestations anti-N-Methyl-D-Aspartic Acid Receptor 2 (Anti-NR2) and anti-ribosomal proteins antibodies can be present64. One of the principal processes responsible for autoantibodies production is apoptosis, defects in the clearance of apoptotic cells can cause inflammation and in fact, in SLE patients this process of clearance is deficient usually because of defects in the complement system65. Autophagy is a degradation mechanism by which cells recycle cytoplasmic components to generate energy. By influencing lymphocyte development, survival, and proliferation, autophagy regulates the immune responses against self and non-self antigens66; normally autophagy is triggered by stress conditions such as senescence and starvation causing cell death. Autophagy is involved in aging, cancer, myocardial infarction and also autoimmunity67. Many studies in fact demonstrated autophagy’s contribution to the presentation of cytosolic antigens in association with MHC class II molecules, playing an important role not only in the acquired immune response but also in the maintenance of self-tolerance.
molecules play a role in the inappropriate inflammatory response characterizing this disease. Increased levels of soluble forms of various adhesion molecules, that have been found in the serum of these patients, reflect cell activation and may have an influence on cell-cell interactions in vivo. For instance, adhesion molecule CD44 enables T lymphocytes’ adhesion to endothelium and during inflammation contributes to T cell migration into target organs. CD44 isoforms seem to be involved in the infiltration of peripheral tissues in SLE. A higher expression of CD44v3 and v6 isoforms has been identified on T cells from SLE patients compared to healthy subjects (HS) and the expression levels seem to correlate with disease activity and associate with specific disease manifestations68.
1.2.3 Clinical manifestations
1.2.3.1 General symptoms
SLE can involve several different organs and systems; the disease can hit the skin, the joints, the kidneys, the lungs, the heart, the nervous system and many other important organs69. The clinical outcome is extremely variable, however, it is characterized by the alternation of flare stages and remission stages. Asthenia, fever, weight loss are common and present in the 50-100% of patients.
Asthenia often is due to anemia, depression or a secondary fibromyalgia70. Fever can represent the disease onset or a disease activity index, but also can be consequent to a hidden infection71. All of these general symptoms can be also due to the use of corticosteroids or immune-suppressive drugs.
1.2.3.2 Muco-cutaneous involvement
The muco-cutaneous involvement is typical of SLE and can be present in several different ways. Skin lesions are influenced by UV light, and in fact, photosensitivity is very common in lupus patients. Skin lesions can be specific or unspecific; specific lesions are characterized by immune-infiltration of the derma-epidermal region and there is a vacuolar degeneration of the basal layer. It is possible to distinguish acute, subacute and chronic lesions. Acute lesions are the malar rash or a diffuse erythema with a significative cutaneous infiltration. Subacute lupus can manifest with diffuse psoriasiform lesions or annular/polycyclic lesions and is associated to the presence of anti-SSA/Ro. Chronic lupus presents atrophy and can interest the face or the scalp: discoid lupus or lupus panniculitis belong to this group72. Unspecific
lesions include alopecia, livedo reticularis, Raynaud phenomenon. Finally, oral aftosis is often present in SLE patients.
1.2.3.3 Musculoskeletal involvement
Arthralgias and arthritis can involve from 70% to 95% of SLE patients and occur several years before the diagnosis. Usually articular symptoms are migrant, can interest several joints and usually can spontaneously disappear in 24 hours. SLE-related arthritis is not an erosive arthritis, when there are joints deformities, they interest hands and feet and can be classified as Jaccoud Arthropathy. This is a deforming arthropathy characterized by ulnar deviation of the second to 5th fingers with metacarpophalangeal (MCP) joint subluxation73. Asthenia and myalgias can be present in 70% of patients, most of the time they are due to a secondary fibromyalgia. In some patients, femoral head osteonecrosis or an early osteoporosis can occur secondary to a strong and continued corticosteroid therapy.
1.2.3.4 Respiratory involvement
Respiratory involvement, even in an asymptomatic form, can be present in 50% of SLE patients. Cough and dyspnea usually are the first symptoms to appear. Pleuritis is the most typical manifestation and it is characterized by chest pain74. Lupic acute pneumonia and alveolar hemorrhage are severe and rare manifestations; interstitial fibrosis can interest often patients with anti-SSA/Ro antibodies75.
