ORIgINAL ARTICLE
Massimo Sandrini [email protected]
1 O.U. of Nephrology, A.S.S.T. Spedali Civili di Brescia, Piazzale Spedali Civili, 1, 25123 Brescia, Italy 2 Università di Brescia, Brescia, Italy
Received: 25 June 2016 / Accepted: 11 August 2016 / Published online: 31 August 2016 © The Author(s) 2016. This article is published with open access at Springerlink.com
Incremental peritoneal dialysis: a 10 year single-centre experience
Massimo Sandrini1 · Valerio Vizzardi1 · Francesca Valerio1 · Sara Ravera2 ·
Luigi Manili1 · Roberto Zubani1,2 · Bernardo J. A. Lucca2 · Giovanni Cancarini1,2 DOI 10.1007/s40620-016-0344-z
output (0.392; p = 0.034). Hospitalization rates were sig-nificantly lower in incrPD (p = 0.021). Eight of 29 incrPD patients were transplanted before reaching full dose treatment.
Conclusions IncrPD is a safe modality to start PD;
com-pared to stPD, it shows similar survival rates, significantly less hospitalization, a trend towards lower peritonitis inci-dence and slower reduction of renal function.
Keywords Incremental dialysis · Peritoneal dialysis · Dialysis adequacy · Residual renal function
Introduction
Incremental dialysis consists in prescribing a dialysis dose aimed at maintaining total solute clearance near the targets set by guidelines. In peritoneal dialysis (PD), the total amount of blood purification is equivalent to the sum of residual renal function plus the peritoneal dialysis dose [1, 2]. This makes it possible to start dialysis at less than full dose when there is still a significant renal function; afterwards, the dialysis dose is gradually increased to compensate renal function decline and to meet adequacy targets [3, 4].
The incremental approach to peritoneal dialysis (incrPD) was first developed in the late 90s. At that time and for many of the following years, however, incrPD was sometimes mistakenly presented as a way to start dialysis earlier [3–8]. Moreover, shifting consensus regarding the timing of dialy-sis initiation has increased confusion between “incremental” and “early” dialysis [9, 10]. In 2006, the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines suggested that nephrologists should evaluate the benefits, risks, and disadvantages of beginning kidney replacement therapy when glomerular filtration rate (GFR) is <15 ml/min [11]; Abstract
Introduction Incremental dialysis consists in prescribing a
dialysis dose aimed towards maintaining total solute clear-ance (renal + dialysis) near the targets set by guidelines. Incremental peritoneal dialysis (incrPD) is defined as one or two dwell-times per day on CAPD, whereas standard peri-toneal dialysis (stPD) consists in three-four dwell-times per day.
Patients and methods Single-centre cohort study.
Enrol-lement period: January 2002–December 2007; end of fol-low up (FU): December 2012. Inclusion criteria: incident patients with FU ≥6 months, initial residual renal function (RRF) 3–10 ml/min/1.73 sqm BSA, renal indication for PD.
Results Median incrPD duration was 17 months (I–III Q:
10; 30). There were no statistically significant differences between 29 patients on incrPD and 76 on stPD regarding: clinical, demographic and anthropometric characteristics at the beginning of treatment, adequacy indices, peritonitis-free survival (peritonitis incidence: 1/135 months-patients in incrPD vs. 1/52 months-patients in stPD) and patient survival. During the first 6 months, RRF remained stable in incrPD (6.20 ± 2.02 vs. 6.08 ± 1.47 ml/min/1.73 sqm BSA; p = 0.792) whereas it decreased in stPD (4.48 ± 2.12 vs. 5.61 ± 1.49; p < 0.001). Patient survival was affected negatively by ischemic cardiopathy (HR: 4.269; p < 0.001), peripheral and cerebral vascular disease (H2.842; p = 0.006) and cirrhosis (2.982; p = 0.032) and positively by urine
exported anonymously to an Excel (®Microsoft, Redmond, WA, USA) file: age, sex, primary renal disease, comorbidi-ties, weight and body mass index, RRF and class of perito-neal permeability according to a modified 3.86 % peritoperito-neal equilibration test (PET) done about 3 months after the beginning of PD. Other data exported were: time on PD, adequacy parameters, incidence of peritonitis, hospital-ization rate and outcome. RRF was measured monthly in incrPD patients and quarterly in stPD patients. The com-parison of adequacy values between incrPD and stPD was made at baseline, after 6 months and at the end of PD or when transition from incrPD to stPD took place.
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD) or median and interquartile range (I–III Q) according to their distribution. Comparisons were performed using Student’s t test or Wilcoxon test as appropriate. Dichoto-mous variables were analyzed using the Chi square test. A p value <0.05 was considered as statistically significant. Comparison of survival was done by Kaplan–Meier curves and log-rank test. Multivariate analysis was done by Cox proportional hazards model. The statistical program used was SPSS® version 19 (SPSS Inc., Chicago, IL, USA).
