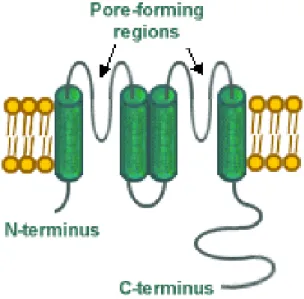
1. 1 Potassium channels: general properties
Potassium channels are a diverse and ubiquitous family of membrane proteins present in both excitable and non excitable cells. Members of this channel family play a critical roles in cellular signaling processes regulating neurotrasmitter release, heart rate, insulin secretion, neuronal excitability, epithelial electrolyte transport, smooth muscle contraction, and cell volume regulation and cell proliferation. A hightly diverse set of K+ channels has
evolved in order to serve such a wide variety of roles (Trauner, 2003).
They are membrane-spanning proteins that selectively conduct K+ ions across
the cell membrane proteins along its electrochemical gradient at a rate of 106
to 108 ions/s. In the resting state, the concentration of K+ ions outside the cell
membrane is some 25-fold lower than the concentration in the intracellulaar fluid, and consequently, an outward current due to the efflux of positively charged ions is generated by the opening of K+ channels. This efflux of K+ is a
mechanism that permits recovery (repolarization) and/or lowering (hyperpolarization) of the resting potential of the cells. Thus, opening of K+
channels offers a mechanism to contract, dampen, or restrict depolarizing activity triggered by influx of cations (Na+ and Ca++) or efflux of anions (Cl-).
Activators of K+ channels tend to dampen or stabilize cellular excitability or
lower the effectiveness of excitatory inputs whereas blockers of K+ channels
have the opposite effect. In addition to controlling cellular excitability, K+
channels are also critical to fluid and electrolyte transport and cell proliferation (Shieh, 2000).
Basically, ion channels are membrane protein complexes composed of a set of salient features: 1) a water filled permeation pathway (pore) that allows K+ions to flow across the cell membrane; 2) a cavity and internal pore (filter)
that specifies K+ permeant ion species; and 3) a gating mechanism (sensor)
that serves to switch between open and closed channel conformation ( Fig. 1) (Hille, 2001).
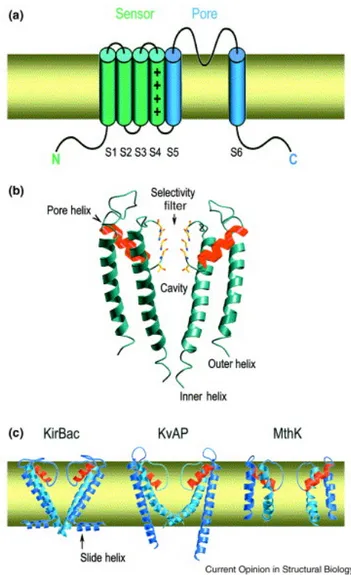
Figure 1.K+ channel architecture. (a) Most K+ channels have six membrane-spanning elements in each subunit. All have at least two, corresponding to the S5–S6 region. The ion conduction pathway forms at the centre of a symmetric tetramer of these subunits. (b) Two diametrically opposed subunits of KcsA are depicted to show the cavity in the membrane. (c) Conformational plasticity of the pore. Two subunits of each of three K+ channel pores are shown. PDB codes: KirBac, 1P7B; KvAP, 1ORQ; MthK, 1LNQ.
The pore is responsible for the transit of the ion. The open pore has the important property of selective permeability, allowing some restricted class of small ions to flow passively down their electrochemical activity gradients at a rate that is very high (> 106 ions per second).
The ion conducting (or pore containing) subunit is generally referred to as the principal or α-subunit. In some cases, the auxiliary subunits (for example, β-subunits) coassemble with the α-subunit serves as the principal binding site for potassium channel openers and blockers although there are clear exceptions where ligad-binding sites resides within an auxiliary subunit. The overall length of the pore is 45 Å, and its diameter varies along its distance. From inside the cell (bottom) the pore begins as a tunnel 18 Å in length (the internal pore) and then opens into a wide cavity (10 Å across) near the middle of the membrane. A K+ ion could move throughout the internal
pore and cavity and still remain mostly hydrated. In contrast, the selectivity filter, separating the cavity from the extracellular solution, is so narrow that a K+ ion would have to shed its hydrating waters to enter (fig.2A and B). The
chemical composition of the wall lining the internal pore and cavity is predominantly hydrophobic (Fig. 2A, yellow). The selectivity filter, on the other hand, is lined exclusively by polar main chain atoms belonging to the signature sequence amino acids. The distinct mechanisms operating in the cavity and internal pore versus the selectivity filter will be discussed below, but first we introduce the determination of K+ ion positions in the pore.
Potassium channels exclude the smaller alkali metal cations Li+ (radius 0.60
Å) and Na+ (0.95 Å) but allow permeation of the larger members of the series
Rb+ (1.48 Å) and Cs+ (1.69 Å). In fact, Rb+ is nearly a perfect K+ (1.33 Å)
analog because its size and permeability characteristics are very similar to those of K+. Because they are more electron dense than K+, Rb+ and Cs+ allow
visualization of the locations of permeant ions in the pore. By difference electron density maps calculated with data from crystals transferred into Rb+
-containing (Fig. 3a) or Cs+-containing (Fig. 3b) solutions, multiple ions are
well defined in the pore. The selectivity filter contains two ions (inner and outer ions) located at opposite ends, about 7.5 Å apart (center to center). In the Rb+ difference map, their actually are two partially separated peaks at the inner aspect of the selectivity filter. These peaks are too close to each other (2.6 Å) to represent two simultaneously occupied ion binding sites. Probably they represent a single ion (on average) in rapid equilibrium between adjacent sites. The single inner ion peak in the Cs+ difference map undoubtedly reflects
the lower resolution which the map was calculated (to 5 Å for Cs+ versus 4.0
Å for Rb+), because the Rb+ difference map, when calculated at the same
lower resolution, also shows only a single peak at the Cs+ position. The Rb+
native electron density map (not shown). Thus, the selectivity filter contains two K+ ions.
A third weaker peak is located below the selectivity filter at the center of the large cavity in the Rb+ difference map (Fig. 3a, cavity ion) and in the Cs+
difference map at a lower contour (not shown). Electron density at the cavity center is prominent in MIR maps, even prior to averaging (Fig. 3c, lower diffuse peak). The difference electro density maps show this to be related to the presence of one or more poorly localized cations situated at least 4 Å away from the closest protein groups.
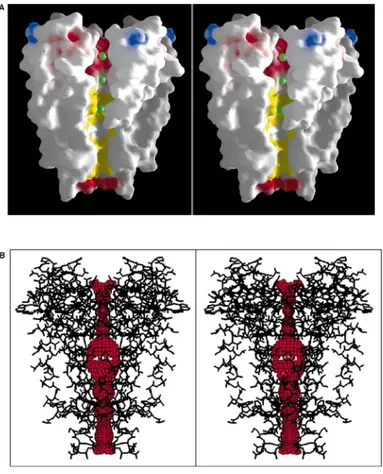
Fig. 2.(A) A cutaway stereoview displaying the solvent-accessible surface of the K1 channel
colored according to physical properties. Electrostatic potential was calculated with the program GRASP, assuming an ionic strength equivalent to 150 mM KCl and dielectric constants of 2 and 80 for protein and solvent, respectively. Side chains of Lys, Arg, Glu, and Asp residues were assigned single positive or negative charges as appropriate, and the surface coloration varies smoothly from blue in areas of high positive charge through white according to carbon atoms of the hydrophobic (or partly so) side chains of several semi-conserved residues in the inner vestibule ( Thr75, Ile100, Phe103,Thr107, Ala108, Ala111, Val115). The green CPK spheres represent K1 ion positions in the conduction pathway. (B) Stereoview of the entire internal pore. Within a stick model of the channel structure is a three-dimensional representation of the
minimum radial distance from the center of the channel pore to the nearest van der Waals protein contact.