In some patients shrinking lung syndrome, can occur; it has probably a muscular involvement with respiratory insufficiency and progressive dyspnea. A rare complication is pulmonary hypertension, due to vasculitis and ventricular hypertrophy or to thromboembolic phenomena caused by anti-phospholipid antibodies76. Infective pneumonia represents an
important warning in SLE; lupus patients in fact are more susceptible to infections because of the dysregulation of the immune response and the immunosuppressive therapy77.
1.2.3.5 Cardiovascular involvement
Cardiovascular involvement is one of the most important clinical manifestations of SLE and often associated to disease mortality. Atherosclerosis in SLE is one of these manifestations; inflammation in fact, has a pivotal role in atherosclerosis and lupus patients which are under a constant chronic inflammation state, have an accelerated atherosclerosis compared to the general population78.
However, pericarditis is the most frequent cardiac manifestation in SLE and can be asymptomatic or being associated to chest pain79. Myocarditis are usually rare and asymptomatic. Some patients might also have valvulopathies or Libman-Sacks endocarditis. Moreover, congenital heart block during neonatal lupus can occur in children of patients with anti-SSA/Ro and anti-SSB/La antibodies80.
1.2.3.6 Gastro-intestinal involvement
The most frequent gastro-intestinal manifestations in lupus patients are peptic ulcer and gastritis. Dysphagia is a common symptom. The causes are probably the inflammation of esophageal mucosa or ischemic damage. More frequent is abdominal pain with nausea or emesis due for instance to pancreatitis. Rare cases of autoimmune hepatitis can also occur in SLE patients81.
1.2.3.7 Lupus nephritis
Lupus nephritis is one of the most important and most frequent clinical manifestations in SLE, mostly because of its influence on disease prognosis. Usually lupus nephritis occurs in the first three years after the diagnosis of SLE; and only in 3-6% of patients can be the first clinical manifestation. In 13-26% of patients there is proteinuria (>3,5 gr per day) and creatinine is increased in 30% of patients82.
There are several different clinical manifestations of lupus nephritis:
• Latent nephritis, including hematuria and proteinuria (<1 gr per day) with edema and hypertension;
• Nephritic syndrome, including hematuria, moderate proteinuria, (between1 gr and 3,5 gr per day), presence of casts in the urines, edema and hypertension;
• Nephrotic syndrome, including severe proteinuria (≥ 3,5 gr per day), reduction of serum albumin, severe edema and increase of lipids;
• Chronic kidney failure, including persistently increase of creatinine with hypertension, edema and anemia.
Every single part of the kidneys can be interested by the pathological process: glomeruli, tubules, vessels, interstitial space. Kidney biopsy is fundamental to determine the grade of organ involvement, the prognosis, the disease activity and also to decide the correct therapeutic approach. However, the most frequent and most severe lesions are in the glomeruli (glomerulonephritis). In 2004 the International Society of Nephrology and the
I) Minimal mesangial glomerulonephritis
Histologically normal on light microscopy but with mesangial deposits on electron microscopy.
II) Mesangial proliferative lupus nephritis
Mesangial hypercellularity and mesangial matrix expansion. Presence of subepithelial and subendothelial deposits on electron microscopy and immunofluorescence but not on light microscopy.
III) Focal proliferative nephritis
Focal active or non-active segmental or global endo- or extra-capillary with involvement of <50% of glomeruli, typically with subendothelial deposits of immunocomplexes, with or without mesangial alterations.
Subclassification:
II (A): focal proliferative nephritis with active lesions;
III (A/C): focal or sclerosant proliferative nephritis with active and chronic lesions; III (C): focal sclerosant nephritis with chronic lesions and glomerular scars.
