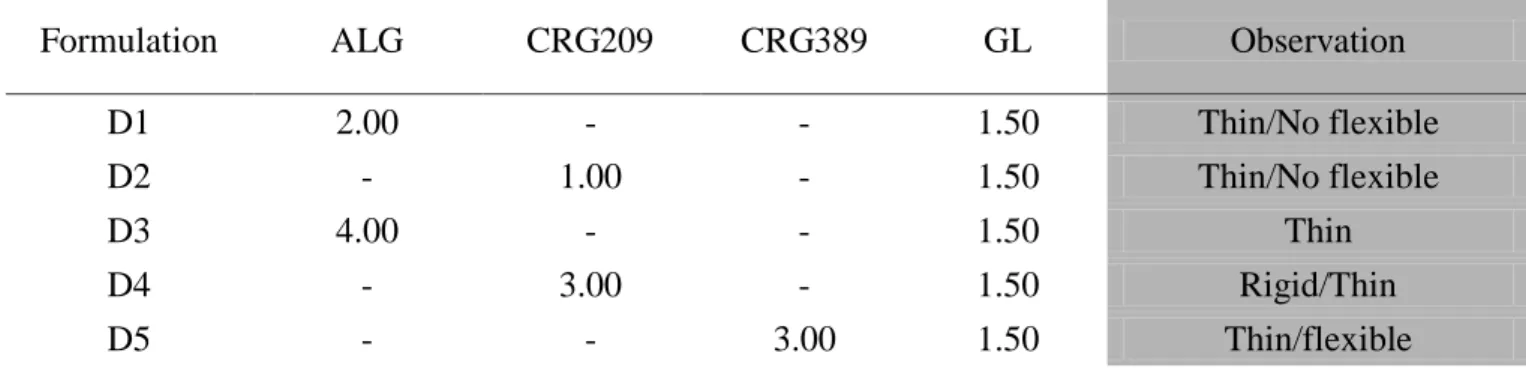
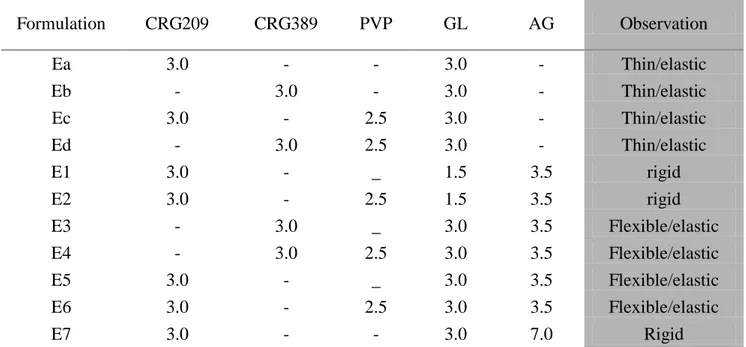
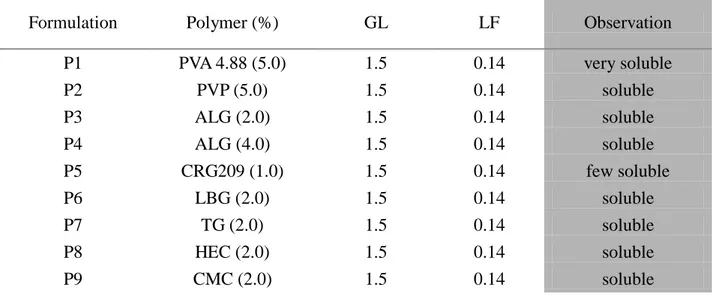
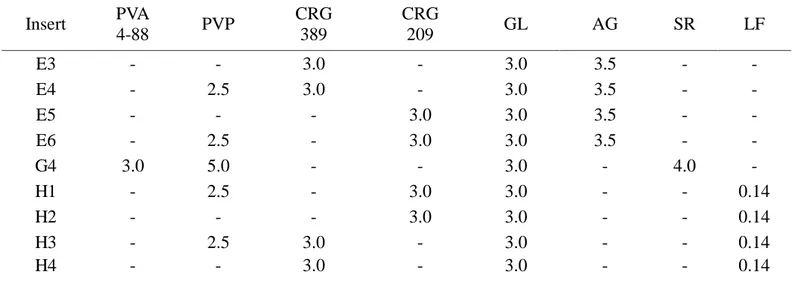
2
3
Abstract
The conventional ocular dosage forms are eye drops and suspensions. These dosage forms are easy to instil but they are also rapidly drained away from pre-corneal area by constant tear flow and lacrimo-nasal drainage. Despite the poor bioavailability the cornea is considered to be a major pathway for ocular penetration of topically applied drugs. Recently, hydrogels, viscous liquid formulations, solid delivery devices (inserts, drug-soaked contact lenses), have been investigated in the attempt to improve the efficacy of ocular medications, applying also the concepts of mucoadhesion. The ocular inserts offer many advantages over conventional dosage forms, like increased ocular residence, possibility of releasing drugs at a slow and constant rate, accurate dosing, exclusion of preservatives and increased shelf life. Moreover, the use of these devices reduces systemic absorption, which otherwise freely occurs with eye drops; it also ensures better patient compliance due to lower frequency of administration and lower incidence of side effects.
The aims of this study were: a) the preparation of bioadhesive ocular inserts based on natural or synthetic polymers (cellulose derivatives, polyvinyl alcohol, natural gums and polysaccharidic polymers) containing appropriate amount of drugs (levofloxacin), natural extract (arabinogalactan and sericin), blood components (platelet lysate) or protein drugs (bevacizumab). b) The technological characterization of the prepared inserts such as: in vitro mucoadhesion test; in vitro drug release study; contact angle measurements; rheological behaviour; swelling studies. c) An in vivo evaluation on albino rabbits of the best experimental formulations to assess the biological compatibility with the ocular structure and the related therapeutic activity.
The AG, SR and LF matrices were prepared by film casting method using film forming polymers (PVP and PVA), natural bioadhesive polymers (CRGs) and glycerin (GL) as a plasticizer. The prepared ocular insert based on Sericin, as active substance, showed very good mucoadhesive properties, useful for retention on the eye surface, and increased the healing rate of corneal wound in rabbits with respect to untreated lesion.
Ocular inserts of levofloxacin showed good technological properties, demonstrated sustained release of the drug for up to 8 h after in vitro studies, and increasing the availability in cul-de-sac as compared to eye drops of the same drug after in vivo studies on albino rabbits.
Lyophilized ocular matrices were prepared for delivering new active substances such as PL and BVZ, which are thermally unstable. The unloaded matrices showed good technological properties, suitable for an ophthalmic application, good mucoadhesive properties, and good in vivo wound healing properties. The addition of PL to the formulations produced matrices still good for the ocular administration. Both freeze-drying process and polymers employed did not disturb the activity of PL, how confirmed by proliferation test on HCE.
All inserts were well tolerated by the rabbit eye and did not produce any irritation. BVZ loaded matrices has the potential to provide an effective and time constant drug release, even if the kinetics of inserts must be investigated.