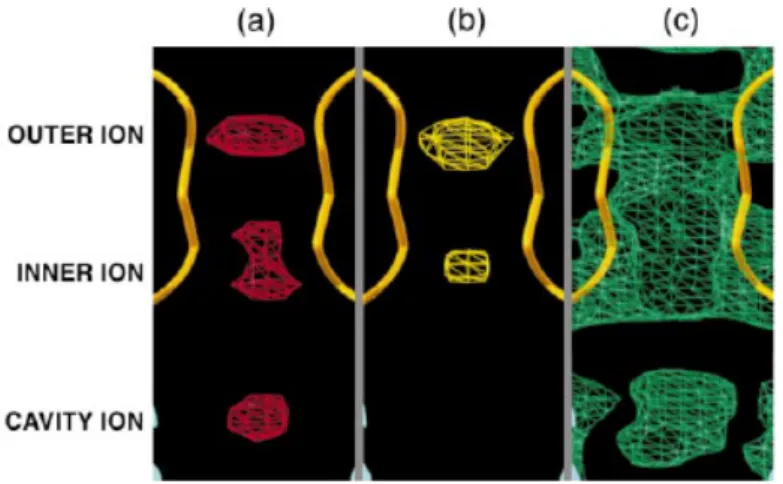
Fig. 3. Identification of permeant ion positions in the pore. (a) A Rb1 difference Fourier map calculated to
4.0 Å and contoured at 6 s identifies two strong peaks corresponding to ions in the selectivity filter (inner and outer ions) and a weaker peak corresponding to ions in the cavity (cavity ion). The inner ion density has two closely spaced peaks. (b) A Cs1 difference Fourier
map calculated to 5.0 Å and contoured at 6 s shows the inner and outer ion peaks in the selectivity filter. Both difference Fourier maps were calculated with Fourier coefficients: F(soak) – F(native-unsharpened) and MIR phases. (c) Electron density map contoured at 1 s showing diffuse density at the cavity ion position. This map was calculated with the following Fourier coefficients: unsharpened native amplitudes and MIR solvent-flattened phases (no averaging information was included).
The Filter Why is there a 10 Å diameter cavity in the center of the channel with an ion in it? (Fig. 2B and Fig. 3). Electrostatic calculations show that when an ion is moved along a narrow pore through a membrane, it must cross an energy barrier that is maximum at the membrane center (Parsegian et al, 1975 ). The electrostatic field emanating from a cation polarizes its environment, bringing the negative ends of dipoles closer to it and thereby stabilizing it. At the bilayer center, the polarizability of the surrounding medium is minimal and therefore the energy of the cation is highest. Thus, simple electrostatic considerations allow us to understand the functional significance of the cavity and its strategic location. The cavity overcomes the electrostatic destabilization resulting from the low dielectric bilayer by simply surrounding an ion with polarizable water.
A second feature of the K+ channel structure also stabilizes a cation at the
bilayer center. The four pore helices point directly at the center of the cavity (Fig.4, A, B, and D). The amino to carboxyl orientation of these helices will impose a negative electrostatic (cation attractive) potential via the helix dipole effect (Sali et al, 1988; Aquist et al, 1991; Lockhart et al 1992). The ends of the helices are rather far (8 Å) from the cavity center, but all four contribute to the effect.
Therefore, two properties of the structure, the aqueous cavity and the oriented helices, help to solve a fundamental physical problem in biology: how to lower the electrostatic barrier facing a cation crossing a lipid bilayer.
Thus, the diffuse electron density in the cavity center (Fig. 3C) likely reflects a hydrated cation cloud rather than an ion binding site (Fig. 5). Alternatively, the channel could have overcome the destabilizing electrostatic effects of the bilayer center by lining the entire pore with a polarizable surface, putting ion binding sites along its entire length. But the structure shows that, with the exception of the selectivity filter, the pore lining is mainly hydrophobic, a general property of K+ channels . This conclusion was anticipated by the
landmark experiments of Armstrong, which showed that hydrophobic cations bind in the pore of K+ channels (Armstrong et al, 1966).
The structure of the selectivity filter exhibits two essential features. First, the main chain atoms create a stack of sequential oxygen rings and thus afford numerous closely spaced sites of suitable dimensions for coordinating a dehydrated K+ ion. The K+ ion thus has only a very small distance to diffuse from one site to the next within the selectivity filter. The second important structural feature of the selectivity filter is the protein packing around it. The Val and Tyr side chains from the V-G-Y-G sequence point away from the pore and make specific interactions with amino acids from the tilted pore helix. Together with the pore helix Trp residues, the four Tyr side chains form a massive sheet of aromatic amino acids, twelve in total, that is positioned like a cuff around the selectivity filter (Fig. 6)
The hydrogen bonding, for example between the Tyr hydroxyls and Trp nitrogens, and the extensive van der Waals contacts within the sheet, offer the immediate impression that this structure behaves like a layer of springs stretched radially outward to hold the pore open at its proper diameter ion with its smaller radius. We propose that a K+ ion fits in the filter precisely so
that the energetic costs and gains are well balanced. The structure of the selectivity filter with its molecular springs holding it open prevents the carbonyl oxygen atoms from approaching close enough to compensate for the cost of dehydration of a Na+ ion.
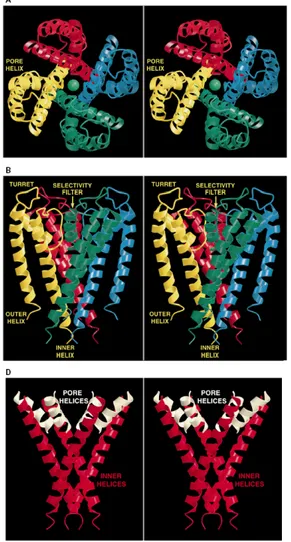
Fig. 4.(A) Stereoview of a ribbon representation illustrating the three-dimensional fold of the KcsA
tetramer viewed from the extracellular side. The four subunits are distinguished by color. (B) Stereoview from another perspective, perpendicular to that in (A). (C) Ribbon representation of the tetramer as an integral membrane protein. Aromatic amino acids on the membrane-facing surface are displayed in black. (D) Inverted teepee architecture of the tetramer.
Fig.5 Two mechanisms by which the K1 channel stabilizes a cation in the middle of the membrane. First, a large aqueous cavity stabilizes an ion (green) in the otherwise hydrophobic membrane interior. Second, oriented helices point their partial negative charge (carboxyl end, red) towards the cavity where a cation is located.
Fig. 6 A section of the model perpendicular to the pore at the level of the selectivity filter and viewed from the cytoplasm. The view highlights the network of aromatic amino acids surrounding the selectivity filter. Tyrosine-78 from the selectivity filter (Y78) interacts through hydrogen bonding and van der Waals contacts with two Trp residues (W67, W68) from the pore helix.
The gate Another part of the channel is the gate. Only when the gate is open the ions can be recognized by the selectivity filter and pass through the channel. Gating requires a conformational change of the pore that moves a gate into and out of an occluding position.
The probabilities of opening and closing of the gate depend on a variety of stimuli, which are controlled by a sensor. Each channel may be so regarded as an excitable molecule, which is specifically responsive to some stimuli; a membrane potential change, a neurotrasmitter or other chemical stimuli, a mechanical deformation and so on.
When open, the channel forms a water-filled pore extending fully across the membrane. The pore is much wider than an ion over most of its length and may narrow to atomic dimensions only in a short stretch, the selectivity filter, where the ionic selectivity is established. Channels are named by what ion(s) they allow to pass: calcium channels, potassium channels, sodium channels, chloride channels, etc.
Regarding the mechanism of activation, ion channels can be even classified according to which chemical or physical modulator controls their gating activity. In fact, besides genetic criteria, a functional classification into superfamilies is also possible (voltage-gated, ligand-gated channels), and even the channel names themselves often include an indication of what controls the gate, i.e. voltage-gated potassium channels greatly diverge in their structural, physiological, pharmacological properties (MacKinnon, 2003).
1.2 Potassium channels: classification
Since the first postulation of a selective K permeability in excitable cells by Julius Bernstein in 1902 , (Bernstein, 1902)., much has been learned about the
nature, diversity, architecture, and function of K+channels. Over 50 human genes enconding various K+ channels have been cloned in the
past and precise biophysical properties, subunit stoichiometry, channel assembly and modulation by second messenger and ligands have been addressed to a large extent. Recently, the crystal structure of a K+ channel from Streptomyces lividans has become available (Doyle et al. 1998).
The amino acid sequences of potassium channels are very easy to recognize because they contain a highly conserved segment called the potassium channel signature sequence (Heiginbotham et al , 1994). It has been suggested that this sequence motif, conserved across all potassium channels, corresponds to the selectivity filter of the pore-forming of the channel protein which allows potassium ions. Since the first gene enconding a potassium channel was cloned from Drosophila Shaker mutant (Papazian et al,1987), more than 200 genes enconding a variety of potassium channels have been identified, all containing the tripeptide sequences Gly-Tyr(Phe)-Gly, which
constituted the signature motif for determining potassium ion selectivity (Shieh et al , 2000).
The ionic channels are generally classified, on the basis to their ionic selectivity, to their conductance, to the mechanisms of regulation (activation/inactivation), to the characteristics of opening, to the kinetics or to the their pharmacology properties (Lawson et al1996, Calderone, 2002).
However, the adopted standard nomenclature for the potassium channels in the last years on phylogenetic relationships is based. The channels characterized at least by 65% of sequence identity were assigned to the same subfamily.
