Scuola Superiore Sant'Anna di Studi Universitari
e di Perfezionamento
PhD Program in Biorobotics
Curriculum Biorobotics
Synthesis and functionalization of innovative
nanomaterials for biomedical applications
PhD Candidate: Daniela Pignatelli
Tutor: Supervisors:
Prof. Paolo Dario Dr. Virgilio Mattoli Prof. Pietro Favia
2
A mia madre che mi ha sostenuto con sacrificio e coraggio in tutto questo percorso.
A mio padre che mi ha sempre vegliato dall'alto e a cui dedico queste parole:
"Quando qualcuno che abbiamo amato viene a mancare, Niente sarà più lo stesso. Tu non sarai più lo stesso. Perché chi se ne va… porta via anche qualcosa di te. Quella spensieratezza, quella sicurezza, quella fiducia … Tutto ciò che avevi affidato alle sue mani così care. Ma chi va via ti lascia qualcosa di sé. Quei ricordi spensierati che ti saranno di conforto nei momenti di maggiore tristezza.
Preziosa memoria che non ti abbandonerà mai. Tua per sempre. Vostra. Come un tempo. Come sempre. Quella sicurezza che ritroverai nelle giornate di pianto, guardando una stella, lasciando che il vento ti scuota e ti accarezzi. Infine. Quella fiducia che ora dovrai avere in te stesso, forte di quei consigli, di quelle parole scolpite ormai nella tua memoria. E che saranno balsamo sulle ferite e sostegno. Saranno motivazione. Un’indicazione puntuale ad ogni bivio della tua vita. E coraggio. E ti lascerà quel sorriso che ritroverai in un passante, nella tua mano quando si fermerà a compiere un gesto di compassione. Ti lascerà la sua voce che ritroverai tra le note di una canzone che non smetterai di ascoltare e che non smetterà di scandire i tuoi passi. Colonna sonora di un affetto cui la morte ha dato solo l’immortalità… Ti lascerà quel brivido improvviso che sarà il suo abbraccio caro, quel profumo improvviso che verrà a raccontarti una vicinanza che non ha fine mai. Che va oltre tutto quel dolore che ora ti stringe il petto. Oltre quello schianto, quell’esplosione che senti nel cuore. No, quando qualcuno muore, non ti lascia per sempre. Cambia forma, cambia il suo modo di dimostrarti amore. Ma resta con te.
E non porta tutto via con sé. Ti lascia la vita. Una vita di cui essere degno, in sua memoria. Per amor suo oltre che tuo.”
3
To my mother who supported me with sacrifice and courage throughout this journey.
To my father who is always watching over me and to whom I dedicate these words:
"When Someone We Loved Comes to Fail, Nothing will be the same anymore. You will never be the same again. Because whoever goes away ... takes away something of you, too. That carefree, that security, that trust ... Everything you had entrusted to his hands so dear. But who goes away leaves you something of himself. Those carefree memories that will comfort you in moments of greater sadness. Precious memory that will never leave you. Yours forever. As before. As always. That safety that you will find again in the days of weeping, looking at a star, letting the wind shake you and caress you. Finally. That trust that you must now have in yourself, strong of those advices, of those words already carved in your memory. And that will be a balm on wounds and a support.
They will be a motivation. A timely indication at every crossroads of your life. And courage. And it will leave you with the smile you will find in a passer-by, in your hand when you stop and make a gesture of compassion. It will leave his voice that you will find among the notes of a song that you will not stop listening and that will not stop to mark your steps. Soundtrack of an affection to which death has only given immortality ... It will leave you with that sudden shiver that will be his dear embrace, that sudden scent that will come to tell you a closeness that never ends. That goes beyond all the pain that now tightens your chest. Beyond that crash, that explosion that you feel in your heart. No, when someone dies, it does not leave you forever. It changes shape, the his way of showing you love. But stay with you. And it does not take it all with it. It leaves you life. A life to be worthy of, in his memory. For his sake as well as yours”
4
Acnowledgments
A conclusione di questo importantissimo traguardo, è doveroso ringraziare tutte le persone che in questi 4 anni mi sono state vicine e mi hanno sempre sostenuto e supportato sia scientificamnete che umanamente.
Ringrazio prima di tutto il Prof. Dario come Tutor di questa attività di ricerca. Per chi come me è ancora giovane nel modo della ricerca, la figura del Prof. Dario rapprensenta un esempio di grande valore scientifico.
Vorrei ringraziare il Dott. Virgilio Mattoli che mi ha accolto nel suo gruppo di ricerca e mi ha sostenuto scientificamente e spesso a distanza in questa attività di ricerca. Vorrei ringraziare di cuore la Dott.ssa Silvia Taccola per il suo prezioso aiuto durante gli esperimenti e il Dott. Francesco Greco per il prezioso contributo con commenti e suggerimenti.
Un grazie speciale al Prof. Pietro Favia che ha permesso la realizzazione di una preziosa collaborazione che ha portato alla nascita di questa attività di ricerca. Lo ringrazio perchè i suoi laboratori sono stati per me la mia seconda casa.
Un semplice “grazie” è riduttivo per ringraziare il Dott. Roberto Gristina per quello che da 8 anni mi ha dato umanamente e scientificamente. Lo ringrazio per gli anni trascorsi a lavorare assieme, per aver cercato sempre di tirare fuori il meglio di me e per avermi aiutato e sostenuto nei vari momenti di difficoltà che non sono mai mancati. Lo ringrazio per avermi aiutato a crescere anche nelle incomprensioni e nei momenti di scontro. In tanti momenti è stato per me come un secondo padre.
5
Grazie alla Dott.ssa Sardella il cui contributo umano e scientifico è stato fondamentale alla realizzazione di questa attività di ricerca.
Vorrei ringraziare il Dott. Comparelli per l’aiuto che ho ricevuto con molte analisi.
Un grazie speciale ai tecnici Danilo e Savino per il lavoro che svolgono con cuore ed efficienza, per l’infinità disponibilità e per la loro grande umanità. Grazie per le pause pranzo trascorse assieme.
Grazie al Porf. Santin che mi ha accolto per 7 mesi nei laboratory dell’Università di Brighton dove ho svolto la mia esperienza all’estero.
Ma sopra ogni cosa ringrazio di cuore i miei familiari, parenti ed amici.
Ringrazio con profonda gratitudine la mia storica e grande amica Marice. La nostra solida amicizia è uno dei più regali che la vita mi ha fatto. La ringrazio per essermi sempre stata vicino, anche a tantissimi km di distanza. Un qualsiasi momento di di difficoltà lei c’è stata, sempre pronta ad ascoltarmi, consigliarmi e se necessario anche rimproverarmi. La ringrazio per la sua grande generosità, la sua nobiltà d’animo e la sua sincerità. Abbiamo condiviso assieme tantissimi avvenimenti importanti delle nostre vite e sono felicissima di condividere con lei anche questo ennesimo traguardo. Grazie per avermi insegnato che nessuno è così importante da portarmi via il sorriso.
Ringrazio con tutto il cuore i miei amici Ciccio, Paolo, Monica e Rossella che mi hanno aiutato a superare un momento molto critico della mia vita. Ringrazio specialmente Monica e Rossella per le lunghe chiacchierate, per aver asciugato le
6
mie lacrime e accolto tutti i miei sfoghi. Ringrazio tutti loro per le bellissime serate trascorse assieme in cui mi hanno regalato momenti di spensieratezza Un sincero e profondo “grazie” va alle mie carissime amiche Roberta e Piera per la splendida amicizia che ho instaurato con loro, per avermi sostenuto nella stesura di questa tesi e per tutti i sorrisi che mi hanno regalato davanti ad un calice di buon vino.
Ringrazio inoltre, Annamaria, Alessandro e Viviana, la cui amicizia, nata durante le gare di corsa, è stata per me un regalo preziosissimo.
Un enorme e sentito grazie va a quella che in quest’anno è diventata la mia seconda famiglia. Grazie con tutto il cuore “Ostuni Runner’s” per tutte le belle domeniche trascorse assieme tra gare ed allenameneti, per tutte le belle serate a cui ho preso parte in vostra compagnia, per aver condiviso con me un anno che mi ha dato delle bellissime e importanti soddisfazioni a livello umano e sportive, e infine perchè condividete con me anche questo importantissimo traguardo personale.
