1
UNIVERSITY OF PISA
Research Doctorate School in
BIOLOGICAL AND MOLECULAR SCIENCES
Course in
MOLECULAR AND EXPERIMENTAL ONCOLOGY
SSD: MED/06
XXVI CYCLE (2011-2013)
THESIS TITLE
ISOLATION AND CHARACTERIZATION OF HUMAN
MAMMARY TUMOR VIRUS (HMTV) IN HUMAN SPORADIC
BREAST CANCER
Candidate: Ivana Armogida
Supervisor: Prof. Generoso Bevilacqua
2
To my family
for your love, support and
for you to enjoy with me my success!
3
ACKNOWLEDGMENTS
I would like to express my deep and sincere gratitude to my supervisor Prof. Generoso Bevilacqua for giving me the opportunity to do exciting research in the field of the Molecular Oncology. His extensive knowledge of the topic has been of fundamental importance for the development of this project.
I’m particularly grateful to my tutor Dr. Chiara Maria Mazzanti for her continuous encouragement, support and guidance while I have been carrying out my research project and writing the thesis.
I wish to thank Dr. Katia Zavaglia for her invaluable support and helpful advices.
I thank the group of Pathologists including Prof. Antonio Giuseppe Naccarato, Dr. Giovanni Fanelli, Dr. Valerio Ortenzi, Dr. Cristian Scatena and Dr. Francesco Carbone for helping me in sample collection.
I especially thank Dr. Giovanni Fanelli for having carefully selected all samples and for the long and enjoyable chats.
I would like to thank Dr. Francesca Lessi, with whom I shared this ambitious project, for her guidance.
I would like to express my heartiest gratitude to Dr. MariaPia Cioni for all her help and for her sincere friendship.
I would also like to thank my PhD collegues, Dr. Federica Panebianco, Dr. Sara Franceschi and Dr. Alessandro Apollo with whom I shared lessons, concerns and frustrations as well as ideas and satisfaction.
Thanks to you all for allowing me to grow professionally.
It was a great pleasure to spend several years at the Division of Pathological
4
TABLE OF CONTENTS
1. ABSTRACT...7
2. INTRODUCTION...9
2.1 The organ in which the tumor arises: human breast cancer histopathology...9
2.2 TNM classification...15
2.3 Histological grading...19
2.4 Risk factors of breast cancer...20
2.5 Viral etiology of mammary tumor...21
2.6 Methods used in the past for viral detection...22
3. MOUSE MAMMARY TUMOR VIRUS...26
3.1 History...26
3.2 Taxonomy and Classification...26
3.3 Properties of the virion...26
3.4 Viral genome...27
3.5 Super antigen………....28
3.6 MMTV life cycle...28
3.7 MMTV distribution in different tissues...30
3.8 Recombination of exogenous and endogenous MMTV...30
3.9 MMTV and tumorigenesis...30
3.10 Replication cycle of MMTV………...32
3.11 Human endogenous retrovirus………....………..35
3.12 Classification………35
3.13 The biological function of HERV……….…....35
3.14 HERV-K family………....36
4. AIM...38
4.1 HMTV involvement in Hereditary and Sporadic breast cancers...38
4.2 Geographical distribution of HMTV...38
4.3 Virus detection in lymph nodes of HMTVes+ and HMTVes- breast cancers...39
5
4.5 HMTVes detection in saliva and salivary glands...39
4.6 Primary cultures of human breast cancer...40
4.7 FISH and Electron Microscopy analyses...40
4.8 Immunocytochemistry and Western Blot analyses...40
5. MATERIALS AND METHODS………...………....41
5.1 Formalin fixed and paraffin embedded sample collection……...41
5.2 Fresh tissue sample collection………..41
5.3 Laser microdissection………...42
5.4 DNA extraction from microdissected tissue………...43
5.5 DNA extraction from fresh tissue and FFPE………....43
5.6 DNA extraction from cell culture….……….…...44
5.7 RNA extraction from cell culture and human saliva...45
5.8 DNase treatment...46
5.9 cDNA synthesis………..…..46
5.10 miRNA extraction from FFPE samples...47
5.11 miRNA reverse-transcription...48
5.12 Housekeeping gene control PCR………..48
5.13 HMTV sequence detection………...49
5.14 Analysis of PCR products……….…49
5.15 Gene expression Real Time PCR assay in HMTVes+/- samples….50 5.16 miRNA analysis...52
5.17 Breast cancer PCR array………...53
5.18 Tissue cultures………...55 5.19 Day 1 protocol………...56 5.20 Day 2 protocol………...56 5.21 Subculture………...57 5.22 MCF7 cell lines………...58 5.23 FISH analysis………....58 5.24 Electron Microscopy………..…...59 5.25 Immunocytochemistry………..59 5.26 Western Blot……….60 5.27 Statistical analysis...60
6
6. RESULTS………..…62
6.1 HMTVes frequency among Hereditary and Sporadic cancers…...62
6.2 HMTVes frequency in Sardinia, Jordan and Australia SBC…..…..62
6.3 HMTVes detection in the early stages of breast cancer.…….…...65
6.4 HMTVes frequency in lymph nodes………..…...66
6.5 Gene expression analysis………...68
6.6 HMTVes frequency in human saliva………...75
6.7 HMTVes frequency in human salivary glands...75
6.8 Primary culture of human breast cancer………..……...76
6.9 HMTV detection by FISH analysis and Electron microscopy…...76
6.10 Detection of the p14 viral protein by Immunocytochemistry…...81
6.11 Detection of the viral protein gp52 by Western Blot…………...82
7. DISCUSSION………..………...83
8. CONCLUSIONS………..…....91
9. FUTURE PERSPECTIVES………....93
7
1. ABSTRACT
Breast cancer is the most common cancer in women and in the 35-55 age range is the principal cause of death amongst women worldwide. It has become clear that the clinical onset of breast cancer is influenced by several factors. Among these, the potential involvement of a virus of the family Retroviridae, the Human Mammary Tumor Virus (HMTV), has represented a major focus of investigation whose existence has been proposed and disputed for many years after the identification of the MMTV (Murine Mammary Tumor Virus). Several scientists were able to demonstrate the presence of HMTVenv sequence (HMTVes) in 30-40% of invasive human breast carcinomas and very recently our laboratory was able to prove that its presence is strictly associated with sporadic breast cancer progression.
In the present study we decide to add more evidence to the possible viral etiology of human breast cancer by approaching the problem from different angles:
1) the fact that in hereditary breast cancer (HBC) inherited gene mutations are recognized as initiating event led us to hypothesize that in this group an involvement of an oncogenic virus is not expected. The presence of MMTVes was investigated in a group of HBC from patients hosting a BRCA1 or BRCA2 mutations as well as in a group of SBC, with the aim to prove this hypothesis. 2) we decided to investigate the frequency of HMTVes in a set of sporadic breast cancer (SBC) samples collected in three different and geographically distant countries such as Sardinia, Jordan and Australia. In the last population when possible we collected also for each SBC case, a normal and pre-invasive lesion in order to assess the presence of the virus before cancer onset.
3) because the infiltrative carcinoma of the breast initially spreads via lymphatics giving metastasis firstly to the axillary and internal mammary lymph nodes, it was interesting to analyze lymph nodes of HMTVes positive and negative (HMTVes+ and -) tumors. From each metastatic lymph node was microdissected the tumoral epithelial cell population and the lymphocytes.