A parallel nomenclature—KCN—was developed by the Human Genome Organisation (HUGO) (White et al,1997). Since then, the K+ channel
superfamily of genes has greatly expanded, requiring an update of the naming system that considers more parameters and in this section will considered.
1.3 The Standardized K+ Channel Nomenclature
K+ channels are classified on the basis of primary amino acid sequence of the
pore-containing unit (α-subnit) into three major families.: 1) voltage-gated potassium channels (Kv) containing six transmembrane regions (S1-S6) with a single pore. 2) inward rectifiers (Kir) containing only two transmembrane regions and a single pore (Fig. 7a-d and Fig 3) two-pore tandem K+ channels
containing four transmembranes with two pores (Fig. 8a-d), (Coghlan et al, 2001).
Fig. 7. Schematic representation of the structure of voltage-gated (Kv), small and large conductance calcium-activated K+ channels (SK and BK, respectively). (A) The structure of the
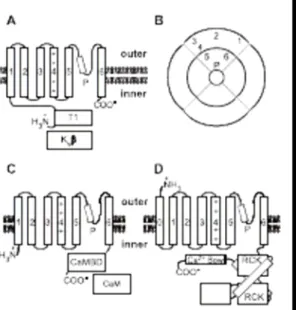
Kv channel a subunit contains six transmembrane domains (1–6), a pore-forming loop (P) and an N termini intracellular domain (T1), which permits interaction with the h subunits (Kvh). (B) Schematic quaternary structure of the Kv channels: The channel is a tetramer of a subunits, each α subunit has α pore-forming motif constituted by the P-loop and transmembrane domains 5 and 6. (C) The structure of the α subunit of SK channels is similar to that of the Kv channels; however, the fourth transmembrane domain is less charged and the C-terminal intracellular domain contains a calmodulinbinding domain (CaMBD), which permits interaction with calmodulin (CaM) and its regulation by calcium. The quaternary structure of SK channels is similar to that of the Kv channels illustrated in B. (D) The BK a subunits contain seven transmembrane domains (0–6), a P-loop and a complex C termini intracellular domain that contains two RCK (regulated conduction of K+) domains and a Ca2+ bowl that permits regulation of the BK channel by Ca2+. The quaternary structure of the BK channels is similar to that shown in B.
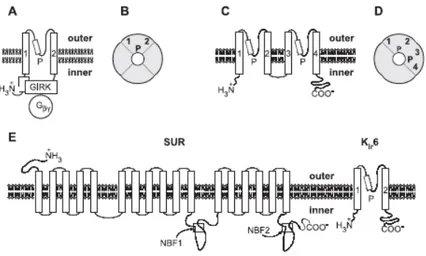
Fig. 8. Schematic representation of the structure of inward rectifier (Kir) and two-pore (K2P) K channels. (A) The structure of the G-protein-regulated inwardly rectifying K+ channel (GIRK or Kir3 channel) comprises only two transmembrane domains and a P-loop. The N and C termini are intracellular and contain domains that interact with the Ghg subunits of G-proteins, which regulate channel opening. (B) Schematic quaternary structure of the GIRK channels: The channel is a tetramer of a subunits (equivalent to that shown in grey in Fig. 7B). (C) The K2P channel α subunits are constituted by four transmembrane domains and two P-loops, with intracellular N and C termini. (D) The quaternary structure of K2P channels consists of a dimer of a subunits. (E) Structure of the KATP channels: The functional channel is constituted by a Kir6 channel and a SUR (SUlfonylurea Receptor) subunit. The Kir6 channel has two transmembrane domains, a P-loop and intracellular N and C termini. The N termini of the Kir6 channel interact with the C termini of the SUR subunit. The SUR subunit has 17 transmembrane domains and two nucleotide binding folds (NBF). The SUR subunit permits the regulation of the channel by G-proteins and several KATP channel openers and blockers. The quaternary structure of the KATP channel consists of a tetramer of Kir6-SUR complexes. The four Kir6 subunits form a pore similar to that shown in B, which is surrounded by four SUR subunits.
1.4 Voltage-gated K channels
The voltage-dependent channel KvAP (Jiang et al 2003) has six transmembrane helical segments, organised into two structural domains (pore and voltage sensor), in each monomer (Fig. 8). The structure of the integral membrane assembly was crystallographically determined to moderate resolution and was accompanied by a high-resolution structure of the isolated voltage sensor. To improve diffraction, each protein was crystallised in complex with an antibody to the S3–S4 loop. It is enlightening to compare the sequence-identical regions of the two KvAP structures. Despite similar secondary structures, the two proteins do not superimpose. To understand why, it may help to look at K+ channels as being formed by a series of lipophilic α-helices separated by more polar sequences amenable to forming sharp turns or structured loops. Some of these separating sequences have the propensity to form helices as well as turns. In the lipid bilayer, many of these segments are functionally important and reside at the membrane–solution interface. Once extracted into micelles, however, any functional role is unimportant, and whether and where helix disruption occurs is dependent on the local environment of the protein and will be strongly influenced by the need to shield exposed hydrophobic residues. In the two KvAP structures, the helical periodicity is disrupted such that turns occur at different relative points in the linear sequence. The isolated voltage sensor is relatively compact, but its counterpart in the channel assemblage is convoluted and makes extensive interactions with the antibody. The KvAP structure, although providing a remarkable first view of a voltage-gated channel, has proved controversial, conflicting with a swathe of biophysical data. Although antibody binding clearly compromises the structure, simple removal of the channel from the membrane also contributes to the confusion. Correct juxtaposition of the pore and sensor domains requires a planar lipid environment. The S4–S5 hinge that connects them is susceptible to angular distortion, particularly if the two domains are incorporated into separate micelles. Because of these complicating factors, it is not possible to draw any firm conclusions about the relationship between the pore and the voltage sensor from the KvAP structure. (Coghlan et al. 2001).
1.5 Two transmembrane one-pore channels
The inward rectifier K channels (Kirs) belong to a distant superfamily of channels with four subunits each containing a two-transmembrane segment (M1 and M2) and a pore loop in between (Fig. 9), (Ho et al. 1993, Kubo et al. 1993). These channels conduct K+ currents more in the inward direction than
This inward rectification is attributed to gating mechanism by internal Mg2+
and polyamines (spermine, spermidine, etc.) that occlude access of K+ to the
internal vestibule of a conducting pore (Matsuda,1991;Ficker et al.,1994; Lu and McKinnon,1994; Wible et al. 1994; Shieh et al. 2000). Like the voltage-gated K+ channels, these channels are organised as tetramers (Yang et al.
1995), although a more complex octameric arrangement has been described, as in the case of ATP-sensitive K+ channels involving four inward rectifiers
contributing to ion conducting pore and four peripheral sulfonylurea receptors as regulatory subunits (Clement et al, 1997; Inagaki et al. 1997; Shyng and Nichols,1997).
Fig. 9 Schematic representation of the structure of a two transmembrane one-pore K+ channel. 1.6 Four transmembrane two-pore channels
The more recently discovered tandem-pore domain family are weak inward rectifiers with four putative transmembrane domains and two-pore domains (Fig. 10), (Ketchum et al 1995; Lesage et al. 1996a). They represent perhaps the most abundant class of K+ channels (at least in C. elegans), with >50
distinct members (Wang et al. 1999). The G(Y/F)G residues of K-selective motif is preserved in the first pore loop of the two-pore channel, but it is replaced by GFG or GLG in the second pore loop.
Although all the two-pore channels have a conserved core region between transmembrane segments M1 and M4, the amino and carboxyl-terminal domains are quite diverse. With two-pore domain subunits, two such subunits would presumably form a channel to retain the tetrameric arrangement
Fig 10. Schematic representation of the structure of a two-pore K+ channel.
1.7 Human Gene Nomenclature Committee System
The KCN system established by the Human Gene Nomenclature Committee (HGNC) of HUGO (Table 1) suffers from a lack of any rational basis for nomenclature and, in particular, ignores the structural and phylogenetic relationships of these proteins. For example, the KCNA–KCND subfamilies refer to 6TM/1P an unrelated protein that functions as an accessory subunit to some families of K+channels. Subfamilies encoding other 6TM/1P proteins
include KCNF, KCNG, KCNH, KCNN, KCNQ, and KCNS, whereas KCNJ contains the 2TM/1P proteins, the KCNK subfamily corresponds to the 4TM/2P proteins, and the 7TM/1P is subfamily KCNMA1. Even though the Eag, Erg, and Elk channels are very different in their sequences, they are grouped together as KCNH; several members of this group, namely Elk1, Erg2, and Erg3, have not yet been assigned HGNC names. Similarly, the small-conductance (SKCa1–SKCa3) and intermediate- conductance (IKCa1) Ca2+activated K+ channels are grouped together in the KCNN subfamily even
though they share only 45% amino acid sequence identity and therefore are best considered as belonging to distinct subfamilies (Gutman et al. 2003).
