CHAPTER 4
INFLUENCE OF HYPOXIA AND NACL SALINITY ON
SWEET BASIL (OCIMUM BASILICUM L.) GROWN
HYDROPONICALLY FOR THE PRODUCTION OF
ROSMARINIC ACID
4.1 INTRODUCTION
Rosmarinic acid (RA) is one of the most abundant caffeic acid derivatives (CADs) widely distributed in the plant kingdom and has a multitude of biological activities, acting for instance as an astringent, antioxidant, antiinflammatory, antimutagen, antibacterial and antiviral compound (Petersen, Simmonds, 2003). Rosmarinic acid is mainly found in species of the families Boraginaceae and Lamiaceae. In the latter family, Ocimum basilicum L. (sweet basil) is an important source of RA and an agro-industrial production of this compound from sweet basil, as grown either in vivo and
in vitro, has been proposed (Juliani et al., 2008; Kiferle et al., 2011).
Likewise the majority of medicinal and aromatic plants, sweet basil is commonly cultivated in the field and this results in large seasonal variability of crop yield as well as of the content of active compounds (Putievsky, Galambosi, 1999). In order to overcome the drawbacks of field crops, there is an increasing interest towards greenhouse soilless cultivation of medicinal plants, as it provides a number of advantages such as higher yield, all-year round production (also where soil is not suitable due to excessive salinity or pollution, for instance) and higher quality of harvested material (Canter et al., 2005). On the other hand, due to the lower buffering capacity of hydroponic culture compared to soil, inappropriate management of irrigation and/or fertilisation may rapidly result in severe plant stress, which in turn reduces crop yield and product quality (Gorbe, Calatayud, 2010; Pardossi et al., 2006).
Among different hydroponic techniques, the floating raft system seems the most suitable system for greenhouse production of leafy vegetables, herbs and some medicinal plants (e.g. Echinacea spp; Maggini et al., 2011), including sweet basil (Kiferle et al., 2011).
In floating system, hypoxia conditions may occur in the stagnant nutrient solution, especially in warm season, as high temperature may reduce oxygen solubility while increasing root respiration (Gorbe, Calatayud, 2010). Adequate oxygen level is necessary to ensure root functionality and oxygen deficiency reduces the uptake of both water and nutrients and restricts plant growth (Morard, Silvestre, 1996). Moreover, oxygen deficit enhances the formation of reactive oxygen species (ROS; Colmer, Voesenek, 2009), which cause several damages to cellular components (Gill, Tuteja, 2010). The detrimental effect of low oxygen in the growing medium solution on the plant grown in hydroponics was observed in several species such as bean (Incrocci et al., 2000), spinach (Tesi et al., 2003), rocket salad (Ferrante et al., 2002) and tomato (Shi et al., 2007). Low oxygen supply in the roots zone may affect nitrate uptake and assimilation (Horchani et al., 2010; Ferrante et al., 2003), thus influencing the content in edible organs of free nitrates, which are potentially toxic to human health (Santamaria, 2006).
Moreover, ions (for instance Na+ and Cl−) dissolved in the irrigation water at concentration higher than root uptake concentration (i.e., the ratio between the ions and the water taken up by the plants) may accumulate in the nutrient solution and induce salinity stress to the crop (Massa et al., 2011). The adverse effects of high salinity in the root zone are associated with osmotic stress, nutritional deficiency and ion toxicity (Munns, Tester, 2008). The excess of salinity may also cause oxidative stress that enhances both the activity of antioxidant enzymes and the production of antioxidant compounds, including phenolics (Gill, Tuteja, 2010).
In sweet basil, NaCl salinity stress altered the biosynthesis of essential oils (Prasad et al., 2007; Hassanpouraghdam et al., 2011) and phenolics (Tarchoune et al., 2009). Other authors observed an increase in phenolic compounds in medicinal plants
exposed to high salinity in the growing medium (Said, Omer, 2011; Sabra et al., 2011; Taârit et al., 2011).
