In Mammals transsulfuration pathway enables the conversion of homocysteine (Hcy), deriving from methionine (Met) transmetilation, into cysteine (Cys) through two steps, the first catalyzed by cystathionine β-synthase (CBS) and the second one by cystathionine-γ-lyase (CGL). The latter enzyme is considered to catalyze the rate limiting step of the whole process and one of its products, Cys, is the limiting amino acid for glutathione (GSH) synthesis. GSH, is a tripeptide that has the prominent function to balance the cell redox state. This role is particularly important in the lens where the oxidative stress is at the base of senile cataract onset. Experimental evidences obtained in our research unit point out the attention on the possible role of cysteinylglycine (CysGly) (an intermediate of GSH catabolism), in modulating the transsulfuration pathway in cultured bovine lens. Blocking γ-glutamyl transpeptidase (γ-GT), upon action of serine borate, appears to induce an extrasynthesis of GSH, which is released into medium without any change in intralenticular levels; while the supplementation of culture medium with CysGly, impaired the observed GSH extrasynthesis, as the use of PPG that inhibits CGL. Aim of this work was the accomplishment of CGL purification from calf lenses in order to assess the possible regulatory role of CysGly on CGL activity. The purification of CGL from bovine lens resulted a very hard task, since the extremely low activity detectable in the lens extracts. The assembled purification protocol did not allowed the availability of sufficient enzymatic sample to proceed to a characterization. Thus cultured bovine lens epithelial cells (BLEc), for which a CGL specific activity two order of magnitude higher than that measured in the lens extract, were selected as a better source to isolate CGL.
The purification of the enzyme from BLEc, even though did not allow to obtain an electrophoretically homogeneous sample provided information on the quaternary structure of the enzyme. A modest, even though of debateable statistical significance, inhibitory effect of CysGly on partially purified CGL was observed.
Cultured bovine lens epithelial cells
BLEc
Cystathionine-γ-lyase CGL
Cystathionine-β-synthase CBS
Cysteine Cys
Cysteinylglycine CysGly
Dithiothreitol DTT
Ethylenediaminetetraacetic acid
EDTA
Homocysteine Hcy
Glutathione GSH
Glutathione synthase
GSH synthase
γ-glutamylcysteinyl synthase
γ-GC synthase
γ -Glutamyl transpeptidase
γ-GT
Methionine Met
PropargylGlycine PPG
Chapter1:INTRODUCTION
1.1) Lens Structure and function.
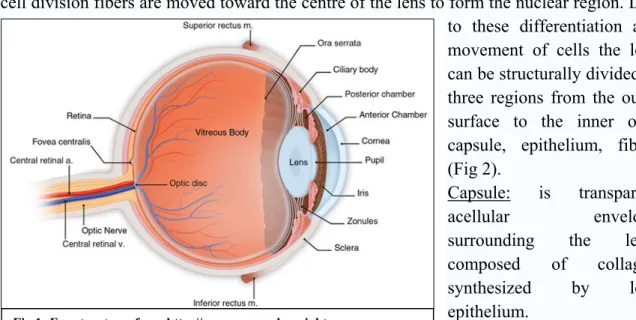
Lens is a transparent avascular structure with a biconvex shape having the anterior surface less curved than the posterior one. Lens is placed between the iris (anterior face) and the vitreous body (posterior face), and is suspended in place by the zonular fibers, which attach to the lens near its equatorial line and connect the lens to the ciliary body (Fig.1). The equatorial zone is mitotically active giving rise to epithelial cells that differentiate into lens fibers moving through the anterior and posterior poles; with a further cell division fibers are moved toward the centre of the lens to form the nuclear region. Due to these differentiation and movement of cells the lens can be structurally divided in three regions from the outer surface to the inner one: capsule, epithelium, fibers (Fig 2).
Capsule: is transparent
acellular envelope surrounding the lens, composed of collagen synthesized by lens epithelium.
Epithelium: is a monolayer of cuboidal cells underling the capsule only in the anterior portion of lens, responsible for the maintenance of lens osmolarity through the action of Na+/K+ ATPase pumps. Moreover, besides collagen synthesis for capsule composition, epithelium serves as progenitor for lens fibers, that constantly produces during the lifetime. Fibers: are constituted by long cells tightly packaged, connected to each other through gap junctions which reflect the importance of a close net between the large bulk of fiber cells and the relatively scant number of metabolically active epithelium cells (Fitzjerald P.J. et al 1985). Two regions can
be distinguished on the basis of fibers age:
- cortex, the outermost and youngest layer
- nucleus, the inner and oldest layer of lens fibers (Fig 2). The mature lens fibers lack nucleus, endoplasmic reticulum
and mitochondria. Lens cells, Fig 2: Lens structure, by http://www.photobiology.info/Roberts.html
together with corneal cells, are responsible for the transmission and focusing of light onto the retina; the lens can fulfil this task thanks to its plasticity and transparency. In fact the lens is a dynamic structure since it is able to change its curvature through the action of ciliary muscles; the change of curvature is a key event in accommodation phenomena that is at the base of focusing ability of the lens. Regarding transparency, this feature is fundamental to maintain refractive power, and for this reason mature lens fibers lack light scattering organelles. Both transparency and plasticity tend to decrease during aging thus affecting lens function; for example loss of transparency within the lens, via disruption of cells membrane or protein aggregation, results in cataract onset.
1.2) Glutathione.