Results
Baseline data
The total number of incident patients in PD in 2002–2007 was 178, of whom 39 had a follow-up <6 months, 28 had a RRF <3 ml/min, at start, 5 a non-renal indication for PD, and 1 patient was lost to follow-up. As a consequence, the eligible patients were 105: 42 (40 %) were women and 63 (60 %) men; 29 (28 %) were in the incrPD group and 76 (72 %) in the stPD group; 57 (75 %) patients of the stPD group were on APD and 19 (25 %) were on CAPD. Main baseline data of the two groups are reported in Table 1: no statistically significant difference was found regarding age, gender, weight, body mass index, comorbidity, primary renal disease, RRF or class of peritoneal permeability. Change of PD modality
Median duration of incrPD was 17 months (I–III Q: 10; 30); 21 incrPD patients shifted to stPD after a median of 17 months (I–III Q: 10; 27). Causes of transition were a reduction in RRF in 19 patients and abdominal hernia in two. Eight patients stopped PD: 5 due to renal transplanta-tion and 1 due to recovery of renal functransplanta-tion; 2 patients died. Median duration of stPD was 36 months (I–III Q: 18; 55); in 2013, dialysis initiation was recommended whenever the
patient becomes symptomatic (independently of GFR) [12]; in 2014, instead, it was advised to start dialysis when GFR is <15 ml/min/1.73 m2 body surface area (BSA) and/or the patient becomes symptomatic, but, in any case, before GFR falls to 6 ml/min/1.73 m2 BSA [13].
A comparison between the results of incrPD as opposed to standard PD (stPD) (full dose) must be done using similar GFR values and excluding cases with an early start of dialy-sis or with an extra-renal indication for dialydialy-sis (i.e. heart failure and end-stage liver disease). The first clinical experi-ences with incrPD date back to 1999–2002 [7, 8, 14, 15]; initial prescription consisted of 1–2 dwell-times per day. In 2003, incremental automated peritoneal dialysis (APD) was suggested; it consisted of a full daily dose of APD, but only for 3–4 nights per week [16].
Based on these reports, in 2002 we started an incrPD program. This paper analyzes our clinical experience and attempts to provide further information regarding this new approach to the start of dialysis.
Patients and methods
This cohort study attempts to define the results of the incre-mental PD approach. All patients who started PD from January 1st 2002 to December 31st 2007 in our center were included. End of follow-up was December 31st 2012, or when the patient stopped PD because of death, shift to hemodialysis (HD), renal transplantation or recovery of renal function.
Residual renal function (RRF) was measured as the mean of creatinine renal clearance and urea renal clearance. Inclu-sion criteria were as follows: follow-up lasting at least 6 months, RRF at start of PD >3 ml/min/1.73 m2 BSA and <10 ml/min/1.73 m2 BSA and a “renal indication” for PD. Standard dialysis dose (stPD) was defined as 3–5 dwell-times per day, 7 days a week, for continuous ambulatory peritoneal dialysis (CAPD) and nightly dialysis sessions, seven nights a week, for APD. Incremental dialysis dose (incrPD) was defined as one or two dwell times per day on CAPD.
The eligible patients were divided into two groups according to the dialysis dose with which they started: stPD or incrPD. Choice of PD modality, either CAPD or APD, was made according to patient preference following adequate information and exclusion of clinical contraindica-tions. IncrPD was suggested only to patients who had cho-sen CAPD, because at that time incremental APD had not been defined yet.
All demographic and clinical data were prospectively recorded in a database (File Maker®, File Maker Inc., Santa Clara, CA, USA). The following baseline data were
of 1/58 over a follow-up period of 2267 patient-months. The incidence of peritonitis was 1/39 on CAPD vs. 1/49 on APD when all periods at risk were taken into consideration. Even though the absolute incidence was quite different, the cumulative probability of being peritonitis-free (log-rank test on Kaplan–Meier curves) showed no significant differ-ence between incrPD and stPD or between CAPD and APD. Hospitalization
Among the patients on incrPD, 30 admissions occurred in 677 patient-months (1/23 patient-months) for a total of 322 days of hospitalization (5.7 days/patient-years). Among the patients on stPD there were 325 admissions in 2932 patient-months (1/9 patient-months) and 3784 days of hos-pitalization (15.5 days/patient-years). At the “as treated” analysis, the cumulative probability to be hospitalization-free was higher in the incrPD group than in the stPD group (p = 0.021) (Fig. 1); only 20 % of the stPD patients were hospitalization-free after 24 months vs. 45 % of the incrPD group.