Work is in progress at the University of Pisa to develop a hydroponic production system of sweet basil for the extraction of RA. In a previous work, sweet basil seedlings grown in floating raft system was found to produce large amount of biomass with RA concentration up to nearly 30 mg/g DW (Kiferle et al., 2011). The present paper reports the results of two experiments carried out to investigate the effects of either oxygen deficiency or NaCl salinity (2.0, 25.0 and 50.0 mmol/l) on growth and RA accumulation in sweet basil plants (cv. Genovese) cultivated in floating system.
Very few information is available on the response to root hypoxia and NaCl salinity in sweet basil grown hydroponically for the production of RA. To our knowledge, only a study was conducted on this topic by Tarchoune et al. (2009), who investigated the effect of 0 and 50 mmol/l NaCl in the nutrient solution on the accumulation of RA and other caffeic acid derivatives in sweet basil grown in floating system.
4.2. MATERIALS AND METHODS
PLANT MATERIAL AND GROWING CONDITIONS
Two experiments were conducted in a glasshouse in summer 2010, under the typical climatic conditions of Mediterranean regions. The ventilation air temperature was 25.0°C; during sunny hours the maximum temperature reached up to 30–32°C notwithstanding a movable shading net above the roof, which was closed between 11.00 a.m. and 03.00 p.m.. Daily global radiation and mean air temperature averaged, respectively, 11.6 MJ/m and 26.6°C.
Sweet basil seeds (cv. Genovese) were germinated in rockwool tray plugs in a growth chamber (25 ± 1°C; 250 μmol/s/m2 PAR; 12 h photoperiod) and, at the
second leaf stage, the seedlings were transferred to a glasshouse located at the University of Pisa. Two weeks after sowing, the plants were transferred in 12 separate hydroponic systems, each consisting of a polystyrene plug tray floating in a 15-L plastic tank with fairly stagnant nutrient solution. Forty plants were planted in each tank and crop density was approximately 160 plants/m2 (on a ground area
basis).
The nutrient solutions were prepared by dissolving in tap water (with approximately 2.0 mmol/l NaCl) appropriate amounts of technical-grade inorganic salts [KNO3, KH2PO4, Ca(NO3)2 and MgSO4] plus chelated trace elements. The concentration of macronutrients and micronutrients was the following: 5.0 mmol/lN-NO3; 1.0 mmol/l P-H2PO4; 6.0 mmol/lK; 2.5 mmol/lCa; 1.5 mmol/lMg; 3.50 mmol/lS-SO4; 40.6 mmmol/l Fe; 35.0 mmmol/l B; 3.6 mmmol/l, Cu; 4.6 mmmol/l, Zn; 10.9 mmmol/l Mn; 1.0 mmmol/l Mo. The pH of all nutrient solutions was adjusted to 6.0 with diluted H2SO4 and the electrical conductivity (EC) of newly-prepared nutrient solutions was 1.71 mS/cm.
In the second experiment, the control nutrient solution (containing 2.0 mmol/l NaCl) was supplemented with 0, 23 and 48 mmol/l NaCl to reach EC equivalent to 1.71, 4.10 and 6.35 mS/cm, respectively. In this experiment all the tanks were continuously aerated, while in the first one air was insufflated only to half the tanks. The nutrient solution in each tank was checked for pH, EC and oxygen concentration almost daily using portable instruments; they were completely replaced every five days. In most cases, the exhausted nutrient solutions were analysed for the concentration of macronutrients.
