UNIVERSITÀ DEGLI STUDI DEL MOLISE
Department of Agricultural, Environmental and Food Sciences
International PhD Course in:
AGRICULTURAL TECHNOLOGY AND BIOTECHNOLOGY
CURRICULUM: SUSTAINABLE PLANT PRODUCTION AND PROTECTION(CYCLE XXXI)
Related disciplinary scientific section: AGR/11 (Entomologia generale e applicata)
PhD Thesis
Development of attractant devices and an automatic trap
for the monitoring of the Olive fruit fly
Bactrocera oleae (Rossi) (Diptera: Tephritidae)
Coordinator of the PhD Course: Prof. Giuseppe MaioranoSupervisor: Prof. Andrea Sciarretta Co-Supervisor: Prof. Pasquale Trematerra
PhD Student: Pasquale Calabrese
155799
____________________________________________________________________ ACADEMIC YEAR 2017/2018
CONTENTS
ABSTRACT pag. 5
RIASSUNTO 6
Chapter 1
INTRODUCTION
1.1 GENERAL ASPECTS RELATED TO INTEGRATED PEST
MANAGEMENT (IPM) 7
1.2 PEST POPULATION MONITORING 11
1.3 E-MONITORING 14
1.4 TEPHRITID FRUIT FLIES 16
1.5 OLIVE FRUIT FLY BACTROCERAOLEAE (Rossi) 18
1.5.1 DISTRIBUTION 18 1.5.2 HOST PLANT 20 1.5.3 MORPHOLOGY 24 1.5.3.1 Adult 24 1.5.3.2 Eggs 25 1.5.3.3 Larva 25
1.5.3.4 Pupa and puparium 26
1.5.4 LIFE CYCLE 26
1.5.5 DAMAGE AND ECONOMIC IMPORTANCE 28
1.5.6 NATURAL LIMITING FACTORS 30
1.5.6.1 Physical factors 30 1.5.6.2 Biological factors 31 1.5.7 MANAGEMENT OF INFESTATION 32 1.5.7.1 Preventive methods 32 1.5.7.2 Monitoring 33 1.5.7.3 Mass-trapping 35
1.5.7.4 Lure and kill methods 36
1.5.7.5 Biological control 37
Chapter 2
SELECTION OF MONITORING SYSTEM
2.1 INTRODUCTION 41
2.2 MATERIAL AND METHODS 42
2.2.1 EXPERIMENTAL AREA 42
2.2.2 ATTRACTANT DEVICES COMPARISON TESTS 43
2.2.2.1 Experimental design 43
2.2.2.2 Trapping device and attractants 46
2.2.2.3 Sampling and data analysis 51
2.2.2.4 Field Trials 53
2.2.2.4.1 Case 1: Comparison of different traps baited with ammonium
carbonate or protein baits 53
2.2.2.4.2 Case 2: Performance of AC-baited trapping devices 54
2.2.2.4.3 Case 3: Trap size effect 55
2.2.2.4.4 Case 4: Trap colour effect 55
2.2.2.4.5 Case 5 Performance of pheromone-baited trapping devices 56
2.2.2.4.6 Case 6: Evaluation of trap saturation 57
2.2.3. SEASONAL MONITORING OF BACTROCERA OLEAE CAPTURES 58
2.2.3.1 Traps and food baits for seasonal monitoring 58
2.2.3.2 Sampling and data analysis 59
2.3 RESULTS 60
2.3.1 ATTRACTANT DEVICES COMPARISON STUDIES 60
2.3.1.1 Case 1: Comparison of different traps baited with ammonium
carbonate or protein bait 60
2.3.1.2 Case 2: performance of AC-baited trapping devices 66
2.3.1.3 Case 3: trap size effect 69
2.3.1.4 Case 4: trap colour effect 72
2.3.1.5 Case 5 Performance of pheromone trapping device 75
2.3.1.6 Case 6: Evaluation of trap saturation 80
2.3.2 SEASONAL MONITORING OF BACTROCERA OLEAE CAPTURES 83
2.4.1 ATTRACTANT DEVICE COMPARISON STUDIES 88 2.4.2 SEASONAL MONITORING OF BACTROCERA OLEAE CAPTURES 92
Chapter 3
DEVELOPMENT OF E-TRAPPING
3.1 INTRODUCTION 94
3.2 MATERIAL AND METHODS 95
3.2.1 E-TRAPS COMPONENTS 95
3.2.2 E-TRAP IMPLEMENTATION 99
3.2.3 E-TRAPS FIELD OPERATIONS 101
3.3 RESULTS 103
3.3.1 TEST A. DATA TRANSMISSION 104
3.3.2 TEST B. REMOTE VS. IN-FIELD COUNT OF FLIES 104
3.3.3 TEST C. CONVENTIONAL JACKYY VS E-TRAP CAPTURES 106
3.4 DISCUSSION 108
Chapter 4
CONCLUSION AND FUTURE DIRECTIONS 110
Chapter 5
GENERAL REFERENCES 113
Appendix 128
ABSTRACT
The Olive fruit fly Bactrocera oleae (Rossi) (Diptera: Tephritidae) is considered the most serious olive pest in many areas throughout the word, affecting the quality and quantity of oil and table olives during the infestation events occurring in late summer and autumn. The management of its infestations in commercial olive groves is vital, also accounting for the large registered fluctuations in the populations from one year to the other. Monitoring pest populations is currently one of the key elements in Integrated Pest Management (IPM) programs, including the monitoring of adult flies with the use of traps. The present thesis focused on the comparison of some commercial or modified traps, baited with various combination of food or sex attractants, to be selected for the development of a semiautomatic monitoring system, contributing to optimize and rationalize B. oleae pest control operations.
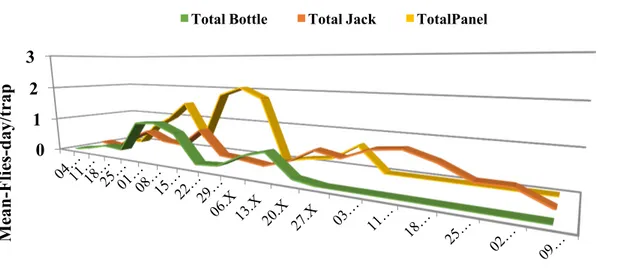
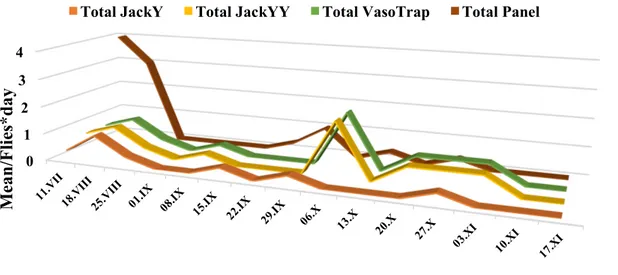
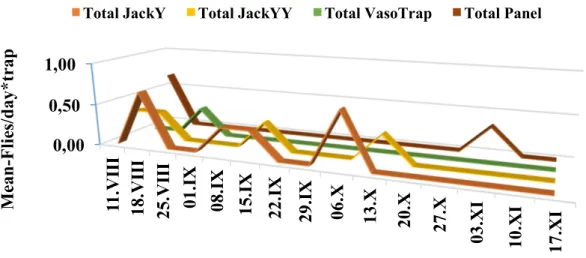
The experimental sites were located in an inner hilly area of the Campania region, in Southern Italy. The area was divided into multiple fields of work chosen using a Latin square experimental design; in these fields the following devices, baited with various attractants, were compared: a bottle trap, a yellow panel, and a Jackson trap, with this latter modified using different colours of support/sticky surface and sizes. The traps were also evaluated for catch selectivity towards predators and parasitoids. Preliminary results showed that the catches in the tested traps depended on the extension of the sticky surface and on the yellow colour, and strongly increased in presence of an attractant (like ammonium carbonate). When different traps were compared, the yellow panel captured significantly more individuals, but applying the correction to make traps equivalent in relation to the size of the sticky surface, no differences for total and female catches were observed between the yellow panel and the standard Jackson trap with a sticky yellow panel inside. When looking at the capture of natural enemies, we found that the size of the trap and the presence of an attractant increased the trapped parasitoids and predators. The extent of the yellow colour directly influenced the catches for parasitoids, whereas for predators only a combined effect with size was significant. The effect of pheromone, alone or in combination with ammonium carbonate, on total catches was evaluated in two types of Jackson traps. In both cases, the best performance was observed for Jackson baited with pheromone and ammonium carbonate, followed by Jackson baited with only the ammonium carbonate or the pheromone. Results were different for the two sexes: for males, the Jackson trap with pheromone had the highest catches, although not statistically different from the Jackson trap baited with both attractants. For females, the Jackson trap with ammonium carbonate, alone or with pheromone, had higher catches. A different test was carried out to evaluate a possible decrease of captures in the Jackson trap due to the saturation of the sticky surface over time. Two all yellow Jackson traps and two green Jackson traps with a yellow panel were used for this test. For each type of trap, the panel was cleaned at each control in one of the two traps and never cleaned in the other one. Not cleaned Jacksons traps had highest B. oleae adult catches compared to the cleaned ones. The results also showed a decrease in total catches as the checking days progressed, whereas the relative proportion of catches in cleaned traps increased over time. Based on our results, we selected a complete yellow Jackson trap baited with ammonium carbonate to be incorporated in the manufacturing of the automatic device.
