Alma Mater Studiorum – Università di Bologna
DOTTORATO DI RICERCA IN
Scienze ambientali: tutela e gestione delle risorse naturali
Ciclo XXV
Settore Concorsuale di afferenza: 02/B3
Settore Scientifico disciplinare FIS/07
TITOLO TESI
The challenges and the limitations in
Life Cycle Impact Assessment
for metal oxide nanoparticles,
a case study on nano- TiO
2
Presentata da : Dott.ssa Beatrice Salieri
Coordinatore di dottorato Relatore
Prof. Enrico Dinelli Dott.ssa Serena Righi
Acknowledgements
I would like to thank everyone who has, in one way or another, contributed to the completion of
this PhD project. In particular I wish to express my appreciation to my supervisor, Dott.ssa. Serena
Righi , for her mentorship and her continuous scientific support. A special thanks to Prof. Andrea
Pasteris who has provided technical, experimental assistance as well as inspirational discussions
and enthusiastic guidance. My thanks to Prof. Stig Irving Olsen who has offered me the
opportunity to spend a period of study at DTU, where I enjoyed a special work experience and a
personal “adventure”. My thanks also to the PhD students of DTU-QSA session for providing such
a positive and inspiring working atmosphere. A special thanks to Mikolaj “il mio compagno di
merenda” who had shared with me evenings in café writing the thesis or projects. A big thanks to
Alexis too, for the wonderful and happy time spend togheter.
Thank you to Prof. Juliane Fielser for giving me the opportunity to spend a period of work at
Zentrum für Umweltforschung und Nachhaltige Technologien (UFT), University of Bremen,
(Germany); thank to you all the colleagues of UFT, Bremen for their technical support and
hospitality in laboratory. A special thanks to my friends Jonas Baumann, Jan Koeser and Carole
Bertrand.
My heartfelt gratitude goes to my friends for their encouragement and patience. Finally I would
like to thank my mother and my father for their loving support and for being my foundation.
Background and aim
Life Cycle Assessment (LCA) is a chain-oriented tool to evaluate the environment performance of products focussing on the entire life cycle of these products: from the extraction of resources, via manufacturing and use, to the final processing of the disposed products. Through all these stages consumption of resources and pollutant releases to air, water, soil are identified and quantified in Life Cycle Inventory (LCI) analysis. Subsequently to the LCI phase follows the Life Cycle Impact Assessment (LCIA) phase; that has the purpose to convert resource consumptions and pollutant releases in environmental impacts. The LCIA aims to model and to evaluate the selected environmental issues, called impact categories (Climate change, Human toxicity, Ecotoxicity, etc.); through the use of category indicators it portrays the overall potential environmental impact of a product system in an aggregated manner.
The LCA methodology is widely applied in several industrial sectors to evaluate the environmental performances of processes, products and services. Several reports and studies emphasises the importance of LCA in the field of engineered nanomaterials (ENMs) The ENMs offer enormous potential for the development of new products and application with improved performance as well as reduction of energy and materials. There are however unanswered questions about the impacts of nanomaterials and nanoproducts on human health and the environment. In the last decade the increasing production, use and consumption of nanoproducts, with a consequent release into the environment, has accentuated the obligation to ensure that potential risks are adequately understood to protect both human health and environment. Due to its holistic and comprehensive assessment, LCA is an essential tool to analyse, evaluate, understand and manage the environmental and health effects of nanotechnology. The evaluation of health and environmental impacts of nanotechnologies, throughout the whole of their life-cycle by using LCA methodology, is mentioned in a number of EU policy documents including the Sixth Community Environment Action Programme and the Communication on Integrated Product Policy (IPP) as well. Currently, only few LCA’s studies on nanotechnology are carried out, and only fewer studies assess the aspects relating to (eco)toxicity (Chapter II). This is due to the lack of knowledge in relation to risk assessment. In fact, to date, the knowledge on human and environmental exposure to nanomaterials, such nanoparticles (ENPs) is limited. This bottleneck is reflected into LCA where characterisation models and consequently characterisation factors for ENPs are missed. Therefore, the PhD project aims to assess limitations and challenges of the freshwater aquatic ecotoxicity potential evaluation in LCIA phase for ENPs and in particular for metal oxide nanoparticles as n-TiO2.
In the Life Cycle Impact Assessment phase the characterisation models have been developed to quantitatively overview the environmental pathway of a substance once released into the environment. The Characterization Factor (CF) of a chemical product for toxic impact category is evaluated as the
product of Effect Factor (EF), Fate Factor (FF) and Exposure Factor (XF), [CF=EF*FF*XF].(Chapter V) Since the exposure assessment of a substance requires the evaluation of fate, behavior and transport in the environmental media, currently environmental multimedia models are used to evaluate the fate factors and exposure factors of pollutants. Despite the usefulness of these models for organic substances, the exposure assessment of ENPs is still critical due to the scarce knowledge of the environmental behaviour of ENPs, of the ENP’s proprieties that affect the behaviour and transport among media compartments and therefore, of their fate processes in the environment.
Therefore the evaluation of the predicted environmental concentration (PEC) of ENPs is currently mainly based on material flow analysis (MFA) where the ENPs are treated as bulk material. In such studies the important particulate nature of the material has not been considered (Chapter III ). A correct evaluation of the environmental exposure, in principle, needs to consider all the environmental fate processes in order to estimate the bioavailable fraction. Two processes that seem to be significant are the fate processes of aggregation (incl. sedimentation) and dissolution. Environmental scientists have recently encouraged modeling of ENPs fate in freshwater based on colloidal chemistry and it has also been recognized that abiotic factors such as ionic strength and pH could influence the colloidal behavior of ENPs in freshwater (Chapter III).
As mentioned above, the assessment of the potential toxic impact of a substance requires the knowledge of its toxic effect. Therefore, acute toxicity tests with n-TiO2 on Daphnia magna (crustaceans) and algae
have been carried out. Furthermore, an extensive bibliographic review of the toxicity of metal oxide nanoparticles (in particular n-TiO2) on freshwater organisms has been performed. The review is focused
on aquatic organisms representative of reflecting the overall topic of this thesis and the experimental work undertaken during the PhD project period. The review aims to describe the current state of knowledge as well as to highlight potential relationships between particle properties and observed effects, while also drawing attention to knowledge gaps and uncertainties. The bibliographic review aims, as well, to collected the effect concentrations of n-TiO2 used into the calculation of the effect factor
(Chapter IV).
The extensive knowledge acquired on the environmental behaviour of ENPs, on the current approach to model their environmental fate, on their ecotoxicity has induced to propose a framework for the calculation of both fate factor and effect factor for metal oxide nanoparticles such n-TiO2 (Chapter VI).
Following the aim of the PhD research, limitations and challenges for LCIA have been highlighted and discussed in each chapters of this thesis.
