Dottorato in Fisiopatologia ed Imaging Cardio-Toraco-Vascolare
Tesi di Dottorato
Utilizzo di un innovativo Gonio-Probe nella chirurgia radioguidata del torace
con traccianti PET per il tumore del polmone
Innovative Gonio-Probe in radioguided chest surgery with PET tracers for lung
cancer
RELATORE DOTTORANDO
Prof. Giuseppe De Vincentis Dr.ssa Viviana Frantellizzi
Abstract
Background. A leading cause of cancer deaths worldwide in both men and women is lung cancer.
PET scanning is a sensitive modality for the detection of NSCLC. The major limitation of surgery in lung cancer is resection of all margins. Patients with microscopic involvement of the resection margin with tumor (R1) following seemingly complete resection have a significantly poorer prognosis than those with negative microscopic margins (R0). The optimal resection of the margins seems therefore to be a debated and fundamentally important question, which is associated with the precise operative location of the tumor. This accuracy that must be achieved is reflected in the length of the intervention, which must be as short as possible even if a very accurate procedure must be guaranteed. Different medical devices can help reduce operating times by ensuring the accuracy of tumor detection and removal of all margins. Intratumoral accumulation of the PET agent [18F]FDG can be used as a guide to identifying lesion margins of NSCLC pulmonary nodules by means of probes for radio-guided surgery, capable of detecting the high energy γ rays. The purpose of this thesis is to realize and validate a new concept, innovative probe, characterized by an active collimation system for fast radio-guided surgery, to date never conceived, using PET tracers, for the precise identification and on-going removal of the lung tumor. The goal of subsequent clinical application of this thesis is to be able to reduce operating times and to ensure negative margins in lung tumor removal, thanks to a new experimental technology of revolutionary conception.
Materials and methods. A prototype of a probe for radio-guided surgery was developed. The
instrument is classified in an application field between a portable gamma camera and a gamma-probes, combining a high efficiency two-dimensional view of the region of interest and a high spatial resolution. The GonioProbe comprises two integrated systems: the first one, the "Navigator", has the function to reach the gamma-rays emitting tissues, the second one, the "Lock-on-Target" system, can quickly identify the exact position of the tumor. Pre-clinical tests of the GonioProbe prototype have been performed using phantoms that reproduce the surgical theater and the position of radioactive sources (chest and lung tumor). Subsequently, tests and measurements were performed on BALB/c mice with the GonioProbe prototype. Three nude BALB/c mice (4-6 weeks old healthy female mice) were used for the experiment. The human lung cancer cell lines in the form of the solid tumor were implanted subcutaneously over the upper side of the right thigh of immune-compromised BALB/c mice. When the tumor diameters reached 6~7 mm, 250 μCi of 18F-FDG was injected intraperitoneally to the animals. The mice were scanned with the microPET (microPET Focus 120, Siemens CTI). During tests, various characteristics had been evaluated: precision in localization in terms of spatial resolution, range of radioactivity useful for the correct
functioning of the instrument, instrument response time, effectiveness, time to identify the tumor lesion, accuracy in localization.
Results. Mice were scanned to verify that the radiotracer is bound to the tumor tissue. The
implementation of algorithms for goniometric tomography for reconstruction and visualization of images in real-time was done. The counts detected by the Gonioprobe are extremely high, this ability to effectively detect the counts is the characteristic that determines the possibility of having the directionality of the navigation system, with a short time to identify the tumor lesion. Even in most extreme conditions, the Gonioprobe, given its high sensitivity, is able to record the minimum variations in counting and to hook itself to the target after having directed the operator in the correct direction of radiation. From the statistical analysis of data collected it was determined that the expected statistical error of the Gonioprobe is less than 1 mm and therefore the Gonioprobe with respect to a commercial probe has proved to be more reliable.
Conclusions. In this thesis, we have shown that thanks to the high efficiency of the GonioProbe, the
amount of radioactivity to be injected in patients would be much lower than normal. Furthermore, the reduction in the time required for surgery would make it possible to further lower the dose to the operators. The problem of negative margins is fundamental, this issue could be easily solved thanks to the GonioProbe. In fact, due to the radical possibility of discriminating the lesion from the background, it will be possible to verify that there are no tissue residues in the operating bed. In conclusion, this technological advancement would allow a more effective and faster thoracic surgery in order to help the surgeon to definitively eradicate lung cancer.
Introduction
The leading cause of cancer deaths worldwide in both men and women is lung cancer [1]. Non-small cell lung cancer (NSCLC) accounts for the majority (approximately 85%) of lung cancers with the remainder as mostly small cell lung cancer (SCLC). Most patients present for diagnostic evaluation because of symptoms suspicious for lung cancer or an incidental finding on chest imaging. For each patient with suspected lung cancer, the overall goal is timely diagnosis and accurate staging so appropriate therapy can be administered. The major goals of the initial evaluation of a patient with suspected lung cancer are to assess the clinical extent and stage of disease, the optimal target site and modality for the first tissue biopsy, the specific histological subtype and the presence of comorbidities, secondary complications, and paraneoplastic syndromes that influence treatment options and outcome. The preferred approach uses imaging as a road map and invasive biopsy as a tool to confirm both the histopathological diagnosis and the stage of disease. When feasible, diagnosis and staging should be established concurrently by targeting for an invasive biopsy the abnormality that would yield the most advanced stage. However, some patients will require multiple imaging studies and/or invasive procedures for tissue sampling. Although imaging and sampling procedures are often described separately, in practice, the pathways to diagnosis and staging are often synchronous. As an example, thoracentesis with cytology examination of fluid or transbronchial needle aspiration biopsy of mediastinal lymphadenopathy may provide both diagnosis and staging data. No single diagnostic algorithm sufficiently addresses the complexity and variation in disease patterns of lung cancer. The local expertise and resources, as well as institution and health system factors, may influence the approach taken. Multi-disciplinary teams may help facilitate an investigative plan so that therapy can be implemented in a timely fashion. Despite conflicting data, there is a consensus that the initial evaluation of patients with suspected lung cancer would be performed in a timely and efficient manner [2]. Most patients can be investigated in an outpatient setting. However, patient factors including comorbidities (i.e. respiratory failure, hemoptysis, debilitating metastases to the brain or bone) may lead clinicians to conduct the work-up in a hospital setting. Expedient diagnosis is especially important when there is a concern for small cell carcinoma, such as in patients with large, central tumors or evidence of bulky mediastinal disease. In clinical practice asymptomatic patients may come to clinical attention during screening or following the incidental detection of imaging abnormalities. Patients with symptoms suggestive of primary or metastatic lung cancer should undergo initial imaging with a chest radiograph. Every attempt should be made to obtain and review any prior chest imaging
studies to determine the age and growth pattern of identified abnormalities. Solid-appearing lesions on chest computed tomography (CT) that are stable in size for at least two years are highly unlikely to represent lung carcinoma [3]. Findings suggestive of cancer or cancer-related complications on chest radiograph should be further evaluated with contrast-enhanced CT chest. The clinical staging of patients with suspected lung cancer, NSCLC, starts with radiographic imaging [4]. Determining the highest radiographic stage prior to biopsy facilitates the selection of a modality that optimizes tissue sampling for diagnosis. Every patient with suspected NSCLC should undergo imaging of the chest with contrast-enhanced computed tomography (CT) scan, imaging of the upper abdomen including liver and adrenal glands, usually by extension of the chest CT through this region and imaging directed at sites of potential metastasis when symptoms or focal findings are present or when chest CT shows evidence of advanced disease. There are conflicting data regarding the harms and benefits (improved survival or reduction in futile thoracotomy) of routinely performing whole-body positron emission tomography (PET) or integrated PET/CT in every patient with suspected NSCLC [5-6]. Until large randomized trials provide more convincing data demonstrating improved survival or a clear reduction in thoracotomies with routine PET or PET/CT, is preferred an approach that is symptom and/or CT-directed. (Figure 1).