After the assembly of the various components, the e-trap prototypes were installed in the field and the functioning of various components tested. The image quality was evaluated by comparing the remote counting of B. oleae adults in the digital images and the number of flies counted in the field in the same trap and day. Linear regressions indicated a very high correlation between the two types of counts. The catches of olive fruit flies in the e-traps were contrasted with numbers of flies captured in conventional Jackson traps, with same colour and size, run in parallel. Results showed higher catches in e-traps than in conventional Jackson traps, but differences were not statistically significant. Overall, the developed prototype proved to be reliable and innovative, revealing considerable potential for its use in IPM olive programs.
RIASSUNTO
La mosca delle olive Bactrocera oleae (Rossi) (Diptera: Tephritidae) è una specie diffusa nella maggior parte dei paesi dove viene coltivato l’olivo, ed è causa di notevoli danni alle drupe nei periodi di tarda estate e autunno, determinando una significativa perdita di prodotto in termini qualitativi e quantitativi. Il contenimento delle sue infestazioni è di fondamentale importanza, considerate anche le ampie fluttuazioni delle popolazioni da un anno all’altro.
L’uso di trappole per il monitoraggio degli adulti è una componente essenziale nei programmi di gestione integrata dei Ditteri Tefritidi. Nella presente Tesi si sono confrontate alcune tipologie di trappole, attivate con varie combinazioni di attrattivi alimentari e sessuali, da selezionare per lo sviluppo di un dispositivo semi-automatico (e-trap) per il monitoraggio degli adulti di B. oleae. Si è inoltre valutata la selettività delle trappole nei confronti di predatori e di parassitoidi. I siti sperimentali sono situati in un'area collinare interna della regione Campania (Italia). Nelle prove si è utilizzato il disegno sperimentale del quadrato latino, mettendo a confronto: bottiglia, pannello giallo e trappola Jackson modificata con differenti tipologie di colore, dimensione e formato o superficie collante.
I risultati preliminari hanno mostrato che le catture degli adulti dipendono dall'estensione della superficie adesiva e dal colore giallo e aumentano in modo significativo in presenza di carbonato di ammonio. Il pannello giallo è risultato avere maggiori performance ma, applicando una correzione alle catture per equiparare le superfici collanti, non risultano esserci differenze significative con la trappola Jackson tutta gialla, mentre le altre tipologie hanno catturato un minor numero di individui. Nei riguardi dei nemici naturali, la dimensione della trappola e la presenza di un attrattivo aumentano le catture. In particolare, una maggiore estensione del colore giallo ha incrementato il numero di parassitoidi, mentre per i predatori solo un effetto combinato con le dimensioni ha determinato un aumento significativo degli individui intrappolati. Due prove hanno riguardato il confronto di trappole Jackson attivate con feromone sessuale, carbonato di ammonio oppure con entrambi. Nel caso dei maschi, le catture più elevate si sono avute nelle trappole con feromone, con o senza carbonato di ammonio. Nelle femmine il maggior numero di individui sono stati osservati nelle trappole attivate con carbonato di ammonio, con o senza feromone.
Un ulteriore test è stato effettuato per valutare la possibile diminuzione delle catture di B. oleae nella trappola Jackson con il passare del tempo, dovuta alla saturazione del pannello collante. Si sono confrontate due trappole Jackson gialle e due trappole Jackson verdi con pannello adesivo giallo. Di ciascuna tipologia, una trappola veniva pulita ad ogni controllo e l’altra no. Le Jackson pulite hanno presentato minori catture rispetto a quelle non pulite. I risultati hanno inoltre mostrato un decremento delle catture totali con il progredire dei giorni. La proporzione relativa di catture nelle trappole pulite sono aumentate nel tempo rispetto a quelle non pulite, rilevando un effetto saturazione di circa 30% dopo 28 giorni. La trappola Jackson gialla attivata con carbonato di ammonio è stata utilizzata per lo sviluppo della e-trap. Dopo l'assemblaggio dei vari componenti, i prototipi sono stati installati e saggiati in campo, evidenziando un’ottima affidabilità del sistema nella trasmissione delle informazioni ad un server remoto. La qualità delle immagini è stata valutata comparando il conteggio di B. oleae effettuato da un computer remoto con quello realizzato direttamente in campo. I risultati avuti hanno mostrato una elevata correlazione tra le due tipologie di controllo. Le catture nelle e-trap sono state infine confrontate con quelle ottenute in trappole Jackson convenzionali aventi uguali caratteristiche e poste negli stessi oliveti. Le maggiori catture osservate nelle trappole automatiche non sono risultate statisticamente significative. Complessivamente, il prototipo di e-trap sviluppato si è dimostrato affidabile e innovativo, rivelando una notevole potenzialità per il suo utilizzo in programmi di gestione integrata dell’olivo.
1. INTRODUCTION
1.1 GENERAL ASPECTS RELATED TO THE INTEGRATED PEST MANAGEMENT (IPM).
The Integrated Pest Management (IPM) is a pest control program using all approaches and techniques available (tactics) in respect to the economic, environmental and toxicological requirements, giving priority to natural limiting agents, such as predators and parasitoids, and defining some thresholds under which to maintain the levels of the pest population. The decision support system for the selection and use of pest control tactics, singly or harmoniously coordinated into a management strategy, based on cost/benefit analyses that take into account the interests of and impacts on producers, society, and the environment (Kogan, 1998; Baumgartner et al., 2007).
Most of the IPM principles (Fig. 1) have been incorporated in the EU Directive 2009/128/EC which provides a common framework for the sustainable use of pesticides. The principles on which IPM is based are:
1. The prevention and/or suppression of harmful organisms should be achieved or supported, among other options, especially by:
o crop rotation,
o use of adequate cultivation techniques (e.g., stale seedbed techniques, sowing dates and densities, under-sowing, conservation tillage, pruning and direct sowing),
o use, where appropriate, of resistant/tolerant cultivars and standard/certified seed and planting material,
o use of balanced fertilisation, liming and irrigation/drainage practices,
o preventing the spreading of harmful organisms by hygiene measures (e.g., by regular cleansing of machinery and equipment),
o protection and enhancement of important beneficial organisms, e.g., by adequate plant protection measures or the utilisation of ecological infrastructures inside and outside production sites.