DETERMINATIONS
Sweet basil plants were sampled for growth determination and laboratory analysis at bloom stage (eight weeks after transplanting), as the content of RA reached the maximum level during this developmental stage (Kiferle et al., 2011).
inflorescences) and roots were measured. The content of RA and other selected CADs (caffeic acid, caftaric acid, chlorogenic acid, cicoric acid, cynarin, ferulic acid,
t-cinnamic acid, p-coumaric acid) was determined in root and leaf tissues. Each
sample, consisting of the organs collected from two individual plants, was rapidly washed in tap water, rinsed in deionised water, gently dried with a towel, frozen in liquid nitrogen and stored at -80°C before analyses, which were performed within a few weeks after sampling. The samples were not dried before extraction as desiccation reduced markedly the RA content of sweet basil tissues (Kiferle et al., 2011). The concentration of CADs in HCl-methanol extracts was determined by means of HPLC, as previously described (Kiferle et al., 2011) and expressed per gram dry weight (DW) on the basis of the dry matter content determined in an aliquot of each sample after desiccation in a ventilated oven at 85°C. Peak identification was accomplished by LC-MS and LC-MS-MS, as previously reported (Kiferle et al., 2011). The detection limit of the analytical method was 0.05 mg g-1 DW.
STATISTICAL ANALYSIS
The experimental design was completely randomized. Data were subjected to analysis of variance (ANOVA) using the Statgraphics Centurion XV.II (Manugistic Co., Rockville, Maryland, U.S.A.) software. Each experiment was repeated twice with similar findings; the paper reports the results from a representative run.
4.3. RESULTS AND DISCUSSION
ROSMARINIC ACID CONTENT
In both experiments, among the CADs of interest RA was the only compound found in plant tissues at concentrations higher than the detection limit, as found previously (Kiferle et al., 2011). Other authors reported that RA was the most important CAD in sweet basil (Javannardi et al., 2002; Jayasinghe et al., 2003). The levels of RA found in sweet basil plants (10 to 97 mg/g DW - Fig.s 4.1, 4.2) were similar to those found
previously (Kiferle et al., 2011) and within those reported in the literature for sweet basil, which range from less than 0.1 mg g-1 DW (e.g. Sgherri et al., 2010) to nearly 100 mg g-1 DW (e.g. Javanmardi et al., 2002). This is likely a result of differences in plant genotype, growing conditions as well as the method used for RA determination.
EFFECT OF HYPOXIA (EXPERIMENT 1)
No significant variations in pH and EC were detected in both aerated and not aerated nutrient solutions (data not shown). This was likely the result of large volume and frequent replacement of the nutrient solutions in all the treatments.
In aerated nutrient solution, the oxygen content remained always above 7.0 mg/l with a mean value of daily measurements of 7.8 mg/l. In contrast, in the non-areated nutrient solution, the oxygen level never dropped below 2.2 mg/l throughout the whole experiment and averaged 3.1 mg/l. In both treatments, lower oxygen levels were found in the last 3-4 weeks, when the plants exhibited a large root system and presumably respiratory oxygen demand was higher compared to the initial stage of cultivation.
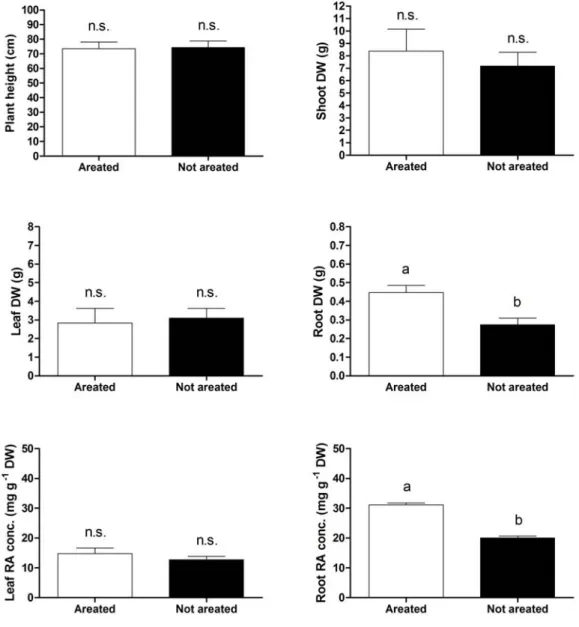
Shoot growth was not affected by the aeration of nutrient solution while, at the end of the experiment, root dry weight was reduced by about 38% in the plants grown at lower oxygen level (Fig. 4.1).