CT and PET provide a non-invasive assessment of tumor size (T), mediastinal node enlargement (N), and potential intra- or extra-thoracic metastases (M) [7-8]. Although confirmation by tissue biopsy must be pursued, these imaging tests provide the basis for the initial assessment of the TNM stage of disease and help guide the clinician in choosing the optimal site(s) for tissue sampling. There is no perfect threshold for what is considered metastatic lymphadenopathy by CT or PET. Small lymph nodes can harbor occult malignancy and some lesions that are not highly fluorodeoxyglucose (FDG)-avid are malignant. A tumor's metabolic activity can be measured using the standardized uptake value (SUV) to assess the tumor uptake of fluorodeoxyglucose (18F-FDG). However, cut-offs worrisome for metastasis to mediastinal lymph nodes are size >1 cm by short-axis diameter on transverse CT scan and/or FDG uptake greater than that of mediastinal blood pool on PET imaging [9]. The major limitation of CT and PET is that neither can stage NSCLC with a high degree of accuracy. The prevalence of granulomatous diseases such as tuberculosis or histoplasmosis in the local patient population is an important factor in the interpretation of PET results [10]. Consequently, except for bulky and confluent mediastinal disease, the identification of suspicious mediastinal metastases by CT or PET does not bypass the need for histologic assessment of mediastinal lymph nodes for accurate staging and exclusion of alternative diagnoses [11]. The relatively low sensitivity and specificity of CT (55 and 81 percent) and PET (80 and 88 percent) can miss occult cancer (false negatives) and result in missed opportunities for potentially curative thoracotomy (false positives). Thus, the major use of CT and PET as staging tools is to facilitate the optimal approach to biopsy so that patients can be accurately staged histologically, and futile surgery avoided[12-13]. Routine extrathoracic imaging aimed at detecting metastases is not necessary for all patients and should be directed at symptoms and abnormal physical examination findings. Because of the higher prevalence of occult brain metastasis in patients with stage III or IV NSCLC, some experts believe that routine brain imaging is warranted, although there is little evidence that early detection and treatment of occult metastasis improves outcomes. Brain CT scan should be used when MRI is not available. The poor sensitivity of PET for brain metastases limits this modality in the detection of brain NSCLC. PET scanning has limited anatomic resolution but does provide information on the metabolic activity of the primary tumor, mediastinal involvement, and potential distant metastases. There are no standardized criteria defining what constitutes a positive PET result and no ideal cut-off point for the standardized uptake value (SUV). However, lymph nodes with FDG uptake greater than that observed in the mediastinal blood pool are highly suspicious for metastatic disease [9]. PET is more accurate in the evaluation of mediastinal disease (N) when compared to contrast-enhanced chest CT and sometimes detects occult disease (i.e. liver, adrenal, bone, and pleural metastases) outside the thoracic cavity (M) that is not radiologically
evident by CT scanning. With respect to mediastinal staging by PET, one systematic review of 45 studies which included 4105 patients reported sensitivity, specificity, positive predictive value and negative predictive values of 80, 88, 75, and 91 percent, respectively [4]. Another meta-analysis of 39 studies reported increased sensitivity of PET (100 percent) when lymph nodes were also enlarged (>1 cm) on CT [14]. The rationale for the use of PET in operable patients with potentially resectable cancers is derived from randomized trials that included patients with radiographic (CT) stage I, II, and IIIA that suggest a reduction in futile thoracotomy when PET is performed[15]. While we favor surgical resection with mediastinal sampling, it is also reasonable to perform PET scanning pre-operatively in those at high surgical risk. Importantly, patients and clinicians must be aware that limiting preoperative staging to imaging alone without a biopsy of radiographically positive lymph nodes sampling carries a risk of a false-positive result that may result in a missed opportunity for surgical cure. When staging the mediastinum, errors associated with PET that should be considered are false positives, that can occur with benign FDG-avid lesions such as infections, inflammation, and granulomatous disease [16] and false negatives, that typically occur when there are microscopic foci of metastasis, and in non-enlarged lymph nodes (<10 mm) [17-18]. As an example, although PET can be positive in small lesions, the detection of occult malignancy in normal-sized lymph nodes for early-stage cancer is lower than for enlarged nodes. PET or PET/CT are indicated to identify occult metastasis in patients with clinical stage IB/II disease. Both modalities have been shown in small prospective observational studies to result in the avoidance of unnecessary thoracotomy in patients with solid tumors [19-20]. Except for brain metastases, whole-body PET or PET/CT scanning are more accurate than conventional scanning (abdominal CT, bone scan) for the detection of unsuspected pleural and extra-thoracic metastases. PET scanning is a sensitive modality for the detection of adrenal metastases in patients with NSCLC[21]. A diagnosis of lung cancer should not be made without definitive pathology. At a minimum, this involves selecting a biopsy site and obtaining an adequate sample for microscopic examination. Additional consideration needs to be given to obtaining a large enough sample for supplemental immunohistochemical and genetic analysis. Acquiring tissue for microscopic examination is necessary for the diagnosis and staging of patients with suspected lung cancer. Most data are derived from studies of patients with non-small cell lung cancer (NSCLC). Although not absolute, for patients with higher disease stage, minimally invasive modalities (i.e. endoscopic procedures) are typically preferred over more invasive modalities (eg, video-assisted thoracic surgery and mediastinoscopy) for the initial biopsy[22-23]. Conversely, for patients with peripheral early-stage disease, a surgical biopsy is sometimes preferred because diagnosis and curative resection may be achieved simultaneously. Patients who undergo a complete resection for NSCLC may develop
recurrent and/or metastatic disease. Multiple factors influence survival following disease recurrence. Surgery is the standard treatment for medically operable patients with clinical stage I and II NSCLC, in whom there is no evidence of mediastinal involvement prior to surgical resection[24-25]. Although the role of surgery has not been validated through randomized trials, the favorable results reported in surgical series and the long-term survival data in these patients have established surgery as the treatment of choice [26]. Lobectomy, the surgical resection of a single lobe, is generally accepted as the optimal procedure for early-stage NSCLC[27]. In patients with early-stage NSCLC, video-assisted thoracoscopic surgery (VATS) or robotic-assisted thoracoscopic surgery (RATS) are alternatives to open thoracotomy for patients undergoing lobectomy [28]. A sublobar resection consists of the removal of one or more anatomic segments (segmentectomy) or, more commonly, of a nonanatomic wedge resection. Limited (sublobar) resection may be an option for patients who cannot tolerate a full lobectomy because of severely compromised pulmonary function, advanced age, or another extensive comorbidity. This approach should probably be limited to primary tumors ≤3 cm. Advances in VATS facilitated the utilization of limited resections in selected high-risk patients[29]. Data regarding the relative efficacy of limited resection compared with lobectomy are limited, with studies generally demonstrating an association between limited resection and worse local control and survival rates compared with lobectomy [30]. However, there may be certain subsets of patients for whom limited resection yields similar results as lobectomy, for example, patients with small, peripheral tumors or older adult patients, particularly those with adenocarcinoma histology. The major limitation of surgery in lung cancer is resection of all margins. Patients with microscopic involvement of the resection margin with tumor (R1) following seemingly complete resection have a significantly poorer prognosis than those with negative microscopic margins (R0) [31-32]. The incidence of local recurrence following surgery for stage I or stage II NSCLC varies from 6 to 55 percent in different studies. Local failures were defined as recurrence at the surgical margin, in the ipsilateral hilum, or in the mediastinum. The five-year incidence of local recurrence was 23 percent, with a median time to recurrence of 14 months. The five-year risk of any treatment failure was 42 percent, including local and/or distant relapses, as well as second primary lung cancers. The initial sites of treatment failure were local, combined local and distant, and distant only in 25, 29, and 46 percent of cases, respectively. The optimal resection of the margins seems therefore to be a debated and fundamentally important question, which is associated with the precise operative location of the tumor. This accuracy that must be achieved is reflected in the length of the intervention, which must be as short as possible even if a very accurate procedure must be guaranteed. Different medical devices can help reduce operating times by ensuring the accuracy of tumor detection and removal of all margins. The concept of