2. Harmful organisms must be monitored by adequate methods and tools, where available. Such adequate tools should include observations in the field as well as scientifically sound warning, forecasting and early diagnosis systems, where feasible, as well as the use of advice from professionally qualified advisors.
3. Based on the results of the monitoring, the professional user has to decide whether and when to apply plant protection measures. Robust and scientifically sound threshold values are essential components for decision making. For harmful organisms threshold levels defined for the region, specific areas, crops and particular climatic conditions must be taken into account before treatments, where feasible. 4. Sustainable biological, physical and other non-chemical methods must be preferred
to chemical methods if they provide satisfactory pest control.
5. The pesticides applied shall be as specific as possible for the target and shall have the least side effects on human health, non-target organisms and the environment. 6. The professional user should keep the use of pesticides and other forms of
intervention to levels that are necessary, considering that the level of risk in vegetation is acceptable and they do not increase the risk for development of resistance in populations of harmful organisms.
7. Where the risk of resistance against a plant protection measure is known and where the level of harmful organisms requires repeated application of pesticides to the crops, available anti-resistance strategies should be applied to maintain the effectiveness of the products. This may include the use of multiple pesticides with different modes of action.
8. Based on the records on the use of pesticides and on the monitoring of harmful organisms the professional user should check the success of the applied plant protection measures.
The importance of integrating several tactics lies in the desire for sustainability or durability of a management program. Although the use of a single management tactic may be successful in the short term, such programs often fail in time. This is because pest population can adjust to a tactic, particularly when it reduces a pest’s ability to reproduce and survive.
This practice depends on a series of well-designed programs that apply to important pest in the production system that is, the development of a strategy. A pest management strategy is an overall plan to eliminate or alleviate a real or perceived pest problem. The particular strategy developed depends on the particular life of the pest and the crop involved. In addressing problems using pest management, we aim to reduce pest status considering both the insect and the crop, and our management program may emphasize modification of either or both of them.
An important tool for evaluating an insect problem is the economic threshold of the pest. The economic threshold is the pest level at which management action should be taken to prevent an increasing pest population from doing economic damage to the crop. The economic threshold takes into consideration market value of the crop, injury and damage, insect density, lag time before implementation occurs, and cost of management. This threshold information is important in distinguishing between trivial plant injury and true economic damage. The real goal of the economic threshold is to prevent a pest population from reaching the point where its damage causes monetary losses that equal the cost of the pest control. This level is defined as “economic injury level” and the economic threshold is always set lower than the economic injury level to provide a “lead time” before reaching the break-even point and before an economic loss occurs.
A key element of a pest management strategy is therefore a plan developed to lower pest status to a tolerable level, i.e., below the “economic injury level”. The strategy developed will depend on the biology of the insect and the cropping system involved. Since the pest status is determined by both insect and crop, a management strategy may focus on modification of either or both of these (Pedigo & Rice, 2009). We have: • Do-Nothing strategy: It is possible that insect injury only seems as if it is causing a
loss, when in reality the crop species tolerates the injury without economic damage. The most frequent need for this strategy usually arises when insects cause indirect injury. In this instance, only surveillance of the resulting pest population is required. Indeed, considerable sampling is required to assure that taking no action is appropriate,
and significant pest suppression may have occurred as a result of natural environmental factors.
• Reduce – number strategy: reduce the insects number to alleviate or prevent problems is probably the most widely used strategy in pest management. It is usually employed in a therapeutic manner when densities reach the economic threshold or in a preventive manner based on a history of problems. The tactics used in this strategy are many and varied, most of these increase mortalities in pest population by creating or intensifying hazards to insects in their environment.
• Reduce – Crop – susceptibility strategy: it is one of the most effective and environmentally desirable strategies available. The insect population is not modified at all, we rather rely on changes made to the host plant or animal that render it less susceptible to an otherwise damaging pest population.
• Combined strategy: this strategy combines objectives of all the other strategies to produce a pest management program with several tactics.
The appropriate strategy and subsequent complexity of a pest management program is primarily determined by the status of a pest in the production system. Four pest types may be identified according to status (Pedigo & Rice, 2009):
Subeconomic pests: these insects are pests in a true sense, even if they cause
insignificant losses.
Occasional pests: these pests may be present on a crop most years but, more often
than not, do not cause economic damage, therefore the pest management programs for these pests tend to be less complex than those of more serious pests.
Perennial and severe pests: only few insects belong to this category, and these are
often referred to as key pest. These pests are characterized by a very high average density in relation to the economic injury level.
1.2 PEST POPULATION MONITORING
The continuous assessment of pest presence and population levels in the crop is mandatory in any IPM program for decision-making purpose, i.e., to evaluate if the economic threshold has been reached and tactics are requested. This continuous process is called Pest monitoring. Since assessment of the total number of pests or their damage symptoms is generally impractical, sampling procedures are employed to estimate densities per sampling unit or proportions of occupied sampling units in a sampling universe (Madden et al., 1996). Monitoring of pest population occurs for many reasons, it is important in any case to identify and define the problem.
Selection of an appropriate sampling technique depends initially on the kind of estimate desired. Generally, estimates can be divided in two categories: absolute and relative. The absolute estimation measures the actual density in the insect population, expressed as number per ground surface area. These are often the most difficult estimates to make and frequently the most costly. Relative estimates differ greatly from absolute estimates because they do not translate directly into densities. Instead, relative estimates are based on the kind of sampling technique used and can be later converted into absolute estimates. Relative estimates have the great advantage of being less expensive and are often the most appropriate for insect pest management.
Many techniques have been used to obtain relative estimates. Among them, the use of traps is the most convenient and most widespread technique, i.e., devices able to attract, capture and retain a pest, allowing the inspector to count the number of caught individuals. There are different types of traps that can be used to catch insects according to the stimuli used to attract insects (light traps, visual traps, pheromone traps, food traps, and so on) or to the type of method used to capture and retain them (sticky traps, pitfall traps, funnel traps, etc.). The efficiency of traps depends partly on the mobility of the insects; therefore, traps are usually used to catch adults (males and/or females). Efficiency depends also on the physiological state of individuals, because in some phases they can not respond to the stimuli that the trap uses to attract them (Pedigo & Rice, 2009).
Figure 1. Diagram showing the main components of IPM (by Pedigo & Rice, 2009).
On a large geographical scale, quarantine services monitor presence absence of arthropod pest on imported goods and initiate strategies of containment and eradication whenever their presence is recorded. Pest control methods generally require information on population densities or proportion of infested units with references to crop growth stages. Detection requires only a sensitive trapping method that provides qualitative information for early warning of emergence, such as arrival or departure of a pest within a crop, and for survey quarantine work (Howse et al, 1998).
When traps are used for monitoring purpose in field crop protection, monitoring usually requires quantitative information from the trap catches, obtained with consecutive sampling data collection, to provide information on the dynamics of target population development (Schaub et al., 1998) and to assist the farmer in making decisions. However, the analysis of the results should be interpreted with caution. A sudden increase in insects caught in a trap does not always mean that an outbreak is occurring: information obtained from traps should be used in combination with other observations to take crop management decisions properly. In particular, additional biological and meteorological data with which to predict the occurrence of the susceptibility stage in the life cycle are
and subsequent damage needs to be established with specific experiments or using forecasting models.