IV) Diffuse proliferative nephritis
Active or non-active nephritis, segmental or global endo- or extra-capillary with involvement of >50% of glomeruli, typically with diffuse subendothelial immunocomplexes, with or without mesangial alterations. This kind of nephritis can be distinguished in (IV-S) diffuse
segmental nephritis when more then 50% of glomeruli is involved and (IV-G) diffuse global nephritis when more then 50% of glomeruli present global lesions. A segmental lesion is a
lesion that affects less than half a glomerulus. Subclassification:
IV-S (A/C): diffuse proliferative segmental and sclerosant nephritis with active and chronic lesions;
IV-G (A/C): diffuse proliferative global and sclerosant nephritis with active and chronic lesions;
IV-S (C): diffuse sclerosant segmental nephritis with chronic lesions and scars; IV-G (C): diffuse global sclerosant nephritis with chronic lesions and scars.
V) Membranous nephritis
Presence of subepithelial deposits of global or segmental immunocomplexes on light microscopy, electronic microscopy or immunofluorescence, with or without mesangial alterations.
This class can be present in combination with class III or IV. Class V can also present advanced sclerosis.
VI) Glomerulosclerosis
More than 90% of glomeruli are globally sclerosant without any residual activity.
For the histological exam and a correct classification of lupus nephritis it is important to have at least 10 glomeruli. The specimen must be studied at light microscopy, by immunofluorescence to check the presence of immunoglobulins or complement and immunocomplexes, and if it is possible also by electronic microscopy. Usually class I and II are associated to a better outcome, on the contrary, class III and IV without treatment are highly progressive. Patients with class III, IV and V have a worse prognosis compared to class I and II. In clinical practice, kidney biopsy is performed in SLE patients with
in order to start an immediate and correct therapy to prevent chronic and irreversible damage84.
1.2.3.8 Neuropsychiatric Lupus
Neurological involvement can be present in 10 to 90% of SLE patients. Nineteen neuropsychiatric syndromes have been designed by the American College of Rheumatology (ACR) of 199985. These can involve both central and peripheral nervous system.
Pathogenesis of these manifestations is complex, however vasculitic processes are involved but also specific autoantibodies such as anti-neuronal, anti-ribosomal and anti-phospholipids which are directed again the nervous system86 play a role.
Patients can present cognitive deficit such as memory loss and loss of attention87. Ischemic phenomena can occur in 19% of SLE patients and are caused by traditional factors such as atherosclerosis, dyslipidemia, hypertension and also by the use of corticosteroids and the presence of anti-phospholipid antibodies. Epilepsy is also common and can be associated to psychosis. Headache is very frequent as much as peripheral neuropathy. Rarer manifestations are transversal myelitis or chorea88. Meningitis can also occur probably because of the immunosuppressive therapy.
1.2.3.9 Hematological involvement
SLE patients can present anemia, leucopenia and thrombocytopenia, Anemia is very common in this disease and can be due to inflammation, kidney failure, hemorrhages, hemolysis, infections and medullary aplasia89. In 10% of SLE patients it is possible to describe a condition of autoimmune hemolytic anemia, characterized by a positive Coombs test, increase
Leucopenia is also frequent in lupus patients. Lymphopenia in particular is often present when there is an active disease. Thrombocytopenia can be present in 50% of SLE patients. Most of the time this condition is caused by the presence of anti-platelets antibodies sometimes can be due to immunosuppressive therapy. Thrombocytopenia might also be a manifestation of anti-phospholipid syndrome. These conditions can be all present at the same time causing pancytopenia. Lymphadenopathy can be present in 50% of SLE patients and it is usually associated to asthenia, fever and hepatosplenomegaly. However, it can be also caused by infections or concomitant lymphoproliferative disorders90.
1.2.4 Classification criteria
ACR (American College of Rheumatology) proposed in 1971 the first non-diagnostic, classification criteria for SLE; these criteria were then revised in 1982 and 199791,92. According to 1997 classification criteria, a patient is affected by SLE when 4 or more criteria are satisfied not necessarily at the same time. In 2012 a new classification system was introduced by the group Systemic Lupus International Collaborating Clinics (SLICC), characterized by a broader number of criteria (from 11 to 17, distinguished in clinical and immunological criteria)93. According to this new classification, a patient can be classified as an SLE patient, when there are 4 criteria, at least one clinical and/or one immunological. Compared to the ACR criteria from 1997 SLICC criteria demonstrated more sensitivity but less specificity. Recently, new classification criteria have been published by the EULAR (European League Against Rheumatism) and the ACR. The 2019 EULAR/ACR classification criteria for SLE include positive ANA at least once as obligatory entry criterion; followed by additive weighted criteria grouped in seven clinical (constitutional, hematological,
(antiphospholipid antibodies, complement proteins, SLE-specific antibodies) domains, and weighted from 2 to 10. Patients accumulating ≥10 points are classified as affected by SLE161.