Patient survival
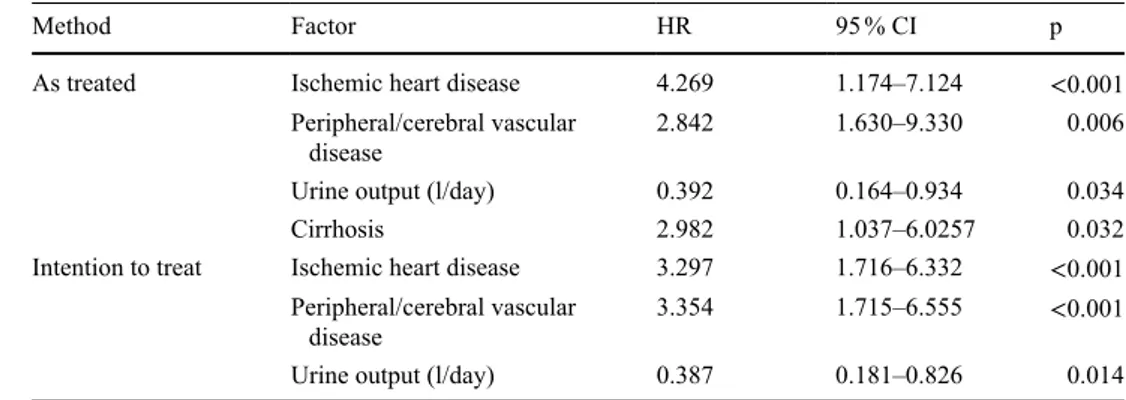
At the end of the follow-up, 41 patients (40 %) had died. In the incrPD group, 2 patients died of peritonitis, 1 died due to sepsis/severe infection, 2 due to ischemic heart disease, 1 due to a stroke, 2 due to cachexia and 1 died due to hem-orrhagic shock. In the stPD group, 3 patients died of peri-tonitis, 12 patients died of sepsis/severe infections, 3 from ischemic heart disease, 1 from stroke, 4 due to cachexia, 1 of malignancy, 6 due to other causes and in two cases the cause of death was unknown. Patient survival was not sig-nificantly different between incrPD and stPD, according to both the “as treated” and the “intention to treat” analysis (Fig. 2). The Cox hazards regression model was applied to identify those factors (PD modality, age, sex, comorbidity, RRF and urine output) significantly affecting patient sur-vival. Ischemic cardiopathy, peripheral and cerebral vascu-lar disease and cirrhosis (the latter only with “as treated” analysis) were detrimental factors. On the contrary, urine output significantly improved survival (Table 4). PD modal-ity did not affect survival.
Discussion
The basic assumption of incremental dialysis is to reach the minimal targets for adequate dialysis by summing renal function and dialysis dose. Mehrotra et al. and Gol-per first described an early and incremental approach to peritoneal dialysis [3, 4]. Some clinical experiences in incrPD were reported in the 90s; the main features of those 5 patients shifted from CAPD to APD, two because of
per-sonal choice, two due to abdominal hernias and one because of inadequate dialysis. A total of 69 stPD patients stopped PD: 27 (39 %) due to renal transplantation, one (1 %) recov-ered renal function, 10 (14 %) shifted to HD and 32 (46 %) died.
Adequacy data
A comparison of dialysis adequacy data is shown in Table 2: total (renal + peritoneal) wKt/V (twKt/V) and wClCr (twClCr) at the beginning, after 6 months and at the end of the treatment. Student’s t test for paired samples was carried out only between initial data and the 6th month, since at the end of treatment the follow-up periods were dramati-cally different among patients. At the sixth month Kt/V and wCrCl were significantly lower in stPD (p = 0.012 and 0.004, respectively), but stable in incrPD (p = 0.672 and 0.485). The changes were associated with a significant reduction of the renal contribution to both the urea and creatinine clear-ance in stPD (p < 0.001 for both), whereas changes were not statistically significant in incrPD.
Changes in peritoneal clearances occurring in stPD mainly depended on changes in the prescribed dose of dialysis.