4
1. INTRODUCTION ... 7
2. THE EYE AND THE OPHTHALMIC ADMINISTRATION OF DRUGS ... 9
2.1.OCULAR ANATOMY AND PHYSIOLOGY ... 9
2.2.OPHTHALMIC DISEASES ... 10
2.3.OCULAR DRUG DELIVERY ROUTES ... 11
2.4.OPHTHALMIC DRUG DELIVERY SYSTEMS... 13
2.4.1.OCULAR INSERTS ... 14 2.4.2.BIOADHESIVE POLYMERS ... 17 2.4.2.1.CELLULOSE DERIVATIVES. ... 19 2.4.2.2.ACRYLATES ... 19 2.4.2.3.HYALURONAN ... 20 2.4.2.4.POLYSACCHARIDES ... 20
2.4.3.MUCOADHESIVE OCULAR INSERTS ... 22
3. AIMS OF THE STUDY ... 25
4. MATERIALS ... 26 4.1. ANIMALS ... 27 4.2. ARABINOGALACTAN ... 28 4.3. LEVOFLOXACIN ... 29 4.4. SERICIN ... 30 4.5. PLATELET LYSATE ... 33 4.6. BEVACIZUMAB ... 33 5. METHODS ... 35
5.1. PH AND OSMOLALITY MEASUREMENTS ... 35
5.2. MUCOADHESION TEST ... 35
5.3. CONTACT ANGLE MEASUREMENTS ... 35
5.4. DSC ANALYSIS ... 36
5
5.6. INDUCTION OF AN EXPERIMENTAL CORNEAL LESION ON RABBIT ... 36
5.7. CYTOTOXICITY TEST ... 37
5.8. PREPARATION AND TECHNOLOGICAL CHARACTERIZATION OF BIOADHESIVE INSERTS PREPARED BY FILM CASTING METHOD ... 38
5.8.1. PREFORMULATION STUDIES ... 38
5.8.2.PREPARATION OF OCULAR INSERTS ... 38
5.8.3.MUCOADHESION TEST ... 39
5.8.4.DSC ANALYSIS ... 39
5.8.5.SWELLING STUDIES... 39
5.8.6.CONTACT ANGLE MEASUREMENTS ... 39
5.8.7.IN VITRO LF RELEASE STUDY ... 40
5.8.8.ANALYTICAL METHOD OF LEVOFLOXACIN ... 41
5.8.9.FORMULATIONS FOR IN VIVO PHARMACOKINETIC STUDIES. ... 41
5.8.10.EVALUATION OF THE PHARMACOKINETIC IN THE PRECORNEAL AREA ... 41
5.8.11.CORNEAL WOUND HEALING ... 42
5.9. PREPARATION AND TECHNOLOGICAL CHARACTERIZATION OF POLYMERIC MATRICES PREPARED BY FREEZE-DRIED METHOD: PRELIMINARY STUDIES ... 42
5.9.1.POLYMERIC VEHICLES UNDER STUDY ... 42
5.9.2.PREPARATION OF LYOPHILIZED MATRICES ... 42
5.9.3.WATER SORPTION TIME ... 43
5.9.4.TECHNOLOGICAL CHARACTERIZATION OF POLYMERIC VEHICLES UNDER STUDY ... 43
5.9.4.1. RHEOLOGICAL BEHAVIOUR ... 43
5.9.4.2. CONTACT ANGLE MEASUREMENTS ... 43
5.9.5.MUCOADHESION PROPERTIES OF THE POLYMERS UNDER STUDY ... 44
5.9.6.CORNEAL WOUND HEALING ... 44
5.10. PREPARATION AND CHARACTERIZATION OF FREEZE-DRIED POLYMERIC MATRICES CONTAINING PL ... 44
5.10.1.FORMULATIONS UNDER STUDY ... 44
5.10.2.MATRICES UNDER STUDY... 45
6
5.10.4.CELL PROLIFERATION ... 45
5.11. PREPARATION AND CHARACTERIZATION OF FREEZE-DRIED POLYMERIC MATRICES CONTAINING BVZ... 46
5.11.1.PREPARATION OF MATRICES FOR IN VITRO RELEASE STUDY ... 46
5.11.2.IN VITRO RELEASE STUDY ... 47
5.11.3.ANALYTICAL METHODS OF FITC-DEXTRANS... 48
5.11.4.ANALYTICAL METHODS OF BEVACIZUMAB ... 48
5.12. STERILIZATION ... 48
6. RESULTS AND DISCUSSION ... 49
6.1. CYTOTOXICITY TEST ... 49
6.2. BIOADHESIVE INSERTS PREPARED BY FILM CASTING METHOD ... 49
6.3. POLYMERIC MATRICES PREPARED BY FREEZE-DRIED METHOD ... 53
6.3.1.PRELIMINARY STUDIES ... 53
6.3.2.STERILIZATION ... 56
6.3.3.FREEZE-DRIED POLYMERIC MATRICES CONTAINING PL ... 56
6.3.4.FREEZE-DRIED POLYMERIC MATRICES CONTAINING BVZ... 57
7. CONCLUSIONS ... 59
REFERENCES ... 60
7
1. Introduction
The eyes provide the majority of information of the outside world to humans, and therefore ocular disorders easily disturb daily life. The eye is effectively protected against foreign substances by its structural features, which is extremely important in the case of microbes and for the maintenance of vital ocular functions. However, these barriers also complicate medical treatment by preventing drug delivery into the eye, thus presenting a constant challenge to the formulator to circumvent the protective barriers of the eye without causing permanent tissue damage. The conventional ocular dosage forms are eye drops and suspensions. These dosage forms are easy to instil but suffer from the inherent drawback that the majority of the medication they contain is immediately diluted in the tear fluid as the solution is instilled into the cul-de-sac and is rapidly drained away from pre-corneal area by constant tear flow and lacrimo-nasal drainage. Therefore, the target tissue absorbs a very small fraction of the instilled dose. For this reason, concentrated solutions and frequent dosing are required for the instillation to achieve an adequate level of therapeutic effect. Despite the poor bioavailability the cornea is considered to be a major pathway for ocular penetration of topically applied drugs (Doane et al., 1978). Drug permeability across the ocular surface is highly dependent on the features of the drug molecule; the drug should be neither extremely hydrophilic nor lipophilic. Thus small lipophilic drugs are absorbed into the eye via the cornea whereas large hydrophilic molecules absorb into the eye through the conjunctiva and sclera (Ahmed and Patton 1985). Systemic administration of drugs is not effective due to the blood/aqueous and the blood/retinal barriers. Furthermore, the use of high doses of administrated drugs to compensate a poor bioavailability may cause systemic and local adverse effects. Ophthalmic drug delivery can also be achieved by periocular and intraocular injections, but these methods are painful, inconvenient and can cause complications in the eye. Moreover, application of many potentially active ophthalmic compounds is seriously limited because of their very low water solubility, or water stability. For example, fast degradation of protein drugs effectively prevents their delivery into target cells regardless of the administration method used.
8
Recently, hydrogels, viscous liquid formulations, solid delivery devices (inserts, drug-soaked contact lenses), have been investigated in the attempt to improve the efficacy of ocular medications, applying also the concepts of mucoadhesion.
The ocular inserts offer many advantages over conventional dosage forms, like increased ocular residence, possibility of releasing drugs at a slow and constant rate, accurate dosing, exclusion of preservatives and increased shelf life. Moreover, the use of these devices reduces systemic absorption, which otherwise freely occurs with eye drops; it also ensures better patient compliance due to lower frequency of administration and lower incidence of side effects.
The eye is prone to a number of diseases, including conjunctivitis (or red eye), uveitis, iritis, cytomegalovirus infection, corneal lesions, age-related macular degeneration, diabetic macular edema, cataract, glaucoma.
Pathogenic bacteria are the cause for a large number of eye disorders and among the antibiotics, levofloxacin is a drug of choice in the treatments of bacterial infections (Prakash Gorle and Gattani, 2010).
Corneal lesions, represented by cut, scratches or abrasions of the thin protective layer of corneal epithelium, are treated by a lot of actives like antiseptic, anti-infective and anti-inflammatory drugs. Recently, new molecules were discovered and employed in ocular lesions like polysaccharides (arabinogalactan) (Burgalassi et al., 2011) and the glycosaminoglycans (Yamada and Sugahara, 2008), growth factors released from platelets (platelet lysate) (Vecchio et al., 2006; Geremicca et al., 2010), and some natural extracts (sericin) (Nagai et al., 2009), which positively influencing attachment and cell adhesion to specific substrates of extracellular matrix or migration and proliferation of epithelial cells, promote the neo-formation of an intact epithelial layer improving the whole healing process.
Vascular endothelial growth factor (VEGF) and its receptors play an important role in the neo-vessel formation that is observed in diabetic retinopathy, venous retinal occlusion, age-related macular degeneration and corneal neovascularization. Recent studies have shown that anti-VEGF drugs (bevacizumab) can provide beneficial effects after intra-vitreous injection in age-related macular degeneration neovascularization, diabetic retinopathy and glaucoma, with minimal toxicity or side effects (Reggiani Mello et al., 2011).
