New strategies for the synthesis of
functionalized substituted bisphosphonates:
chemistry and biological activity
by
MULANI Iqbal Mubarak
DISSERTATION
Presented to the Chemistry Department
of the University of Calabria
In Partial Fulfilment of the Requirements
For the Degree of
DOCTOR OF PHILOSOPHY
The University of Calabria
Rende (CS), ITALY
December, 19, 2012
This work is dedicated to my family, close friends,
well-wishers and everyone who supported me and
believed in my capabilities.
Declaration
I hereby certify that this thesis has not been submitted before, in
whole or in part, to this or any other university for any degree and
is, except where otherwise stated, the original work of the author.
Signed: __________________ Date: ______________
Mulani Iqbal Mubarak
Acknowledgment
Before introducing the findings of my research, First and foremost, I would like to thank Almighty for his immense blessings and help provided to me throughout my life. You have given me the power to believe in myself and pursue my dreams. I could never have done this without the faith I have in you, the Almighty.
I would like to express my gratitude and appreciation to university of Calabria for the priceless opportunity to pursue my doctoral research degree in the Italy. The experience I have gained for the three years I have spent at the university while communicating with bright, knowledgeable and professional people was helpful and incomparable to any other experiences. I take immense pleasure to express my sincere and deep sense of gratitude to Prof. Giovanni Sindona, Director and Head of chemistry department, for allowing me to explorer the field of synthetic organic chemistry as I pleased. I offer my sincerest gratitude to Prof. Olga Bortolini under whose supervision I chose this topic and began the research. Her much appreciated guidance, support, patience and most of all her friendship throughout the course of this research will not be forgotten. I learnt a lot from her, which I am sure, will be useful in different stages of my life. One could not wish for a better or friendlier supervisor.
I would like to take this opportunity to thank, Prof. Antonio De Nino and Prof. Loredana Maiuolo, who have supported me throughout my thesis with their patience and knowledge whilst allowing me the room to work in my own way. I salute them for their encouragement and effort and without them this work would not have been completed. One simply could not wish for a better or responsive co-supervisor.
ACKNOWLEDGMENT
IV
A grateful thanks to Prof. Olga Bortolini’s research team at university of Ferrara and Prof. Giuseppina De Luca, university of Calabria for their assistance in ESI-MS plus biological activity experiments and 31P NMR experiments respectively.
The members of Prof. Sindona’s group have contributed immensely to my personal and professional time at the unical. The group has been a source of friendships as well as good advice and collaboration. I am especially grateful to Beatrice (Bea), Gaetano (Gedha), Alessandro (Alex), Monica (Moni), and other master students. In my daily work, I have been blessed with a friendly and cheerful group of fellow students.
Nevertheless, I would like to thank Dr. Naim for shared his experience about professional as well as personal life and his continuous help during my degree. Nonetheless, I would like to thanks to Vito and Fitim for their help, suggestions and informations. I enjoyed all the friendly conversation on science and social aspects with you. I wish you all nothing but the best in the future. Moreover, I would like to thank to Prof. Roberto Bartilino, Director and Head of “Bernardino Telesio School” and Prof. Bartolo Gabriele, PhD coordinator of OMPI doctorate school for examined me carefully during my presentations and workshops. I expresaly would like to thanks to Silvio for his time to thesis arrangement and printing.
Beyond chemistry (which sometimes seemed to be nothing more than a distant dream), I owe my deepest gratitude to Montalto family (Alice, Pasquale and Devid) for their support and help during my stay in university campus. I will never forget your assistance in my life, salute to you. I would like to extend my sincere thanks to all my Indians specially, Mauli, Yogesh, Prasad, Rakesh, Sumit, Navin and International friends, here in this university for their help, support and love.
Expressly, I will not forget to say thanks to cricket players who played cricket with me on Indo-Calabria ground. I cherish the friendship I had and
ACKNOWLEDGMENT
take this opportunity to thank each one of them. My friends Sachin, Mauli, Raju, Sameer, Vijay, Sambhaji, Nivrutti, Anil, Gajanan, Mahesh and more have been an encouragement every time and their motivation my confidence that had helped me reach here.
It’s my pleasure to thanks Dr. Iliyas, Dr. Muballigh, Dr. Hamid and Dr. Yunnus for sharing their experience and helping me. They have always been a tremendous help no matter the task or circumstance. Friends, thanks for your continuous love, support and for all the good wishes.
Of course no acknowledgments would be complete without giving thanks to my parents and grandparents. My Abba and Mummy have instilled many admirable qualities in me and given me a good foundation with which to meet life. They’ve taught me about hard work and self-respect, about persistence and about how to be independent. Mom, especially, is a great role model of resilience, strength and character. Both have always expressed how proud they are of me and how much they love me. I too am proud of them and love them very much. I am grateful for them both and for the ‘smart genes’ they passed on to me.
Last, but certainly not least, I must acknowledge with tremendous and deep thanks my life, love, and one and only my younger brother, Sikandar (Munnya). I couldn’t be at this stage without his support, salute dude. Nevertheless, I am very grateful to my life partner my Megha for her patience, sympathy and inspiration. Moreover, I would like to thanks to Mrs Shabnam (wife of my bro), being member of Mulani family.
If I did not mention someone’s name here, it does not mean that I do not acknowledge your support and help. Again, I would like to thank everyone who supported and helped me during my Ph.D. study.
Abbreviations
aq aqueous
Ar aryl (substituted aromatic ring) ATP adenosine triphosphate
Bn benzyl bp boiling point BPs Bisphosphonates br broad (NMR signal) ca circa (approximately) °C degrees Celcius cat. Catalytic conc. Concentrated 1,3-DC 1,3-dipolar cycloaddition DEXA Dexamethasone DCM Dichloromethane DMSO Dimethylsulfoxide E+ Electrophile
EDG Electron-donating group e.e. Enantiomeric excess
e.g. exempli gratia (for example)
Equiv equivalent Fig. figure
FPPS farnesyl pyrophosphate syntheses ESI electronspray ionization
Et ethyl etc etcetera
ABBREVIATIONS
VIII
EWG Electron-withdrawing group FMO Frontier Molecular orbital δ chemical shift
g gram
gem geminal
GC Gas liquid chromatography GTP guanosine triphosphate h hour
HA hydroxyapatite H-Bonding Hydrogen bonding
HOMO Highest occupied molecular orbital HPLC High performance liquid chromatography hν irradiation with light
J coupling constant (NMR signal) i.e. for example
LAH lithium aluminum hydride LA Lewis acid
LUMO Lowest unoccupied molecular orbital m multiplet (NMR signal) mg milligram MHz megahertz min minutes mL milliliter mmol millimole m/z mass/charge Me methyl MO Molecular orbital
ABBREVIATIONS
MS mass spectrometry
NMR nuclear magnetic resonance Nu nucleophile (general)
o ortho
Ph phenyl
p para
PEG Poly-(ethylene glycol) PPi pyrophosphate
ppm parts per million (NMR signal) Py pyridine
q quartet (NMR signal)
Rf retention factor in chromatography RT room temperature s singlet (NMR signal) sec secondary t triplet (NMR signal) TS Transition state THF Tetrahydrofuran