radio-guided surgery using radiation detection probe systems was first introduced in 1949 by Selverstone et al. [33]. Intraoperative radiation detectors are subdivided into two general categories: gamma probes and beta probes [34]. Gamma probes detect photons, while beta probes detect electrically charged radiation consisting of either positrons or electrons. The latter category includes beta probe systems with γ photon background rejection capabilities [35]. The nature of the radiation and its different kinds of interaction in tissues define the requirements for intraoperative probes. Although γ rays undergo exponential attenuation in tissue, they can penetrate large distances before being significantly absorbed. Because collimation has a relevant impact on the outcome of radioguidance, the thickness and design of the collimation and shield depend on the energy of the radionuclide used [36]. Likewise, γ rays are monoenergetic, and so can be discriminated according to their energy using dedicated processing electronics (thresholding or energy windowing techniques). Beta particles (positrons and electrons) undergo many inelastic collisions before reaching a complete stop in tissue (a few mm, depending on the beta energy). They are easy to collimate and much less sensitive to background uptake from surrounding tissues, making the probe more specific than gamma probes. However, the continuous energy distribution spectrum does not allow energy discrimination and the short range of the β particle limits the range of the probe to a few mm under the skin or tissue surface, thus making it difficult to locate deeper tumors. The most significant parameters defining the performances, especially for gamma probes, consist of overall sensitivity (efficiency), spatial resolution (radial and lateral), energy resolution and signal to noise ratio [37]. The sensitivity is the detected count rate per unit of activity and is determined at the tip of the probe. Radial resolution is the width of the measurement cone where the radiation is detected at a defined distance. With a wider cone, the background signal may overcome the target source. With a narrower cone, the background will be reduced, and the detection of the target source will be more accurate. Lateral spatial resolution is the capability to accurately localize the position of a target source and to separate two adjacent sources. The energy resolution is the capacity of the gamma detection system to discriminate between radiations of different energies. This property is essential to distinguish between two simultaneously administered radionuclides that have different energies and to discriminate scattered photons from primary photons. The signal to noise ratio relates to the ability of the probe to discriminate the signal arising from the target with respect to the noise represented by the background radiation within the surrounding tissue. Gamma radiation detectors are generally based on the use of scintillation crystals. The scintillator absorbs the radiation and emits several visible photons proportional to the energy absorbed. The visible light is measured using a photon detector, usually a PMT. The crystals used in scintillator detector probes include NaI(Tl), CsI(Tl), cerium activated LSO, BGO and cerium doped GSO. The high penetration power
of γ rays means that background events could come from parts of the patient far away from the target volume. Although a relevant fraction of these events is attenuated within the patient’s body, in order to further reduce the background, the gamma probes are equipped with a shield (material such as lead, tungsten, gold or platinum) and collimators (designed with different lengths and apertures for different FOVs) to prevent attenuated radiation from non-target locations (i.e. scattered radiation) from accessing the detector head and thus producing spurious counts. Several factors determine the choice of an intraoperative probe. From the point of view of the surgeon, there are many desirable design features of the detection probe systems that are important [38]. In particular, the specific surgical application is an important decision aspect for the choice of the appropriate intraoperative probe. Gamma probes for radio-guided surgery require high spatial resolution to allow for more precise localization of small lesions [39]. On the other hand, gamma probes for radio-guided surgical resection of tumors require a high sensitivity to guide the surgeon to the specific site of the target over a relatively large surgical field. Other features, such as the shape, weight and ergonomic design of an intraoperative probe, are also critical. The audible signal and digital display of the detector control unit are important additional variables for providing critical output information to the surgeon, such as the quick and accurate localization of the radionuclide, without distracting his/her attention from the surgical field. Flexibility and adaptability of the system are also functional to different clinical issues, such as removable side shielding, interchangeable collimators, interchangeable detector probes and adjustable energy windows for different radionuclides. Recent developments of hand-held self-contained gamma detection probes based on wireless technology (Bluetooth) eliminate the need for cables connecting the probe to the control unit [40], which is a possible confounding factor within the surgical field. The use of gamma probe for lung cancer is still to be validated, even if it seems that in the future it may be an excellent resource for the surgeon. Video-assisted thoracic surgery is an interesting and emerging procedure for the diagnosis and treatment of peripheral pulmonary nodules. However, when the nodule is either too small or too deep beneath the pleural surface, failure in localization and, therefore, conversion to open surgery may be necessary. When relying only on thoracoscopic exploration and endoscopic palpation, this event is more likely if the distance between the nodule and the nearest pleural surface is more than 5 mm and/or if the nodule is less than 10 mm in size [41]. To overcome such limitations, several techniques have been developed for pre-operative localization of deeper nodules. Percutaneous hookwire placement and methylene blue injection under CT guidance has been widely used, either alone or together. However, these techniques do have some failures (because of displacement of the wire or intraparenchymal diffusion of the dye) and/or complications (pneumothorax and/or hemothorax). Moreover, when employing such
localization techniques, the time elapsed between pre-operative positioning of the hookwire (or blue dye injection) and the surgery itself must be kept to a minimum, while intralesional injection of 99mTc MAAs for radio-guided surgery can be performed even 24–36 h before surgery. The radioguided approach described, for example in solitary pulmonary nodules, can yield the exact location of the nodule and its depth from the pleural surface, to ensure safe surgical margins of the excision and avoid thoracotomy for palpation to search for the lesion. On the other hand, the possibility remains to perform radioguided surgery after systemic administration of a tumor seeking radiopharmaceutical, with optimal tumor localizing properties yielding sufficiently high TBRs. [18F]FDG constitutes one such potential radiopharmaceutical, as it has already been explored for other regions of the body [42-45]. Intratumoral accumulation of the PET agent [18F]FDG can be used as a guide to identify lesion margins of solitary pulmonary nodules by means of probes capable of detecting the high energy γ rays. The purpose of this thesis is to realize and validate a new concept, innovative probe, characterized by an active collimation system for fast radio-guided surgery, to date never conceived, using PET tracers, for the precise identification and on-going removal of the lung tumor. The goal of subsequent clinical application of this thesis is to be able to reduce operating times and to ensure negative margins in lung tumor removal, thanks to a new experimental technology of revolutionary conception.