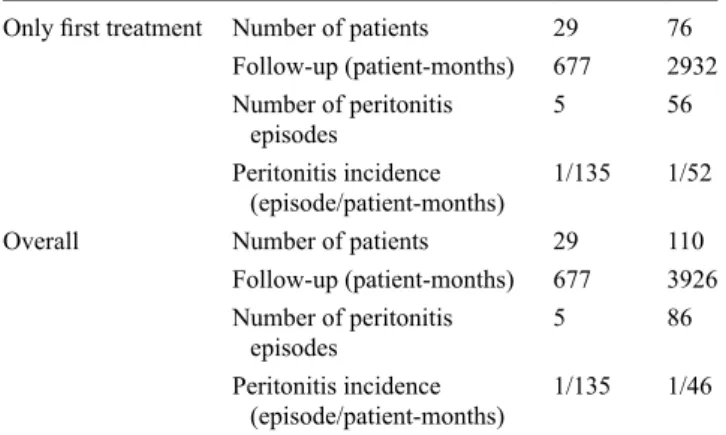
Incidence of peritonitis
During a total follow-up of 4603 patient-months, 91 epi-sodes of peritonitis occurred, with an incidence of 1/51 patient-months. When considering only the time spent on the first modality, incrPD or stPD, the incidence of perito-nitis was 1/135 patient-months in the incrPD group vs. 1/52 patient-months in the stPD group (Table 3). If all periods at risk are taken into consideration, the incidence of peritonitis in the stPD group becomes 1/46 patient-months (Table 3). In the stPD group, the 19 patients on CAPD had a follow-up of 665 patient-months with an incidence of peritonitis of 1/39, while the 57 on APD had an incidence of peritonitis
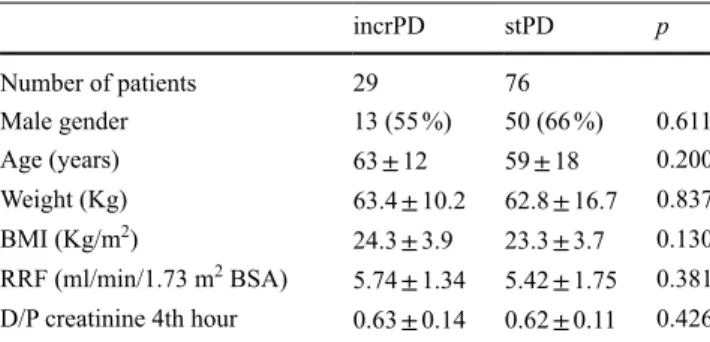
Table 1 Baseline data of the two groups: incrPD and stPD
incrPD stPD p Number of patients 29 76 Male gender 13 (55 %) 50 (66 %) 0.611 Age (years) 63 ± 12 59 ± 18 0.200 Weight (Kg) 63.4 ± 10.2 62.8 ± 16.7 0.837 BMI (Kg/m2) 24.3 ± 3.9 23.3 ± 3.7 0.130 RRF (ml/min/1.73 m2 BSA) 5.74 ± 1.34 5.42 ± 1.75 0.381 D/P creatinine 4th hour 0.63 ± 0.14 0.62 ± 0.11 0.426
incrPD incremental peritoneal dialysis, stPD standard peritoneal
dialysis, BMI body mass index, RRF residual renal function, BSA body surface area, D/P dialysate/plasma
Table 2
Results of renal and peritone
al clearances of creatinine and urea in incrPD and stPD groups
Initial data
6th month
End of treatment
a
p value 6th month vs. initial data
incrPD stPD p incrPD stPD p incrPD stPD p incrPD stPD Number of patients 29 75 25 66 25 65 twKt/V 2.08 ± 0.38 2.40 ± 0.58 0.008 2.13 ± 0.45 2.20 ± 0.43 0.527 1.77 ± 0.50 2.01 ± 0.35 0.007 0.672 0.012 twCrCl (l/w/1.73 m 2) 81 ± 15 85 ± 18 0.341 83 ± 19 77 ± 20 0.192 66 ± 27 62 ± 21 0.460 0.485 0.004
Residual renal creatinine clearance
7.55 ± 1.94 7.36 ± 2.21 0.690 7.74 ± 2.97 5.81 ± 2.72 0.004 5.39 ± 3.85 2.62 ± 3.35 0.001 0.797 < 0.001
Peritoneal creatinine clearance
1.98 ± 0.70 2.80 ± 0.93 < 0.001 2.02 ± 0.63 3.22 ± 1.21 < 0.001 2.16 ± 0.68 4.08 ± 1.37 < 0.001 0.297 < 0.001 Renal +
peritoneal creatinine clearance
9.53 ± 1.99 10.16 ± 2.52 0.231 9.77 ± 2.87 9.04 ± 2.63 0.255 7.55 ± 3.66 6.71 ± 2.82 0.246 0.600 0.015
Peritoneal contribution to total creatinine clearance (%)
21 ± 7 28 ± 8 < 0.001 22 ± 10 38 ± 19 < 0.001 32 ± 13 70 ± 30 < 0.001 0.448 < 0.001
Residual urea renal clearance
4.55 ± 1.23 3.81 ± 1.35 0.014 4.63 ± 1.42 3.12 ± 1.67 < 0.001 3.27 ± 2.09 1.4 ± 1.85 < 0.001 0.778 < 0.001
Peritoneal urea clearance
2.44 ± 0.78 4.50 ± 1.38 < 0.001 2.54 ± 0.79 4.68 ± 1.18 < 0.001 2.66 ± 0.81 5.66 ± 1.52 < 0.001 0.393 0.155 Renal +
peritoneal urea clearance
6.99 ± 1.15 8.30 ± 1.93 < 0.001 7.17 ± 1.32 7.80 ± 1.54 0.073 5.94 ± 1.80 7.06 ± 1.42 0.246 0.490 0.018
Peritoneal contribution to total urea clearance (%)
35 ± 11 54 ± 11 < 0.001 36 ± 11 61 ± 16 < 0.001 47 ± 16 82 ± 21 < 0.001 0.757 < 0.001
Creatinine renal clearance/urea renal clearance
1.70 ± 0.43 2.08 ± 0.92 0.007 1.69 ± 0.55 2.16 ± 1.24 0.007 1.65 ± 0.33 2.17 ± 1.58 0.051 0.678 0.080
Residual renal function
6.08 ± 1.47 5.61 ± 1.49 0.160 6.20 ± 2.02 4.48 ± 2.12 < 0.001 4.36 ± 2.96 2.03 ± 2.55 < 0.001 0.792 < 0.001 A ll v al ue s e xc ep t K t/V a re n or m al iz ed t o 1 .7 3 m 2 of b od y s ur fa ce a re a St at is tic al ly s ig ni fic an t v al ue s a re i n b ol d St at is tic al ly n on -s ig ni fic an t v al ue s a re i n i ta lic twK t/V to ta l w ee kl y K t/V , t w C rCl to ta l w ee kl y c re at in in e c le ar an ce aLa st v al ue b ef or e c ha ng in g f ro m i nc rP D t o s tP D o r s to pp in g P D
cost reduction and increased PD penetration; it is also con-sidered as an ideal bridge to renal transplantation.