Contents
Declaration ... I Acknowledgment ... III Abbreviations ... VII Abstract ...XV Chapter 1Bisphosphonates: structure and biological activity ... 1
1.1 Introduction ... 1
1.2 History ... 9
1.3 Bisphosphonates ... 11
1.3.1 Structure ... 25
1.3.2 Chemistry ... 26
1.3.3 Biological activity included structure-activity relationship ... 26
1.4 Pharmacological relevant bisphosphonates ... 31
1.5 References ... 33
Chapter 2 1, 3-dipolar cycloaddition reaction ... 41
2.1 Introduction ... 41
2.1.1 Definition and history of 1, 3-dipolar cycloaddition ... 43
2.1.2 Dipoles or ylides ... 44
2.1.3 The dipolarophile ... 46
2.1.4 Frontier Molecular Orbital Interactions ... 47
2.2 Overview of the Nitrone Reactivity Profile ... 48
2.2.1 1,3-Dipolar Cycloaddition Reaction to Nitrone ... 50
2.2.2 Selectivity of the 1,3-Dipolar Cycloaddition Reaction Between Alkenes and Nitrones ... 51
XII
2.2.4 Diastereoselectivity and Enantioselectivity of the 1,3-Dipolar
Cycloaddition Reaction with Alkenes ...54
2.3 Methods for Synthesis of Isoxazolidine ...56
2.4 References ...59
Chapter 3 1,3-DC with hetero substituted alkenes and nitrones. ...67
I. Oxa substituted alkenes ...67
3.1 Nitrones Activated by Electron-withdrawing Groups ...67
3.1.1 Thermal conditions ...68
i Acyclic Nitrone ...68
ii Cyclic Nitrones ...72
iii brønsted acid-catalyzed conditions ...73
3.1.2 Lewis Acid-catalyzed Condition ...74
i. Europium (III) Catalyst ...74
ii. Copper (II) and Zinc (II) Catalysts ...75
3.2 C-Aryl-substituted Nitrones ...77
3.2.1 Thermal conditions ...77
3.2.2 Lewis Acid-catalyzed Conditions ...78
i TMSOTf-promoted Reactions ...78
ii Boron(III) Catalyst ...79
iii Aluminium(III) Catalyst ...80
iv Brønsted Acid-catalyzed Conditions ...82
II Aza substituted alkenes ...83
3.3 Enamines as Dipolarophile ...83
3.4 Enamides as Dipolarophile ...84
3.4.1 N-Vinylnucleobases ...84
3.4.2 Simple N-vinylamides and hetero derivatives ...90
Chapter 4
5-Heterosubstituted Isoxazolidine Ring Opening Methods ... 99
4.1 Introduction ... 99 4.2 5-Oxa-substituted Isoxazolidines ... 100 4.2.1 via reduction ... 100 4.2.2 Via disproportionation ... 106 4.3 5-Aza-substituted Isoxazolidines ... 110 4.4 References ... 111 Chapter 5 Result and discussion ... 115
5.1 INTRODUCTION ... 115
5.1.1 Synthesis of Nitrones ... 116
5.1.2 Synthesis of tetraethylvinylidene-1, 1-bisphosphonate ... 117
5.1.3 Performed reactions to obtain 1, 3 dipolar cycloadduct ... 118
5.1.4 Synthesis and regeiochemistry of 1, 3 dipolar cycloadduct ... 119
5.1.5 Hydrolysis of isoxazolidines bisphosphonates and salt formation ... 122
5.1.6 Biological Activity results ... 124
5.2 Isoxazolidiene ring reduction using transition metal carbonyls ... 126
5.2.1 Reaction mechanism of N-O bond cleavage ... 128
5.2.2 Hydrolysis of ring opened products and their salt formation ... 129
5.3 Synthesis of Zoledronic acid ... 130
5.4 Synthesis of vinyl thymine and substituted isoxazolidiene ... 131
5.5 Biological activity result of substituted thymine isoxazolidiene ... 133
5.6 Referances ... 139 Conclusion ... 143 Chapter 6 Experimental ... 145 Chapter 7 Spectral Data ... 179
Abstract
The ever expanding cutting edge technologies in medicine for the benefit of society, the orthopedic branch is one among those significant branches in medicine pertaining to bone. Bisphosphonates (BPS) are being increasingly and successfully used to prevent bone fractures and the concerning problems of bone diseases such as Paget’s diseases, osteoporosis and tumour bone disease. In view of this specific problem, BPS are well established in the treatment of osteoclast -mediated resorbtive bone diseases including osteoporosis, Paget's disease and tumor-induced osteolysis. Recent studies suggest that, besides inhibiting bone resorbtion, BPS may also exert a direct antitumor effect, and this class of drugs has been shown to inhibit proliferation and to induce apoptosis in vitro in different human tumor cell lines. BPs are classified into two groups according to their chemical structure and mechanism of action: (i) non nitrogen containing BPS such as etidronate and clodronate that are of low potency and inhibit osteoclast function via metabolism into toxic ATP-metabolites and (ii) nitrogen-containing BPS (NBPS), such as pamidronate, alendronate, risedronate, ibandronate and zoledronate which is the most potent antiresorptive agent.
Hence in present investigation we synthesized some several
bisphosphonates bearing a substituted isoxazolidine ring by direct 1, 3-dipolar cyclization reaction in the absence of solvent and good yield under novel, promising and low cost microwaves catalysis. The method allows the simultaneous incorporation on the geminal position of the bisphosphonate framework, of basic nitrogen and of an oxygen atom, as third hook. The studies on the inhibitory potency of cyclic nitrogen
ABSTRACT
XVI
containing bisphosphonates indicate that the presence of two geminal phosphonate groups is responsible for interaction with the molecular target. In addition, basic nitrogen in the heterocyclic side chain affects potency and its orientation is critical for effective inhibition of bone diseases.
For the synthetic point of view, different aryl and alkyl substituents on the isoxazolidine ring prompt us to investigate the ring opening of these compounds through cleavage of the N-O bond. This strategy represents a novel access to new gem-hydroxyl bisphosphonates, bearing aryl substituents on the lateral chain. The reductive cleavage of the N-O bond in isoxazolidines represents a simple and direct access to N-substituted aminoalcohols, valuable intermediates in many synthetic strategies. Moreover, additional reaction path way have been envisaged leading to the formation of non-hydroxyl bisphosphonates.
Abstract in lingua italiana
I derivati bisfosfonati (BPs) sono stati impiegati con successo nella prevenzione delle fratture ossee e nei problemi legati alle malattie ossee come la malattia di Page, l’osteoporosi e il tumore osseo. Più in dettaglio i bisfosfonati sono utili per il riassorbimento mediato degli osteoclasti nelle malattie descritte in precedenza. Secondo studi recenti, i BPs possono esercitare un effetto antitumorale diretto e questa classe di composti inibisce la proliferazione, inducendo apoptosi in vitro in differenti linee cellulari tumorali umane. I BPs sono classificati in due gruppi in base alla struttura chimica e il loro meccanismo d’azione: (i) BPs non contenenti azoto, a bassa potenza inibitoria; (ii) BPs contenenti azoto, più potenti e con una migliore risposta. Di conseguenza, nel seguente lavoro di tesi di dottorato sono stati sintetizzati diversi BPs contenenti un anello isossazolidinico, tramite cicloaddizione 1,3-dipolare e utilizzo di microonde (buone rese in ogni caso). Questo metodo permette
ABSTRACT
l’incorporazione simultanea di un atomo di azoto e di ossigeno nella struttura dei bifosfonati. Studi sulla potenza inibitoria dell’azoto ciclico presente sui BPs indicano che la presenza di due gruppi fosfonati geminali è responsabile generalmente dell’interazione con il target molecolare. Dal punto di vista sintetico, dopo avere inserito diversi sostituenti arilici e alchilici sull’anello isossazolidinico, ci siamo spinti a investigare l’apertura dell’anello attraverso la rottura del legame N-O. Questa strategia rappresenta un approccio innovativo alla sintesi di bisfosfonati gem-idrossilici con sostituenti arilici sulla catena laterale. La rottura riduttiva del legame N-O rappresenta, infatti, un diretto accesso all’ottenimento di amminoalcoli N-sostituiti, intermedi interessanti in molti processi sintetici. Inoltre, è stato investigato anche il percorso meccanicistico alternativo che porta alla formazione di bisfosfonati non idrossilati.