Materials and methods
Gamma probe prototype
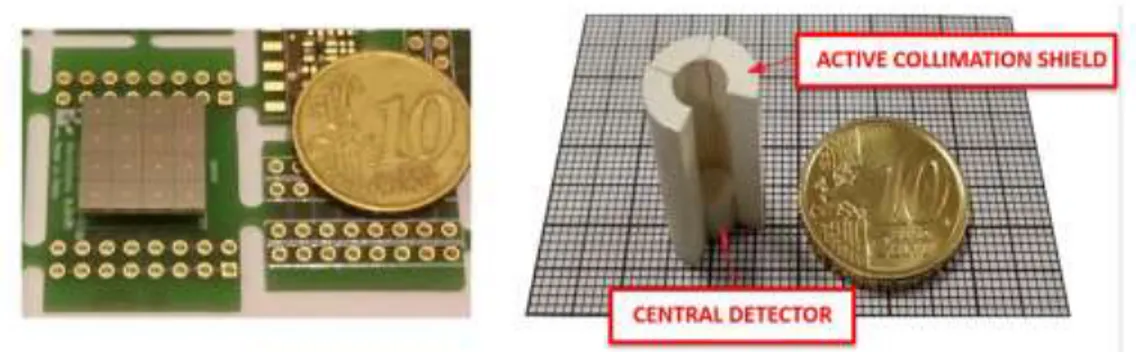
The prototype of probe for radio-guided surgery developed for this thesis is the GonioProbe. The instrument is classified in an application field between a portable gamma camera and a gamma-probes, combining a high efficiency two-dimensional view of the region of interest and a high spatial resolution. The GonioProbe comprises two integrated systems: the first one, the "Navigator", has the function to reach the gamma-rays emitting tissues, the second one, the "Lock-on-Target" system, can quickly identify the exact position of the tumor. The probe consists of a circular disc divided into four independent scintillation crystals, which replaces the passive collimation of traditional gamma-probes, and a smaller crystal in the center. The scintillation ring provides the Navigation Functions, guiding the surgeon along the direction of the tumor location, by an automated searching procedure. The exact identification of the tumor is then confirmed by the combined information of the central crystal and the outer detection system (Lock-on-target). The outer detection ring increases the field of view of the system up to 20x20 cm2 FOV radially and 180° in azimuth direction and the sensitivity of the device (reducing the searching time). Thanks to crystals combination it can measure the depth of emitting tissue, nowadays obtained only from PET imaging. The size of the detection head is a cylinder 20mm height and 12 mm diameter, the overall size is a bit greater than a filter pen (Figure 2).
The maximum detection sensitivity is close to 10%, the highest measured for radio-isotopic molecular imaging (1000 times more than Anger camera) it can allow lower radiotracer activity administration. Moreover, the best spatial resolution is about 6 mm. Finally, using the gamma-rays energy information it can discriminate at the same time multiple sources generated by multiple radio-labeled molecules. A further innovation was the creation of an image system based on newly concept of a Goniometric Tomography approach, starting from the existing miniaturized prototype. The basic concept consists of a novel assembly of detection crystals resulting from a combining of radiation absorption techniques with DOI (Depth of Interaction) encoded techniques currently used in PET and based on light single-ended readout of scintillation arrays (encoded imaging). The detector consists of about twenty distinct parts, where each one has a defined angle of view and its counting is closely related to the solid angle subtended at the position taken by the probe (position chosen by the surgeon) with respect to the radiation source. The detection system is a hollow cylinder divided into three layers. A SiPM-based photodetection system can read each individual gamma interaction with the LYSO scintillation crystals. (Figure 3).
Fig. 3 Current GonioProbe detection head: SIPM photodetector and Detector assembly
The whole apparatus works with the same principles of a small Anger camera providing encoded images where a single image spot corresponds to a counting relative to a position/direction of the incident radiation. The height of the detection cylinder is roughly divided into three layers corresponding to the detection of three whole solid angle portions, front, side and back. In the
sources) in spherical polar coordinates. The identification of sources, side or back located, allows to localize the inoculum source and to evaluate and subtract in real-time the background counting of the detection system. This allows us to eliminate the shielding window currently present in commercial probes and to automatically calibrate the detection system in real-time. In addition, it is possible to estimate the source to cylinder distance by using the source self-collimation method, which offers an increasingly precise response to reduced distances (number of crystals involved in irradiation). The expected estimate of the source to cylinder distance is about 2-cylinder diameters, which corresponds to a FOV of the imaging system of about 5 cm in diameter. The front detection area allows precise identification of the source position in three zones: outside and in the inner cylinder ring (detection crown) and in the central area. From preliminary simulations and measurements, it is possible to state: the crown of the crystals is arranged in such a way that it accurately distinguishes external sources from those inside the detection face. The spatial resolution expected is about 6 mm. Thanks to the different solid angles subtended by the crystals from the same source, it is possible to identify the source depth (distance from detector face) up to 2 cm with a position error within 2 mm along the vertical axis of the cylinder. The most important feature of this device is the very high detection efficiency (100 cps / kBq max) that allows any update on the position of the source in less than one second for the activities typically required in these techniques.
Procedures and tests on Gamma probe prototype
The following procedures have been performed: implementation of new algorithms for the goniometric characteristics of the probe for the entire solid angle; implementation of algorithms for goniometric tomography for reconstruction and visualization of images in real-time. Pre-clinical tests of the GonioProbe prototype have been performed using phantoms that reproduce the surgical theater and the position of radioactive sources (chest and lung tumor).
Animals
Three nude BALB/c mice, (4-6 weeks old healthy female mice) weighing 16–20 g, were used for the experiment. All the animals were maintained as per the principles and guidelines of the local ethical committee. The animals were housed in a specific‐pathogen‐free environment in opaque polypropylene cages in a standard 12 hr/12 hr light/dark cycle at 22 ± 2°C and 55–60% humidity. Food and water were available ad libitum. The mice could adapt to the above‐mentioned conditions for 7 days before experimentation. The human Lung cancer cell lines in the form of the solid tumor
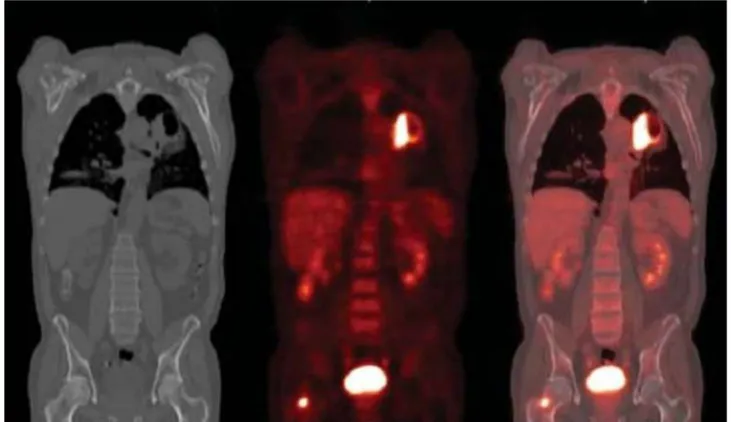
were implanted subcutaneously over the upper side of the right thigh of immune-compromised BALB/c mice. When the tumor diameters reached 6~7 mm, the mice were used for the experiments and 250 μCi of 18F-FDG were injected intraperitoneally to the animals. Mice were maintained conscious in their cages during 18F-FDG uptake. After the complete uptake of the tracer, animals were immediately anesthetized with inhaled isoflurane mixed with oxygen (3-4% for anesthetic induction and 2- 3% for anesthesia maintenance). The mice were placed in a headfirst prone position and scanned with the microPET (microPET Focus 120, Siemens CTI) to verify that the radiotracer is bound to the tumor tissue.
