Contents lists available atScienceDirect
Journal of Archaeological Science
journal homepage:www.elsevier.com/locate/jasQuantitative ultrasonometry for the diagnosis of osteoporosis in human
skeletal remains: New methods and standards
Natascia Rinaldo
a, Alba Pasini
a,∗, Roberta Donati
a, Maria Giovanna Belcastro
b,c,
Emanuela Gualdi-Russo
aaDepartment of Biomedical and Specialty Surgical Sciences, University of Ferrara, Corso Ercole I d’Este 32, 44121, Ferrara, Italy bDepartment of Biological, Geological and Environmental Sciences, University of Bologna, Via Selmi 1, 40126, Bologna, Italy cAix Marseille University, CNRS, EFS, ADES, Marseille, France
A R T I C L E I N F O Keywords: Bone density Ultrasonometry Phalanx Ultrasound Paleopathology Osteoporosis A B S T R A C T
Osteoporosis, a complex and heterogeneous disorder with a multi-factorial etiology, is characterized by ab-normal bone loss leading to an increased risk of fractures.
In recent years, the study of osteoporosis and bone mineral quality has received increasing interest by bio-logical anthropologists. In particular, the study of bone quality in ancient populations in relation to sex, age and cultural background can provide important insights into the diachronic evolution of a seemingly modern pa-thology. However, a number of challenges remain in the determination of bone loss in ancient remains, partly due to the methodological approaches applied in the anthropological analysis. This underlines the need for a new methodology and new standards, specifically created and adapted to human skeletal remains.
The current study aims to develop a new methodology to assess bone quality in modern and ancient human skeletal remains using Quantitative Ultrasonometry, applied for the first time to a skeletal sample of known age-at-death and sex (Frassetto collection, University of Bologna). After the assessment of intra- and inter-observer reliability, new ultrasonometric standards based on the analysis of age-related and sex-related changes in bone quantity and quality were created, providing a reference point for the analysis of osteoporosis and bone loss in skeletal remains. The applicability of the method was tested in a medieval sample including both males and females. The low intra- and inter-observer errors suggest that the Phalangeal Ultrasonometry is a reliable and valid technique that can be applied to modern and ancient human skeletons.
1. Introduction
Osteoporosis is a metabolic bone disorder characterized by a com-promised bone strength due to low bone density and microarchitectural deterioration of bone tissue, leading to an increasing risk of fracture (Curate, 2014; Dede and Callan, 2018; Golob and Laya, 2015; NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, 2001). The most common consequences of this condition are three different types of fractures occurring after a moderate trauma, such as a fall: fractures of the proximal femur (hip), vertebral com-pression fractures, and fractures of the distal junction of the radius (Colles’ fracture, Smith fracture) (Curate, 2014;Golob and Laya, 2015; Johnell and Kanis, 2006).
Bone tissue constantly undergoes modeling and remodeling pro-cesses throughout life through the action of several types of bone cells (osteoblasts, osteocytes and osteoclasts), whose relative activity
determines bone balance (Boyd, 2009;Brickley and Ives, 2008;Curate, 2014;Fleisch, 2000;Golob and Laya, 2015;Gosman and Stout, 2010). During the modeling process, bone tissue is constantly modified in size, shape and position to mechanically adapt bone during the initial ske-letal formation; after the end of puberty, remodeling becomes the prevailing metabolic skeletal process (Curate, 2014; Prestwood and Raisz, 2000). Bone tissue is always subject to remodeling processes during the life of the individual in order to respond to new stresses and replace older bone tissue. However, osteoblastic activity decreases with senescence. (Curate, 2014; Gilsanz, 1999; Golob and Laya, 2015; Madimenos, 2015;International Osteoporosis Foundation, 2017). Age-related bone density loss begins after peak bone mass (PBM) and the balance between modeling and remodeling is interrupted, leading to a prevalence of bone resorption over bone formation (Golob and Laya, 2015). Skeletal disorders such as osteopenia (i.e., generalized loss of bone) and osteoporosis occur when excessive osteoclastic activity leads
https://doi.org/10.1016/j.jas.2018.09.013
Received 5 July 2018; Received in revised form 28 September 2018; Accepted 28 September 2018
∗Corresponding author. Department of Biomedical and Specialty Surgical Sciences, University of Ferrara, corso Ercole I d’Este 32, Ferrara, 44121, Italy. E-mail address:[email protected](A. Pasini).
Available online 11 October 2018
0305-4403/ © 2018 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
to a bone density loss (Curate, 2014;Golob and Laya, 2015). Osteoporosis is a multiple-etiological disorder influenced by en-dogenous and exogenous factors, including senescence, sex, hormonal factors, dietary and behavioral habits, genetics, reproductive and lac-tation factors and physical activity (Brickley and Ives, 2008;Dede and Callan, 2018;Golob and Laya, 2015). Aging is known to be the primary risk factor of osteoporosis, due to decreases in osteoblastic activity and in the intestinal absorption of calcium and other nutrients useful to bone formation (Curate, 2014;Madimenos, 2015;Recker et al., 2004; Riggs and Melton, 1986).
The study of osteoporosis in ancient populations could be helpful to understand the patterns and prevalence of this disease in the past and the present (Agarwal and Grynpas, 1996). Although osteoporosis was first described about 250 years ago (Duverney, 1751), several studies have subsequently underlined how this condition (and a more gen-eralized decline in bone strength) has always affected human groups, especially after the sedentarism following agriculture and domestica-tion (Agarwal et al., 2004;Agarwal and Grynpas, 1996;Beauchesne and Agarwal, 2017;Brickley, 2002;Dewey et al., 1969;Larsen, 2003;Mays, 2016;Ruff et al., 2006,2015;Ryan and Shaw, 2015;van Gerven et al., 1969). Although it is quite difficult to establish a clear origin of this disease (Curate, 2014;Curate et al., 2013;Mays, 2008), several ana-lytical approaches to the study of ancient evidence of osteoporosis have revealed clear signs of patterns of bone loss. A preliminary diagnosis of osteoporosis in archaeological samples is mainly based on the presence of characteristic fractures in elderly groups of a population, which are usually perceived as osteoporotic, or due to the fragility of the bone structure (Curate, 2014).
In order to apply a more standardized and quantitative metho-dology in the paleopathological study of osteoporosis, several biome-dical techniques have been tested in skeletal samples (Agarwal and Grynpas, 1996; Beauchesne and Agarwal, 2017; Curate, 2014). The most common methods of bone mass evaluation are dual X-ray ab-sorptiometry (DXA) and radiogrammetry. DXA, considered a very ac-curate method to assess osteoporosis in archaeological skeletal samples (Beauchesne and Agarwal, 2017;Golob and Laya, 2015), calculates the amount of hydroxyapatite in grams of mineral per unit area on a bone through the transition of two radiation stems across the bone; however diagenetic processes can affect the outcomes, and the costs of this method are also quite substantial (Curate, 2014). Radiogrammetry quantifies the amplitude or geometry of the cortical bone in long bones by calculating the ratio between medullary cavity thickness and total width of the diaphysis directly on radiographic images; nevertheless the measurement of the medullary cavity is not always accurate, with a precision of the method around 5–10% (Beauchesne and Agarwal, 2017;Curate, 2014;Ives and Brickley, 2004).
