Development of the Central Nervous System
The vertebrate Central Nervous System (CNS) is one of the most complex known structures; it’s results from a series of cell-cell interactions in which groups of cells induce their neighbors to acquire new differentation fates (embryonic induction process) (Lewis., 1904).
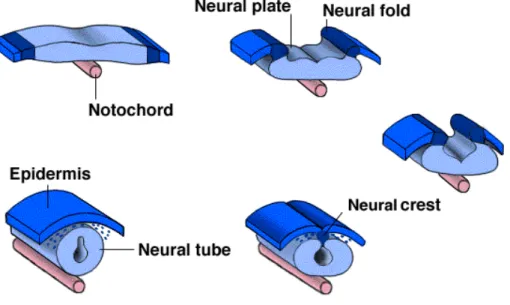
CNS development starts earlier at blastula stage when a portion of dorsal ectoderm is induced by the underlying chordomesoderm signals to become neural ectoderm and its cells become distinguishable by their columnar appearance Fig.1 (Holtfreter 1933; Speaman 1938; Nieukoop and Koster 1995; keller et al., 1992). This region of the embryo is called the neural plate and the process by which this tissue forms a nural tube, the rudiment of the CNS, is called neurulation (Gilbert 2003).
Neural induction requires the combined activity of two distinct signaling centers. One is the well-known Nieuwkoop center, located in dorsal-vegetal cells, which expresses Nodal-related endomesodermal inducers. The other is a blastula Chordin- and Noggin-expressing (BCNE) center located in dorsal animal cells that contains both prospective neuroectoderm and Spemann organizer precursor cells. Both centers are downstream of the early β-Catenin signal (E.M. De Robertis et al., 2004). Cell lineage studies demonstrated that BCNE cells give rise to a large part of the brain and retina and, in more posterior regions of the embryo, to floor plate and notochord.
After neural plate induction, the edges thicken and move upward to form the neural folds, while a U-shaped neural groove appears in the center of the plate, dividing the future right and left sides of the embryo (Balinsky and Fabian, 1981) (Fig.1).
The differentiation of the neural tube into various regions occurs simultaneously in three different ways. On the gross anatomical level along the antero-posterior and dorsal-ventral axes by means of specific regional espression of several “patterning genes”, (Lupo et al., 2002; Wilson and Rubenstein, 2000), where the neural tube and its lumen bulge and constrict to form the chambers of the brain and spinal cord. At the tissue level, the cell populations within the wall of the neural tube rearrange themselves to form the different functional regions of the brain and the spinal cord. Finally, on the cellular level, where proliferative processes and apoptosis control the cell population, the neuroepithelial cells themselves differentiate into numerous types of nerve cells (neurons) and supportive cells (glia) present in the body (McConnell and Kaznowski, 1991).
The anterior-posterior axis
The early neural tube is a straight structure. However, even before the posterior portion of the tube is formed, the most anterior part is undergoing drastic changes. In this region, the neural tube balloons into the three primary vesicles: forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon).
By the time the posterior end of the neural tube closes, secondary bulges – the optic vesicles – have extended laterally from each side of the developing forebrain (Fig.2). The prosencephalon becomes subdivided into the anterior telencephalon and the more caudal diencephalon. The telencephalon will eventually form the celebral hemispheres, and the diencephalon will form the thalamic and hypothalamic brain regions, which receive neural imput from the retina. Indeed the retina itself is a derivative of the diencephalon. The mesencephalon does not become subdivided, and its lumen eventually becomes the cerebral aqueduct. The rhombencephalon becomes subdivided into a posterior myelencephalon and more anterior metencephalon. The myelencephalon eventually becomes the medulla oblongata, whose neurons generate the nerve centers responsible for pain relay to the head and neck, auditory connection, tongue movement, and balance control, as well as respiratory and cardiovascular movements. The metencephalon gives rise to the cerebellum, the part of the brain responsible for coordinating movements, posture, and balance.
The rhomboencephalon develops a segmental pattern that specifies the places where certain nerves originate.
Fig. 1. Vertebrate Neural Tube formation (neurulation; Lewis Wolpert., 1986)
The Neural Retina is a part of the Central Nervous System
A wide range of neuronal and glial cell types is generated from a pool of multipotent progenitor cells during development in both vertebrates and invertebrates. Progenitor cells, defined here as dividing cells, of the CNS generate neurons in an order that is typically conserved for a given area of the CNS. A second property of CNS progenitor cells is their ability to integrate extracellular signals with their intrinsically defined cellular state to decide, or at least partially direct, the fates of their progeny. In addition, the cell fate decisions made by progenitor cells differ at different developmental stages in the same tissue and also between different parts of the nervous system. Therefore, for example, retinal progenitor cells make certain cell types early in development, a different set of cell types late in development, and a completely different set of cell types compared to those found in other parts of the nervous system. The production of different cell types at different times appears to derive from differences in the intrinsic properties of progenitor cells, referred to as their competence to make different cell types, and provides the basis for our current model of retina development (Liversey and Cepko, 2004).
The peculiarity of the neural retina is to condense a really considerable structural and functional complessity in a small area. In this purpose the neural retina is a suitable model to study in vivo the CNS development, because it is an simple accessible part of it and it form a relatively easy structure if compared to other neural derived fields (Cepko et al., 1996).
In fact, its relative accessibility to embriological, genetic and molecular manipulations make the neural retina a powerful tool to investigate how cellular diversity in the central nervous system is achieved.
Particularly, in these last years scientists focused their attention on the molecular mechanisms underlying proliferation, survival, differentiation and neural retinal precursor placement.
The Vertebrate Neural Retina
The Vertebrate neural retina is a complex structure coming from different parts of the embryo such as ectoderm, anterior neuroectoderm, and neural crest cells.
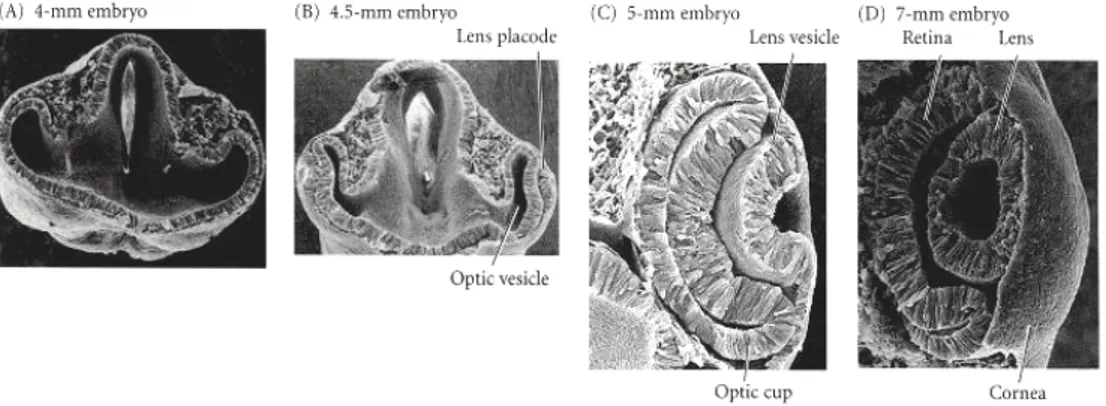
The first morphological signal of eye development in vertebrates is the appearance of a bilateral evagination of the diencephalon; in mammals, this is marked by the appearance
of the optic pit, while in fish and amphibians a bulging of the optic primordia occurs. Upon continuous evagination of the optic primordia, two optic vesicles are generated which extend towards the overlying ectoderm, which will ultimately give rise to the lens and the cornea. The optic vesicle invaginates and gives rise to a double layered optic cup: the inner layer (facing the lens) will give rise to the neural retina, while the outer layer will differentiate into the retinal pigmented epithelium (reviewed in Chow and Lang, 2001; Fig. 3).
The retinal progenitors in the inner layer of the optic cup are proliferating and share an uniform morphology; they are initially arranged as a pseudostratified neuroepithelium, whereby cells contact both surfaces of the layer. This layer is apposed at its outer (scleral) surface to the retinal pigmented epithelium (RPE), but remains separated by the potential space of the obliterated neural tube lumen (optic ventricle). The nuclei of progenitors undergo S-phase distal to the neural tube lumen but enter M-phase at the luminal surface. During mitosis retinal progenitors likely retain their basal process, divide and subsequently re-establish contact with both sides of the layer (Cayouette and Raff., 2003).
It is interesting to note that during retinal progenitor proliferation there is a change of cell cycle length and all studies of cell cycle timing in the retina are in accordance with the fact that it slows during development (Rapaport, 2006; Decembrini et al., 2006). During histogenesis the retinal progenitors division mode changes.