9
2. The eye and the ophthalmic administration of drugs
2.1. Ocular anatomy and physiology
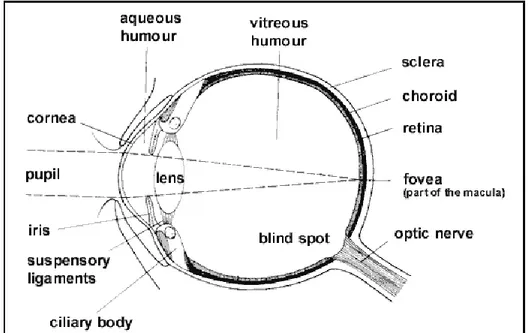
Although it is small in size, the eye (Figure 2.1.) provides us with the most important of the five senses: vision.
The eyeball has a wall consisting of three layers of tissue. The outer region consists of the cornea and the sclera. The cornea is a transparent and avascular structure that refracts and transmits the light to the lens and the retina and protects the eye against infection and structural damage to the deeper parts. The sclera forms a connective tissue coat that protects the eye from internal and external forces and maintains its shape. The cornea and the sclera are connected at the limbus. The visible part of the sclera is covered by a thin transparent membrane, the conjunctiva. It is vascular and moistened by the tear film. The goblet cells are an important anatomical element of the conjunctiva, which synthesize secretory mucins and TFF-peptides or trefoil factors. A volume of about 2 to 3 μl of mucus is secreted daily and a turnover of the mucus layer in approximately 15 to 20 h is reported. Mucus consist of glycoproteins (whose primary component is mucin), proteins, lipids, electrolytes, enzymes, mucopolysaccharides and water.
The middle layer of the eye is composed of the iris, the ciliary body and the choroid. The iris, in the front of the eye, is pigmented and is responsible for eye color. It controls the size of its opening, the pupil, and thus the amount of light reaching the retina: in dim light, the pupil opens wider letting more light into the eye, in bright light the pupil closes down. This not only reduces the amount of light entering the eye but also improves its image-forming ability. The ciliary body controls the power and shape of the lens and is the site of aqueous production. The choroid is a vascular layer that provides oxygen and nutrients to the outer portions of the retina.
The inner layer of the eye is the retina. It contains the light receptors, the rods and cones, and many interneurons that process the signals arising in the rods and cones before passing them back to the brain.
10 aqueous, the vitreous and the lens.
The aqueous is a water-like fluid which fills the front part of the eye between the lens and cornea. This fluid is produced by the ciliary body and drains back into the blood circulation through channels in the anterior chamber angle. It is turned over every 100 minutes.
The vitreous is a jelly-like, clear fluid which fills most of the eye (from the lens back). This tends to liquefy with age, and its separation from the retina can lead to retinal tears and detachment.
The lens is located just behind the iris, and helps to focus light: a "capsule" surrounds its "nucleus". The nucleus can become cloudy, and this is termed cataract.
Fig. 2.1. The structure of the eye
2.2. Ophthalmic diseases
The eye is prone to a number of diseases including:
- conjunctivitis: inflammation of the conjunctiva that can be allergic or bacterial. On the basis of the structures affected they can be classified in blepharoconjunctivitis (inflammation of eyelids), keratoconjunctivitis (inflammation of the cornea) and episcleritis (inflammation between conjunctiva and sclera). The signs and symptoms are: redness, irritation, grittiness and watering of eyes;
11
in anterior uveitis, intermediate uveitis, posterior uveitis, pan-uveitic uveitis;
- glaucoma: obstruction to the outflow of aqueous humor from the anterior segment. It is classified in primary open-angle and angle-closure glaucoma;
- age-related macular degeneration: (AMD) it can be classified in dry (non-exudative) and wet (exudative). In dry AMD there is breakdown of photoreceptors, retinal pigment epithelium and choriocapillaris. In wet AMD there is growth of abnormal blood vessels behind the retina, in the macula, disruption of Bruch’s membrane and degeneration of retinal pigment epithelium leading to a complete loss of vision;
- macular edema: is classified in cystoids macular edema and diabetic macular edema. The pathology is associated with the leakage of fluids, from retinal blood vessels, and deposition of lipids and proteins beneath the center of the macula resulting in swelling;
- cataract: lens opacities that obstruct the passage of light. It can be age-related, congenital, secondary, traumatic;
- proliferative vitreoretinopathy: consists in simple scar formation and proliferation of cells in vitreous and retina. On the basis of the inflammation of retina it is classified in focal, diffuse, subretinal, circumferential;
- cytomegalovirus: is an infection of the retina that can leads to retinal detachment and complete blindness.
- corneal lesions: are cuts, scratches or abrasions of the thin protective layer of corneal epithelium. The sign and symptoms are pain, tearing, photophobia, foreign body sensation and a gritty feeling. Corneal lesions may be caused by many factors: unexpected contact with toxic substances (alkali or acid burns), infections (bacterial, viral and fungal), mechanical factors (accidental trauma), altered physiologic functions, such as a reduced tears production in dry eye syndrome, and the use of contact lenses, both rigid and soft.
2.3. Ocular drug delivery routes
Depending on the physicochemical characteristics of the compounds, delivery of drugs from the surface of the eye can occur through the corneal route or the conjunctival/scleral route.
The corneal route is the main route for delivery of drugs to the anterior chamber and for most ocular drugs, passive diffusion is the main transport process across the
12
cornea. Passive diffusion depends on their lipophylicity, molecular weight, charge and ionization degree. Permeation of hydrophilic drugs and macromolecules through the corneal epithelium is limited by the presence of tight junctions between adjacent outer superficial epithelial cells: the corneal surface epithelial intercellular pore size has been estimated to range between 20 Å and 30 Å and only very small ionic and hydrophilic molecules penetrate corneal epithelium through the paracellular pathway (Toropainen, 2007). The corneal epithelium is negatively charged and because the isoelectric point is 3.2, the paracellular space is more permeable to cations than to anions at physiological pH (Rojanasakul and Robinson, 1989; Liaw et al., 1992). Most drugs that are used clinically are sufficiently lipophilic to permeate across the cornea via transcellular route (Sasaki et al., 1999). Drug lipophilicity is one of the most important factors and it has been reported that the log (partition coefficient) of 2-3 is optimal for corneal penetration (Schoenwald and Ward, 1978; Huang et al., 1983; Schoenwald and Huang, 1983).The abundant presence of hydrated collagen in the stroma may hamper the diffusion of highly lipophilic agents. The endothelium is lipophilic in nature (transcellular permeability) and allows the passage of hydrophilic drugs and macromolecules through the paracellular pathway due to the presence of ‘leaky tight junctions’ called desmosomes or macula adherens.
The non-corneal or conjunctival/scleral route involves penetration across the conjunctiva and sclera into the intraocular tissues. It is usually less efficient for drug delivery, but may be used for the delivery of hydrophilic and larger molecules, which cannot easily diffuse through the corneal epithelium: the conjunctival tissue is permeable to molecules up to 20,000 Da, whereas the cornea is impermeable to molecules larger than 5000 Da (Ludwig, 2005). Unlike the cornea, the conjunctiva has a rich vasculature and a large amount of the administered drug crossing it is removed by the systemic circulation. The remaining drug penetrates through the sclera, which is more permeable than the cornea, but less permeable than the conjunctiva.
The passage of drugs from the anterior to the posterior segments of the eye is not very efficient due to the aqueous turnover: ocular surface administered drugs usually do not reach the posterior segments of the eye (retina, vitreous, choroid) in sufficient therapeutic concentrations. Therefore, topical application is useful in the treatment of disorders affecting the anterior segment of the eye and in order to maintain a therapeutic drug level, the agents need to be frequently dosed resulting in poor patient
13
compliance, toxic side effects and cellular damage at the ocular surface (Ludwig, 2005).
Unlike topical administration, systemic or intravenous dosing helps in the treatment of diseases affecting posterior segment of the eye but only 1-2% of administered drug reaches to vitreous cavity (Gaudana et al., 2009): the tight junctions of blood retinal barrier restrict the entry of systemically and intravenously administered drugs into posterior segment of the eye. This results in frequent administration of high amounts of drugs leading to systemic side effects. Intravitreal administrations appear to be the main strategies for treating posterior segment infections/diseases. This method involves injection of drug solution directly into vitreous via pars plana using a 30 G needle. Unlike other routes, intravitreal injection offers higher drug concentrations in vitreous and retina. Elimination of drugs following intravitreal administration depends on their molecular weight. Linear and globular shaped molecules (especially protein and peptide drugs) with molecular weight greater than 40 and 70 kDa, respectively, tend to cause longer retention in vitreous humor. Though intravitreal administration offers high concentrations of drugs in retina, it is associated with various short term complications such as retinal detachment, endophthalmitis and intravitreal hemorrhages. Moreover, patients need to be carefully monitored following intravitreal injections.