Infine vorrei ringraziare tutta la mia famiglia a cominciare da mia madre per il suo grande coraggio e per gli enormi sacrifici che ha fatto per non farmi mai mancare nulla. Nonostante la perdita di mio padre ha messo da parte il suo dolore e mi ha aiutato e sostenuto con tutte le sue risorse possibili in questi 4 anni. Grazie perchè mi ha fatto sia da madre che da padre.
Ringrazio lo straordinario uomo di mio padre. La vita lo ha portato via una settimana esatta dall’inizio di questo percorso e, nonostante all’inizio sia stata
7
durissima, penso che lui non mi abbia mai abbandonato. In ogni momento di sconforto so che mi ha dato il coraggio per proseguire lungo la mia strada e a credere in me stessa e nei valori che lui mi ha insegnato. Le persone che abbiamo amato non smettono di vivere fino a quando il loro ricordo vive nei nostri cuori. Ringrazio di cuore mia sorella. Anche se le circostanze ci portano ad essere spesso lontani so che su di lei potrò sempre contare.
Rigranzio tutti i parerenti e gli amici, che non ho menzionato, ma che mi sono sempre stati vicini nei momenti di difficoltà e che gioiscono con me per tutti I traguardi raggiunti nel corso della mia vita.
Per concludere vorrei fare un enorme in bocca al lupo a me stessa. Sono profondamente orgogliosa e felice per la conclusione di questo percorso che mi ha contribuito in maniera fondamentale alla mia crescita professionale, scientifica ed umana. Sono stati anni caratterizzati da momenti davvero duri ma sono fiera per non aver mai mollato nonostante le circostanze esterne lo portassero a fare. Faccio un enorme augurio a me stessa perchè la fine di questo percorso è solo l’inizio di altri traguardi che spero di raggiungere senza perdere mai il coraggio, la determinazione e specialmente il sorriso.
1
Abstract
In the last years nanomaterials technologies have been used to fabricate high performance biomaterials with tailored physical, chemical and biological properties. These technologies, indeed, represent an area of interest for emerging biomedical applications such as scaffolds, devices for tissue regeneration and drug delivery system, and offer newer fabrication protocols of a variety of original materials as well as a novel range of tissue engineering applications.
The research described in this thesis involves the development of two particular classes of nanomaterials with peculiar properties and very interesting applications in biomedical field and regenerative medicine.
The main part of this thesis regards the fabrication and the functionalization of self-supporting ultrathin films, known as Free-standing Nanofilm (FsNFs).
FsNFs are a new class of polymeric nanomaterials with thickness in the order of few tens – hundreds of nanometers, characterized by peculiar features as ultra-conformability, and the possibility of being injected through a syringe without losing integrity(Fujie, Okamura et al. 2007). FsNFs can be fabricated by a single step spin-assisted deposition in combination with a sacrificial layer and/or a supporting layer (Fujie, Saito et al. 2010, Okamura, Kabata et al. 2013). In the first part of this thesis is described a new and versatile method to obtain self-supported free-standing plasma-deposited NFs using atmospheric (AP) and low pressure (LP) plasma enhanced chemical vapor deposition (PE-CVD) processes. In the case of LP deposition/sputtering processes, the deposition was performed
2
directly on sacrificial layer of polyvinyl alcohol (PVA) realized by spin coating deposition. In the case of AP processes a supporting layer made of PolyLactic Acid (PLA) was utilized.
In the second part of these thesis the synthesis of polymeric nanoparticles of hydroxyapatite modified with different amounts of selenium (npHA_Se), has been optimized. From the performed experiments it was noticed that there is a clear correlation between the synthesis experimental parameters and the structure of the synthesized nanoparticles. The cytocompatibility of both FsNFs and npHA_Se has been tested with two different cell types: SAOS2 cells derived from a human osteosarcoma, and BMSC, ovine mesenchymal stem cells.
3
Summary
Acnowledgments ... 4 Nomenclature ... 6 1 Introduction ... 10 1.1 Thesis outline ... 132 Free-standing Nanofilms containing molecules of Vancomycin produced by atmospheric pressure plasma process ... 18
2.1 Introduction ... 18
2.1.1 Uses of Vancomycin in biomedical fields... 18
2.1.2 Atmospheric pressure plasma process to produce bio-composite coating ... 21
2.2 Materials and Methods ... 26
2.2.1 Spin coating deposition parameters to produce sacrificial layer for nanofilm release in water ... 26
2.2.2 Plasma processes reactor setup ... 27
2.2.3 Experimental plasma parameters ... 28
2.2.4 Release of free-standing nanofilms in water ... 30
2.2.5 NFs Characterization (FT-IR, profilometer, SEM analysis, NFs release test) ... 30
2.3 Results and discussion ... 32
2.3.1 Study of delamination, morphology and chemical properties. ... 32
2.3.2 Study of VAN release in water ... 35
2.4 Conclusion ... 36
Chapter 3 ... 37
3 NFs embedding silver nanoparticles and NFs with unfouling and cell-adhesive properties produced by low pressure plasma processes ... 37
3.1 Introduction ... 37
3.1.1 Nanofilm embedding silver nanoparticles for antibacterial applications ... 37
Nanofilm with unfouling cell-adhesive properties in biomedical field produced by PE-CVD ... 41
3.2 Materials and Methods ... 44
4
3.2.3 Experimental Plasma Parameters ... 46
Characterization of NFs ... 47
3.3 Results and discussion ... 48
3.3.1 Study of morphology and chemical properties. ... 48
3.4 Conclusion ... 55
4 Low pressure plasma enhanced chemical vapour deposition of chemically different films for biomedical applications ... 56
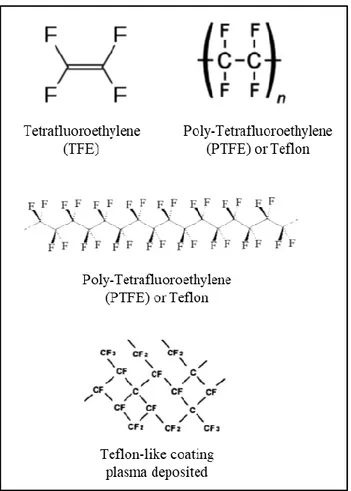
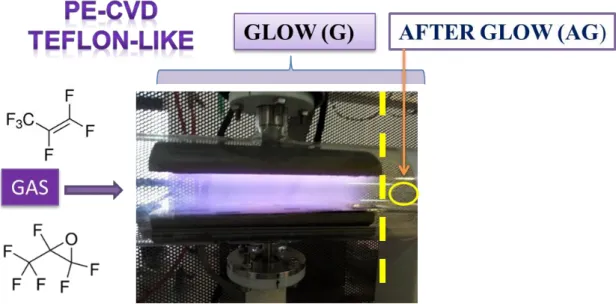
4.1 Hydrophobic Teflon like films ... 57
4.1.1 Introduction ... 57
4.1.2 Materials and Methods ... 61
4.1.3 Results and discussion ... 65
4.2 Films with a different density of COOH on their surface ... 74
4.2.1 Introduction ... 74
4.2.2 Materials and Methods ... 76
4.2.3 Results and discussion ... 78
4.2.4 Conclusions ... 94
5 Biological characterization of films produced by plasma processes... 96
5.1 Introduction ... 96
5.1.1Primary cells and Immortal Cell line Cultures ... 96
5.1.2 In vitro Study of Cells: Evaluation of Adhesion and Viability ... 101
5.2 Materials and Methods ... 105
5.2.1 Saos2 cell line ... 105
5.2.2 Coomassie Blue staning assay... 105
5.2.3 MTT test ... 105
5.3 Results and discussion ... 107
5.3.1.2. Study of the effect on Saos2 cells of hydrophilic surfaces produced by plasma deposition: effect of CO2 in the feed gas mixture ... 110
5.4 Conclusion ... 119
6 Synthesis of Hydroxyapatite Nanoparticles doped with different amount of selenium for tissue engineering applications ... 120
6.1 Introduction ... 120
6.1.1 Role of selenium in the biomedical field ... 120
6.1.2 Synthesis and functionalization of hydroxyapatite nanoparticles ... 125
5
6.2.1 Synthesis of hydroxyapatite nanoparticles (npHA) ... 131
6.2.2 Synthesis of hydroxyapatite nanoparticles doped with different percent of selenium (npHA_Se) ... 132
6.2.3 Chemical-physical characterization of nanoparticles: TEM, SEM, XRD ... 134