Early progenitors go through a period of symmetrical divisions during which each daughter reenters the cell cycle. This mode allows the pool of progenitors to expand exponentially. Later, retinal progenitors divide asymmetrically, one daughter returning to the cell cycle, the other exiting, migrating and differentiating. At some late stage, progenitors go through a terminal symmetrical division in which both daughters become post-mitotic and differentiate in the correct retinal cell types (Rapaport, 2006).
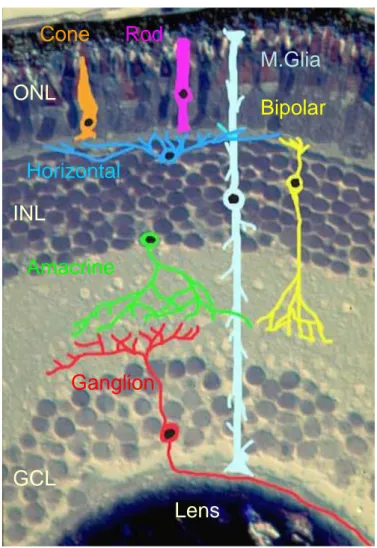
The mature neural retina is a laminar network composed of specialized sensory neurons (rods and cones), interneurons (amacrine, bipolar and horizontal cells) and projection neurons (the ganglion cells), intermingled with Müller glia cells. Of these six major classes of neurons, some can be subdivided into several subtypes, which differ from each other in morphology, physiological properties and functions (Wassle and Boycott, 1991; Vaney, 2002). The retinal neurons are organized in three nuclear layers: ganglion cells and some displaced amacrine cells are located in the ganglion cell layer (GCL, closer to the lens); the interneurons are located in the inner nuclear layer (INL); finally
the photoreceptors are located in the outer nuclear layer (ONL), adjacent to the pigmented epithelium. In addition, in the mammalian retina, a small number of ganglion cells are displaced in the inner nuclear layer (Drager and Olsen, 1981). Between the nuclear layers are two plexiform layers (IPL) (Fawcett et al., 1994).
Within the plane of each retinal layer, cells are distributed in a non-random fashion and, as for the case of photoreceptors, they form very regular patterns (Fig.4).
The visual stimuli are detected by the photoreceptors and are transmitted via the bipolar interneurons to the ganglion cells, which relay the signals to the brain (Wassel and Boycott, 1991). Further elaboration of visual input is due to the horizontal and amacrine cells (Wassel and Boycott, 1991; Taylor et al., 2000) forming specialized subcircuits, working together in parallel to process different features of the visual image. For example, rods are sensitive to low-light levels and a rod-driven circuit exists for visualizing objects under dim light conditions. In most vertebrates, this circuit involves connections among rod photoreceptors, rod bipolar cells and a specialized type of amacrine cells, the AII amacrine cells connecting to ganglion cells (Strettoi et al., 1990). On the other hand, under high light level conditions, cones work involving two vertical pathways, giving also a chromatic information of the visual stimulus. Cones contact a variety of cone bipolar cells, some of which are depolarized (ON) and others hyperpolarized (OFF) by increased illumination. ON and OFF-cone bipolar cells contact ganglion cells, which respond to changes in illumination according to their bipolar input. Together, the ON and OFF pathways provide contrast information. In addition to these basic features, the retina has also specialized circuits that can compute other features of the visual scene, such as the direction of motion and orientation of edges (Wong, 2006).
Despite the differences among each vertebrate class, two common features are shared by the newly generated neurons during development of the retina. First, as shown by birthdating analyses, the seven major retinal cell types are generated in an extremely conserved histogenetic order, with ganglion cells born first and Müller cells born last (Cepko et al., 1996). Second, as demonstrated by means of lineage tracing analyses and cell ablation studies, retinal progenitors are multipotent at the different developmental stages, as the progeny derived from a single precursor cell comprises different cell types (from one to six). Even two-cell clones are constituted by cells adopting different fates, suggesting that the cell fate choice can be made during or after the last mitotic division (Holt et al., 1988; Wetts and Fraser, 1988; Turner et al., 1990). So, retinal histogenesis
follows a precise and evolutionarily conserved order which suggests the conservation of the underlying molecular mechanisms among the Vertebrates. Moreover, progenitors are multipotent and their differentiation is influenced by extrinsic cues.
Despite their being multipotent, progenitors are also heterogeneous: early and late progenitors display distinct differentiative capacities when placed in similar environments and, moreover, they show different gene expression profiles as development proceeds. In this respect, progenitors can be subdivided at least into two distinct subpopulations on the basis of the expression of distinct markers, like VC1.1 and sintaxin (Alexiades and Cepko, 1997). VC1.1 epitope and syntaxin are two markers of amacrine and horizontal cells; they were found to be expressed in a subset of progenitors in a temporally regulated manner that closely paralleled the birthdays of these cell types. Early in retinal development, VC1.1+ progenitors generated a high percentage of amacrine and horizontal cells, but no cone photoreceptors. During this same period, a comparable number of cone photoreceptors were generated by VC1.1- progenitors. In the late embryonic and early postnatal period, VC1.1+ progenitors continued to generate
predominantly amacrine cells, but also gave rise to an increasing number of rod photoreceptors. These findings demonstrate that expression of these two markers by progenitors is highly correlated with a bias towards the production of amacrine and horizontal cells.
The data from lineage studies indicate that amacrine cells do not derive from a progenitor committed to making only amacrine cells.
Fig. 3. Development of theVertebrate eye.
The optic vesicle evaginates from the brain and contacts the overlying ectoderm (A). The overlying ectoderm differentiates into lens cells as the optic vesicle folds in on itself (B). The optic vesicle becomes the neural and pigmented retina (C;Lewis Wolpert., 1986)
Fig. 4. Structure of the Mammalian Retina.
Cross-sectional microscopic drawing of the nerve cells in the retina made by Santiago Ramon y Cajal (1900). http://hubel.med.harvard.edu/12.jpg
Xenopus laevis as retinal model system
The model system used in this thesis work is the well-characterized clawed frog Xenopus laevis (Nieuwkoop and Faber, 1956).
The advantages of using this gentle African frog as an experimental model come from the ability to easily obtain embryos at different stages. The females, indeed, can be induced to ovulate at any time of the year by means of a simple hormone injection. Usually, from 1000 to 1500 eggs are laid each time, which are easily fertilized in vitro using testis homogenates. The embryos can easily grow in a Petri dish in simple saline solutions; moreover, they are “large” and can be microinjected and micromanipulated with relative ease. In addition, development is rapid: it takes about three days from fertilization to reach the tadpole stage (st. 42), at which retina development and in generalorganogenesis, is completed.
During the past several years, many new techniques have been devised or adapted for Xenopus. For example, in situ hybridisation or immunocytochemistry, which allow to visualize gene or protein expression domains in the whole embryo or on sections. Analysis of gene function can be performed by means of two complementary approaches, in both gain-of-function and loss-of-function experiments. Inducible gene expression systems or stage-specific transfection of constructs, by means of the lipofection technique, allow to control timing of gene expression in the gain-of-function assays; whereas dominant negative proteins (for example dominant negative ligands or transcription factors), or antisense oligo “morpholino” technology, prove to be useful tools to inactivate gene function.
Moreover, the large size of the embryos, the ability of the explanted tissues to survive without requirement for added nutrients and the availability of detailed fate maps make Xenopus a very interesting model for studies of lineage commitment or induction.
Anyway, despite these many advantages compared to mammalian or bird model organisms, Xenopus laevis shows some features, like a long generation time (1-2 years) and tetraploidy, which are an obstacle for functional genomic studies or transgenesis. Nevertheless, these difficulties may be overcome with the diploid Xenopus tropicalis. The Xenopus retina (Fig. 5):
Photoreceptors. Rod photoreceptors represent 53% of total photoreceptors (Wilhelm and Gabriel, 1999). Rods are rather uniform regarding their morphology, although a minor population (2-3%) of blue-sensitive rods, with a thinner outer segment, has been
described on the basis of its visual pigment content (Witkovsky et al., 1981). Cones have also been classified on the basis of their visual pigment content and can be divided into three types: miniature, ultraviolet-wavelength-sensitive (UWS) (4% of all cones); large, shortwavelength- sensitive (SWS) (10%); and large, long-wavelength-sensitive (LWS) cones (86%) (Rohlich et al., 1989).