Periocular route has been considered as the most promising and efficient route for administering drugs to posterior eye segment. Periocular refers to the region surrounding the eye. It is a broad term which includes peribulbar, posterior juxtascleral, retrobulbar, subtenon and subconjunctival routes. Drug solutions are placed in close proximity to the sclera and this results in high retinal and vitreal concentrations. Anterior segment complications have been observed in some patients following periocular injections: these include rise in intraocular pressure, cataract, hyphema,strabismus and corneal decompensation.
2.4. Ophthalmic drug delivery systems
Ocular drug delivery has remained as one of the most challenging task for pharmaceutical scientists.
Topical application of drugs to the eye is the most popular and well-accepted route of administration for the treatment of various eye disorders. The traditional ophthalmic
14
formulations, as aqueous solutions and ointments, are the common method for the treatment of ocular diseases, but the bioavailability of ophthalmic drugs administered by conventional eye-drops is extremely low (2-10%) due to efficient protective mechanisms of the eye: blinking, baseline and reflex lachrymation, and drainage remove rapidly foreign substances, including drugs, from the surface of eye reducing the contact time of the medication with the corneal surface. Moreover, application of many potentially active ophthalmic compounds is limited because of their very low water solubility or their low stability in aqueous formulations. Nowadays it is well-established that prolongation of ocular contact and controlled drug release to the tear fluid may increase the ocular bioavailability. Moreover, once-a-day formulations improve patient compliance.
Novel ocular drug delivery systems have been investigated in the attempt to improve the efficacy of ocular medications, applying also the concepts of mucoadhesion. Recent delivery systems include:
a) Ocular inserts, corneal shields and contact lenses that provide controlled delivery of drug to the eye;
b) In situ gelling systems, which provide ease of administration as drops and are
converted to gel form in the eye, thereby providing some sustained effects of drug; c) Vesicular systems, which offer advantages of targeted delivery, biocompatibility, and freedom from blurring of vision;
d) Mucoadhesive systems, which provide better and longer retention in the eye; e) Prodrugs;
f) Penetration enhancers; g) Lyophilized carrier systems;
h) Innovative ophthalmic drug delivery systems, wherein additive beneficial effects of two delivery systems were obtained.
2.4.1. Ocular inserts
Ocular inserts are defined as preparations with a solid or semisolid consistency, whose size and shape are especially designed for ophthalmic application. These inserts are placed in the lower fornix and, less frequently, in the upper fornix or on the cornea. They are usually composed of a polymeric vehicle containing the drug and are mainly used for topical therapy.
15
Ocular inserts offer several advantages,which can be summarized as follows:
4. Increased ocular residence time, hence a prolonged drug activity and a higher bioavailability with respect to standard vehicles;
5. Possibility of releasing drugs at a slow, constant rate;
6. Accurate dosing (contrary to eye drops that can be improperly instilled by the patient and are partially lost after administration, each insert can be made to contain a precise dose which is fully retained at the administration site);
7. Reduction of systemic absorption (which occurs freely with eye drops via the naso-lacrimal duct and nasal mucosa);
8. Better patient compliance, resulting from a reduced frequency of administration and a lower incidence of visual and systemic side-effects;
9. Possibility of targeting internal ocular tissues through non-corneal (conjunctival scleral) routes;
10. Increased shelf life with respect to aqueous solutions;
11. Exclusion of preservatives, thus reducing the risk of sensitivity reactions; 12. Possibility of incorporating various novel chemical/technological approaches, such as pro-drugs, mucoadhesives, permeation enhancers, microparticulates, salts acting as buffers, etc.
But the ocular inserts are not free from disadvantages.
A capital disadvantage resides in their 'solidity', i.e., in the fact that they are felt by the (often oversensitive) patients as an extraneous body in the eye. This may constitute a formidable physical and psychological barrier to patients acceptance and compliance. Moreover, other factors are unfavourable:
5. the occasional inadvertent loss during sleep or while rubbing the eyes;
6. their interference with vision, and
7. difficult placement and removal, for insoluble types, complicated by the possible unwanted migration of the insert to the upper fornix.
The mechanisms by which inserts control the drug release to the eye are: diffusion, osmosis, and bio-erosion.
In the diffusion mechanism, the drug is released continuously at a controlled rate through the membrane into the tear fluid. If the insert is formed of a solid non-erodible body with pores and dispersed drug the release of drug can take place via diffusion through the pores. Controlled release can be further regulated by gradual dissolution
16
of solid dispersed drug within this matrix as a result of inward diffusion of aqueous solutions.
In a soluble device, true dissolution occurs mainly through polymer swelling. In swelling-controlled devices, the active agent is homogeneously dispersed in a glassy polymer. Since glassy polymers are essentially drug-impermeable, no diffusion through the dry matrix occurs. When the insert is placed in the eye, water from the tear fluid begins to penetrate the matrix, then swelling and consequently polymer chain relaxation and drug diffusion take place. The dissolution of the matrix, which follows the swelling process, depends on polymer structure: linear amorphous polymers dissolve much faster than cross-linked or partially crystalline polymers. Release from these devices follows in general Fickian 'square root of time' kinetics; in some instances, however, known as case II transport, zero order kinetics has been observed.
In the osmosis mechanism, the insert comprises a transverse impermeable elastic membrane dividing the interior of the insert into two compartments; the first compartment is bounded by a semi-permeable membrane and the impermeable elastic membrane, and the second compartment is bounded by an impermeable material and the elastic membrane. There is a drug release hole in the impermeable wall of the insert. The first compartment contains a solute which cannot pass through the semi-permeable membrane and the second compartment provides a reservoir for the drug
which again is in liquid or gel form.
When the insert is placed in the aqueous environment of the eye, water diffuses into the first compartment and stretches the elastic membrane to expand the first compartment and contract the second one so that the drug is forced through the release hole.
In the bioerosion mechanism,the configuration of the body of the insert is constituted from a matrix of bioerodible material in which the drug is dispersed. Contact of the insert with tear fluid results in controlled sustained release of the drug by bioerosion of the matrix. The drug may be dispersed uniformly throughout the matrix but it is believed a more controlled release is obtained if the drug is superficially concentrated in the matrix.
In truly erodible or E-type devices, the rate of drug release is controlled by a chemical or enzymatic hydrolytic reaction that leads to polymer solubilization, or degradation to
17
smaller, water-soluble molecules. Erodible inserts undergoing surface hydrolysis can display zero order release kinetics, provided that the devices maintain a constant surface geometry and that the drug is poorly water-soluble.
Ocular inserts are classified in: insoluble ocular, soluble and bio-erodible.
Only the insoluble types can usually deliver drugs by a variety of methods at a controlled, predetermined rate, but need removal from the eye when empty. Bio-erodible inserts are formed by bio-erodible polymers (cross-linked gelatin derivatives, polyester derivatives) which undergo hydrolysis of chemical bonds and hence dissolution.
The soluble inserts offer the advantage of being entirely soluble so that they do not need to be removed from their site of application. They can be broadly divided into two types, the first one being based on natural polymers (collagen) and the other on synthetic (PVA) or semi-synthetic (cellulose derivatives) polymers. The soluble inserts offer the additional advantage of being of a simple design, of being based on products well adapted for ophthalmic use and easily processed by conventional methods. The main advantage is a decreased release rate, but it is still controlled by diffusion. However, the inherent problems encountered with this type of inserts are the rapid penetration of the lachrymal fluid into the device, the blurred vision caused by the solubilization of polymeric components and the risk of expulsion due to the initial dry and glassy consistency of the device (Karthikeyan et al., 2008).