6.2.4 Testing for nanoparticles cytotoxicity ... 136
6.3 Results and discussion ... 139
6.3.1 Effect of different amount of selenium on morphology, cristalline structure and chemical composition of npHA_Se... 139
6.3.2 Effect of hydroxyapatite nanoparticles doped with different percent of selenium on two different cell types: Saos2 and BMSC. ... 142
6.4 Conclusion ... 147
7 Conclusions and Prospectives ... 148
8 References ... 150
6
Nomenclature
AA: Acrylic Acid
AA-APPD: Aerosol Assisted AP PE-CVD Process AG: After Glow
Ag: Silver
AP: Atmospheric pressure
AP_VAN:CHO NFs: Nanofilms embedding vancomycin plasma deposited BMSC: Bone Marrow Stem Cells
CBB: Coomassie Brilliant Blue CPP: Calcium Polyphosphate CW: Continuous Wave
DBD: Dielectric Barrier Discharge
DEGDME: Di-ethylene Glycol Dimethyl Ether DMEM: Dulbecco’s Modified Medium
ECM: Extra-Cellular Matrix
EDTA: Ethylenediaminetetraacetic acid EDX: Energy-Dispersive X-ray Spectroscopy EGDMA: Ethylene Glycol Dimethacrylate FsNFs: Free-standing Nanofilms
FT-IR: IR Fourier Transform Spectroscopy G: Glow
7 GS-SeH: Glutathione Selenopersulfide GS-Se-SG: Selenodiglutathione
H2Se: Hydrogen Selenide
HA: Hydroxyapatite HPF: Hexafluoropropene
HPFO: Hexafluoropropylene Oxide
hTERT-BJ1: Human Telomerase Cancer Cells i-CVD: Initiated-Chemical Vapour Deposition IDD: Iodothyronine Deiodinases
LbL: Layer-by-layer
LDH: Lactate Dehydrogenase LP: Low pressure
LP_Ag:CHO: Organic nanofilms embedding silver nanoparticles plasma deposited
LP_CHO: Organic nanofilms with low content of ether groups plasma deposited LP_PEO: Polyethylene Oxide nanofilms plasma deposited
MMA: Methacrylic Acid MRSA: Staphylococcus aures MW: Modulated Wave
npHA: Nanoparticles of Hydroxyapatite
npHA_Se: Nanoparticles of Hydroxyapatite modified with different amounts of Selenium
8 NK: Natural Killer
PBS: Phosfate Buffer Saline PDLLA: Poly(D,L)-lactic Acid
PE-CVD: Plasma Enhanced Chemical Vapor Deposition PEO: Polyethylene Oxide
PES: Polyethersulfone
PET: Polyethylene Terephthalate PFA: Paraformaldeyde
PG: Post Grafting PLA: PolyLactic Acid
PLLA/b-TCP: poly-L- lactic/b-tricalcium Phosphate PS: Polystyrene
PTFE: Polytetrafluoroethylene or Teflon PVA: Polyvinyl Alcohol
RGD: Arginine-Glycine-Aspartic Acid RNS: Reactive Nitrogen Species
ROS: Reactive Oxygen Species SAM: Self-Assembled Monolayer Se: Selenium
SeCys: Seleno-Cysteine
SEM: Scanning Electron Microscopy SeMet: Seleno-Methionine
9 SeMSC: Se-Methylseleno- Cysteine TCPS: Tissue Culture Polystyrene
TEM: Trasmission Electron Microscopy TrxR: Thioredoxin Reductase
VAN: Vancomycin
WCA: Water Contact Angle
XPS: X-ray Photoelectron Microscopy XRD: X-ray Powderd Diffraction
10
Chapter 1
1 Introduction
The development of functional biomaterials is of particular interest for many biomedical applications, such as the fields of regenerative medicine and tissue engineering.
Accordingly, engineered scaffolds or patches should allow cells to attach, grow, and, if necessary, be transplanted into a specific wounded area of the human body (Hosseinkhani, Hosseinkhani et al. 2010, Khang, Carpenter et al. 2010, Jurgens, Kroeze et al. 2011). In order to obtain a successful engineered biomaterial acting as a substrate for tissue regeneration, the following main requirements are demanded (Chevrier, Baert et al. 1995).
1. biocompatibility with the host tissue
2. specific morphological, mechanical and chemical properties promoting suitable interactions with cells (e.g. adhesion, proliferation and/or migration).
Moreover, the fabrication process should be easy, controlled and reproducible. Among these requirements, when coupling desired cell type with a flexible substrate, controlling the surface morphology and chemistry must be considered and deeply studied.
In fact, it has been demonstrated that cellular events (adhesion, proliferation, migration and differentiation) are sensitive to and can be affected by the surface
11
properties of the material (Cho, Char et al. 2001, Dalton, Walboomers et al. 2001, Bush, Nayak et al. 2011).
Concerning the first cellular event occurring when there is contact between cells and substrates, Raffa et al. (Raffa, Pensabene et al. 2007) proved that surfaces with nanometer-scale topography directed the PC12 pheochromocytoma cells adhesion, while, as Washburn et al. (Washburn, Yamada et al. 2004) reported, the proliferation of MC3T3-E1 osteoblastic cell was sensitive to the nanometer scale roughness of the polymeric materials. Furthermore, the differentiation process can also be affected by altering both the surface and bulk structure of the materials(Washburn, Yamada et al. 2004, Stevens and George 2005). Engler et al(Engler, Sen et al. 2006). reported the critical role of stiffness of micropatterned collagen scaffolds on polyacrylamide gels in striated muscle differentiation, showing that myotube differentiation was strongly dependent on the substrate's elastic modulus.
In the last decades, many tissue engineering technologies have been developed in order to direct cellular growth as well as morphogenesis.
In the recent years increasing interest is arising regarding a new class of nanomaterials called “Free-standing Nanofilms”. FsNFs are “almost-two-dimensional” structures, characterized by a thickness ranging from ten to one hundred nanometers, and by aspect ratio higher than 106 (Sinibaldi, Pensabene et
al. 2010), where aspect ratio describes the proportional relationship between its surface area and its volume. These peculiar properties, indeed, drive the
12
mechanical properties of these kinds of nanomaterials. Among other properties, FsNFs are ultra-conformable, show good adhesive properties and in some case are transparent. Another very interesting property of FsNFs is the possibility to inject them through a syringe (Zhang and Takeoka 2012, Fujie, Mori et al. 2014, Fujie 2016, Taccola, Pensabene et al. 2017). Moreover, FsNFs can easily detach from a supporting substrate without influencing their mechanical integrity. The peculiar characteristics of these structures make them suitable for different applications; in the biomedical field in particular, ultrathin film have been developed as artificial skin (Hajicharalambous, Lichter et al. 2009), scaffolds for regenerative medicine and tissue engineering (Fujie, Furutate et al. 2010, Fujie, Desii et al. 2012), drug delivery systems (Berg, Zhai et al. 2006, Redolfi Riva, Desii et al. 2013), biosensors (Kittle, Wang et al. 2012), bio-interfaces for improved adhesion (Zhang and Takeoka 2012), proliferation and migration of cells (Ventrelli, Fujie et al. 2014), and as antimicrobial surfaces (Tai, Ma et al. 2012).
Different fabrication approaches exist for FsNFs: layer-by-layer deposition (LbL) (Decher, Hong et al. 1992, Lvov, Decher et al. 1993, Decher 1997), Langmuir-Blodgett method (Corkery 1997) with crosslinkable amphiphilic copolymers, spin coating method (Forrest, Dalnoki-Veress et al. 1996, Hall, Underhill et al. 1998), cross-linking of self-assembled monolayer (Beyer, Godt et al. 2008) (SAM) and electrophoretic deposition (Injeti and Leo 2008). All these methods influence the resulting FsNFs for two general aspects: the degree of control over the final
13
thickness, composition and stability; and the detachment step of the nanosheet from the supporting substrate.