Research programs generally require intensive sampling programs for estimation of population parameters; on the contrary, extensive and cost-efficient sampling procedures are needed for IPM-related monitoring.
The basic components of an attractive trap-based monitoring system are the attractant source, the trap design, and of course, a sufficient knowledge of the pest biology to interpret the catches. Attractive source can be of different types: a colour, a light source or a chemical substance. The latter has been most used in IPM programs, because very selective substances can be chosen against a particular species. In this case, the attractive source or lure consists basically of two components: the active ingredient and its controlled-release device. Active ingredients are usually semiochemicals, i.e., substances which influence the behaviour of pest insects such as pheromones, or food attractants. Whatever attractants are used, the objective is to sample a representative and selective number of insects from the population at any given time. Minor components may therefore be added to the lure not to enhance the attraction of the target species but to inhibit the capture of non-target species.
Most attractive components are volatile substances and would volatilize very quickly unless they are formulated in some sort of controlled-release device. The objective in most cases is to slow down the release of attractive compost, and in any case, the device protects the active ingredient from the degradation due to the ultraviolet light.
Trap designs are the result of empirical selection and very few systematic studies of insects behaviour and semiochemical plume formation with relation to trap design have been undertaken.
Entrapment system falls in two groups: those that involve a non-drying adhesive or water to retain the insects caught and those that have a one-way entrance system with no exit. The size of the traps is often related to the size and the number of insects that is intended to catch. Trap colour will sometimes influence the number of insects caught, especially for fruit flies (Katsoyannos, 1989).
1.3 E-MONITORING
In recent years, information and communication technologies have opened many opportunities to modern agriculture systems. Monitoring the fruit farm is one of the potential applications that may help improving fruit farm profitability through observing the fluctuation of the pest population and environmental conditions in the field. These data can be used to provide knowledge or warning for the farmers and other agricultural stakeholders to accurately respond to the observed variations, allowing to incorporate spatial variability and perform precision treatments (Sciarretta & Trematerra, 2014) Automatic or Electronic traps (e-traps) have been suggested as part of this modern tool for pest monitoring (Shaked et al., 2014).
An automatic trapping device consists of a trap equipped with sensors for data collection in the field and hardware for the transmission of data to a remote server accessible online to store the information in geo-referenced databases.
Although the automatic trap by itself is a working-alone tool, usually the automated monitoring in the context of IPM is a multi-modular process. First of all, it is necessary to digitalize the geo-referenced information, such as the geographical position of the traps and the link to GIS layers such as roads, houses, trees, field borders, land uses and background, so that information arriving from the trap can also be visualized or elaborated in a GIS tool.
Secondly, we have the catching module, adapted from existing devices or newly developed, and including, an attractant, in order to increase attractiveness or to make the trap more selective. The sensors can be different and connected to each other in wireless networks; for the count of the captured insects, sensors are usually optical or infrared. Additional sensors, such as temperature, humidity, rainfall, etc., can be considered. Remote connection (Wi-Fi, 3G, radio waves, etc.) is an important component of the automatic trapping device to transmit data to the cloud. A power unit is also necessary to sustain all electronic components: when energy supply is not directly available, batteries and solar panels represent possible source of energy. A graphical user interface (GUI) allows to read and interpret the information from automatic trap remotely. Additional software can be fed with data coming from automatic traps, such as a decision support system (DSS), which can, for example, provide information on the selection of control
tools or assist the operator in a spraying process, i.e. when, how and where to do a treatment.
Among others, first examples of automatic image-based traps were developed for whiteflies, or other small-bodied insects (Qiao et al., 2008; Chung et al., 2014). Nevertheless, these e-traps were mainly aimed for greenhouses and annual crops. E-traps based on image analysis have also been recently developed, and marketed, especially for moths pests (Cho et al., 2007; Guarnieri et al., 2011). Regarding fruit flies, in recent years many research groups focused their research activities on the development of an automatic or semi-automatic method for the monitoring (Philimis et al., 2013; Potamitis
et al., 2017; Shaked et al., 2017).
In semiautomatic systems, an optical sensor collects the images at certain times and transmits them via internet to a remote operator who can check and count insects directly in a remote device and this can be done in real time. In case of a fully automated system, the insects are recognized and counted using a software, usually based on machine learning algorithms. In this case, discriminating features of the insect, for example dimension of body, can be used to differentiate the target species from other species. An adequate number of examples are collected and the algorithm accumulate sufficient experience allowing the recognition of the correct species.
A wireless automatic trap has been developed by modifying a McPhail model for the monitoring of Ceratitis capitata Wiedemann and Bactrocera oleae (Rossi). In this case, a camera captures images of moving insects entering into the trap along a transparent funnel; a software processes the images for fruit fly identification (Philimis et al., 2013). An additional e-trap for B. dorsalis (Hendel) employs a species-specific attractant and an infrared interruption sensor to count attracted male flies passing through the electronic funnel (Jiang et al., 2008; Deqin et al., 2016).
Other technologies of e-monitoring use location-aware systems, based on a real-time Wireless Multimedia Sensor Network (WMSN), integrated with a semi-automatic trapping and insect counting, based on existing traps, able to acquire and transmit data to a remote server, and a Decision Support System (DSS) that will perform the final optimization of the control treatments (Tsiligiridis et al., 2014). A McPhail trap for fruit fly based on images and other advanced sensors technologies has also been, recently, developed in Australia, and a McPhail trap based on sensors detecting differences in light transmission resulting from entering insects into the trap was also developed for B. oleae (Potamitis et al., 2017).
Shaked et al., (2017) developed two e-traps, both versions were based on the wireless transmission of images of trapped insects on the glue surfaces of e-traps. The development and validation focus on following specific species in different agro-ecosystems: Medfly in peach orchards, cherry fruit fly in cherry orchards, olive fruit fly in olive orchards and the Ethiopian fruit fly in melons growing in plastic tunnels. The comparison between the e-traps and the standard traps was conducted directly in the field. The verification of the e-trap was performed comparing the e-trap captures data to the standard-trap captures and showed a similar trend between the two counts, with the number of flies caught in the ReTIC traps similar to the one obtained with the standard traps.
1.4 TEPHRITID FRUIT FLIES
The fruit flies belonging to the family Tephritidae represent a major group of phytophagous Diptera, which causes widespread economic losses to fruit crops in the tropical and temperate areas of the world. They count at least 3500 species and numerous subspecies or local variations in morphology or ecological adaptations.
“From an economic point of view, true fruit flies are, by far, the most important insect family attacking horticultural crops. Tephritid flies cause both direct and indirect losses as their presence can result in major international trading constraints. Total damage caused in all production, harvesting, packing, and marketing worldwide is estimated to amount to more than 2 billion dollars annually. Their economic and trading importance is so high that in many fruit growing countries there is at least one unit dedicated to fruit fly detection and control under the National Plant Protection Organizations” (Shelly et al., 2014).
Among the most notorious members of the Tephritidae family there are: the Mediterranean fruit fly, Ceratitis capitata (Wiedemann); the oriental fruit fly, Bactrocera
dorsalis (Hendel) and its close relatives; the Queensland fruit fly, Bactrocera tryoni
(Froggatt); the peach fruit fly, Bactrocera zonata (Saunders); the melon fly, Bactrocera
cucurbitae (Coquillett); the olive fruit fly, Bactrocera oleae (Rossi); the South American
fruit fly, Anastrepha fraterculus (Wiedemann); the Mexican fruit fly, Anastrepha ludens (Loew); the West Indian fruit fly, Anastrepha obliqua (Macquart); the apple maggot fly,
Rhagoletis pomonella (Walsh), and the European cherry fruit fly, Rhagoletis cerasi (L.)
the fruit surface with eggs laying. This may also result in biochemical or physiological changes in the fruit at the oviposition site, which can produce callus tissue to varying degrees. On hatching, the larvae bore into the fruit tissue and open the way for bacterial and fungal pathogens as secondary invaders. These in turn cause rapid and extensive necrosis and complete loss of the fruit(Belcari et al., 2003).