1.2.5 Clinimetric indices
Evaluation of disease activity is of crucial importance for the management of SLE patients, in order to prevent organ damage. However, currently there is not yet a gold standard index which can be preferentially used. In 1996 the Systemic Lupus International Collaborating Clinics (SLICC) pointed out the need of a more complete index which could evaluate disease activity, chronic damage and quality of life94. The most used disease activity index is the Systemic Lupus Erythematosus Activity Index (SLEDAI) first published in 1992, then revised in 2002 (SLEDAI-2K)95,96. This index includes 24 objective variables (16 clinical variables and 8 laboratory variables) present in the patient in the last 30 days. The sum of these variables corresponds to the disease activity. To evaluate also the improvement and so the response to therapy the SLEDAI-2K RESPONDER INDEX 50 (SRI-50) was proposed97,98. Moreover, the Safety of Estrogen in Lupus Erythematosus National Assessment trial (SELENA—SLEDAI)99 proposed to modify the SLEDAI introducing the concept of flare. The British Isles Lupus Assessment Group (BILAG), proposed in 1988, was revised and validated in 2005100,101. In this last version the clinical variables refer to disease activity in 9 different organs in the last 30 days; the BILAG is certainly the most complete index, and for this reason is preferentially used in clinical trials to evaluate the efficacy of new therapies for SLE patients. The European Consensus Lupus Activity Measurement (ECLAM), was first published in 1992102. This index includes 15 clinical and laboratory parameters. Since the number of SLE patients in pregnancy is increased, in 1999 several clinimetric indices to
P-DAI)103; the BILAG-2004 Pregnancy Index104 and the Lupus Activity Index in Pregnancy (LAI-P)105.
The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index (SDI) is a useful clinimetric index to evaluate chronic damage106.
1.2.6 Quality of life indices
Evaluation of quality of life in SLE patients can be measured by several indices such as LupusQoL, this is a specific index for lupus patients recently validated for Italian patients. This is a survey including 34 variables divided in 8 categories: physical health, pain, ability to make projects, intimacy, relationships, psychological health, body perception and fatigue107.
1.2.7 Therapy
In 2008 the first recommendations for the treatment of SLE patients were published by the EULAR (European League Against Rheumatism)108 and in 2010 the first recommendations for the neuropsychiatric lupus were also proposed109 and in 2012 the EULAR/ACR recommendations for lupus nephritis were also published110. In 2019, an update of the EULAR recommendations on the management of systemic lupus erythematosus has been published162.
In the clinical management of lupus patients, the therapeutic choice must be done considering the kind of organ that is involved. General symptoms such as, fever, arthralgias, weight loss
In case of SLE-related arthritis initially low dose steroids or antimalarial drugs can be used. Hydroxychloroquine and chloroquine are immunomodulatory drugs with several beneficial effects: anti-inflammatory effects, anti-thrombotic effects, anti-osteoporotic effects and also anti-viral effects, moreover they can protect against UV light and help to reduce levels of cholesterol and glycemia. In case of more severe arthritis, methotrexate, leflunomide, cyclosporine can be used112.
Skin lesions can be treated with corticosteroids and antimalarial drugs. Patients must avoid smoke and sun exposure and must use a high protection sunscreen113. Skin lesions that do not respond to antimalarial drugs and steroids can be treated with thalidomide114 or immunosuppressants such as methotrexate, azathioprine, ciclosporin, mycophenolate mofetil, dapsone or tacrolimus.