Chapter 1
Bisphosphonates: structure and biological
activity
1.1 Introduction
Recently, the development of organophosphorus chemistry has been characterized by a great interest in bisphosphonates (BPs) and
bisphosphonic acids. The discovery and development of the
bisphosphonates (BPs) as a major class of drugs for the treatment of bone diseases has been a fascinating story that has extended over three decades. Nowadays, the ultimate goal of many chemists all around the world has become rational or at least semi-rational drug discovery. Furthermore, the development of the medicinal chemistry as both a pure and an applied science is considered to have a significant impact upon it 1. A boost of medicinal chemistry is based on development of such disciplines like combinatorial chemistry, compounds identification (e.g. NMR), automated synthesis etc. Organo-phosphorus chemistry, which, recently have taken an important effect on the synthesis and design of a wide variety of biologically active compounds. This chemistry, being ignored for many years, recently has achieved important and well-recognized place in the search for new drugs 2. Organo phosphorus chemistry, as a discrete area of study, is the study of compounds containing a C-P bond 3. Its present impact on the field of medicinal Chemistry is even difficult to quantify. Among the list of all organo phosphorus compounds the main place is occupied by phosphonates and bisphosphonates, which have found huge
INTRODUCTION
2
application as pharmaceuticals. For instance, derivatives of phosphonic acid are used in the synthesis of different α-amino phosphonic acids which are considered to be structural analogues of the corresponding α-amino acids. It is noteworthy that, their negligible mammalian toxicity, and the fact that they very efficiently mimic amino carboxylic acids makes them extremely important anti metabolites in the process of designing new drugs 4
. On the other side, bisphosphonates have been found recently as an ideal therapeutic agent for treatment of different bone diseases. Their capability to chelate metal ions and inhibit crystals growth but also a strong affinity to bone was used in synthesizing new drugs by many pharmaceuticals companies5. Bone tissue constitutes our bodily scaffold around which our organs are compartmentalized. It is a dynamic tissue that maintains the mineral balance in an organism, as well as providing an environment for cellular machinery involved in different physiological functions6. Bone tissue undergoes constant remodelling, where tightly regulated anabolic and catabolic processes enable bone adaptation during the lifespan of an organism. In addition to the cells involved in regulating bone tissue mass, i.e., bone-depositing osteoblasts, bone resorbing osteoclasts and regulatory osteocytes, bone tissue provides a home for a diverse array of cells involved in systemic functions. Immune regulatory cells involved in host defence, mesenchymal stem cells involved in tissue healing repair, and hematopoietic precursors destined for systemic gas transport, are distinct cell populations residing in the bone tissue. Bone tissue is distinguished from the rest of our tissues by the presence of a massive mineral phase, i.e., biological apatite. Approximately 3–4 kg of mineral mass is present in our bodies, and two-thirds of this mineral mass is estimated to be present in the bone tissue7. More than 99% of bodily calcium deposits are located in bone. With the exception of dental tissue and pathological calcifications, such as kidney stones and calcified atherosclerotic plaques, no other tissue
INTRODUCTION
systems contain such a concentrated mineral phase. It is the mineral phase in bones that can serve as a unique receptacle for absorption of molecules from the systemic circulation, and molecules in circulation that display a preferential affinity to biological apatite have the potential to seek and concentrate in the bone tissue. This provides a unique opportunity for developing magic bullets for bone diseases, following on from Paul Ehrlich’s idea that an ideal drug will act specifically on a disease-causing agent, in this case in bones, without affecting other tissues in an organism. Only a limited number of molecules exhibit a strong affinity to bone. These include heavy metals, such as strontium, rhenium and lead, and the well-known antibacterial agent tetracycline8. The conventional therapeutic agents, except one class of molecules (bisphosphonates, the subject of this critical review), do not exhibit any particular affinity to bone. The systemic administration of these molecules accordingly results in non-specific distribution throughout an organism. For developing bone-specific therapeutic agents, the critical challenge becomes the design of molecules that display a preferential affinity to biological apatite with no affinity to other tissues. Systemic administration of such molecules will result in specific deposition to bone tissue with no accumulation at other tissues. This goal is likely to be difficult to achieve, since all therapeutic molecules will display a certain degree of affinity to other tissues, given the diverse array of functional groups (e.g., hydrophobic, polar, charged, etc.) found in biological membranes and surfaces. However, a step towards this goal is to engineer the currently utilized therapeutic agents for an appetite affinity. The benefits of this end ever will be two-fold. First, the molecules that are currently acceptable for treatment of bone diseases (i.e., where the therapeutic action overshadows the undesired activities) will be more effective, since bone targeting will concentrate the pharmacological agents at the desired site of activity. This will allow a more potent activity without
INTRODUCTION
4
increasing the administered dose, which is not always possible due to undesirable activities of the therapeutic agents at extra-skeletal sites.
Secondly, promising molecules not previously tested for bone diseases due to unacceptable side effects (i.e. where the undesired activities outweigh the therapeutic action) may become effective on bone diseases after being concentrated in the bone tissue. Modifying the therapeutic agents for bone affinity, of course, should not alter the inherent pharmacological activity of the agents. In this way, a given therapeutic agent can be tailored to have a higher specificity by concentrating it to bone sites and, possibly, to display lower toxicity by reducing its exposure to extra-skeletal sites.
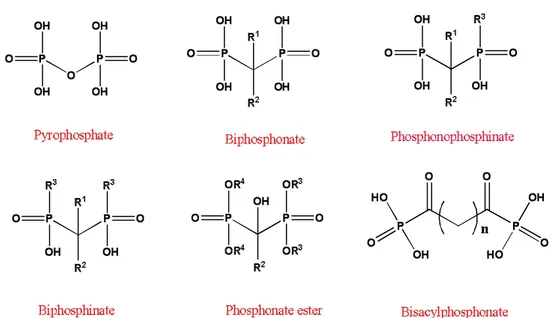
Efforts in this direction were set into motion in the early 1960s while probing the physiological function of an endogenous molecule, pyrophosphate (Fig. 1).
Fig. 1 Structure of the endogenous pyrophosphonate, and its synthetic analogue, bisphosphonate (BP), which exhibit a strong bone affinity. The geminal (a) carbon in BPs typically contains two separate substituents, R1 and R2, which may significantly affect both the mineral affinity and the pharmacological activity.
INTRODUCTION
Pyrophosphate is localized throughout an organism, and displays a dual activity on the formation and dissolution of biological apatite, a carbonated form of the tachometric hydroxyapatite [Ca10(PO4)6(OH)2]
9,10
. The strong affinity of the pyrophosphate to nucleating HA crystals was considered to be the underlying basis of this dual activity11. On one hand, the pyrophosphate appeared to become localized on the growing crystal surfaces, preventing the growth of the HA (i.e., ‘poisoning’ the fledgling crystal growth). On the other hand, this pyrophosphate coating on HA surfaces provided a protective layer against the dissolution of the already nucleated crystals. The ability of the pyrophosphate to suppress crystal growth is put into constant use in our bodies where preventing aberrant calcification from the supercritical solutions found in the tissues is an enduring process. Indeed, pyrophosphate administration was found early on to be beneficial in an animal model of aberrant calcification, namely the rat aortic calcification model12. The ability of the pyrophosphate to suppress apatite dissolution, on the other hand, suggested a means to prevent the loss of tissues already mineralized (i.e. deposited bone). Unlike its beneficial effect in suppressing aortic calcification, pyrophosphate was not beneficial in suppressing bone loss, and this lack of activity in bone resulted in a search for pyrophosphate analogues that displayed superior stability in the bone milieu, the presumed shortcoming of the pyrophosphate in this environment. The search led to identification of phosphonate-based molecules, where the hydrolysis-resistant –C–P (O)–(OH)2 moieties replaced the labile –O–P(O)–(OH)2 moieties in the pyrophosphate
13-16 . Such diphosphonates were shown to be capable of controlling HA dissolution13, 14, as well as preventing bone loss induced by immobilization15 and parathyroid extract injection in animal models14.