Many fruit flies are invasive species and can have far reaching ecological and economic impacts worldwide (Mack et al., 2000; Davis, 2009). One of the best ways to reduce the likelihood of exotic species invasions is to prevent their establishment, but this relies on being able to initially identify those invasive species with the highest potential to establish. This can be challenging considering that there are hundreds, or even thousands of species that have the potential to invade and establish in any particular region or country.
Fruit flies represent one of the best study systems any researcher can find. These insects are well suited for interspecific comparisons, field and laboratory studies, and experiments under semi-natural conditions. Since adults of many species are relatively easy to obtain and rear, there is no difficulty in quantifying their behaviour and performing long-term studies on marked individuals. This combination of features has motivated entomologists worldwide to study this group for more than a century.
The genus Bactrocera Macquart is among the largest genera within Tephritidae, with about 500 described species arranged in 28 subgenera (Drew, 1989a; Drew & Hancock, 2000). The relationships among Tephritid subfamilies and tribes are still being developed and improved (Drew, 1989b; White & Elson-Harris, 1992; Aluja & Norrbon, 2001; White, 2006). Bactrocera differentiates from its sister group Dacus, Fabricius based on the following morphological synapomorphies: radial veins of wings crowded anteriorly and medial cells very broad, female abdominal tergite 6 separate from preceding tergites, and tergite 5 of both sexes with glandular ceromae (Munro, 1984; White, 2000), as well as mitochondrial DNA (Shelly et al., 2014).
Drew (1989a) proposed a subgeneric classification of Bactrocera based on present/absent permutations of just five morphological characters. He also assembled the subgenera of Bactrocera into four, presumably monophyletic, groups based on all possible permutations of just two male characters, the shape of the 5th male sternite and
the length of male surstylus lobe. Drew’s (1989a) subgeneric classification was not based on cladistics principles and as such, may not necessarily reflect the evolutionary history of the genus.
1.5 OLIVE FRUIT FLY BACTROCERAOLEAE (ROSSI)
The olive fruit fly, Bactrocera oleae (Rossi), belongs to the order Diptera, suborder Brachycera, Tephritidae family, Dacinae subfamily. It was recorded attacking olives in biblical times and considered the major pest of olive groves in the Mediterranean basin (Manousis & Moore, 1987a; Tzanakakis, 2003) and other areas throughout the world, wherever there is the presence of Olea europea L. (Fig. 2). As such, the olive fruit fly’s distribution is primarily limited to regions where cultivated and wild olive trees are found (Daane & Johnson, 2010). The damages caused by the olive fruit fly, seen from an economic perspective, affect the quality and quantity of oil and table olives during the infestations occurring in late summer and autumn (Michelakis & Neuenschwander, 1983; Manousis & Moore, 1987a; Economopoulos, 2002). Due to its importance, many aspects of its bio-ecology, management and impact on olive production have been investigated in the last few decades.
1.5.1 DISTRIBUTION
The olive fruit fly is widely distributed in the Mediterranean basin, where approximately 95% of the estimated worldwide more than 10 million ha of olive trees are cultivated (Russo et al., 2016). In Europe, B. oleae is widely distributed in Greece, Crete Island, Italy and the islands of Sicily and Sardinia, and in the Iberian Peninsula. It is also present in Azores Islands, Balearic Islands, Cyprus, France, Malta, Switzerland, Turkey, and in Balkans region, mainly Croatia, Montenegro and Slovenia. Despite its abundance and notoriety on cultivated olives in the Mediterranean region, the olive fruit fly most likely originated in regions of sub-Saharan Africa where wild olive varieties are found and from which domesticated cultivars were derived (Zohary, 1994).
In the African continent, B. oleae is largely distributed in the Northern part, near to the Mediterranean Sea: Algeria, Egypt, Libya, Morocco and Tunisia. This fly is also present in sub-Saharan Africa, mainly in Eastern Africa: Angola, Eritrea, Ethiopia, Kenya, and Sudan. South Africa and Canary Islands also report B. oleae presence, probably as an invasive pest. In Asia, the Middle-East region is the most affected, being
B. oleae present in Israel, Palestine, Jordan, Lebanon, Saudi Arabia and Syria. The olive
fly is also present in China, India and Pakistan, where was reported as a morphological variety B. oleae var. asiatica (Silvestri, 1916) (Nardi et al., 2006, 2010). In North
Figure 2. Olive fruit fly distribution in the world (from CABI, 2014).
America, B. oleae was accidentally introduced in 1998 in California, more specifically in the Los Angeles Basin (Burrack & Zalom, 2008; Zalom et al., 2008). Since that time, B. oleae spread through the region and become present in all growing region of the state by 2004. The pest also spread to Mexico, occupying part of Central America. In South America, B. oleae is reported for Argentina, Chile, Peru and Uruguay (Augustinos
et al., 2002), due to the introduction of olives cultivation or production intensification. So
far, Australia is one of the few areas in the world where olives are cultivated and well established for commercial purposes, without B. oleae infestation.
In fact, in the last century, olive cultivation has expanded to North, Central and South America, South Africa, China and Australia. B. oleae has followed this expansion and untill now the insect’s infestations have been reported everywhere the tree grows except Australia (Augustinos et al., 2005). In Italy and other Mediterranean countries, the olive fly is expected to follow the expansion of the range of olives to higher elevations and northern areas following a global warming scenario (Ponti et al., 2014).
Figure 3. Distribution of Olea europea in the Mediterranean basin (from Oteros Jose, 2014)
1.5.2 HOST PLANT
Bactrocera oleae is an oligophagous insect, developing on several species of Olea
plants, such as Olea verrucosa (Willd.), Olea chrysophylla Lam., Olea ferruginea Royle, but mostly preferring the cultivated species Olea europea ssp. europaea, so much that many authors consider the fly practically monophagous.
The genus Olea belongs to the family Oleaceae and includes about 30 to 40 species distributed in Oceania, Asia, Africa, America and Europe (Rohwer, 1996).
The type of olive tree which produces edible olives and olive oil belongs to the species Olea europaea L. The cultivated variety, O. europaea var. europaea, has become more flexible to climatic and environmental conditions. It penetrates towards higher, colder and more continental lands, mainly on calcareous soils, and sandy marls (Carriòn
et al., 2013). The olive is one of the most extensively cultivated fruit crops in the world.
In 2016, there were more than 10 million hectares planted with olive trees (Russo et
al., 2016), mostly located in the Mediterranean region where 95% of the world's olives
were produced (Fig. 3).
Since late Prehistory, the olive has been grown for its oil-rich fruit. For thousands of years, the olive tree has been the tree for excellence in the Mediterranean region and has coexisted with the people of the area, connected to their daily lives and customs; it has gone beyond the boundaries of the landscape, leaving its traces in all the civilizations which have developed on its shores.
Figure 4. A commercial olive orchard (left); an olivaster three (right).