Horizontal cells. The Xenopus retina differs from the pattern in mammals, in which horizontal cell dendrites contact only cones, while the axon terminal contacts rods (Steinberg, 1969; reviewed in Wassle and Boycott, 1991). There are two types of horizontal cells in Xenopus retina (Stephan and Weiler, 1981). In the axon-bearing cell of the luminosity type (i.e. lights of any spectral composition elicit a hyperpolarization), both dendrites arise in the cell body and axonal branches contact both rods and cones. The other horizontal cell type lacks an axon and is of the chromatic type (depolarized by red light, hyperpolarized by blue light) (Stone et al., 1990). Its dendrites contact short-wavelength sensitive cones and rods (Witkovsky et al., 1995).
Bipolar cells. The main point in the structure of the lower vertebrates is that retinal circuits did not evolve separate rod-dedicated and cone-dedicated pathways. So, bipolar cells receive direct rod and cone inputs and specific rod bipolar cells do not exist. In intermediate light condition (mesopic state), when both cones and rods are active, it is probably disadvantageous for an animal to receive double information concerning the same object (due to, for instance, the different latency of the two kinds of photoreceptors). However, the mutual antagonism between rod and cone signals decreases the magnitude of this problem; this mechanism of mutual inhibition involves the neuromodulator dopamine, synthesized by the dopaminergic amacrine cells (Krizaj et al., 2000).
Amacrine cells. Sporadic attempts to note down the cell types by their morphology have been made by early researchers, particularly by Cajal (1892). He distinguished 13 amacrine cell types, mostly on the basis of ramification pattern in the IPL. In the Anuran retina, the majority of the amacrine cells uses GABA and glycine as neurotransmitters (Voaden, 1974). Moreover, dopaminergic cells are present at low density, representing about 0.5% of the total amacrine cell number. In addition, in the Xenopus retina other amacrine cell types are present, identified bydifferent markers: serotonin-immunoreactive, nitric-oxide producing cells, neuropeptide Y, substance P, somatostatin and colecystokinin immunoreactive (reviewed in Vigh et al., 2000). The importance of diverse amacrine populations in retinal information processing can be
explained by the relative simplicity of the brain visual system in lower vertebrates (Vigh et al., 2000).
Ganglion cells. It can be cautiously considered the presence of 12 types of ganglion cells in the frog retina. There has been a long-standing debate on the existence of direct bipolar to ganglion cell contacts in the lower vertebrate retina. Definite bipolar to ganglion cell synapses have been identified in Xenopus laevis (Buzas et al., 1996), representing about 10% of all ganglion cell inputs in the IPL. This fact can be explained by considering that there is a higher divergence of bipolar cell output to amacrine cells in frog than in mammals (reviewed in Gabriel et al., 2000).
Retinal pigment epithelium. The RPE is juxtaposed to the neural retina; it is composed of a single layer of hexagonal cells that are densely packed with pigment granules. When viewed from the outer surface, these cells are smooth and hexagonal in shape. When seen in section, each cell consists of an outer non-pigmented part containing a large oval nucleus and an inner pigmented portion which extends as a series of straight thread-like processes between the photoreceptors.
The RPE in adulthood essentially acts soaking spurious visible radiations, is involved in the phagocytosis of the outer segment of photoreceptor cells and it is also involved in the vitamin A cycle where it isomerizes all trans retinol to 11-cis retinal.
The retinal pigment eptihelium also serves as the limiting transport factor that maintains the retinal environment by supplying small molecules such as amino acids, ascorbic acid and D-glucose while remaining a tight barrier to choroidal blood borne substances. Homeostasis of the ionic environment is maintained by a delicate transport exchange system. During development its function consists in driving neural retina morphogenesis and successively in substaining the luminar organizzation ( Raymond and Jackson, 1995)
Fig. 5. The Xenopus neural retina (Poggi et al., 2005)
Cone
Rod
M.Glia
Bipolar
Horizontal
Amacrine
Ganglion
ONL
INL
GCL
Lens
The Ciliary marginal zone (CMZ): a recapitulation of embryonic retinogenesis
A peculiarity of the amphibian and fish retina is that its peripheral portion, termed ciliary marginal zone (CMZ), is a pseudo-stratified neuroepithelium from which retinal precursors differentiate, allowing growth of the retina throughout the whole life of the animal (Wetts and Fraser, 1988)(Fig. 6).
Bromodeoxyuridine (BrdU) or tritiated-thymidine, that are compounds able to identifiy DNA in mitotic cells, highlight a ring of marked cells, suggesting a migration of new borned cells starting from the CMZ which are added into the mature retina. In fact after the embryonic phase of retinogenesis, new cells are added to the central retina from this peculiar proliferative region (Wetts et al., 1989).
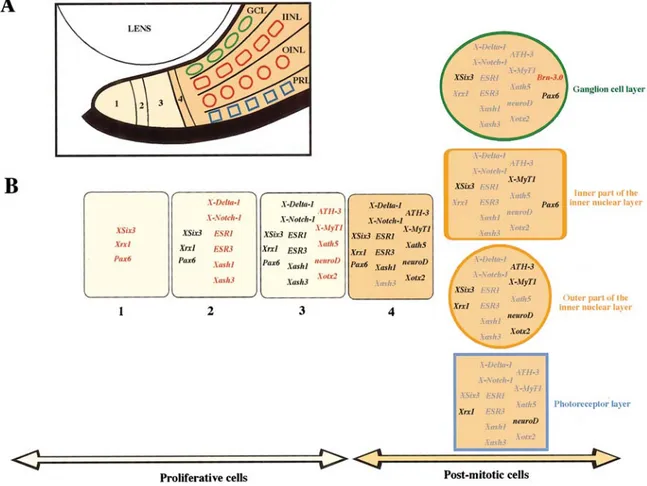
The main feature of this region is that the retinoblasts are ordered along the CMZ, from its peripheral edge towards the centre, according to their grade of commitment; stem cells are located in the most peripheral region of the ciliary margin, post-mitotic precursors are adjacent to the central retina, and proliferating neuroblasts are distributed between these two regions. This spatial gradient in the CMZ recapitulates embryonic retinogenesis and provides a powerful system to examine the relative order of gene expression during this process. A number of neurogenic and proneural genes have been described to have interacting roles in the development of the vertebrate nervous system, and so it is of major importance to put these genes in a hierarchical pathway (Perron et al., 1998).
Therefore, because each set of precursors in each state of commitment, is characterized by the expression of an unique combination of genes, the consequence of such a defined spatial distribution of the precursors is that the CMZ recapitulates spatially the temporal sequence of gene expression during retinogenesis (Wetts and Fraser, 1988; Perronet al., 1998).
Zone 1 the most peripheral part of the CMZ is composed by stem cells. Cells in this
zone do not express any of the neurogenic or proneural genes but do express Xrx1, XSix3, and a low level of Pax6. One might imagine that these genes are important for giving the stem cells a retinal identity. Zone 2 includes the neurogenic genes X-Notch-1, X-Delta-1, ESR1, ESR3 and proneural genes such as Xash1 e Xash3. Since expression of the ESR genes might be indicative of functional inhibition through the neurogenic pathway, the coordinated expression of all these neurogenic genes throughout this zone implies that the cells here are involved in mutual inhibition, rather than directional
inhibition in which there would be distinct subdomains of X-Notch-1 and X-Delta-1 expression. Zone 3 comprises the proneural genes homologs of the “atonal “ complex in Drosophila: ATH-3, Xath5, neuroD, and X-MyT1 are turned on. neuroD is a bHLH protein that has been identified as a neuronal differentiation factor (Lee et al., 1995). These genes are normally espressed also during embryogenesis after Xash (Harris and Perron, 1998). Finally Zone 4 where the cells in the CMZ stop dividing. Differentiation of neurons will occur only after neurogenic genes are turned off and some proneural genes maintained, which is consistent with the model that cells must escape the neurogenic signaling to be able to proceed to the next developmental step. In addition, together with the neurogenic genes, some other proneural genes are also turned off, depending on the neural layer where cells will differentiate. Among the transcription factors we studied, only Brn-3.0 is expressed solely in the central retina in stage 40 Xenopus retina. Brn-3.0 is a member of the Brn-3 family of POU domain transcription factors. It is expressed in retina ganglion cells in Xenopus (Hirsch and Harris, 1997a). A spatially arrayed ciliary margin does not exist in the mammalian and bird retina, even if both types of retina contain stem cells. The mouse retinal stem cells are located in the pigmented ciliary margin (PCM) (Tropepe et al., 2000). These cells may be the evolutionarily homologs of the amphibian and fish CMZ precursors, but their location is completely different; in fact, the nonpigmented iris margin of the mammalian retina (corresponding to the amphibian and fish CMZ) is devoid of stem cells. The PCM stem cells can differentiate into various retinal neuronal types including photoreceptors, bipolar neurons and Müller glia (Tropepe et al., 2000).