4) a gene expression study, using specific breast cancer arrays and a panel of selected genes, was performed to assess the genetic background in SBC
8
HMTVes+ and – tumors and matched normal.
5) because of the strong similarities between the human and the murine disease, the presence of the MMTV virus in all murine exocrine glands led us to investigate if we could find HMTVes also in human saliva and salivary glands. 6) we have created HMTVes+ breast cancer cell primary cultures, from fresh human breast cancer tissues collected from the surgery-room in order to isolate the virus and FISH, Electron microscopy, Immunocytochemistry and Western experiments were performed to confirm the presence of the virus.
The results obtained from all experiments support our recent findings and also our new hypotheses. We were able to obtain only one HMTVes+ primary cancer cell line which seemed to be HMTV positive also by electron microscopy, FISH, immunocytochemistry and Western Blot analysis.
9
2. INTRODUCTION
2.1 The organ in which the tumor arises: human breast cancer histopathology
Breast cancer is a malignant tumor that originates from the epithelial cells of the mammary gland which is in charge of the production of milk.
From an anatomical point of view, breast consists of 15-18 lobes each one containing more lobules and a main duct which opens into the nipple and allows the passage of milk from the lobules to the nipple.
The lobules are surrounded by fatty tissue which gives the breast its consistency. Each main duct is divided into ducts of progressively lower caliber; all these structures are coated, inside them, by a thin layer of epithelial cells (Figure 1).
Figure 1: Anatomy of the female breast.
Breast cancer has two main variants, the ductal and the lobular cancer that represent about 75% and 5% respectively of the infiltrating tumors.
These two terms were introduced a few decades ago, believing that the first form was derived from the main ducts and the second from the lobules.
10
(DLTU) and subsequently, for mechanisms still not well known, give rise to different tumours, not only for their morphology but also for their biological behavior. In particular, the ductal apparence is secondary to a process of carrying out of the normal lobular architecture.
For both types, an in situ and an infiltrating form are recognized.
The ductal type represents approximately 75% of infiltrating tumors, while the
lobular about 5%.
Less frequent infiltrating histotypes are represented by the medullary (15%),
colloid or mucinous (2%) and tubular (1-2%) carcinoma, to which several other
rare forms are added.
The transition from the normal structures to in situ carcinoma takes place through the formation of intermediate lesions, different for the two main types of cancer, referred to as pre-neoplastic lesions. The process from the pre-neoplastic lesions to metastasis, through the stages of in situ and infiltrating carcinoma, represents the morphological appearance of the so-called tumor progression (Figure 2).
Figure 2: Main stages of breast cancer progression.
The stages of the progression that precede the ductal carcinoma in situ are represented by usual hyperplasia and atypical hyperplasia caused by an hyperplasia of the ephitelial cells of the DLTU.
11
The usual hyperplasia form consists in the formation of several cell layers (above 34) with the creation of bridges that cross the lumen and of solid areas that fill and relax the ducts themselves; in the athypical hyperplasia to these characters is added the presence of cytological atypia. At this stage the risk to develop an invasive carcinoma is still moderate.
The Ductal Carcinoma In Situ (DCIS), consists of malignant epithelial cells confined to the mammary ducts, without microscopic evidence of invasion through the basement membrane into the surrounding tissue (Figure 3).
Figure 3: Ductal Carcinoma In Situ (DCIS).
According to the tumor differentiation, DCIS can be further divided into low, intermediate, and high grade. Such stratification has prognostic implications. DCIS can have a different morphology so that we can distinguish two main groups: comedo and non-comedo. The latter may have solid, cribriform, papillar or micropapillar aspects.
The comedo subtype carries the higher probability of high nuclear grade and microinvasion while the non-comedo tumors have cells with small, round and monomorphic nuclei with few mitotis and generally don’t have necrosis.
This division is useful as the comedo carcinomas have an higher malignancy. The lobular carcinoma in situ (LCIS) is preceded from the atypical lobular
12
hyperplasia, from which it differs essentially for an increased severity of the
cytological and architectural atypia. The presence of pre-neoplastic lesions increases the risk of developing an invasive carcinoma.
The cells of a lobular carcinoma in situ, proliferate filling the lumens of the DLTU (Figure 4): the presence of this lesion increases of 9 times the risk of invasive cancer in the same or in the controlateral breast.
The pre-neoplastic lesions of the less common histological types are not defined yet.
Figure 4: Lobular Carcinoma In Situ (LCIS).
The most frequent invasive carcinoma is the ductal, caracterized of an abundant amount of fibrous or scleroialin stroma, that confers it a very hard consistency, for this reason it is also called scirrhous carcinoma (that is hard); the neoplastic population is disposed in solid islets, in coarse cords, in tubular structures. It is often used to add at the term invasive ductal carcinima the words “not otherwise specified” (NOS) to distinguish it from the rarer histotypes and with a more favorable prognosis such as medullary and mucinous. Invasive ductal carcinoma commonly spreads to the regional lymph nodes and carries the poorest prognosis among various ductal types. Nuclear and histological grade have shown to be effective predictors of prognosis.
13
The invasive lobular carcinoma (ILC) is characterized by the fact that the cells invade the stroma in thin chains, unicellular (in single row) often arranging concentrically around the ductal or lobular structures.
The lesions tend to have not well-defined margins, and occasionally the only evidence is a subtle thickening or induration. Patients with infiltrating lobular carcinoma are especially prone to have bilateral carcinomas. Invasive lobular carcinoma has a similar prognosis to infiltrating ductal carcinoma.
Among the less frequent infiltrating histotypes examples are given by the medullary, colloid and tubular carcinomas.
The medullary carcinoma is a rare type of IDC that has the characteristic of presenting well-circumscribed margins, a widespread infiltration of lymphocytes and tumor cells of large size (Figure 5). When it is pure, that is not commingled with other variants, has a better prognosis than IDC and ILC.
Figure 5: Medullary carcinoma.
The colloid (mucinous) carcinoma is a rare IDC composed of cells that produce mucus. Generally has a better prognosis and a lower risk of metastasis compared to IDC and ILC of the same size (Figure 6).
14 Figure 6: Colloid (mucinous) carcinoma.
The tubular carcinoma is a particular type of IDC characterized by the proliferation of small irregular tubules (Figure 7). Often it is multicentric and bilateral and sometimes is accompanied with lymph nodes metastasis. The pure form has a very favorable prognosis, being curable in more than 90% of cases.
15
Half of all cases of carcinoma arise in the outer upper quadrant of the breast, 20% in the central or subareolar quadrant, 10% in each of the remaining three quadrants.
The higher incidence in the outer upper quadrant of the breast is likely related to the fact that in this area there is the largest glandular part. In its invasive growth, a carcinoma of any kind, can infiltrate the overlying skin and retract it (is the nipple that is retracted) or can infiltrate the chest wall and then remain fix during palpation. Following a widespread neoplastic infiltration of lymphatic vessels a lymphedema of the breast skin can occurs which becomes thickened and hard assuming a finely granular aspect called “orange peel” for the expansion of the outlet orifices of the annexal glands; the lymphangiosis, if widespread, can cause an intense inflammatory reaction of the entire breast which becomes red, swollen, sore and tender giving rise to the so-called inflammatory carcinoma.
Both invasive and in situ carcinomas can contain some microcalcifications, small, numerous and grouped in clusters, which are very useful for the mammographic diagnosis of malignancy.