Root zone hypoxia was found to inhibit root growth earlier and/or to larger extent than shoot growth (Drew, 1983; Incrocci et al., 2000; Shi et al., 2007). Growth reduction is considered one of the first adaptive plant responses to hypoxia as this allows to conserve energy, inhibiting a wide range of ATP-consuming processes and hence reducing O2 demand (Geigenberger, 2003). A number of reasons may explain why in our study hypoxia conditions affected only root growth. Firstly, the growing period was too short (less than two months) to induce a significant decrease in shoot biomass accumulation. Secondly, we induced a moderate hypoxia stress as the oxygen level never dropped below 2.1 mg/l. The tolerance to low oxygen condition depends on plant genotype; for instance, the values of oxygen level described as limiting factor ranged from 2.7 and 4 mg/l in other crops such tomato (Zeroni et al.,
1983) and cucumber (Gilerod, Adams, 1983).
Moreover, plant sensitivity to hypoxia conditions may vary in different cultivars of the same crop species. In some cultivars of Medicago sativa root growth was severely restricted by waterlogging, whereas shoot biomass was not affected (Smethurst, Shabala, 2003); in other cultivars, however, the growth of both roots and shoot was limited by waterlogging stress. The cultivar of sweet basil used in our work appeared moderately tolerant to hypoxia stress.
Finally, differential response to root zone hypoxia of the root system and the aerial organs may be also associated to the ethylene entrapment in submerged plant tissues, as a consequence of the 10-4-fold decrease in the gas diffusion rate in water compared to air (Visser, Vosenek, 2004). Ethylene plays a key role in the plant’s adaptation to hypoxia, for instance by regulating the formation of adventitious roots and aerenchima, shoot elongation, epynasty etc. (Licausi, 2011). On the other hand, the hormone is known to inhibit root growth, even at relatively low concentration (Abeles et al., 1992).
Similarly to the effect of plant growth, hypoxia reduced significantly RA accumulation only in the roots, whereas leaf content of this metabolite was not modified by the oxygen level (Fig. 4.2).
In contrast to our findings, some authors observed an increase in the level of phenolics in plants grown under root zone hypoxia, for instance in the shoots and the roots of Hypericum brasiliense grown under waterlogging (Nacif de Abreu, Mazzafera, 2005), and in the stems of Eucalyptus marginata (Burgess et al., 1999). In the latter work, the higher phenolics concentration was associated to increased activity of some enzymes involved in their biosynthesis, such as phenylalanine ammonium-lyase (PAL), 4-Coumarate coenzyme A ligase (4-CL) and Cinnamyl alcohol dehydrogenase (CAD) (Burgess et al., 1999). Correspondingly, Aguilar et al. (2000) reported that hypoxia stimulated PAL activity in banana roots.
Figure 4.1. The effect of hypoxia on plant growth and the rosmarinic acid (RA) in
leaf and root tissues of basil (O. basilicum L.) plants (cv. Genovese) grown hydroponically and sampled eight weeks after transplanting, at full bloom. Mean values and standard errors of ten (growth data) or four (RA data) replicates, each consisting of one individual plant. One-way ANOVA was performed. Values followed by different letter differs significantly (P ≤ 0.05).
Recent studies have provided evidences that oxygen deficiency led to a decrease in transcripts for genes encoding proteins associated with ATP-consuming processes, such as the biosynthesis of secondary metabolites, in order to conserve ATP for other metabolic processes involved in the adaptive responses to low oxygen (van Dongen et al., 2012). Genes involved in the phenylpropanoid pathway were found to be down-regulated under hypoxia conditions in soybean seedlings (Nanjo et al., 2011), potato tuber discs (Geigenberger, 2003) and Arabidopsis roots (van Dongen et al., 2012).