Table 1
New nomenclature system introduced by the International Union of Pharmacology
With the completion of the mapping of the human genome, the time may be ripe for a re-evaluation of these issues, and the development of a uniform and rational nomenclature for all K+-selective channels. Such a naming system
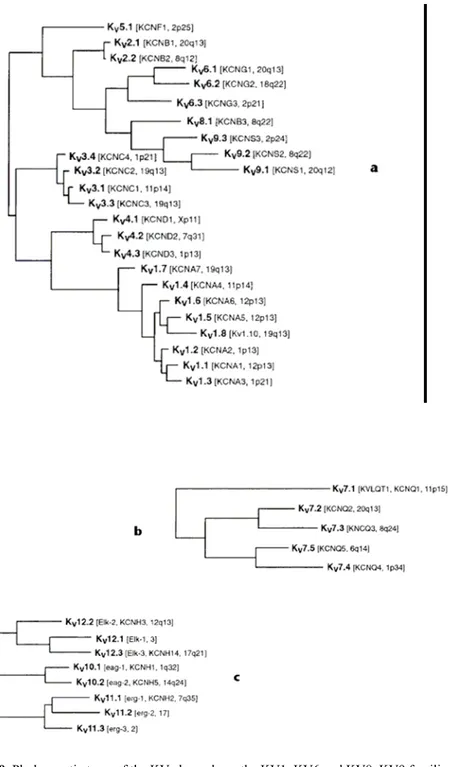
could incorporate the architectural similarities between different channel families, as well as their phylogenetic relationships. For example, the numerous 6TM/1P proteins and the sole 7TM/1P channel listed in Table 1 could be grouped into a single large family comprising multiple subfamilies defined according to their phylogenetic relatedness. (Gutman et al. 2003). The 6TM/1P channels have been organized into two distinct groups based on their structural relatedness and predominant functional characteristics, namely the voltage-gated (KV) and calcium-activated (KCa) channels. The two-pore (K2P) and inward rectifier (Kir) channels likewise form two additional groups. Figure 1a shows the voltage-gated K+ channels of families KV1–KV6
and KV8–KV9, in a phylogenetic reconstruction using maximum parsimony based on an amino acid sequence alignment. Among this group, only KV1.8 currently lacks an HGNC name.
FIG. 9. Phylogenetic trees of the KV channels. a, the KV1–KV6 and KV8–KV9 families; b, the KV7 family; c, the KV10–KV12 families. Only the hydrophobic core region of the alignment was used for analysis.
FIG. 10 Two phylogenetic trees showing the KCa1–KCa5 families
FIG. 11 Phylogenetic tree showing the K2P families.
1.8 Concluding remarks
K+ channels play a critical role in a great variety of physiological processes.
As such, they have been recognised as potential and interesting therapeutic drug targets.
In the last years, the human genome project, together with an intense cloning effort, has identified more than 200 K channel-related genes.
The most impressive diversification has occurred among voltage-dependent potassium channels. Most open only after the membrane depolarised. Some open rapidly, other slowly. Often these channels could be modulated by neurotrasmitters or intracellular messengers. Each excitable membrane uses a different mix of these several potassium channels.
References
Trauner, D. (2003). Angew. Chem. Int. Ed. Engl. 42, 5671-5.
Shieh, C., Coghlan, M., Sullivan, J. P., Gopalakrishnan, M. (2000).
Pharmacol. Rev. 52, 557-94.
Hille, B. (2001). In: Ion Channels of Excitable Membranes, 3th Edn., Sinauer Associates, Sunderland, MA.
Parsegian V. A , (1975) Ann. N.Y. Acad. Sci. 264, 161 Sali D., Bycroft M. and Fersht A R, (1988) Nature 335, 740
Aqvist J., Luecke H., Quiocho F. A., Warshel A., (1991) Proc. Natl.
Acad. Sci. U.S.A. 88, 2026
Armstrong C. M. and Binstock L. (1965). 48, 859 Armstrong C. M., (1966) J. Gen. Physiol 50, 491 MacKinnon, R. (2003). FEBS Letters 555, 62-65.
Papazian, D. M., Timpe, L. C., Ian, Y. N., Jan, L. Y. (1991). Nature 349, 305-10.
Bernstein, J. (1902). Pflugers Arch., 92, 521-94
Doyle, D. A., Morais Cabral, J., Pfuetzner, R. A., Kuo, A., Gulbis, J. M., Cohen, S. L., Chait, B. T.,
Mackinnon, R. (1998). Science 280, 69-77.
Heginbotham L., Lu Z., Abramson T and MacKinnon R. (1994).
Biophys. J. 66, 1061-67.
Calderone V. (2002) Curr. Med. Chem. 9, 1385-95
Papazian, D. M., Schwarz, T. L., Tempel, B. L.; Jan, Y. N.; Jan, L. (1987) Y. Science 237, 749-53.
Coghlan M. j., Carroll W. A., Gopalakrishnan M. (2001) J. Med.
Chem. 44, 1627-53.
MacKinnon R. (1991) Nature, 350, 232-35. MacKinnon R. (1995) Neuron 14, 889-92.
Jan L.Y. and Jan Y.N. (1997) Ann. Rev. Neurosci. 20, 91-123. MacKinnon R.and Miller C. (1988) J. Gen. Physiol. 91, 335-49. MacKinnon R., Heginbotham L. and Abramson T. (1990) Neuron. 5,
767-71.
Yellen G., jurman ME., Abramson T. and MacKinnon R. (1991)
Science 251, 939-42.
Goldstein SA., Pheasant DJ. And Miller C. (1993) Neuron 12, 1377-88.
Pascual JM., Shieh CC., Kirsch GE. And Brown AM. (1995) Biophys.
J. 69, 428-34.
Choi Kl., Mossman C., Aube J. and Yellen G. (1993) Neuron 10, 533-41.
Lopez GA., Jan YN. and Jan LY. (1994) Nature 367, 179-82. Shieh CC.and Kirsch, GE. (1994) Biophys. J. 67, 2316-25.
Yeola Sw, Rich TC., Uebele VN, Tamkun MM. and Snyders DJ. (1996) Circ. Res. 78, 1105-14.
Isacoff EY., Jan YN and Jan LY. (1991) Nature 353, 86-90.
Perozo E., Santacruz-Toloza L., Stefani E., Bezanilla F. and Papazian DM. (1994) Biophys. J. 66, 345-54.
Papazian DM., Timpe LC., Jan YN and Jan Ly. (1995) Neuron 349, 305-10.
Seoh SA., Sigg D., Papazian DM. And Benzanilla F (1996) Neuron 16, 1159-67.
Reimann, Curr. Top. Membr. 1999, 46, 1-5.; Nichols Annu. Rev. Physiol. 1997, 59, 171-191.
Liu Y., Holmgren M., Jurman ME. And Yellen G. (1997) Neuron 19, 175-84.
Kanevsky M. and Aldrich RW. (1999) J. Gen. Physiol. 114, 215-42. Ho K., Nichols CG., Lederer WJ., Lytton J., Vassilev PM.,
Kanazirska MV. And Hebert SC. (1993) Nature 362, 31-8
Kubo Y., Reuveny E., Slesinger PA., Jan YN and Jan LY. (1993)
Nature 364, 802-6.
Matsuda H. (1991) Ann. Rev. Physiol. 53, 289-98.
Ficker E., Taglialatela M., Wible BA., Henley CM. and Brown AM. (1994) Science 266, 1068-72.
Lu Z. and McKinnon R.(1994) Nature 371, 243-46.
Wible BA., Taglialatela M., Ficker E. and Brown AM. (1994) Nature 371, 246-49.
Clement AJ. Kunjilwar K. Gonzalez G., Schwanstecher M., Panten U., Aguilar-Bryan L. and Bryan J. (1997) Neuron 18, 827-38.
Inagaki N., Gonoi T and Seino S (1997) FEBS Lett. 409, 232-236. Shyng S.and Nichols CG. 1997) J. Gen. Physiol. 110, 655-64.
Ketchum KA., Joiner WJ., Sellers AJ., Kaczmarek LK. And Goldstein SA.(1995) Nature 376, 690-95.
Wang ZW., Kunkel MT., Wei A., Butler A. and Salkoff L. (1999)
Ann. NY Acad. Sci. 868, 286-303.