INDEX
1. What are nanoparticles? ... 1
1.1 Definition and classification ... 1
1.2 Nanoparticles applications ... 4
1.3 Engineered nanoparticles: environmental concerns ... 6
1.4 Environmental exposure ... 6
1.5 Ecotoxicity... 8
1.6 Are ENPs environmental sustainable? ... 9
1.7 Bibliography...11
2 Life Cycle Assessment Methodology ...13
2.1 LCA: The four phases ...13
2.1.1 Goal and scope definition ...14
2.1.2 Life cycle Inventory ...14
2.1.3 Life cycle Impact Assessment ...14
2.1.4 Interpretation ...17
2.2 LCA and nanotechnology ...18
2.2.1 Goal and scope in the nanotechnology LCA study ...18
2.2.2 Life cycle inventory in the nanotechnology LCA study...20
2.2.3 Life Cycle Impact Assessment in the nanotechnology LCA study...21
2.2.4 Importance of evaluating fate and effect of nanoparticles ...24
2.3 Conclusion and outlook ...25
2.4 Bibliography...27
3 Engineered nanoparticles (ENPs) environmental fate processes in freshwater and environmental exposure assessment ...31
3.1 Introduction ...31
3.2 Environmental fate processes ...31
3.2.1 Dissolution ...32
3.2.2 Chemical transformation: oxidation and reduction ...33
3.2.3 Physical transformation: aggregation ...34
3.3 Aggregation kinetics ...39
3.3.1 Particle collision ...40
3.3.2 Interparticle force ...45
3.3.3 DLVO theory ...48
3.4 A briefly application of the DLVO theory to predict the environmental behaviour on –TiO2 in freshwater archetypes ...50
3.4.1 Material and method ...50
3.4.2 Result ...52
3.4.3 Discussion and conclusion ...53
3.5 Environmental assessment ...53
3.6 Discussion and conclusion ...57
3.7 Bibliography...60
4 Ecotoxicity of metal oxide nanoparticles(n-TiO2) on crustaceans, algae and fish ...64
4.1 Introduction ...64
4.2 The ecotoxicity of n-TiO2: a case study on D.magna ...65
4.2.1 Material and Method ...66
4.2.2 Acute toxicity test...69
4.2.3 Result ...70
4.2.4 Discussion ...73
4.2.5 Conclusion ...76
4.3 The ecotoxicity of n-TiO2 on algae: a case of study with P.subcapitata ...77
4.3.1 Materials and methods ...77
4.3.2 Ecotoxicological test with algae ...77
4.3.3 Result ...78
4.3.4 Discussion and Conclusion ...79
4.4 Standard toxicity test and preparation of nanoparticles suspensions ...79
4.5 A bibliographic review on n-TiO2 toxicity ...83
4.5.1 Toxicity of n- TiO2 to algae ...83
4.5.2 Toxicity of n - TiO2 to freshwater invertebrates ...91
4.5.3 Toxicity of n-TiO2 to freshwater vertebrates (fish) ...100
4.6 Discussion and conclusion ...101
4.7 Bibliography...105
5 The characterisation of freshwater toxic impact: the USEtox model ...109
5.1 The characterisation step: a qualitative description ...109
5.2 USEtox model ...110
5.2.1 Historical context ...110
5.2.2 The characterisation of freshwater toxic impact ...112
5.3 The fate model ...114
5.4 Exposure and effect model ...118
5.6 Bibliography...120
6 The characterisation of freshwater ecotoxicity for n-TiO2: an open issue ...123
6.1 A framework to evaluate the fate factor for n-TiO2 in freshwater in accordance with USEtox requirements ...123
6.1.1 Introduction ...123
6.1.2 Material and method ...125
6.1.3 Result ...133
6.1.5 Discussion and conclusion ...135
6.2 The effect factor for freshwater toxic impact for n-TiO2 in accordance with USEtox model 138 6.2.1 Introduction ...138
6.2.2 Ecotoxicity effect indicator (EEI) ...138
6.2.3 Material and method ...142
6.2.4 Result ...143
6.2.5 Discussion and conclusion ...147
1
1. What are nanoparticles?
1.1 Definition and classification
Nanoparticles (NPs) belong to the wider group of nanomaterials, where the prefix ‘nano’ refers to infinitesimal physical dimensions and where particles are defined as a “minute piece of matter with defined physical boundaries” where “physical boundary can also be described as an interface” (ISO, 2008) Many definitions have been proposed for nanoparticles and nanomaterials and in literature the terms “engineered nanoparticles (ENPs)”, “engineered nanomaterials (ENMs)” and “nanoproducts” are not used in a uniform manner.
The chemical composition may be the same as with bulk material, but nanoparticles display totally new characteristics due to the high surface to- volume ratio and their small size (Oberdörster et al., 2007) at which quantum mechanics come into play. Thus, it is difficult to find a sound definition.
Some efforts are seen in the scientific literature to define nanoparticles based on their novel size-dependant properties. A common definition of engineered nanoparticles, combining both size and property characteristics, refers to particles with dimensions of about 1 to 100 nm, purposefully manufactured to have unique properties (Kreyling et al., 2010; Auffan et al., 2009). Hence nanoparticles possess properties that are “qualitatively or quantitatively distinctly different from their of other physical
forms” (SCHENIHR 2006), such as those of larger-sized particles (bulk particles) made from the same
materials and their water-soluble/ionic form. Size-related differences in particle properties may be due to the larger surface area per mass, resulting into an increased ratio of surface-to-core atoms and increased number of corner and edge atoms. This results in an increased reactivity (Feldheim, 2007) or an increased ion release which enables their use in novel applications.
The Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) was set up in 2004 by the European Commission to provide scientific input on elements to consider when developing a definition of the term "nanomaterial". The SCENHIR concluded that size is universally applicable to nanomaterials. A defined size range would facilitate a uniform interpretation. A lower limit of 1 nm1 and an upper limit of 100 nm were proposed.
Also, the number size distribution should also be considered using the mean size and the standard deviation of the size to refine the definition. The size distribution of a material should be presented as size distribution based on the number concentration (i.e. the number of objects within a given size range divided by the number of objects in total).
2
Recently, the European commission, on October 18th, 2011 adopted the Recommendation on the definition of a nanomaterial. According to this Recommendation "Nanomaterial" means:
“A natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50 % or more of the particles in the number size distribution, one or more external dimensions is in the size range 1 nm - 100 nm.”
Where:
…”particle", "agglomerate" and "aggregate" are defined as follows:
(a) "Particle" means a minute piece of matter with defined physical boundaries;
(b) "Agglomerate" means a collection of weakly bound particles or aggregates where the resulting external surface area is similar to the sum of the surface areas of the individual components;
(c) "Aggregate" means a particle comprising of strongly bound or fused particles.
and also “In specific cases and where warranted by concerns for the environment, health, safety or
competitiveness the number size distribution threshold of 50 % may be replaced by a threshold between 1 and 50 %”.
The nanoparticles can be classified, according to their origin, in natural and anthropogenic and then, intentional or unintentional (Oberdörster et al., 2007; Bhatt et al., 2011).
Fig.1.1 Categories of nanoparticles (Source: Bhatt et al, 2011)
The natural nanoparticles are assumed to be derived: From natural combustion processes;
3
From geological mechanism, e.g. physic-chemical weathering, autogenesis/neoformation and volcanic eruptions;
Many biological molecules/entities (e.g. DNA, nucleic acids, viruses) are typically nano-sized. Some of these are released into the environment directly from the organism by biological processes as nucleoprotein exudates from algae, dispersion of viruses from animals;
From degradation of biological matters, e.g. humic and fulvic acids.
The unintentional anthropogenic nanoparticles derive from combustion processes (e.g. diesel exhausts) or waste and corrosion of products containing nanoparticles. The intentional source of nanoparticles are described by the class of engineered nanoparticles (ENPs). The ENPs are intentionally produced for dedicated applications and manufactured by two distinct methods: down and bottom-up. In the top-down method, particle with size lesser than 100 nm and 30 nm are produced by cutting larger pieces of source material (lithographic techniques). The bottom-up methods are based on physicochemical principles of molecular or atomic self-organization. This approach produces selected, more complex structures from atoms or molecules, better controlling sizes, shapes and size ranges. It includes aerosol processes, precipitation reactions and sol-gel processes (Bhatt et aI., 2011).