The Layer-by-layer method, developed by Decher et al. (Decher, Hong et al. 1992, Lvov, Decher et al. 1993, Decher 1997) in the early 1990s, consists in alternate deposition of oppositely charged polyelectrolytes by non-covalent bonds such as electrostatic interactions, hydrogen-bonding or hydrophobic interactions. This approach is very simple and allows to obtain a high degree of control over FsNFs thickness. Stroock et al. (Stroock, Kane et al. 2003) extensively described the synthesis of polymeric FsNFs 10–15 nm thick and well-defined lateral size and shape. Another interesting approach deals with the preparation of FsNFs with the spin-coating technique. This simple technique was proposed by different groups such as Jiang et al. (Jiang and Tsukruk 2006) and Takeoka (Takeoka, Okamura et al. 2008) group from Waseda University in Tokyo. In spin-coating technique method an excess amount of a solution is placed on the substrate, which is then rotated at high speed in order to homogenously spread on the substrate the fluid by centrifugal force.
1.1 Thesis outline
Motivated by the need to obtain a method to obtain FsNFs characterized by a specific surface chemistry and with desired biological properties, this thesis explores different types of deposition processes by plasma, at low and atmospheric pressure.
14
Plasma Enhanced Chemical Vapor Deposition (PE-CVD) at low (LP) and at atmospheric (AP) pressure are potentially able to produce FsNFs of many different kinds. Nevertheless, in literature very few examples of this approach can be found.
In the following cases plasma techniques have been used at some extent to fabricate polymeric FsNFs: LP air glow discharge crosslinking of spin-casted polyethylene oxide (PEO) polymers (Suzuki, Takaku et al. 2013); Amorosi et al.(Amorosi, Mustin et al. 2012) have fabricated 300–600nm thick free-standing films by means of plasma-induced polymerization of methacrylic acid (MAA) and ethylene glycol dimethacrylate (EGDMA) followed by delamination of the coatings in water from glass substrates. Furthermore, plasma etching and functionalization processes were also used to improve the fabrication of free-standing micrometer films synergistically with other methods to cultivate neural cells (Cesca, Limongi et al. 2014). FsNFs were prepared with an elegant but complicated multistep CVD approach specific for xylene-based materials (Bally-Le Gall, Friedmann et al. 2015). In another example, initiated CVD allowed to produce free-standing films at the surface of drops of ionic liquids used as sacrificial layer; this versatile method, though, is restricted to monomers with a double C=C bond and leads only to coatings characterized by conventional chemical composition (Haller, Frank-Finney et al. 2011).
PE-CVD processes developed in our lab were deposited on substrates decorated with a sacrificial layer, to originate many different FsNFs as shown in the Figure
15
1. In the second chapter an aerosol assisted AP PE-CVD process (AA-APPD) was optimized to obtain organic NFs embedding Vancomycin (VAN), an antibiotic drug; LP PE-CVD processes utilized to prepare NFs with unfouling/ cell adhesive properties and nanocomposite silver-containing NFs, are the protagonist of Chapter 3. All coatings have been easily peeled off in few minutes from silicon and glass substrates after the dissolution of the sacrificial layer in water.
Figure1.1- Scheme of plasma assisted FsNFs fabrication.
In the chapter 4 the possibility of obtaining FsNFs characterized by a different degree of hydrophilicity is described. Different processes were investigated, varying process parameters and assessing how they influenced the surface chemistry and water delamination of the obtained FsNFs. After process optimization three different kind of NFs were obtained, characterized by a different density of COOH groups at their surface and, consequently, by a
16
different hydrophilicity. The synthesis of hydrophobic NFs with Teflon-like composition was also optimized.
In the chapter 5 cytocompatibility assays, with the Saos2 cell line, on all types of NFs, characterized by different contact angle, values are shown.
In the last chapter of these thesis the synthesis of polymeric nanoparticles of hydroxyapatite (nHA) modified with different amounts of selenium has been developed for application in tissue engineering.
Hydroxyapatite (HA) is the main inorganic component in the hard tissues of animals and humans. HA is used as a bone replacement material in orthopedic implants due to its exceptional biocompatibility and bioactivity properties(Ferraz, Monteiro et al. 2004).
Biological apatite, except that found in tooth enamel, differs from the synthetic HA in stoichiometry, and is composed of carbonate hydroxyapatite containing different ions (ie, Mg2 +, Na +, K +, Zn2 +, F-, Cl-, HPO4 2 -) which influence its
physical-chemical and biological properties. This natural characteristic has led to different studies about the possibility of doping HA with different ions to implement important properties of this material such as osteo-integration (Laurencin, Almora-Barrios et al. 2011).
In our experiments HA was doped with selenium (Fernández-Martínez and Charlet 2009) since it plays an important role in various metabolic processes as a constituent of the seleno-proteins, and can prevent carcinogenesis and inhibit the growth of tumor cells (Combs and Gray 1998, Drake 2006, Zeng, Cao et al. 2013).
17
In this thesis, selenium-substituted selenium nanoparticles (npHA_Se) with different Se/P ratios were synthesized to study how the effect of different concentrations of npHA_Se were able to affect the adhesion and growth of two cell types.
The npHA_Se were synthesized with a coprecipitation, using sodium selenite (Na2SeO3) as a selenium source. The chemical and physical properties of the
nanoparticles have been studied through transmission electron microscopy (TEM) and X-ray powderd diffraction (XRD) characterization. the obtained particles are needle-like and have a size and crystallinity similar to that of pure HA nanoparticles. Moreover, when it has been P / Se ratio increases, the crystallinity is reduced, miming in this way the biological HA.
Cytocompatibility studies, using MTT and Coomassie blue staining, showed that nanoparticles npHA_Se induced different cytotoxic effects when incubated with primary cells, ovine BMSC or with Saos2 cells. This cytotoxicity depends on both the type of nanoparticles, i.e. the percentage of Selenium with respect to HA, and the concentration used.
The obtained results clearly showed a cytotoxic effect for the immortal cell lines but not for primary cells.
18
Chapter 2
2 Free-standing Nanofilms containing molecules of Vancomycin
produced by atmospheric pressure plasma process
2.1 Introduction
In this chapter a new method consisting of the combination of the AA-APPD technique with a spin coating deposition technique, to develop an organic FsNFs embedding Vancomycin, is described.
2.1.1 Uses of Vancomycin in biomedical fields
Vancomycin is a glycopeptide antibiotic produced by Streptococcus orientalis, used in the biomedical field to treat a number of infections. It is characterized by a high molecular weight of 1449.3 g.mol-1, able to kill Gram positive but not Gram
negative bacteria, since VAN acts by inhibiting proper cell wall synthesis in Gram-positive bacteria. VAN is administered intravenously as a treatment for complicated skin, bone, joint and bloodstream infections, endocarditis and meningitis caused by methicillin-resistant Staphylococcus aureus (MRSA). VAN is also recommended for treating infections of the intestine caused by Clostridium difficilis as well as to treat infections that can cause inflammation of the colon.
Common side effects include pain in the area of injection and allergic reactions, as well as, occasionally, hearing loss, low blood pressure, and bone marrow suppression.
19
VAN was first sold in 1954 (Estée Török 2009). It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. In the United States, the capsules are more expensive than the intravenous solution. The intravenous solution may be safely taken orally for the treatment of C. difficile colitis to reduce costs.