The many studies on osteoporosis in ancient populations highlight the absence of a standardized methodology, a diversified panorama of analytical approaches and several methodological limits often due to age-at-death estimation methods in archaeological samples (Brickley, 2002; Curate, 2014). As pointed out by Curate (2014), the lack of a reference model for ancient populations should be overcome by the creation of a standardized methodology based on a skeletal collection with known age-at-death and sex. Quantitative ultrasonometry (QUS) is based on measurement of the velocity of ultrasound and on inter-pretation of the characteristics of the ultrasound signal (Wüster et al., 2000). This technique is currently used by clinicians for the assessment of bone mineral status in modern populations (Giavaresi et al., 2004; Hans and Baim, 2017). Phalangeal ultrasonometry applied since 1992, has proved to be a reliable method to diagnose osteoporosis and predict fractures (Baroncelli et al., 2006;Glüer, 1997;Guglielmi et al., 2009; Wüster et al., 2000); based on ultrasound propagation, it provides im-portant information about bone health, such as bone density, bone elasticity and risk of fractures in children, adolescents and adults in-dependently of bone mineral density (Baroncelli et al., 2010;de Terlizzi
et al., 2000; Guglielmi et al., 2009). This technique is also useful in assessing properties of bone microstructure, such as elasticity (Barkmann et al., 2000;de Terlizzi et al., 2000;Guglielmi et al., 2009; Wüster et al., 2005), correlated to bone density quality and risk of fractures. The reliability and potential of QUS have also been high-lighted in a cadaver study (Wüster et al., 2005), showing how quanti-tative ultrasound can provide better information than DXA concerning the architecture and mechanical resistance of bone tissue (Wüster et al., 2005). Limitations of the QUS technique are usually associated with the monitoring and correct maintenance of the devices and with the re-producibility of measurements; indeed, precision and rere-producibility of measurements are essential for the correct clinical use of QUS devices (Hans and Krieg, 2009;Wuster et al., 1998). Moreover, QUS is most effective when combined with an assessment of clinical risk factors, especially at the start of the monitoring process (Hans and Krieg, 2009). Therefore, this method provides several advantages, in that it is non-invasive, radiation-free, easy-to use, portable and computer-as-sisted, and automatically collects a large amount of data (Baroncelli et al., 2006; Guglielmi et al., 2009,2003; Hans et al., 1998;Wüster et al., 2005,2000). However, despite its advantages, it has never been applied to skeletal material, as far as we know. The aim of the present study was to develop new quantitative reference standards for the di-agnosis of osteoporosis in skeletal human remains by application of Quantitative Ultrasound analyses to phalanges of a large sample of individuals belonging to a modern skeletal collection with known age-at-death and sex (Frassetto collection, University of Bologna). Fur-thermore, archaeological specimens were used to assess osteoporosis based on comparisons with the new standard curves.
2. Materials and methods
2.1. Osteological samples
Our study included 110 subjects of known sex and age-at-death from the Frassetto collection (Museum of Evolution, Department of Biological, Geological and Environmental Sciences, University of Bologna). These individuals, who died in the early 1900s, were buried in the cemetery of Bologna and their remains were exhumed during the first half of the 20th century to be kept in the museum (Belcastro et al., 2017). In particular, we randomly selected approximately a dozen male subjects and a dozen female subjects for each of the following age groups: 21–30; 31–40; 41–50; 51–60; > 60, until a sample of 100 in-dividuals was reached. Furthermore, ten inin-dividuals were randomly chosen from the same collection for cross-validation.
Individuals who died of tuberculosis (cause of death was generally known as well; Appendix A,Supplementary Table 1) were excluded from the analysis due to the well-known destructive effect of this pa-thology on the bone tissues of several skeletal districts (Choi et al., 2017;Mariotti et al., 2015).
At the end of the experimentation, we applied QUS to a medieval sample of 20 adult subjects from different sites in the Po Valley to ex-emplify their placement in the new reference curves and the resulting interpretation. These specimens are now part of the osteological col-lections of the University of Ferrara (Laboratory of Archaeo-Anthropology and Forensic Archaeo-Anthropology, Dep. Biomedical and Specialty Surgical Sciences). Sex and age-at-death of the adult in-dividuals were estimated by traditional anthropological methods (Acsádi and Nemeskéri, 1970; Brooks and Suchey, 1990; Brothwell, 1981; Buikstra and Ubelaker, 1994;Ferembach et al., 1980; Gualdi-Russo, 2007; Lovejoy, 1985; Phenice, 1969; Todd, 1921, 1920). We used the mean age value of each individual (assessed by different methods) to plot the points in the graph of the AD-SoS reference curves, drawing through these points a bar parallel to the x-axis to represent the uncertainty in age determination.
2.2. Measurement protocol
Ultrasound measurements were performed with a DBM Sonic device 1200 (Igea, Carpi, Italy) that transmits an ultrasound wave at a fre-quency of 1.2 MHz through the proximal phalanges of the hand. The instrument consists of two probes acting as signal generator and re-ceiver mounted on a high precision caliper. The probes were applied on the metaphysis of the proximal phalanges of fingers II-IV in a medio-lateral direction following the standard protocol suggested by the manufacturer (Baroncelli et al., 2001). The distal end of the diaphysis of the proximal phalanx is an optimal site because it contains both cortical and trabecular bone (Wuster et al., 1998).
The QUS parameters considered in this study were AD-SoS (m/s) (amplitude-dependent speed of sound), BTT (μs) (bone transmission time), and UBPI (ultrasound bone profile index). AD-SoS (m/s) is de-fined as the distance between the transducers divided by the time of flight, that is the time from emitted pulse to received signal. It depends both on velocity and amplitude of the signal received considering the signal that reaches a predetermined minimum amplitude value (2 mV) for the first time (Baroncelli et al., 2001;Cadossi and Canè, 1996). BTT (μs) is the difference between the time when the first peak of the signal received attains its maximum and the time when the signal reaches 1700 m/s. BTT depends only on bone properties and is not influenced by the soft tissue (Baroncelli et al., 2006). UBPI is an index directly calculated by the instrument, whose value is between 0 and 1. It quantifies the signal characteristics and can be considered the “fracture-predictive value” (Wüster et al., 2000).
The final values considered for the analysis were the mean values obtained for each of the three parameters over the four fingers (Baroncelli et al., 2001,2006). When possible, the measurements were taken on the left hand. However, there were no significant differences between the two hands in previous studies (Baroncelli et al., 2001; Ventura et al., 1996). Before the experimentation, an instrument cali-bration was carried out by the manufacturer by means of a composite mother phantom.
Prior to the measurement, phalanges were immersed in water for five hours to remove the air inside the spongy bone. The amount of time needed was experimentally defined.
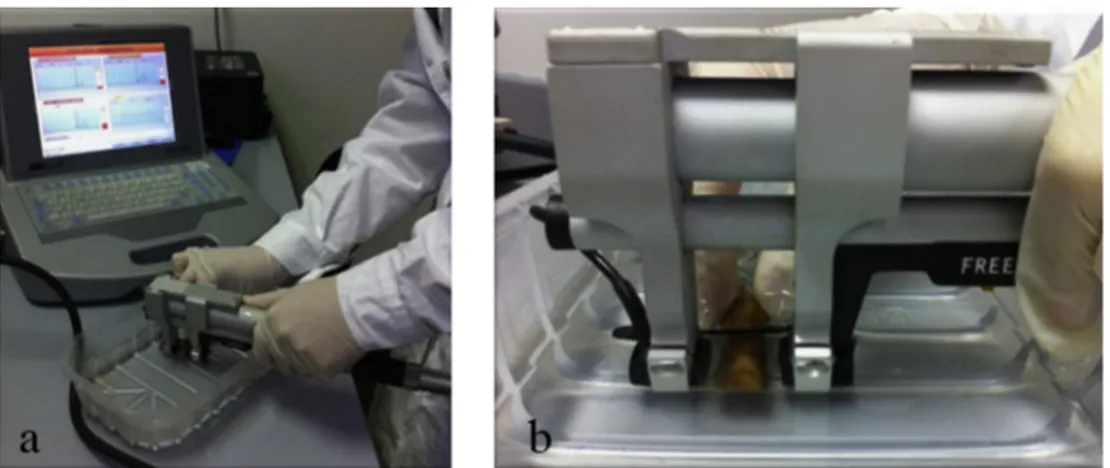
On living subjects, the coupling of the probes with the skin is mediated by standard ultra-sound gel. This method was ineffective when applied to skeletal material. Therefore, we decided to keep the phalanges in a tray filled with water during the measurement to allow the passage of the ultrasound signal through the phalanges and to im-prove the acoustic coupling (de Terlizzi et al., 2000; Wüster et al., 2005). (Fig. 1a). Due to the absence of soft tissues where probes adhere in the case of measurements on the living, a fixed distance of 18 mm between the probes was experimentally defined to allow measurements of both male and female phalanges (Fig. 1b).