2.4.2. Bioadhesive polymers
To improve the ocular bioavailability of drugs, numerous natural and synthetic viscosifying agents were added to the vehicle in order to increase the viscosity of the preparation, to reduce the drainage rate and to improve the therapeutic efficacy but no significant bioavailability enhancement was reported in humans, as was obtained in rabbits, due to differences in blinking frequency and tolerance. Viscous semi-solid preparations, such as gels and ointments, provide a sustained contact with the eye, but they cause a sticky sensation, blurred vision and induce reflex blinking due to discomfort or even irritation. A further approach to optimize the ocular dosage form was the implementation of the mucoadhesive concept, which was successful in many other fields, such as buccal and oral, nasal and vaginal (Burgalassi et al., 1996;
18
Bertram et al., 2010; Bonferoni et al., 2006). Due to interactions with the mucus layer or the eye tissues, an increase in the precorneal residence time of the preparation was observed. Some mucoadhesive polymers showed not only good potential to increase the ocular bioavailability of the drug applied, but also protective and healing properties to corneal epithelial cells (Burgalassi et al, 2007; Chung et al., 1989).
Polymer-related factors influencing mucoadhesion are hydration or degree of swelling, molecular weight, functional groups, molecular conformation or chain flexibility and mobility, and concentration. Polymer hydration results in the relaxation of stretched, entangled or twisted macromolecules, exposing the adhesive sites. Furthermore, chain interdiffusion is favoured by polymer–water interactions dominating the corresponding polymer–polymer interactions. A critical chain length is necessary to obtain interpenetration and molecular entanglement between the polymer and the mucus glycoproteins. The threshold required for successful mucoadhesion is a molecular weight of at least 100,000 Da. Excessive cross-linking in the polymer, however, decreases the chain length available for interfacial penetration. Also, excessive formation of interchain physical entanglement and hydrogen bonding within the polymer itself can lead to conformation hindering polymer diffusion into the mucus network. As a result, chain flexibility is critical for interpenetration and entanglement with the mucus gel. The higher the mobility of the chain segment, the greater the interdiffusion and the interpenetration of the polymer within the mucus network. Coiling of polymer chains, due to pH or osmolality of the medium, can result in the shielding of active groups necessary for the adhesion process. Many high molecular weight polymers with different functional groups (such as carboxyl, hydroxyl, amino, and sulfate) capable of forming hydrogen bonds, and not crossing biological membranes, have been screened as a possible excipient in ocular delivery systems. Unfortunately, in vivo studies in humans were not always performed. A general conclusion is that charged polymers both anionic and cationic demonstrate a better mucoadhesive capacity in comparison to non-ionic cellulose-ethers or polyvinyl alcohol (PVA). In fact some viscosifying polymers with excellent mucoadhesive capacity are: polyacrylic acid (neutralized); Carbomer (neutralized); Hyaluronan; Na carboxymethylcellulose; Na alginate and pectin, all with anionic charge, and chitosan with cationic charge (Ludwig, 2005).
19
2.4.2.1. Cellulose derivatives.
The first cellulose polymer, methylcellulose, was introduced in pharmaceutical field over 50 years ago. Subsequently, a number of substituted cellulose-ethers have been employed for artificial tear solutions and as viscosity-enhancing ophthalmic vehicles. Methylcellulose also possesses wound healing properties and is a suitable tear substitute for dry eyes, especially for those with punctate lesions. All cellulose-ethers impart viscosity to the solution, have wetting properties and increase the contact time by virtue of film forming properties. Some cellulose-ethers (e.g. hydroxypropylmethylcellulose and hydroxypropylcellulose) also exhibit surface active properties, interact with components of the tear film and alter the physicochemical parameters governing the tear film stability. Surface active viscosifying agents can influence the blinking rate, which in turn influences the elimination of the drug instilled. They cause irritation and extensive lachrymation, provoking a rapid wash out of the ophthalmic solution and consequently a poor bioavailability. Generally, less surface active hydroxyethylcellulose is better tolerated (Ludwig et al., 1992), but the mucoadhesive properties of non-ionic cellulose-ethers are rather poor (Meseguer et al., 1993; Séchoy et al., 2000). Sodium carboxymethylcellulose (NaCMC), however, exhibits a mucoadhesive capacity comparable to that of poly(acrylic acid) (PAA).
2.4.2.2. Acrylates
The first mucoadhesive polymers proposed were poly(acrylic acid) derivatives.
The mucoadhesive properties of poly(acrylic acid) are due mainly to hydrogen bonding, while hydrophobic interaction with mucin is not significant. When anionic polymers interact with mucin, the maximum interactive adhesive force occurs at an acidic pH, suggesting that the mucoadhesive polymer in its protonated form is responsible for the mucoadhesion. The swollen polymer entangles with mucin on the eye surface, stabilizing a thick hydrogel structure. In contrast, in the precorneal area, the neutral pH value of the tears and the shielding of the carboxyl groups by cations present in the tear fluid diminish the interaction of carbomer with the functional groups on mucin. A decrease in mucoadhesion is measured. Rheological studies performed with various kinds of Carbopol® (974P NF, 980 NF, 1342 NF) demonstrated no significant differences in the interaction between these different carbomers and mucin. It was demonstrated that the interaction depends on the mucin
20
concentration, which implies that this interaction is only possible close to the corneal/conjunctival epithelium. Physical entanglement and secondary bond formation between poly(acrylic acid) derivatives and diluted mucin in the tear film can be excluded (Ceulemans and Ludwig, 2002). Polyanionic polymers such as polyacrylates or carbomers were proposed as long-lasting artificial tears for the relief of dry eye syndrome and traumatic injury. The use of these high molecular weight polymers is based on inherent mucuslike and lubricating properties, shear thinning behaviour, and good retention on the ocular surface. Concentration-dependent blurring of vision and uncomfortable feeling are sometimes reported (Calonge, 2001).
2.4.2.3. Hyaluronan
Besides synthetic polymers, natural macromolecules such as hyaluronan (HA), present in the vitreous body of the eye, were proposed as viscosifying agents. Sodium hyaluronate molecules have physical properties and a composition comparable to tear glycoproteins, and easily coat the corneal epithelium. Polymers adsorbed at the mucin/aqueous interface extend into the adjacent aqueous phase, thereby stabilizing a thick layer of water. The non-Newtonian rheological behaviour of sodium hyaluronate combines the advantage of high viscosity at rest between blinks with those of lower viscosity during blinking. Diluted solutions of sodium hyaluronate have been employed successfully as tear substitutes in severe dry eye disorders. The beneficial effects are attributed to biophysical properties similar to mucins, providing a long-lasting hydration and retention and a good lubrication of the ocular surface. Hyaluronic acid is an important constituent of the extracellular matrix and may play a role in inflammation and wound healing and may promote corneal epithelial cell proliferation (Aragona et al., 2002). Gurny et al. (1987) confirmed the positive influence of hyaluronate vehicles on the bioavailability of pilocarpine. High molecular weight of the polymer is an essential requirement for the prolonged precorneal residence time of the preparation (Saettone et al., 1991).