Serositis can be treated with NSAIDs or prednisone associated to antimalarial drugs; for resistant cases, methotrexate can be used. Hematological involvement, such as leucopenia, thrombocytopenia or neutropenia are usually treated with corticosteroids. Hemolytic anemia is a severe disease manifestation and must be treated with high dose corticosteroids with an immunosuppressive therapy, such as azathioprine or ciclosporin. In more severe cases high dose intravenous immunoglobulins can be used115. Lupus nephritis therapy depends on the results of kidney biopsy, which give information about the histological class and the disease activity. There are different protocols for each class of lupus nephritis, and they all include an inductive treatment and a maintenance therapy; they include high dose corticosteroids and immunosuppressant like mycophenolate mofetil, cyclophosphamide, azathioprine, cyclosporine and rituximab (as an off-label therapy). Neurolupus is treated with high dose corticosteroids and symptomatic drugs. Anticoagulants and antiaggregant drugs are used
hemorrhage or lupic pneumonia and myocarditis or pericarditis are treated with very high dose of corticosteroids in association with azathioprine or cyclophosphamide116.
In the last few years, new biological therapies have been used in SLE treatment: the two most important drugs of this group target B cells: rituximab and belimumab. Rituximab is a monoclonal antibody anti-CD20, belimumab is a monoclonal antibody which targets Blys (b-lymphocyte stimulator). Rituximab probably acts by inducing apoptosis or ADCC of CD20+ B cell, while belimumab targets Blys which is a fundamental cytokine for B cell survival. Rituximab is still an off-label therapy for SLE despite its success in refractory cases of SLE and the efficacy as a treatment of lupus nephritis; belimumab is approved as SLE therapy, and it is the first new drug to be approved for SLE after 50 years117.
1.3 KLRG1
The killer-cell lectin like receptor G1 (KLRG1) is expressed on NK cells and antigen-experienced T cells and has been proposed to be a marker of senescence and cellular differentiation, however, data indicating that KLRG1 plays an inhibitory role, have also
emerged118. In both mice and humans, KLRG1 expression is found on NK cells and
antigen-experienced T cells119. Human KLRG1 is also found on γδ T cells120 and on a large proportion
of CD4+ and CD8+ T cells found in cord blood121. In young adults, the expression of KLRG1
is about 40% on CD8+ T cells, 20% on CD4+ T cells and 60% on NK cells122. The expression
of KLRG1 rises with age, with more than 90% expression of KLRG1 being seen on CD8+ T
tyrosine-in mice, with antibody-mediated cross-ltyrosine-inktyrosine-ing of KLRG1 betyrosine-ing shown to tyrosine-inhibit cytolytic
activity and IFNγ production in NK cells124. In murine T cells, the cross-linking of TCR and
KLRG1 by platebound antibodies was shown to lower Ca2+ influx125 and to decrease IL-2
production126. Data suggesting that KLRG1 plays an inhibitory role in human NK cells and T
cells have been also published. KLRG1-mediated inhibition of NK cell function revealed that KLRG1/ligand interactions inhibit the cytolytic activity of polyclonal human NK cells by
interfering with both degranulation and IFNγ release127; however, the authors showed that the
degree of inhibition was modest and required high expression levels of KLRG1’s ligand, E-cadherin. A few studies demonstrated a role for KLRG1 as an inhibitory receptor also in T
cells128. KLRG1 exerts its inhibitory effects after binding to its ligands: E-, N- and
R-cadherin129,130. The cadherins represent a family of transmembrane glycoproteins that mediate
Ca2+ dependent cell-cell adhesion131. E-cadherin is expressed on epithelial cells and
Langerhans cells, whereas N- and R-cadherin are expressed by the nervous system. E-cadherin is also expressed on peripheral blood cells, notably on myeloid DCs, demonstrating that E-cadherin is found not only in the epithelium but on a wide range of antigen-presenting
cells suggesting a larger range of scenarios for immune control by KLRG1/cadherin
interactions. KLRG1 contains one ITIM motif in its cytoplasmic domain, which mediates its effects through the recruitment of SHIP-1 and SHP-2 phosphatases and a tyrosine residue at
position 7 in the ITIM132. KLRG1 forms both monomers and dimers, with a substantial
fraction of KLRG1 being found on the cell surface as disulfide-linked trimeric and tetrameric
complexes. It has been demonstrated that, in contrast to KLRG1-tetramers128,129 monomeric
KLRG1 shows little binding to E-cadherin-expressing cells, suggesting that KLRG1 binds to E-cadherin with relatively low affinity. (Fig.1-2)
1.4 KLRG1 and SLE
KLRG1 gene emerged as a disease susceptibility gene for Systemic Lupus Erythematosus in four different ethnic groups: European Americans, African Americans, Asian Americans and Hispanic Americans in childhood-and adult-onset SLE cases133. Because of its inhibitory functions, some authors have proposed a role in autoimmunity prevention, by increasing the activation threshold of NK and T cells.