Within genus Olea only the species O. europaea bears edible fruit from which oil can be extracted. Wild forms of O. europaea ssp. europaea are commonly referred to as ‘oleasters’ and are often assumed to be simply cultivated olives that have gone feral. Oleaster fruit tends to be more elongate in shape and smaller than most cultivated varieties. Oleaster is a shrub or small tree (Fig. 5) found in most countries of the Mediterranean basin, usually it shows a marked preference for calcareous soils and the proximity of the sea. Several other subspecies with highly localized disjunctive distributions in the Canary Islands, the Madeira archipelago, Morocco, and in the Sahara, are also recognized, such as O. e. ssp. cerasiformis G. Kunkel & Sunding (Green, 2002). In sub-Saharan Africa, the fruit fly is associated to Olea europaea var. silvestris.
The olive tree is a long-lived evergreen tree, which develops its new shoots and leaves mostly in spring; growing in the wild it exhibits a shrub habit of growth and can reach a height of 10 m, and sometimes, after some agronomic practice, up to 15 meters of height (Fig. 4). It is easy to find in in semi-arid to sub-humid warm-temperate regions with winter rainfall and with relatively hot summers. It can be also found in grassland, woodland and riparian habitats. The crown in the past was left free to vegetate, but nowadays it is common to find different kind of tree crowns, shaped by pruning. The axillary flower buds develop into inflorescence in the late spring, the fruit grows in size from early to late summer, a period of fruit development then follows until the fruit collection is due in late autumn. The olive tree is well adapted to withstanding drought, attributed in part to the nature of its leaves, relatively small with a thick cuticle on the upper surface and masses of peltate hairs on the lower surface. The inflorescences have a cluster shape with a variable number of at least 20/25 single flowers. The flower is small and white. After the period of pollination, the olive fruits start to be complete, they will
have different forms and sizes and different weights mostly depending on the cultivar implanted. The fruit is at first green with an elliptical form of the drupe with different size according to the different cultivar used, when ripe the olive is brownish-black in colour. The Mediterranean climate, where the olive tree originates and has grown for thousands of years, and in which olive insects and mite must also have evolved, is characterised by mild yet distinct winters and dry, often hot summers. Furthermore, certain parts of the tree fed upon by a number of pests such as tender leaves, tender shoots, inflorescence, or young fruits are not available for most of the year.
The cultivated olive plant produces extra virgin olive oil and table olives. The global success of these typical components in everyday meals, consumed in the Mediterranean diet, relies on the mythical food, selected as a goodness triad.
Today about 97% of the world supply of olives is produced in Spain, Italy, Greece, Portugal, Turkey, Tunisia, Morocco, Syria, Algeria, France, former Yugoslavia, Jordan, Cyprus, Israel, Libya and Egypt. The remainder is produced in Argentina, USA, Chile, Peru, Mexico, Australia, South Africa and Japan.
The phytopagous insect known to feed and develop on olive trees exceeded 100 species, belonging to different orders of insects such as Homoptera, Hemiptera, Thysanoptera, Coleoptera, Diptera and Lepidoptera, both oliphagous and poliphagous. In specific areas, some of them have had the population evolved or strain adapted to olive so that in those areas olive is preferred to another host. In particular, about a dozen species are considered in the Mediterranean countries major pests of economic importance in agricultural and industry business.
Among them, B. oleae is considered the key pest of olive crops. Other olive pests are (Fig. 5):
- The olive moth, Prays oleae (Bernard, 1788) during its three generation attacks flowers, buds, small fruits and leaves. The damage is caused by the larvae of first generation that enter inside the buds destroying the reproductive organs, and the larvae of second generation that enter inside the young fruit and start to dig galleries in the mesocarp causing the premature falling of the fruit.
- The black scale, Saissetia oleae (Oliver, 1791), causes defoliation sucking sap and its abundant honeydew supports the development of sooty mould fungi. The severity of resulting damage depends on the level of infestation, in extreme cases there may be complete defoliation of the tree and die-back of twigs.
Figure 5. (1) Bactrocera oleae, (2) Prays oleae, (3) Saissetia oleae, (4) Otiorhynchus cribricollis, (5) Palpita unionalis. Images retrieved from www.teatronaturale.it,
www.agrochem.es, www.agraria.org .
Figure 7. Olive fruit fly eggs: female laying an egg (left); egg inside olive fruit (middle and right).
- Otiorhynchus cribricollis (Gyllenhal, 1834) adults cause the damage feeding on the leaves during the night leave, leaving the daily refuge. The damages are most severe on the young olive plants and in nursery.
- The olive moth Palpita vitrealis (Hübner, 1796), larvae cause damages by feeding on young shoots. Injury to leaf bud, grafts and new growth by young larvae can be serious in nurseries and newly planted olive groves.
- The leopard moth, Zeuzera pyrina (Linneaus, 1761), larvae cause galleries in branches, stems or trunk.
Figure 6. Olive fruit fly adults: male (left) and female (right).
Figure 8. Olive fruit fly larvae damaging beneath or inside the fruit.
1.5.3 MORPHOLOGY 1.5.3.1 Adults
The adult of the olive fruit fly is about 5 mm long, with a wingspan of 10 mm. The wings are positioned horizontally and are held away from the body (Fig. 6). Olive fruit flies may be distinguished from related fruit flies by the presence of black spots on the wing tips and the lack of banding across the wings.
The thorax and abdomen of the adult fly are mostly dark-brown to black, with yellow-brown markings and short, silvery hairs. The female has a serrated ovipositor that is used to pierce the skin of fruits during oviposition. The female differs from male for the presence of the ovipositor and the biggest dimension of the abdomen, the male presents a hardening at the apex of the anal rib whose narrow stretch is longer than the female one.
Figure 9. Puparium inside the olive fruit.
1.5.3.2 Eggs
The female fly deposits an egg inside the developing olive fruit, which it is not visible unless the fruit is cut open. Females typically deposit one egg per olive (Ant et al., 2012) on smaller fruit (< 1 cm3) (Yokoyama et al., 2006). Newly deposited eggs of the olive fruit fly are opaque and creamy white in colour. Eggs are about 0.74 mm long and 0.21 mm wide (Genc, 2014). The shape is typical of Tephritid fruit fly eggs: elongated and somewhat curved. They maintain this appearance until hatch time, when the first instar larva is visible through the chorion (the membrane surrounding the egg) (Genc, 2014) (Fig.8).
1.5.3.3 Larva
Larvae emerge from the anterior end of the egg and move deep within the olive fruit to feed (Genc, 2014). As with the egg, the larva will not be visible until the fruit is cut open. Larvae are typical Tephritid fruit fly maggots: small (5-6 mm long, 1.5 mm wide), elongated, and slightly tapered at each end (Phillips, 1946). The different shapes of the frontal stigmas allow determination of the larvae of the second and third stages, while the larva at its first stage is metapneustic. The larvae spend their life tunnelling galleries in the olive mesocarp. The gallery at first is filiform and then progressively widens towards the core (Fig. 8). In the field, B. oleae larval development is largely temperature-dependent and the resulting number of annual generations depends on humidity as well as the availability and quality of olive fruits (Burrack & Zalom, 2008; Kounatidis et al., 2008).
1.5.3.4 Pupa and puparium
The pupation takes place inside the exuvia of a mature larva, which hardens forming the puparium. Pupation usually occurs within the olive fruit but may also occur in the soil depending on the time of the year and the number of generations. Pupation is more likely to take place in the soil at the end of the season in areas where there are many generations per year (Rice, 2000). The puparium, similar to a barrel with rounded ends, is at least 3.5 – 4.5 mm long. It is yellow at the beginning of the development and then reddish. Inside the puparium the larva becomes an adult and the change in colour of the puparium can determine the age of the pupa (Fig. 9).