INTRODUCTION
6
The diphosphonates were also active in preventing pathological aorta calcification16, similar to the first beneficial use of the pyrophosphates. The diphosphonates used in these early studies were dichloromethylene diphosphonate14, 15, methylene diphosphonate14 and 1-hydroxyethylene-1, 1-diphosphonate16. The two phosphonate moieties in these compounds were located on the same carbon (α-carbon), in fact forming the basis of the bisphosphonate (BP) class of compounds. This promising work spurred intense research activity where the end-goal was to identify pharmacologically active analogues of BPs, i.e. potent compounds where a predictable inhibition of bone loss could be obtained when delivered in a convenient, clinically acceptable fashion without significant side-effects. Human use of the BPs immediately followed with almost no lag time for clinical entry17. As with the first generation of BPs, contemporary BPs display an exceptional affinity to HA; once localized to the bone tissue, however, they exert their respective pharmacological activities primarily by modulating local cellular activities, and rather than affecting the physicochemical properties of the apatite. It must be pointed out that Fleisch’s early work also recognized the possibility of cellular effects by the early BPs, in addition to their effects on inhibition of HA dissolution14. It was within a few years of realization of the pharmacological activities of BPs that their utility as bone carriers was also demonstrated. The initial use of BPs for bone targeting was for delivering the radio nucleotide 99mTc to skeletal tissues for imaging purposes18, 19. Complexes formed between a BP and 99mTc did not compromise the bone-seeking capability of the compounds, providing a means to visualize skeletal tissues via the c-emitting isotopes. Several critical observations were immediately noted from this collective activity: heterogeneity in bone uptake of the labelled complexes, ability to detect osteolytic metastasis in bones, as well as locating neoplastic tissues extra-skeletally in soft tissues (presumably due
INTRODUCTION
to local spots of calcification) spurred a diagnosis-centred BP research20. These studies initially established the existence of a structure–function relationship for BPs, and inspired subsequent studies to further elucidate this relationship. It was not until 1986, however, when the development of a bone targeted therapeutic (i.e. the synthesis of BP-incorporating molecules with pharmacological activities distinctly different from the BP action) was first reported. Two of the earliest examples of bone-seeking therapeutics, which relied on a BP moiety for bone targeting and a distinct moiety for pharmacological activity, were an 131I-containing BP21, and the anti-neoplastic drug 1,2,4-triglycidylurazol chemically linked to a BP22. A wide spectrum of bone-seeking therapeutic agents has subsequently been pursued.
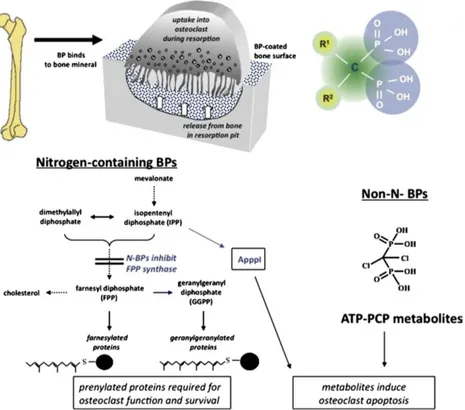
Over the years there were many attempts made to explain how bisphosphonates work on cells, especially via inhibitory effects on enzymes. Various studies suggested possible effects on glycolysis, or direct or indirect inhibition of the osteoclast proton pumping H+ATPase , phosphatases, or lysosomal enzymes, and even effects on osteoblasts to produce an osteoclast inhibitory factor 23. The contemporary view is that there are two major but distinct molecular mechanisms by which bisphosphonates affect osteoclasts, and that bisphosphonates can be classified into at least two major groups based on these different modes of action which is shown in Fig.2.
The first group comprises the non-nitrogen bisphosphonates, such as clodronate and etidronate that seem able to most closely mimic pyrophosphate. They behave as PPi analogues by being metabolically incorporated into non-hydrolysable analogues of ATP though the reversal of the actions of amino acyl- tRNA synthetases. The resulting metabolites contained the P-C-P moiety in place of the β, γ-phosphate groups of ATP, thus resulting in non-hydrolysable (AppCp) nucleotides 24,25,26,27. It is likely
INTRODUCTION
8
that intracellular accumulation of these metabolites within osteoclasts 28, 29 inhibits their function and may cause osteoclast cell death, probably by interference with mitochondrial ATP translocases 30. This group of non-nitrogen-containing bisphosphonates therefore appears to act essentially as prodrugs, being converted to active drug metabolites following intracellular uptake by osteoclasts in vivo.
Fig. 2 The cellular and biochemical mechanisms of action of bisphosphonates.
In contrast, the second group of bisphosphonates contains all of the more potent, nitrogen-containing compounds (N-BPs), which are not metabolised to AppCp-type metabolites as described above. In contrast, members of this group of N-BPs interfere with specific metabolic reactions, notably in the mevalonate biosynthetic pathway that leads to the synthesis of cholesterol and other sterols. The enzymes in this pathway metabolise pyrophosphate-containing isoprenoid lipids, which are progressively condensed into longer chains. Bisphosphonates are able to inhibit several enzymes in this pathway to varying extents31, 32, but the major target for the anti-resorptive N-BPs is farnesyl pyrophosphate syntheses (FPPS).
1.2 History
Bisphosphonates have been known to chemists since the middle of the 19 th century, the first synthesis dating back to 1865 in Germany33. Due to its ability to chelate metals, the early uses of bisphosphonates were not only for soften water in irrigation systems used in orange groves but also for industrial, mainly for corrosion prevention, and largely used in the textile, fertilizer and oil industries as well as in washing powders. And, because of their property of inhibiting calcium carbonate precipitation, as preventers of scaling.
The study and development of bisphosphonates as a major class of drugs for the treatment of bone diseases began only three decades ago. The first report of the biological characteristics of bisphosphonates was published in 196834 (Fig.3). At that time, scientists discovered that bisphosphonates have a marked ability to inhibit bone resorption. The initial rationale for their use in humans was their potential in preventing the dissolution of hydroxyl apatite, the principal bone mineral, thus arresting bone loss. The concept was derived from earlier studies in Prof. H. Fleisch laboratory on inorganic pyrophosphate35, in which it was found that plasma and urine contained compounds inhibiting calcium phosphate precipitation in
vitro and it was found that part of this activity was due to inorganic
pyrophosphate, a Substance that had not been described previously in these fluids.
HISTORY
10
Fig. 3 The history of bisphosphonates
The research group of Prof. H. Fleisch then found that pyrophosphate also inhibited calcium phosphate dissolution in vitro. In vivo, this compound prevented ectopic calcification but had no effect on normal mineralization and on bone resorption, possibly because it was destroyed locally by phosphatises. This prompted scientists to look for analogs of pyrophosphate that were not destroyed enzymatically. The bisphosphonates fulfilled these conditions 36,37. Only in the 1990s was their actual
mechanism of action demonstrated with the initial launch
of Fosamax (alendronate) by Merck.
A recent search in PubMed under the term ‘bisphosphonates’ revealed over 19,000 publications, and even this large list this does not cite abstracts, nor all publications and the many books and review articles available that describe the chemistry, pharmacology, and Clinical applications of bisphosphonates.
1.3 Bisphosphonates
Bisphosphonates (BPs) are class of drug that used for over 30 years in treatment of various disorders of mineral metabolism apart from this it
serves to regulate calcium, and prevent bone breakdown38. The
bisphosphonates are powerful, they cause dramatic changes in the bone physiology, and they deserve respect. In women or men whose bone density T-score is lower than -2.5, or who already have a vertebral fracture, these medicines reduce the incidence of fractures and improve the quality of life39. In addition to this, a significant number of these compounds are currently being used for the treatment of several bone disorders such as Paget’s disease, myeloma, bone metastases and osteoporosis40, as well as in some childhood diseases41. Recently, bisphosphonate drugs have also been found to have activity against the in vitro proliferation of several protozoan parasites, including Trypanosoma brucei which causes African
trypanosomiasis or sleeping sickness in human and animals42.