Due to its antibacterial properties, VAN is used for different kind of applications in the biomedical field, such as the development of controlled release drug delivery systems, or of biomaterials embedding antibiotic molecules. A simple and powerful way to synthesize antibacterial biomaterials with applications as implants in orthopedic surgery is described in the literature. Such implants are obtained by covalently grafting VAN-functionalized nanoparticles at the surface of implants made of Ti90A16 V4 alloy (Pichavant, Carrié et al. 2016). VAN is an interesting candidate of the use in the field of implant associated infection since it acts on bacterial walls. As a consequence, VAN does not need to be released to be active. Radin et al.(Radin and Ducheyne 2007) have developed a thin sol-gel film from which VAN can be released in a controlled way. It was described the synthesis of thin, resorbable, controlled release bactericidal sol-gel films on a Ti-alloy substrate and determined the effect of processing parameters on its degradation and VAN release. A close correlation between release and degradation rates suggests that film degradation is the main mechanism underlying the control of release. Some studies showed the possibility to use calcium phosphates, including hydroxyapatite and tricalcium phosphate as
20
potential VAN delivery matrices (Dion, Berno et al. 2005). Gauthier et al. (Gautier, Daculsi et al. 2001) have incorporated VAN into a biphasic calcium phosphate matrix by dynamic compaction process that leads to low-temperature inter-particle bonding throughout the matrix. VAN release was sustained over 4– 6 days in distilled water at 37 °C. However, the incorporated VAN expressed only 28% of regular VAN activity, suggesting that the rise in temperature during dynamic compaction may have caused the VAN to denature. In another work, calcium polyphosphate (CPP) VAN delivery matrices were prepared using a unique processing technique involving the exposure of antibiotic-loaded CPP pastes to high humidity for 0, 5, or 24 h (Dion, Berno et al. 2005). After the designated gelling period, samples were dried for a minimum of 24 h. In orthopaedic surgery using poly(D,L)-lactic acid (PDLLA) as a carrier, PDLLA coatings carrying VAN were prepared on the surface of titanium alloy plate substrates for internal fixation of fractures using solvent-casting technology (Tang, Zhao et al. 2010). The bacteriostatic activity toward Staphylococcus aureus and the drug-release profile of the plates were evaluated in vitro.
VAN-loaded plates showed a sustained in vitro drug release character in the experiment. The in vitro inhibition of S. aureus showed that the plates had an inhibitory effect on S aureus, and the antibacterial activity was maintained for at least 15 days. These findings may have important clinical significance for preventing acute infections of internally implanted fixation plates after the fracture. Another important application of VAN regards the treatment of osteomyelitis caused by
21
MRSA, that often requires surgery and/or prolonged systemic antibiotic treatment. For this aim it was developed a VAN-containing poly-l-lactic acid/b-tricalcium phosphate (PLLA/b-TCP) composite to control antibiotic release and stimulate bone formation (Kankilic, Bayramli et al. 2011). In a work of Ravelingien M. et al. (Ravelingien, Mullens et al. 2010), the influence of the poly (d,l-lactic acid) (PDLLA) coating thickness on the in vitro VAN release from a hydroxyapatite (HA) carrier was studied. Microporous hydroxyapatite fibers were fabricated, loaded with VAN, and spray-coated with PDLLA. The spray coating technique allowed to deposit uniform coating thicknesses. It was clear that the in vitro VAN release rate can be adjusted by varying the PDLLA coating thickness. In the present Thesis is shown how to realize FsNFs embedded with molecules of VAN by means of atmospheric plasma deposition. The presence of VAN was verified, and its release in liquid was studied after 24h of water immersion.
2.1.2 Atmospheric pressure plasma process to produce bio-composite coating
The synthesis of bio-composite coatings, containing an organic/inorganic synthetic matrix embedding, or grafted with, molecules or nanoparticles, represents an important technological issue: bio-composite coatings, in fact, bring added value to the material they are deposited on, without affecting its bulk characteristics. This kind of materials is very versatile since, by properly choosing the nature of the matrix and of the coupled biomolecule and their relative amount, the composite properties can be finely tuned, thus matching specific technological requirements in several type of applications.
22
Bio-composite coatings have been developed as biosensor in the industry field, with the aim of increasing the selectivity of transistor-based array devices towards specific target biomolecules such as oligo- and poly-nucleotide sequences (Miyachi, Ikebukuro et al. 2004) saccharides (Yoshimura and Hozumi 1996, Muguruma, Hiratsuka et al. 2000) , proteins (Kojima, Hiratsuka et al. 2003, Injeti and Leo 2008, Lisboa, Villiers et al. 2011, Magliulo, Mallardi et al. 2013)and, sometimes, cells or viruses (Patolsky, Zheng et al. 2004, Mao, Liu et al. 2009). In bioreactors, the long-term maintenance and the differentiation of human cells have been improved with the introduction of oligo-peptides at the polymer surface of porous inner membranes (De Bartolo, Morelli et al. 2007). The same strategy has been used to produce antibacterial or cell growth enhancing coatings either on 3D scaffolds (Ryu, Ku et al. 2010, Kim, Jung et al. 2013) or on flat substrates (Sun, Thian et al. 2012, Kim, Lee et al. 2013), for tissue engineering and regenerative medicine. In food packaging bio-composite coatings have been fabricated to prolong the shelf life of meal, such as fresh cheese(Quintieri, Pistillo et al. 2013). Some works in literature reported the possibility to realize coatings embedding synthetic drugs that can be used as drug delivery patches (Panchaxari, Pampana et al. 2013, Lim and Lee 2014).
Since biocomposite coating can be applied in different fields, many research groups have focused their attention on developing versatile processes for making biocomposite films. For this purpose, several types of strategies are reported in literature, as Photo-polymerization (He, Cao et al. 2004), initiated-Chemical
23
Vapour Deposition (i-CVD)(Bardon, Bour et al. 2009), 3D printing (Kim, Lee et al. 2013), micro-dispensing (Sun, Thian et al. 2012), and many wet chemistry methods (Ji, Zhu et al. 2004, Patolsky, Zheng et al. 2004, Ryu, Ku et al. 2010, Zhang, He et al. 2013). All these methods present drawbacks and limits related to their applicability on industrial scale because multi-step processing of substrates or reagents are generally needed, and potentially harmful solvents and reagents are often required. To overcome all these problems strategies based on plasma processes have been developed. In literature examples are present of bio-conjugating active biological compounds to plasma-functionalized substrates. These processes usually include at least two-steps: an initial phase, carried out either with low- or with atmospheric-pressure plasma, aimed to enrich the topmost surface of the substrates with functional groups (e.g. grafting oxygen- or nitrogen-containing reactive groups or depositing functionalized films). The process continues with the adsorption or with the covalent binding of the bioactive molecule at the surface of the substrate. In alternative, covalent coupling reaction steps involving carbodiimides and molecular ‘‘spacer’’ arms (e.g., bis-amine PEG, alkyl diamines, etc.) are needed to enhance and properly drive the coupling reaction, thus increasing the complexity of the synthesis. This strategy has allowed, for instance, the preparation of anticoagulation (Favia, Palumbo et al. 1998), antibacterial (Chen, Zhou et al. 2011, Quintieri, Pistillo et al. 2013, Mauchauffé, Bonot et al. 2016), and cell-growth enhancing composite coatings
24
(Baquey, Palumbo et al. 1999, Lopez, Gristina et al. 2005) as well as highly selective biosensors for the detection of specific DNA strains or proteins.
Recently, a new strategy for plasma deposition of bio-composite coatings has been developed, called aerosol-assisted atmospheric pressure plasma deposition (AA-APPD), consisting in coupling an atomizer directly to a plasma reactor. This experimental set-up, so far developed mainly for plasma processing at atmospheric-pressure, allows to efficiently spray low vapor-pressure pure liquids, salt or biomolecule solutions or even nanoparticles suspensions (Heyse, Roeffaers et al. 2008, Fanelli, Mastrangelo et al. 2014). A solution or a dispersion containing the molecule is sprayed, through an atomizer, inside the chamber of an atmospheric pressure plasma reactor in which the gas precursor for the matrix is inlet as well. When the complex feed passes through the discharge, the precursor is plasma polymerized forming the matrix. Mild plasma conditions and the formation of a thin watery shell all around the solute during atomization can limit damages of the biomolecule that, consequently, can embed the growing film without loss of structure and activity (Heyse, Van Hoeck et al. 2011). In contrast with the more common two-steps strategy of securing the biomolecule to the substrate surface, this process allows either the retention of the bioactive compound in the coating, or its release in the surrounding medium according to the characteristics of the matrix. Moreover, spacer molecules or enhancer are not needed.
25
These peculiar characteristics allow this technique to be suitable for biomolecules. If a bioactive molecule is embedded the coatings can be defined bio-composite. Biocomposite coatings embedding lysozyme, a natural antibiotic molecule, were developed with the AA-APPD technique obtaining a drug delivery system(Palumbo, Camporeale et al. 2015). Feeding the plasma with ethylene and an aerosol of aqueous solution of lysozyme carried by helium is possible to obtain a polymeric matrix in which the drug molecules lay embedded.