Jiang Y., Lee A., Chen J., Ruta V.,. Cadene M,. Chait B.T and. MacKinnon R, 2003 Nature 423, 33–41
Gutman G. A. Chandy K. G., Adelman J., Clapham D., Covarrubias M., Desir G. V., Furichi K.,, Ganetzky B., Garcia M.L., Grissmer S., MacKinnon D., Nichols C. G., O’Kelly I., Robbins J., Robertson G., Rudy B., Vandenberg c.a., Wei A., Wulff H., and Wymore R. S. (2003) Pharmacological Review 55, 583-6.
2.1 Potassium channels: from the Crystal Structure to a Nobel Prize
Since the end of the 1990s, Roderick MacKinnon and co-workers (TheRockefeller University, New York) have shed light on most of the potassium channels functional properties by analyzing a series of progressively role complex and more informative X-ray crystal structures, and have thus essentially elucidated how potassium channels work.
These seminal studies were recently rewarded with the Nobel Prize in Chemistry (only five years after the first crystal structure appeared in the literature!)
The Nobel Prize in Chemistry 2003, awarded for “Discoveries concerning channels in cell membranes”, was shared equally between MacKinnon and Peter Agre (Johns Hopkins University, Baltimore, USA). Agre identified the long-sought-after water channels in 1988.
2.2 The first crystal structure
Water channels, also known as aquaporins, pass water molecules across lipid membranes while maintaining the pH gradient not a simple task when one considers that protons can be transferred along water networks by “proton hopping”. Aquaporins have little in common with potassium channels in terms of structure or function, but are of equal physiological importance. The first X-ray crystal structure to emerge from the MacKinnon laboratory was of the relatively simple bacterial potassium channel KcsA, with a resolution of 3.2 Å (Figure 1)( Doyle et al., 1998).
Its disclosure made a big splash, as at that time nobody had thought the crystallization and structural elucidation of ion channels possible. KcsA contains only two transmembrane helices per subunit (Figure 1b), in contrast to more complex eukaryotic potassium channels, which have been traditionally assigned six. As shown in Figure 1a, four subunits form the central pore, which consists of a narrow region known as the selectivity filter and a water-filled wider inner cavity. This tetrameric quaternary structure is a general feature of potassium channels. The selectivity filter is lined with the carbonyl groups of the peptide backbone, the amino acid residues of which are given by the highly conserved TXXTXGYG signature sequence.
A second, higher-resolution structure of KcsA (2.0 Å) resolved some of the remaining questions and doubts and provided further insight into how the potassium ions pass through the selectivity filter (Zou et al. 2001). Furthermore, it gave clues as to how the ions are stabilized in the middle of the membrane. The new electron-density map even showed a potassium ion residing in the central
cavity of the channel, complete with its inner hydration sphere, an array of eight water molecules in a square antiprismatic orientation around the ion (Figure 1b). A remarkable feature of these studies was the cocrystallization of
antibodies with the channel protein to stabilize the crystal lattice. This strategy has also been used in subsequent structure determinations.
With the next X-ray crystal structure disclosed by the MacKinnon research group, they were able to explain the basic gating mechanism of potassium channels (a,b Jang et al.2002 ).
The MthK channel, identified in the genome of Methanobacterium
thermoautotrophicum, was expressed in the presence of Ca2+ ions and was
crystallized in its open form. The X-ray crystal structure was solved at a resolution of 3.3 Å (Figure 2).
MthK is a ligand-gated channel that opens and closes in response to intracellular changes in Ca2+ concentration. The intracellular
calcium-sensitive domain is connected to the C-terminal end of the transmembrane domain, which forms the actual channel. In the tetrameric state interact to form a “gating ring”, which opens up like the shutter of a camera when calcium ions bind to it. From the X-ray crystal structure and concomitant sequence-alignment studies, MacKinnon and co-workers identified a highly conserved glycine residue situated in the middle of the S6 helix, the transmembrane helix closest to the pore. According to MacKinnon’s gating model, this residue acts like a hinge. Upon gating, the lower section of the inner helix appears to bend backwards at the glycine hinge, away from the central axis of the channel, creating an opening on the intracellular side of the channel. In the closed state, the four S6 helices of the subunits form a tight bundle, which has been likened to an “inverted tepee”. Thus, the structure of KcsA (Figure 1) represent the closed state.
In MthK the shutterlike expansion of the gating ring is mechanically coupled to the bending movement of the four inner helices. Unfortunately, the amino acid residues linking the gating ring with the transmembrane domain of the channel are disordered in the crystal structure. Some of the details of the gating mechanism have therefore not been fully clarified. However, the gating mechanism proposed for MthK appears to be general for potassium channels, including voltage-gated channels (see below). Flexible domains of a channel can be allosterically coupled to the bending of the lower part of the S6 helix and can thus effect the opening or closing of the gate.
MacKinnon and co-workers recently disclosed the structure of a complete voltage-gated channel.(a,b Jang et al. 2003). According to hydrophobicity plots, voltage-gated channels contain six helical transmembrane segments per subunit (S1–S6), as opposed to their simpler congeners KcsA and MthK, which have just two.
The fourth segment, S4, typically contains an array of four to seven positively charged amino acids, most often arginine residues, which had long been thought to act as the “voltage sensor”. Remarkably, charged lysine residues are rarely found in the voltage-sensor region. As the membrane is depolarized, that is, as the extracellular side becomes more negative, the positively charged voltage sensor physically moves across the cell membrane, a movement that is mechanically coupled with the opening of the gate. After many attempts with more common voltage-gated channels, which were ill fated as a result of the inherent flexibility of these proteins, a change in strategy brought about a breakthrough, when a channel from an unusual organism was chosen. The voltage-gated channel KvAP was identified by sequence comparison in the genome of Aeropyrum pernix, a thermophilic archaebacterium found near deep-sea hot springs (black smokers). Genetic and electrophysiological studies showed that KvAP behaves essentially as a voltage-gated channel and
is very similar to the previously mentioned eukaryotic Kv channels. The idea was that a channel that operates at 96 °C in its native environment would be less flexible at or below room temperature and thus should be easier to crystallize. To further stabilize the channel and provide hydrophilic contacts for the crystal lattice, antibodies were raised against the channel and crystallized in the complex with the channel protein.
The X-ray crystal structure of KvAP, solved at a resolution of 3.2 Å, provided some surprises (Figure 3).
Although the transmembrane helices closest to the pore, S5 and S6, showed the now canonical fold and closely resembled the open MthK structure, the remaining four “transmembrane” segments were not oriented as anticipated, that is, perpendicular to the (imaginary) membrane. In fact, S3 and S4 were not even situated in a region corresponding to the membrane, and S1 and S2 were oriented almost parallel to the plane of the membrane. The question now was how, with this structure, which clearly did not represent a physiologically relevant conformation of the channel in the membrane voltage gating could be explained.
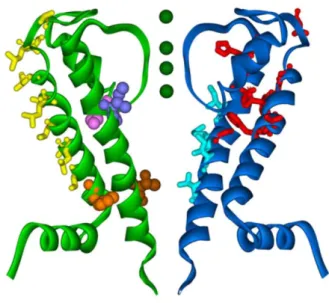
Further supported by an independent crystal structure of the S1–S4 segment and by a series of sophisticated biochemical experiments, MacKinnon proposed a refined model for voltage gating (Figure 4). According to his model, S4 and parts of S3 form a “voltage paddle”, which is not embedded in the protein core but resides at the periphery of the channel in contact with the membrane. Upon depolarization, the four voltage paddles on the tetrameric channel cross the membrane to display their edges on the extracellular side. The delocalized positive charges on the arginine residues could render this movement through the lipophilic membrane energetically feasible. As mentioned previously, this membrane crossing is mechanically coupled with the bending of S6 and opening of the gate.
Figure 4. MacKinnon's model for voltage-gating: Upon depolarization the voltage paddle moves towards the extracellular side of the membrane.
According to this model, the positively charged residues of S4 are embedded in the core of the protein, thus avoiding direct contact with the membrane lipids, and move up and down in response to changes in the membrane potential. This movement would essentially occur perpendicular to the membrane. It will be interesting to see whether the new voltage-paddle model can be reconciled with the results of previous biophysical experiments, which were carried out with different voltage-gated channels (Cohen et al 2003, Miller 2003).
Although the essential structural and functional features of potassium channels have been mapped out, many questions remain: For example how the S1–S6 domain folded in the open and closed states of a voltage-gated channel is. How does the voltage sensor move across the membrane. What are the energetic details of this process. What role does the β subunit associated with some potassium channels play.
Perhaps all these questions will be answered through the elucidation of the three-dimensional structure of the Shaker channel. Shaker is the archetypical potassium channel and the model for voltage-gated channels of the Kv class in the human genome. Its name is derived from a Drosophila melanogaster phenotype whose legs shake “under the influence” of ether. Shaker was the first potassium channel to be cloned, an achievement that opened the door for all the structural elucidations described above (Papazian et al. 1997).