EFFECT OF SALINITY (EXPERIMENT 2)
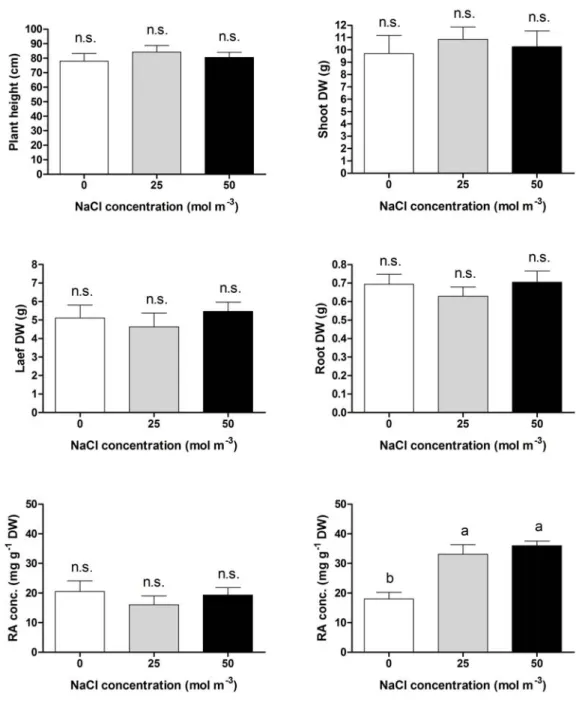
At harvest, plant height, shoot, leaf and root dry biomass (Fig. 4.2) did not differ significantly in the plants grown at 2.0, 25.0 and 50.0 mmol/l NaCl. The leaf tissue water content was also unaffected by NaCl salinity, averaging 9.61 g g-1 DW.
Other authors found that NaCl concentrations up to 50 mmol/l did not affect growth of sweet basil grown in water culture (Attia et al., 2009; Tarchoune et al., 2009, 2010). In contrast, Bernstein et al. (2010) reported the NaCl salinity reduced significantly root and shoot growth in hydroponically-grown sweet basil; however, remarkable growth suppression was observed only at concentration higher than 50 mM. Therefore, sweet basil can be considered moderately tolerant to NaCl salinity, although the response to this stress may change in different cultivars (Attia et al., 2011; Omer et al., 2008).
The tolerance of sweet basil to NaCl salinity was ascribed to the plant’s ability to control the upward flux of Na+ absorbed by the roots, desalinization of xylem sap along its ascending path and re-translocation of Na+ towards the roots within the phloem (Attia et al., 2009, 2011; Bernestein et al., 2010). The maintenance of adequate leaf tissue water content, as found in our work, is also considered an indication of salt tolerance (Attia et al., 2009, 2011; Bernestein et al., 2010).
Figure 4.2. The effect of NaCl concentration in the nutrient solution on plant growth
and the rosmarinic acid (RA) in leaf and root tissues of basil (O. basilicum L.) plants (cv. Genovese) grown hydroponically and sampled eight weeks after transplanting, at full bloom. Mean values and standard errors of ten (growth data) or four (RA data) replicates, each consisting of one individual plant. One-way ANOVA was performed. Values followed by different letter differs significantly (P ≤ 0.05).
A strong correlation between salt tolerance and antioxidant capacity has been demonstrated in several plant species (Gill, Tuteja, 2010). Tarchoune et al. (2010) observed that sweet basil was more tolerant to 50 mmol/l Na+ toxicity when the accompanying anion was Cl- instead of SO42-. The superior tolerance to NaCl than to Na2SO4 was associated to higher activity of antioxidant enzymes such as ascorbate peroxidase (POX), glutathione reductase (GR) and peroxidase (POX).