Using the same process but changing the drug or molecule is possible to obtain other type of bio-composite coatings. In a recent work, AA-APPD processes were used for one-step synthesis of VAN-containing nano-capsules (Lo Porto, Palumbo et al. 2018). It represents an absolute novelty since, for the first time, a one-step, easy to- handle, eco-friendly, plasma process is shown for the synthesis of nano-capsules embedding a bioactive molecule. As for previous works (Yang, Camporeale et al. 2014, Palumbo, Camporeale et al. 2015, Lo Porto, Palumbo et al. 2017)ethylene was selected as precursor of the shell of the capsules; VAN was selected to be embedded in the capsules from an aqueous aerosol, helium was used as buffer and aerosol-forming.
In this thesis, combining the AA-APPD technique with the spin coating deposition technique, an organic FsNFs embedding VAN was developed. The procedures and the obtained results were discussed in the next paragraphs.
26
2.2 Materials and Methods
2.2.1 Spin coating deposition parameters to produce sacrificial layer for nanofilm release in water
The experimental procedure to realize AA-APPD biocomposite nanofilms contaning molecules starts from the spin coating deposition of a sacrificial layer. The sacrificial layer is fundamental for the release step in order to obtain self-standing NFs.
Silicon wafer shards (Silicon Materials, Kaufering, GER) 1-4 cm2 wide and 13
mm diameter glass disks were used as substrates after 10 min cleaning in ethanol and rinsing in de-ionized (DI) water.
A spin coating procedure was used for the deposition of a sacrificial layer of polyvinyl alcohol (PVA, a biocompatible water-soluble polymer), on top of which is plasma-deposited the functional layer that must be released in water as free-standing film. Before the deposition of the functional layer, an additional layer of Polylactic Acid (PLA, a biocompatible water-insoluble polymer) can be spun as supporting layer in order to improve the mechanical properties of the functional coating. In details, a 10 mg/ml PVA (PVA, Sigma–Aldrich, Mw 13 000–23 000 Da) water solution was spin coated (WS-650 spin processor, Laurell Technologies Corp. North Wales, PA; 3000 rpm, 60 s, 500 rpm s-1) onto the substrates to obtain a PVA sacrificial layer about 20 nm thick, according to a procedure optimized for conventional FsNFs (T. Fujie et all., 2011; S.Taccola et all. 2011; E. Ridolfi et all., 2013. The plasma process deposition is directly performed on the top of the
27
prepared substrate, obtaining a layer that can be released in water as FsNFs following the procedure reported in section 2.2.4.
If an additional supporting layer is needed, the PVA-coated substrate is spin coated with a PLA layer. In details, a 20mg/ml PLA (Sigma Aldrich, Mw ~ 60000 Dalton)-water solution was prepared in chloroform and spin coated as support layer (3000 rpm, 60 s, acceleration of 500 rpm s-1) on the top of the PVA layer. The PLA support layer was used to reinforce the subsequent AP_VAN:CHO coating, characterized by weak mechanical properties, thus avoiding breaking during the release process.
Before performing the plasma process deposition, the spin coated substrates were heated on a hot plate at 80°C.
2.2.2 Plasma processes reactor setup
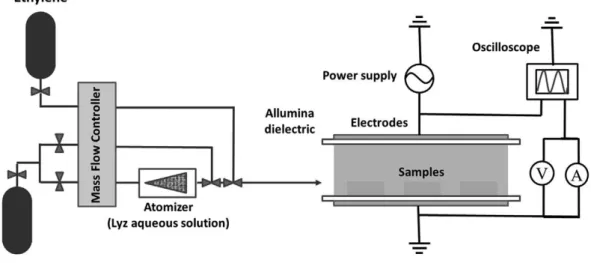
Bio/nano-composite NFs, in which VAN is embedded in a hydrocarbon matrix were deposited in an aerosol-assisted AP PE-CVD process performed in the Dielectric Barrier Discharge (DBD) reactor schematized in Figure 2.1. The DBD consists of two parallel plates silver electrodes 8 x 13 cmwide, 5 mm apart, both covered with 0.63 mm thick alumina dielectric sheets. The gas feed is injected through the gap between the electrodes (short side), and it is pumped out by an aspirator from the opposite side. The reactor is confined in a sealed Plexiglas chamber. An aerosol of a 10-20 mg/ml VAN solution was generated with an atomizer (mod. 3076, TSI) working with H at a flow rate of 2-5 slm. According to the literature (Somers, Dubreuil et al. 2014), the average diameter of the
28
droplets produced is typically around 50 nm. Ethylene was fed at a flow rate of 10-20 sccm, as well as 5 slm helium. The gas flow rates were controlled by means of electronic mass flow controllers (MKS instruments). Silicon substrates were placed on the lower electrode. Before any deposition the chamber was purged with 5 slm He for 5 min. The discharges were ignited using an AC power supply consisting in a function generator (TG1010A, TTi), an amplifier (Industrial Test Equipment Powertron 1 000 A) and a high-voltage transformer (Amp Line). The average power was obtained by multiplying the energy per voltage cycle by the frequency; the energy per cycle was calculated from the time integral of the current multiplied the voltage in one cycle.
Figure 2.1-Scheme of the aerosol-assisted atmospheric-pressure DBD deposition
system.
2.2.3 Experimental plasma parameters
To produce free-standing bio-composite coatings, substrates coated with the PVA layer and with the PVA/PLA bilayer were placed onto the lower electrode. 10mg/mlVAN/water solution was fed in the discharge as aerosol with He (5 slm)
29
through a pneumatic atomizer (TSI 3076); C2H4 (20 sccm flow rate) was used as
precursor of the hydrocarbon matrix.
According to a protocol optimized to control the timing of the VAN release, and to prevent excessive leaching of the drug in water during the in-water release step, the biocomposite coating was encapsulated between two plasma deposited barrier layers deposited from feeds without aerosol. The final NFs consisted of three layers deposited in the same reactor: bottom and top barrier layers with hydrocarbon structure, with the biocomposite VAN-containing layer in the middle. The top and bottom hydrocarbon coatings were produced feeding the DBD with He (5 slm) and C2H4 (20 sccm) for 3min. The biocomposite interlayer
was deposited by adding the VAN/water aerosol to the He/C2H4 feed, with the
discharge ignited for 15min in pulsed mode (tON 40ms, tOFF 80ms) at 24 kHz and
3.8 kVpp (about 1.38W cm-2). To investigate the influence of deposition time on
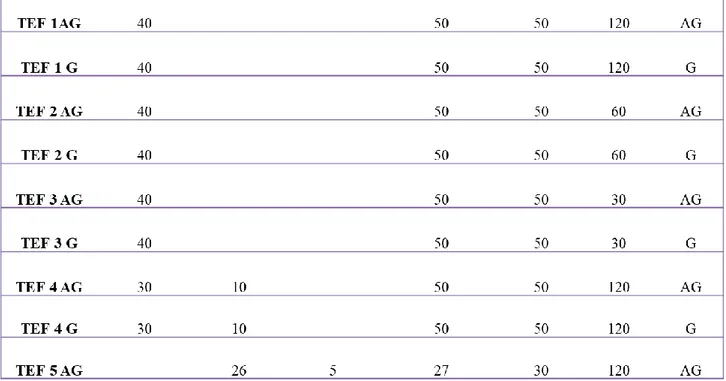
chemical structure and morphology of AP_VAN:CHO/PLA NFs, the biocomposite coating were deposited at 3 different times of plasma treatments: 5, 10 and 15 minutes. Plasma parameters used to obtain the biocomposite NF containing VAN are reported in Table 2.1.
Parameters TOP / BOTTOM COATING BIOCOMPOSITE COATING He 5 slm 5 slm C2H4 20 sccm 20 sccm Frequency 24 kHz 24 kHz Tension 3.8 kVpp 3.8 kVpp
30
Duty Cycle 100% 33%
TON n.a 40 ms
TOFF n.a. 80 ms
Regime Continuous mode Pulsed Mode
Time treatment 3 minutes 5, 10, 15 minutes
Table 2.1 - Experimental plasma parameters used to PE-CVD deposition of
biocomposite NF containing VAN.