Our experience with incrPD began in the late 90s and is based on two assumptions: (a) advanced renal failure and renal replacement therapy are a continuum and should be treated as such [18]; (b) in accordance with the stud-ies regarding peritoneal dialysis adequacy [2], total blood purification is considered as the sum of renal and peri-toneal clearance, even though they have different blood purification profiles. Furthermore, we have to clarify the enrolment modality: the choice of PD modality between CAPD and APD, as well as between incrPD and stPD, was up to the informed patient (in the absence of clinical contraindications). The incremental approach was sug-gested only to patients who had chosen CAPD because, at that time, incrAPD had not been recommended in the studies are summarized in Table 5. The aforementioned
studies enrolled only a few patients, without a control group and without any statistical comparison. De Vecchi et al. first reported working activity, degree of rehabilita-tion and quality of life in incrPD patients; quality of life and social rehabilitation were better preserved with incrPD than with stPD [15].
As of today, there are no papers which delineate the features, efficacy, feasibility and safety of incrPD. Despite these considerations, incrPD is used more and more often: in Italy, 54 % of PD centers use incrPD and 29 % of patients start PD with the incremental approach [17]. The hypotheti-cal benefits of incrPD may explain its widespread use: better quality of life, reduced glucose exposition, better peritonitis-free survival, longer preservation of residual renal function,
Table 3 Prevalence of peritonitis in the two groups of patients: incrPD
and stPD
Only first treatment Number of patients 29 76 Follow-up (patient-months) 677 2932 Number of peritonitis
episodes 5 56
Peritonitis incidence
(episode/patient-months) 1/135 1/52
Overall Number of patients 29 110
Follow-up (patient-months) 677 3926 Number of peritonitis
episodes 5 86
Peritonitis incidence
(episode/patient-months) 1/135 1/46 Overall: calculated over the entire follow-up period according to the modality in use at that time and considering the patient who changes modality as a new patient
Log-rank test: p = 0.021 100 80 60 40 20 0 Follow-up (months) 48 24 0 12 36 60 incrPD stPD Cumulative
survival from hospitalization
(%)
Fig. 1 Cumulative probability to be hospitalization-free in incrPD and
stPD Log-rank test: p = 0.057 100 80 60 40 20 0 Follow-up (months) 96 72 48 24 0 12 36 60 84 108 120 incrPD stPD Patient survival (%) Log-rank test: p = NS 100 80 60 40 20 0 96 72 48 24 0 12 36 60 84 108 120 incrPD stPD Patient survival (%) AS TREATED ANALYSIS
INTENTION TO TREAT ANALYSIS
a median duration of incrPD of 17 months, which could positively affect the patients’ quality of life on PD due to a lesser burden of dialysis procedures. On this ground it is also clear that patients on incrPD need a closer clini-cal follow-up to reduce the risk of under-dialysis; in our center, RRF was measured monthly and total clearances quarterly in incrPD.
Peritonitis is a major complication of PD and remains an important cause of drop-out. According to the recommen-dations of the International Society for Peritoneal Dialysis, the peritonitis rate should be lower than one episode every 18 months. In the literature regarding incrPD, peritonitis rates range from zero (follow-up: 84 patient-months) to one episode/21 patient-months (Table 5). De Vecchi et al. reported that the risk of peritonitis was associated to the number of exchanges [15]. In our study the peritonitis rate was 1/135 patient-months in the incrPD group and 1/52 patient-months in the stPD group (Table 3). The reduced number of connections and the “dry” period could have played a role in reducing the peritonitis rate in incrPD even though it was not statistically significant according to the Kaplan–Meier curves. On the other hand, the low frequency of exchanges of APD, in incrPD, could reduce patient experience, increase the risk of errors and, conse-quently, the occurrence of peritonitis. According to our results, however, this effect does not appear to be so impor-tant. Hospitalization-free survival was significantly better in the incrPD group than in the stPD group; however, it must be considered that the treatment of our patients suf-fering from peritonitis is done on an inpatient basis. We did not find any papers comparing patient survival in incrPD vs. stPD. In our study, incrPD showed a trend towards bet-ter survival at the “intention to treat” analysis, but it was marginally non-significant (Fig. 2). The Cox analysis did not indicate incrPD as a risk factor for mortality (Table 4). The data we collected do not suggest a clear survival ben-efit with incrPD, but at least they support a non-inferiority of incrPD vs. stPD.