To assess the reproducibility of the technique, measurements of 40 phalanges (ten individuals) were recorded by two trained observers (A and B) who worked independently from each other. Once all ten ske-letons were measured, the assessment was repeated by observer A, six days later.
Once the validation phase was completed, observer A measured the sample of 110 modern skeletons following the established protocol. Finally, observer A measured the phalanges of 20 medieval skeletons from the human skeletal collection of the Laboratory of Archeo-Anthropology and Forensic Archeo-Anthropology (University of Ferrara). 2.3. Statistical analysis
We assessed the intra- and inter-observer agreement in the mea-surements of bone density by computing the intra- and inter-observer error, Intraclass Correlation Coefficient (ICC) and Bland Altman plot, including mean differences and limit of agreement. ICC was based on a two-way model considering single measures and the same rater for all subjects. Discrepancies between the intra-observer measurements were identified by the Wilcoxon test and between inter-observer measure-ments by the Mann-Whitney test. The coefficient of variation (CV%), an expression of bone mineral density (BMD) variability, was calculated following the formula reported byEl Maghraoui and Roux (2008).
Descriptive analyses were reported as mean and standard deviation of QUS parameters by sex and age. Associations of age with QUS parameters were determined using the Pearson correlation coefficient (r) and the coefficient of determination (r2). Reference curves were plotted for AD-SoS by age, separately for sex, using a computational procedure of polynomial curve fitting.
The statistical significance was set at P < 0.05. All analyses were performed using “Statistica” for Windows, version 11 (StatSoft, Tulsa, OK, USA). We carried out the study in accordance with the guidelines for Reporting Reliability and Agreement Studies (GRRAS) (Kottner et al., 2011).
3. Results
The repeated intra- (A1-A2) and inter-observer (A1-B) measure-ments of the two considered QUS parameters (AD-SoS tot and BTT tot) showed no statistical differences (Table 1). The Bland Altman plot (Fig. 2), representing the total agreement between the repeated mea-sures of AD-SoS, showed a mean difference of −22.1 m/s between the repeated measurements of the same operator (A1-A2), with 95% limits of agreement between 153.8 and −109.7 m/s. All the measurements fell within the limits of agreement. The ICC resulted in an intra-varia-bility of 0.823 (95% CI 0.463–0.952) (Table 2), confirming the ex-cellent repeatability of the QUS method in the skeletal material. The measurements comparisons between two different operators (A1-B)
displayed similar results, with broader limits of agreement in the Bland Altman plot (169.4 and −221.6) and a slightly higher difference be-tween the two measurements (22.1 m/s) (Fig. 3). Moreover, only one measurement is slightly below the lowest limit of agreement. However, the ICC value between the two operators is nearly the same (0.823 95% CI 0.463–0.952) (Table 2) as the value obtained with the repeated measures by the same operator, signifying excellent reproducibility of the method. The coefficient of variation (CV%) calculated for AD-SoS was 2.54% for intra-observer repetition and 3.65% for the inter-ob-server repetition, proving good precision and a very small difference in reproducibility and repeatability in the application of QUS to the phalanges of the skeletal material.
Table 3 reports the mean and SD values of the three principal parameters detected/calculated by the Sonic Bone Profiler, AD-SoS, BTT and UBPI, reported for both the different age groups (overall range from 20 to 60+) and the total sample, for males and females separately. The linear association between QUS parameters and age was
investigated. AD-SoS showed a significant negative correlation with age in both sexes (the parameter decreases with age), with a coefficient of determination slightly higher in females (13% of the variance explained in males vs 18% of the variance explained in females) (Table 4). In contrast, BTT showed no significant linear association with age and UBPI showed significant correlation with age only in males, although the explained variance was low for both sexes (Table 4). We decided to create the reference curves for the modern skeletal collection based only on AD-SoS values (Fig. 4A and 4B) because in our study it was the only parameter significantly correlated with age in both sexes. More-over, in the scientific literature on QUS, it is the most widely used parameter, being considered a diagnostic tool for osteoporosis diag-nosis. Therefore, the use of the same parameter allows a comparison
Table 1
Intra-observer and inter-observer error between measurements derived from QUS.
Intra-observer error (n = 10) Parameter A1 Mean
(SD) A2 Mean (SD) Difference 95% CI p value AD-SoS tot (m/s) 1890(121) 1868(104) −22.1 −70.1 to 26.0 0.1688 BTT tot (μs) 1.41 (0.67) 1.38 (0.58) −0.04 −0.30 to 0.22 0.9528 Inter-observer error (n = 10) AD-SoS tot (m/s) 1890(121) 1916 (201) 26.1 −45.3 to 97.4 0.8785 BTT tot (μs) 1.41 (0.67) 1.60 (0.96) 0.18 −0.06 to 0.43 0.1029
Fig. 2. Bland Altman plot evaluating the absolute agreement between two
measures of the same observer (A1 vs A2) for the parameter AD-SoS m/s) X-axis: average of the two measures; Y-X-axis: difference between the two measures.
Table 2
Intraclass Correlation Coefficient (ICC) values resulting from the repeatability and reproducibility tests of the AD-SoS tot (m/s) and their related 95% CI.
Intra-variability (n = 10) Inter-variability (n = 10) A1 – A2 A1 – B
ICC values 0.823 0.824 95% CI 0.463 to 0.952 0.459 to 0.953
Fig. 3. Bland Altman plot evaluating the absolute agreement between measures
of two different observers (A1 vs B) for the parameter AD-SoS m/s) X-axis: average of the two measures; Y-axis: difference between the two measures.
Table 3
Mean and Standard Deviation values for AD-SoS, BTT and UBPI in each age range of males and females.
Age range N AD-SoS (m/s) BTT (μs) UBPI Mean ± SD Mean ± SD Mean ± SD Males 21–30 12 1829 ± 56 1,07 ± 0,36 0,33 ± 0,10 31–40 11 1842 ± 54 1,24 ± 0,25 0,32 ± 0,12 41–50 11 1819 ± 72 1,11 ± 0,35 0,27 ± 0,09 51–60 10 1790 ± 46 1,00 ± 0,20 0,27 ± 0,09 61 + 11 1772 ± 56 0,95 ± 0,29 0,25 ± 0,19 Total 55 1811 ± 61 1,08 ± 0,30 0,29 ± 0,12 Females 21–30 14 1839 ± 42 1,00 ± 0,28 0,37 ± 0,09 31–40 9 1799 ± 29 0,74 ± 0,18 0,36 ± 0,16 41–50 9 1799 ± 41 0,95 ± 0,29 0,27 ± 0,10 51–60 10 1798 ± 42 1,03 ± 0,30 0,38 ± 0,12 61 + 13 1779 ± 61 0,91 ± 0,38 0,32 ± 0,09 Total 55 1805 ± 49 0,94 ± 0,30 0,34 ± 0,11 Table 4
Linear correlation between age and QUS parameters (AD-SoS, BTT and UBPI) in males and females.
QUS parameter Males Females
r r2 p r r2 p
ADSoS (m/s) −0.366 0.134 0.0061a −0.419 0.176 0.0014a
BTT (μs) −0.217 0.047 0.1121 −0.029 0.001 0.8346 UBPI −0.268 0.072 0.0475a −0.158 0.025 0.2541
between our new curves and other reference curves from the literature, (Hamidi et al., 2008;Hayman et al., 2002;Wüster et al., 2000).
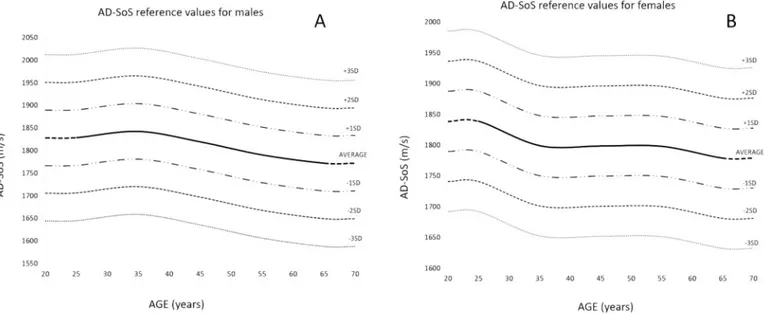
In males, the peak value of AD-SoS was found in the 31–40 age group, whereas in females the peak value was in the first group aged 21–30 (Table 3). The lowest value in both males and females was ob-served in the last group aged 61 or more (Table 3).Fig. 4shows the average AD-SoS trend in the two sexes. Several reference lines have been drawn to display −1 SD, −2 SD and −3 SD from average. These cutoffs were chosen to indicate, respectively, mild bone loss (from < -1SD to ≥ -2SD), moderate bone loss (from < -2SD to ≥ -3SD) and severe bone loss (< -3SD). Moreover, we completed the graphs re-presenting +1 SD, +2 SD and +3 SD lines from the average (Figg. 4A and 4B).