Consequently, the well-proven clinical utility of bisphosphonates has fostered the development of several methodologies for the preparation of novel derivatives, structure -activity studies have actually indicated that bioactivity is highly dependent on the nature of substituents linked to the bisphosphonic skeleton43. Some cancers can cause bone pain and weakness. These are most often cancers that have started in another part of the body and have spread to the bone such as myeloma, breast cancer, protest cancer and lung cancer therefore BPs are also of interest in the context of cancer and immunotherapy, as they possess potent Effects against the parasites responsible for sleeping sickness, chagas’ disease, malaria and leishmaniasis44. Etidronate (Didronel), pamidronate (Aredia, Novartis Pharmaceuticals Corp.; East Hanover, NJ), alendronate (FosamaxR; Merck and Company, Inc.; West Point, PA), risedronate
BISPHOSPHONATES
12
(Actonel; Proctor and Gamble Pharmaceuticals,Inc.;Cincinnati,OH) zoledronic acid (Zometa or Reclast Novartis Pharmaceuticals Corp.), ibandronate (Bondronat or Boniva; Hoffmann-La Roche Inc.; Nutley, NJ), these are the names of biphosphonate which are available in market. These medicines reduce the incidence of fractures and improve the quality of life. Their common name and uses are mentioned in Table 1. A report by Schousboe et.al found that alendronate is not cost-effective in treating women with "osteopenia" who do not already have an osteoporotic fracture. We still don't know the effects of long-term suppression of bone formation45.
Table 1. List of bisphosphonates used in clinical studies and under clinical development
Bisphosphonates R1 R2 Main current uses
Etidronate OH CH3 Osteoporosis, Paget’s disease
Clodronate Cl Cl Metastases, myeloma
Pamidronate OH CH2CH2 NH2 Hypercalcaemia,
myeloma, Paget’s disease
Alendronate OH (CH2)3 NH2 Osteoporosis and other indications Residronate OH CH2-3-pyridine Registration pending
for osteoporosis
Tiludronate H CH2-S-phenyl-CI Paget’s disease
Ibandronate OH CH2CH2 N(CH3) Pentyl In development, osteoporosis and several diseases YH529 OH CH2CH2 N(CH3) 2 Icadronate H N-(cyclo-heptyl) Olpadronate OH CH2CH2N(CH3) 2 Neridronate OH (CH2)5 NH2 EB1053 OH CH2-1-pyrrolidinyl
BISPHOSPHONATES
INDICATIONS (Who can take)
Postmenopausal women with vertebral compression fractures
Postmenopausal women with total hip bone density T-score below -2.5 Elderly men with non-traumatic fractures
Some patients with secondary osteoporosis due to corticosteroids Paget's disease
Cancer metastatic to bone
Other bone diseases with high bone resorption CONTRA INDICATIONS (Who can’t) Women who are pregnant or planning pregnancy Chronic kidney disease stages 4 or 5
Low serum calcium Osteomalacia
Vitamin D deficiency (until it is corrected) Oral bisphosphonates should not be used in: Patients with serious esophageal disease
BISPHOSPHONATES
14
Use with Caution
Patients with abnormal white blood cells Patients with high PTH
Patients with gastric or intestinal bypass surgery Children (no long-term safety data) SIDE EFFECTS
Oral or IV forms
Hypocalcaemia Mild decrease common; severe decease unusual
Increased PTH Usually modest
Skin rash Rare
atrial fibrillation FDA found no association
Bone pain Unusual
Subtrochanteric
fractures Unusual, after long-term use
Oral forms
Upper GI irritation Common
Esophageal ulceration Unusual
Esophageal cancer Unusual
Intravenous forms
Fever Common
Transient leukopenia Mild, no symptoms
Acute-phase reaction Common, lasts 1-3 days
Eye inflammation Rare
Nephrotic syndrome Rare
Jaw osteonecrosis With very high doses in cancer patients.
Etidronate (Didronel)
Osteomalacia Common with high doses
Hyperphosphatemia Usually mild effect
BISPHOSPHONATES
Dose for osteoporosis
In the large trials the fracture rates with the lower doses were not significantly different from the rates in higher doses, despite greater increases in the DEXA measurements with the higher doses (Fig 4). In the risedronate study, with 5445 women between 70 and 79 yrs, the relative risk reduction for a hip fracture with the 2.5mg/d dose was statistically significant (CI 0.3 to 0.9) but for the 5mg/d dose it was not statistically significant (CI 0.4 to 1.1). Nevertheless, the company decided to market the higher dose.The FIT trial of alendronate documented significant fracture reduction at 2 years with the 5mg/day dose. After 2 years the dose was increased to 10mg/day, but the study design precluded actual dose comparisons.Subsequent studies have shown that doses given once a week are as effective as those given daily. Almost all patients prefer this approach. Zoledronic acid continues to suppress bone formation and resorption for at least 2 years. A single dose increased the bone density at two years as well As two annual doses46.
BISPHOSPHONATES
16
DOSE FOR OSTEOPOROSIS PATIENT
Everybody needs adequate CALCIUM and VITAMIN D
Alendronate 35 mg once a week (this is the dose approved for prevention, and the official dose for treatment is 70mg a week)
Risedronate 15 mg a week more logical but approved dose is 35mg/week
Ibandronate 150mg/month oral OR 3mg/3 months I.V. push
Zoledronic acid: 5mg I.V. in 100ml NS over 15 minutes. A single dose lasts at least 2 years. Adjust for kidney function.
Pamidronate 30 to 60mg I.V. in 200-300ml D5W over 2 hrs every 6 months in special cases, with calcium>9, normal creatinine, WBC and
vitamin D. This is NOT APPROVED for osteoporosis.
How to take oral doses
This is an important!! These medications are poorly absorbed and any food in the stomach will reduce the amount aborted. They also can cause esophageal erosions so must be "flushed down" with about 4 oz of water. In the FIT trial, a full glass of water caused more reflux and esophageal problems than 1/2 glass of water according to a recent lecture by Dr. David Karpf. Although it has not been carefully studied with newer medicines, calcium supplementation following the doses of etidronate reduced effectiveness,
NOT WITH FOOD IN YOUR STOMACH
NOT WITH TEA, COFFEE or CHOCOLATE MILK
NOT WITH A LITTLE SIP OF WATER (DRINK ABOUT 4 oz)
NOT BEFORE YOU GO TO BED
BISPHOSPHONATES
Alendronate
The Fracture Intervention Trial enrolled 6,000 postmenopausal women aged 55-79, with a bone density at the hip lower than 0.68g/cm2 (T-score about -2). There were two arms of the study: 2000 women who already had vertebral compression fractures and 4000 women who did not. The women who had a fracture took alendronate for three years, the others 4.2 years. Dose was 5mg/day for the first 2 years and then 10mg/day. The alendronate group showed increases in bone density of about 8% at the spine and 5% at the hip47. Summary of results of the Fracture Intervention Trial: Bone density increased in over 90% of women taking the medication, whether or not they already had a fracture. Notice how the incidence of new fractures depends on presence of baseline fracture shown in fig 5.
Fig. 5 Vertebral fractures at baseline
In women who did not have a vertebral fracture at baseline, the decrease in vertebral fractures was significant but of low magnitude and the decrease in clinical fractures was not significant. In women who already had fractures (established osteoporosis) there was a decrease in fractures that was both statistically significant and clinically important.
BISPHOSPHONATES
18
Risedronate
The largest risedronate study included 9331 women: 5445 were 70-79 years old with T-score lower than -3 and 3886 were older than 80 with either low T-score or a clinical risk factor. BMD was done on 31% of the women older than 80. Fig.6 shows the rates of hip fractures.
Fig. 6 Hip fractures in 3 years
The incidence of hip fractures over time is shown in Fig.7. The older women had more fractures but no statistically significant benefit from the risedronate 48.