Tests on BALB/c mice with GonioProbe prototype
Through tests and measurements on BALB/c mice with GonioProbe prototype, the various characteristics had been evaluated: precision in localization in terms of spatial resolution, range of radioactivity useful for the correct functioning of the instrument, instrument response time, effectiveness, time to identify the tumor lesion, accuracy in localization. Considering the type of technical experiments carried out for this thesis, the number of animals was considered adequate for a proof of concept model.
Results
Tests on Gamma probe prototype
The implementation of new algorithms for the goniometric characteristics of the probe for the entire solid angle and the analysis of the angular response of the detection system had been done. To understand if the prototype is suitable for the purposes for which it was designed, that is to be able to identify exactly one radioactive source located in a certain position, it was decided to study the response through two different irradiation geometries: one at 360 ° on the plane perpendicular to the probe axis, to irradiate the entire lateral surface, and the other at 180 ° on the plane parallel to the probe axis, to cover the entire front visual field. In both configurations a 99mTc source collimated to 7 mm was used and placed at 10 cm from the detector, to define on the probe a parallel beam of about 7 mm in diameter on an irradiation field of about 20 mm, with an angular opening of about 12° degrees. This condition is very important for the definition of the error committed to determining the direction of the source. In the first phase of measurements, the experimental setup was constructed to leave the probe and the acquisition system fixed in space in a vertical position and the source located on the side, placed on a rotating base such as to allow it to sweep an angle 360 ° all around the detector. It was thus possible to study the response of the system under different angles, keeping the source-detector distance fixed. The acquisitions were made at constant steps of 15 ° around the detector and for variable duration, starting from an initial fixed acquisition time (150 s) for later adapt it to each subsequent acquisition following the physical decay of the source radioactive (which is halved after just 6 hours), in order to have the same statistics of counts at each measure. The data was then processed and for each acquisition the energy spectra were analyzed, and the images constructed using the algorithm of the center of gravity. The responses produced by the individual crystals were discriminated through the selection of appropriate ROI around the 5 spots present in the images; each of these shows a peak produced by 140 keV photoelectric absorption of gamma, which varies in intensity depending on irradiation conditions of the different sectors of the probe. The percentage of counts recorded in each crystal, shown in figure 4 when the source rotates with respect to probe, was estimated by selecting only those events that released theirs energy due to photoelectric effect inside the crystals, therefore through appropriate energy windows around each individual photopeak (between channels 50 ÷ 250 for the crystals of LYSO); although not visible in the spectra, a small percentage (1%) of the gamma pass successfully the active collimator and reach the crystal located in the center, therefore a sign that the collimation system is working properly.
Fig. 4 Percentage of counts recorded by individual LYSO crystals compared to
total recorded by the probe when the position of the source varies around the detection system.
The implementation of algorithms for goniometric tomography for reconstruction and visualization of images in real-time was done. The radionuclide used and the quality of the radiation are important aspects to consider in the design of a detector. The prototype analyzed was initially designed to be used with radioisotopes characterized by low energy emissions, like the 99mTc, but the measurements made with the 137Cs showed that the probe can be able to identify also the annihilation radiation produced by the PET tracers. (See Figure 5 and 6)
Fig. 5 Front irradiation of the probe with 57Co source (Eγ = 122 keV)
Pre-clinical tests of the GonioProbe prototype have been performed using phantoms that reproduce the surgical theater and the position of radioactive sources (chest and lung tumor). The model was composed of several sources of radioactivity representative of the uptake of generic tumors (target). For the simulation of the lesions were made paper bibs sealed with parafilm of various sizes. The 8 sources built had a diameter between 0.5 and 2 cm and were placed at a variable distance (6-12 cm, due to the finite size of the phantom) inside the anthropomorphic phantom. The sources were placed at different depths to best simulate the real location of the tumors. The radioactivity of the target generally depends on the physiological and morphological characteristics of the patient, and results to be a small percentage with respect to the radioactivity of the whole body, normally variable between 0.1 and 5%, for this reason, it was chosen to use sources with amount of radioactive similar to tumor lesions that occur in the patient in vivo. For a precise localization, in terms of spatial resolution, the anthropomorphic phantom containing the previously prepared sources was placed on a plane. Subsequently, measurements were performed using the GonioProbe and the positions of the sources found, and the distances measured were marked on the surface of the phantom. (Figure 7).
Tests on BALB/c mice with GonioProbe prototype
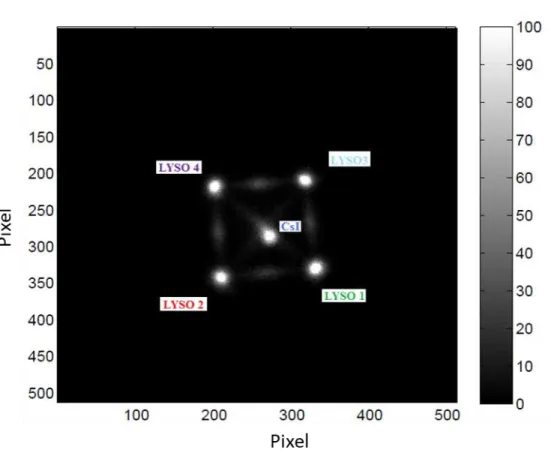
The measurements made on mice were of various types: navigation and coupling of the target in the presence of a shield (1 cm of lead) placed to verify and assess the influence of the background of radioactivity generated by the different tissues/organs and navigation and coupling of the target in the absence of shielding. In order to verify the range of radioactivity useful for the correct functioning of the instrument, the data were stored, following each session, as the counts recorded on each crystal and sector of the Gonioprobe. Let us briefly recall that the navigation and the identification of the target in the GonioProbe occur through five crystals: four sectors of cylindrical crown that reconstruct the direction of origin of the radioactivity and a central crystal, shielded by the first four, which records the radioactivity only when the GonioProbe is positioned above the target (See Figure 8).
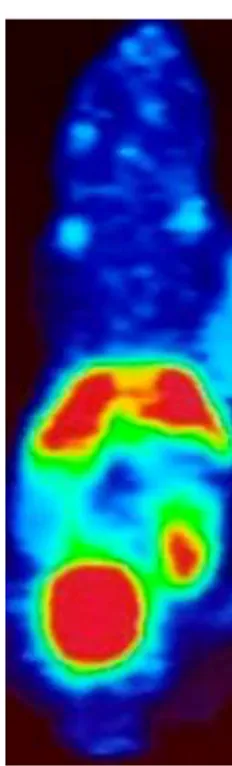
Fig. 8 BALB/c mice PET target lesion.