More recently, retinal stem cells have been isolated from the human retina. These cells display self-renewal properties and, above all, when transplanted into post-natal mouse or embryonic chick eyes, are able to survive and differentiate in the host retina (Coles et al., 2004). Thus, the adult mammalian eye harbours stem cells, which can be induced to re-enter the cell cycle and initiate neuronal differentiation.
Moreover, it has been demonstrated that in the injuried adult mammals retina , Müller glia cells display a neurogenic activity that results in the production of retinal neurons (Ani V. Das et al., 2006). These results confirm the hypothesis that Müller glia has neural stem cells properties, as first proposed in lower vertebrates (Bernardos et al., 2007; Raymond et al., 2006) .
Fig. 6. Reconstruction of a hierarchical genetic cascade during retinogenesis.
A)The CMZ is subdivided into four zones, noted 1, 2, 3, and 4, and cells in the central retina are schematized with a different shape for each layer. The region where cells are dividing, accordingto BrdU staining, is in yellow, and the region where cells are postmitotic is in orange. GCL, ganglion cell layer; INL, inner par of the inner nuclear layer; OINL, outer part of the inner nuclear layer; PRL, photoreceptor layer. (B) Gene expression in cells of the four zones of the CMZ are presented (1, 2, 3, and 4) (Perron et al., 1998)
Neural retina cells fate: the competence model
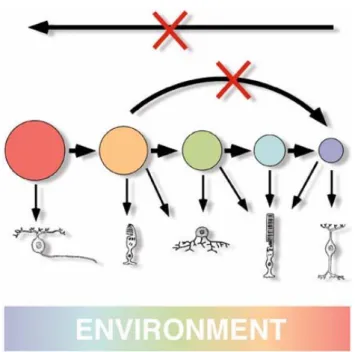
Several observations as birthdating studies, heterochronic coculture experiments (Belliveau and Cepko 1999; Belliveau et al. 2000; Rappaport et al. 2001), heterochronic transplantation (Rappaport et al. 2001), and lineage analysis (Turner and Cepko 1987; Holt et al. 1988; Wetts and Fraser 1988; Turner et al. 1990), have given rise to the competence model of retinal cell fate specification (Cepko et al. 1996).
In this model, retinal progenitors pass through a series of competence states. During every state progenitors are competent to produce one or a restricted subset of retinal cell types. Within a given competence state, the generation of a particular type of cell depends by the intrinsic ability of a progenitor to respond to the extrinsic cues present in the changing environment (Cepko et al., 1996; Harris, 1997; Edlund and Jessel, 1999; Livesey and Cepko, 2001; Fig. 7).
The competence model was also formulated to explain cell fate choice in many other regions of the developing nervous system including - neural crest (Selleck and Bronner- Fraser, 1996), spinal cord (Ericson et al., 1996), and cerebral cortex (McConnell,1988; Qian et al., 2000) where changes in progenitor competence over time, frequently result in altered sensitivity to extrinsic factors (Blackshaw et al.,2001).
The model of temporal changes in competence is strongly supported by elegant studies of Drosophila CNS development (Isshiki et al., 2001; Pearson and Doe, 2003), where the sequential expression of the transcription factors Hunchback, Krüppel, Pdm and Castor was found to change cell fate competence (Isshiki et al., 2001). Noteworthy, cdc25 mutants, whose cell cycle is arrested at the G2-M transition, fail to progress through the normal sequence of gene expression (Isshiki et al., 2001), suggesting the importance of a cell cycle-dependent clock (Decembrini et al., 2006).
At this point several questions need answers: what are the cues that defines the cellular differences among progenitors at different times and moreover, how those differences define different competences and how passage between one state and the next is regulated?
A diverse range of neural cell types is generated from a pool of dividing stem and progenitor cells in an orderly manner during development.
Little is known about the intrinsic changes at the mRNA and protein level, even though developing techniques of functional genomics are supplying new data about the transcriptional program of progenitors and its modulation during retinogenesis (Livesey
and Cepko, 2001). Several projects have recently been started aimed at identifying genes involved in the transcriptional program correlated with retinal progenitor differentiation. In particular, Cepko ad colleagues have used a nonbiased method to purify populations of neural progenitor cells from the murine CNS to characterize the gene expression program of mammalian retinal progenitor cells. Analysis of these data led to the identification of a core set of >800 transcripts enriched in retinal progenitor cells compared to both their immediate postmitotic progeny and to differentiated neurons (Livesey et al., 2004). Again, in order to identify genes that might regulate retinal development, Cepko and coworkers performed a gene expression study in the developing mouse retina using serial analysis of gene expression (SAGE). SAGE is a technique that provides a comprehensive profiling of gene expression; data obteined by means of SAGE have been analyzed by in situ hybridization. In this way, a molecular atlas of the expression patterns of 1051 genes in the developing and mature retina thereby constructed (Blackshaw et al., 2001).
During early stages of retinal development, mitotic progenitors form the outer neuroblastic layer (ONBL), while newborn neurons reside in the inner neuroblastic layer (INBL). The position of mitotic progenitors within the ONBL varies depending upon their progress through the cell cycle, with S phase cells being found on the vitreal side of the ONBL near the border with the INBL and M-phase cells being found on the scleral side of the ONBL, near the retinal pigment epithelium. Blackshaw, analyzed gene expression in the scleral and vitreal portions of both the ONBL and INBL separately. Virtually every gene previously reported to regulate retinal development was detected in this analysis and showed dynamic expression during development. For instance, NeuroD1, which regulates rod photoreceptor survival, as well as possibly rod differentiation (Morrow et al. 1999; Wang et al. 2001), is overexpressed at P4.5.
In the case of genes previously shown to be required for production of certain cell types in the developing retina, such as Ath5 and Chx10 - which are required for ganglion cell and bipolar neurons, respectively (Burmeister et al., 1996; Brown et al., 2001; Wang et al., 2001) - peak expression typically occurred around or just after the peak time of exit from mitosis for that cell type (Blackshaw et al., 2001).
On the other hand, the authors have identified a number of genes that show temporally restricted expression in early ONBL. By analyzing the expression of a large number of genes that were highly expressed early in development they found that they are expressed in broad but temporally restricted subsets of mitotic progenitor cells. For
example, sFrp2 (secreted Frizzled related protein 2, a modulator of the Wnt signalling pathway) RNA was found to be broadly expressed in the ONBL until E16, after which it rapidly decreased. Expression of Fgf15 (Fibroblast growth factor 15) was seen to persist longer, but never was easily detected after P0. Lhx2, by contrast, was weakly expressed in subsets of cells in the ONBL until P0, when it was dramatically and transiently upregulated throughout the ONBL.
From this study it has been confirmed that the population of progenitors is complex at any time, as there is evidence for progenitor heterogeneity at several points during development (Livesey and Cepko, 2001) (Fig. 8). In fact, a limited number of genes have previously been reported to be expressed in subsets of mitotic retinal progenitor cells, including genes such as Ath5, and have been shown to be required for retinal ganglion cell development (Brown et al. 2001; Wang et al. 2001).
Anyway, given that a competence state seems to be intrinsically defined, how a progenitor moves among competence states is still an open question.
Another possibility is that the generated neurons can alter the competence of the remaining progenitors. Data on this point are contrasting but we must precise that considering the short time window in which differentiation happens, is really difficult to determine the correct time in which these events take place. For example, studies in chick have shown that post-mitotic ganglion cells inhibit the differentiation of further retinal ganglion cells and that this effect might be mediated by NGF signalling (Waid and MacLoon, 1998). In zebrafish, however, newly generated retinal ganglion cells (RGCs) secrete Sonic hedgehog (Shh) and promote differentiation of further RGCs from adjacent retinal precursor cells (RPCs), which in turn start secreting Shh. In any case, during retinogenesis, the continuous increase in post-mitotic and differentiated neurons will produce alterations in the extracellular environment which somehow influence cellular diversification. Upon a population of intrinsically different precursors (in terms of their bias towards a certain cell fate), the extrinsic factors can act into two ways: 1) by promoting or inhibiting the acquisition of a particular cell fate by a post-mitotic cell; 2) by triggering the progression of progenitors through the sequential states of competence. These possibilities are not mutually exclusive.