1.4.4 LIFE CYCLE
The olive fruit fly has three to eight generations per year depending upon local conditions. Overwintered adult populations decline to low levels by February or March, however new adults from overwintered pupae begin to emerge in March and April. These females lay eggs inside the previous year’s fruits left on the tree. The first-generation of adults appears in the spring and the second generation appears in mid-summer. The susceptibility of the olives increases at the time of pit hardening. There can be several generations and in some cases, continuous adult emergence throughout the whole year.
In Italy, the species can complete up to six generations per year, depending on latitude, altitude, climatic conditions and fruit availability in spring. In coastal areas, the olive fruit fly adults are observed all year round, with the highest densities in spring and autumn. Females have immature ovaries in winter, due to low temperatures, and in late spring-early summer, due to unavailability of fruits suitable for oviposition. In spring, B. oleae can complete 1-2 generations on unharvested olives, especially in high yield years, and 3-4 generations in summer-autumn. In olive orchards at higher altitudes and in Central Italy, adults emerge in spring from overwintering pupae and oviposit on olives in summer, completing 1-3 generations per year depending on climatic conditions. Population levels and intensity of fruit infestation greatly vary depending on biotic and abiotic factors, of which the most important are the climate, yearly meteorological conditions, plant cultivar, crop load, and natural enemies (Delrio & Lentini, 2016).
Figure 10. Olive fruit fly oviposition (left), exit hole (middle) and exit of a new individual (right).
The number of olive fly generations varies according to different factors: geographical region, agronomic and climatic conditions, quality of the fruits, among others. High populations can develop very rapidly when ideal temperature favors rapid development.
In most cases, the greatest damage occurs as the fruit begins to soften and turn colour from September to November. The optimum temperature for the insect development is between 20 and 30° C. In practice, the air temperature during the spraying process must be between 12 and 28° C and the wind speed must be less than 28.8 km/h. High wind speed inhibits the insect flights. During the favourable climate for oviposition, females prefer to visit the south-west part of the tree foliage and fruits at 1.5 meter of height. The growing of the olives can accelerate the eggs maturation, but oviposition can be stopped or slowed by high temperature.
The plant selection behaviour of B. oleae has been widely investigated. It is known that chemoreception in fruit flies is crucial for host-plant finding and selection (Levinson & Levinson, 1984; Drew, 1987; Drew & Fay, 1988). In addition, the olive juice released from the olive fly oviposition wound normally spread on the olive surface, preventing further oviposition on the same fruit (Cirio, 1971). Despite the importance of shape, size and colour for orientation of olive fruit flies, optic tactile stimuli are insufficient for host recognition, and olfactory and gustatory stimuli are required. It has been observed that the oogenesis and the oviposition are caused or at least regulated by chemical stimuli. Volatile metabolites including ammonia and unidentified emanates from the olive fruit encourage oviposition, while benzaldehyde, 4-hydroxy-3-methoxybenzoic acid and several phenol derivatives recovered from olive juice were found to deter egg laying by
B. oleae. These stimuli are detected only after the direct contact of the B. oleae female
with the olive, probably identified through contact or olfactory chemoreceptors activated only near the olive fruit.
The olive fruit fly establishes permanent symbiosis with the bacterium Erwinia
dacicola, necessary for larvae to feed on olives and neutralize the negative effects of the
oleuropein and for adults as it metabolizes complex nitrogen compounds (Sacchetti et al., 2008 and 2016).
In optimal temperature condition, the oviposition started 7-9 days after the adult emerging often preceded by sterile ovipositions, i.e., laying without eggs. On the green olive fruits, the egg laying appears like a triangular stain of brown colour of at least 1-1.5 mm long, due to transparency of the underlying tissues incised and blackened by oblique laying. After about twenty days on young fruits, the wound becomes a small outcrop point.
Each female can oviposit about 12 eggs a day and about 200 - 250 eggs in a lifetime (Mavragani-Tsipidou, 2002), with a high pick of egg laying when the temperature is between 20 and 27°C degree and high relative humidity around 80-90%. The olive fruit fly eggs do not develop below 7.5-10°C and are unviable above 30-32°C (Tsitsipis, 1977). Under optimal conditions, eggs can hatch in 1 day, giving origin to larvae that feed on olive mesocarp, creating galleries inside the fruit. Larvae are classified according to their developing stage as L1, L2 and L3. For larvae development, temperatures can not be below 10-12.5°C or above 30-32°C (Tsitsipis, 1977). Under optimal conditions, larvae of B. oleae can fully develop in 8 days, with an interval from 8 to 37 days, consuming from 45 to 150 mg of olive pulp (Neuenschwander & Michelakis, 1978, 1979; Tsitsipis, 1977). Once ready for pupae formation, larvae from B. oleae open an exit hole in the olive epicarp and either escape from the fruit (Fig. 10) to pupate in the soil or pupates inside the fruit and open an exit hole for the adult. Under optimal conditions, adults emerge after 9 days, ranging from 9 to 49 days (Tsitsipis, 1977; Neuenschwander & Michelakis, 1979). Contrarily to larvae, adults have diversified sources of nutrients for their diet, feeding on insect honeydews, nectar and pollens from the available plants, fruits exudates, as well as bird feces, bacteria and yeasts (Daane & Johnson, 2010).
1.4.5 DAMAGE AND ECONOMIC IMPORTANCE
As olive trees cultivation is increasing and spreading to new areas of the globe, the olive fruit fly is increasing its importance as a global spread pest, causing increasingly damages each year (Bueno & Jones, 2002). It is predicted that the direct consequence of
Figure 11. Examples of damages on olives caused by B. oleae.
Economic losses due to B. oleae affect the entire olive chain. First of all, the olive farmers have to handle productivity losses due to fruit drop, rot fruits, and lower income in table olives and olive oil production. Infestation of the olive fruits by the larvae causes premature fruit drop and reduces fruit quality for both table olive and olive oil production (Michelakis & Neuenschwander, 1983). In table olives, the presence of few larvae can lead to the rejection of an entire crop. Some infestation levels can be tolerated in olive oil production. However, the presence of larvae and associated microorganisms raises oil acidity and thereby reduces the quality of the oil.
For the table olives, the damage can be serious, generally receiving more attention in
B. oleae management, as oviposition punctures cause serious reduction of the value of
the crop (Kapatos, 1989), in fact the presence of some holes on the surface of the olive can compromise the sale in the market. For these reasons the economic threshold is set at around 2% (Tremblay, 2005).
For the production of oil, the damage consists mostly in the larval activities inside the olive pulp, increasing the premature fruit drop and affecting the quality of olive due to the increase of oil acidity (Fig. 11). Olive oil quality decreases as levels of acid increase (Gomez–Caravaca et al., 2008). This relationship is influenced by the presence of microorganisms such as bacteria (Xanthomonas), yeasts, and molds (Fusarium or
Penicillium), with a positive logarithmic relationship between microflora populations and
oil acidity (Torres–Villa et al., 2003). The economic threshold varies from 4% to 18% of infested fruits depending on the cultivar but is usually set at about 10%. (Tremblay, 2005). The olive plant usually has a biennial cycle of olive production, with one year of high olives production and one year with low olives production (Lavee, 2006). However, the
olive production is also influenced by climate conditions, the age of the plants and the agronomic practices. In the olive groves with a strong alternation of production, the quantity of olives affects the olive fruit fly presence during the current year and also during the following season.
1.5.6 NATURAL LIMITING FACTORS 1.5.6.1 Physical factors
Temperature is one of the most important factors for the B. oleae development. Under 6-7°C or above 36°C the adults are completely inactive, for a longer period they stop their life cycle. The adults start to fly around 13-14°C, while for reproduction they need at least 16-17°C (Lucchese, 1954; Girolami, 1979). They can also tolerate temperatures around 0°C during the night but only if during the day, the temperature increases to 14-16°C. Lethal temperatures for all the B. oleae stages are under 9°C and above 42°C.