Also, ENPs can be distinguished in five classes:
Metal oxide nanoparticles: CeO2, TiO2, ZnO, Fe2O2, Al2O3, MgO, ZrO, SnO.
Carbon based nanoparticles: These nanomaterials are composed mostly of carbon, most commonly taking the form of a hollow spheres, ellipsoids, or tubes. These particles have many potential applications, including improved films and coatings, stronger and lighter materials, and applications in electronics. This group comprises both fullerenes (C60) and carbon nanotubes
(CNTs). Two classes of carbon nanotubes are distinguished: single walled (SWNTs) and multi-walled nanotubes (MWNTs). SWCNTs are structurally single-layered graphene sheets rolled up in cylindrical shapes of approximately 1 nm diameter and several micrometers of length, whereas MWCNTs possess two or more concentric layers with varying length and diameters (Bhatt et al., 2011).
Zero-valent metals: They are usually prepared by reduction of metal salts, e.g. zero-valent iron is
made through the reduction of ferric (Fe 3+) or ferrous (Fe 2+) salts with a sodium borohydride. Similarly, the chemical synthesis of gold and silver ENPs involves dissolution of the metal salt in an appropriate solvent and its subsequent reduction to the zero valency.
4
Quantum dots: They are a closely packed semiconductor crystal comprised of hundreds or thousands of atoms, and whose size is on the order of a few nanometers to a few hundred nanometers. Usually they are nanoparticles made of semiconductor materials with fluorescent properties, crucial for biological applications.
Dendrimers: They are complex, multifunctional polymers with 1–10 nm diameter. The surface of a dendrimer has numerous chain ends which can be tailored to perform specific chemical functions. This property could also be useful for catalysis. Also, because three-dimensional dendrimers contain interior cavities into which other molecules could be placed, they may be useful for drug delivery.
For the aim of this thesis I will refer to ENPs, to the group of metal oxide nanoparticle and I will focus on nanoparticles of titanium dioxide (n-TiO2).
1.2
Nanoparticles applications
In the last decade an increasing interest has been shown in nanoparticles due to their physicochemical properties that differ from those of the bulk material. The novel physicochemical properties of the ENPs are attributable to their small size, chemical composition, surface structure, solubility, shape, aggregation (Nel, 2006). The advent of nanotechnology2 has unleashed enormous potential for the development of new products, and their applications in a number of industrial and consumer sectors (cosmetic, medicine and drugs, optical engineering). The nanomaterials and nanoparticles are widely applied in several sectors; their applications are so extended that, to date, they are largely applied in daily consumer products (clothes, sunscreens and others).
The Nanotechnology Consumer Product Inventory of the Woodrow Wilson Institute (WWI) highlights that the number of nano-enabled consumer products is increasing rapidly, where the production and distribution of nanotechnology products is increasingly global. As a result of the continuously increasing of applications of nanomaterials in consumer products, a total of 858 consumer products containing nanomaterials (nano-consumer products) were identified in the European market (2010); this result represents a six fold increase when compared to the number of 143 products in the European market
2
Nanotechnology is the intentional and controlled generation, or modification of materials at a nanometer (nm) scale level (Handy et al., 2008).
5
(2007). The product categories with the largest growth are the ‘Personal care products and cosmetics’ like sunscreens and various ‘Coating products’ such as anti-rain products for shoes and textiles.
In the following the main applications of the ENPs area described. Metal oxide nanoparticles (n-TiO2):
Commercial production of nano-TiO2 between 2006 and 2010 has been estimated at 5000 tons per year,
more than 10 000 tons per year between 2011 and 2014 and approximately 2.5 million tons by 2025 (Menard et al., 2011).
Titanium dioxide is a naturally occurring mineral that can exist in three crystalline forms, known as rutile, anatase and brookite, and in amorphous form. The element titanium is also found in ilmenite (FeTiO3) and
other minerals and ores; rutile phase is the most common form of TiO2 found in nature (EPA, 2010).
Anatase phase exhibits the highest photocatalytic activity and therefore it is used in catalysis and photocatalysis applications; rutile is known as white pigment providing opacity to paints, papers, inks, and consumer products such as toothpaste. Anatase and brookite are used as electrodes in dye-sensitized solar cells (Jiang et al., 2002). Such properties have led to the use of nano-TiO2 for a wide variety of
applications, including self-cleaning surface coatings, light-emitting diodes, solar cells, disinfectant sprays, sporting goods, sunscreens (EPA, 2009). For environmental applications, suspended TiO2 nanoparticles
have been largely used as efficient catalysts for the decomposition of organic contaminants present in water and aqueous wastes (Zhang et al., 2007).
A surface coating, for example silica and other compounds, can also be added to nanosized TiO2 to
decrease its photo-reactivity so that nano-TiO2 can be used to protect human skin, plastic, and other
objects from UV radiation (Menard et al., 2011).
Carbon based nanoparticles:
Fullerenes are applied for the sorption of organic compounds (e.g. naphthalene) and for the removal of organometallic compounds. CNTs and their derivates are used for the sorption of metals such as copper, nickel, cadmium, lead, silver, zinc, americium and rare earth metals in: electronics and computers, plastics, catalysts, battery, fuel cell electrodes, water purification systems, orthopedic implants, conductive coatings, adhesives and composites, sensors, and components in the electronics, aircrafts, aerospace, and automotive industries, as well as in sporting goods.
Quantum dots:
They are applied in medicine, e.g. medical imaging and targeted therapeutics, in solar cells, photovoltaic cells, security inks, photonics and telecommunications.
6 Zero-valent metals:
Zero-valent ions are used in nitrate removal from water, soil and sediments and also for detoxification of organochlorine pesticides and polychlorinated biphenyls, in bioremediation for the decomposition of molinate (a carbothionate herbicide).
Dendrimers:
They are applied in manufacture of macro-capsules, coloured glasses, chemical sensors, modified electrodes, as DNA transfecting agents, therapeutic agents for prion diseases, in drug delivery and DNA chips, in tumor treatment (used as a powerful anticancer drug).
It is worth noting that nanomaterials are associated with presumably revolutionary contributions to environment and sustainable development in terms of (Rickerby et al., 2007):
Environmental monitoring: more sensitive detection systems for air and water quality monitoring; Replacement in the use of hazardous chemical substances;
Energy and resource saving, thanks to lighter and stronger materials f vehicle production and to more efficient fuel cells;
Environmental remediation and treatment: for example zero-valent nanoparticles (as zero valent iron) are used in water remediation nanotechnology for in situ application to remove a wide variety of contaminants (heavy metals, pesticides, chlorinated organic solvents ect.).
1.3
Engineered nanoparticles: environmental concerns
With the increasing production of nanomaterials and the escalating promise of new and unique nanotechnology materials, concerns of occupational, safety, and environmental hazards are raising leading to some controversies in the nanotechnology debate. In fact, with the expected benefits of nanomaterials and nanoparticles, nanotechnology is still a largely unknown area and the consequences due to the widespread production and utilization of nanomaterials are difficult to predict.
Several important aspects in regard to the environment and risk assessment of ENPs are addressed: 1) to exposure assessment, and 2) to ecotoxicity.