2.4.2.4. Polysaccharides
Numerous polysaccharides were evaluated as mucoadhesive ophthalmic vehicles: polygalacturonic acid, xyloglucan, xanthan gum, gellan gum, pullulan, guar gum, scleroglucan and carrageenan. Also, in the case of polysaccharides, the formation of
21
macromolecular ionic complexes with drugs improved the bioavailability and lengthened the therapeutic effect when compared to drug solutions (Saettone et al., 1994; Saettone et al., 1992). Toxicological studies indicate that xyloglucan is very well tolerated by conjunctival cells, has cell protective properties and is able to reduce drug-related toxicity (fluoroquinolones, timolol, merthiolate) probably due to its mucin-like structure. Timolol, in association with xyloglucan, has a prolonged duration of action, and is suitable for ocular administration in cases of elevated intraocular pressure (D’Amico et al., 1999). In rabbits, high timolol concentrations in the ocular tissues were measured, but with low systemic absorption. The performances were comparable to the in situ gelling system of Timoptic® XE containing gellan gum (Burgalassi et al., 2000). Xanthan gum interacts moderately with mucin: a small viscoelastic synergistic effect can be observed, but the effect is due to physical entanglement of both components. Xanthan gum should exist as an ordered double-stranded helix in the precorneal tear film, due to ions present in the lacrimal fluid. Results of an in vivo study in healthy volunteers confirm that an increase in viscosity of xanthan gum solutions delays the clearance of the instilled solution. Xanthan gum is therefore more suitable as a viscosifying agent when compared to poly(vinyl alcohol), hydroxyethylcellulose and hydroxypropylmethyl cellulose (Ludwig, 2005). Carrageenan is a high-molecular-weight, sulphated polygalactan derived from several species of red seaweed of the class Rhodophyceae, that is used for the textural stabilization of foods. The most common forms of carrageenan are designated as kappa, iota and lambda. Kappa carrageenan is mostly the alternating polymer of D-galactose-4- sulphate and 3,6-anhydro-D-galactose. Iota carrageenan is similar but with the 3,6-anhydro-D-galactose being sulphated at the second carbon. Lambda carrageenan has alternating monomeric units composed mostly of D-galactose-2-sulfate (1,3-linked) and D-galactose-2,6-diD-galactose-2-sulfate (1,4-linked) (Cohen and Ito, 2002). Only a limited number of studies have been performed on carrageenan systems with a substantial lack of studies comparing the various polymer formulations. Rupenthal et al., (2011) evaluated a variety of ion-activated anionic polysaccharides in terms of their gelling behaviour, rheological and textural properties, gel microstructure, contact angle and in vitro release characteristics and to compare them to an uncharged as well as a positively charged polymer system. Formulations based on gellan gum and carrageenan exhibited the most favourable characteristics in terms of phase transition,
22
rheological and textural properties, as their viscosity remarkably increased upon contact with cations of the tear fluid, thus prolonging corneal residence time and reducing nasolacrimal drainage.
The rheological behaviour and mucoadhesive properties of various carrageenan fractions were studied and compared to gellan gum (Gelrite) in order to evaluate their use as ophthalmic viscolysers (Verschueren et al., 1996). The iota and kappa fractions and the mixture of carrageenans exhibited gelling and viscoelastic properties, which depend, however, on the composition of the vehicle or the lachrymal fluid of the patient after instillation. Their properties resemble the characteristics of gellan gum.
2.4.3. Mucoadhesive ocular inserts
Inserts were developed more than 30 years ago to treat the symptoms of dry eyes. Inserts dissolve and/or erode on contact with the ocular surface and therefore need to be used in addition with other artificial tears to initiate the dissolving process. Although the sustained release effect is very pronounced, insert use is severely diminished by the high cost, as well as the difficulty in handling for elderly people, and the intense foreign body sensation. Considering the various mucoadhesion mechanisms, hydration or degree of swelling of the polymers involved play an important role. In the case of dry or partially hydrated dosage forms, water movement from the mucus layer to the formulation can be a significant factor in mucoadhesion, being more important than molecular interpenetration (Mortazavi and Smart, 1993). However, excessive water content leads to an abrupt drop in adhesive strength due to the formation of slippery non-adhesive mucilage at the interface. The contact time between the mucoadhesive dosage form and the mucus layer determines the extent of swelling and interpenetration of the polymer chains. The swelling time is important for the assessment of the adhesiveness. Decreasing the swelling time results in an improvement of the interpenetration phenomena. Hydrophilic polymers with poor mucoadhesive properties may be added to a mucoadhesive polymer with poor swelling characteristics to ensure fast swelling. Some additional polymers can hinder the formation of bonds between the mucoadhesive polymer and mucus by preferentially binding to the hydrated mucoadhesive polymer. There is also a reduction in the strength of the bond between the mucoadhesive polymer and mucin (Tobyn et al., 1996). Karatas and Baykara (2000) evaluated various water-soluble
23
cellulose derivatives and polyvinyl alcohol in preparing inserts by film casting and compression-moulding. The release from these inserts was controlled by a diffusion mechanism. The release rate of indomethacin can be modified by using polymers with different solubility and viscosity grade, and by changing the blending ratio and the preparation method.
Inserts made by compression moulding exhibit higher release rates. The swelling of the inserts, the rate of water penetration and erosion in vivo were not investigated. Lux et al. (2003) developed a lyophilisate based on hydroxypropylmethylcellulose (Methocel E50) deposited on a flexible poly(tetrafluoroethylene) carrier strip. They measured the bioavailability of fluorescein after deposition of the device in the cul-de-sac. Upon contact with the conjunctiva, the lyophilisate is hydrated rapidly by the tear fluid. Compared to conventional eye drops, this new dosage form results in significantly higher cornea and aqueous humour concentrations for up to 7 h after application. No discomfort, good tolerability and excellent safety were reported. Chetoni et al. (1998) prepared rod-shaped mucoadhesive inserts (diameter 0.9 mm, length 6–12 mm, weight 3–8 mg, drug content 0.8 mg) from appropriate blendings of silicone elastomer, oxytetracycline hydrochloride and sodium chloride as release modifier. Mucoadhesion of the device depended on the composition and thickness of the interpenetrating polymer network present at the surface of the insert, which was realised by grafting of polyacrylic acid or polymethacrylic acid onto polydimethylsiloxane. In rabbits, the ocular retention of grafted devices was significantly higher when compared to ungrafted inserts. All grafted inserts tested ensured a prolonged drug release, and zero-order release kinetics. Oxytetracycline tear levels of 20–30 g/ml were measured for several days. This concentration is 10- to 30-fold above the MIC90 values for common ocular pathogens.
Polyethyleneoxide (PEO) exhibits good compressibility and thus is easy for the manufacturing of matrix tablets. In contact with an aqueous medium, polyethyleneoxide hydrates and gels superficially, the polyether chains of PEO forming strong hydrogen bonds with water. Drug release from PEO matrices is controlled by polymer swelling and erosion, or drug diffusion through the gel, or by both processes. Various release patterns can be achieved depending on the polyethyleneoxide molecular mass and physicochemical properties of the drug. Considering these characteristics, Di Colo et al. (2001) selected PEO to develop
gel-24
forming erodible inserts for controlled delivery of ofloxacin. Inserts of 6 mm diameter, 20 mg weight and medicated with 0.3 mg drug were prepared by direct compression of plain linear PEO (400 kDa PEO) or mixed with Eudragit L100R (EUD) neutralized at 17% or 71% with NaOH. EudragitR was added in order to modulate the swelling and erosion rate of the insert, because the polymer should interact via hydrogen bonding between its non-ionized carboxyl groups and the ether oxygens of PEO. After application in the cul-de-sac of rabbits eyes, the inserts adhered almost instantaneously to the mucosa, and gradually formed well-tolerated mucoadhesive gels which spread over the cornea and eroded within 60 (PEO/EUDNa71) to 180 min. Compared to commercial eye drops, the transcorneal drug permeation was retarded by the PEO/EUDNa71 inserts, but both retarded and prolonged by the PEO/EUDNa17 inserts, while concentration values in the aqueous humour were significantly not influenced. In contrast concentration values in the aqueous humour and bioavailability were greatly increased, up to 11-fold, plain PEO inserts. The authors ascribed the increase of bioavailability to PEO mucoadhesion, which could increase the corneal permeability, and/or increased tear viscosity, which limit drainage and consequently drug clearance. Further studies demonstrated that the mucoadhesion was maximal for poly(ethylene oxide) 400.
25
3. Aims of the study
The overall aim of the present study was to develop new ocular therapeutic platforms in order to increase the performance of ophthalmic preparations such as the residence time in the application sites, the bioavailability of the active agents and their release at a controlled rate for several hours. The specific aims of the study were:
1.
The preparation of bioadhesive ocular inserts based on natural or synthetic polymers (cellulose derivatives, polyvinyl alcohol, natural gums and polysaccharidic polymers) containing appropriate amount of drugs (levofloxacin), natural extract (arabinogalactan and sericin), blood components (platelet lysate) or protein drugs (bevacizumab).2.
The technological characterization of the prepared inserts such as: in vitro mucoadhesion test; in vitro drug release study; contact angle measurements; rheological behaviour; swelling studies.3.
A preliminary evaluation on application of gamma-ray sterilization method on freeze-dried ocular inserts.4.