1.5 IL1R8
IL1R8 is a member of the interleukin-1 receptor family (ILRs) which, together with the toll-like receptors (TLRs) are characterized by the presence of a conserved intracellular domain and the toll-IL-1resistance (TIR) domain. ILRs and TLRs have a major role in the immune system, in fact, they are responsible for the initiation and amplification of several events leading to inflammation and innate and adaptive immune responses. TLRs basically work as sensors for pathogens and host tissue injury, recognizing specific pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). Considering the capacity of these receptors of triggering immune responses and inflammation, the modulation of their activity is of crucial importance in both physiological and pathological conditions. This modulation acts by several and diverse mechanisms at different levels; in fact, all cell types of the innate immune system express ILRs and TLRs134. IL1R8, also known as TIR8 or SIGIRR, is a member of this family that behaves as a negative regulator of the
signaling pathway leading to signal transduction135. In addition, IL1R8 is a component of the receptor recognizing the anti-inflammatory cytokine IL-37136. IL-37 is an anti-inflammatory cytokine that acts as a natural brake of inflammation, signaling through IL-1R5/IL-18Rα and IL1R8 was recently described as a coreceptor, required for the formation of the tripartite complex IL-37–IL-1R5/IL-18Rα–IL1R8. IL1R8 is widely expressed in several epithelial tissues such as kidney, digestive tract, liver, lung, and in lymphoid organs. Among leukocytes, it is expressed by monocytes, B and T lymphocytes, dendritic cells, and NK cells137. Little is known about the regulation of IL1R8 expression and the pathways involved. IL1R8 exerts its regulatory activity by inhibiting NFκB and JNK activation induced by TIR-containing ILRs or TLRs upon ligand binding, but not by other receptors such as TNF receptors. There are some evidences about a role of IL1Rs and TLRs in autoimmunity development. IL-1 regulates the differentiation and function of Th17 cells, which are involved in inflammatory diseases such as rheumatoid arthritis (RA), multiple sclerosis, psoriasis, and inflammatory bowel disease (IBD)138, moreover Gulen et al. recently showed that IL1R8 was induced during Th17 cell polarization, differentiation, expansion, and effector functions through the direct inhibition of IL-1 signaling in T cells139. Some data have been published, showing reduction of both IL1R8 mRNA and protein in inflammatory conditions like ulcerative colitis or psoriatic arthritis140,141.
IL1R8 seems to be also involved in RA, in fact, it was shown to suppress the release of pro-inflammatory cytokines in human RA synovial cells in vitro, suggesting its involvement in the modulation of chronic inflammation in RA. In vivo experiments supported this evidence, since IL1R8-deficient mice developed a more severe disease in a collagen antibody-induced arthritis model. IL1R8 deficiency is also associated to increased susceptibility to psoriasis,
IL1R8 was shown to be involved also in sterile kidney diseases, by regulating TLR activation by DAMPs, released during ischemic cell necrosis in pathological conditions, such as lupus nephritis, post-ischemic acute renal failure, or kidney transplantation143-146.
L-1R8 is widely expressed in the brain by neurons, microglia, and astrocytes, and it was shown to be involved in the regulation of LPS responsiveness in the brain147-149 and its deficiency was associated with a massive LPS-induced inflammation in the brain.
It is largely known that chronic inflammation can increase the risk of cancer; inflammatory cells and mediators are present in the tumor tissue, and they are involved in tissue repair, remodeling, and angiogenesis. Several studies have revealed a crucial role of ILR and TLR signaling in this context, in which they seem to play a protective role in the pathogenesis of cancer-related inflammation in different murine models of colon cancer150 (Fig.3).