Hydroponics facilitates root uptake of both water and nutrients (Pardossi et al., 2006), thus limiting the osmotic and nutrition stress imposed by NaCl (Munns, Tester, 2008). When mineral nutrition was improved by the mycorrhizal colonization and/or high fertilization, pot-grown sweet basil plants exhibited a greater tolerance to irrigation with saline water (approximately 50 mmol/l NaCl) compared to un-inoculated plants grown with standard fertilisation level (Zuccarini et al., 2008). On the other hand, NaCl salinity stress was more severe in barley plants grown in hydroponics than in those grown in soil, as lower uptake of both Na+ and Cl- was observed in the latter plants (Tavakkoli et al., 2010).
The content of RA in leaf tissues was not affected by NaCl salinity, while root content increased significantly in the presence of 25 and 50 mmol/l NaCl (Figure 4.2). In a study with different cultivars of sweet basil grown in water culture, Tarchoune et al. (2009) observed that 50 mmol/lNaCl reduced leaf concentration of RA and other CADs in one cultivar without significant effect in another genotype. Increased levels of several CADs were observed in the roots of hydroponically-grown Echinacea angustifolia with 50-75 mmol/l NaCl in the nutrient solution (Montanari et al., 2008; Sabra et al., 2012). Higher CAD biosynthesis was associated with increased PAL activity (Montanari et al., 2008).
4.4. Conclusions
The floating raft system provides a suitable growing method of sweet basil for agro-industrial production of RA, in agreement with previous findings (Kiferle et al.,
2011). Both plant growth and RA accumulation in the leaves were not affected by hypoxia conditions and NaCl salinity. In contrast, the content of this metabolite in root tissues decreased or increased in response to hypoxia and salinity stress, respectively. These results have some implications from the operational point of view, as they suggest that poor quality irrigation water can be used and that the aeration of nutrient solution is not a crucial factor for optimal plant growth and RA production in floating raft system.
4.5. LITERATURE
Abeles, F.B., Morgan, P.W., Saltveit, M.E. Jr. (1992). Ethylene in Plant Biology. Academic Press, San Diego, CA, U.S.A.
Aguilar, E.A., Turner, D.W., Sivasithamparam, K. (2000). Fusarium oxysporum f.sp
cubense inoculation and hypoxia alter peroxidase and phenylalanine ammonia
lyase activities in nodal roots of banana cultivars (Musa sp.) differing in their susceptibility to Fusarium wilt. Aust. J. Bot. 48: 589–596
Attia, H., Karray, N., Ellili, A., Msilini, N., Lachaâl, M. (2009). Sodium transport in basil. Acta Physiol. Plant. 31: 1045-1051.
Attia, H., Ouhibi, C., Ellili, A., Mslini N., Bouzaïen, G., Karray, N., Lachaâl, M. (2011). Analysis of salinity effects on basil leaf surface area, photosynthetic activity, and growth. Acta Physiol. Plant. 33: 823-833.
Bernstein, N., Kravchik, M., Dudai, N. (2010). Salinity-induced changes in essential oil, pigments and salts accumulation in sweet basil (Ocimum basilicum) in relation to alterations of morphological development. Ann. Appl. Biol. 156: 167–177.
Burgess, T., McComb, J.A., Colquhoun, I., Hardy, G.E. StJ. (1999). Increased susceptibility of Eucalyptus marginata to stem infection by Phytophthora
cinnamomi resulting from root hypoxia. Plant Pathol. 48: 797–806.
Canter, P.H., Thomas, H., Ernst, E. (2005). Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 23: 180-185.
Colmer, T.D., Voesenek, L.A.C.J. (2009). Review. Flooding tolerance: suites of plant traits in variable environments. Funct. Plant. Biol. 36: 665–681.
Drew, M.C. (1983). Plant injury and adaptation to oxygen deficiency in the root environment: a review. Plant and Soil. 75: 179-199.
postharvest strategies for reducing nitrate content in rocket (Eruca sativa). Acta Hort. (ISHS). 628: 153-159.
Geigenberger, P. (2003). Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 6: 247-256.