A low level of humidity, usually during summer, leads to high mortality of eggs and larvae (Delrio & Prota, 1976), caused by the reduction of the watery content of the olives. In dry conditions, mortality of pupae and adults in clay soils is also high (Neuenschwander et al., 1981) due to the formation of a superficial coating.
The seasonal climatic trend represents a very important factor that can limit the development of B. oleae. Hot and dry summers, particularly during the months of July and August, with temperatures constantly above 32-33°C, result in the stop of the adult activities and an increased larval mortality that is reflected in the low levels of the autumn generations. Warm autumns and winters increase the risk of high infestation levels, in fact higher temperatures during these months determine an advance in the vegetative development of the olive trees and the drupes became receptive to the first attack of the pest in advance.
Cultural practices are a manipulation of the cropping environment to increase pest mortality or reduce rates of pest infestation or damage. Some cultural controls may also increase or decrease pests' natural enemy populations. Good cultural control methods often become stable farming practices that serve multiple purposes. When available, use of resistant crop varieties is another useful tactic in managing insect pests. By using resistant varieties, other insect control measures may not be needed because the crop is less likely to be economically injured by the insect pest.
1.5.6.2 Biological factors
In CABI (2014) 34 natural enemies are listed, including some pathogen viruses. In the Mediterranean basin, most indigenous parasitoids attacking B. oleae are generalist ectoparasitoids, such as Eupelmus urozonus Dalm. (Eupelmidae), Pnigalio mediterraneus Walk. (Eulophidae) and Eurytoma martellii Dom. (Eurytomidae). Nevertheless, their significance varies through space and time, and needs further assessment of their host ranges. Attempts of flood releases using Opius concolor (Szepl.) did not succeed (Monastero & Genduso, 1964; Liotta & Mineo, 1969; Fimiani, 1982). Sufficiently untouched ecosystems, preferably with a cover grass, preserve a considerable cohort of fly’s natural enemies with a steady consistence (Fimiani, 1982). The lack of real knowledge and understanding of parasites' bio-ethology, their rarefying because of chemical treatments, the agro-ecosystems’ biotic potential strongly benefiting the phytophages, the need to work on a large scale, are just some of the reasons that led to a poor efficiency of the biocontrol methods traditionally applied. How abiotic factors affect the parasitization level of the fly’s instars, particularly the temperature inside the drupes, was also investigated (Pucci & Forcina, 1981). A possible explanation for the poor performance of parasitoids is the relative size of the commercial olive fruit compared to the wild olive fruits. Wild fruits are small, usually 1 cm in diameter, whereas cultivated varieties can be thicker-skinned and are significantly larger, usually 2-3 cm in diameter (Tzanakakis, 2003). Species that parasitize B. oleae in natural environments, though evidently well adapted to attacking small wild olives, may have difficulty reaching close to the pit of the fruit in larger cultivated olives (CABI, 2014).
A variety of predators attacks B. oleae in southern Europe. These include many staphylinid and carabid beetles, earwigs, the cecidomyiid Prolasioptera berlesiana Paoli, chrysopids and other neuropterans, many species of ants, diplurans, spiders and myriapods (Neuenschwander et al., 1983, 1986). Some studies have been published regarding the predatory role of P. berlesiana, which may also act as a pest due to introduction of decay agents in addition to feeding on fly eggs. The role of P. berlesiana as both predator and pest was disputed by Harpaz & Gerson (1966) and requests more studies to be clarified.
A study by Orsini et al. (2007) showed that in California, ants like Formica aerata (Francoer) contributed significantly to the mortality of B. oleae pupae in the soil.
Pathogens of Tephritid fruit flies have received comparatively little attention to date. Several bioassay studies reported varying levels of toxicity of the bacteria Bacillus
thuringiensis Berliner against B. oleae (Karamanlidou et al., 1991; Navrozidis et al.,
2000) and other Tephritids (Robacher et al., 1996, 2000).
Furthermore, there is potential for the use of the mitosporic entomopathogenic ascomycetes for Tephritid control; they are not only virulent against both preimaginal and adult flies, but they also secrete new molecules with natural insecticidal properties for the control of adults (Konstantopoulou et al., 2006).
1.5.7 MANAGEMENT OF INFESTATION 1.5.7.1 Preventive methods
Agronomic practices are able to influence the development of B. oleae. The alternation of olive production affects the infestation levels: during a discharge year, a high infestation can lead to the total fall of the olives with consequence lack of olive on the ground for the spring generation and then without generation in the following autumn generations (Delrio & Prota, 1990). In case of high infestation, a competition can lead to larvae mortality and a decrease of pupae weight (Cirio & Gherardini, 1984).
In olive groves, some very important practices that can help the B. oleae control are pruning, irrigation, fertilization, tillage, and all the other actions in the agro-ecosystem that allow the limiting action of natural enemies against B. oleae. The complete fruit collection in all olive groves reduces the number of fly wintering forms and hinder the numerical increase of the spring generations of the fly (Delrio et al., 1978 and 1995; CABI, 2014)
Olive cultivars in Mediterranean countries show varying susceptibility to infestation by olive fly. In general, large size olives and olives with higher water content (mostly table cultivars) are more susceptible than small olives with lower water content (many oil cultivars).
Kaolin is a clay inert dust used as a physical barrier on the olive, creating a film on the fruits that prevents the fly oviposition activity (Caleca et al., 2010). Kaolin is distributed over the trees, with no observed effects on plants and oil (Khalegi et al., 2015).
1.5.7.2 Monitoring
An efficient method to assess the presence of the pest in the olive groves, is the use of various kind of traps for B. oleae. These traps are located on a branch of the tree, in the external part of the crown, clearly visible at a height of 1.5 m or above. For data collection and for the action plan development, 4-6 traps per hectare are usually needed, depending on the type of trap used(Varikou et al., 2014).
Adult monitoring helps us to have various information on the population in the olive groves, for example on the mating status of the individuals. When the diameters of the young fruits have not reached a sufficient size for oviposition, the flies are usually not sexually active. As the summer progresses, the fruits come to the point at which oviposition is possible and sexual activities is triggered in the adult flies. This can be observed by comparing the catches of male flies in pheromone baited or not baited traps. When there is no sexual activity, catches are more or less equal in both types of traps. When mating activities begin, the catches in baited traps suddenly become up to 10 times higher, signalling the need to take a look to the state of development of the ovaries in the females. The two factors are then taken together as an indication of risk of infestation to the olive fruits in that particular grove (Montiel Bueno, 1989).
Various traps have been tested for B. oleae, alone or in combination with attractants. Depending on the attractant, solid or dissolved in water, the traps are called “dry” or “wet”, respectively. Dry traps usually are open and the flies are captured by a sticky surface. Wet traps are closed boxes containing the liquid bait. The flies enter through small openings and drop in the liquid. Example of dry traps are Delta trap, ChamP trap, Yellow panel. Examples of wet traps are McPhail trap, Bottle trap, Easy trap (IAEA, 2003) (Fig. 12).
The types of attractant devices used for the monitoring action are:
• Chromotropic devices: usually a sticky panel, with yellow colour and different size, covered by glue. This trap uses the chromotropic action of the yellow colour that attracts the adults, but also other species of insects, including natural enemies such as lacewings and parasitoids. The glue on the panel, by a mechanic action, captures the flies; the application of these traps can be indicated also for mass-trapping of adults.
• Pheromone devices: they have the advantage of using the attraction by the synthetic pheromone lure, called spiroketal (1-7dioxaspiro- 5,5undecans), also in