1.4
Environmental exposure
Among nanoproducts not all will lead to environmental exposure (e.g. a semiconductor is unlike to lead to direct exposure during its use), but materials and products with the potential to release nanoscale
7
materials into environment, such as aereosol, powders, or suspensions of nanometer-diameter particles may lead to relevant exposure. Metal oxide nanoparticles are among the most used nanomaterials and receive attention over their potential effects. The widespread use of metal oxide nanoparticles (e.g. TiO2)
could lead to significant release of nanoparticles into the environment leading to a potential increased environmental exposure to nanoparticles (Hall et al., 2009). Particular attention has been posed on a freshwater ecosystem that seems to be an environmental compartment expecially affected by the release of these particles (Lovern and Kapler, 2006). The route of exposure of ENPs into the aquatic environment can be possible by accidental and intentional release (e.g. through environmental remediation efforts). The potential fate of nanoparticles in the aquatic environment and their interactions with aquatic organisms is illustrated in Fig. 1.2. Once there, their fate will depend on a number of factors such as presence of natural organic matter (NOM), ionic strength and pH. Currently very few data exist regarding observed environmental concentrations of TiO2 nanoparticles.
Fig.1.1: Possible route of environmental exposure of ENPs after realse into acquatic environment (Source: Baun et al., 2008) Kiser et al.,(2009) have measured the levels of titanium nanomaterial removed and released from wastewater treatment plants. They found out that raw sewages contain 100–3000 μg/L of Ti whereas its concentrations in effluents from wastewater treatment plants ranged from <5 to 15 μg/L. As Ti is removed, it accumulates in settled solids with concentrations ranging from 1 to 6 μg/mg. Mueller and Nowack, (2008) and Gottschalk et al., (2009) modelled the quantities of TiO2 nanoparticles released into
8
Reports show that metal oxide nanoparticles (e.g. TiO2) once introduced into water, will most probably
aggregate and partition onto sediments and suspended particulate matter (Boxall et al., 2007; Praetorius et al., 2012). Aggregated particles are generally less mobile and can interact with filter feeders and sediment-dwelling organisms (Farré et al., 2009). It has been argued that the environmental behavior of ENPs is strongly affected by the environmental chemical condition (e.g. pH, ionic strength, humic acids), in function of which different environmental behaviours may be expected (Navarro et al., 2008; Domingos et al., 2010).
Therefore, it is not clear, at this stage, how predicted environmental concentrations for nanoparticles can be calculated. The commonly used mathematical models will need adaptation for the assessment of the environmental distribution and dispersal of nanoparticles. This implies incorporation into the models of the key physic-chemical characteristics relevant to nanoparticles such as: surface area and morphology, charge, number of particles, size, solubility and potential chemical and physical conversion into other forms, as described earlier
(SCHENIHR, 2006)
Table 1.1: Predicted environmental concentrations of n-TiO2 into environmental compartments in different countries; a Mueller and Nowack, 2008; b Gottschalk et al., 2009. (Source: Menard et al., 2011)
Environmental compartment
Predicted environmental concentration
Switzerland Europe U.S.
Water 0.7–16 μg/L a 0.012–0.057μg/Lb 0.002–0.010μg/Lb 0.016–0.085 μg/Lb Soil 0.4–4.8μg/kg a 1.01–4.45μg/kgb 0.43–2.3 μg/kgb 0.21–1.04μg/kgb
Sludge treated soil / 70.6–310μg/kgb 34.5–170 μg/kgb
Sediment 426–2382μg/kgb 273–1409μg/kgb 44–251 μg/kgb
Air 0.0015–0.042μg/m
3a
0.0005μg/m3b 0.0005 μg/m3b
0.0007–0.003μg/m3b
Sewage treatment plant effluent 3.50–16.3μg/Lb 2.50–10.8μg/Lb 1.37–6.70 μg/Lb
Sewage treatment plant sludge 172–802mg/kgb 100–433 mg/kgb 107–523 mg/kgb
1.5
Ecotoxicity
The toxic potential of materials is different on a nano-scale for several reasons. Nanomaterials are theoretically expected to be more toxic than their bulk counterparts due to their greater surface reactivity and the ability to penetrate into and accumulate within cells and organisms. This can make materials more chemically reactive, and affect their functional properties such as mechanical strength or electrical properties. For example, as the size decreases, the number of atoms on the surface increases, with a conseguent increase of the biological reactivity, offering potential use in pharmaceutical industry as drugs delivers (Lovern and Kapler, 2006). Also, it is important to note that nanomaterials can be on the same
9
scale as elements of living cells, including proteins, lipids, nucleic acids, and organelles. Therefore, one must focus particular attention on how nanoparticles can interact with or influence biological systems, which may be desirable for certain medical applications, but may cause unanticipated hazardous effects upon occupational or environmental exposure to nanomaterials. For istance, the small size of the nanoparticles increases the rate of uptake and interaction with biological tissue, raising adverse biological effects; this wouldn’t be possible with the bulk material.
To date, the precise mechanisms of toxicity of metal oxide nanoparticles are largely unknown (Griffitt et al., 2008). Anyway, recent reports have shown that the toxicity of nanoparticles is generally governed by properties such as particle size, shape, chemical composition and surface properties (Crane et al., 2008; Navarro et al., 2008). For instance, n-TiO2 is photo-inducible, redox active and thus a generator of
potential reactive oxygen species (ROS) at its surfaces have been argued. However, the precise mechanisms of toxicity of nanosized TiO2 and other metal nanoparticles are largely unknown (Griffitt et
al., 2008).
1.6
Are ENPs environmental sustainable?
ENPs are expected to affect living organisms but due to the high variability of the toxic data reported, it is difficult to characterize their ecotoxicological hazard. The environmental fate of ENPs is far to be modelled and predicted, and it is still uncertain how ENPs would behave in the environment. Although environmental concentrations of manufactured nanoparticles (ENPs) have yet to be routinely measured, there are concerns that ENPs will be released from these products over their life (e.g., by erosion of the materials with use, or deliberate introduction during remediation of contaminated environmental media), or that product applications could generate wastes containing nanomaterials (e.g., domestic waste-water containing nanomaterials from household products). It is also unclear whether or not sewage treatment works could completely remove ENPs from final effluents. Therefore, despite the fact that nanoproducts are already released into environment (Som et al., 2010 ), environmental concerns on production, use and end of life of nanomaterials are raised. It cannot be overlooked that methods are needed to assess whether the potential benefits of nanotechnology outweigh the risks.
The benefits and potentials are currently neither completely substantiated by an assessment of ecological and human health risks or by a holistic assessment of all aspects along the life cycle of nano based products and services (Som et al., 2010).
An holistic environmental sustainability assessment of products requires the evaluation of both material and energy input and environmental releases of the life-cycle stages. Moreover, to minimise the environmental impact and achieve sustainability, material loops must be closed and it is essential to
10
obtain an accurate estimate of the full environmental impact. Life cycle assessment (LCA) is a useful technique for calculating energy and raw material requirements for a product’s manufacture, use and final disposal or re-use and for assessing the true environmental impacts (Rickerby and Morrison, 2007). In fact, due to its holistic and comprehensive perspective LCA has been recognized as a key tool for assessing the environmental performance of nanoproducts and, furthermore, for comparing a product that includes ENMs with similar products without ENMs (Klopffer, 2007).
11
1.7
B
ibliography
Auffan M., Rose J., Bottero J.Y., Lowry G.V., Jollivet J.P., Wiesner M.R. «Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective.» Nature Nanotechnology 4, n. 10 (2009): 634-641.
Baun A., Hartmann N.B, Grieger K., Kusk KO. «Ecotoxicity of engineered nanoparticles to aquatic
invertebrates: a brief review and recommendations for future toxicity testing.» Ecotoxicology 17 (2008): 387-395.
Bhatt I., Tripathi B.N. «Interaction of engineered nanoparticles with various components of the
environment and possible strategies for their risk assessment.» Chemosphere 82 (2011): 308-317. Boxall A., Tiede K., Chaudhry Q. «Engineered nanomaterials in soils and water: how do they behave and
could they pose a risk to human health?.» Nonomedicine 2, n. 6 (2007): 919-927. Crane M., Handy R.D., Garrod J., Owen R. «Ecotoxicity test methods and environmental hazard
assessment for engineered nanoparticles.» Ecotoxicology 17 (2008): 421-437.