Gill, S.S., Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48: 909-930. Gorbe, E., Calatayud, Á. (2010). Optimization of Nutrition in Soilless Systems: a
Review. Adv. Bot. Res. 53: 193–245.
Hassanpouraghdam, M.B., Gohari, G.R., Tabatabaei, S.J., Dadpour, M.R., Shirdel, M. (2011). NaCl salinity and Zn foliar application influence essential oil composition of basil (Ocimum basilicum L.) Acta Agriculturae Slovenica. 97: 93-98.
Heidari, M. (2012). Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L.) genotypes. Afr. J. Biotechnol. 11: 379-384.
Horchani, F., Aschi-Smiti, Samira, A.S., Renaud, B. (2010). Involvement of nitrate reduction in the tolerance of tomato (Solanum lycopersicum L.) plants to prolonged root hypoxia. Acta Physiol. Plant. 32: 1113-1123.
Incrocci, L., Pardossi, A., Vernieri, P., Tognoni, F., Serra, G. (2000). Effects of heat stress and hypoxia on growth, water relations and ABA levels in bean (Phaseolus vulgaris L.) seedlings. Acta Hort. (ISHS). 516: 31-40.
Javanmardi, J., Khalighi, A., Kashi, A., Bais, H.P., Vivanco, J.M. (2002). Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J. Agric. Food Chem. 50: 5878-5883. Jayasinghe, C., Gotoh, N., Aoki, T., Wada, S. (2003). Phenolics Composition and
Antioxidant Activity of Sweet Basil (Ocimum basilicum L.). J. Agric. Food Chem. 51: 4442−4449.
Juliani, H.R., Koroch, A.R., Simon, J.E. (2008). Basil: a new source of rosmarinic acid. In: Ho, C.T., Simon, J.E., Shahidi, F., Shao, Y. (eds), Dietary Supplements, American Chemical Society Symposium Series 987. Washington D.C., USA A.C.S. Pp. 129-143.
Kiferle, C., Lucchesini, M., Mensuali-Sodi, A., Maggini, R., Raffaelli, A., Pardossi, A. (2011). Rosmarinic acid content in basil plants grown in vitro and in hydroponics. Cent. Eur. J. Biol. 6: 946-957.
Licausi, F. (2011). Regulation of the molecular response to oxygen limitations in plant. New Phytol. 190: 550–555.
Maggini, R., Tozzini, L., Pacifici, S., Raffaelli, A., Pardossi, A. (2011). Growth and accumulation of caffeic acid derivatives in Echinacea angustifolia DC. var
angustifolia grown in hydroponic culture. Ind. Crop Prod. 35: 269-273
(2010). Strategies to decrease water drainage and nitrate emission from soilless cultures of greenhouse tomato. Agricult.Water Manag. 97: 971–980.
Montanari, M., Degl’Innocenti, E., Maggini, R., Pacifici, S., Pardossi, A., Guidi, L. (2008). Effect of nitrate fertilization and saline stress on the contents of active constituents of Echinacea angustifolia DC. Food Chem. 107: 1461–1466.
Morard, P., Silvestre, J. (1996). Plant injury due to oxygen deficiency in the root environment of soilless cultures: a review. Plant and Soil. 184: 243-254.
Munns, R., Tester, M. (2008). Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 59: 651–681.
Nacif de Abreu, I., Mazzafera, P. (2005). Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 43: 241-248.
Nanjo, Y., Maruyama, K., Yasue, H., Yamaguchi-Shinozaki, K., Shinozaki, K., Komats, S. (2011). Transcriptional responses to flooding stress in roots including hypocotyl of soybean seedlings. Plant Mol. Biol. 77: 129–144.
Omer, E.A., Said-Al Ahl, H.A.H., Hendawy, S.F. (2008). Production, chemical composition and volatile oil of different basil species/ varieties cultivated under Egyptian soil salinity conditions. Res. J. Agr. Biol. Sci. 4: 293-300.