An in vivo evaluation on albino rabbits of the best experimental formulations to assess the biological compatibility with the ocular structure and the related therapeutic activity.26
4. Materials
The following materials were used: Alginate (ALG) (Protanal® SF 120, Savini, Italia); Arabinogalactan (AG) (FiberAid®, Larex, USA); Benzalkonium chloride (Carlo Erba, Italy); Carrageenans (CRG209 and CRG389) (Viscarin®209 and Viscarin®389, FMC BioPolymer, Ireland); Cell proliferation reagent (WST-1) (Roche Diagnostics GmbH, Germany); Dialysis membrane (MWCO 3.5 kD, MWCO 50 kD and MWCO 300 kD) (SpectrumLabs, Holland); Ethylenediaminetetraacetic acid (EDTA) (Carlo Erba, Italy); Fluorescein isothiocyanate-dextran 40 kDa and 150 kDa (D40 and FITC-D150) (Sigma-Aldrich, Sweden); Glycerin (GL) (Carlo Erba, Italy); Hyaluronic acid (HA) (Chemofin, Italy); Hydroxyethylcellulose (HEC) (Natrosol® 250 MR, Aqualon, Italy); Hydroxypropylcellulose (HPC) (Klucel® MF, Hercules, USA); Hydroxypropylmethylcellulose (HPMC15 and HPMC4) (MethocelTM K15M and MethocelTM K4M Premium EP, Colorcon, Italy); Hog gastric mucin (HGM) (Roth, Germany); Lactose (Carlo Erba, Italy); Levofloxacine (LF) (Sigma-Aldrich, USA); Locust bean gum (LBG) (Vidogum® L-250, General Trading and Consulting s.r.l., Italy); Mannitol (Carlo Erba, Italy); n-heptanol (Sigma-Aldrich, USA); Polyacrylic acid (PAA) (Carbopol® 980, Goodrich, USA); Polyvinylalcohol (PVA 4-88, 8-88 and 26-88) (PolyViol®, Merck, Germany); Polyvinylpyrrolidone (PVP) (Kollidon® 90F, Basf, Germany); Sericin (SR) (J and C, Italy); Sodium carboxymethylcellulose (CMC) (Blanose® 7MF, Hercules, USA); Tara gum (TG) (Vidogum® SP-250; General Trading and Consulting s.r.l., Italy); Vitamin D-α-Tocopheryl Polyethylene Glycol 1000 Succinate (Vitamin E-TPGS) (Eurochem Asia Ltd, China); Xanthan gum (XG) (Xantural® 75, Giusto Faravelli SpA, Italia). Milli-Q water was used in all experiments.
The artificial tear fluid (ATF) had the following composition: MgCl2 (4.75 mg/100ml);
CaCl2 (7.97 mg/100ml); KHCO3 (260.0 mg/100ml) and NaCl (754.0 mg/100ml).
66.7 mM phosphate buffer solution (PBS) consisted of 9.2 g/l NaH2PO4.H2O and 9.47
g/l Na2HPO4 mixed in different ratios to obtain the desidered pH value.
A commercial solution containing 25 mg/ml of bevacizumab (BVZ) (Avastin®, Roche, Swiss) was used for in vitro drug release study.
The growth medium had the following composition: DMEM/F-12 (1:1) supplemented with fetal bovine serum (15% v/v), L-glutamine (1% v/v, 2 mM), penicillin (100 UI/ml), streptomycin (0.1 mg/ml), and amphotericin B (0.25 μg/ml) (Invitrogen,
27
Milan, Italy), insulin (5 μg/ml), and epidermal growth factor (10 ng/ml) (Sigma-Aldrich®, Milan, Italy).
The human corneal epithelial cell line (HCE) was provided by the Riken BRC (no. RCB2280, HCE-T, Japan) through the National Bio-Resource Project of the MEXT. The platelet lysate (PL) was obtained as follow: blood samples were taken from rabbits, centrifuged at 1500 rpm (IEC MicroCl 17, Thermoelectron Corporation, Germany) for 5 min and the platelet-rich plasma was collected and freezed at -18°C until use (Vecchio et al., 2006).
All other chemicals used are of reagent grade.
4.1. Animals
In vivo studies were carried out in albino New Zealand rabbits, weighing 2.5-3.0 Kg (Pampaloni Rabbitry, Fauglia, Italy) used and treated as prescribed in the publication “Guide for the Care and Use of Laboratory Animals” (NIH Pubblication No. 92-93, revised 1985). All experiments were conformed with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research: they were carried out under veterinary supervision, after approval of the protocols by the Ethical-Scientific Committee of the University of Pisa.
The animals were housed in standard single cages in a room with controlled lighting, at 19 ± 1°C and 50 ± 5% R.H., with no restriction of food or water. During the experiments, the rabbits were placed in restraining boxes to which they have been habituated, in a room with dim lighting; they were allowed to move their heads freely, and their eye movements were not restricted.
28
4.2. Arabinogalactan
Fig. 4.1 Structure of Arabinogalactan
Arabinogalactans are a class of long, densely branched high-molecular polysaccharides MW: 10,000-120,000 Da. In nature, arabinogalactans are found in several microbial systems, especially acid-fast mycobacteria where it is complexed between peptidoglycans and mycolic acids as a component of the cell wall and influences monocyte-macrophage immunoreactivity of tubercular antigen. Many edible and inedible plants are rich sources of arabinogalactans, mostly in glycoprotein form, bound to a protein spine of either threonine, proline or serine (arabinogalactan protein) but the major commercial source of arabinogalactan is the Larch tree. Two sources are Western Larch (Larix occidentalis) and Mongolian Larch (Larix dahurica). Most commercial arabinogalactan is produced from Western Larch, a renewable resource, through a counter-current extraction process. Pharmaceutical-grade larch arabinogalactan is a fine, dry, off-white powder with a slightly sweet taste and mild pine-like odor. As compared with other natural polysaccharides, the unique properties of larch arabinogalactan are: ease of solution, complete solubility; good body properties without viscosity buildup; excellent dispersant and surfactant properties; and stability over a wide range of concentrations, pH and temperature. It is composed of galactose and arabinose molecules in a 6:1 ratio, with a small amount of glucuronic acid. Lower molecular weight polysaccharides typically exhibit an anti-inflammatory, anti-complement, antiallergy effect, while those of higher weights stimulate natural
29
killer (NK) cell cytotoxicity and reticuloendothelial cells. In the case of larch arabinogalactan, molecular weights of the two major fractions are 16,000 and 100,000, perhaps accounting for its wide range of therapeutic properties (D’Adamo, 1996).
Larch arabinogalactan is used for infections, including the common cold, flu, H1N1 (swine) flu, ear infections in children, and HIV/AIDS. It is also used to treat liver cancer, as well as a brain condition caused by liver damage (hepatic encephalopathy). Some people use it to provide dietary fiber, lower cholesterol, and to boost the immune system. Burgalassi et al (2011) proved that natural polysaccharide arabinogalactan (AG) was well tolerated after ocular administration and exerts a high restoring effect on corneal epithelium abrasions. For Mano et al., (2007) the high solubility in water, biocompatibility, biodegradability and the ease of chemical modification in aqueous media make arabinogalactan an attractive polymer for the synthesis of scaffolds for application in tissue engineering.
4.3. Levofloxacin
Fig. 4.2. Structure of Levofloxacin
The quinolones comprise a series of broad-spectrum synthetic antibacterial agents derived from nalidixic acid. They were discovered casually in 1962 and since then are essentially used in the treatment of wide range of infectious diseases. The fluoroquinolones are quinolones with fluorine at position 6 of naphthyridine ring: published structure-activity data show that fluorine atom help broadens their activity spectrum against both Gram-negative and Gram-positive pathogens (Santoro et al., 2006). Levofloxacin (LF) is the L-enatiomer of the fluoroquinolone ofloxacin.
30
Levofloxacin 0.5% ophthalmic solution is approved for the treatment of bacterial conjunctivitis in Japan, US and in several European countries. LF has greater solubility in water than ofloxacin, norfloxacin or ciprofloxacin at neutral pH, although it is sufficiently lipophilic to penetrate the eye. Levofloxacin uptake is concentration dependent; maximal corneal penetration occurred at a pH of 6.5. LF concentrations tended to be lower in the aqueous humor than in the conjunctiva or cornea and low LF concentrations were detected in the vitreous humor. Microbial eradication and clinical cure rates were significantly higher with LF than with placebo or than with ofloxacin 0.3% ophthalmic solution at endpoint.