The main biases of our study are: (1) it is a retrospective, one-center study, and (2) the patients were not randomized. clinical literature. This study’s comparison of incrPD vs.
stPD was made only in those patients who had started dialysis before 2008 as, after the end of 2007, the positive clinical results yielded by incrPD urged us to suggest it to all patients with RRF ≥3 ml/min/1.73 m2 BSA. Because of this, a stPD group for comparison was not available any more.
For a comparison between incrPD and stPD to be made, it is necessary that: groups of patients are comparable, the start of dialysis occurs at similar values of RRF and patients who started PD due to non-renal indication are excluded. The two groups of our study were comparable (Table 1). RRF at the beginning of PD, calculated as the mean of measured creatinine and urea clearance, was in line with the guidelines in force at the time of the study [19]. RRF was not different between the incrPD group and the stPD group, so incrPD was not started at an earlier stage than stPD. It should be noted that to start with incrPD it is necessary to have a good pre-dialysis program and a timely initiation of dialysis; several studies have shown that a good pre-dialysis education program increases the prevalence of patients opting for self-care dialysis [19–
25]. As far as adequacy is concerned, patients in both the incrPD group and the stPD group were always above the minimal targets of adequacy [2] even though, as expected, adequacy values were higher on stPD at the beginning of treatment (Table 2).
It is interesting to note that total Kt/V and wCrCl in the incrPD groups remained stable over the first 6 months, whereas they decreased significantly in the stPD group. This could be due to a better preservation of residual renal function in incrPD both for creatinine and urea whose ratios did not significantly change. It should also be noted that the peritoneal contribution to the total clearance is always higher for urea than for creatinine, due to differ-ences in the renal handling and peritoneal permeability of those molecules. The results of this study suggest a protec-tive role of incrPD on RRF which was stable in incrPD in the first 6 months whereas it significantly decreased in stPD (Table 2). This stability could be the reason for
Method Factor HR 95 % CI p
As treated Ischemic heart disease 4.269 1.174–7.124 <0.001
Peripheral/cerebral vascular
disease 2.842 1.630–9.330 0.006
Urine output (l/day) 0.392 0.164–0.934 0.034
Cirrhosis 2.982 1.037–6.0257 0.032
Intention to treat Ischemic heart disease 3.297 1.716–6.332 <0.001 Peripheral/cerebral vascular
disease 3.354 1.715–6.555 <0.001
Urine output (l/day) 0.387 0.181–0.826 0.014
HR hazard ratio, CI confidence interval
Table 4 Results of the Cox
hazard regressions on patient survival
Author , year Period of time Study design No. pts on incrPD incrPD schedule Initial GFR (ml/ min) Time on incrPD (patient-months) Peritonitis rate (episode/patient- months)
Results/outcomes W illiams, 1999 [ 14 ] NA Pilot study 15 CAPD 1 dwell/day 9.8 ± 1.9 90 1/30
Adequacy (good) Hospitalization (3 admissions) Survival (patients and technique)
De
Vecchi et al., 2000 [
15
]
1995–1999
Pilot prospective, not controlled
25
CAPD 1–2 dwells/ day
6–10
262
1/21
Good rehabilitation with incrPD Better quality of life with incrPD Adequacy (good) Exit-site infections (8 episodes) Complications Hospitalization (3 days/ year) Survival (patients and technique)
Burkart et al., 2000 [
7
]
1997–1999
Non randomized, prospective
13
CAPD 1–3 dwells/ day
6.7
±
2.4
159
1/53
Adequacy (good) Complications Survival (patients and technique)
Foggensteiner et al., 2002 [
8
]
1997–2000
Pilot, not random
-ized, prospective 39 CAPD 1 dwell/day 10 422 1/30
Adequacy (good) Complications Hospitalization (3.6 days/ year) Survival (patients and technique)
Neri et al., 2003 [ 16 ] 2000–2001 Preliminary experience 5
APD 3–4 sessions/ week
7–9
84
none
Adequacy Peritonitis Compliance Complications Survival (patients and technique)
V iglino et al., 2008 [ 27 ] 2004–2007 Retrospective 11 CAPD 2 dwells/day 7.3 ± 2.7 106 NA
Choice of dialysis modality RRF and adequacy (good) Technique survival
Table 5
Papers published regarding c
As a consequence, the results cannot be generalized. How-ever, its results could be of some help in designing future multicenter randomized studies.