In order to demonstrate the use of the new reference curves in the archaeological skeletal material, we applied the same methodology to a sample of medieval skeletons of various age-at-death.Table 5reports the list of medieval individuals analyzed for osteoporosis by site. Then we plotted the resulting AD-SoS values of medieval skeletons on the previously created graphs inFig. 5. The results of four males (Fig. 5A) fell between plus and minus 1SD from the average curve (ID no. 9, 6, 1, 8), indicating a normal range of bone mineral density. Mild and mod-erate bone loss resulted for ID no. 5, 3, 10 and for ID no. 2, 4 respec-tively. Severe bone loss resulted only for ID no. 7 that fell slightly un-derneath of -3SD line. Among females (Fig. 5B), four of them fell within the normal range (ID no. 2, 3, 5, 6). Among the remaining females, three had values slightly lower than -1SD, indicating mild bone loss (ID no. 1, 4, 10), other two fell under -2SD, indicating moderate bone loss (ID no. 7, 8), whereas just an old female resulted to have a severe bone loss (ID no. 9).
4. Discussion
The main objective of this study was to test whether QUS can be considered a reliable method for the determination of bone loss and bone quality in ancient and modern skeletal material. To create new standard curves that could be used as reference values, we applied the technique to a sample of modern healthy skeletons of known age-at-death and sex.
Although QUS can be applied to various skeletal sites (e.g tibia, metacarpal, phalanges) (Stieglitz et al., 2017) we decided to use the phalanges for several reasons. As observed byWüster et al. (2005), the human phalanx is one of the most metabolically active parts of the
skeleton, being subject to morphological and structural changes of its trabecular and cortical bone during the bone remodeling process oc-curring with aging. Its epiphyseal and metaphyseal trabecular and cortical structure are directly involved in the transmission of ultrasound impulses, since the trabecular bone of the proximal meta-diaphyseal region is the first one to undergo the resorbing process (Barkmann et al., 2000; Wüster et al., 2000, 2005). Furthermore, the common discovery of phalanges in ancient burials makes phalangeal QUS a very useful method to assess the bone mineral status of individual bones in ancient populations.
In the first part of this study, we assessed the reliability of QUS applied to the phalanx of modern skeletal material. This was necessary because, to our knowledge, the applicability of QUS to skeletal material had never been proven. Our results show a low, non-significant abso-lute difference between the repeated measures, both between the same operator and between two different operators. Moreover, ICC values demonstrate an excellent repeatability and reproducibility of the method, with a lower intra-observer than inter-observer error. Further steps should be taken to ensure agreement if the method were to be used by multiple observers. The precision of the method was evaluated through the Coefficient of Variation (CV%). The results obtained for AD-SoS values are slightly higher than those obtained in cross-sectional studies that used the same instrument applied to a living population (mean 1.0%; range 0.4%–2.5%) (Krieg et al., 2008). This is true espe-cially for the inter-observer precision (3.65%). On the other hand, the intra-observer precision is very similar to that observed in the living (2.54%). This is likely due to the greater difficulties in measuring dry bone of the phalanx in comparison to fingers with soft tissue.
After testing for reliability and agreement, we statistically verified the correlation between QUS parameters and age and created new re-ference curves based on mean AD-SoS values. The creation of these standard curves for AD-SoS will be a useful tool to assess the bone mineral status of a skeletal individual in comparison to a skeletal re-ference population from the same geographic area rather than in comparison to living populations. Moreover, these curves may be fun-damental in the diagnosis of osteopenia and osteoporosis using the established cut-offs. An AD-SoS value below −2 SD (corresponding to < −2 z score or < 3rd percentile) may identify a condition of “low mineral status” and therefore osteopenia, while a value under −3 SD (corresponding to < −3 z score or < 0.1st percentile) should indicate osteoporosis. The need of new reference curves based on skeletonized material is underlined by the risk of bias between dry bones and living
Fig. 4. AD-SoS references values for males (A) and females (B). Curves are smoothed with a moving average of ± 10 years, with reference lines included
subjects with soft tissue. As an example of the applicability of the re-ference curves, we tested the technique on some medieval skeletons, obtaining important indications of their bone mineral status compared to the reference sample.
Our results confirm that age is an important factor affecting QUS parameters, especially AD-SOS. As it is well known, there is a peak of BMD at age 25–35 and then a natural decrease after a certain age (approximately 40) (Guglielmi et al., 2015; Maalouf et al., 2007;
Wüster et al., 2000), consistently with our reference curves. In our fe-male sample, there is a peak of BDM at age 25, followed by a slight decrease from 25 to 35 and a rapid decrease after 55 (probably due to menopause). This trend is partially consistent with the AD-SoS data obtained in a sample of living European women byWüster et al. (2000). In particular, the curve ofWüster et al. (2000)showed a small increase in the age range 19–30, a decrease until 40, then a rapid decline after 50. The reference curve for Lebanese females showed a slightly
Table 5
List of medieval subjects of different sex and age examined by QUS.
ID number Age Archaeological site Dating Archaeological context References Males
1 50–60 Chiunsano 2nd -4th
Century The burial area consisted in 31 inhumations from the Early Middle Ages,distributed along the Roman settlement area in Gaiba municipality (Rovigo, Italy).
(Büsing et al., 1994;Büsing and Büsing Kolbe, 1996) 2 45–55 Crocetta Early 15th
Century Single inhumation in rectangular pit located at the center of the Oratoryof Cento (Ferrara, Italy); the body was placed in a supine position, with the head lodged within a niche in the south wall of the pit and covered by a tile; other tiles were found located close to the lower extremities.
(Balboni et al., 2005;Lorenzini, 2001;Onisto and Gualdi-Russo, 2011)
3 53–63 S. Maria in Padovetere 4th- 7th
Century Simple pit burials referring to the Early Medieval period and referring tothe area of the VI Century church of S. Maria in Padovetere (Comacchio, Ferrara, Italy).
(Corti, 2007) 4 57–63 S. Maria in Padovetere
5 54–68 S. Maria in Padovetere
6 23–28 Ferrara S. Anna 15th-16th
Century Several Medieval inhumations where found in the area of the cloister andthe church of S. Anna (Ferrara, Italy), founded in 1295 as a convent and lately converted in hospital and asylum.
(D'Angelo, 2005;Onisto et al., 2006)
7 30–40 Imola – Via Maghinardo 13th Century Two coffer graves inhumations discovered in the city of Imola (Bologna, Italy), siding two masonry structures from the same chronological frame. 8 53–68 Chiesazza 4th-6th
Century The burial area of Chiesazza (Ficarolo, Rovigo, Italy) consisted in 59inhumations in simple pit burial or in wooden coffin. Only one burial (grave no. 28) showed the presence of grave goods.
(Büsing et al., 1994;Büsing and Büsing Kolbe, 1996) 9 23–34 Chiesazza
10 30–39 Chiesazza Females
1 50–59 Chiesazza 4th-6th
Century See description above. (Büsing Kolbe, 1996Büsing et al., 1994);Büsing and 2 37–49 Chiesazza
3 30–40 Chiesazza 4 30–34 Chiesazza 5 23–39 Chiesazza
6 18–21 Chiunsano 2nd-4th
Century The burial area consisted in 31 inhumations from the Early Middle Ages,distributed along the Roman settlement area (Büsing Kolbe, 1996Büsing et al., 1994);Büsing and 7 18–22 Ferrara S. Anna 15th-16th
Century See description above. (D'Angelo, 2005;Onisto et al., 2006) 8 25–35 Imola – via Emilia 7th-8th
Century Single inhumations in brick coffins. These burials were part of a group ofthree graves dated to the Lombard era, discovered along the Via Emilia (Imola, Bologna, Italy).