Fluoroquinolones inhibited the proliferation of corneal cells in a dose-dependent manner. LF 100 μg/ml did not significantly affect the wound healing rate after a portion of the bovine corneal epithelial cells were scraped, although wound healing rates were significantly (p<0.05) decreased by gatifloxacin, moxifloxacin, tosufloxacin and ciprofloxacin 100 μg/ml. After corneal epithelial debridement in rabbits, wound healing did not significantly differ between levofloxacin 0.5%, ofloxacin 0.3% and saline ophthalmic solutions, instilled three times daily for 3 days (Keating, 2009).
4.4. Sericin
Fig. 4.3. Filament of silk
Sericin is a protein created by Bombyx mori (silkworms) in the production of silk. Silk emitted by the silkworm consists mainly of two proteins, sericin and fibroin: in the serigene glands, the two distinct types of proteins (fibroin and sericin, like highly concentrated watery solutions) are synthesized in two separate areas and forced outward through special canals. The sericin forms a layer enveloping the nucleus, the
31
fibroin mass. A little before the expulsion, the filaments of fibroin produced by the two glands, go to the sides and at the exit the crude silk is the sum of the two secretions: two filaments of fibroin enveloped in a layer of sericin. Sericin is the second silk protein. Besides the functions of covering, adhesion and protection for the fibroin, it lubricates and promotes the biological process of wrapping of the thread in the construction of the cocoon. The sericin serves as a bivalent cation donor and acceptor of water molecules that free themselves from the fibroin crystalline region (Rigano et al, 2005). It is a complex protein molecule with isoelectric point 4.0. The chemical composition of sericin is C30H40N10O16. Five principal fractions of sericin
have been isolated with glycoproteins of different molecular weight (65-400 kDa) and other smaller figures. The amino acids that make up sericin, which are more distinct than those in fibroin, are reported in Table 4.1. To be noted is the high content of oxydrilated polar serine amino acids (33.5%) (Figure 4.4.), which are non-essential for human development and an important source of carbon atoms for the intercellular synthesis of purin, as well as the scarce quantity of non-polar amino acids. Sugars of the saccharidic chain are also present (3%) linked to the polypeptidic part with O-glucosidic and N-O-glucosidic links (Komatsu K, 1972).
Table 4.1. Amino acidic composition of Sericin (residue per molecule) Polarity Amino acid %
Non-polar aliphatic Alanin 6.0 Glycin 13.5
Isoleucin 0.8 Leucin 1.0 Prolin 0.7
Valin 3.0
Non-polar aromatic Tirosin 2.6 Polar amidic Asparagin 13.7
Glutamin 4.4 Polar basic Arginin 3.1
Istidin 1.3
Lisin 3.7
Polar oxdrilated Serin 33.5 Treonin 9.7
32
Figure 4.4. Structure formula of anti-parallel serin
(Rigano et al, 2005)
Sericin contains random coil and -sheet structure; random coil structure is soluble in hot water and as the temperature lowers the ramdom coil structure converts to -sheet structure, which results in gel formation. The solubility of sericin increases by addition of poly-Na-acrylate and decrease by the addition of polyacrylamide, formaldehyde, or resin finishing agents (Padamwar and Pawar, 2004). Cosmetic applications of sericin, once considered a scrap material of the woven thread industry, have been rather weak in the past. Today, the rediscovering of its essential properties and the availability of new processes to keep them unaltered have led to obtain a more intact and functional active ingredient (Rigano et al, 2005). Sericin has recently been investigated for its activities in biotechnological fields. Silk sericin due to its proteinous nature is susceptible to the action of proteolytic enzymes present in body and hence it is digestible. This property makes it a biocompatible and biodegradable material (Padamwar and Pawar, 2004). Hydrogels with good mechanical strength and water resistance are produced by casting aqueous solution containing sericin and dimethylurea on a glass plate and heating at 80 and 120°C for 1 and 3 h, respectively (Nakamura and Koga, 2001). Sericin has been found to possess wound-healing property and can be used as wound covering material in the form of film (Wu et al., 1996). Sericin also has adhesive property due to its chemical composition: it has affininity to keratin (Voegeli et al., 1993). Nagai et coll. (2009) reported that sericin instillation had a potent effect in promoting wound healing and wound-size reduction in rats. They also applied sericin as eye drops for corneal wound repair in diabetic patients (Nagai, Murao et al., 2009). Kurioka (1998) has explained silk sericin as a biomaterial. The silk sericin has the potential to find application in the development of contact lenses. Kato et al. (1998) provided the first evidence for an antioxidant action of the silk protein sericin by showing that sericin suppressed in vitro lipid
33
peroxidation. Furthermore, sericin was found to inhibit tyrosinase activity.
4.5. Platelet lysate
Platelets are specialized secretory cells that release from intracellular alpha granules, in response to activation, a large number of biologically active substances and in particular growth factors (GFs). The most intensively investigated GFs derived from platelets are PDGF (platelet derived growth factor), TGF-α and (transforming growth factors alpha and beta), FGF (fibroblast growth factor), EGF (epidermal growth factor), VEGF (vascular endothelial growth factor). By concentrating the platelets at the site of a lesion, there is in situ release of a large amount of GFs and other biochemical mediators that manifest their full regenerative potential, triggering a chain reaction that starts and then amplifies a virtuous cicle and leads to the final result of healing of the lesion (Vecchio et al., 2006). Moreover it is also recognized that the efficacy of the GFs critically depends on the way they are made available to the injured tissue. Recently platelet lysate (PL) which is an haemoderivative based on bioactive molecules (especially GFs) released by platelets after freeze-thawing destruction usually starting from a platelet rich plasma (PRP), has been proposed in clinical practice for the treatment of oral mucositis (Del Fante et al., 2011). In a previous work (Sandri et al., 2011) PL was loaded in a mucoadhesive vehicle based on polyacrylic acid or chitosan (well known mucoadhesive polymers) as viscous eye drops. The formulation based on PL/chitosan was able to enhance cell growth while that containing polyacrylic acid was less efficient. Geremicca et al (2010) studied the use of PL, in the form of eye drops, for the treatment of corneal ulcers caused by neurotrophic keratitis and of epithelial and stromal loss following physical or chemical trauma. The results were satisfactory in terms of both tissue regeneration and healing time.
4.6. Bevacizumab
Bevacizumab (149 kDa) is a humanized monoclonal antibody, and was the first commercially available angiogenesis inhibitor. Its main action is the inhibition of the function of a natural protein called vascular endothelial growth factor (VEGF). that stimulates new blood vessel formation. Because most malignant tumors are highly dependent on angiogenesis it was expected that bevacizumab (BVZ) can stop or delay
34
growth of tumors. BVZ binds directly to VEGF to form a protein complex which is incapable of further binding to VEGF receptor sites (which would initiate vessel growth) effectively reducing available VEGF. BVZ/VEGF complex is both metabolized and excreted directly.
VEGF and its receptor also play an important role in the neovessel formation that is observed in diabetic retinopathy, venous retinal occlusion, age-related macular degeneration, and corneal neovascularization (Tolentino et al., 1996). High VEGF expression was observed in neovascularized corneas after penetrating keratoplasty in corneal inflammatory diseases and in corneas that were burned by alkalis during the healing process (Philipp et al., 2000; Gan et al., 2004). Anti-VEGF drugs have sparked a revolution in the treatment of neovascular diseases by reducing neovascularization and also by their supposed action on fibroblasts (Kahook and Lin, 2008). These drugs can provide beneficial effects after intra-vitreous injection in age-related macular degeneration (AMD) neovascularization, diabetic retinopathy and neovascular glaucoma (Reggiani Mello et al., 2011). However, the intraocular intervention needed to deliver this macromolecular agent is associated with the risk of an infectious endophthalmitis, vitreous hemorrhage and retinal detachment. Kim et al., (2010) demonstrated that BVZ was detected in the anterior chamber after single subconjunctival injection, suggesting that subconjuctival injection of BVZ might be one alternative administration route, less prone to complications.