Domingos R.F., Peyrot C., Wilkinson K.J. «Aggregation of titanium dioxide nanoparticles: role of calcium and phosphate.» Environ. Chem. 7 (2010): 61-66.
(EPA 2010), United States Environmental Protection Agency. «Nanomaterial Case Studies:Nanoscale Titanium Dioxide in Water Treatment and in Topical Sunscreen.»
Farré M., Gajda-Schrantz K., Kantianu L., Barcelò D. «Ecotoxicity and analysis of nanomaterials in the aquatic environment.» Anal. Bioanal. Chem. 393 (2009): 81-95.
Feldheim D.L. «The new face of catalysis.» Science 316, n. 5825 (2007): 699-700.
Gottschalk F., Sonderer T., Scholz R.W., Nowack B. «Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions.» Environ. Sci. Technol. 43
(2009): 9216-9222.
Griffitt R.J., Luo J., Gao J., Bonzongo J-C., Barber, DS. «Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms.» Environ.Toxicol. Chem. 27, n. 8 (2008): 1972-1978.
Hall S., Bradle T., Moore J.T., Kuykindalla T., Minella, L. «Acute and chronic toxicity of nano-scale TiO2
particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on TiO2 toxicity.» Nanotoxicology 3, n. 2 (2009): 91-97.
Handy R.D., Von der Kammer F., Lead J.R., Hassellov M., Owen R., Crane M. «The ecotoxicology and chemistry of manufactured nanoparticles.» Ecotoxicology 17 (2008): 287-314.
(ISO, 2008) International Organisation for Standardisation. Nanotechnologies-Terminology and definitions for nano-objects. Nanoparticle, nanofibre and nanoplate. ISO/TS 27687, Geneva: International Organisation for Standardisation.
12
Kiser M.A., Westerhoff T.B., Wang Y., Perez-Rivera J., Hristovski K. «Titanium nanomaterial removal and release from wastewater treatment plants.» Environ. Sci. Technol. 43 (2009): 6757-6763. Klopffer W., Curran M.A., Frankl P., Heijungs R., Kohler A., Olsen S.I. «Nanotechnolgy and life cycle
assessmnet-a systems approach to nanotechnolgy and the environment.» Washinton DC, 2007. Kreyling W.G., Semmler-Behnke M., Chaudry Q. «A complementary definition of nanomaterial.» Nano
Today 5, n. 3 (2010): 165-168.
Lovern S.B., Kapler R. «Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles.» Environ.Toxicol.Chem. 24, n. 4 (2006): 1132-1137.
Menard A., Drobne D., Jemec A. «Ecotoxicity of nanosized TiO2. Review of in vivo data.» Environ. Pollut.
159, n. 3 (2011): 677-684.
Mueller N.C., Nowack B. «Exposure modeling of engineered nanoparticles in the environment.» Environ.
Sci. Technol. 42 (2008): 4447–4453.
Navarro E., Baun A., Renata B., Hartmann N.B., Filser J., Miao AJ., Quigg A., Santschi PH., Laura S.
«Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi.»
Ecotoxicology 17 (2008): 372-386.
Nel A., Xia T., Madler L., Li N. «Toxic potential of materials at the nanolevel.» Science 311, n. 622 (2006): 622-627.
Oberdörster G., Stone V., Donaldson K. «Toxicology of nanoparticles: a historical perspective.»
Nanotoxicology 1, n. 1 (2007): 2-25.
Praetorius A., Scheringer M., Hungerbuhler K. «Development of environmental fate models for engineered nanoparticle-a case study of TiO2 nanoparticles in the Rhine river.» Environ.Sci.Technol. 46, n. 12
(2012): 6705–6713.
Project on Emerging Nanotechnologies, Woodrow Wilson International Center for Scholars (WWI).Available at
http://www.nanotechproject.org/process/assets/files/8278/pen_submission_cpsc.pdf [Accessed 13/01/2013]
Rickerby D.G., Morrison M. «Nanotechnolgy and the environment: an european perspective.» Sci.
Techn.Adv. Mat. 8, n. 1-2 (2007): 19-24.
(SCHENIR, 2006) Scientific Committee on Emerging and Newly Identified Health Risk. «The
appropriateness of existing methodologies to assess the potential risk associated with engineered and adventitious products of nanotechnologies.» 2006.
Som. C., Berges, M., Chaundhry, Q., Dusinka, M., Fernandes, T.F., Olsen, S.I., Nowack, B. «The importance of life cycle concenpts for the developmnet of safe nanoproducts.» Toxicology 269 (2010): 160-169.
Zhang X., Sun H., Zhang Z., Niu Q., Chen Y., Crittenden JC. «Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles.» Chemosphere 67 (2007): 160-166.
13
2
Life Cycle Assessment Methodology
Life Cycle Assessment (LCA) is a standardized methodology (ISO, 2006a,b) for determining and assessing the environmental impacts of products across their whole life cycle, for comparing different options/products with respect to their potential impacts on the environment, and for identifying the critical points within the product life cycle that contribute most to these impacts. The key environmental issues which are considered in an LCA include the following: climate change, stratospheric ozone depletion, tropospheric ozone (smog) creation, eutrophication, acidification, toxicological stress on human health and ecosystems, depletion of resources, water use, land use, noise, and ionizing radiation. This framework is applied to any kind of product and to any decision where environmental impacts are of interest and by a broad variety of actors – from governmental organisations to industry.
According to ISO Standards 14040 (ISO, 2006a,b), LCA is conducted in four main phases (Fig.1): (i) defining the goal and scope of the study, (ii) establishing a life-cycle inventory which aggregates all inputs from and outputs to the environment within the system boundaries, (iii) performing a life-cycle impact assessment which translates the inventory into potential impacts of the system on the environment and (iv) interpreting the results from the assessment to provide consistent support to decision-makers in relation to the goal and scope of the study.
Figure 2.2: Phase and application of an LCA (Source: ILCD Handbook, 2011)
2.1 LCA: The four phases
In accordance with ISO:14040 (ISO, 2006a) the procedure of carrying out an LCA is organized in the following four steps.
14
2.1.1 Goal and scope definition
The goal and scope of an LCA shall be clearly defined and shall be consistent with the intended application. In defining the goal of an LCA, the following items shall be unambiguously stated: the intended application, the reasons for carrying out the study, the intended audience, whether the results are intended to be used in comparative assertions intended to be disclosed to the public. In defining the scope of an LCA. The scope of the study must clearly describe the system of the studied product or process and its boundaries, the included items and the items to be evaluated, the system functions, the functional unit, the impact categories, the methodology applied, and finally, the necessary assumptions and restrictions. Where:
Functional unit: the functional unit defines what precisely is studied and quantifies the service delivered by the product system, providing a reference to which the inputs and outputs can be related.
System boundaries: boundaries define which processes in the products life cycle are included in the LCA. Data: the data should include all inputs and outputs from the processes. Inputs are, for example, the use of energy, water, materials, etc. Outputs are the products, co-products and emissions. Emissions can be divided into four categories: air, water, soil and solid waste depending on what the emissions affect. A lot of databases with LCA data exist and they are suitable with the LCA software. Data can also be collected through national statistics or bibliographic reviews.
2.1.2 Life cycle Inventory
In this phase all mass and energy flows into and out of the system are balanced. All these flows are listed and calculated in relation to the functional unit. For all activities throughout the product life cycle (production, transportation, use and waste treatment processes ), the required materials and energy and the emissions and solid waste are assessed.