2.2.4 Release of free-standing nanofilms in water
After the plasma processes, the AP_VAN:CHO NFs samples were cut with a razor blade at the edges of the silicon/glass substrates and immersed in DI water at room temperature for dissolving the sacrificial PVA layer; this process make possible the release of the coatings as freestanding NFs. Rinsing in water for 5–10 min by gentle shaking, and the use of tweezers, aided the detachment.
2.2.5 NFs Characterization (FT-IR, profilometer, SEM analysis, NFs release test)
To investigate the chemical composition and structure of the films, different characterization techniques have been used directly on the films deposited on silicon substrates.
The chemical composition of the films was evaluated by IR Fourier transform spectroscopy (FT-IR), the morphology of the surface by SEM (scanning electron microscopy), the drug release by spectrophotometric analysis and the thickness with a profilometer.
31
IR spectroscopy provides information on the vibrations of chemical bonds and is a useful method to detect qualitative information on chemical composition and structural orientation of molecules and polymers. The acquisitions were performed on coatings deposited on silicon shards, using a Bruker spectrometer mod. Vertex 70V. The spectra acquired from 400 to 4000 cm-1 were normalized for the contribution to the intensity of the band with highest absorption. The subtraction of the baseline and normalization was performed using the Opus 5.0 program (Bruker Optics).
Profilometer
To determine the thickness of the deposited films the α-step 500 Tecnor Instruments profilometer was used. The instrument measures the profile of the
sample by sliding a tip on its surface after the scratching grooves in the coating deposited on silicon or glass with a scalpel. For each sample three measurements were performed on three different points.
SEM analysis
SEM images were acquired with an EVO MA 10 SEM instrument (Carl Zeiss, Oberkochen, Germany) at 5.00 kv of magnification using an acceleration voltage of 5 kV. Before SEM, the samples were coated with a Au layer of about 8 nm with a Q150R BS sputter coater (Quorum Technologies, West Sussex, UK).
32
The release of Vancomicin was determined from AP_VAN:CHO NFs deposited in 15 min, with the barrier layers at the two sides. The samples were cut with a razor blade at the edges of the silicon substrates and immersed in DI water within a Petri dish at room temperature. After the complete detachment, in about 10 minutes, the NFs were collected on a new silicon substrate, then transferred in a 6 cm diameter Petri dish with 1 ml of DI water and left for 2h. The surnatant was collected and transferred in a quartz cuvette for the spectrophotometer analysis. The release solution was analyzed with a spectrophotometer UV-2401PC (Shimadzu) set at 280 nm, for detecting the released vancomicyn.
2.3 Results and discussion
2.3.1 Study of delamination, morphology and chemical properties.
The aim of the present study is to obtain FsNFs embedding VAN molecules. For this purpose, optimizing the release protocol of the nanofilm from the substrate in water is quite important, in order to avoid lacerations. The delamination in DI water was tested for AP_VAN:CHO coatings deposited on a PVA, and for coatings deposited on PLA/PVA layer, capable of providing robustness and elasticity to the NF. After 10 minutes of gentle shaking, only the detachment of the coating, with no lacerations, deposited on PVA/PLA layer could be observed, as shown in Figure 2.2. No detachment, instead, was obtained for the coating directly deposited on the PVA layer, even after 24 hours in DI water, confirming the important role of the PLA layer in case of fragile NFs.
33
Figure 2.2 - Free-standing AP_VAN:CHO/PLA NFs obtained after 10 minutes of
immersion in distilled water. The withish color of the NF confirms the presence of the drug embedded.
The SEM morphological analysis was performed on 3 samples of AP_VAN:CHO/PLA NFs with the middle VAN containing coating deposited in 5, 10 and 15 minutes in pulsed mode. The top and the bottom coating were deposited in the same conditions in all 3 samples. In the SEM images shown in Figure 2.3, morphological features can be observed on the NFs, whose sub micrometric dimensions increase with the deposition time, along with the size of their aggregates. The features have been described in (Lo Porto, Palumbo et al. 2018) as core-shells nano-capsules, with the polymer-like shell mainly composed by hydrocarbon moieties, and the core containing the water solution of VAN.
34
Figure 2.3 - SEM images of coatings deposited in pulsed mode (15 mg mL−1
VAN solution, 20 sccm ethylene) at (a) 5, (b) 10 and (c) 15 min deposition time of the drug-containing layer.
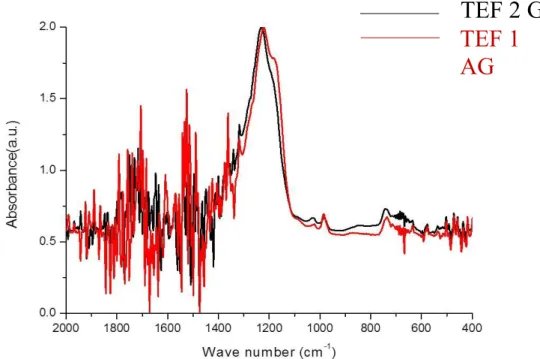
The FT-IR spectrum of this composite AP_VAN:CHO coating, prepared directly on silicon substrate without any PVA or PLA layers, is reported in Figure 2.4, along with other spectra relevant to the investigation. All deposited coatings exhibit a common hydrocarbon backbone, revealed by the absorption bands relative to CH2 and CH3 stretching (2964–2933 cm−1), and CH2 bending (1430–
1370 cm−1). The samples show also bands relative to C=O (1712–1658 cm−1) and OH stretching (3371 cm−1), testifying the presence of oxidized carbon moieties in the coating, certainly due to the action of oxidized water fragments (O atoms, OH radicals) in the deposition mechanism. The spectra of the drug containing coatings exhibit the characteristic absorption bands of VAN, due to N-containing chemical moieties: in particular, the bands at 3300 cm−1 and 1502 cm−1, due to NHx group,
and that at 1232 cm−1. Such bands, though not specific, are evidently related to the presence of VAN. As these absorption bands are more intense in the spectra of coatings deposited in pulsed mode, it can be inferred that in this case films with higher VAN (or VAN-like)/hydrocarbon relative content are obtained.
35
Figure 2.4 - FT-IR transmission spectra of coating deposited (20 sccm of C2H4)
in different conditions on Silicon substrates: (a) feeding the CW plasma with C2H4 and water aerosol, (b) feeding the CW plasma with C2H4 and VAN containing aerosol (15 mg mL−1), (c) feeding the PM (DC 33%) plasma with C2H4 and VAN containing aerosol (15 mg mL−1), d) drop-casted VAN. * bands characteristic of VAN.
2.3.2 Study of VAN release in water
The possibility to use AP_VAN:CHO NFs for drug delivery was assessed with a simple release test; nanofilms 1 cm2 wide were dipped in 1 ml DI water and after
24 h the liquid was analyzed by UV/Vis spectroscopy. It was found that 130±6 μg of VAN were released, for a total amount of 58±3 μg/cm−2 releasable antibiotic. Keeping the NF longer in water resulted in no further release. UV/Vis spectroscopy analysis of the water used in the peel-off revealed that only about 2–3% of the total VAN released in 24 h was lost during the peel-off step.
36
2.4 Conclusion
This work presents one of the first case of FsNFs containing biomolecules obtained by means of an aerosol-assisted atmospheric pressure PE-CVD process. By varying the plasma parameters, it is possible to tune the dimensions and the aggregation of the nano-capsules. These structures seem to release the contents of their cores after immersion in water. The AP aerosol-assisted PE-CVD allows the one-step synthesis of NFs containing core/shell nano-capsules of different kinds and offers the possibility to embed a biomolecule in a matrix without altering its structure and activity. Such a process, optimized for a hydrocarbon polymeric shell embedding VAN, is clearly not restricted only to these components: the composition of the core and of shell can be tuned, in principle, by properly selecting different feed precursors for the shell and different bioactive compounds for the aerosol.