1.6 IL1R8 and SLE
Several evidences in both animal models and humans demonstrated a reduction in IL1R8 expression in inflammatory conditions, enlightening how this receptor is a fundamental modulator of autoimmunity151. IL1R8 knock-out lupus mice develop massive lymphoproliferations, serositis, glomerulonephritis an increased production of autoantibodies152. IL1R8 was also protective in a model of hydrocarbon oil-induced lupus, in which it modulated TLR7-mediated activation of DCs and expansion of autoreactive lymphocyte clones. IL1R8 is therefore involved in the regulation of DC and B cell activation, by preventing exacerbated autoimmune reactions, lymphoproliferation, and tissue damage in SLE144. In humans there are a few data, especially from genetic studies, which revealed a
in a large European-descent population showed no correlation between IL1R8 polymorphisms and SLE, but the analysis was restricted to a single missense SNP (rs3210908)153. However, another genetic variant of IL1R8 (rs7396562) was identified, and it was demonstrated to correlate with the susceptibility to SLE, in a Chinese population154. A recent study showed in a Chinese cohort of SLE patients a reduced frequency of IL1R8+ CD4+ T cells in the
peripheral blood of SLE patients compared with healthy subjects. Moreover, the frequency of IL1R8+ CD4+ T cells was further reduced in SLE patients with nephritis, compared with those
without nephritis155. On the contrary, another study showed an increased frequency of IL1R8+ B cells in SLE patients; in this study B cells from SLE patients displayed an upregulation of TLR7 and TLR9 compared with healthy subjects, but the response to corresponding ligands was normal or even reduced. The authors suggested that this could be explained by the enhanced IL1R8 expression in SLE B cells, even though the pathological significance of IL1R8 increase in this context is still unclear156.
Chapter 2
2.1 AIM
KLRG1 and IL1R8 are both inhibitory receptors involved in several immune functions; a few data have been published about their role in SLE, therefore, a better understanding of their function in this disease might give useful information for developing new therapeutic strategies. The principal aim of this project was to characterize KLRG1 and IL1R8 expression on NK cells, NKT cells, CD56+CD3bright cells (which mostly include gamma delta T cells), CD4+ and CD8+ T cells subpopulations (naïve, central memory, effector memory and effector) from SLE patients compared to healthy subjects. Correlations of these results with clinical data, including SLE disease activity score (SLEDAI-2K), have been also investigated. As secondary aims, functional in vitro studies about KLRG1-mediated signaling on NK cells have been performed.
Chapter 3
3.1 Materials and methods
3.1.1 Patients and controls
Eighteen patients (18F, median age 38 years IQR 17, median disease duration 7.5 years IQR 12.75) affected by SLE according to the 1997 ACR criteria, and twelve healthy subjects (12F, median age 34 years IQR 18.5) were enrolled for the evaluation of KLRG1 expression. Eighteen SLE patients (18F, median age 38 years, IQR 22.5, median disease duration 7 years,
the characterization of IL1R8. One SLE patient and two healthy subjects were enrolled for the
in vitro functional studies about KLRG1-mediated signaling on NK cells. All patients were
enrolled from Sapienza Lupus Cohort while healthy subjects were enrolled among the medical and non-medical team of the Rheumatology Unit of Sapienza University. Clinical and laboratory information were also registered at time of enrollment: disease history, physical examination, ongoing therapies, disease manifestations at the moment of enrollment, levels of ANA, anti-dsDNA, ENA, LA, aCL, anti-beta2GPI and C3 and C4. Disease activity was measured by SLEDAI-2K157. The study was performed in agreement with the declaration of Helsinki and it was approved by the Ethics Committee of Sapienza University of Rome, and informed written consent was obtained from all patients prior to enrollment.
3.1.2 PBMCs purification
Blood samples were obtained by antecubital venipuncture from lupus patients and healthy subjects
.
Peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation (Lympholyte-H; Cedarlane Laboratories, Hornby, Ontario, Canada).3.1.3 Assessment of KLRG1 and IL1R8 by flow cytometry
Cells were treated with FcR-blocking reagent and then incubated for 30 min at 4°C with CD56, CD3, CD4, CD8, CD45 RA, CD62L and KLRG1 or anti-IL1R8 antibodies. After incubation, cells with anti-anti-IL1R8 primary antibody, were washed and stained with the streptavidin for 15 minutes at 4°C. Appropriate isotype controls were used.