In this phase the allocation of sub-products may occurs; it is defined as the partitioning of the input and/or output flows of a process to the product system under study. It is required where a single production system produces more than one good, to proportion the environmental impacts of the production system to those different economic goods. Also, it becomes necessary when waste materials are recycled and reused instead of the primary materials.
2.1.3 Life cycle Impact Assessment
Life cycle impact assessment (LCIA) is the phase in LCA where the inputs and outputs of elementary flows collected and reported in the LCI are translated into impact indicator results related to human health, natural environment, and resource depletion” (ILCD Handbook, 2011). Thus, in the Life Cycle Impact Assessment phase the potential environment impacts in a number of impact categories are calculated through relating the single input and output flows to the environmental impact they may cause.
15
The potential impact for each of the impact category (e.g. acidification, global warming) are expressed in terms of Impact Score:
ISj = CFi,j x mi,J
Where:
CF: is the characterisation factor (for human toxicity case/kg) for the substance i for the impact category j (e.g. human toxicity); m i,j is the mass (kg emitted) of the substance emitted and classified within the
impact category j
The phase of Life Cycle Impact Assessment is composed by a series of steps, some of which are compulsory whereas other are optional according to the ISO standard.
Classification (obligatory): The inventory results are classified according to the type of environmental impact that they may cause. The impact categories are identified (e.g. global warming potential, acidification, human toxicity, etc.).
Characterisation (obligatory): In this step the impacts are quantitatively characterized; all the substances contributing to the same impact category have to be translated from a mass or energy load into an impact load, ending up with one specific unit for each category.
In this step the so-called characterisation factors (CF) are applied. The characterisation factors are substance-specific and are based on models of cause-effect chains that describe the behaviour of a substance in the environment. For example, for the impact category of Global Warming Potential (GWP), all the GHG emissions (in the LCI phase express in kg) will be converted to the common unit of this impact category (e.g. CO2 eq.). This results in a numerical indicator outcome, i.e. the LCIA profile for the product
16
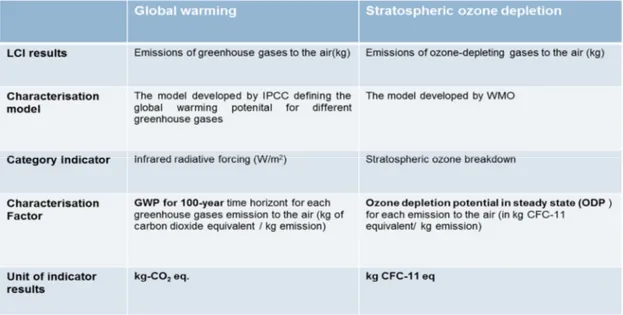
Table 2.2: Example of the characterisation steps for the impact category of Global warming and Stratospheric ozone depletion
Normalization (optional): The impact per category can be normalized to a certain magnitude, for example to the total impacts arising in a country.
Grouping (optional): It possible to summarize the specific impact categories (e.g. human toxicity, freshwater toxicity, acidification, etc.) into the three Areas of Protection: human health, ecosystem quality and resource.
Weighting (optional): The environmental effects of chosen impact categories can be weighted; where the weights are assigned to the different impact categories and resources reflecting the relative importance, they are assigned in the study in accordance with the goal of the study.
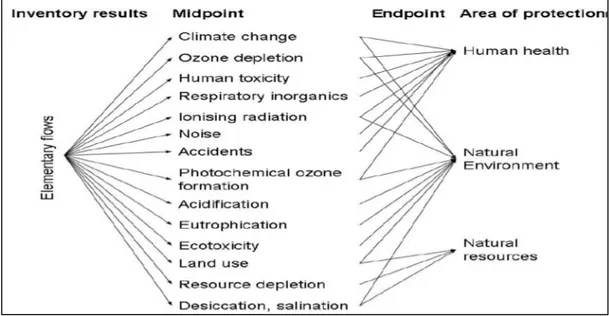
The life cycle impact assessment covers impact categories that have been recognized as non-toxic or toxic. Within the first group for example the climate change, acidification, and resource depletion are classified. In contrast, human toxicity and ecotoxicity represent the toxic impact categories. Furthermore, the impact categories are distinguished in midpoint or endpoint. The distinction among midpoint or endpoint categories is based on the point in which the indicator is chosen along the impact pathway. Characterisation at midpoint level models the impact using an indicator located somewhere along (but before the end of) the environmental impact pathway of a substance. Characterisation at the endpoint level requires modelling all the way to the impact on the entities described by the Area of Protection i.e. on Human Health, on the Natural Environment and on Natural Resources (Fig.2.2). Also, the environmental impacts on different geographical scale are referred:
Global Impacts
Global Warming: Polar melt, soil moisture loss, longer seasons, forest loss/change, and change in wind and ocean patterns.
17 Ozone Depletion: Increased ultraviolet radiation.
Resource Depletion: Decreased resources for future generation. Regional Impacts
Photochemical Smog: decreased visibility, eye irritation, respiratory tract and lung irritation, and vegetation damage.
Acidification: building corrosion, water body acidification, vegetation effects, and soil effect. Local Impacts
Human Health: increased morbidity and mortality.
Terrestrial Toxicity: decreased production and biodiversity and decreased wildlife for hunting or viewing.
Aquatic Toxicity: decreased aquatic plant and insect production and biodiversity and decreased commercial or recreational fishing.
Eutrophication: nutrients (phosphorous and nitrogen) enter water bodies, such as lakes, estuaries and slow-moving streams, causing excessive plant growth and oxygen depletion.
Land Use: loss of terrestrial habitat for wildlife and decreased landfill space.
Fig. 2.2: LCIA, midpoint and endpoint impact categories (ILCD Handbook, 2011)
2.1.4 Interpretation
After establishing the inventory and assessing the environmental impacts based on the inventory the results have to be further analysed, presented and discussed. In this phase, sensitivity analysis can be performed in order to check the robustness of the result; also uncertainty analysis may be applied to interpreting the results of the life cycle assessment.
18
2.2 LCA and nanotechnology
As argued, LCA has been recognized as a powerful “tool” to assessing the environmental performance in the field of nanotechnology. The state of art on “LCA and nanotechnology“ in the following sub-chapter is presented and discussed.
2.2.1 Goal and scope in the nanotechnology LCA study
In an LCA study all relevant resource and energy inputs and all relevant outputs of a system are related to the functional unit which serves as the object of investigation. This functional perspective allows establishing a denominator to compare the performance of alternatives which are not comparable in absolute terms.
Among the studies conducted on nanoproducts, most of them adopt the mass as functional unit e.g. 1 kg of nanomaterial. Whereas, for comparative LCA study with the aim to compare one product based on nanomaterial with a conventional product, the weight-based functional unit is not correct since the comparison has to be carried out on the basis of a system function. For instance, the study [14] is focused on the application of carbon nanofibres in a polymer composite (before the same authors studied the production of carbon nanofibers [10]). The objective of the study is to compare traditional material such as steel with a polymer composite with a mechanical stiffness or strength equal to those of steel. The author shows that to achieve the same functionalities only 0.38 kg of polymer nanocomposite is necessary instead of 1 kg of steel. Thus, a comparison of nanofibers and steel on an equal weight basis does not reflect the actual replacement; a comparison at the level of the functionality shall be performed. However, other studies may focus on specific applications (case) of the respective materials and uses a context –related functional units (e.g. 1 m2 of photovoltaic cell in study [15]).