37
Chapter 3
3 NFs embedding silver nanoparticles and NFs with unfouling and
cell-adhesive properties produced by low pressure plasma
processes
3.1 Introduction
3.1.1 Nanofilm embedding silver nanoparticles for antibacterial applications
In the last decades many researcher focused their attention on the prevention on bacterial adhesion on biotic and abiotic surfaces that represent a widespread problem in many fields. In the biomedical field, non-specific adsorption of proteins and adhesion of cells or bacteria onto device surfaces can be compromise the use of therapeutic and diagnostic devices during long-term in vivo and ex vivo exposure to physiologic fluids. For example, when a prosthesis was implanted, a competition exists between integration of the device into the surrounding tissue and adhesion of bacteria at the implant surface. To obtain a successful implants, tissue integration should occur before bacterial adhesion in order to prevent the colonization at the implant. In many cases host defenses are often unable to prevent bacteria adhesion and colonization (Hetrick and Schoenfisch 2006). In this case, bacteria first adhere to the biomaterial interface, then actively bind to the extra-cellular matrix (ECM) surrounding the implant and form a protein layer. In this process, bacteria progressively form a biofilm, i.e., a well-defined metabolic state of bacteria life cycle where microbial cells, driven by a quorum sensing mechanism, lower their growth rate and baseline metabolism and express
38
mechanisms leading to cellular aggregation in an amorphous polysaccharide matrix, also called slime. Biofilms allow bacteria to survive in harsh environmental conditions (Campoccia, Montanaro et al. 2010). Infections related to the use of medical devices can be derived when biofilms adhering to biomaterials surfaces and, in most of the case, the bacteria can evade the host defenses (Stewart and William Costerton 2001). To avoid this problem one approach consists in loading the material with antibiotics, as we have previously shown with the development of inorganic FsNF embedding VAN deposited by plasma process. As it's known the use of antibiotics presents some limits since the ongoing release of antibiotics may promotes the development of resistant strains and the risks for the spread of such resistance, following the biomaterial prophylactic and therapeutic clinical use (Reidy, Haase et al. 2013).
An alternative to antibiotics for reducing bacterial viability and adhesion on medical devices is to focus on antimicrobial coatings that release antimicrobial agents such as silver (Ag) able to repel and/or kill bacteria .
The antibacterial activity of Ag and Ag+ in proper concentrations, accompanied
by low toxicity to human cells, is well known since a long time ago; in addition, bacterial resistance is not developed. Silver has been incorporated into the surface of a variety of medical devices, such as vascular, urinary and peritoneal catheters, vascular grafts, prosthetic heart valve, sewing rings, surgical sutures and fracture-fixation devices (Bayston, Vera et al. 2010). During the last years,
39
nanotechnology has produced a new route to take advantage of the antimicrobial behavior of metals by synthesizing highly active metal nanoparticles (NPs) (2008).
Moreover, studies in literature have suggested that impregnation of Ag into a coating can be more effective than direct surface coating alone, since surface Ag can be readily deactivated by protein anions, while Ag release cannot be controlled in the case of Ag coatings (Furno, Morley et al. 2004, Stobie, Duffy et al. 2008). Polymers that release Ag in the oxidized form have shown a strong antibacterial activity and would act as reservoirs of Ag and be capable of releasing Ag for extended periods (Hetrick and Schoenfisch 2006).
LP PE-CVD processes represent an efficient method to fabricate composite coatings embedding Ag NPs. Polymer-like coatings represent a potentially interesting approach to controlled drug release of inorganic antimicrobial compounds (Katsikogianni, Foka et al. 2013). Plasma polymerization of an organic film while adding an antibacterial agent by sputtering from a target is one of the most widely used and well characterized approach in this field. In this research polyethylene oxide (PEO) -like coatings embedding silver nanoparticles (PEO-like/Ag nanocomposite coating) have been produced following this approach. The formation of Ag clusters in different matrices is promoted by the high mobility of Ag atoms in the growing coating, which leads to aggregation of Ag clusters (Venkata Sai Kiran, Christian et al. 2012), whose size is strongly dependent on the Ag content (Drábik, Pešička et al. 2015); often, the silver
40
distribution is not uniform along the thickness of the coatings (Escobar Galindo, Manninen et al. 2013).
To deposit Ag/PEO-like nanocomposite coatings we used an LP plasma deposition system with parallel asymmetrical plate electrodes. Adjusting the process parameters, the coatings could be tailored (e.g., Ag content, dimensions distribution and morphology of Ag clusters) to different requirements for specific applications, where they act as Ag reservoirs. Bacterial adhesion on such samples was evaluated both in static and under flow conditions to investigate the combined effect of flow and surface chemistry on the ica gene expression (Katsikogianni, Foka et al. 2013). By a comparison between Ag/PEO-like coatings and highly cross-linked PEO-like ones, but with no silver embedded, prepared in the same conditions, only 30% of adherent bacteria on Ag/PEO-like coatings remained alive. It is known that assessment of icaA DBC operon gene expression is crucial to the understanding of the pathogenesis of S. epidermidis infections (Kajiyama, Tsurumoto et al. 2009). Higher expressions of icaA genes were observed for bacteria in contact with Ag/PEO-like coatings with respect to those on the corresponding highly cross-linked PEO-like ones with no silver.
In one study, to preserve the antibacterial efficiency while protecting eukaryotic cells from the toxic effect of the excessive Ag+ release, a barrier layer was
deposited on top of plasma deposited Ag/PEO-like coatings (D’Agostino R.; Favia) to limit the release of Ag+ ions in water media. The barrier layer can control and prolong the antibacterial effect. To check the toxicity of Ag/PEO-like
41
coatings for eukaryotic cells, in vitro cyto-toxicity tests were performed with SAOS2 cells grown on coatings loaded with 5% of silver, with and without barrier layers. Coatings without the barrier resulted somewhat toxic and could promote the adhesion of only few round shaped cells, due to the high release of Ag+ but, maybe, also to the direct contact of cells with Ag clusters protruding from the surface.
In this part of the Thesis, using the same LP PE-CVD processes and the same plasma reactor, FsNFs embedding silver nanoparticles has been developed depositing the plasma coatings on sacrificial layer that dissolve in water.
Nanofilm with unfouling cell-adhesive properties in biomedical field produced by PE-CVD
In this work of research is described how, using the same plasma process used to deposit Ag/PEO-like nanocomposite coatings but using the steel target, it is possible to obtain FsNFs PEO-like coatings with others specific bio-functionality as unfouling properties. Polymers as PEO have been shown to resist to protein and cell (including bacteria) attachment (Harris, Tosatti et al. 2004). Such surfaces are defined as “non-fouling” or “anti-fouling”(Brétagnol, Lejeune et al. 2006, Feng, Zhu et al. 2006, Choukourov, Gordeev et al. 2010). This property is believed to correlate strongly with the hydration layer at the PEO surface (Morra 2000), attributed to the presence of hydrophilic ether (CH2-CH2-O)n.
functionalities. These groups create a water-solvated structure which forms a liquid-like surface with highly mobile disordered molecular chains (Favia and
42
d’Agostino 1998, Chen, Li et al. 2010). For protein adsorption to occur, there must be a reduction in the dehydration entropic energy associated with the removal of surface bound water. Due to this effect, the tightly bound water molecules entrapped in the PEO surface through hydrogen bonds form a physical and energetic barrier that cannot be displaced by proteins and cells. Due to the characteristic properties of PEO, the researchers have been focused the attention to the development of different approaches (i.e., covalent immobilization, physical adsorption, self-assembled monolayers and plasma deposition) to fabricate PEO-like thin films (Illum, Jacobsen et al. 1987, Lee, Lee et al. 1995, Beyer, Knoll et al. 1997, Favia, Sardella et al. 2003, Sardella, Gristina et al. 2004, Sardella, Gristina et al. 2005, Sardella, Detomaso et al. 2008, Krsko, Kaplan et al. 2009).
Compared to other methods, LP PE-CVD processes from monomers with CH2CH2O moieties in their structure have been widely applied as a versatile tool
to impart non-fouling properties with PEO-like coatings on a large variety of substrates (Baquey, Palumbo et al. 1999, Rodriguez-Emmenegger, Kylián et al. 2011, Chen, Chen et al. 2013, Quintieri, Pistillo et al. 2013, Fanelli, Mastrangelo et al. 2014). Three important features must be achieved for these coatings: good adhesion to substrates, stability in water media, and high retention of the CH2CH2O functionalities of the monomer. This latter parameter is known as PEO
character and can be evaluated by measuring the relative importance of the ether carbon component C1 at ~286.5 eV of Binding Energy in the C1s X-ray