Another intriguing hypothesis is that the differences in the response of retinal progenitors to extrinsic signals are mediated by intrinsic changes in the expression levels of cell surface receptors. One interesting example is the observation, in mouse, that levels of the EGF receptor (EGF-R) increase from late embryonic to post-natal
stages, causing a growing responsiveness to EGF (Lillien, 1995; Lillien, 1998). Anyway, the question remains, as it has not yet been identified what regulates EGF-R expression. Therefore, it has still to be clarified how the sequential production of the different retinal cell types is achieved, and what are the relative contributions of extrinsic and intrinsic signals to the process of retinogenesis.
Fig. 7. The competence model.
Progenitors competence (represented in different colours) changes over time. b) From a single precursor all the main neural cell types are generated (Livesey and Cepko, 2001)
Fig. 8. The competence model of retinal cell fate determination.
Retinal progenitors comprise a dynamic mixture of mitotic cell types that interact with the environment to make the different postmitotic cell types. Each progenitor cell is thought to be controlled by a complex of transcription factors that define its competence state. Retinal progenitors are modulated to progress from one state of competence to another in only one direction. The environment is shown to be changing over time (from Cepko et al., 1999).
Intrinsic versus extrinsic signals during retinogenesis
As mentioned above, several studies in the vertebrate retina showed that a single RPC is potentially able to give rise to both neural and glial cells (Holt et al., 1988; Wetts and Fraser, 1988; Turner et al., 1990).
How do progenitors choose among different cell fates?
Evidence coming from in vitro heterochronic transplantation experiments supports the hypothesis that intrinsic mechanisms play a fundamental role in retinal cell types differentiation. For istance, early chick progenitors, which normally generate ganglion cells in vivo, generate retinal ganglion cells in vitro regardless of the environment they are placed in (Austin et al., 1995). Similar experiments in the rat indicate that mid-stage precursors, that normally produce amacrine cells and cone photoreceptors, and late-stage progenitors, that produce almost exclusively rod photoreceptors, do not change the type of progeny they produce even when placed in different environments (Belliveau et al., 1999). When mixed with an excess of post-natal retinal cells in vitro, embryonic progenitors do not show a bias towards photoreceptor cell fate, which is the main fate adopted by post-natal precursors (Morrow et al., 1998). Another example comes from Cayouette et al. (2003), who showed that E16-E17 rat retinal precursors produce clones of similar size and cellular composition when cultured under two different conditions and that they behave as their in vivo counterparts. These results suggest that the retinal progenitors are intrinsically determined to give only some cell types rather than others. In these last years numerous intrinsic factors have been discovered to be necessary and sufficient to establish and moreover to change the competence of a retinal prognitor. Recently, Foxn4 a winged helix/forkhead transcription factor has been isolated which is necessary and sufficient for commitment to the amacrine and horizontal cells (Li et al., 2004). Mutations in Foxn4 result in the elimination of horizontal cells and in a great reduction of amacrine cells (Li et al., 2004).
Several homeobox genes such as Crx/otx5 are involved in photoreceptor differentiation in mouse and Xenopus (Furukawa et al., 1997; Viczian et al., 2003) and the functional ablation of this gene promotes a decrease of photoreceptor cells . They It has also been demonstrated that CRX/OTX5 works in synergism with NRL, a leucine zipper transcription factor, in order to promote rod differentiation (Mitton et al., 2000; Mears et al., 2001).
Another homeobox gene coding for a transcription factor involved in retina cell types specification is Chx10. In mice the loss of Chx10 leads both to reduced proliferation of retinal progenitors and to a specific absence of differentiated bipolar cells (Burmeister et al., 1996).
Even though we are still far from a complete understanding of the mechanisms at the basis of retinogenesis, in this last years many different experiments demonstrated the pivotal role of several intrinsic factors.
In fact, recent evidence suggests that RPCs competence to differentiate into specific cell types can be more fixible than previously thought, raising the hypothesis that, at least in vitro, progenitors can be driven to a cell fate they would not be competent to give in vivo. For istance Watanabe and Raff demonstrated that E15 (embryonic stage at which ganglion cells are born in mouse) retinal cells display the ability to differentiate as rods when mixed with retina cells that are several days older (Watanabe and Raff, 1990). This work shows that the same cells can differentiate into different cell types thus corroborating the hypothesis that fate restriction of the progenitors may depend from the external environment rather than from an intrinsic clock.
James and co-workers have shown that a small percentage of E18 chick cultured retinal progenitors, express RGCs markers like Islet- 1 and RPF1 and display the ability to differentiatiate into RGCs (E18 in chick is the stage at which more photoreceptors differentiate). Morever, a considerable percentage of the E18 precursors are driven towards the acquisition of ganglion cell fate when co-cultered with chick E3 progenitors (E3 is the stage at which ganglion differentiation reaches its peak) (James et al., 2004). Again, Belliveau and Cepko demonstrated that previously generated amacrine cells produce a feedback signal that inhibits the production of the amacrine cell themselves and at the same time, this inhibition is compensated by a second signal affecting the production of cones. No changes in other cell type frequency are observed (Belliveau and Cepko, 1999). On the other hand, signals in the embryonic retinae inhibit rod and favour bipolar cell generation from postnatal progenitors (Belliveau et al., 2000).
These and other experiments suggested to us that extrinsic signals can influence progenitor decision, in order to control the number of differentiated cells, but the choice of the cell fate is restricted by the intrinsic biases of progenitor cells (Belliveau and Cepko, 1999).
Several exemples where extrinsic farctors play a role during retinal cell types differentiation are known.
In zebrafish it has been demonstrated that during the initial stages of vertebrate retinogenesis, Shh signals produced by RGCs play a fundamental role in triggering the switch from a proliferative state to a neurogenic state. The roles of Shh in retinogenesis are not limited to the expansion of the neurogenic wave into the undifferentiated retinal territory. At the neurogenic wave front of vertebrate retina, newly postmitotic RGCs emerge in non-random arrays (McCabe et al., 1999), while behind the wave front increasing numbers of RGCs differentiate and begin to express Shh (Zhang and Yang, 2002; Stenkamp et al., 2002; Shkumatava et al., 2004).
This is somewhat similar to what happens in the Drosophila eye disc, where hedgehog (Hh) controls the timingand rate of photoreceptor differentiation at the morphogenetic furrow (Ma et al., 1993).
Increasing Shh signal levels lead to a reduction of differentiated RGCs, whereas decreasing Shh signals lead to an increased production of RGCs genesis during the peak period of RGCs production in the chick retina (Zhang and Yang, 2002). Moreover, by monitoring responses of cohorts of early progenitor cells, it has been established that Shh signaling negatively affects progenitor cell specification towards the ganglion cell fate during or soon after their last mitotic cycle (Zhang and Yang, 2002). Thus, secreted Shh molecules derived from differentiated RGCs act as negative feedback signals to modulate the further production of RGCs from the early retinal progenitor pool.
Also FGF signals are involved in the specification of retinal cell types. During the initial stage of chick retinogenesis, FGF1 is expressed at high levels in the peripheral retina. Blocking FGF signaling with a protein kinase inhibitor retards the progression of the RGCs wave in retinal explants, while FGF1, but not FGF8, treatment accelerates the RGCs specification wave (McCabe et al., 1999). This result highlights the proneural activity associated with FGF in vertebrate eye formation. In the developing Xenopus embryo, inhibiting FGF signalling by expressing a dominant negative form of the Xenopus FGFR causes a 50% loss of both photoreceptor and amacrine cells, accompanied by a 3.5-fold increase of Müller glia (McFarlane et al., 1998).
Furthermore, overexpressing FGF2 in RPCs causes a 35% increase of RGCs and a 50% increase of Müller cells. Despite the unaltered proportion of photoreceptors among total cells, the ratio of rod versus cone photoreceptors is also affected by FGF2 overexpression (Patel et al., 2000). Interestingly, transgenic tadpoles expressing a dominant-negative FGFR4a receptor under the control of the Xenopus Rx1A (XRx1A) promoter, which is active in retinal progenitors, show disorganized retinas that either
specifically lack photoreceptors or contain a few ectopic photoreceptors (Zhang et al., 2003). Injecting toxin-treated chick eye with a combination of insulin and FGF2 enhances the number as well as the differentiation of neurons that express ganglion cell markers (Fischer et al., 2002). These findings provide evidence that FGF signaling during retinogenesis participates in RPCs fate choice.
It has been demostrated that several other secreted factors are involved in the alteration of retinal cell fates.