(Pasini et al., 2018) 9 58–72 Imola – via Emilia
10 23–39 S. Maria in Padovetere 6th- 7th
Century See description above. (Corti, 2007)
different trend, with an increase between 25 and 35 followed by a rapid decrease (Maalouf et al., 2007).
In our study the trend of AD-SoS values of males, with a peak at age 35, followed by a rapid decrease, is consistent with the values obtained byMontagnani et al. (2000)in a sample of living Italian males: the AD-SoS peak was reached at age 31–40, with a subsequent decrease reaching its lowest values in the last decade, and the BTT values fol-lowed the same trend. Similar trends have been obtained for other male samples (Hayman et al., 2002). More generally, our curves are con-sistent with the physiological decrease of BMD during aging in both males and females (Wüster et al., 2000; Drozdzowska et al., 2005; Baroncelli et al., 2010;Maalouf et al., 2007). However, the mean values of QUS parameters in the modern skeletal material, especially AD-SoS, are lower than those of the living population. Although some studies did not report differences between bone or finger (soft tissue plus bone) in AD-SoS, suggesting that this parameter reflects only the structural characteristic of the bone (Guglielmi et al., 2003;Sakata et al., 2004), they probably did not consider the difference between fresh bone and dry bone.
The evaluation of osteoporosis and bone quality in archaeological skeletal remains has become a great challenge in paleopathology, mainly due to a lack of methodological standardization, the lack of reference values and methodological limitations in the age-at-death estimation of archeo-anthropological skeletal remains (Curate et al., 2016;Genant et al., 1993;Gonzalez-Reimers et al., 2007;Hirata and Morimoto, 1994;Mays, 2006). Although a large number of methods can offer different views of bone remodeling and maintenance (Brickley and Agarwal, 2003), results from different methodologies are not directly comparable (Curate, 2014); therefore, the use of standardized metho-dology and reference models are necessary for the paleopathological study of osteoporosis.
One of the commonly used tools for measuring BMD and evaluating osteoporosis and fracture risks is the dual energy X-ray absorptiometry (DXA). However, this method has some limitations, particularly in its applicability to skeletal material. First, data comparisons between skeletal specimens and living individuals can cause several inter-pretative errors (Curate, 2014). Second, its reliability can be distorted due to the effects of taphonomic processes (Sutlovic et al., 2016). However, quantitative ultrasonometry also presents these limitations, as reported in the following section.
In recent years, QUS has been used by clinicians to the assessment of BMD and to evaluate osteoporosis and fracture risks (Baroncelli et al., 2006; Glüer, 1997; Hamidi et al., 2008; Hayman et al., 2002; Montagnani et al., 2000; Zhu et al., 2008). Research has also been conducted to determine how the density and elasticity of the spongy bone from decalcified pig phalanges and fresh human phalanges in-fluenced the propagation of ultrasound waves, the results being com-pared with those obtained from DXA, micro-computed tomography and mechanical testing. They showed that transmission of the ultrasound signal is closely linked to the degree of mineralization, and thus is a powerful tool for the diagnosis of osteoporosis (de Terlizzi et al., 2000; Wüster et al., 2005). However, these studies had several limitations, mainly due to the impossibility of comparing the values obtained with those of other methodologies and to the low number of specimens used. QUS techniques applied to osteological remains have several ad-vantages over other classically used techniques (i.e. DXA), such as lower equipment and operational costs, the lack of any need for a radiographic technologist or designated room, the absence of ionizing radiation (Hamidi et al., 2008;Hans and Baim, 2017) and the lack of any need to sacrifice skeletal parts as required by some destructive analytical techni-ques (i.e. histological analysis). Hence, it would appear to be a more ac-cessible method for the paleopathological assessment of osteoporosis.
Nonetheless, whatever technique is applied, there are several lim-itations to the study of bone density in archaeological remains. In particular, post-mortem changes in bones due to taphonomic factors may lead to incorrect results (Sutlovic et al., 2016). In addition to a
general loss of bone density after death, the inhumed bone may be severely damaged by humic acids (Schultz, 2003). Moreover, plant roots, fungi and algae, insects and larvae can attack the skeleton, de-stroying bone and producing holes and tunnels (Hackett, 1981; Schotsmans et al., 2017;Schultz, 2003). With regard to QUS, in addi-tion to these limitaaddi-tions, particular cauaddi-tion is required in the case of specimens with poor preservation, as the procedure involves dipping the specimen in water for five hours before taking the measurement. The absence of soft tissue and the presence of air inside the bones make it impossible to follow the standard protocol, which needs to be adapted accordingly. The use of deionized water can reduce the exchange of minerals between bone and water.
In conclusion, to our knowledge this is the first study to address the use of QUS to assess osteoporosis in skeletal remains and to propose reference standards. A key strength of the present investigation is that all the modern skeletons examined came from an osteological collection with known age-at-death and sex; the subjects referred to the same modern period and came from the same geographic area. In addition, almost all the individuals had the same low social status. Application of QUS to a medieval sample of both sexes from some northern Italian archaeological sites indicated promising results, as we were able to measure AD-SoS values of all the individuals and to estimate their bone density according to the new reference graphs; thus confirming the applicability of QUS even on archaeological skeletal material.
This study also has limitations that need to be taken into account. The first is the impossibility to estimate the real effect of diagenesis on the loss of bone density, at least without the use of an invasive analysis. Another limitation is the lack of comparison between the results ob-tained using QUS and those of other techniques, such as anthro-pological and radiographic analysis and diagnosis of osteoporotic fractures or DXA analysis. Moreover, although the new standards have been developed without including individuals in the sample who died of tuberculosis, we cannot exclude that other diseases may have af-fected bone density. Finally, despite the fact that the Frassetto collec-tion has proved to be a good reference sample for several methodolo-gical studies (Gualdi-Russo, 2007;Gualdi-Russo et al., 1999; Gualdi-Russo and Galletti, 2004;Gualdi-Russo and Russo, 1995;Hens et al., 2008;Zampetti et al., 2016), the applicability of the curves based on this collection to other non-Mediterranean populations must be de-monstrated by further research, since ethnic differences in bone density have been observed with higher values in Africans than in whites and lower values in Asians than in whites (Manifold, 2014).
Future studies should address the diagnosis of osteoporosis in ske-letal remains by means of QUS compared to other diagnostic techni-ques. Thanks to our new reference curves, this technique could also be useful to identify the pathological status related to increased bone mi-neral density (hyperostosis).
Declarations of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
We should like to thank Dr. Francesca De Terlizzi for her support and several useful suggestions given during the analysis.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps:// doi.org/10.1016/j.jas.2018.09.013.
References
Acsádi, G., Nemeskéri, J., 1970. History of Human Life Span and Mortality. Akadémiai Kiadó, Budapest, Hungary.
Agarwal, S.C., Grynpas, M.D., 1996. Bone quantity and quality in past populations. Anat. Rec. 246, 423–432.
Agarwal, S.C., Dumitriu, M., Tomlinson, G.A., Grynpas, M.D., 2004. Medieval trabecular bone architecture: the influence of age, sex, and lifestyle. Am. J. Phys. Anthropol. 124, 33–44.https://doi.org/10.1002/ajpa.10335.
Balboni, B., Borghi, R., Guarnieri, C., Tassinari, V., 2005. L’oratorio della Crocetta: sco-perte e prospettive di ricerca. Contributi centesi n°2, collana di studi storici curata dal comune di Cento. Siaca Editore, Cento (Fe).
Barkmann, R., Lüsse, S., Stampa, B., Sakata, S., Heller, M., Glüer, C.C., 2000. Assessment of the geometry of human finger phalanges using Quantitative Ultrasound in vivo. Osteoporos. Int. 11, 745–755.https://doi.org/10.1007/s001980070053. Baroncelli, G.I., Federico, G., Bertelloni, S., De Terlizzi, F., Cadossi, R., Saggese, G., 2001.