Conclusions
Incremental peritoneal dialysis, which must not to be con-fused with an early start of dialysis, provides adequate dia-lytic doses, has a reduced hospitalization rate compared to stPD, yields a similar patient survival to that observed with stPD, and is time saving for the patient. Therefore, incrPD is a safe modality to begin dialysis and should be offered to most patients with significant RRF (3–6 ml/min/1.73 m2 BSA) at the start of dialysis. In order to obtain good compli-ance, patients should be given adequate information about the future need to increase the dose of dialysis when RRF declines. A longer preservation of RRF could be a further positive effect of this modality and favor its choice among patients on the waiting list for renal transplantation.
Two things are necessary to start dialysis with incrPD: (1) a well-organized pre-dialysis outpatient clinic able to postpone the start of dialysis [26] as well as to “build” an informed and compliant patient; (2) a close clinical and lab-oratory follow-up to avoid that a sudden reduction in RRF could precipitate the patient towards a condition of under-dialysis. Moreover, last but not least, some patients could receive a kidney transplant while on incrPD before switch-ing to full-dose PD. Finally, one cannot exclude than incrPD could favor the diffusion of PD, a dialysis modality that is cost-saving in comparison to HD.
Compliance with ethical standards
Conflict of interest Author GC has received research lecture fee/
expertise from Baxter SpA and Sigma Tau, grants to the institution by Pfizer and Gambro and financial support for attending symposia by Amgen, all not-related to the present study. The other authors declare that they have no conflict of interest.
Ethical approval The study was conducted in accordance with the
ethical standards of our institution and with the 1964 Helsinki declara-tion and its later amendments or comparable ethical standards.
Research involving human participants and/or animals This
arti-cle does not contain any studies with human participants or animals performed by any of the authors.
Informed consent For this type of study formal consent is not
required.
Open Access This article is distributed under the terms of the
Creative Commons Attribution 4.0 International License ( http://cre-ativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. Author , year Period of time Study design No. pts on incrPD incrPD schedule Initial GFR (ml/ min) Time on incrPD (patient-months) Peritonitis rate (episode/patient- months)
Results/outcomes Domenici et al., 201 1 [ 28 ] 2000–2008 Retrospective 17 ? 6.9 ± 1.1 480 1/48
Reduced rate of loss of RRF
Jeloka et al., 2013 [ 29 ] 2006–201 1 Retrospective 13 CAPD 1 dwell/day 7.8 ± 2.6 244 1/56 Adequacy (good)
Barràs Sans et al., 2016 [
30 ] 2003–2012 Retrospective 46 CAPD 3 dwells/day 8.0 ± 3.2 1035 1/99
Reduced rate of loss of RRF Reduced dose of erythropoietin
APD a ut om at ed p er ito ne al d ia ly si s, C APD c on tin uo us a m bu la to ry p er ito ne al d ia ly si s. F or o th er a bb re vi at io ns , s ee p re vi ou s t ab le s Table 5 (continued)
16. Neri L, Viglino G, Cappelletti A, Gandolfo C, Barbieri S (2003) Incremental dialysis with automated peritoneal dialysis. Adv Perit Dial 19:93–96
17. Gruppo di Studio di Dialisi Peritoneale (2014) Retrieved Jun 9, 2016, from http://www.dialisiperitoneale.org/web/procedure/pro-tocollo.cfm?List=WsIdEvento,WsIdRisposta,WsRelease&c1=C ENGDSDP&c2=30&c3=1
18. Burkart JM, Golper TA (2000) Should we treat patients with incremental dialysis prescriptions? Blood Purif 18:298–303 19. EBPG Working Group (2002) Section I: measurement of renal
function, when to refer and when to start dialysis. 1.3 When to start dialysis. Nephrol Dial Transplant 17:10–11
20. Mehrotra R, Marsh D, Vonesh E, Peters V, Nissenson A (2005) Patient education and access of ESRD patients to renal replace-ment therapies beyond in center hemodialysis. Kidney Int 68:378–390
21. Manns BJ, Taub K, Vanderstraeten C, Jones H, Mills C, Visser M, McLaughlim K (2005) The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int 68:1777–1783
22. Mason J, Khunti K, Stone M, Farooqi A, Carr S (2008) Educa-tional interventions in kidney disease care: a systematic review of randomized trials. Am J Kidney Dis 51:933–951