For istance they show that FCS (foetal calf serum) arrests rod development in these cultures at a postmitotic, rhodopsin-, pre-rod stage. They present evidence that FCS acts indirectly by stimulating the proliferation of Müller cells, which arrest rod differentiation by releasing leukaemia inhibitory factor (LIF). These findings identify an inhibitory cell-cell interaction, which may help to explain the long delay that can occur both in vitro and in vivo between cell-cycle withdrawal and rhodopsin expression during rod development.
CNTF (Ciliary Neurotrophic Factor) and other cytokines added in postnatal rat retinal explants resulted in a dramatic reduction in the number of differentiating rods. Conversely, the number of cells expressing markers of bipolar cell differentiation was increased to a level not normally seen in vivo or in vitro. In addition, a small increase in the percentage of cells expressing either a marker of amacrine cells or a marker of Müller glia was noted (Belliveau et al.,2000).
Taurine is an extrinsic factor produced from P0 rat retinal cultures. Its addition in retinal explants promotes rod differentiation acting via glycine receptor and (GABA)A receptor (Youngand Cepko, 2004). A competitive antagonist of taurine’s bioactivity was identified and shown to partially inhibit rod development in retinal explants, suggesting that taurine may normally act to stimulate rod development in the retina (Altshuler et al., 1993).
It has also been shown that isolated progenitors differentiate to rods or cones according to the relative amounts of retinoic acid and thyroid hormone (Kelley et al., 1995 and 1999).
Among the extrinsic factors important for retinal development there are neurotrophins, a family of growth factors consisting of NGF (Nerve Growth Factor), BDNF (Brain-Derived Neurotrophic Factor), NT-3 (Neurotrophin-3) and NT-4/5. Besides their critical importance for correct specification and survival of a number of classes of neurons in
the central and peripheral nervous system (Lewin and Barde, 1996), neurotrophins have an important role in earlier stages of development (Pearson, 2006).
For example, NT-3 is expressed in retinal pigmented epithelium and then in neural retina (Rodriguez-Tebar et al., 1993). It has been demonstrated that NT- 3 stimulates the birth of new neurons. By inhibiting NT-3 action using specific antibodies to neutralize endogenous NT-3 (Bovolenta et al., 1996) there is a marked decrease in retinal neuron differentiation, ganglion cells being most affected. Additionally, the impairment of NT-3 signalling causes a decrease in clonal expansion of cells derived from a single retinal progenitor (Das et al., 2000). In contrast, NGF and BDNF have a role during programmed cell death occurring during retinogenesis (Frade et al., 1999).
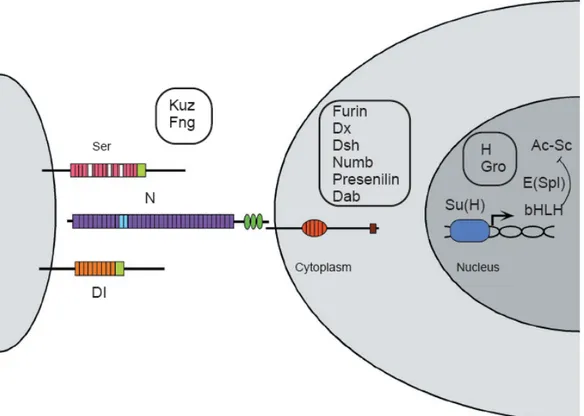
The last but most important point is that once produced, an extrinsic signals needs to be translated into an internal code that will drive a cell towards one fate or another, by switching on a precise transcriptional program. The Notch-Delta pathway is a paradigmatic example of how an extracellular signal can do so (Agathocleos and Harris, 2006). Notch is a transmembrane receptor that transduces an extrinsic cue, the binding of its ligand Delta or Serrate, to directly regulate the transcription of several target genes, in particular repressing proneural genes coding for basic helix-loop-helix (bHLH) transcription factors (Artavanis-Tsakonas et al., 1999) (Fig. 9). Studies in frog, rat, chick and mouse have shown that Notch1 is expressed by proliferating and undifferentiated cells (Dorsky et al., 1995; Bao and Cepko, 1997; Lindsell et al., 1996) and its expression is retained by Müller glial cells (Furukawa et al., 2000; Dorsky et al., 1995). It has been demonstrated that constitutive activation of the Notch pathway in fish, frog, chick and rat retina inhibits neurogenesis (Dorsky et al., 1995; Austin et al., 1995; Bao and Cepko, 1997; Scheer et al., 2001) and promotes gliogenesis (Furukawa et al., 2000; Scheer et al., 2001).
Progress in this regard comes from a recent work where Jadhav et al. (2006) demonstrated, by means of a comprehensive molecular characterization, that activation of Notch in early progenitors allowed them to retain appropriate early progenitor gene expression. When examined at later stages of development, however, the cells exhibited expression of an inappropriate mixture of progenitor genes (like fgf15 and cyclin D1) and glial genes (Jadhav et al., 2006). Moreover, a functional assay showed that these cells could form neurospheres, similar to stem cells derived from the retinal pigmented epithelium of the mammalian peripheral retina (Jadhav et al., 2006). Furthermore, selective reactivation of Notch pathway in newly generated postmitotic cells that had
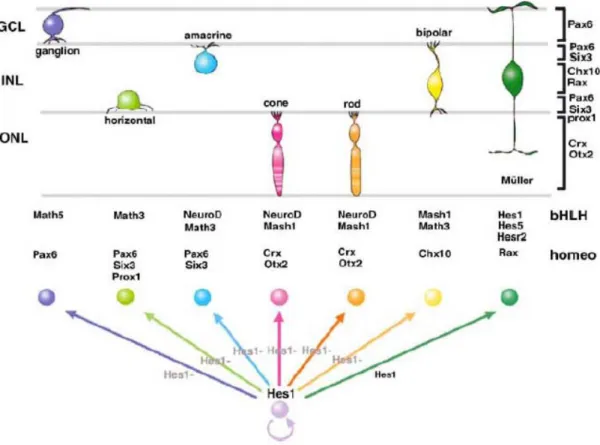
previously released Notch activation during development, led to their differentiation in proper Müller glial cells (Jadhav et al., 2006). In conclusion, prolonged Notch activity in progenitors permits them to progress through multiple states without perturbing temporal identity, promoting early progenitor characteristics early in development and late characteristics later in development. Remarkably, constitutive Notch activation led these cells to acquire both glial and stem cells characteristics (Jadhav et al., 2006) Fig 9. On the other hand, the relationship between lineage and histogenesis is certainly consistent with the idea of an intrinsic developmental clock (Cayouette et al., 2006). Besides the studies of extrinsic regulation of cell fate, we have several evidences on the role of intrinsic factors during retinal neuron determination. bHLH proneural genes, mentioned above as target of Notch signalling, and homeobox genes are basically the best characterized transcription factors involved in generating the diversity of retinal cell fates (Fig. 9). A prime example of a bHLH gene is Ath5. The Xenopus homologue, Xath5, promotes retinal ganglion cell genesis when overexpressed in vivo (Kanekar et al., 1997). It can induce the expression of Xbh1, a homeodomain transcription factor involved in ganglion cell differentiation (Hutcheson and Vetter, 2001; Liu et al., 2001; Poggi et al., 2004). When ath5 gene is non functional, such as in zebrafish lakritz mutants (Kay et al., 2001) or in Math5 mutant mice, there is a depletion of ganglion cells (Brown et al., 2001; Wang et al., 2001). Ath5 has an interesting effect on cell cycle because cells that express Xath5 tend to exit the cell cycle early, at the appropriate time for ganglion cell genesis (Ohnuma et al., 2002a).
Other bHLH have different profiles of activity with respect to cell determination in the retina. NeuroD, for example, promotes amacrine over bipolar cell fate and favour photoreceptor survival (Morrow et al., 1999). Mash1 and Math3 are both expressed in bipolar cells and in their double mutation virtually all bipolar cells are abolished (Tomita et al., 2000). Recently, a bHLH transcription factor, has been identified Bhlhb4, that is required for rod bipolar cell maturation. Bhlhb4-/- mice specifically lack rod bipolar cells, while the other retinal neurons are unaffected (Bramblett et al., 2004). Hes1 inhibits neuronal differentiation and maintains progenitors. Differentiating neurons lose Hes1 expression. Homeodomain and bHLH factors determine the neural fate of retinal cell types. The cells that do not lose Hes1/Hes5 expression during neurogenesis stages adopt the Müller glial fate (from Hatakeyama and Kageyama, 2004).