The invasive carcinomas of the breast initially spread via lymphatics giving metastasis firstly to the axillary and internal mammary lymph nodes, with different frequency depending on the area of the breast in which they are incurred.
2.2 TNM classification
For many decades, breast cancer was characterized and classified on the basis of the:
extension of the primary tumor (T)
lymph nodes involvement (N)
distant metastasis (M)
The staging of breast cancer according to the TNM system allows a description of the extension of the neoplastic disease at a given time and it is universally accepted to estimate the prognosis, to define the best treatment and to evaluate the results.
The cancer stages are described by roman numerals from I to IV.
16
the physical examination and imaging examination (eg. radiography, mammography, etc.).
The pathological stage (pTNM) includes the results of tests performed on the surgical samples and it is the most important because it provides information on the nodal status, that is on whether the cancer has spread to the lymph nodes and it is not generally known until the lymph nodes were not examined histologically (to assess the presence of macrometastasis) and by Immunohistochemical investigations (to assess the presence of micrometastasis).
Clinical classification:
Primary tumor (T)
Tx Primary tumor cannot be assessed T0 No evidence of primary tumor
Tis Carcinoma in situ; intraductal carcinoma, lobular carcinoma in situ, or
Paget's disease of the nipple with no associated tumor
T1 Tumor 2.0 cm or less in greatest dimension
T1mic Microinvasion 0.1 cm or less in greatest dimension T1a Tumor more than 0.1 but not more than 0.5 cm in greatest
dimension
T1b Tumor more than 0.5 cm but not more than 1.0 cm in greatest
dimension
T1c Tumor more than 1.0 cm but not more than 2.0 cm in greatest
dimension
T2 Tumor more than 2.0 cm but not more than 5.0 cm in greatest
dimension
T3 Tumor more than 5.0 cm in greatest dimension
T4 Tumor of any size with direct extension to (a) chest wall or (b) skin,
only as described below.
Note: Chest wall includes ribs, intercostal muscles, and serratus anterior muscle but not pectoral muscle.
17
T4b Edema (including peau d'orange) or ulceration of the skin of the
breast or satellite skin nodules confined to the same breast
T4c Both of the above (T4a and T4b) T4d Inflammatory carcinoma
Regional lymph nodes (N)
Nx Regional lymph nodes cannot be assessed (e.g., previously removed) N0 No regional lymph node metastasis
N1 Metastasis to movable ipsilateral axillary lymph node(s)
N2 Metastasis to ipsilateral axillary lymph node(s) fixed to each other or
to other structures
N3 Metastasis to ipsilateral internal mammary lymph node(s)
Distant metastasis (M)
Mx Presence of distant metastasis cannot be assessed M0 No distant metastasis
M1 Distant metastasis present (includes metastasis to ipsilateral
supraclavicular lymph nodes)
Pathological classification:
Primary tumor (pT)
corresponds to clinical T classification
Regional lymph nodes (pN)
pNX Regional lymph nodes cannot be assessed (not removed for
pathologic study or previously removed)
pN0 No regional lymph node metastasis
pN1 Metastasis to movable ipsilateral axillary lymph node(s) pN1a Only micrometastasis (none larger than 0.2 cm)
18
pN1b Metastasis to lymph node(s), any larger than 0.2 cm pN1bi Metastasis in 1 to 3 lymph nodes, any more than 0.2 cm
and all less than 2.0 cm in greatest dimension
pN1bii Metastasis to 4 or more lymph nodes, any more than 0.2
cm and all less than 2.0 cm in greatest dimension pN1biii Extension of tumor beyond the capsule of a lymph node
metastasis less than 2.0 cm in greatest dimension
pN1biv Metastasis to a lymph node 2.0 cm or more in greatest
dimension
pN2 Metastasis to ipsilateral axillary lymph node(s) fixed to each other
or to other structures
pN3 Metastasis to ipsilateral internal mammary lymph node(s)
Distant metastasis (pM)
corresponds to clinical M classification.
Stage grouping: Stage T N M 0 Tis N0 M0 I T1 N0 M0 IIA T0 N1 M0 T1 N1 M0 T2 N0 M0 IIB T2 N1 M0 T3 N0 M0 IIIA T0 N2 M0 T1 N2 M0 T2 N2 M0 T3 N1 M0 T3 N2 M0 IIIB T4 Any N M0 Any T N3 M0 IV Any T Any N M1 TNM classification.
19
2.3 Histological grading
All invasive breast carcinomas, with the exception of medullary carcinoma, must be marked with the grading.
The grading of a tumor is determined by estabilishing the morphological characteristics, assigning a value from 1 (favorable) to 3 (unfavorable) for each of the features, then adding up all the scores of the three categories. A combined value of 3-5 points marks a grade 1, a combined value of 6-7 points marks a grade 2; a combined value of 8-9 points marks a grade 3.
Gx the differentiation grading can not be defined G1 low combined hystological grading (favorable)
G2 intermediate combined hystological grading (moderately positive) G3 high combined hystological grading (unfavorable).
20
2.4 Risk factors of breast cancer
Breast cancer is the second leading cause of cancer deaths in women today (after lung cancer) and it is the most common cancer among women [1]. Despite the fact that it represents the most frequent cancer in women and that it is largely studied all over the world since many decades, the etiology of breast carcinoma is largely unknown.
However, the strongest risk factors for breast cancer include age, reproductive factors, hormonal status, genetics and lifestyle as well as a potential viral involvement is representing a major focus of investigation but the data are still inconsistent and inconclusive.
Age
Aging is considered as one of the greatest risk factors for the development of breast cancer. Breast cancer incidence is strongly associated with an increase of age, with an estimated 64% of women over the age of 55 at the time of breast cancer diagnosis [2].
Reproductive factors
Breast cancer risk increases in women who experience a later age at menopause, increased age at first pregnancy, and low parity [3].
Menopause: although the risk of breast cancer incidence increaseswith age, postmenopausal women have a lower risk of developing breast cancer than premenopausal women of the same age. Each 1 year delay in the onset of menopause is associated with 3% increased risk of breast cancer [4].
Age at menarche: later age at menarche (age at 15 or more) is associated with reduction in risk of breast cancer compared to earlier age (at 12 or less). 1 year delay in the onset of menarche is associated with 5% reduction risk of developing breast cancer in later life [5].
Parity: an increased parity is associated with a decreased risk for breast cancer compared to nulliparity. Each birth reduces the relative risk of breast cancer by an average of 7%. This reduction in the risk per birth is greater in young women (before age 20 years) compared to older ages [6].
21
Hormonal status
Breast cancer risk has been extensively reported in women with exposure to exogenous sex steroids such as oral contraceptives (OC) and postmenopausal hormone replacement therapy (HRT).
The hormonal effects of OC in breast cancer is complex. They often cause protective anovulatory cycles, but the mixture of progesterone and estrogens may also stimulate the mitotic activity on the breast [7]. One case control study found a relative risk of 1.4 in women who took oral contraceptives for longer than 12 years compared with non users. These results are consistent with the earlier metaanalysis results, which had shown no difference in risk after 10 or more years of discontinuing use of oral–contraceptives [8]. Hormone replacement therapy increase the risk of breast cancer in current users with substantial increased differences between the effects of the only estrogens only and the ones of the estrogens/progesterone preparations [9].