BISPHOSPHONATES
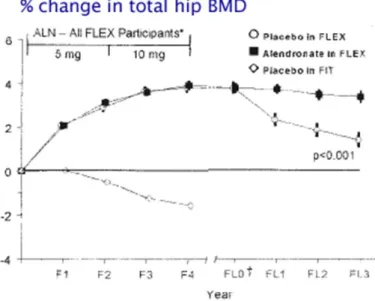
Long term effects
The Fig.8 shows change in bone density in 1099 women from the Fracture Intervention Trial who had taken alendronate for 5 years, and then were randomly given either alendronate or placebo for 5 years. The biochemical markers of bone resorption remained suppressed in both groups for 3 years after discontinuation. The serum formation markers were somewhat different; P1NP increased moderately but was still 24% below baseline 5 years after stopping, whereas the BAP gradually increased towards baseline 49
.
Fig. 8 change in bone density
Despite the difference in bone density during the last 5 years, the women who discontinued alendronate had essentially the same number of fractures as the women who kept taking the drug50. The alendronate group had fewer "clinical" vertebral fractures (those diagnosed by their physician) but when the xrays were measured the total number of "morphometric" fractures was not different. Also, there was no difference in moderate to severe vertebral fractures. Among the women with fractures there was more height loss in the alendronate group (3.5cm) vs the placebo group (2.1cm, p=.02)51.
BISPHOSPHONATES
20
Because there is no benefit to reduce fractures beyond 5 years, it is logical to stop alendronate after 5 years in most patients. Currently there are no practice guidelines about this issue and experts disagree about when to stop biphosphonates52.
Fracture healing
The clinical trials of bisphosphonates have not reported any increased incidence of fracture non-union in patients treated with active drug. However, Solomon et.al recently found an association between non-union of humerus fractures and bisphoshonates. When bisphosphonates are given to patients after joint replacement surgery, there is less loosening of the prosthesis 53, 54, 55 although after 5 years there was no residual positive effect of a dose of pamidronate given at the time of surgery 56. When given to patients 2 weeks after a fracture of the lower leg, bisphosphonates prevented the bone loss that was seen in the proximal femur of placebo control patients 57.Animal studies show that callus forms vigourously in animals who had been given bisphosphonates prior to the fracture 58. However, the callus does not normally remodel. The bisphosphonate-treated fracture callus persists, and woven bone does not get replaced by lamellar bone.
When alendronate was given to pigs after spine fusion with a bone graft, the fusion area had more woven bone and greater amount of fibrous tissue, with no difference in the rate of fusion59 another study in rats, however, found that despite the higher fusion area, the fusion rate was lower in alendronate-treated groups 60. After a fragility fracture (for example, a hip fracture) in an untreated patient with osteoporosis, it makes sense to begin a bisphosphonate. The demonstrated risk of a future fracture is greater than the potential risk of non-union or poor callus remodelling. Of course, these patients need an evaluation for other causes, and concomitant treatment
BISPHOSPHONATES
with calcium and vitamin D and physical therapy. It is possible that treatment with anabolic agents will provide even better benefit for the skeleton, but currently bisphosphonates remain the first choice due to their lower costand greater familiarity.
In the recent study of zoledronic acid following a hip fracture, Eriksen EF et.al found that patients dosed later than 6 wk after hip fracture exhibited a greater increase in total hip and femoral neck BMD at month 12 compared with patients dosed earlier than 6 week.The subjects who were treated immediately after the surgery did not respond as well, although it is possible that they were frailer. An animal study by Amanat N. et.al found better fracture healing when zoledronic acid treatment was delayed.
Use in younger patients
Children
Children with severe osteogenesis imperfecta, who have multiple fractures, show reduction in pain and fracture rates with bisphosphonates. The radiographs of the long bones show a unique striped pattern when pamidronate is given intermittently, and this is caused by layers of thick bone alternating with osteopenic bone. There may be some weakness in these areas. Currently it is unclear when to stop giving these medications. The drugs are still excreted in the urine 8 years after stopping. Because of uncertainties about long-term effects, these drugs should be used only in serious cases 61. Cyclical intravenous pamidronate treatment affects metaphyseal modeling in growing patients with osteogenesis imperfecta62. Used with permission from American Society for Bone and Mineral Research.Children with polyostotic fibrous dysplasia or juvinile Paget's disease may also benefit from bisphosphonates. Again, there are
uncertainties about howlongtousethemedications63. Children with
BISPHOSPHONATES
22
from bisphosphonates. Again, there are uncertainties about how long to use the medications63.
Premenopausal women
Bisphosphonates are not aproved for prevention of osteoporosis in premenopausal women. They should not be used in women who got a DEXA out of curiosity and discovered osteopenia. They are beneficial in other situations, such as prolonged high dose steroid use, organ transplantation, fibrous dysplasia, and metastatic carcinoma. Studies in animals show fetal and maternal abnormalities in bones and calcium metabolism, so it is unethical to study this medication in pregnant women or women who might become pregnant while the bisphosphonate is still in the bones.
Recently postmenopausal women
The following graph shows the effect on bone density in women who are recently postmenopausal. Alendronate 5mg/day increases bone density compared to placebo, but not as well as estrogen with norethindrone or estrogen with medroxyprogesterone. These results are from data in the EPIC study64 (Fig 9). There were very few fractures in this study, and no significant difference in fracture rates could be detected.
BISPHOSPHONATES
Many experts say that bisphosphonates could be used instead of estrogen in women with osteopenia, to prevent osteoporotic fractures. This is based on wishful thinking instead of evidence. It takes decades to reach "the age of fracture" and we don't know if any drugs except estrogen will work that long. In my opinion, bisphosphonates should be used only if the risk of fracture within the next ten years is high enough to justify the potential risks. As more evidence accumulates about long-term benefits, my recommendations may change. I also wonder if very small doses might be better for long-term prevention, but I'm not aware of any ongoing studies.
Use in elderly patients
In the Fracture Intervention Trial the women between 75 and 80 who had established osteoporosis showed improvement in bone density and fracture reduction that was similar to the women aged 55-75. One study of alendronate included women until age 85, but mean age was 71 so most women were still younger than 80. In that study there were not enough women to determine fracture rates, but the bone density increased 6% at the spine and 4% at the femoral trochanter.Risedronate studies shown above did not show fracture benefit to women older than 80, even among the 941 women who had osteoporosis by bone densitometry. Maybe this was just bad luck, with a negative finding due to inadequate power. Perhaps in the older women, other factors (such as quality of bones or falls) become relatively more important so it is more difficult to determine a beneficial drug effect. Some women may have lost so much bone mass by age 80 that they will have fractures despite improvement in the bone strength. There is also a possibility that the bisphosphonate does not work as well in women older than 80, because these women may already have low bone formation rate due to inadequate osteoblasts.In the recent report of zoledronate the average age was 73 and women up to age 89 were enrolled. These women
BISPHOSPHONATES
24
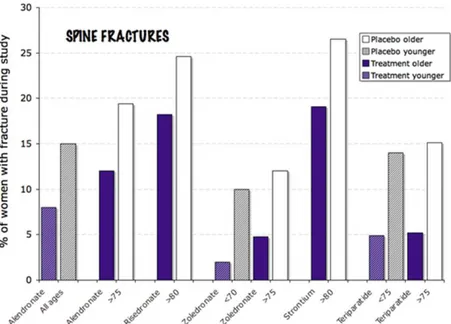
with established osteoporosis had a significant reduction in hip fractures (Fig 10).Bisphosphonates are excreted by the kidneys and are not recommended for persons with stage 4 or 5 chronic kidney disease. Often elderly, thin women with low muscle mass have a deceptively normal creatinine. This should be taken into account when prescribing bisphosphonates. Also, it is especially important to insure adequate vitamin D levels in elderly women before starting bisphosphonates, because the skin is less effective at converting vitamin D after sunlight exposure.
Fig. 10 This image shows the improvement in rate of spine fractures with
various medicines in elderly subjects. Some of the trials compared young to older women, and with alendronate and zoledronate the youn er women
1.3.1 Structure
Biphosphonate (BPs) are hydrolytically stable analogs of a naturally occurring pyrophosphate and constitute an important class of pharmacologically active molecules. In which the labile phosphor anhydride bond (H203P-O-P03H2) of pyrophosphate is replaced by a stable methylene group (H203PCR1R2-P03H2) to which two groups (R1 and R2) are attached. The long side-chain (R2 in the diagram) determines the chemical properties, the mode of action and the strength of bisphosphonate drugs.