Bone quality assessment by quantitative ultrasound of proximal phalanxes of the hand in healthy subjects aged 3-21 years. Pediatr. Res. 49, 713–718.https://doi.org/ 10.1203/00006450-200105000-00017.
Baroncelli, G.I., Federico, G., Vignolo, M., Valerio, G., del Puente, A., Maghnie, M., Baserga, M., Farello, G., Saggese, G., 2006. Cross-sectional reference data for pha-langeal quantitative ultrasound from early childhood to young-adulthood according to gender, age, skeletal growth, and pubertal development. Bone 39, 159–173.
https://doi.org/10.1016/j.bone.2005.12.010.
Baroncelli, G.I., Battini, R., Bertelloni, S., Brunori, E., de Terlizzi, F., Vierucci, F., Cipriani, P., Cioni, G., Saggese, G., 2010. Analysis of quantitative ultrasound graphic trace and derived variables assessed at proximal phalanges of the hand in healthy subjects and in patients with cerebral palsy or juvenile idiopathic arthritis. A pilot study. Bone 46, 182–189.https://doi.org/10.1016/j.bone.2009.09.010.
Beauchesne, P., Agarwal, S.C., 2017. A multi-method assessment of bone maintenance and loss in an Imperial Roman population: implications for future studies of age-related bone loss in the past. Am. J. Phys. Anthropol. 164, 41–61.https://doi.org/10. 1002/ajpa.23256.
Belcastro, M.G., Bonfiglioli, B., Pedrosi, M.E., Zuppello, M., Tanganelli, V., Mariotti, V., 2017. The history and composition of the identified human skeletal collection of the certosa cemetery (Bologna, Italy, 19th–20th century). Int. J. Osteoarchaeol. 5, 912–925.https://doi.org/10.1002/oa.2605.
Boyd, S., 2009. Micro-computed tomography. In: Sensen, C.W., Halgrímsson, B. (Eds.), Advanced Imaging in Biology and Medicine: Technology, Software Environments, Applications. Springer-Verlag, Berlin, pp. 3–25.
Brickley, M., 2002. An investigation of historical and archaeological evidence for age-related bone loss and osteoporosis. Int. J. Osteoarchaeol. 12, 364–371.https://doi. org/10.1002/oa.635.
Brickley, M.B., Agarwal, S.C., 2003. In: Agarwal, S.C., Stout, S.D. (Eds.), Techniques for the Investigation of Age-related Bone Loss and Osteoporosis in Archaeological Bone BT - Bone Loss and Osteoporosis: an Anthropological Perspective. Springer US, Boston, MA, pp. 157–172.
Brickley, M., Ives, R., 2008. The Bioarchaeology of Metabolic Bone Disease, first ed. Elsevier.
Brooks, S., Suchey, J.M., 1990. Skeletal age determination based on the os pubis: a comparison of the Acsádi-Nemeskéri and Suchey-Brooks methods. Hum. Evol. 5, 227–238.https://doi.org/10.1007/BF02437238.
Brothwell, D.R., 1981. Digging up Bones. The Excavation, Treatment and Study of Human Skeletal Remains. British Museum (Natural History). Cornell University Press, London.
Buikstra, J.E., Ubelaker, D.H., 1994. Standards for data collection from human skeletal remains. Arkansas Archaeol. Surv. Res. Ser. Series 44.
Büsing, H., Büsing Kolbe, A., 1996. Sei anni di ricerche archeologiche a Ficarolo Gaiba, vol. XXXI. Padusa, pp. 7–17.
Büsing, H., Büsing Kolbe, A., Bierbrauer, V., 1994. Comuni di Ficarolo e Gaiba, Chiunsano, Rovigo: due tombe ostrogote. I Goti, pp. 186–188.
Cadossi, R., Canè, V., 1996. Pathways of transmission of ultrasound energy through the distal metaphysis of the second phalanx of pigs: an in vitro study. Osteoporos. Int. 6, 196–206.https://doi.org/10.1007/BF01622735.
Choi, C.-J., Choi, W.-S., Kim, C.-M., Lee, S.-Y., Kim, K.-S., 2017. Risk of sarcopenia and osteoporosis in male tuberculosis survivors: korea national health and nutrition ex-amination survey. Sci. Rep. 7, 13127.https://doi.org/10.1038/s41598-017-12419-y.
Corti, C., 2007. Santa Maria in Padovetere: la chiesa, la necropoli e l’insediamento cir-costante. In: Berti, F., Bollini, M., Gelichi, S., Ortalli, J. (Eds.), Genti Nel Delta Da Spina a Comacchio. Uomini, Territorio e Culto Dall’antichità All'Alto Medioevo. Corbo Editore, Ferrara, pp. 531–552.
Curate, F., 2014. Osteoporosis and paleopathology: a review. J. Anthropol. Sci. 92, 119–146.https://doi.org/10.4436/JASS.92003.
Curate, J.F.T., Albuquerque, A., Correia, J., Ferreira, I., de Lima, J.P., Cunha, E.M., 2013. A glimpse from the past: osteoporosis and osteoporotic fractures in a Portuguese identified skeletal sample. Acta Reumatol. Port. 38, 20–27.
Curate, F., Silva, T.F., Cunha, E., 2016. Vertebral compression fractures: towards a standard scoring methodology in paleopathology. Int. J. Osteoarchaeol. 26, 366–372.
https://doi.org/10.1002/oa.2418.
de Terlizzi, F., Battista, S., Cavani, F., Canè, V., Cadossi, R., 2000. Influence of bone tissue density and elasticity on ultrasound propagation: an in vitro study. J. Bone Miner. Res. 15, 2458–2466.https://doi.org/10.1359/jbmr.2000.15.12.2458.
Dede, A.D., Callan, M., 2018. Treatment of osteoporosis: whom, how and for how long? Br. J. Hosp. Med. 79, 259–264.https://doi.org/10.12968/hmed.2018.79.5.259.
Dewey, J.R., Armelagos, G.J., Bartley, M.H., 1969. Femoral cortical involution in three
Nubian archaeological populations. Hum. Biol. 41, 13–28.
Drozdzowska, B., Pluskiewicz, W., Halaba, Z., Misiołek, H., Beck, B., 2005. Quantitative ultrasound at the hand phalanges in 2850 females aged 7 to 77 yr: a cross-sectional study. J. Clin. Densitom 8, 216–221.
Duverney, J.G., 1751. Traité des maladies des os. De Bure, Paris.
D'Angelo, F., 2005. In: Archeologia Medievale, vol. XXXII All’insegna del Giglio, Firenze. El Maghraoui, A., Roux, C., 2008. DXA scanning in clinical practice. QJM 101, 605–617.
https://doi.org/10.1093/qjmed/hcn022.
Ferembach, D., Schwydeski, I., Stloukal, M., 1980. Recommendations for age and sex diagnoses of skeletons. J. Hum. Evol. 9, 517–549.
Fleisch, H., 2000. Bisphosphonates in Bone Diseases: from the Laboratory to the Patient. Academic Press, San Diego.
Genant, H.K., Wu, C.Y., van Kuijk, C., Nevitt, M.C., 1993. Vertebral fracture assessment using a semiquantitative technique. J. Bone Miner. Res. 8, 1137–1148.https://doi. org/10.1002/jbmr.5650080915.
Giavaresi, G., Borsari, V., Fini, M., Martini, L., Tschon, M., De Terlizzi, F., Nicolini, A., Carpi, A., Giardino, R., 2004. Different diagnostic techniques for the assessment of cortical bone on osteoporotic animals. Biomed. Pharmacother. 58, 494–499.https:// doi.org/10.1016/j.biopha.2004.08.017.
Gilsanz, V., 1999. Accumulation of bone mass during childhood and adolescence. In: Orwoll, E. (Ed.), Osteoporosis in Men. Academic Press, San Diego, pp. 65–85. Glüer, C.C., 1997. Quantitative ultrasound techniques for the assessment of osteoporosis:
expert agreement on current status. J. Bone Miner. Res. 12, 1280–1288.https://doi. org/10.1359/jbmr.1997.12.8.1280.