Genetics
Women with a family history of breast cancer are at high risk of developing the disease representing 5% of breast cancer cases. The number of breast cancer genes is not yet known. However two autosomal dominant genes, BRCA1 and BRCA2, which are located on chromosome 17 and 13 respectively, have been account for most of the cases of familial breast cancers. The lifetime risk of developing breast cancer for BRCA1 and BRCA2 mutation carriers is 80-85% [10]. According to a combined analysis of 22 studies, the average cumulative risk in BRCA1-mutation carrier aged 70 was 65%, and 45% in BRCA2-mutation carriers [11].
2.5 Viral etiology of mammary tumour
The etiological role of the Murine Mammary Tumor Virus (MMTV) in the development of tumors of the mammary gland in mice is demonstrated since a long time [12]. It is interesting to note that much of what is known about the pathogenesis of human breast carcinoma was learned by the experimental model of the MMTV-induced mouse mammary tumors [13, 14].
22
called preinvasive lesions as morphological steps of its development are based on this murine model [15]. Moreover, the promotional role of estrogens was built on the observations conducted in mice [16, 17].
These strong similarities between the human and the murine disease represented the reason for the quest of a possible viral etiology of breast carcinoma in women since more than half century: MMTV viral antigens were found in human breast tumors [18], MMTV particles were described in human cells and milk [19, 20], MMTV sequences were found in humans [21]. Unfortunately, these data were never considered conclusive mainly because of their scarce reproducibility. Moreover, the less sensitive and specific techniques used at that time are considered the main reason of the variability of results.
2.6 Methods used in the past for viral detection
In the '70s, most of the experiments were based on immunohistochemical techniques. The envelope protein of MMTV (MMTV gp52) was studied in human breast tumor, and in human breast cancer cell line T47D [18], but there were conflicting results, that may be due to the low sensitive techniques used.
The electron microscopy results were also conflicting. Some laboratories reported the presence of morphologically related retrovirus particles, called RVLPs (Retrovirus-Like Particles) in samples of human milk [22], and in macrophages of breast cancers tissues as well as in human breast cancer cell line T47D, after stimulation with estradiol, followed by stimulation with progesterone [23]. Other researchers considered those particles as cellular debris with no correlation with the onset of breast cancer [24].
A subsequent wave of interest came in the early '80s, when some laboratories reported the presence of reverse transcriptase in samples of breast cancer or in the serum of patients. An essential characteristic of retroviruses is to encode a DNA-dependent RNA polymerase (called reverse transcriptase) that retrotranscribes the viral RNA into a double helix of DNA. The reverse transcriptase enzyme is therefore considered a marker of retroviruses and can be used as an indicator of their presence [25]. Again, there were conflicting results obtained [20, 26] by different working groups due to the low sensitivity of the techniques used. The
23
same authors concluded that if the human variant of MMTV exists, it must be present in amount so low to be detectable with the techniques used. In addition, the methods discussed did not allow a clear discrimination between the endogenous retroviral sequences and the exogenous virus. In subsequent years it has been shown that certain endogenous sequences (type HERV-K) are capable of producing complete viral particles, related to MMTV [27, 28].
The molecular biology techniques significantly contributed in detecting the MMTV sequences in human breast cancer.
Sequences homologous to MMTV were first shown in human DNA by using hybridization experiments [21, 29], but there was the doubt that these sequences were due to the presence of endogenous retrovirus.
In 1995, Wang et al [30] selected a region of 660 bp of the MMTV envelope gene (MMTV env), with a homology of only 16% to HERVK10, the prototype human endogenous retrovirus highly similar to MMTV. Later other authors, by using MMTV-specific primers located in the 660 bp region, identified a MMTV envelope gene-like sequence (MMTVels) in 38% of human infiltrating breast carcinomas, with a 90–98% of homology to the MMTVels. On the other hand, MMTVels was found in only 2% of normal human breast samples.
Several other groups were able to confirm these data [31, 33], whereas negative results were published too [34, 35]. This discrepancy could be consequence of differences in technical procedures, of tissue heterogeneity, and of the fact that env sequences are present in few copies.
More in favor of the exogenous origin of these env-like sequences, is the amplification of the whole proviral structure from human breast carcinomas (Figure 8) that shows an homology of 95% to MMTV in 9.9 Kb and of 57% to HERVK10 in 3.5Kb. Homology to the endogenous retrovirus was seen primarily in the pol gene, which is known to be conserved among different retroviruses and partially in the gag gene [36].
In 2006 Zammarchi et al [37] from this laboratory designed a rigorous methodological approach able to overcome these difficulties, based on the association of a laser microdissection procedure and a highly sensitive fluorescent nested PCR. The MMTV env-like sequence was detected in 33% of a series of
24
human breast carcinomas, whereas normal breast tissues and other types of human tumors resulted negative.
Figure 8: Structure of proviral sequences in human breast cancer showing
genes, ORFs, and sequence homology to MMTV and HERV-K10.
Subsequently, in 2007, Pogo et al. isolated HMTV particles from the ascitic and pleural fluids of a patient with metastatic breast cancer whose primary tumor was positive for the env sequence and expressed the env protein. They demonstrated the presence of retroviral particles by electron microscopy. The viral particles in culture medium showed retroviruses of type B, with size between 100 and 110 nm, a spherical shape, an eccentric dense core and the presence of mature or immature capsid [38] (Figure 9).
Figure 9: Electron microscopy of MSSM3 cell associates and PF viral particles.
A and B, selected examples of cell-associated viral particles. C and D, selected examples of viral particles in PFs. Note that the nucleoids are condensed to varying degrees. Original magnification, 130,000 (bar, 0.2 Am).
25
carcinoma is receiving much more attention than in the past. Several papers go towards this direction, even if in general they restrict their research to demonstrate viral particles and proteins in tumor cells [38, 39].
26
3. MOUSE MAMMARY TUMOR VIRUS
3.1 History
Mouse Mammary Tumour Virus (MMTV) was first reported in 1933 by the Jackson Memorial Laboratory and by Korteweg in 1934 as an extrachromosomal influence on the incidence of breast cancer in inbred mouse strains [40]. Subsequently, in 1936 Bittner showed that a cancerous agent, that he called “milk factor”, could be transmitted by cancerous mother to young mice while nursing [41]. In 1966 it was proven that Bittner’s milk factor was a virus that remained dormant during the early stages of life in mouse but produced cancer in the middle age when the sexual hormones were in right conditions [42].
3.2 Taxonomy and Classification
MMTV is a prototype specie of the genus B Betaretrovirus in the family Retroviridae. These viruses previously were referred to as type B retroviruses based on their appearance by electron microscopy (a characteristic acentric core within particles of c.100 nm). Multiple double-stranded DNA copies are found in the chromosomal DNA of most commonly used laboratory strains of mice (called integrated or endogenous proviruses) [43]. These endogenous proviruses presumably are present as viral insertions into chromosomal DNA of germline cells and are referred to as Mtv followed by an Arabic number, for example, Mtv8.
Most endogenous Mtvs have defect in one or more genes and therefore these proviruses often fail to produce infectious viruses [44]. Currently, MMTV is classified with other betaretroviruses including Mason–Pfizer monkey virus (MPMV), Jaagsiekte sheep retrovirus (JSRV), and human endogenous retrovirus type-K (HERV-K) [45].
3.3 Properties of the virion
The mature MMTV virion is 100nm in diameter, containing a single stranded positive-sense RNA, which exists as a dimer, and is encapsidated as a helical ribonucleoprotein (RNP) by the nucleocapsid (NC) protein; reverse transcriptase
27
(RT) and integrase (IN) are closely associated with the RNP.