Immunocytometry Systems). To quantify the percentage of cells positive for KLRG1 and IL1R8 a gate was set according to the staining intensity of an isotype control and analysis was done on each population of CD56+ CD3- NK cells, on CD56+CD3+ NKT cells, CD56+CD3bright cells, and CD4+ and CD8+ T cells and their subpopulations CD45RA+ CD62L+ (naïve), CD45RA- CD62L+ (central memory), CD45RA-CD62L- (effector memory), CD45RA+CD62L- (effector). The following reagents were purchased: FcR-blocking reagent (Miltenyi Biotec), Allophycocyanin (APC)-labeled anti-KLRG1 (Biolegend), peridinchlorophyII protein complex (PerCP)-labeled anti-CD8 and anti-CD4 (Biolegend), Phycoerythrin (PE)-labeled anti-CD62L (biolegend), Phycoerythrin (PE)-labeled anti-CD56, Fluorescein isothiocyanate (FITC)-labeled anti-CD3 (biolegend) and anti-CD45 RA (BD), biotinylated anti-IL1R8 (R&D Systems), streptavidin Alexa Fluor 647 cojugate (Invitrogen Thermo Fisher Scientific), Allophycocyanin (APC)-labeled IgG2a, k isotype control (biolegend), normal goat biotinylated IgG control antibody(R&D Systems).
3.1.4 NK cells assay: CD107a mobilization and intracellular IFN-g production
Freshly isolated PBMCs (1x106 /well) were stimulated for 6 hours at 37°C by plastic-immobilized mAb, in the presence of conjugated anti-CD107a mAb.
To this end, a 24-well plate was coated overnight at 4°C with goat F(ab')2 anti-mouse IgG (H+L) (1µg/well) alone or combined with 1 µg/well of E-cadherin, and then coated for 1 hour at 37° with anti-CD16 (3G8), used at the minimum saturating dose (1 µg/106).
After the first hour, 50µM Monensin (Golgi-stop; Merck, code M5273) and 10µg/ml Brefeldin A (Merck code B7651) were added to each sample.
At the end of stimulation, cells were transferred in FACS tubes, washed in PBS, incubated for 10 min at room temperature with Cell Vital Dye (1ul/sample) and washed twice with 5% FBS containing PBS.
Before fixing (2% paraformaldehyde, Merck, code 158127), cells were stained with anti-CD56, anti-CD3 and anti-KLRG1 antibodies. Samples were then permeabilized by incubating for 30 minutes at room temperature with 0.5% saponin (Sigma-Aldrich, code S4521) in PBS supplemented with 1% FCS and then stained with anti-IFN-g mAb.
CD107a expression levels and intracellular IFN-g production were simultaneously analyzed in KLRG1-expressing NK cells by FACSCanto II (BD) and data were obtained with FlowJo v9.3.3 (Treestar) software; a total of 50.000 events per sample were recorded (Fig.4).
The following reagents were used: AffiniPure F(ab’)2 fragment goat anti-mouse IgG (Jackson laboratories immunoresearch), leaf purified anti-CD16 (clone 3G8) (Biolegend), Brefeldin A (Merck), Monensin solution (Merck), Fixable viability stain 780 (BD HorizonTM), Phycoerythrin- Cyanin 7 (PECy7)-labeled CD56 (biolegend), peridinchlorophyII protein complex (PerCP)-labeled anti-CD3 (biolegend), Allophycocyanin (APC)-labeled anti-KLRG1 (biolegend), Fluorescein isothiocyanate (FITC)-labeled anti-CD107a (biolegend), Phycoerythrin (PE)-labeled anti-IFNg (biolegend), Saponin Quillaja sp (Sigma Life Science), recombinant human E-cadherin (R&D Systems).
3.1.5 PBMCs in vitro treatment by hydroxychloroquine
Three lupus patients with an active disease (SLEDAI-2K=12±4.3 mean±SD) who were not taking HCQ, were enrolled for the in vitro study about hydroxychloroquine influence on KLRG1 expression. PBMCs were purified and treated with two different concentrations of