Pardossi, A., Malorgio, F., Incrocci, L., Tognoni, F. (2006). Hydroponic technologies for greenhouse crops. In: Dris R. (ed.), Crops: Quality, Growth and Biotechnology, vol. 23. Helsinky, WFL Publisher: 360-378.
Petersen, M., Simmonds, M.S.J. (2003). Molecules of interest: rosmarinic acid. Phytochem. 62: 121–125.
Prasad, A., Lal, R. K., Chattopadhyay, A., Yadav, V. K., Yadav, A. (2007). Response of Basil Species to Soil Sodicity Stress. Comm Soil Sci Plant Anal. 38: 2705–2715.
Putievsky, E., Galambosi, B. (1999). Production systems of sweet basil, in: Hiltunen, R., Holm, Y. (eds.), Basil: The Genus Ocimum. Amsterdam, The Netherlands, Harwood Academic Publishers. Pp. 39-65.
Sabra, A., Adam, L., Daayf, F., Renault, S. (2011). Salinity-induced changes in caffeic acid derivatives, alkamides and ketones in three Echinacea species. Environ. Exper. Bot. 77: 234-241.
Said, H.A.H., Omer, E.A. (2011). Medicinal and aromatic plants production under salt stress. A review. Herba Polonica. 57: 72-87.
Santamaria, P. (2006). Nitrate in vegetables: toxicity, content, intake and EC regulation. J. Sci. Food. Agric. 86: 10–17.
Sgherri, C., Cecconami, S., Pinzino, C., Navari-Izzo, F., Izzo, R. (2010). Levels of antioxidants and nutraceuticals in basil grown in hydroponics and soil. Food Chem. 123: 416-422.
poor growth in root-restricted plants o tomato (Lycopersicon esculentum Mill.). Environ. Exper. Bot. 61: 181–189.
Smethurst, C.F., Shabala, S. (2003). Screening methods for waterlogging tolerance in lucerne: comparative analysis of waterlogging effects on chlorophyll fluorescence, photosynthesis, biomass and chlorophyll content. Funct. Plant Biol. 30: 335-343.
Tâarit, M.B., Msaada, K., Hosni, K., Marzouk, B. (2011). Physiological changes, phenolic content and antioxidant activity of Salvia officinalis L. grown under saline conditions. J. Sci. Food. Agric. DOI: 10.1002/jsfa.4746.
Tarchoune, I., Incerti, A., Lachaal, M., Ouerghi, Z., Izzo, R., Navari, F. (2009). Relations between antioxidant activity and salinity in basil (Ocimum basilicum Mill.). Agrochimica. 53, 56-65.
Tarchoune, I., Sgherri, C., Izzo, R., Lachaal, M., Ouerghi, Z., Navari-Izzo, F. (2010). Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol. Biochem. 48: 772-777.
Tavakkoli, E., Rengasamy, P., McDonald, G.K. (2010). The response of barley to salinity stress differs between hydroponic and soil systems. Funct. Plant Biol. 37, 621–633.
Tesi, R., Lenzi, A., Lombardi, P. (2003). Effect of different O2 levels on spinach (Spinacea oleracea L.) grown in a floating system. Acta Hort. (ISHS). 614: 631-637.
Van Dongen, J.T., Fröhlich A., RamÍrez-Aguilar, S.J., Schauer, N., Fernie, A., Erban, A., Kopka, J., Clark, J., Langer, A., Geigenberger, P. (2009). Transcript and metabolite profiling of the adaptive response to mild decreases in oxygen concentration in the roots of Arabidopsis plants. Ann. Botany 103: 269–280. Visser, E.J.W., Voesenek, L.A.C.J. (2004). Acclimation to soil flooding–sensing and
signal-transduction. Plant and Soil. 254: 197–214.
Zuccarini, P; Okurowska, P. (2008). Effects of mycorrhizal colonization and fertilization on growth and photosynthesis of sweet basil under salt stress. J. Plant Nutrition. 31: 497-513.