The RNP is surrounded by an icosahedral shell composed of capsid (CA) protein. MMTV capsids are bound via the matrix (MA) proteins to the viral envelope, a portion of the cellular plasma membrane that has been modified by the insertion of the surface (SU) and transmembrane (TM) proteins [46] (Figure 10).
Figure 10: The structure of MMTV.
3.4 Viral genome
The viral RNA is bound at either ends by a short direct repeat (R) of 15 bp. The R regions are adjacent to a region of approximately 120 and 1200 bp, respectively, present at the 5 (U5) or 3 (U3).
U5: a unique, non-coding region which forms the 3' of the proviral genome and it is the first part of the genome to be reverse transcribed.
U3: a unique, non-coding region which forms the 5' of the proviral genome and after reverse transcription contains the promoter elements responsible for the transcription of the provirus.
A cellular tRNA (tRNA3Lys) is bound through 18 bp of complementarity to each copy of the viral RNA at the primer-binding site (PBS) located just downstream
28
of U5.
The first splicing donor (SD) site precedes the group-specific antigen (gag) region that encodes a Gag precursor. The virus also encodes two other precursor polypeptides, gag-pro, gag-pro-pol from genomic RNA. Gag-pro encodes the gag proteins, a dUTPase (DU), and the viral protease (PR), whereas the gag-pro-pol protein encodes RT, including a ribonuclease H (RNase H) activity and IN and finally the env region that encodes the envelope protein which compose the surface (SU) and transmembrane proteins (TM).
3.5 Super antigen
The long terminal repeat (LTR) of MMTV harbor an Open Reading Frame (ORF) that encodes a superantigen (Sag), essential for its life cycle [47]. The superantigens are different from normal antigens for their ability to stimulate a greater amount of T cells. This property derives from their ability to bind to all T cells expressing a particular Vβ chain of T-cell receptor (TCR), and not only at the groove formed by the α and β chains, as antigens conventionally do. In this way, all T cells that express the chain Vβ, have the ability to recognize and be stimulated by superantigens, which are presented as exogenous antigens [48]. Another characteristic of superantigens is to be present only in the context of the class II Major Histocompatibility Complex molecules (MHCII) [49]. MMTV requires functional MMTV Sag to determine infection of the mammary gland of mice. Laboratory experiments have shown that exogenous virus with impaired function of Sag, are not infectious [50]. MMTV also needs a functional immune system (B and T cells) to complete its infectious cycle [51].
3.6 MMTV life cycle
The virus can be transmitted vertically (endogenous virus) when embryonic germline cells are infected [52] but it is usually transmitted horizontally through maternal milk (called exogenous virus or milk-borne virus) [12]. MMTV particles ingested by newborn mice from maternal milk, cross the epithelium cells through M cells in small intestine. The virion initially encounters and infects dendritic cells and B cells located in Peyer’s patches of gastrointestinal tract [53]. As a
29
result of the virus expression, superantigen (Sag) protein is produced and presented on B cells by MHC II. The Sag binds both MHC II on B cells and the Vβ of the T cell receptors making it a potent T cell stimulus [54]. The stimulated T cells release cytokines, that cause the proliferation of many B cells that subsequently, also become infected with MMTV [55].
These infected lymphoid cells become a reservoir of MMTV that will preserve MMTV infectivity prior to the onset of puberty in mice when a source of susceptible and dividing mammary cells become available [50].
MMTV infection increases during lactation, where high virion production ensures that large amounts of viral particles will increase the probability of newborn offspring infection [56].
The chronic infection of mammary cells induces the formation of malignant tumors including mammary adenocarcinomas. Infected T-cells rarely become tumorigenic [57] (figure 11).
30
3.7 MMTV distribution in different tissues
The pattern of distribution and expression of exogenous and endogenous MMTV, in different tissues, is very similar. Exogenous MMTV can be expresssed only in cells infected with this virus [47]. A high expression of MMTV is found in organs with exocrine function. A strongest expresion is found in normal breast lactating and in mammary tumors; however, high levels of expression are also seen in non-lactating mammary glands and in salivary glands. Much lower levels of expression are found in male seminal fluid and in reproductive organs [58]. In addition, several studies have reported the expression of MMTV lymphoid organs [59]. Extracts obtained from the mammary glands and seminal vescicles are able to infect nude mice following injection, whereas extracts obtained from lymphocytes or plasma are unable to do it [60].
3.8 Recombination of exogenous and endogenous MMTV
The coexpression of exogenous and endogenous RNA molecules in vivo results in viral recombination. Recombination between exogenous and endogenous genomes occurs during cDNA synthesis and is responsible for the generation of variant viruses with new biological characteristics. This recombination occurs becouse the RNA-dependent DNA polymerase, reverse transcriptase, can switch templates during replication [61].
When an exogenous MMTV infects the cells where the endogenous MTV is highly expressed, there is a 'high possibility of recombination between two viral forms. This event generates new viruses that have the ability to infect different strains of mice than the parental forms [62].
3.9 MMTV and tumorigenesis
MMTV does not encode any oncogene but mammary tumorigenesis takes place after proviral insertion near specific cellular proto-oncogenes, that activate them in a process called insertional mutagenesis [63]. Retroviral integration is not sequence-specific, therefore the greater the amount of virus, the greater probability that the proviral DNA integrates near a proto-oncogene. In fact there has been a considerable correlation between viral load in breast milk and the
31
incidence of breast cancer in a given mouse strain [64]. Once a provirus is integrated into the host genome, the expression of the proviral DNA is regulated by specific sequences within the LTR that cause an increased viral transcription in response to glucocorticoid receptor/steroid hormone complexes. The most important steroid hormone that increased the transcription of MMTV during pregnancy is the progesterone [65]. Analysis of mouse mammary tumors induced by MMTV has shown an alteration in six genes [66] belonging to different family members of fibroblast growth factors (FGF) and members of the family Wnt genes. They are: Int-1/Wnt-1, Int-2/Fgf-3, Wnt-3, Hst/kFgf/Fgf-4, Wnt-10b and FGF-8.
An interesting feature common to these genes is that they are all involved in the short-range cellular communication, many of them, in fact, encode secreted proteins [67]. The activation of the expression of these genes, caused by MMTV is mainly due to the presence of enhancer sequences in the 5 'LTR (Long Terminal Repeat) of the genome of MMTV. These regions act on promoters of adjacent genes, located up to 20 kb from the LTR. The MMTV provirus integrates generally outside the coding region of the protooncogene and only rarely within it [68]. The Wnt genes are members of a family that consists of structurally related genes which encode secreted signaling proteins. These proteins have been implicated in oncogenesis and in several developmental processes, including the morphological development of the tissues during embryonic and adulthood stages [69]. The other common target for the integration of MMTV is the fibroblast growth factors (FGF) gene family. The proteins encoded by these genes are potent angiogenic factors in vivo, and are involved in cell differentiation [70]. Three of the eight members of the FGF family, namely, Int-2 (FGF-3), FGF-4 and FGF-8, are activated by the insertion of the proviral MMTV. As in the case of the genes Wnt-1 and Wnt-3, the three FGF genes are not normally expressed in the mammary gland, but are found only in embryonic life or in adult tissues other than in breast [71, 72]. It has been shown that the onset of the murine breast cancer is associated with the activation of targeted genes (int-2 3), hst
(Fgf-4), and Fgf-8) by MMTV proviral insertion. These observations suggest therefore
32
3.10 Replication cycle of MMTV
MMTV replication cycle begins when the viral surface glycoprotein, gp 52, binds to the tranferrin receptor (tfr1) that is expressed in many rodent cells. Human tfr1 doesn’t allow the viral entry, however MMTV bounding to trf1 enters the cell via endocytosis of clathrin-coated pits, and receptors are recycled to the cell surface where they allow the entry of additional MMTV particles into previously infected cells.