Golob, A.L., Laya, M.B., 2015. Osteoporosis: screening, prevention, and management. Med. Clin. 99, 587–606.https://doi.org/10.1016/j.mcna.2015.01.010. Gonzalez-Reimers, E., Velasco-Vázquez, J., Arnay-de-la-Rosa, M., Machado-Calvo, M.,
2007. Quantitative computerized tomography for the diagnosis of osteopenia in prehistoric skeletal remains. J. Archaeol. Sci. 34, 554–561.https://doi.org/10.1016/ j.jas.2006.06.004.
Gosman, J.H., Stout, S.D., 2010. Current concepts in bone biology. In: Larsen, C.S. (Ed.), A Companion to Biological Anthropology. Wiley-Blackwell, Chichester, pp. 465–484. Gualdi-Russo, E., 2007. Sex determination from the talus and calcaneus measurements. Forensic Sci. Int. 171, 151–156.https://doi.org/10.1016/j.forsciint.2006.10.014.
Gualdi-Russo, E., Galletti, L., 2004. Human activity patterns and skeletal metric indicators in the upper limb. Coll. Antropol. 28, 131–143.
Gualdi-Russo, E., Russo, P., 1995. A new technique for measurements on long bones: development of a new instrument and techniques comparison. Anthropol. Anzeiger 53, 152–182.
Gualdi-Russo, E., Tasca, M.A., Brasili, P., 1999. Scoring of nonmetric cranial traits: a methodological approach. J. Anat. 195, 543–550.https://doi.org/10.1017/ S0021878299005543.
Guglielmi, G., Njeh, C.F., De Terlizzi, F., De Serio, D.A., Scillitani, A., Cammisa, M., Fan, B., Lu, Y., Genant, H.K., 2003. Phalangeal quantitative ultrasound, phalangeal mor-phometric variables, and vertebral fracture discrimination. Calcif. Tissue Int. 72, 469–477.https://doi.org/10.1007/s00223-001-1092-0.
Guglielmi, G., Adams, J., Link, T.M., 2009. Quantitative ultrasound in the assessment of skeletal status. Eur. Radiol. 19, 1837–1848. https://doi.org/10.1007/s00330-009-1354-1.
Guglielmi, G., De Terlizzi, F., Nasuto, M., Sinibaldi, L., Brancati, F., 2015. Quantitative ultrasound at the phalanges in a cohort of monozygotic twins of different ages. Radiol. Medica 120, 277–282.https://doi.org/10.1007/s11547-014-0440-x. Hackett, C.J., 1981. Microscopical focal destruction (tunnels) in exhumed human bones.
Med. Sci. Law 21, 243–265.https://doi.org/10.1177/002580248102100403. Hamidi, Z., Sedaghat, M., Hejri, S.M., Larijani, B., 2008. Defining cut-off values for the
diagnosis of osteoporosis in postmenopausal women by quantitative ultrasonography of the phalanx. Gynecol. Endocrinol. 24, 546–548.https://doi.org/10.1080/ 09513590802340548.
Hans, D., Baim, S., 2017. Quantitative ultrasound (QUS) in the management of osteo-porosis and assessment of fracture risk. J. Clin. Densitom. 20, 322–333.https://doi. org/10.1016/j.jocd.2017.06.018.
Hans, D., Krieg, M.-A., 2009. Quantitative ultrasound for the detection and management of osteoporosis. Salud Publica Mex. 51, s25–s37. https://doi.org/10.1590/S0036-36342009000700006.
Hans, D., Njeh, C.F., Genant, H.K., Meunier, P.J., 1998. Quantitative ultrasound in bone status assessment. Rev. Rhum. Engl. Ed. 65, 489–498.
Hayman, S.R., Drake, W.M., Kendler, D.L., Olszynski, W.P., Webber, C.E., Rosen, C.J., Genant, H.K., Orwoll, E.S., Pickard, L.E., Adachi, J.D., 2002. North American male reference population for speed of sound in bone at multiple skeletal sites. J. Clin. Densitom. 5, 63–71.https://doi.org/10.1385/JCD:5:1:063.
Hens, S.M., Rastelli, E., Belcastro, G., 2008. Age estimation from the human os coxa: a test on a documented Italian collection. J. Forensic Sci. 53, 1040–1043.https://doi.org/ 10.1111/j.1556-4029.2008.00818.x.
Hirata, K., Morimoto, I., 1994. Vertebral osteoporosis in late edo Japanese. Anthropol. Sci. 102, 345–361.
International Osteoporosis Foundation, 2017. Facts and statistics.https://www. iofbonehealth.org/facts-statistics, Accessed date: 28 September 2018.
Ives, R., Brickley, M.B., 2004. A procedural guide to metacarpal radiogrammetry in ar-chaeology. Int. J. Osteoarchaeol. 14, 7–17.https://doi.org/10.1002/oa.709. Johnell, O., Kanis, J.A., 2006. An estimate of the worldwide prevalence and disability
associated with osteoporotic fractures. Osteoporos. Int. 17, 1726–1733.https://doi. org/10.1007/s00198-006-0172-4.
Kottner, J., Audige, L., Brorson, S., Donner, A., Gajewski, B.J., Hroóbjartsson, A., Roberts, C., Shoukri, M., Streiner, D.L., 2011. Guidelines for reporting reliability and agree-ment studies (GRRAS) were proposed. Int. J. Nurs. Stud. 48, 661–671.https://doi. org/10.1016/j.ijnurstu.2011.01.016.
Krieg, M.A., Barkmann, R., Gonnelli, S., Stewart, A., Bauer, D.C., Del Rio Barquero, L., Kaufman, J.J., Lorenc, R., Miller, P.D., Olszynski, W.P., Poiana, C., Schott, A.M., Lewiecki, E.M., Hans, D., 2008 Jan-Mar. Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD official positions. J. Clin. Densitom. 11 (1), 163–187.
https://doi.org/10.1016/j.jocd.2007.12.011.
Larsen, C.S., 2003. Animal source foods and human health during evolution. J. Nutr. 133, 3893S–3897S.https://doi.org/0022-3166/03.
Lorenzini, L., 2001. L’oratorio della Crocetta e la confraternita di Santa Croce. In: Corti, T., Lorenzini, L. (Eds.), Terra Di Cento. Gherli Editore, pp. 62–72.
Lovejoy, C.O., 1985. Dental wear in the Libben population: its functional pattern and role in the determination of adult skeletal age at death. Am. J. Phys. Anthropol. 68, 47–56.https://doi.org/10.1002/ajpa.1330680105.
Maalouf, G., Wehbe, J., Farah, G., Sawan, D., Tannous, Z., Nehme, A., Chidiac, R.M., Gannage Yared, M.H., Jalkh, S., 2007. Phalangeal osteosonogrammetry age-related changes and assessment of a Lebanese reference population. Bone 40, 1650–1654.
https://doi.org/10.1016/j.bone.2006.02.066.
Madimenos, F.C., 2015. An evolutionary and life-history perspective on osteoporosis. Annu. Rev. Anthropol. 44, 189–206. https://doi.org/10.1146/annurev-anthro-102214-013954.
Manifold, B.M., 2014. Bone mineral density in children from anthropological and clinical sciences: a review. Anthropol. Rev. 77, 111–135. https://doi.org/10.2478/anre-2014-0011.
Mariotti, V., Zuppello, M., Pedrosi, M.E., Bettuzzi, M., Brancaccio, R., Peccenini, E., Morigi, M.P., Belcastro, M.G., 2015. Skeletal evidence of tuberculosis in a modern identified human skeletal collection (Certosa cemetery, Bologna, Italy). Am. J. Phys. Anthropol. 157, 389–401.https://doi.org/10.1002/ajpa.22727.
Mays, S.A., 2006. A palaeopathological study of Colles' fracture. Int. J. Osteoarchaeol. 16, 415–428.https://doi.org/10.1002/oa.845.
Mays, S.A., 2008. Metabolic bone disease. In: Pinhasi, R., Mays, S.A. (Eds.), Advances in Human Palaeopathology. John Wiley & Sons, Chichester, pp. 215–252.