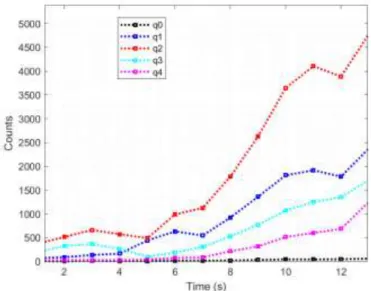
Five sectors of a further back crystal, on the other hand, are used for recording the radioactivity of the background; these last counts are used to correct the navigation data. Figure 9 shows the counts recorded on the five Gonioprobe crystals (panel A) and on the five corresponding sectors of the back crystal (panel B). In the diagrams, you can see the variation in counts as a function of time expressed in seconds. The choice of the detection of radioactivity in terms of 1 second was dictated by the needs of the healthcare professional using the Gonioprobe, a nuclear doctor or surgeon, who
established that a shorter acquisition time would have been impossible to perceive with the human eye, determining the congruence with the clinical practice of the default time of 1 second. At the instant t = 35s there was a sudden increase in the counts, especially those in the back sector, and this occurs at the elimination of the shielding in progress of the measurement. The trend in black in figure 9 corresponds to the central crystal (q0, panel A) and to the corresponding central back sector (q8, panel B). For t = 35 s the lead shielding is eliminated, and the crystal counts increase suddenly. In correspondence with this increase, the counts in the crystals increase unbalancing the navigation towards a major source, but the corresponding counts in the back sectors increase correspondingly, giving a precise indication of the direction of background radiation, thus allowing a correction and guaranteeing correct navigation and accuracy in localization. It should be noted that the counts detected by the Gonioprobe and shown in the graph are extremely high, this ability to effectively detect the counts is the characteristic that determines the possibility of having the directionality of the navigation system, with a short time to identify the tumor lesion.
Fig. 9 Counts recorded on the five crystals of the Gonioprobe (panel A) and on the five back sectors
(panel B) during a single measurement session.
For the measurement of the detection time of a tumor, and to evaluate the instrument response time, the GonioProbe was used by bringing it closer to the surface of the tumor in the mouse, and the maximum counting point (source) was identified and this was marked on the surface itself. During
this phase, the operator (Nuclear Medicine) was timed and the time taken in seconds to identify the exact point was measured. At the beginning of the session, the Gonioprobe is placed in a generic position, for a distance ranging from 5 to 10 cm from the position of the tumor, and, in the meantime, the counts deriving from the crystals are processed in real-time to provide on one screen the direction in which the Gonioprobe must be moved to approach the source "felt" by the crystals (which, in a first phase, in the presence of a shield, coincides with the target only). Figure 9 shows the first seconds of the measurement session; the Gonioprobe is placed about 10 cm from the tumor in the opposite direction to the source simulating another tumor (for which it is about 22 cm from the latter); it is possible to see the unbalanced trend of the counts of the crystals used for navigation: from this unbalance it is possible to reconstruct the direction of origin of the source, determining a high precision in localization in terms of spatial resolution. In the specific case, we note that one of the four crystals (red line) shows a greater number of counts, and this information is processed on-line to give the operator the direction in which to move; as the operator approaches to the lesion, all counts increase over time.
Fig. 10 The unbalanced counts of the four side crystals indicate to the operator the direction in
which to move the Gonioprobe to bring it closer to the source.
When the target is localized, i.e. when the Gonioprobe is placed in contact with the source-tumor, the central crystal records a certain number of counts and the four side crystals are balanced in the counts (Figure 11).
Fig. 11 Target hooking condition: the counts of the four crystals (red, blue, cyan and magenta lines)
are balanced and the central (black line) records a significant number of counts.
Furthermore, it is possible to use the information coming from the back crystal to correct the counts recorded in the side windows of the Gonioprobe and to guarantee correct navigation. Figure 12 shows the counts recorded in an extreme situation, when, for the effect of the decay, the radioactivity of the tumor lesion is very low. We note that the activity recorded by the back sectors is perfectly able to reconstruct the direction of the background activity and can, therefore, be used to correct the unbalanced activity of the crystals. Even in these most extreme conditions, in any case, the Gonioprobe, given its high sensitivity, is able to record the minimum variations in counting and to hook itself to the target after having directed the operator in the correct direction of radiation.
Fig. 12 Target coupling in the session with a poorly radioactive tumor lesion
Statistical analysis
The statistical angular error of the GonioProbe was evaluated with an approximate formula based on the calculation of the statistical error determined by the centroid method applied to the counts on the crystals.
The counts represented in the previous analyzes show a magnitude level of the order of thousands of average counts per crystal, confirmed in the graph of Figure 9, which shows the number of counts as a function of distance. This determines with the Gonioprobe a very minimal angular error. About the temporal choice made for the graphical representation of the Gonioprobe response, the order of magnitude of 1 second for the time factor was determined by the choice of the Medical Doctors, representing the minimum reading time limit of the operators themselves. The difference with PET is noteworthy as this last instrument has acquisition times in the order of one minute. The statistical precision in terms of position is determined by the counting statistic. For the measurements performed with PET and the consequent analysis of the images, it was necessary to determine the centroid of the Gaussian distribution, in which FWHM is about 1 cm and the error is
given by σ / √N, where N = count. This makes it easy to understand how, in order to have reasonable counts, the acquisition time in PET must be very long, in particular it has been calculated that PET times must be lengthened by a factor of 100 to have the same counting statistics as in the Gonioprobe. About the commercial probe, the order of magnitude of the acquisition time is approximately 10 seconds of integration of counts, since on average 10 cps / KBq are detected from its central crystal. The greatest limitation given by the use of the commercial probe in determining the center of radioactivity is given precisely by the angle of vision with which the target object is detected. That is, the radioactive object may be seen and therefore detected only randomly or totally for random reasons of displacement of the probe itself. The result is a very high variation in the counting statistics, position-dependent, with a consequent longer search by the surgeon to identify the maximum count that allows localization. Regarding the efficiency of the Gonioprobe, it has been shown that it has a response time of fewer than 3 seconds, at a distance of 1 meter, with a source of 50 µCi of Cs137. Its dose rate is 0.14 µSv / h with an error of about 20 °, proving to have very high efficiency, much greater than a PET for the same detectors size involved while using the same type of crystal. As regards the analysis of the error in the determination of the position, with the use of PET to increase its accuracy we must increase the acquisition time in the order of minutes to have a good counting statistic, while for the commercial probe, however, there will be a difference of a factor of about 20 with respect to the Gonioprobe and in any case the detection will be linked to random movements. The Gonioprobe measurements on phantom have shown that with the navigation system, near the source simulating the tumor, counts increase considerably up to 1000 average counts per crystal. This leads to an error measurement of less than 1°. The 4 side crystals allow the perfect centering of the Gonioprobe, based on their balance. In fact, the error of less than 1 ° is determined when all 4 crystals are centered. From the statistical analysis of data collected it was determined that the expected statistical error of the Gonioprobe is less than 1 mm and therefore the Gonioprobe with respect to a commercial probe has proved to be more reliable.