Shaker is a voltage-gated channel with characteristics similar to those of KvAP. It undergoes inactivation upon prolonged opening. Despite continuing attempts, the X-ray crystal structure of Shaker or a mammalian Kv channel has not yet been solved. Nevertheless, if the elements from the crystal structures disclosed so far are combined, a functional model of a complete voltage gated potassium channel emerges. The work of MacKinnon and coworkers has provided most of the key components of this model (Figure 5). The selectivity filter of Shaker and related Kv channels contains the characteristic TXXTXGYG signature sequence and presumably closely
resembles the corresponding regions found in the four potassium channel structure elucidate to dates (Kuo et al 2003).
The gate is formed by the lower portion of S6, which bends at the glycine hinge upon opening. The voltage sensor possibly consists of a voltage paddle, whose movements are mechanically coupled to the gate. At the N-terminal end of S1, the polypeptide chain continues with a connector whose three-dimensional structure remains as yet unknown. The connector links S1 with the tetramerization domain (T1 domain), a region that was once believed to be essential for the self-assembly of potassium channels. Finally, at the N teminus of the T1 domain (and of the entire channel) resides a flexible loop and a well-defined short sequence of amino acids, which have been collectively dubbed the “ball and chain”. These domains are responsible for the fast inactivation of the open channel. According to the “ball and chain” mechanism of inactivation, initially proposed by Armstrong, the N-terminal peptide sequence threads into the open pore from the intracellular side, presumably through openings between the four connectors, and literally plugs the channel (Doyle et al 1998).
In the case of certain mammalian channels, a fourfold symmetric b subunit has been shown to bind to the T1 domain. To complete the detailed model of a β-subunit–T1-domain assembly have also been solved by X-ray crystallographic analysis (Gulbis 2000).
Surprisingly, the β subunit was found to contain a binding pocket for NADPH/H+, the functional significance of which remains unclear. It is quite tempting to speculate about the existence of a direct link between the redox state of a cell and its transmembrane potential. The detailed crystal structures of potassium channels disclosed by Mac-Kinnon and co-workers will certainly benefit the design and development of drugs against ion-channel diseases (channelopathies).
2.3 Concluding remarks
With their fourfold symmetry, their relatively uncomplicated protein folding pattern, and their fascinating functional properties, potassium channels appeal to chemists and biologists alike.
They are an excellent example of the selfassembly of supramolecular species with highly complex functions from much simpler components. MacKinnon's work will have an impact beyond neurobiology and medicine on diverse fields, such as supramolecular chemistry, molecular recognition, (Gradl et alo 2003) nanotechnology, and materials science.
Voltage-gated potassium channels are in essence field-effect transistors on a nanometer scale, that is, devices that change their conductance in response to fluctuations in an electromagnetic field. Compared with other nanoscale “molecular machines”, such as ATP synthases, transporters, or ion pumps, their structure and function are now relatively well understood, thus provoking scientists to use them as a starting point for further elaboration. More than fifty years after Hodgkin and Huxley (Nobel Prize 1963) postulated their existence and twenty-five years after Neher and Sakmann (Nobel Prize 1991) provided the necessary techniques to study them on a “single molecule” level, detailed structures of potassium channels have been disclosed and again this research has been rewarded with a Nobel Prize. Many of the lessons learned from studying potassium channels will be applicable to related transmembrane proteins, such as sodium or calcium channels, and perhaps even to ion channels in general. With an improved understanding of the structure and function of ion channels, neurobiology is bound to mature into a “true” molecular science. Chances are that structures of other key elements of neurobiology, in particular G-protein-coupled receptors and ion pumps, will soon follow, and push back even further what promises to be one of the most exciting scientific frontiers of the 21st century.
References
Doyle, D. A., Morais Cabral, J., Pfuetzner, R. A., Kuo, A., Gulbis, J. M., Cohen, S. L., Chait, B. T.,
Mackinnon, R. (1998). Science 280, 69-77.
Zhou Y . F, Morais-Cabral J. H., Kaufman A., R. MacKinnon, (2001)
Nature, 414, 43.
Jiang Y. X.,. Lee AChen, , J. Y. , Cadene M,. Chait B. T, MacKinnon R., (2002) Nature, 417, 515
Jiang Y. X,. Lee A,. Chen J. Y, Ruta V.,. Cadene M,. Chait B. TMacKinnon, R. (2002) Nature 417, 523.
MacKinnon R., (2002), Nature, 417, 523.
Jiang Y. X,. Lee A,. Chen J. Y, Ruta V.,. Cadene M,. Chait B. TMacKinnon, R., (2003) Nature, 423, 33
Jiang Y. X.,. Ruta V,. Chen J. Y, Lee A., MacKinnon R., (2003)
Nature, 423, 42.
Cohen B. E., Grabe M, L., Jan Y, (2003) Neuron, 39, 395 Miller C., (2003) Nat. Struct.Biol. 10, 422.
Papazian D . M.,. Schwarz T. L,. Tempel B. L, Jan Y. N., Jan L. Y., (1987) Science, 237, 749.
Kuo A. L.,. Gulbis J. M,. Antcliff J. F,. Rahman T,. Lowe E. D,. Zimmer J,. Cuthbertson J, Ashcroft F. M.,. Ezaki T, Doyle D. A., (2003) Science, 300, 1922.
Gulbis J . M., Zhou M., Mann S.,.MacKinnon R, (2000) Science, 289, 123.
Gradl S . N,. Felix J. P,. Isacoff E. Y,. Garcia M. L, Trauner D. 2003,
3.1 Potassium Channels as Therapeutic Drug Targets
K channels are increasingly being elucidated as molecular targets in a number of pathophysiologic states, and they continue to trigger considerable enthusiasm as drug targets. The pivotal role of K channels in various physiological processes including neuronal signalling, vascular and nonvascular muscle contractility, cardiac pacing, auditory function, hormone secretion, immune function, and cell proliferation has been underscored by the flurry of discoveries linking K channel mutations to various inherited disorders. Molecular cloning and expression of diverse K channels offer a platform for the medicinal chemist to examine compounds targeting defined subtypes. It will be particularly important in the post-genome era to understand what role each K channel gene product plays in the formation of native currents and what role each molecularly defined K current plays in cellular physiology and pathophysiology.
Nevertheless, the intent of the next chapter is not to review all know K channels but rather to highlight many of the therapeutically relevant members. A special emphasis on BK-channels, their physiological role and therapeutic potential will showed in the next section.
3.2 Potassium channels as universal regulators of cellular function
Potassium channels are of interest to physiologists because they are universal regulators of cellular function. The function of many organs and many different cell types is modulated via activation or inhibition of K+ channels.
Insulin secretion in the pancreas is regulated by ATP-sensitive potassium channels (Beauvais, et al.1995).
The shape and duration of the cardiac action potential is controlled by several different potassium channels (Benham et al 2002; Cahalan et al 1997; Cahalan et al 2001)
The vascular tone of arterioles, and thus the blood supply of various organs, is regulated via ATP-sensitive potassium channels (Daut et al 1994; Dittrich et al 1999)
NO-synthesis in vascular endothelial cells (and thus the tone of the adjacent vascular smooth muscle cells) is regulated by (i) ATP-sensitive, (ii) voltage-activated and (iii) Ca2+ activated K+ channels (Donnelly 1999; Giebisch et al 2001)
The excitability and firing pattern of neurons is regulated by potassium channels and their interacting proteins (Griffith et al 2002; Gulbins et al. 2003)
The immune response is modulated by K+ channels expressed in T and B lymphocytes, macrophages, mast cells and neutrophils (Himmel 1993;,Gunthorpe 2002).
Potassium channels have been implicated in the control of cell proliferation, in apoptosis (Karicheti 2001; Keating, 2001; Khan 2001; Hoenderop 2002) and in the development of tumours (Kunzelmann 2002).
Potassium channels are involved in the electrolyte transport in epithelial, for example in the tubules of the kidney or in the mammalian colon (Lang 2000; Liss 2001).
Potassium channels are involved in the regulation of the contractions of the myometrium (Lopez-Barneo 1999) and in the urogenital tract (Lopez-Barneo 2001).
There are two major mechanisms by which K+ channels influence cellular
function. To illustrate the different types of regulation, two prototypes are described here: (i) the role of K+ channels in vascular smooth muscle cells
(SM-type regulation), and (ii) the role of K+ channels in vascular endothelial
cells (EC-type regulation).
In the SM-type mechanism, typical for electrically excitable cells, an increase in transmembrane calcium influx is initiated by closure of K+ channels
(associated with depolarisation). In the EC-type mechanism, typical for non-excitable cells, an increase in calcium influx is initiated by opening of K+
channels (associated with hyperpolarisation).