Following entry and the partial coating in the cytoplasm, the virally encoded RT is activated. The Primer Binding Sequence (PBS) of the viral RNA is complementary to the 3’ nucleotides of the host tRNA. The host tRNA, lys-3, acts as the DNA primer for the reverse transcriptase to synthesize minus-strand proviral DNA. The initial product of the reverse transcription is a RNA-DNA complex. The RNase H digests the RNA strand, and allows the synthesis of the double-stranded provirus [74]. However, the product of the reverse transcription is different from the starting template so that the U5 and U3 sequences present uniquely in the viral RNA are duplicated to give longer repeats at each end of the provirus (LTRs).
Because the nuclear entry of the preintegration complex containing the provirus is thought to require nuclear envelope breakdown during mitosis, is generally believed that MMTV must infect dividing cells. Once the preintegration complex enters the nucleus the viral integrase randomly inserts the provirus into the host genome [75]. Integrase introduces an asymmetric 2 bp cut (cytosine and adenine) from the linear ends of the provirus as well as an asymmetric break exactly 6 bp apart on the opposite DNA strands of the host DNA. Proviral integrations are not site-specific and may occur at transcriptionally active sites. Following the joining reaction, the repair of virus–cell junctions by cellular enzymes generates a 6 bp direct repeat of cell DNA that flanks the viral LTRs [76].
The integrated MMTV LTR is a stretch of DNA (1195 base pairs in length) at the 5’ end of the viral genome that is responsible for the expression of the virus in the mammary epithelial cells and for the activation of cellular proto oncogenes. The transcription is initiated from the standard promoter in the 5' LTR starting at the U3/R junction and terminating at the R/U5 junction.
33
The transcription of the MMTV LTR produces full-length mRNA, spliced mRNAs, as well as the spliced mRNAs are the env mRNAs and the sag mRNAs [77, 78]. MMTV also produces a doubly spliced mRNA that encodes the RNA export protein, Rem [79]. In most cell types, the levels of gag-pol and env mRNAs greatly exceed Sag and Rem mRNAs.
The viral mRNAs are translated into the viral proteins in the cytoplasm using the host cellular machinery. The gag mRNA is translated into the precursor protein Pr77, a Gag polyprotein. The proteolytic processing of Pr77 by the viral protease produces the structural proteins: the matrix protein p10 or MA; the capsid protein p27 or CA; the nucleocapsid protein p14 or NC, and some smaller proteins p21, p3, and p8 that have no known function [80, 81, 82].
The capsid protein forms the structural shell of the core of the virion, which enclose the ribonucleoprotein complex that contains the genomic RNA. The capsid protein appears to be important in the assembly of immature capsid [83]. The nucleocapsid protein has a sequence that includes many basic amino acids and a cysteine-histidine box that is similar to a zinc finger domain seen in DNA-binding proteins, which explain its packaging role of the viral RNA in the cytoplasm [84].
The gag-pro mRNA is translated as a precursor protein Pr110, a Gag-Pro polyprotein. Within Pr110, the NC sequence of the gag gene and the first part of the pro gene comprise a dUTPase gene (DU gene). DU prevents misincorporation of uracil and mutation of newly synthesized proviruses in nondividing cells [85]. The C-terminus end of the pro gene codes for the viral protease p13 or PR. PR is responsible for the cleavage events that produce the mature virion proteins, MA, p21, p3, p8, CA, and NC. The functions of p21, p3, and p8 are currently unknown [86]. The precursor protein Pr160, a Gag-Pro-Pol polyprotein encodes a viral reverse transcriptase with RNase H activity and an integrase [87].
The env mRNA is translated into a precursor protein, pr73-env, on membranes bounded by polyribosomes. This precursor is modified by glycosylation in both the endoplasmic reticulum and the Golgi, where a cellular protease cleaves the env poly protein to produce gp52 that is a viral surface envelope protein also known as SU and gp36 that is a viral transmembrane envelope protein also known
34
as TM. SU and TM remain attached to each other via disulfide bonds [88]. They travel to the host plasma membrane in vescicles and are then incorporated into the lipid envelope of the mature B type virion when the immature A particle buds from the host cell [89].
The Sag mRNA is translated into a precursor protein Pr48, Sag, a superantigen protein. This protein is glycosylated and cleaved in the Golgi by protease to generate the Sag protein [90]. Sag is associated with the class II Major Histocompatibility Complex (MHC) proteins at the surface of antigen-presenting cells. Sag is essential for the activation of certain T cells subsets containing Vβ TcR [91].
MMTV utilizes a polyprotein strategy for viral assembly. This process insures that none of the individual matrix, capsid, and nucleocapsid proteins, are individually targeted to the membrane. The order of the proteins in the polyproteins corresponds to their relative location in the virion from the outside of the virus to the inside of the virus.
MMTV polyproteins assemble into a capsid shell or immature A particle in the cytoplasm. The formation of the A particle occurs prior to its transport to the plasma membrane [89], then the immature A particles bud from the tips of long filamentous actin projections as mature virions [92]. The viral envelope proteins are transported to the plasma membrane via the host secretory pathway.
The A particles associate with the cell membrane, acquire a viral lipid envelope with viral envelope proteins as the virus buds from the host cell [93]. The budding process of MMTV seems to involve the host actin cytoskeleton. The immature viral A particles have been observed at the tips of long filamentous projections [89]. In addition, cytochalasin D, which disrupts the actin cytoskeleton, reduced the number of mature viral B particles released into the supernatant by 80% [94]. Thus budding of MMTV appears to be an actin-dependent process. Infected human breast cells with MMTV initiated the formation of filamentous projections with virus particles at their tips. These filopodial projections emerged from the cell surface of infected live cells when viral production was hormonally induced with dexamethasone [95].
35
3.11 Human endogenous retrovirus
Human Endogenous Retroviral (HERV) elements comprise 8% of human DNA [96], and are likely to be derived from ancient viral infections during evolution but their biological relevance is largely unknown.
It is thought that exogenous infection allowing HERVs sequences to be inserted into the genome of germ line cells, where they have been replicated along with the genes of the host cell following a Mendelian pattern [97, 98]. Integration of endogenous retroviruses into the human germ line is thought to have occurred 2 to about 70 million years ago depending on the individual retroviruses and were introduced by mechanisms involving reverse transcription. Most of the Human Endogenous Retroviruses (HERVs) are defective, due to multiple mutations or deletions, and therefore none of them is capable of encoding complete viral particles and cause infection [99]. Although many HERVs are transcriptional active, they are not able to produce functional proteins. The high number of copies present in the human genome is attributed to repeated cycles of infection and retrotransposition [100].
3.12 Classification
The known HERVs families have been grouped into classes.
HERV class I shows clustering phylogenetically with Gammaretrovirus, those clustering with the betaretrovirus are class II, and class III HERV clustering with spumaviruses [101].