Looking to the various studies on nanoproducts, the system boundaries: 1) cover all life cycle stages (e.g. cradle to grave) from the extraction of the resource until the end of life (e.g. disposal /recycling) or 2) are defined as cradle-to-gate, with the gate being on the level of factory gate of the production site of engineered nanomaterials (e.g. carbon nanotubes in study [10]) or 3) are defined on the level of the factory gate of the nano-enabled products (e.g. carbon nanofibers polymer composite as in [15]) (Fig.2.3).
19
Fig.2.3: Stages of the nanoproducts life cycle.
The first life stages (extraction of raw materials, production of precursors and production of engineered nanomaterial) are covered by all the studies reported in Table 2.1. On the contrary, few studies cover the use phase and generally information on the use phase is not easily available and therefore not reported. Several environmental advantages (e.g. environmental remediation applications, energy saving) are claimed with the use of nanoproducts. However, potential release of nanoparticles during the use phase has been outlined raising concerns on the human and environmental toxicity (Gottschalk et al., 2010). Thus, further efforts on the analysis of the use phase should be carried out to evaluate the “real” benefits of the use of nanoproducts.
The analysis of the end-of-life (EOL) of nanomaterials, as disposal to landfill or incineration in waste incineration plants, is critical because it has not yet been subject of investigation. Furthermore, significant impacts for the environment may arise due to the ambient emissions and little is known about the environmental degradation (e.g. in landfill) of nanoproducts (Som et al., 2010). To date, only few LCA studies cover the phase of end of life. Meyer et al. (2010) did not consider the EOL phase because the nano-silver was assumed to have been washed off during the use phase and the EOL was assumed to be the same as for non-nano-silver product. Moreover, when a study covers the EOL, several assumptions are performed. For instance, Bauer et al. (2008) reported only a qualitative description of the EOL in the case study of CNT in electronic sector. Most of the studies (cradle-to-grave) assume as end of life the incineration in municipal solid waste incineration plants, where models for traditional chemical incineration are adopted (Hischier and Walser, 2012). Due to the lack of information about the behavior of ENM during the waste incineration, the fate of engineered nanomaterial is not accounted(e.g. Table 2.1 LCA study [6]). It is unknown what ENM fraction remains in the slag and what percentage becomes air bones or degrades under incineration condition (Som et al.,. 2010). To date, the release into atmosphere of nanoparticles due to incineration process is estimated by a fate model based on the removal efficiency of the incineration plant and by treating the nanoparticle as “particulate matter”. In accordance to a fate modeling study (Gottschalk et al., 2010), the release of nanoparticles into atmosphere based on the removal efficiency (99.9%) of multistage flue gas cleaning filters for particles smaller than 100 nm has been estimated being 0.1%. Another open question is the “recyclability” of the nanostructured materials containing ENMs; little literature appears to have been evolved around nanoproducts recycling. The low number of citations may be related to the lack of development for recycling infrastructure technology or to the cost. The recycling technology for nanoproducts might benefit from further development of the technology (Asmatulu et al., 2012).
20
2.2.2 Life cycle inventory in the nanotechnology LCA study
An adequate LCI data on materials is necessary for an appropriate Life Cycle Impact Assessment. Therefore adequate and comprehensive LCI data for engineered nanomaterials are requested. To date, the LCA studies on nanomaterials are based on LCI data of publicly available literature or, in few cases, on pilot/commercial plants.
The input data on the extraction of raw materials, the production of precursors, the request of energy for the production of engineered nanomaterials are available in the current LCI data –bases (ETH database, Simapro7, Ecoinvent databes ecc.). In contrast, the life cycle inventory data on emissions (output data) to air, water or soil is scarcely covered (Hischier and Walser, 2012). Currently, two issues may be drawn about the life cycle inventory analysis concerning the engineered nanomaterials.
Firstly, specific physical-chemical properties may be required on the nanomaterials. The second one concerns the knowledge of the production processes of nanoproducts and the potential emissions during all life stages of the nanoproducts. In standard LCI tables, only the quantity and the chemical composition of releases are reported; generally, few chemicals require additional characteristics such as, their isotope (for radioactive releases), their stereo-isomer (for a chemical like cyclohexane) or their valence (for an ion such as chromium). On the contrary, several parameters influence fate, exposure, and effect of nanoparticles in the environment. For instance, chemical composition, particle size, shape, crystal structure, surface charge, solubility and adhesion properties likely influence the toxicity of nanomaterials. Moreover, as nanoparticles may also be coated, it is important to find out whether to report the pure material or the coated material. In this context, it is also important to know whether nanoparticles change their form (shape, coating, etc.) during their life cycle, for instance, due to aging and other influences such as weather, mechanical stress/pressure, electromechanical fields or catalysis. As a result, the elementary flows characterizing nanomaterials in the inventory may require that these additional characteristics be described. The production of data of nanomaterials and structure shall be based on precise and comprehensive LCI data with high level and representativeness. As nanoproducts are only starting to enter the market, it is at present unclear how processes related to use, maintenance and end-of-life services (e.g. disposal, recycling) will proceed. Some materials will be released during use, both intentionally and unintentionally (e.g., nano-additives in tires or nanoparticles in sunscreen). Exact release rates are not always available, especially when they are condition-dependent and the behaviour of nanomaterials discarded after use is also not yet clear. For instance, their reaction with other materials in an incinerator or at a dump site is uncertain, yet these are required data in an LCA study (Asmatulu et al., 2012).
21
2.2.3 Life Cycle Impact Assessment in the nanotechnology LCA study
As argued before, the LCIA phase requires the knowledge on the toxic effect following the release of nanomaterials/nanoparticles to the environment. For LCIA proposal and for the impact category of aquatic ecotoxicity, the quantification of the toxic effect is based on toxicity values collected from the main databases (e.g. IUCLID, International Uniform Chemical Information Database).
Currently, due to the lack of a specific database for nanoparticles and/or nanoproducts toxicity, data have to be collected by means of bibliographic reviews or literature where a strong variability of the toxic data on ENPs is reported. The high variability of the toxic data for ENPs and the lack of specific ENPs-fate models are both referred to as the reason an incomplete life cycle impact assessment phase on ENMs in the LCA studies performed until now.
The reviews performed by Hischiers and Walser (2012) and Gavankar et al. (2012) show that the phase of impact assessment is not complete in the sense of ISO 14040 series. The LCA studies on ENMs not do cover a complete life cycle of engineered nanomaterials or products. Most of the studies are cradle-to-gate and the environmental impacts are correlated to the energy and material flows for the extraction of raw materials and manufacturing phases (cradle-to-gate analysis). All this without considering the nano-specific fate, transport, and the toxicity and ecotoxicity. Although aspects relating to (eco)toxicity are usually assessed in LCA, the specific potential impacts of ENMs have not been included in the studies done so far, due to a lack of knowledge in relation to risk assessment.
This bottleneck is reflected in LCIA where characterization factors for nanoparticles are completely missed. To my knowledge only two recent studies (Eckelman, et al., 2012; Walser et al., 2011) assessed the potential toxic impact of nanoproducts. Eckelman et al. (2012) quantified and compared aquatic ecotoxicity impacts over the life cycle (production, use and release) of carbon nanotubes (CNTs) by employing the USEtox model. Walser et al. (2011) performed a cradle-to-grave LCA study to compare nanosilver T-shirts with conventional T-shirts with and without biocidal treatment (triclosan), thus assessing global warming potential, freshwater and seawater toxicity (1,4 kg-DCB-eq.).
In LCIA, the characterisation of toxic impact categories (e.g. freshwater ecotoxicity) requires the qualitative and quantitative knowledge of the exposure to a substance.