Biphosphonate Pyrophosphate
Fig. 11 Structure of Biphosphonate and Pyrophosphate
The short side-chain (R1), often called the 'hook', mainly influences
chemical properties and pharmacokinetics. BPs (also called as
Diphosphonate) is synthetic organic compounds characterized by a P-C-P backbone structure. They are called bisphosphonates because they have
two phosphonate (PO3) groups and are similar in structure
BIOLOGICAL ACTIVITY INCLUDED STRUCTURE-ACTIVITY RELATIONSHIP
26
1.3.2 Chemistry
Chemically the bisphosphonates were first synthesized in the 1800s 65, but it is only in the past 40 years that they have been used to treat disorders of calcium metabolism. Diphosphonates and gem-diphosphonates are both correct names for bisphosphonates. However, recently and after the wide application of this class of compounds in therapeutic uses, the single term “bis-“ is generally used for compounds characterized by the [P-(R1 ) C(R2 )-P] structure. This feature allows a great number of possible variations, mostly by changing the two lateral chains (R1 and R2) on the carbon. Small changes in the structure in the R1 or R2 moiety can lead to extensive alterations in their physicochemical, biological, therapeutic, and toxicological characteristics. For these reasons, it appears there is a need for more BP-compounds with a greater margin between the inhibitions of mineralization with accompanying increase in toxicity, and improved oral bioavailability with side effects.
1.3.3 Biological activity included structure-activity
relationship
The evolution of concepts about the structure activity relationships among BPs has been reviewed and some of the key historical aspects summarized in detail by Ebetinoet.al 66.
All bisphosphonates have the same generic structure. Even if their chemical structure closely resembles the chemical structure of pyrophosphate (PPi), there are many features, which differs them from each other. First of all, in PPi two phosphate groups are linked by oxygen being hydrolytically unstable, whereas in BPs they are linked to carbon, which render them hydrolytically stable and also to withstand incubation in acids or with
BIOLOGICAL ACTIVITY INCLUDED STRUCTURE-ACTIVITY RELATIONSHIP
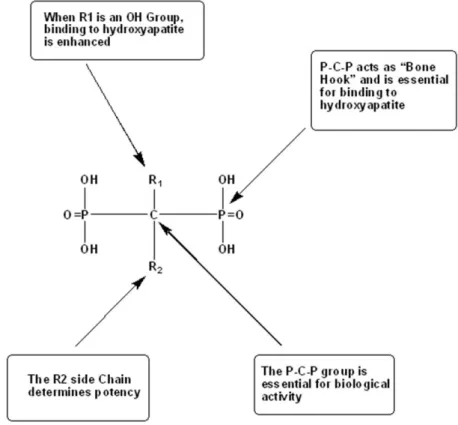
hydrolytic enzymes67.The P-C-P moiety has been called the “bone hook”, namely it is the primary structural feature that endows the molecule its affinity and targets it to the bone 68.The “bone hook” is responsible for giving bisphosphonates high affinity to the bone, which can further be enhanced by the substitution of a hydroxyl group in the side chain R1. Interestingly, the side chain R2 plays an important role in determines the potency of bisphosphonates [Fig.12] 68, 69.
Fig. 12 Structure of a bone-active bisphosphonate to show functional domains
It is noteworthy to add, that the presence of a hydroxyl group in the side chain R1 can increase the ability of BPs to bind to bone mineral by preventing both crystal growth and dissolution70. Moreover, its presence enhances the affinity for calcium and thus bone mineral even further, owing to the ability of bisphosphonates to chelate calcium ions.Like PPi, bisphosphonates form a three dimensional structure capable of binding
BIOLOGICAL ACTIVITY INCLUDED STRUCTURE-ACTIVITY RELATIONSHIP
28
divalent metal ions such as Ca2+, Mg2+, Fe2+ in the bidentate manner 71,72. Below appears figure Fig.13 presenting this mechanism.
Fig. 13 Mechanism of bisphosphonates to bind to divalent metal ions such as Ca2+
More recent studies have explored, that the structure present in R2 side chains of bisphosphonates is the major determinant of anti resorptive potency. A turning point concerning clinical investigation of bisphosphonates was found in BPs containing an additional moiety a nitrogen atom in the side chain R2 (N-BPs). In particular, bisphosphonates such as Pamidronate or Alendronate with a basic primary nitrogen atom were found to be up to 1000-fold (Table 2) more potent than non-amino bisphosphonates (Clodronate or Etidronate). Fig.14 Moreover, it was determined that bisphosphonates containing a secondary amine group in the side chain R2 are more potent up to 300-fold than those containing a primary amine (Incadronate) Fig15. Most importantly, the highest antiresorption potency has been shown by bisphosphonates containing a tertiary nitrogen atom within ring structures 72 in the side chain R2. An example of this kind of N-BPs is Risendronate and Zolendronate Fig.15.
BIOLOGICAL ACTIVITY INCLUDED STRUCTURE-ACTIVITY RELATIONSHIP
These cyclic nitrogen bisphosphonates has proved to be up to 10.000-fold more active than for example Editronate (in experimental systems used for human-beings) 73. The difference in potencies might arise from the different mechanisms of action exhibited by nitrogen containing bisphosphonates and non-nitrogen containing bisphosphonates (Table 2). Non-nitrogen containing bisphosphonates are metabolically incorporated into adenosine triphosphate (ATP) producing non hydrolyzable ATP analogues. Nitrogen-containing bisphosphonates inhibit the enzyme farnesyl diphosphate (FPP) synthase in the melavonate pathway74.
Table 2: Relative potency of Biphosphonate drugs
Biphosphonate Common Name Potency
Etidronate Didronel 1 Tiludronate Skelide 10 Pamidronate Aredia 100 Alendronate Fosamax 1,000 Risedronate Actonel 10,000 Ibandronate Boniva 10,000 Zolendronate Zometa >100,000
The results obtained to date indicate that the key features required for high inhibitory potency of N-BPs include:
The presence of two geminal phosphonate groups responsible for interaction With the molecular target
The presence of a basic nitrogen in heterocyclic side chain affects potency
The three-dimensional orientation of those basic nitrogen atom is critical for an effective inhibition
The geminal hydroxyl group does not influence the ability of the N-BPs to act at the cellular level. The introduction of lipophilic groups
into N-BPs backbone can significantly improve their
pharmacokinetics increasing the availability for soft tissues.
BIOLOGICAL ACTIVITY INCLUDED STRUCTURE-ACTIVITY RELATIONSHIP
30
with the respect to antiresorptive potency, their pharmacokinetics are imilar and characterized by highly elective localization and retention in bone 75.
Fig. 14 Non-nitrogen-BPs currently used in clinical setting
Fig. 15 Structures of the Nitrogen-BPs currently used in clinical studies classified
according to their biochemical mode of action.
The analysis of structure-activity relationships allowed the spatial features of the active pharmacophore to be defined in considerable detail even
BIOLOGICAL ACTIVITY INCLUDED STRUCTURE-ACTIVITY RELATIONSHIP
before the molecular mechanism of action was fully elucidated. For maximal potency, the nitrogen atom in the R2 side chain must be a critical distance away from the P-C-P group and in a specific spatial configuration76. This principle was used successfully for predicting the features required in the chemical design of new and more active compounds.
In summary, studies of the relationships between bisphosphonate structure and antiresorptive potency suggested that the ability of bisphosphonates to inhibit bone resorption depend on two separate properties of the bisphosphonate molecule.