HERV families that reside in human genome are estimated to be between 30 and 50 [102], among them various so-called HERV-K families. The letter K indicates that a primer binding site specific for lysine-tRNA was used to prime reverse transcription. In total, 10 HERV-K families have been defined based on sequence similarities, and they have been named HERV-K1 (HML-1) to HERV-K10 (HML-10) (for human MMTV [mouse mammary tumor virus] like) because of some sequence relationship to the mouse mammary tumor virus [103].
3.13 The biological function of HERV
36
genome of mammals, suggests that they have important biological functions and therefore they have been conserved during evolution. Using reverse transcription and subsequent insertion of their genome into the host DNA, these sequences regulate the plasticity of the genome, accelerating the evolution of new genes and altering the transcription of existing genes [104].
Their biological function is still unknown and there are many assumptions that, to date, are proposed. In analogy with the animal model, it has been hypothesized that these endogenous retroviral sequences are protected in respect of their endogenous counterparts. In the case of MMTV, for example, the expression of the Sag endogenous coding sequences during the formation of the immune system, induces clonal deletion of all T cells reactive to that particular Sag and at the same time, makes it immune to the forms of exogenous MMTV coding Sag with the same specificity to T cells [50, 105].
There are several experimental evidences that show the possibility of recombination between exogenous viruses and endogenous retroviral sequences. It has been identified a variant of MMTV, highly tumorigenic, resulting from recombination between sequences derived from endogenous Mtv, and a strain of exogenous MMTV [62].
3.14 HERV-K family
The majority of HERV, the family of HERV-K genes has an open reading frame (ORF) for retroviral essential genes of retroviruses (gag, pol and env) as well as its ability to synthesize all essential retroviral proteins [106, 107]. This family was originally identified for its high homology with the Mouse Mammary Tumor Virus (hence the nomenclature further HML, human endogenous MMTV-like). The HERV-K are present in the human genome with about 30-50 proviral copies and approximately 10,000 LTRs solitary, probably arising by homologous recombination between LTRs, followed by excision of the intermediate viral genome [105].
HERV-K families have been classified into six subgroups (HML by HML-1 to-6), based on the sequence homology in the conserved regions of the pol gene, encoding the enzyme reverse transcriptase [108, 109]. HERV-K10 has been
37
sequenced and showed to have a complete provirus of 9 Kb, and contains ORFs for all retroviral genes, but showed to have a stop codon in the env gene and a frameshift in the gene gag [110].
38
4. AIM
On the basis of the previous results from our laboratory and of the data published from other research groups worldwide about a possible involvement of HMTV in human breast cancer, in the present study we decided to have a wider vision of the topic by approaching it from different angles.
4.1 HMTV involvement in Hereditary and Sporadic breast cancers
The long-standing familiarity of our laboratory with the molecular aspects of hereditary breast carcinomas offered the opportunity for a theoretical approach at the problem of the meaning of HMTV env sequence (HMTVes) in human breast cancer. Inherited mutations of BRCA1 and BRCA2 tumor suppressor genes account for the largest part of the cases and are recognized as the pathological event able to initiate the neoplastic process in human mammary gland, representing the first “hit” according to Knudson’s hypothesis. The presence of a known etiological molecular event in HBC suggests that a hypothetical etiological viral agent should not play a role in this group of tumors or at most, only occasionally it could act as a possible second “hit” in a small number of cases. With the aim to prove this hypothesis, the presence of HMTVes was investigated in a group of infiltrating ductal Hereditary Breast Carcinomas (HBC) from patients hosting a BRCA1 or BRCA2 mutation, enrolled in the Center for Hereditary Tumors of Pisa University Hospital, as well as in a group of infiltrating SBC.
4.2 Geographical distribution of HMTV
In human breast cancer literature was reported that human breast cancer incidence is higher in the geographical areas where a specific mouse species Mus
Domesticus, is the prevalent one. These areas include West Europe, North Africa,
Australia, South and North America.
To assess the frequency of detection of the viral sequence in different and geographically distant countries, we have conducted an epidemiological study on
39
sporadic breast cancer (SBC) samples collected in three areas such as Sardinia, Jordan and Australia.
In the last population, when possible, for each SBC case we have collected also a normal and pre-invasive lesion in order to assess the presence of the virus before cancer onset.
4.3 Virus detection in lymph nodes of HMTVes+ and HMTVes- breast cancers
Because the infiltrative carcinoma of the breast initially spreads via lymphatics giving metastasis firstly to the axillary and internal mammary lymph nodes, it was interesting to analyze regional lymph nodes of HMTV env positive and negative (HMTVes+ and -) tumors. The detection of HMTVes was assessed in the tumoral epithelial cell population and in the lymphocytes from each metastatic lymph node.
4.4 Gene expression analysis
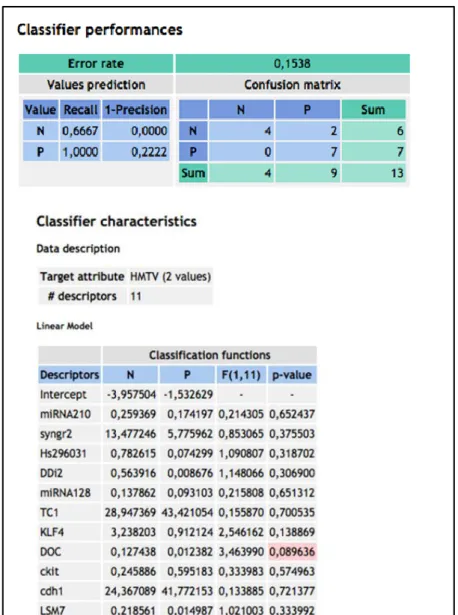
Assuming that SBC HMTVes+ and – tumors and matched normal tissues could have a different genetic background, we have used specific breast cancer arrays to evaluate the expression profiles of 84 key genes commonly involved in the dysregulation of signal transduction and in other biological processes during breast carcinogenesis as well as a panel of selected genes previously used in our laboratory to distinguish among benign and malignant thyroid tumors that include genes also involved in breast cancerogenesis as KLF4, cKIT, SYNGR2, TC1, CDH1 as well as miRNA128 and miRNA210.
Due to the reduced number of samples used, each single gene of the panel has been analysed both in HMTVes+ and HMTVes- tumors as well as in combination with the normal HMTVes+ and HMTVes- samples.
4.5 HMTVes detection in saliva and salivary glands
Looking for possible interhuman routes of HMTV transmission, the fact that several viruses in humans, including the ones involved in human cancers [114], can be transmitted through saliva and on the basis that in the murine disease
40
MMTV is present in all exocrine glands, we have been prompted to test if HMTV could follow this transmission route. Saliva can contain a range of infectious agents and, despite several antimicrobial mechanisms, transmission of these can occur. Therefore several saliva samples from different groups of individuals, both healthy and not, as well as salivary glands were analysed for the presence of the viral sequence.
4.6 Primary cultures of human breast cancer
In order to isolate the virus, fresh human breast cancer tissues collected from the surgery-room were used to create HMTVes+ breast cancer cell primary cultures.
4.7 FISH and Electron Microscopy analyses
FISH and Electron Microscopy analyses were performed to confirm the presence of the virus in the primary breast cancer cell line resulted HMTVes+.