Fig. 9. The Notch-Delta pathway
Extracellular regions of Notch (N) and Delta (Dl) interact to activate the receptor. As a result of activation, the Supressor of Hairless [Su(H)] transcription factor eventually binds to regulatory sequences of the Enhancer of split [E(Spl)] complex genes, which encode bHLH proteins. bHLH products, together with Groucho, can repress the expression of the Achaete-Scute (Ac-Sc) proneural genes. Several additional factors that influence signaling through these core elements and that display molecular interactions are also shown. These include the ligand Serrate (Ser) and its negative regulator Fringe (Fng); the metalloprotease Kuzbanian (Kuz), which acts as a Delta- and potentially as a Notch-processing enzyme; the trans-Golgi convertase Furin, which cleaves Notch; Presenilin, which may cleave Notch in the membrane; and the Notch intracellular domain interacting proteins Deltex (Dx), Disheveled (Dsh), Disabled (Dab), and Numb; and in the nucleus, the two regulators Hairless (H) and Groucho (Gro).
The role of intrinsic factors during retinogenesis
In the last decade have been isoleted many different key genes controlling different developmental stages during reinogenesis such as i) eye field identification ii) specification of its polarity iii) proliferation and cell death control iv) retinoblast specification v) differentiation bias between neuronal or glial retinoblasts fate.
Many of these key genes code for transcription factors most of which are charaterized by a homeodomain. For several homeobox genes the up and down-stream cascade of activation is known. They act by definition in the same cell in which they are expressed or in a “cell-autonomous” way. The most important genes involved in retina formation are listed below.
As mentioned above, retinal precursors are a heterogeneous population. A still unsolved issue regards how this heterogeneity is achieved, as all RPCs derive from the eye field, the most anterior part of the neural plate.
In all the vertebrate species studied so far, the eye field is established by the simultaneous expression of some homeodomain transcription factors which together define the retinal character of neural plate cells (Fig. 10). These factors, namely Pax6, Rx1, Six3, Otx2 and Lhx2, play a fundamental role in initiating eye development.
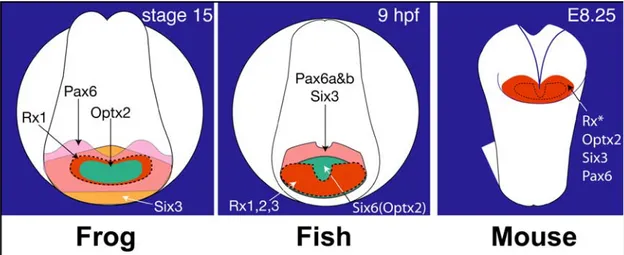
The location and timing of eye primordia specification in the anterior neural plate is synchronized with the coordinated expression of a group of eye field transcription factors or EFTFs. The expression pattern of the Xenopus EFTFs ET, Rx, Pax6, Six3, Lhx2, tll and Optx2 overlap in the presumptive eye primordia during and immediately following its specification (Zuber et al., 2003). The EFTFs of other species have a similar pattern of coordinated expression. For example, at neural plate stages Pax6, Rx, Six3, and Optx2 are also observed in a single band of expression in the chick, zebrafish and mouse embryo (Fig. 10) and (Walther and Gruss, 1991; Li et al., 1994; Oliver et al., 1995; Mathers et al., 1997; Bovolenta et al., 1998; Toy et al., 1998; Ohuchi et al., 1999; Toy and Sundin, 1999; Chuang and Raymond, 2002)). EFTFs have been highly conserved through evolution and genetic evidence from multiple species demonstrates they are required for vertebrate eye formation. Functional inactivation of Pax6, Rx, Lhx2, Tll, Six3 and Optx2 results in frogs, fish, rodents and/or humans with abnormal or no eyes (Hill et al., 1991; Mathers et al., 1997; Porter et al., 1997; Hollemann et al., 1998; Chow et al., 1999).
Fig. 10. Eye field transcription factors (EFTFs) have overlapping expression patterns during eye primordia formation.
Anterior, frontal views of neural plate staged frog (Xenopus laevis), fish (zebrafish and medakafish), and mouse embryos show the expression domains of Rx, Pax6, Six3 and Optx2 homologues (Oliver et al., 1995; Mathers et al., 1997; Seo et al., 1998; Toy and Sundin, 1999; Inoue et al., 2000; Chuang and Raymond, 2002; Zuber et al., 2003; Bailey et al., 2004).
Pax genes
Pax genes contain twoDNA-binding domains: the “homeobox” and the “paired domain”. This last domain codes for a 128 aa sequence that has an α-elix β-sheet β-turn structure (Xu et al., 1995). Generally, in vertebrates Pax6 is expressed during CNS development in the eye and in the olfattive placode.
Pax6 mutations promote “eyeless” (ey) phenotype in Drosophila (Quiring et al., 1994), corroborating its necessity in eye formation. For these reasons, Pax6 was considered the eye master control gene, able by itself to activate the genetic hierarchy to complete eye morphogenesis. eyeless overexpression in Drosphila immaginal disks supports ectopic eyes formation on wings, legs and antennas (Halder et al., 1995).
Moreover it has also been demonstrated that the Drosophila Pax6 overexpression promotes ectopic eye formation in mouse (Halder et al., 1995). Furthermore Pax6 heterozygous mice show small eye (sey) phenotype with a remarkable microphtalmia (Hogan et al., 1988), while homozygous mice die (Hill et al., 1991).
Studies on retinogenesis highlighted a role for Pax6 in the mantainance of the retinal precursor multipotency (Brown et al., 1998). Finally, it has been shown that Pax6 controls bHLH transcription.
At later stages Pax6 functional ablation promotes only amacrine cells generation (Marquardt et al., 2001). This last result highlights a common feature of homeodomain gens; the ability to perform different functions at diverse developmental times. In Xenopus, Xpax6 expression is localized both in neuronal – neural and pigmented retina– and ectodermal derived region –cornea and crystallin-.
Between stage 13 and 33/34, Xpax6 is mostly expressed in the developing retina; after stage 35, its expression is restricted in ganglion and amacrine cells and in proliferant precursors in the CMZ (Harris and Perron, 1998; Hirsch and Harris, 1997).
Six genes
Six family genes code for transcription factors containing two conserved domains: the homeodomain, found in all “homeobox” genes and the “Six-domain”, characteristic of this family. It codes for a 110-115 amminoacidic sequence. Both domains are able to bind specific sequence of DNA. Six3 displays the characteristics expected from an effector of neural inducers involved in specifying and maintaining anterior neural plate properties. Xsix3 overexpression promotes cell proliferation and inhibits neurogenesis at early neurula stage by activating Xhairy2, Zic2, Xrx1 and XBF1 and regulating the expression of p27Xic1 and cyclinD1.
Furthermore, Six3 represses BMP expression in both Xenopus and zebrafish and is able to rescue the anterior neural plate defects of chordino mutants. The effect of Xsix3 on BMP4 appears to be direct as suppression occurs even in the absence of protein synthesis. Although Xsix3 efficiently suppresses BMP4 expression, we observed that it is unable to induce neural tissue, requiring Xotx2 for this activity. Taken together with the recent observation that Six3 is able to repress Wnt expression (Braun et al., 2003; Lagutin et al., 2003), these data indicate Six3 as a crucial factor for anterior neural plate specification. In the vertebrate optic region Six3 is initially expressed in the optic vesicle and in the optic stalk. Afterwards, Six3 mRNA appears in the neural retina, in the pigmented epithelium and in the lens. Finally, this gene expression is restricted in the retina INL . Six3 injection in precociuos Xenopus embryos promotes ectopic retina formation (Loosli et al., 1999).
Sine oculis (so), a member of the six gene family, has been the first gene isolated in Drosophila (Cheyette et al., 1994). so mutation causes riduction or absence of eyes in Drosophila: the reason of that is the lack of retinal progenitor cells, that undergo apoptosis before eye field formation.
In mice have also been isolated six others Six genes (Six1-Six6), among which Six3 e Six6/Optx2, that have an expression domain limited to the antirior region of the brain and in the eyes.
In the developing vertebrate eye Optx2 is expressed in the optic vesicle and in the overlying ectoderm, successively in the neural retina and in the optic stalk (Toy et al., 1998). In Xenopus, its espression is localized in proliferating precursors of the CMZ (Harris and Perron, 1998).
Xoptx2 injected embryos show an expansion of the eye field (“giant eyes”) and of eye field markers such as Rx1 e Pax6 (Zuber et al., 1999).