3.3 The role of potassium channels in vascular smooth muscle cells
The tone of vascular smooth muscle is strongly influenced by the prevailing membrane potential: hyperpolarization (more negative membrane potential) is associated with a relaxation of the smooth muscle cells (vasodilation), depolarization (less negative membrane potential) is associated with a contraction of smooth muscle cells (vasoconstriction). The underlying mechanisms are illustrated in a very simplified scheme:
In the membrane of vascular smooth muscle cells depolarization-activated Ca2+ channels are co-expressed with a variety of potassium channels
(KATP-channels, Kir (KATP-channels, Kv (KATP-channels, K2P channels). Opening of these K+
channels leads to a hyperpolarisation. The steady-state open probability of voltage-activated Ca2+ channels in the steady state is quite low (<< 0,1), but
strongly voltage dependent in the range of membrane potentials occurring in vascular smooth muscle cells in vivo (-55 to -25 mV). Thus, a hyperpolarisation of, for example, 2-3 mV (induced by the opening of 10-20 K+ channels) will lead to an at least two-fold increase the steady-state Ca2+
influx. Now the free cytosolic Ca2+ concentration depends on the balance
between Ca2+ influx (through voltage-activated Ca2+ channels) and Ca2+ efflux
(through the plasma membrane Ca2+ ATPase and through Na+/Ca2+ exchange).
Hyperpolarisation shifts the balance in favour of Ca2+ efflux, which leads to a
decrease in intracellular Ca2+. The free cytosolic Ca2+ concentration, mainly
through its effect on myosin light chain kinase (MLCK), via calmodulin, largely (but not exclusively) determines the tone of the vascular smooth
muscle cells. The signal transduction in vascular smooth muscle cells can be summarised as
follows:
(i) activation of K+ channels
(ii) hyperpolarisation
(iii) decreased Ca2+ influx
(iv) decreased free cytosolic Ca
2+(v) decreased activation of Ca
2+/calmodulin dependent MLCK
(vi) decreased tone of vascular smooth muscle cells
(vii) vasodilation
The tone of vascular smooth muscle cells in resistance arteries is closely correlated with membrane potential: hyperpolarisation leads to relaxation, hyperpolarisation leads to vasoconstriction (Maeno et al. 2000, Miki et al 2001). Thus, potassium channels play a major role in the regulation of blood pressure and in the neural regulation of blood flow in different organs and in metabolic regulation of oxygen supply. K+ channels have also been implicated
in oxygen sensing in the carotid bodies (Miki et al. 2001; Nerbonne et al. 2000; Nerbonne et al 2001; Nilius et al 2001 ; Pardo et al. 1999). Similar signal transduction mechanisms have been found in many electrically excitable cells, for example in neurons, in cardiac muscle cells, as well as in pancreatic b-cells and other endocrine cells. In these excitable cells activation
of K+ channels induces a hyperpolarisation which leads to a decrease in
transmembrane calcium influx and to a subsequent inhibition of cellular activity.
3.4 The role of potassium channels in vascular endothelial cells
The signal transduction of non-excitable cells, for example endothelial or epithelial cells, is quite different from that described above. This is illustrated below for vascular endothelial cells:
In the membrane of vascular endothelial cells a variety of potassium channels is expressed (Pardo et al 1999; Patel et al 2001; Sanguinetti et al 2000), but depolarisation-activated Ca2+ channels are absent. In the endothelium, in contrast to smooth muscle cells, hyperpolarisation leads to an increase in cytosolic free Ca2+. Many groups have found that the synthesis and secretion
of nitric oxide (NO) or prostaglandins by vascular endothelial cells is increased under conditions in which the cells are hyperpolarised (Schnitzler et al 2000; Sobey et al 2001). Thus the opening of potassium channels (which causes hyperpolarisation) stimulates secretion of vasoactive substances by endothelial cells. The reason for this striking difference is the presence of calcium permeable channels that conduct larger currents during hyperpolarisation. The nature of these “calcium leak channels” has not been
unequivocally identified, but they are very likely members of the TRP channel family (Suzuki et al 2001; Tran et al 2000; Tristani-Firouzi et al. 2001; Vennekens et al 2002 ). Thus the signal transduction in endothelial cells can be summarised as follows:
(i) opening of K+ channels via hormones, transmitters or mechanical forces (ii) hyperpolarisation
(iii) increased Ca2+ influx via 'leak channels', probably members of the TRP family
(iv) increased free cytosolic Ca2+.
(v) increased synthesis of vasoactive substances such as NO (vi) vasodilation
(vii) increase in the permeability and hydraulic conductivity of the vascular wall, mediated by changes in the structure of the tight junctions between endothelial cells.
Thus, both in vascular smooth muscle cells and in vascular endothelial cells hyperpolarisation leads to vasodilatation, albeit via entirely different mechanisms. The two mechanisms may be described as SM-type signal transduction and EC-type signal transduction. EC-type signal transduction is found in many non-excitable cells, for example, in intestinal epithelial cells, renal epithelial cells and glial cells EC-type and SM-type signal transduction both play an important role in many organs. In the microvasculature, there is an additional complication since endothelial cells and microvascular smooth muscle cells are coupled by myo-endothelial gap junctions (Von Beckerath et al 1991; Von Beckerath et al 1996).
This implies, that the endothelial cells and the underlying smooth muscle cells sense the same membrane potential and that changes in membrane potential are transmitted rapidly from one cell type to the other. Thus SM-type and EC-type signal transduction co-operate in the regulation of blood flow in
resistance arteries. Hyperpolarisation of the myo-endothelial regulatory unit (Pardo et al 1999) causes vasodilation, albeit by two entirely different mechanisms: the EC-type mechanism present in the endothelium increases the release of NO via an increase in cytosolic calcium, the SM-type mechanism in vascular smooth muscle cells causes relaxation via a decrease in free cytosolic calcium. Thus, both mechanisms co-operate in the regulation of vascular tone. In many cells the basic machinery for stimulating or inhibiting cellular function is relatively simple and invariant, whereas the fine-tuning is achieved via activation or inhibition of potassium channels. In neurons, for example, the variability of voltage-activated Na+ or Ca2+ currents is relatively small and
only a few different genes are involved. On the other hand, the fine-tuning, which determines spontaneous activity, excitability, action potential pattern and frequency, is regulated by the more than 80 different potassium channel genes and their accessory subunits. Another instructive example is cardiac muscle: In the membrane of cardiac ventricular muscle there is only one type of sodium channel and two types of calcium channels (L- and T-type, but the latter plays only a minor role). They provide for the upstroke of the cardiac action potential and the initial calcium influx. In contrast, at least 12 different K+ channels are found in the membrane of cardiac ventricular muscle cells. They control diastolic potential, action potential duration and heart rate are clinically important because they are involved in the generation of arrhythmias or because they represent potential targets of antiarrhythmic drugs.
In conclusion, potassium channels are involved in many types of regulation of cellular function. Many K+ channels are considered as potentially useful drug targets because of (i) their specific regulatory role in many cells, (ii) their specific expression pattern in various tissues and (iii) the specific pharmacology of various channel subtypes.
3.5 K+ channels and apoptosis
Apoptosis is an evolutionarily conserved process that plays a critical role in embryonic development and tissue homeostasis. In humans, disorder of apoptosis has been linked to pathogenesis of cancer, atherosclerosis and other diseases. It is generally accepted that cysteine proteases such as caspases and nucleases, play a major role as effectors of apoptosis. One of the earliest events during apoptosis is cell shrinkage, which usually takes place before cytochrome c release, caspase activation and DNA fragmentation (Beauvais et al 1995; Gomez-Angelats et al 2001; Gomez-Angelats et al 2002; Bortner et al 2001;1 Ekhterae et al 2001). Cell volume is primarily controlled by intracellular ion homeostasis. Since K+ ions are the dominant cations in the
cytosol, cell volume depends to a large extent of the intracellular K+ concentration, and from K+ movements across the plasma membrane. It should be noted, however, that the efflux of K+ ions alone is insufficient to cause a volume change in cells because it has to be accompanied by an efflux of an anion to achieve a change in intracellular osmolarity. Thus, apoptotic cell shrinkage may be elicited by concurrent activation of K+ and Cl-channels (Szabo 1998; Langet al 1999; Lang et al 2000). Furthermore, high intracellular K+ is essential for suppressing the activity of nucleases and
caspases, and in several cell types an increase in K+ permeability is an early
response to apoptotic stimuli (Maeno et al 2000, Hara et al 2002; a,b Okada et al 2001; 9-12). It has been postulated that activation of K+ channels may be
one of the initial triggers of apoptotic cell volume decrease and that, conversely, inhibition of K+ channels may play a role in attenuating apoptosis.
Reference
Beauvais, F., Michel, L., and Dubertret, L. (1995) J Leukoc Biol 57, 851-855
Benham, C. D., Davis, J. B., and Randall, A. D. (2002).