23. Kurella Tamura M, Li S, Chen SC, Cavanaugh KL, Whaley-Connell AT, McCullough PA, Mehrotra RL (2014) Educational programs improve the preparation for dialysis and survival of patients with chronic kidney disease. Kidney Int 85:686–692 24. Ribitsch W, Haditsch B, Otto R, Schilcher G, Quehenberger F,
Roob JM, Rosenkranz AM (2013) Effects of a pre-dialysis patient education program on the relative frequencies of dialysis modali-ties. Perit Dial Int 33:367–371
25. Blake PG, Quinn RR, Oliver MJ (2013) Peritoneal dialysis and the process of modality selection. Perit Dial Int 33:233–241 26. Dattolo P, Michelassi S, Amidone M, Allinovi M, Vignali L,
Antognoli G, Roperto R, Pizzarelli F (2015) Structured clini-cal follow-up for CKD stage 5 may safely postpone dialysis. J Nephrol 28:463–469
27. Viglino G, Neri L, Barbieri S (2008) Incremental peritoneal dialy-sis: effects on the choice of dialysis modality, residual renal func-tion and adequacy. Kidney Int Suppl 73:S52–S55
28. Domenici A, Comunian MC, Fazzari L, Sivo F, Dinnella A, Della Grotta B, Punzo G, Menè P (2011) Incremental peritoneal dialysis favourably compares with hemodialysis as a bridge to renal trans-plantation. Int J Nephrol. doi:10.4061/2011/204216. (Epub 2011
Sep 15)
29. Jeloka T, Sanwaria P, Chaudhari L, Periera A (2013) “Ico-Alone” single nocturnal exchange to initiate peritoneal dialysis in patients with residual renal function. Five year, single center experience. Indian J Nephrol 23:276–279
30. Barràs Sans M, Chacón Camacho A, Cerdá Vilaplana C, Usón Nuño A, Fernández E (2016) Incremental peritoneal dialysis: Clinical outcomes and residual kidney function preservation. Nefrologia 36:299–303
References
1. Canada-USA (CANUSA) Peritoneal Dialysis Study Group (1996) Adequacy of dialysis and nutrition in continuous peri-toneal dialysis: association with clinical outcomes. J Am Soc Nephrol 7:198–207
2. Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawani-shi H, Blake PG (2006) Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int 26:520–522
3. Mehrotra R, Nolph KD, Gotch F (1997) Early initiation of chronic dialysis: role of incremental dialysis. Perit Dial Int 17:426–430 4. Golper TA (1998) Incremental dialysis. J Am Soc Nephrol
9:S107–S111
5. Nolph KD (1998) Rationale for early incremental dialysis with continuous ambulatory peritoneal dialysis. Nephrol Dial Trans-plant 13(Suppl 6):117–119
6. Tzamaloukas AH (1999) Incremental initiation of peritoneal dial-ysis: current practice. Adv Perit Dial 15:175–178
7. Burkart JM, Satko SG (2000) Incremental Initiation of dialysis: one center’s experience over a two-year period. Perit Dial Int 20:418–422
8. Foggensteiner L, Baylis J, Moss H, Paul W (2002) Timely initia-tion of dialysis. Single-exchange experience in 39 patients start-ing peritoneal dialysis. Perit Dial Int 22:471–476
9. Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Demp-ster J, Fraenkel MB, Harris A, Harris DC, Johnson DW, Kes-selhut J, Luxton G, Pilmore A, Pollock CA, Tiller DJ, IDEAL Study Steering Committee (2004) The initiating dialysis early and late (IDEAL) study: study rationale and design. Perit Dial Int 24:176–181
10. Susantitaphong P, Altamimi S, Ashkar M, Balk EM, Stel VS, Wright S, Jaber BL (2012) GFR at initiation of dialysis and mor-tality in CKD: a meta-analysis. Am J Kidney Dis 59:829–840 11. Clinical Practice Guidelines and Clinical Practice
Recommenda-tions, National Kidney Foundation, Kidney Disease Outcomes Quality Initiative (2006) Retrieved Jun 9, 2016, from https:// www.kidney.org/sites/default/files/docs/12-50-0210_jag_dcp_ guidelines-pd_oct06_sectionb_ofc.pdf
12. Rosansky SJ, Cancarini G, Clark WF, Eggers P, Germaine M, Glassock R, Goldfarb DS, Harris D, Hwang SJ, Imperial EB, Johansen KL, Kalantar-Zadeh K, Moist LM, Rayner B, Steiner R, Zuo L (2013) Dialysis initiation: what’s the rush? Semin Dial 26:650–657
13. Nesrallah GE, Mustafa RA, Clark WF, Bass A, Barnieh L, Hem-melgarn BR, Klarenbach S, Quinn RR, Hiremath S, Ravani P, Sood MM, Moist LM, Canadian Society of Nephrology (2014) Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ 186:112–117 14. Williams PF (1999) Timely initiation of dialysis. Am J Kidney
Dis 34:594–595
15. De Vecchi AF, Scalamogna A, Finazzi S, Colucci P, Ponticelli C (2000) Preliminary evaluation of incremental peritoneal dialysis in 25 patients. Perit Dial Int 20:412–417