Rx genes
Rx vertebrate genes have been indipendently discovered in three different laboratories (Casarosa et al., 1997; Furukawa et al., 1997a; Mathers et al., 1997). These genes code for a homeodomain transcription factor of “paired-like”class. Two Rx omologue genes have been discovered in mammals (Wang et al., 2004) and in chick (Ohuchi et al., 1999), while in Zebrafish have been isolated three (Chuang et al., 1999).
In Xenopus, Xrx1 is initially expressed during gastrulation, in the anterior neural plate and successively its expression is localized in the developing eye field (neural retina and pigmented ephitelium), in the epiphysis, in the diencephalon floor by where are found hypothalamus, hypophysis and the optic chiasm. Whole mount in situ experiments show (Casarosa et al., 1997) that Xrx1 is detectable until stage 45.
During retinogenesis, Xrx1 is expressed in undifferentiated progenitors and in CMZ cells, where its expression persists as retinogenesis is terminated (Mathers et al., 1997; Perron et al., 1998; Casarosa et al., 2003).
Gain- and loss-of-function studies of rx in different species have shown this gene to be necessary for the formation of anterior brain structures and eye, and sufficient to induce retina and neural tube hypertrophy (Mathers et al., 1997; Andreazzoli et al., 1999; Winkler et al., 2000; Chuang and Raymond, 2001; Loosli et al., 2001).
Xrx1 lipofection in Xenopus retina promotes clonal proliferation of retinal precursors, while its functional impairment produces the opposite effect.. The decreased clone size
after Xrx1–EnR lipofection is due to an impairment of mitotic activity, to increased cell death, or to both.
Xbh1 genes
XBH1 is one of the Xenopus homologs of Drosophila BarH genes. These genes were first identified because they are involved in the Bar-like eye mutation, in which the anterior part of the compound eye lacks ommatidia (Higashijima et al., 1992a; Kojima et al., 1991).
In the fruitfly, BarH1 and BarH2 are expressed in the developing peripheral nervous system and in the eye (Higashijima et al., 1992b). In particular, in the compound eye, BarH1/BarH2 are necessary for the differentiation of the external photoreceptors (R1/R6), where they are regulated by lozenge (lz), and of primary pigment cells, where they are regulated by sparkling, a homologue of mammalian Pax2 (Daga et al., 1996; Fu and Noll, 1997). In the developing notum, BarH1/BarH2 genes are regulated by the secreted factors decapentaplegic and wingless, and exert their function by modulating the proneural achaete-scute genes (Sato et al., 1999). It has recently been shown that BarH genes are able to suppress transcription of atonal in the differentiating eye imaginal disc, regulating differentiation in the morphogenetic furrow (Lim and Choi, 2003).
As happened for many genes in Drosophila, also for these genes homologs been isolated in different vertebrate species (Saito et al., 1998; Patterson et al., 2000; Poggi et al., 2002).
In mouse, the MBH1 (also called Barhl2) gene is expressed in the developing central nervous system and in the eye. It is potentially involved in the regulation of bHLH factors. As shown by Saito and collaborators, MBH1 is able to elicit overexpression of ngn2 and downregulates Mash1 when transfected in P19 teratocarcinoma cells. It is noteworthy that in vivo, in the neural tube, MBH1 is expressed in the same sites as ngn2 and in mutually exclusive sites compared to Mash1, strongly suggesting a role in the regulation of expression and function of the bHLH factors. Recently, MBH1 function during retinogenesis has been investigated. This gene has a dynamic expression in the retina, localized in amacrine, horizontal and ganglion cells. When overexpressed in the retina, MBH1 leads to a dramatic increase in glycinergic amacrine cells at the expense of Müller and bipolar cells (Mo et al., 2004).
A different effect is shown by XBH1 overexpression in the Xenopus retina, were ganglion cell differentiation is elicited at the expense of photoreceptors (Poggi et al., 2004). A similar role is also suggested for the medaka homolog, whose expression is found in differentiating RGCs (Poggi et al., 2002)
Otx genes
This family incude vertebrate “homeobox” genes homologues to orthodenticle (otd) of Drosophila. Orthodenticle is a cephalic gap gene able to control CNS in Drosophila (Finkelstein and Perrimon, 1990; Finkelstein et al., 1990). Otx genes code for transcription factors with a homeodomain of the K50 Paired-like class, characterised by a lysine residue at position 50 of the homeodomain. The homeodomain is a 60 amino acid module representing a variation on a helix-turn-helix motif of prokaryotic repressor. Three α-helical regions are separated by turns in the protein backbone. Helix 3 (recognition helix) of the homeodomain binds to the major groove of DNA, while helices 1 and 2 lie outside the double helix. Helix 3 contacts both the phosphate backbone and specific bases. An N-terminal arm lies in the minor groove, and makes additional contacts (Lewin, 2003).
The homeodomain is followed by a glutamine-rich region, a basic region (rich in lysine and arginin) and a WSP domain, a highly conserved region of unknown function. The OTX proteins have an OTX-tail, at first identified in CRX (Furukawa et al., 1997) but usually present in tandem repetition. By deletion analysis it has been demonstrated that multiple regions in the C-terminal portion of CRX contribute to its transactivating activity. OTX nuclear trafficking is highly regulated. The pathway of transport to the nucleus is mediated by nuclear localization signal (NLS) sequences that are characterized by one or more clusters of basic amino acids (Fei and Hughes, 2000). By deletion analysis it has been demonstrated that CRX NLS resides in the C-terminal of the homeodomain, between residue 88 and 107 (Fei and Hughes, 2000). Moreover, nuclear translocation of CRX is mediated by Karyopherin 13 (also referred as Importin 13), that directly binds to the CRX homeodomain and to its flanking regions, mediating the nuclear translocation (Ploski et al., 2004). Recently, a structural characterization of OTX2 was carried out. As for CRX, OTX2 nuclear localization is controlled by a nuclear localization sequence located within the homeodomain. Moreover, it works in conjunction with a novel nuclear retention domain, located downstream of the homeodomain (Chatelain et al., 2006). Besides DNA binding and protein trafficking, the homeodomain is involved in protein-protein interactions. Several cofactors have
been identified that interact with OTX proteins. For example, it has been demonstrated that CRX binds to NRL (Mitton et al., 2000) and to NR2E3, forming a trimeric complex able to induce photoreceptor differentiation (Peng and Chen, 2005).
In vertebrates, have been identified many menbers of the Otx family: Otx1, Otx2, Otx3, Otx4, Otx5, Otx5b e crx. All these genes are involved in the development of the most anterior parts of the embryo. The first ones to be identified were Otx1 and Otx2, in mouse (Simeone et al., 1992); Otx2(-/-) mice dies precociously during development because of gastrulation defects (Acampora et al., 1995).
Also in zebrafish have been characterised two orthologhs of Otx1 e Otx2 mouse genes: zOtx1 is expressed in the hypoblast, in the axial mesoderm and in the neuroectoderm anticipating zOtx2 espression (Li et al., 1994).
In Xenopus, Xotx2 supports development of the most anterior part of the head as in mouse. In fact, Xotx2 overexpression (as in Xotx5), produces cement gland, ectopic neural tessue and disfunctions in the development of the posterior part of the body or in neural tube closure (Andreazzoli et al., 1997; Vignali et al., 2000).
During retinal development in mouse, cells of the photoreceptor lineage turn on the expression of Otx2, which is essential but not sufficient for photoreceptor differentiation. This has been established by means of an Otx2 conditional knock-out (CKO), in which Otx2 was inactivated under control of the Crx promoter (Nishida et al., 2003): CKO mice showed a complete loss of retinal photoreceptors. Moreover, it was found that Otx2 is a direct upstream regulator of Crx (Nishida et al., 2003). On the other hand, Crx is able to regulate its own expression and its promoter contains four CRX-binding sites (Furukawa et al., 2002). So, the upregulation of Crx may be a necessary step for the expression of both rod and cone genes (Chen et al., 1997).
Crx is able to activate several specific genes as rhodopsin, IRBP (Interphotoreceptor Retinoid Binding Protein), β-phosphodisterase, arrestin. Crx (-/-) mice don’t develop outer photoreceptors segment losing phototrasduction capability (Furukawa et al., 1999).
Crx mutation in humans are associated with cones and rods dystrophy (Freund et al., 1997), retinitis pigmentosa and leber congenital amaurosis.
In Xenopus, Xotx2 and Xotx5b (the homolog of Crx) are expressed in different patterns during retinal histogenesis: transcription of both genes starts at tailbud stage in a diffuse fashion throughout the retina, but then their expression is progressively restricted and in the mature retina, Xotx2 mRNA is found only in bipolar cells, while Xotx5b is