The characterisation factor of a substance is developed on the basis of 1) “fate and exposure model” (e.g. USEtox, Rosenbaum et al., 2008) which calculates the environmental concentration at which the organisms are exposed and of 2) its toxicity potential (e.g. concentration of toxic effect).
22
Table 2.1 (a): LCA studies of engineered nanomaterials; Source (Gavankar et al., 2012) (Hischier et al., 2012)
A u th o r T y p e o f st u d y C o v e re d n a n o m a te ri a ls F o cu s o f th e s tu d y F u n ct io n a l u n it L C A p h a se Im p a ct a sse ss m e n t [1 ] G re ij e r 2 0 1 1 LCA , c ra d le -t o -g ra v e N a n o cr y s ta ll in e d y e 1 k W h e le c tr ic it y o u tp u t fr o m t h e s o la r c e ll s y s te m A ll p h a s e s G lo b a l w a rm in g [2 ] L lo y d a n d 2 0 0 3 ; L lo y d 2 0 0 4 H y b ri d L CA P o ly m e r n a n o c o m p o s it e ( b a s e d o n n a n o c la y ) R e p la ci n g a u to -b o d y p a n el s ma d e o f s te e l w it h t h o s e i n p o ly m e r c o m p o s it e s a n d a lu m in iu m E x tr a c ti o n a n d m a n u fa c tu ri n g G lo b a l w a rm in g a n d t o x ic r e le a s e s ; T o x ic it y f o r s c e n a ri o b a s ed m a te ri a l c o m p o s it io n o f e v a lu a te d o n n a n o /n o n -n a n o o p ti o n s . T o x ic it y a n d m a n u fa c tu ri n g o f th e n a n o c o m p o s it e i ts e lf a re n o t d is cu ss e d S te in fe ld t 2 0 0 4 a ; S te in fe ld t 2 0 0 4 b [4 ] Ll o y d 2 0 0 5 H y b ri d L CA N a n o s c a le p la ti n u m g ro u p (P G M ) me ta l p a rt ic le s E v a lu a ti n g r e d u c ti o n i n n o n -re n e w a b le r e s o u rc es l ik e P G M v ia g re a te r p ro c e ss c o n tr o l o ff e re d b y n a n o te c h To ta l P G M d e m a n d f o r th e U S v e h ic le f le e t E x tr a c ti o n a n d m a n u fa c tu ri n g G lo b a l w a rm in g a n d v a ri o u s (c u m u la ti v e) i n v e n to ry i te m s . T h e fo c u s i s o n h o w n a n o te c h n o lo g y c a n h e lp r e d u c e t h e u sa ge o f p re c io u s m e ta l w it h in t h e s a m e p ro c e ss w it h th e s a m e o u tp u t a s b e fo re . [5 ] O s te rw a ld e r 2 0 0 6 E n e rg y a n d C 02 a n a ly s is , c ra d le -t o -g a te N a n o p a rt ic le o f ti ta n iu m d io x id e a n d z ir c o n ia E n e rg y c o m p a ri s o n o f w e t a n d d ry s y n th e s is m e th o d s f o r o x id e n a n o p a rt ic le p ro d u c ti o n 1 t o n o f n a n o m a te ri a ls M a n u fa c tu ri n g F o c u s o n t h e l e v e l o f c o n s u m e d e n e rg y a n d p ro d u c e d CO 2 e mi s s io n s . R e le a se a n d i m p a c t o f e m is s io n , a s s o c ia te d t o x ic it y d u ri n g n a n o -m a n u fa c tu ri n g a n d n a n o -s y n th e s is n o t c o n s id e re d . [6 ] R o e s 2 0 0 7 LCA , c ra d le -t o -g ra v e P o ly m er n a n o c o m p o s it e Co m p a re e n v ir o n m e n ta l im p a ct s a d c o s ts w it h n a n o c o m p o s it e p ro d u c ts v is -a -v is t h o s e w it h c o n v en ti o n a l p ro d u c ts (i )a m o u n t o f p a ck a g in g f il m f o r 1 0 0 0 b a g s fo r 2 0 0 g c a n d ie s ( ii ) a m o u n t o f fi ll t o c o ve r a s ta n d a rd g re e n h o u s e o f 6 5 0 m 3 (i ii ) b o d y p a n e ls t o d ri v e 1 5 0 .0 0 0 km A ll p h a s e s V a ri o u s m id p o in t in d ic a to rs ( CM L m e th o d s ). H u ma n a n d e c o to x ic it y n o t c o n si d e re d . U s e p h a s e a s s u m e d to b e t h e s a m e f o r n a n o a n d c o n v e n ti o n a l [7 ] K u sh n ir a n d Sa n d e n 2 0 0 8 E n e rg y a n a ly s is , LC A c ra d le t o g a te C a rb o n n a n o p a rt ic le s e .g . F u ll e re n e a n d n a n o tu b e s Im p li c a ti o n s f o r in d u s tr ia l s c a le p ro d u c ti o n 1 k g CN P E x tr a c ti o n a n d m a n u fa c tu ri n g E n e rg y a n a ly s is ; n o d is c u s si o n o n n a n o -s p e c if ic e n v ir o n m e n ta l im p a c ts [8 ] H e a ly 2 0 0 8 LCA ,c ra d le -t o -g ra te S in g le -w a ll c a rb o n n a n o tu b e s ( S W CN T ) E n v ir o n m e n ta l a s s e ss m e n t o f S W N T p ro d u c ti o n 1 k g S W CN T E x tr a c ti o n a n d m a n u fa c tu ri n g A ir b o rn e i n o rg a n ic s , c li m a te c h a n g e, a c id if ic a ti o n . Q u a li ta ti v e d e s c ri p ti o n o f E H S c o n ce rn s d u e t o n a n o m a te ri a l N o q u a n ti fi e d e v a lu a ti o n a s p a rt o f LCA s tu d y [9 ] S in g h 2 0 0 8 ; A g h o o ia 2 0 0 5 LCA ,c ra d le -t o -g ra te S in g le -w a ll c a rb o n n a n o tu b e s ( S W CN T ) E IA v ia L CA m e th o d ( c ra d le -t o - g a te (? )) 5 9 6 K G O F C A R B O N N A N O T U B E S P E R H O U R E x tr a c ti o n a n d m a n u fa c tu ri n g U s e o f T R A CI m e th o d s [1 0 ] K h a n n a 2 0 0 8 E n e rg y a n a ly s is , LC A c ra d le -t o - g a te Ca rb o n n a n o fi b e r (C N F ) 1 k g CN F E x tr a c ti o n a n d m a n u fa c tu ri n g V a ri o u s m id p o in t in d ic a to rs (G W P ,A P .. )f ro m C M L me th o d . R e le a se a n d i m p a c t o f CN F o n h u m a n s a n d e c o s y s te m d u ri n g m a n u fa c tu ri n g n o t c o n s id e re d V a ri o u s mi d p o in t in d ic a to rs [3 ] LCA , c ra d le -t o -g ra v e (i )N a n o v a rn is h ,( ii ,i ii ) c a rb o n n a n o tu b e s, ( iv ) q u a n tu m d o ts Li g h ti n g , Ch e m ic a l/ p a in ti n g s , Ch e m ic a l/ p la s ti c , E le c tr o n ic s /d is p la y s (i ) s u rf a c e t re a tm e n ts o f 1 m 2 me ta l s u rf a c e , (i i) 1 k g S ty ro l, ( ii i) 1 7 "f la t s c re en , ( iv ) 6 .5 7 9 M io Lu me n h o u rs A ll p h a s e s