Neuropharmacology 42, 873-888
Cahalan, M. D., and Chandy, K. G. (1997). Curr Opin Biotechnol 8, 749-756
Cahalan, M. D., Wulff, H., and Chandy, K. G. (2001). J Clin Immunol 21, 235-252
Coleman, H. A., Tare, M., and Parkington, H. C. (2002) Clin Exp
Pharmacol Physiol 29, 630-637
Daut, J., Maier-Rudolph, W., von Beckerath, N., Mehrke, G., Gunther, K., and Goedel-Meinen, L. (1990) Science 247, 1341-1344 Daut, J., Standen, N. B., and Nelson, M. T. (1994). J Cardiovasc
Electrophysiol 5, 154-181
Dittrich, M., and Daut, J. (1999). Am J Physiol 277, H119-127 Donnelly, D. F. (1999) Respir Physiol 115, 151-160
Giebisch, G. (2001) Kidney Int 60, 436-445 Griffith, T. M. (2002). Biorheology 39, 307-318
Gulbins, E., Dreschers, S., and Bock, J. (2003). Exp Physiol 88, 85-90 Gunthorpe, M. J., Benham, C. D., Randall, A., and Davis, J. B. (2002)
Trends Pharmacol Sci 23, 183-191
Himmel, H. M., Whorton, A. R., and Strauss, H. C. (1993).
Hypertension 21, 112-127
Hoenderop, J. G., Nilius, B., and Bindels, R. J. (2002). Biochim
Karicheti, V., and Christ, G. J. (2001). Curr Drug Targets 2, 1-20 Keating, M. T., and Sanguinetti, M. C. (2001). Cell 104, 569-580 Khan, R. N., Matharoo-Ball, B., Arulkumaran, S., and Ashford, M. L.
(2001). Exp Physiol 86, 255-264
Kunzelmann, K., and Mall, M. (2002). Physiol Rev 82, 245-289 Lang, F., Ritter, M., Gamper, N., Huber, S., Fillon, S., Tanneur, V.,
Lepple-Wienhues, A., Szabo, I., and Gulbins, E. (2000). Cell Physiol
Biochem 10, 417-428
Liss, B., Franz, O., Sewing, S., Bruns, R., Neuhoff, H., and Roeper, J. (2001). Embo J 20, 5715-5724
Lopez-Barneo, J., Pardal, R., Montoro, R. J., Smani, T., Garcia-Hirschfeld, J., and Urena, J. (1999).K+ and Ca2+ channel activity and cytosolic [Ca2+] in oxygen-sensing tissues. Respir Physiol 115, 215-227
Lopez-Barneo, J., Pardal, R., and Ortega-Saenz, P. (2001).Cellular mechanism of oxygen sensing. Annu Rev Physiol 63, 259-287
Maeno, E., Ishizaki, Y., Kanaseki, T., Hazama, A., and Okada, Y. (2000). Proc Natl Acad Sci U S A 97, 9487-9492
Miki, T., Iwanaga, T., Nagashima, K., Ihara, Y., and Seino, S. (2001).
Diabetes 50 Suppl 1, S48-51
Miki, T., Liss, B., Minami, K., Shiuchi, T., Saraya, A., Kashima, Y., Horiuchi, M., Ashcroft, F., Minokoshi, Y., Roeper, J., and Seino, S. (2001). Nat Neurosci 4, 507-512
Nerbonne, J. M. (2000). J Physiol 525 Pt 2, 285-298
Nerbonne, J. M., Nichols, C. G., Schwarz, T. L., and Escande, D. (2001). Circ Res 89, 944-956
Nilius, B., and Droogmans, G. (2001). Physiol Rev 81, 1415-1459 Pardo, L. A., del Camino, D., Sanchez, A., Alves, F., Bruggemann,
A., Beckh, S., and Stuhmer, W. (1999). Embo J 18, 5540-5547 Patel, A. J., and Honore, E. (2001). Eur Respir J 18, 221-227 Sanguinetti, M. C. (2000). J Cardiovasc Electrophysiol 11, 710-712 Schnitzler, M. M., Derst, C., Daut, J., and Preisig-Muller, R. (2000). J
Physiol 525 Pt 2, 307-317
Sobey, C. G. (2001). Arterioscler Thromb Vasc Biol 21, 28-38 Suzuki, M., Li, R. A., Miki, T., Uemura, H., Sakamoto, N.,
Ohmoto-Sekine, Y., Tamagawa, M., Ogura, T., Seino, S., Marban, E., and Nakaya, H. (2001). Circ Res 88, 570-577
Tran, Q. K., Ohashi, K., and Watanabe, H. (2000). Cardiovasc Res 48, 13-22
Tristani-Firouzi, M., Chen, J., Mitcheson, J. S., and Sanguinetti, M. C. (2001). Am J Med 110, 50-59
Vennekens, R., Voets, T., Bindels, R. J., Droogmans, G., and Nilius, B. (2002). Cell Calcium 31, 253-264
Von Beckerath, N., Cyrys, S., Dischner, A., and Daut, J. (1991). J
Von Beckerath, N., Dittrich, M., Klieber, H. G., and Daut, J. (1996). J
Physiol 491 ( Pt 2), 357-365
Beauvais, F., Michel, L., and Dubertret, L. 1995. J Leukoc Biol 57, 851-855
Gomez-Angelats, M., and Cidlowski, J. A. Adv Cancer Res 85, 175-201
Gomez-Angelats, M., and Cidlowski, J. A.. J Biol Chem 276, 44944-44952
Bortner, C. D., Gomez-Angelats, M., and Cidlowski, J. A.2001 Biol
Chem 276, 4304-4314
Ekhterae, D., Platoshyn, O., Krick, S., Yu, Y., McDaniel, S. S., and Yuan, J. X. 2001 Am J Physiol Cell Physiol 281, C157-165
Szabo, I., Lepple-Wienhues, A., Kaba, K. N., Zoratti, M., Gulbins, E., and Lang, F. Proc Natl Acad Sci U S A 95, 6169-6174
Lang, F., Ritter, M., Gamper, N., Huber, S., Fillon, S., Tanneur, V., Lepple-Wienhues, A., Szabo, I., and Gulbins, E. 2000 Cell Physiol
Biochem 10, 417-428
Lang, F., Szabo, I., Lepple-Wienhues, A., Siemen, D., and Gulbins, E. 1999 News Physiol Sci 14, 194-200
Hara, Y., Wakamori, M., Ishii, M., Maeno, E., Nishida, M., Yoshida, T., Yamada, H., Shimizu, S., Mori, E., Kudoh, J., Shimizu, N., Kurose, H., Okada, Y., Imoto, K., and Mori, Y. 2002. Mol Cell 9, 163-173
Okada, Y., and Maeno, E. 2001. Comp Biochem Physiol A Mol Integr
Physiol 130, 377-383
Okada, Y., Maeno, E., Shimizu, T., Dezaki, K., Wang, J., and Morishima, S. 2001 J Physiol 532, 3-16
Maeno, E., Ishizaki, Y., Kanaseki, T., Hazama, A., and Okada, Y. 2000. Proc Natl Acad Sci U S A 97, 9487-9492
4.1 Calcium activated potassium channels
Calcium (Ca2+) flow into the cells triggers a cascade of events such as release
of calcium from the intracellular depots, activation of secondary messenger system, and opening of ion channels. One such phenomenon is the opening of potassium (K+)-selective ion channels, which are triggered by an increase in
intracellular Ca levels.
In the first way, in the 1958 Gardos, (who studied potassium permeability through human erythrocytes in presence of elevated calcium concentration (Gardos, 1958)), observed that minor changes in intracellular Ca levels can alter potassium diffusion across the plasma membrane. The first identification instead of an ionic current activated by a rise in intracellular calcium was made by Meech in 1970 (Meech e Strumwasser, 1970). Currently, it is know that such ionic currents originate in different types of cells and are mediated mainly by the opening of potassium-selective channels: Ca-activated potassium channels.
Calcium-activated K+ channels are not so numerous as the Kv channels and have been classified in three different families on the basis of channel conductance. They are usually named large-conductance 100-300 pS (big, BK) (Marty,1981), intermediate- conductance 25-100 pS (IK) Gardos,1958; Ishii et al., 1997; Logsdon et al., 1997) and small- conductance 2-25 pS (SK) (Blatz e Magleby,1986; Park, 1994). Several subtypes of BK and SK channels have been described (Vergara et al., 1998; Gutman et al., 2003). The common characteristic in all KCa channels is that they are activated when the cytoplasmic concentration of Ca2+ is increased, but BK channels are also voltage sensitive (Vergara et al., 1998; Vogalis et al., 2003).