Discussion
The main purpose of this thesis is to try to solve a current clinical surgical problem through technological advancement. In radio-guided surgery, there are many technological dampers, also due to the currently available commercial probes, which influence the skill of the surgeon. The current handheld devices in radio-guided surgery (gamma probes) are characterized by a central active detection system focused by a passive collimation system. The basic principle of standard technology is very old and presents several limitations: good efficiency but poor spatial resolution, or poor efficiency but high spatial resolution. These characteristics allow a rapid localization of the radioactive area associated with an inability to identify the single lesion or, on the opposite, make it possible to identify a small-sized lesion but at the expense of very long detection time. Indeed, the low sensitivity of the device implies high detection time to localize the source. Furthermore, they have a limited field of view (FOV) which can be widened only by losing spatial resolution. Another characteristic of commercial probes is the high influence of radioactive background, which results in an inability to discern multiple sources. The current devices are characterized by high weight, low manageability that tires the surgeon and affects his efficacy. In addition to the very high weight, the PET probes have the major limitation of being made up of a large collimator, which, together with the efficiency reduction up to 15 times compared to a gamma probe, becomes decidedly unusable. The inability to provide to the surgeon the direction on which to move the device implies a time consuming and highly manual search time where the surgeon has to scan many times over the operating theater. In addition, the counting is very sensitive to the probe position, so it has poor reproducibility. Consequently, current surgery needs preliminary use of an Anger camera or a PET to mark sensitive areas on the patient before the intervention. The commercially available gamma-probes are unable to measure the gamma-rays direction, and a passive collimation system is needed. The innovation of the GonioProbe is to make the gamma-rays collimator sensitive to radiation [46]. The most innovative aspect of this instrument is represented by the potential detection capability of multiple sources with their imaging representation. This is a key point for the full effectiveness of the surgical technique for lung cancer. In fact, in many cases, the taking up lymph nodes are more than one and add to the primary tumor lesion, so a detection instrument able to define a prompt high-resolution picture, that guides the surgeon to a complete solution, is the successfully crucial point of this surgical technique. The detection cylinder consists of about 17 detection sectors. Each crystal is arranged in such a way to have a different angular point of view and in some cases, the detection is obscured by the absorption of the radiation of other adjacent crystals. The position of a
source is so univocally determined to start from the direction in spherical polar coordinates (ro, theta and phi). Starting from preliminary leading information of some reference crystals, it is possible to roughly define source position, to limit the main viewing angle of each crystal and to attribute to each crystal a bundle of parallel flight lines as large as the crystal dimensions. Back projection reconstruction algorithms, typically used for variable Slant collimator systems [47], are used to identify a reconstructed image matrix of 10x10x7 pixels on an FoV of at least 30 mm in diameter and 21 mm in depth. This, in order to guide the surgeon towards perfect centering of the main source, to proceed with its removal and to continue the search of the remaining sources. The system, maintaining gamma spectrometry characteristics similar to a PET scintillation array module, is able to discriminate gamma emissions of different isotopes at the same time and consequently to carry out contemporary imaging information even in a very complex operating theatre. The innovation introduced with this new device consists in making the collimator itself sensitive to radiation so that it can generate a signal every time a photon is absorbed. An apparatus of this type makes it possible to operate with extremely high acquisition speeds (at least 100 times higher than imaging systems), similarly to existing device, but with the advantage of being able to provide further information on the direction of origin of the radiation within a few tens of seconds, depending on the size of the display field and the accumulated radioactivity [48-49]. In summary, the basic principle of detection of absorbed gamma radiation events is that of a micro range camera. This represents substantial technological progress for intraoperative activities and involves one series of significant advantages in terms of time reduction and surgical invasiveness, as well as the certainty of the material to be removed, with the consequent increase of benefits provided to the patient for the regression of possible post-surgical complications linked to the duration of the intervention and to the certainty of the success of the intervention itself. The use of the position-sensitive photodetector, thanks to the possibility of obtaining an image from the five probe sectors (four collimators plus the central crystal), allowed to accurately evaluate the answers of the single elements as if they were independent units. The response obtained showed the ability of the system to identify the direction toward the radiation point with an error (<10°) always less than that connected to manual operation (± 30°), in less time than those required by the operator in making decisions. It is estimated that the goal can be achieved in just over one minute, compared to several minutes needed with commercial probes. Radioguided surgery involving the use of PET tracers such as 18F-FDG has been under study for a long time [50- 52]. Unfortunately, it also includes many limitations, for example, the fact that the patient must be injected with the radioactive just before entering the operating room, with consequent exposure as if he were doing a PET. In this thesis, we have shown that thanks to the high efficiency of the GonioProbe, the amount of
radioactivity to be injected would be much lower than normal. Furthermore, the reduction in the time required for surgery would make it possible to further lower the dose to the operators. The problem of negative margins is fundamental, this issue could be easily solved thanks to the GonioProbe. In fact, due to the radical possibility of discriminating the lesion from the background, it will be possible to verify that there are no tissue residues in the operating bed. In conclusion, this technological advancement would allow a more effective and faster thoracic surgery in order to help the surgeon to definitively eradicate lung cancer.
References
1) Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87.
2) Ost DE, Yeung SC, Tanoue LT, Gould MK. Clinical and organizational factors in the initial evaluation of patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143:e121S.
3) Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143:e93S.
4) Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143:e211S.
5) De Wever W. Role of integrated PET/CT in the staging of non-small cell lung cancer. JBR-BTR 2009; 92:124.
6) Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med 2009; 361:32.
7) Cheng G, Huang H. Prognostic Value of 18F-Fluorodeoxyglucose PET/Computed Tomography in Non-Small-Cell Lung Cancer. PET Clin. 2018 Jan;13(1):59-72. doi: 10.1016/j.cpet.2017.08.006.
8) Cheng G. Non-Small-Cell Lung Cancer PET Imaging Beyond F18 Fluorodeoxyglucose. PET Clin. 2018 Jan;13(1):73-81. doi: 10.1016/j.cpet.2017.09.006.
9) Paesmans M, Garcia C, Wong CY, et al. Primary tumour standardised uptake value is prognostic in nonsmall cell lung cancer: a multivariate pooled analysis of individual data. Eur Respir J 2015; 46:1751.
10) Pak K, Park S, Cheon GJ, et al. Update on nodal staging in non-small cell lung cancer with integrated positron emission tomography/computed tomography: a meta-analysis. Ann Nucl Med 2015; 29:409.
11) Lardinois D, Weder W, Roudas M, et al. Etiology of solitary extrapulmonary positron emission tomography and computed tomography findings in patients with lung cancer. J Clin Oncol 2005; 23:6846.
12) Cherkashin M, Aniskhin M, Berezina N, Puchkov D. CT and PET/CT fusion for lung cancer biopsy planning. BMJ Case Rep. 2017 Oct 4;2017. pii: bcr-2017-221972. doi: 10.1136/bcr-2017-221972
13) Walker R, Deppen S, Smith G, Shi C, Lehman J, Clanton J, Moore B, Burns R, Grogan EL, Massion PP. 68Ga-DOTATATE PET/CT imaging of indeterminate pulmonary nodules and lung cancer. PLoS One. 2017 Feb 9;12(2):e0171301. doi: 10.1371/journal.pone.0171301. 14) Gould MK, Kuschner WG, Rydzak CE, et al. Test performance of positron emission
tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 2003; 139:879.
15) Vansteenkiste JF, Stroobants SG. Positron emission tomography in the management of non-small cell lung cancer. Hematol Oncol Clin North Am. 2004 Feb;18(1):269-88.
16) Gupta NC, Graeber GM, Bishop HA. Comparative efficacy of positron emission
tomography with fluorodeoxyglucose in evaluation of small (<1 cm), intermediate (1 to 3 cm), and large (>3 cm) lymph node lesions. Chest 2000; 117:773.
17) Higashi K, Ueda Y, Seki H, et al. Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med 1998; 39:1016.
18) Yamamoto Y, Nishiyama Y, Kimura N, et al. Comparison of FLT PET and (18)F-FDG PET for preoperative staging in non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2008; 35:236.
19) Subedi N, Scarsbrook A, Darby M, Korde K, Mc Shane P, Muers MF The clinical impact of integrated FDG PET-CT on management decisions in patients with lung cancer. Lung Cancer. 2009 Jun;64(3):301-7. Epub 2008 Nov 11.