1.4
Pharmacological relevant bisphosphonates
As drugs bisphosphonates display a few unusual features. Their remarkable selectivity for their target organ of bone is paramount among these and accounts for much of the efficacy and safety of the drug class, as reviewed by Cremers and Papapoulos77. Secondly unlike many drugs, BPs are not metabolized to inactive products, and drug derivatives do not appear in urine. Intracellular conversion of some non-N-BPs to ATP derivatives does occur however, as discussed elsewhere. Thirdly their oral bioavailability is extremely low, characteristically below 1% for many BPs, and rarely above 5% for others. Nonetheless the property of being active by mouth in early animal studies was key to their future use in man. The mechanism of intestinal absorption of BPs has been ascribed to paracellular transport. BPs are highly charged molecules, and no transporters have been identified. Absorption appears to be enhanced by EDTA, an effect attributed to calcium chelation that opens up gap junctions between intestinal mucosal cells78. Finally, the overall safety profile of BPs is good, but the issues of safety are much discussed and debated79-81 as described by Pazianas and Abrahamsen et.al 82.
1.5 References
1. P. W. Erhardt, Pure & Appl. Chem, 2002, 74, 703.
2. L. D. Quim, A Guide to Organophosphorus Chemistry,
Wiley-Interscience. A John Wiley & Sons. Inc. Publication.
3. P. J. Murphy, Organophosphorus Reagents. A Practical Approach
in Chemistry, Oxford. University Press.
4. P. Kafarski, B. Lejczak, Curr. Med. Chem. Anticancer Agents, 2001, 1, 301.
5. S. Zhang, G. Gangal, H. Uludang, Chem. Soc. Rev, 2007, 36, 507.J.
P. Bilezikian, L. G. Raisz and G. A. Rodan, Academic Press, San Diego, USA, 2nd edn, 2002.
6. S. F. Siconolfi, R. J. Gretebeck, W. W. Wong, S. S. Moore and J.
H. Gilbert, III, J. Appl. Physiol, 1998, 85, 1578.
7. D. Stepensky, L. Kleinberg and A. Hoffman, Clin.Pharmacokinet,
2003, 42, 863.
8. H. Fleisch and S. Bisaz, Am. J. Physiol, 1962, 203, 671.
9. H. Fleisch, J. Maerki and R. G. G. Russell, Proc. Soc. Exp.
Biol.Med, 1966, 122, 317.
10. H. Fleisch, R. G. G. Russell and F. Straumann, Nature, 1966, 212,
901.
11. H. Fleisch, D. Schibler, J. Maerki and I. Frossard, Nature, 1965, 207, 1300.
REFERENCES
34
12. H. Fleisch, R. G. G. Russell, S. Bisaz, P.A. Casey and R. C. Muhlbauer, Calcif. Tissue Res, 1968, S2, 10.
13. H. Fleisch, R. G. G. Russell and M. D. Francis, Science, 1969, 165, 1262.
14. H. Fleisch, R. G. G. Russell, B. Simpson and R. C. Muhlbauer,
Nature, 1969, 223, 211.
15. M. D. Francis, R. G. G. Russell and H. Fleisch, Science, 1969,165, 1264.
16. C. A. Bassett, A. Donath, F. Macagno, R. Preisig, H. Fleisch and M. D. Francis, Lancet, 1969, 2, 845.
17. H. P. Pendergr, M. S. Potsaid and F. P. Castrono, Radiology (Oak
Brook, IL, US), 1973, 107, 557.
18. E. B. Silberstein, E. L. Saenger, A. J. Tofe, G. W. Alexander, and H. M. Park, Radiology (Oak Brook, IL, US), 1973, 107, 551.
19. I. Fogelman, Eur. J. Nucl. Med, 1980, 5, 473.
20. M. Eisenhut, J. Nucl. Med, 1984, 25, 1356.
21. F. Wingen, H. Sterz, H. Blum, H. Moller, W. Pittermann,B. L. Pool, H. J. Sinn, H. Spring and D. Schmahl, J. Cancer Res.Clin.
Oncol, 1986, 111, 209.
22. R. Graham and G. Russell, Bone, 2011, 49, 2.
23. M.J. Rogers, R.J. Brown, V. Hodkin, R.G.G. Russell, D.J. Watts,
Biochem Biophys Res Commun, 1996, 9, 224.
24. S. Pelorgeas, J.P. Martin, M. Satre, Biochem Pharmacol, 1992,44,
REFERENCES
25. J.C. Frith, J. Monkkonen, G.M. Blackburn, R.G.G. Russell, M.J. Rogers. J Bone Miner Res, 1996, 12, 1358.
26. A.S. Frith, M.J. Rogers, A. Koivuniemi, J. J. Mönkkönen, B.
Chromatogr, 1997, 704, 187.
27. J.C. Frith, J. Monkkonen, S. Auriola, H. Monkkonen, M.J. Rogers.
Arthritis Rheum, 2001, 44, 2201.
28. M.J. Rogers, X. Xiong, X. Ji, J. Mönkkönen, R.G.G. Russell, G.M.
Blackburn, Pharm Res, 1997,14, 625.
29. P.P. Lehenkari, M. Kellinsalmi, J.P. Napankangas, K.V. Ylitalo, J.
Monkkonen, M.J. Rogers, Mol Pharmacol, 2002, 61,1255.
30. D. Amin, S.A. Cornell, M.H. Perrone, G.E. Bilder, Drug Res, 1996, 46, 759.
31. D. Amin, S.A. Cornell, S.K. Gustafson, S.J. Needle, J.W. Ullrich,
G.E. Bilder. J Lipid Res, 1992, 33, 1657.
32. M. Menschutkin, Ann Chem Pharm, 1865, 133, 317.
33. H. Fleisch, R.G.G. Russell, S. Bisaz, P. Casey, R. Mühlbauer,
Calcif Tissue Res, 1968, 2, 10.
34. H. Fleisch, R.G.G. Russell, F. Straumann, Nature (London),
1966, 212, 901.
35. M.D. Francis, R.G.G. Russell, H. Fleisch, Science, 1969, 165,
1264.
36. H. Fleisch, R.G.G. Russell, M.D. Francis, Science, 1969, 165, 1262.
REFERENCES
36
37. K. Yokoyama, P. Trobridge, F. S, Buckner,. J. Scholten, K. D. Stuart, W. C. Van Voorhis, M, H. Gelb, Mol. Biochem. Parasitol, 1998, 94, 87.
38. a) G. H. Ebetino, A. V. Bayless, L. Amburgey, K. J. Ibbotson, S. Dansereau, A. Ebrahimpour, Phosphorus Sulfur Silicon, 1996, 109, 220; b) E. van Beek, C. Lowik, I. Que, S. Papapoulos, J. Bone
Miner. Res. 1996, 11, 1492.
39. S. Zhang, G. Gangal, H. Uludag, Chem. Soc. Rev, 2007, 36, 507.
40. T. Srivastava, U. S. Alon, Eur. J. Pediatr, 2003, 162, 735.
41. E. Kotsikorou, Y. Song, J. M. W. Chan, S. Faelens, Z. Tovian, E.
Broderick, N. Bakalara, R. Docampo, E. Olfield, J. Med. Chem, 2005, 48, 6128.
42. a) G. H. Ebetino, A. V. Bayless, L. Amburgey, K. J. Ibbotson, S. Dansereau, A. Ebrahimpour, Phosphorus Sulfur Silicon, 1996, 110, 217; b) E. van Beek, C. Lowik, I. Que, S. Papapoulos, J.Bone
Miner. Res. 1996, 11, 1492.
43. a) S. H. Szajnman, A. Montalvetti, Y. Wang, R. Docampo and J.
B.Rodriguez, Med.Chem. Lett., 2003, 13, 3231, b) L. R. Garzoni, A. Caldera, L. Nazareth, M.Meirelles, S.L.Castro, R. Docampo, G. A. Meints, E. Oldfield and J. A. Urbina, Int. J. Antimicrob. Agents, 2004, 23, 273, c) L.R. Garzoni, M. C. Waghabi, M.M. Baptista, S.
L.Castro, L.Nazareth, M.Meirelles, C.C.Britto,
44. R. Docampo, E. Oldfield and J. A. Urbina, Int. J. Antimicrob.