Mays, S.A., 2016. Bone-formers and bone-losers in an archaeological population. Am. J. Phys. Anthropol. 159, 577–584.https://doi.org/10.1002/ajpa.22912.
Montagnani, A., Gonnelli, S., Cepollaro, C., Mangeri, M., Monaco, R., Bruni, D., Gennari, C., 2000. Quantitative ultrasound at the phalanges in healthy Italian men. Osteoporos. Int. 11, 499–504.https://doi.org/10.1007/s001980070092. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy,
2001. Osteoporosis prevention, diagnosis, and therapy. J. Am. Med. Assoc. 285, 785–795.https://doi.org/10.1001/jama.285.6.785.
Onisto, N., Gualdi-Russo, E., 2011. Osservazioni antropologiche sui resti scheletrici medievali: analisi osteologica della sepoltura presso la Crocetta. In: Lambertini, R., Tassinari, V. (Eds.), L'Oratorio Della Crocetta Tra Storia e Restauri. Siaca Editore, Cento (Fe), pp. 34–40.
Onisto, N., Marzi, M., Forlani, L., Gualdi-Russo, E., 2006. Osservazioni tafonomiche su una sepoltura medievale: la donna con il mazzo di fiori. In: Atti Del XVI Congresso Degli Antropologi Italiani (Genova, 29-31 Ottobre 2005). Edicolors Publishing, Milano, pp. 731–740.
Pasini, A., Manzon, V.S., Gonzalez-Muro, X., Gualdi-Russo, E., 2018. Neurosurgery on a pregnant woman with post mortem fetal extrusion: an unusual case from medieval Italy. World Neurosurg 113, 78–81.https://doi.org/10.1016/j.wneu.2018.02.044. Phenice, T.W., 1969. A newly developed visual method of sexing the os pubis. Am. J.
Phys. Anthropol. 30, 297–301.https://doi.org/10.1002/ajpa.1330300214.
Prestwood, K., Raisz, L., 2000. Consequences of alterations in bone remodeling. In: Henderson, J., Goltzman, D. (Eds.), The Osteoporosis Primer. Cambridge University Press, Cambridge, pp. 199–210.
Recker, R., Lappe, J., Davies, K.M., Heaney, R., 2004. Bone remodeling increases sub-stantially in the years after menopause and remains increased in older osteoporosis patients. J. Bone Miner. Res. 19, 1628–1633.https://doi.org/10.1359/JBMR. 040710.
Riggs, B., Melton 3rd, L.J., 1986. Involutional Osteoporosis. N. Engl. J. Med. 314, 1676–1684.
Ruff, C., Holt, B., Trinkaus, E., 2006. Who's afraid of the big bad Wolff?: “Wolff's law” and
bone functional adaptation. Am. J. Phys. Anthropol. 484–498. https://doi.org/10. 1002/ajpa.20371.
Ruff, C.B., Holt, B., Niskanen, M., Sladek, V., Berner, M., Garofalo, E., Garvin, H.M., Hora, M., Junno, J.-A., Schuplerova, E., Vilkama, R., Whittey, E., 2015. Gradual decline in mobility with the adoption of food production in Europe. Proc. Natl. Acad. Sci. Unit. States Am. 158, 312–324.https://doi.org/10.1073/pnas.1502932112.
Ryan, T.M., Shaw, C.N., 2015. Gracility of the modern Homo sapiens skeleton is the result of decreased biomechanical loading. Proc. Natl. Acad. Sci. Unit. States Am. 112, 372–377.https://doi.org/10.1073/pnas.1418646112.
Sakata, S., Barkmann, R., Lochmüller, E.M., Heller, M., Glüer, C.C., 2004. Assessing bone status beyond BMD: evaluation of bone geometry and porosity by quantitative ul-trasound of human finger phalanges. J. Bone Miner. Res. 19, 924–930.https://doi. org/10.1359/JBMR.040131.
Schotsmans, E.M., Marquez-Grant, N., Forbes, S. (Eds.), 2017. Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment. Wiley, Chichester.
Schultz, M., 2003. Differential diagnoses of intravitam and postmortem bone loss at the micro-level. In: SC, A., SD, S. (Eds.), Bone Loss and Osteoporosis: an Anthropological Perspective. Kluwer Academic Publishers, New York, pp. 172–187.
Stieglitz, J., Trumble, B.C., Kaplan, H., Gurven, M., 2017. Horticultural activity predicts later localized limb status in a contemporary pre-industrial population. Am. J. Phys. Anthropol. 163, 425–436.https://doi.org/10.1002/ajpa.23214.
Sutlovic, D., Boric, I., Sliskovic, L., Popovic, M., Knezovic, Z., Nikolic, I., Vucinovic, A., Vucinovic, Z., 2016. Bone mineral density of skeletal remains: discordant results between chemical analysis and DXA method. Leg. Med. 20, 18–22.https://doi.org/ 10.1016/j.legalmed.2016.03.008.
Todd, T.W., 1920. Age changes in the pubic bone. I. The male white pubis. Am. J. Phys. Anthropol. 3, 285–334.https://doi.org/10.1002/ajpa.1330030301.
Todd, T.W., 1921. Ages changes in the pubic bone: III the pubis of the white female. IV the pubis of the female white negro hybrid. Am. J. Phys. Anthropol. 4, 1–70. van Gerven, D.P., Armelagos, G.J., Bartley, M.H., 1969. Roentgenographic and direct
measurement of femoral cortical involution in a prehistoric Mississippian population. Am. J. Phys. Anthropol. 31, 23–28.https://doi.org/10.1002/ajpa.1330310105. Ventura, V., Mauloni, M., Mura, M., Paltrinieri, F., De Aloysio, D., 1996. Ultrasound
velocity changes at the proximal phalanxes of the hand in pre-, peri- and post-menopausal women. Osteoporos. Int. 6, 368–375.https://doi.org/10.1007/ BF01623010.
Wuster, C., Heilmann, P., Pereira-Lima, J., Schlegel, J., Anstatt, K., Soballa, T., 1998. Quantitative ultrasonometry (QUS) for the evaluation of osteoporosis risk: reference data for various measurement sites, limitations and application possibilities. Exp. Clin. Endocrinol. Diabetes 106, 277–288.https://doi.org/10.1055/s-0029-1211986. Wüster, C., Albanese, C., De Aloysio, D., Duboeuf, F., Gambacciani, M., Gonnelli, S.,
Glüer, C.C., Hans, D., Joly, J., Reginster, J.Y., De Terlizzi, F., Cadossi, R., Barkmann, R., Baroncelli, G.P.I., Boivin, C.M., Boschitsch, E., Federico, G., Franceschi, C., Gennari, C., Guglielmi, G., Mauloni, M., Pini, G., Nijs, J., Njeh, C.F., Ortolani, S., Pluskiewics, W., Sabatier, J.P., Sili Scavalli, A., Theiler, R., 2000. Phalangeal osteo-sonogrammetry study: age-related changes, diagnostic sensitivity, and discrimination power. J. Bone Miner. Res. 15, 1603–1614.https://doi.org/10.1359/jbmr.2000.15.8. 1603.
Wüster, C., de Terlizzi, F., Becker, S., Cadossi, M., Cadossi, R., Müller, R., 2005. Usefulness of quantitative ultrasound in evaluating structural and mechanical prop-erties of bone: comparison of ultrasound, dual-energy X-ray absorptiometry, micro-computed tomography, and mechanical testing of human phalanges in vitro. Technol. Health Care 13, 497–510.
Zampetti, S., Mariotti, V., Radi, N., Belcastro, M.G., 2016. Variation of skeletal degen-erative joint disease features in an identified Italian modern skeletal collection. Am. J. Phys. Anthropol. 160, 683–693.https://doi.org/10.1002/ajpa.22998.
Zhu, Z.Q., Liu, W., Xu, C.L., Han, S.M., Zu, S.Y., Zhu, G.J., 2008. Reference data for quantitative ultrasound values of calcaneus in 2927 healthy Chinese men. J. Bone Miner. Metabol. 26, 165–171.https://doi.org/10.1007/s00774-007-0801-9.