20) Nosotti M, Castellani M, Longari V, et al. Staging non-small lung cancer with positron emission tomography: diagnostic value, impact on patient management, and cost-effectiveness. Int Surg 2008; 93:278.
21) Schuurbiers OC, Tournoy KG, Schoppers HJ, Dijkman BG, Timmers HJ, de Geus-Oei LF, et al. EUS-FNA for the detection of left adrenal metastasis in patients with lung cancer. Lung Cancer. 2011 Sep;73(3):310-5. doi: 10.1016/j.lungcan.2010.12.019
22) Woodard GA, Jablons DM. The Latest in Surgical Management of Stage IIIA Non-Small Cell Lung Cancer: Video-Assisted Thoracic Surgery and Tumor Molecular Profiling. Am Soc Clin Oncol Educ Book. 2015:e435-41. doi: 10.14694/EdBook_AM.2015.35.e435. 23) Citak N, Buyukkale S, Kok A, Celikten A, Metin M, Sayar A, Gurses A. Does
compared with standard cervical mediastinoscopy? Thorac Cardiovasc Surg. 2014 Oct;62(7):624-30. doi: 10.1055/s-0033-1358656.
24) Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015 Jun;16(6):630-7. doi: 10.1016/S1470-2045(15)70168-3.
25) Vannucci F, Gonzalez-Rivas D. Is VATS lobectomy standard of care for operable non-small cell lung cancer? Lung Cancer. 2016 Oct;100:114-119. doi: 10.1016/j.lungcan.2016.08.004. 26) Lv X, Cao J, Dai X, Rusidanmu A. Survival rates after lobectomy versus sublobar resection
for early-stage right middle lobe non-small cell lung cancer. Thorac Cancer. 2018 Aug;9(8):1026-1031. doi: 10.1111/1759-7714.12782.
27) Thomas PA. Use of minimally invasive approaches for stage I non-small cell lung cancer: A surgeon's point of view. Cancer Radiother. 2015 Oct;19(6-7):365-70. doi:
10.1016/j.canrad.2015.06.012.
28) Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009; 27:2553. 29) Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell
Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016; 34:3175.
30) Vallières E, Van Houtte P, Travis WD, et al. Carcinoma in situ at the bronchial resection margin: a review. J Thorac Oncol 2011; 6:1617.
31) National Comprehensive Cancer Network guidelines
http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (Accessed on March 19, 2014).
32) Kawaguchi T, Watanabe S, Kawachi R, et al. The impact of residual tumor morphology on prognosis, recurrence, and fistula formation after lung cancer resection. J Thorac Oncol 2008; 3:599.
33) Selverstone B, Sweet WH, Robinson CV. The clinical use of radioactive phosphorus in the surgery of brain tumours, Ann. Surg. 130 (1949) 643–651.
34) Hoffman EJ, Tornai MP, Janeck M, et al. Intra-operative probes and imaging probes, Eur. J. Nucl. Med. 26 (1999) 913–935.
35) Yamamoto S, Matsumoto K, Sakamoto S, et al., An intra-operative positron probe with background rejection capability for FDG-guided surgery, Ann. Nucl. Med. 19 (2005) 23–28.
36) Kawamura K, Shimoda Y, Yui J, Zhang Y, Yamasaki T, Wakizaka H, et al. A useful PET probe [11C]BU99008 with ultra-high specific radioactivity for small animal PET imaging of I2-imidazoline receptors in the hypothalamus. Nucl Med Biol. 2017 Feb;45:1-7. doi:
10.1016/j.nucmedbio.2016.10.005.
37) Povoski SP, Neff RL, Mojzisiket CM, al., A comprehensive overview of radio-guided surgery using gamma detection probe technology, World J. Surg. Oncol. 7 (2009) 11. 38) Schillaci O. PET probes and oncological surgery: A productive new marriage for nuclear
medicine? Eur. J. Nucl. Med. Mol. Imaging 34 (2007) 1530–1533.
39) Park HM, Joo KS. Feasibility of a wireless gamma probe in radioguided surgery. Phys Med Biol. 2016 Jun 21;61(12):N311-21. doi: 10.1088/0031-9155/61/12/N311.
40) Ciriaco P, Negri G, Puglisi A, et al., Video-assisted thoracoscopic surgery for pulmonary nodules: Rationale for preoperative computed tomography-guided hookwire localization, Eur. J. Cardiothorac. Surg. 25 (2004) 429–433.
41) Essner R., Hsueh EC, Haigh PI ,et al., Application of an [18F]fluorodeoxyglucose-sensitive probe for the intraoperative detection of malignancy, J. Surg. Res. 96 (2001) 120–126. 42) Zervos ee, Desai DC, Depalatis LR, et al. 18F-labeled fluorodeoxyglucose positron emission
tomography-guided surgery for recurrent colorectal cancer: A feasibility study, J. Surg. Res. 97 (2001) 9–13.
43) Meller, B, Sahlmann C, Horstmann O, et al., Conventional gamma and high energy probe for radio-guided dissection of metastases in a patient with recurrent thyroid carcinoma with 99mTc-MIBI and 18F-FDG, Nuklearmed. 44 (2005) 23–25.
44) Manca, G, Biggi E, Lorenzoni A, et al., Simultaneous detection of breast tumour resection margins and radio-guided sentinel node biopsy using an intraoperative electronically-collimated probe with variable energy window: A case report, Clin. Nucl. Med. 36 (2011) 196–198.
45) Pani, R., Pellegrini, R., Cinti, M.N., et al. Development of a novel gamma probe for detecting radiation direction (2016) Journal of Instrumentation, 11 (1), art. no. C01002, . DOI: 10.1088/1748-0221/11/01/C01002
46) Pellegrini, R., Pani, R., Cinti, M.N., et al. Gamma emission tomosynthesis based on an automated slant hole collimation system (2015) Journal of Instrumentation, 10 (3), art. no. C03003, . DOI: 10.1088/1748-0221/10/03/C03003
47) Polito C, Pani R, Frantellizzi V, et al. Imaging performances of a small FoV gamma camera based on CRY018 scintillation crystal. Nucl Inst and Methods in Physics Research, A. 2017 DOI: 10.1016/j.nima.2017.10.023
48) Cinti MN, Frantellizzi V, Pani R, et al. Innovative LuYAP:Ce array for PET imaging. JINST 2017, DOI:10.1088/1748-0221/12/03/C03069
49) Polito C, Pani R, Frantellizzi V, et al. Imaging characterization of a new gamma ray detector based on CRY019 scintillation crystal for PET and SPECT applications. JINST 2017DOI: 10.1088/1748-0221/12/02/C02034
50) Mancini-Terracciano C, Donnarumma R, Bencivenga G, Bocci V, Cartoni A, Collamati F, et al. Feasibility of beta-particle radioguided surgery for a variety of "nuclear medicine" radionuclides. Phys Med. 2017 Nov;43:127-133. doi: 10.1016/j.ejmp.2017.10.012
51) Orsaria P, Chiaravalloti A, Fiorentini A, Pistolese C, Vanni G, Granai AV, Varvaras D, et al. PET Probe-Guided Surgery in Patients with Breast Cancer: Proposal for a
Methodological Approach. In Vivo. 2017 Jan 2;31(1):101-110.
52) Gulec SA, Daghighian F, Essner R. PET-Probe: Evaluation of Technical Performance and Clinical Utility of a Handheld High-Energy Gamma Probe in Oncologic Surgery. Ann Surg Oncol. 2016 Dec;23(Suppl 5):9020-9027.