4.8 Immunocytochemistry and Western Blot analyses
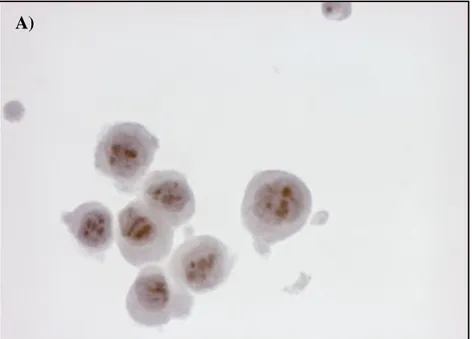
To further highlight the presence of HMTV in human breast cancer, the detection of the p14 and gp52 viral proteins was performed by Immunocytochemistry and Western Blot analyses on the HMTVes+ primary breast cancer cell line using antibodies anti viral-protein kindly borrowed from Beatriz G T Pogo, Professor of Microbiology at Mount Sinai School of Medicine, Division of Hematology and Medical Oncology, NY, USA and from Jacob Hochman, Professor of Cell Biology, Institute of Life Sciences, The Hebrew University of Jerusalem, Israel. HMTVes- MCF7 cell lines were used as negative control.
41
5. MATERIALS AND METHODS
5.1 Formalin fixed and paraffin embedded sample collection
1. 47 cases of infiltrating ductal Hereditary Breast Carcinoma (HBC), 25 of them were from patients hosting a BRCA1 gene mutation and 22 from patients hosting a BRCA2 gene mutation, and 56 cases of infiltrating ductal Sporadic Breast Carcinoma (SBC), were collected at the Division of Surgical, Molecular and Ultrastructural Pathology – University of Pisa.
2. 80 Jordan SBC, including 62 Infiltrating Ductal Carcinomas (IDC) and 18 Ductal Carcinomas In Situ (DCIS), were collected at the King Addullah University Hospital (KAUH), at the King Hussein Cancer Center (KHCC) and at the Basma Hospital (BA); 42 Sardinia infiltrating ductal SBC and 12 Sardinia liver cases from patients affected by biliary cirrhosis and chronic hepatitis, used as negative controls, were collected at the “S. Giovanni di Dio”, Cagliari Hospital; 53 Australian cases, from 23 patients, including 23 normal, 12 DCIS and 18 IDC lesions were collected at the Albury-Wodonga Hospital.
3. 12 and 15 cases of non-metastatic and metastatic lymph nodes from 27 HMTVes+ breast cancers and 10 and 6 cases of non-metastatic and metastatic lymph nodes from 16 HMTVes- breast cancers, were collected at the Division of Surgical, Molecular and Ultrastructural Pathology – University of Pisa. 4. 4 normal (adjacent to the breast cancer) HMTVes- breast samples, 4
infiltrating HMTVes- breast cancer samples, 4 normal (adjacent to breast cancer) HMTVes+ breast samples and 4 infiltrating HMTVes+ breast cancer samples, were collected at the Division of Surgical, Molecular and Ultrastructural Pathology – University of Pisa.
5. 40 parotid glands were collected at the San Rossore Clinic and at the Division of Surgical, Molecular and Ultrastructural Pathology – University of Pisa.
5.2 Fresh tissue sample collection
1. 5 samples of fresh human infiltrating ductal breast carcinoma were collected at the Breast Unit of the University Hospital of Pisa and tumoral
42
pieces were selected by an experienced pathologist of the Division of Surgical, Molecular and Ultrastructural Pathology – University of Pisa. 2. Saliva samples from: 17 newborn babies, 30 pediatric patients (3 month-14
years age range), 132 healthy donors, 56 women with breast cancer were collected in sterile tubes at the Neonatological, Pediatric, Transfusional and Senological Unit respectively.
5.3 Laser microdissection
A Leica ASLMD automatic laser microdissector was used to select the epithelial cell population to be studied (Figure 12). Two µm thick sections were cut from each case using each time a new microtome blade, and applied on microdissecting slide, followed by staining with haematoxylin and eosin, then obtaining a total of 10,000–15,000 cells (figure 13). Stromal and inflammatory cells were carefully excluded. Due to the long experience of the laboratory with this method, no difficulty was found in selecting areas of interest.
43
Figure 13: Laser capture microdissection, A) before applying microdissection,
B) after applying microdissection.
5.4 DNA extraction from microdissected tissue
Microdissected cells were suspended in buffer containing 10 mM Tris-HCl, 1 mM ethylenediamine tetraacetic acid (EDTA), 1% Tween 20, and 0.1 mg/ml proteinase K, pH 8.0, and incubated overnight at 56°C. Higher concentrations of proteinase K have been reported to improve the quality of DNA recovered from fixed tissue sections. The sample was subsequently heated at 95°C for 10 minutes to inactivate the proteinase K and the DNA was ready for PCR analysis. To avoid cross-contamination, blank DNA samples (lysis buffer with proteinase K) were processed in parallel with the tissue samples.
5.5 DNA extraction from fresh tissue and FFPE
DNA extraction from fresh human breast cancer sample and from saliva was performed following the manufacturing instructions of the Genomic DNA from Tissue (Macherey-Nagel):
1. Cut 25 mg human tissue into small pieces or cut 2 sections of 10 µm thick. Using sterile forceps and blade for each sample, place it in sterile 1.5ml eppendorf tube.
2. Add 180 µl Buffer T1 and 25 µl proteinase K solution and mix by vortexing.
3. Incubate overnight at 56° C.
4. Vortex the samples. Add 200 µl Buffer B3. Vortex vigorously and incubate at 70°C for 10 minutes. Vortex briefly.
5. Add 210 µl ethanol (96-100%) to the sample and vortex vigorously.
6. Apply the sample to the NucleoSpin Tissue column. Centrifuge for 1 min
44
at11000 x g. Discard the flow-through and place the column back into the Collection Tube.
7. Add 500 µl Buffer BW. Centrifuge for 1 min at 11000 x g. Discard the flow-through and place the column back into the Collection Tube.
8. Add 600 µl Buffer B5 to the column and centrifuge for 1 min at 11,000 x g. Discard the flow-through and place the column back into the Collection Tube.
9. Centrifuge the column for 1 min at 11,000 x g.
10. Place the NucleoSpin Tissue Column into a 1.5 ml microcentrifuge tube and add 100 µl prewarmed Buffer BE (70°C). Incubate at room temperature for 1 min. Centrifuge 1 min at 11,000 x g.
5.6 DNA extraction from cell culture
DNA extraction from cultured cells was performed following the manufacturing instructions of the Genomic DNA from Tissue (Macherey-Nagel):
1. Resuspend up to 107 cells in a final volume of 200 µl Buffer T1. Add 25 µl proteinase K solution and 200 µl Buffer B3. Incubate the sample at 70°C for 10-15 min.
2. Add 210 µl ethanol (96-100%) to the sample and vortex vigorously.
3. Apply the sample to the NucleoSpin Tissue column. Centrifuge for 1 min at11000 x g. Discard the flow-through and place the column back into the Collection Tube.
4. Add 500 µl Buffer BW. Centrifuge for 1 min at 11000 x g. Discard the flow-through and place the column back into the Collection Tube.
5. Add 600 µl Buffer B5 to the column and centrifuge for 1 min at 11,000 x g. Discard the flow-through and place the column back into the Collection Tube.
6. Centrifuge the column for 1 min at 11,000 x g.
7. Place the NucleoSpin Tissue Column into a 1.5 ml microcentrifuge tube and add 100 µl prewarmed Buffer BE (70°C). Incubate at room temperature for 1 min. Centrifuge 1 min at 11,000 x g.