Glutathione (GSH) (Fig 3) is a tripeptide of cysteine (Cys), glutamate, and glycine whose peculiarity is the γ-peptide bond between glutamate and Cys. The γ-carboxyl bond is more stable with respect to -carboxyl bond and for this reason GSH easily resists the hydrolytic action of most
peptidases. Mammalian lenses contain millimolar
concentration of GSH with the highest concentration in epithelial layer (Reddy 1990). In physiological conditions the greatest part of GSH is in reduced form thanks to the action of glutathione disulfide reductase. GSH is quantitatively the most important intracellular thiol redox buffer and also serves as a major reservoir for cysteine (Meister et al., 1983). The main physiological functions exerted by GSH are:
-Maintenance of reduced environment: maintaining the reduced state of protein thiol residues and low molecular weight compounds such as ascorbic acid and vitamin E. -Antioxidant defence: GSH acts as scavenger of free radicals and reactive oxygen species (ROS) both reacting with them directly, or acting as cofactor in the reaction catalyzed by glutathione peroxidase that generates GSSG.
-Detoxification of xenobiotic compounds: the conjugation of GSH with a xenobiotic compound is essential for the detoxification of such molecules. Conjugation can be non enzymatic or enzymatically driven by GSH- transferase.
-Modulation of redox regulated signal transduction : GSH can be covalently linked to protein thus regulating their function, through a phenomenon known as glutathionylation (Pompella, A. Et al., 2003).
-Reserve of cysteine: Cys undergoes rapid auto-oxidation producing radical species potentially toxic (Onley et al., 1990) to avoid this phenomenon cellular cysteine is stored as GSH.
The maintenance of high levels of GSH seems to play a key role in preserving lens transparency as demonstrated in several studies on experimental induced cataract. In such studies the decrease of lens transparency was preceded by a severe fall in GSH (Harding J.J., 1991). The maintenance of appropriate levels of GSH seems to be related not only to de novo synthesis , but also to transportation of intact GSH from acqueous humor to lens (Zlokovic et al.,1994; Mackic et al., 1996). A sort of gradient in GSH concentration from
the outermost layer of the lens to the nucleus was observed in several experimental models such as rabbit and guinea pig: this can be due not only to a similar gradient in levels of enzyme such as glutathione reductase, but also to the impaired ability to transport GSH to the nuclear region and could explain the onset of nuclear cataract in aging lenses. In fact in the nucleus extended protein oxidation can lead to cross-linking and protein precipitation, two phenomena which affect the transparency of lens.
1.3) GSH metabolism and catabolism: the γ-glutamyl-cycle.
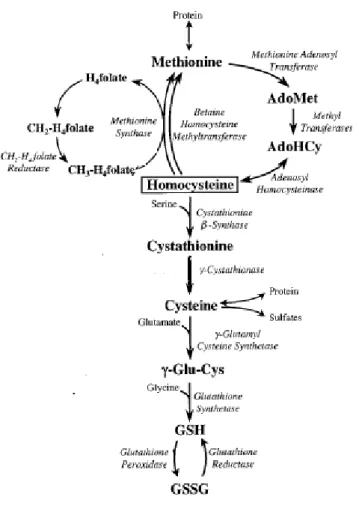
In the cell GSH levels depend on the balance between GSH metabolism and catabolism which form γ-glutamyl-cycle (Fig. 4). GSH is synthesized in cytosol upon consecutive action of γ-glutamylcysteinyl synthase (γ-GC synthase) and GSH synthase. The rate of the process depend on Cys availability (Jackson, 1969; Taylor et al., 1975), and the feed-back regulation of GSH on γ- GC synthase (Jackson, 1969; Richman and Meister, 1975). γ- GC synthase is trascriptionally up-regulated under oxidizing conditions (Griffith OW. 1999; Iwata-Ichiwata E., et al. 1999; Shukla GS. Et al., 2000; Stover SK. Et al., 2000) thus increasing GSH production.
Fig.4: γ-glutamyl cycle
GSH synthase seems to have not a regulatory role; indeed once synthesized, glutamylcysteine is promptly converted into GSH.
GSH is constantly secreted in the extracellular space where the membrane bound enzyme γ-glutamyl-transpeptidase (γ-GT) transfers the γ-glutamyl fraction to an acceptor that could be represented by an amino-acid, a dipeptide or GSH itself (Tate and Meister, 1981). The internalization of γ- glutamyl amino acid, through the action of γ-GT, implies the release of cysteinylglycine (CysGly), which is substrate of dipeptidase activities. γ- glutamyl amino acid is then converted to free amino acid and proline by a cyclotransferase, proline is linearized to yield glutamate in an ATP dependent reaction catalyzed by 5-oxo-prolinase (Wang e Ballatori, 1998). Thus at end of the cycle GSH is converted to its aminoacidic components which can be used for several aims, as the re-synthesis of GSH.
1) γ-glutammyltranspeptidase 2) γ-glutamylciclotransferase 3) 5-oxo-prolinase 4) γ-Glu-Cys synthetase 5) GSH synthetase 6) Dipeptidase
1.4) Sulfur containing amino acids and transsulfuration pathway
.In Mammals Cys derives from diet and from methionine (Met) and serine (Ser) via transulfuration pathway, for this reason Cys is considered to be a semiessential amino acid while Met is an essential one (Stipanuk, 2004). Met and Cys are metabolically linked by the unidirectional transsulfuration pathway that uses Met as a source of sulphur atom for Cys synthesis, Met is the precursor of homocysteine (Hcy), the conjunctive amino acid between transmethylation and transsulfuration pathway (Fig. 5). ATP dependent activation of methionine (catalysed by Met-adenosyltransferase), produces S-Adenosyl methionine and subsequent demethylation and removal of the adenosyl moiety yields Hcy. At this point Hcy can be committed in transulfuration pathway, or in remethylation pathway to re-synthesize Met. The transulfuration pathway of Hcy to Cys is catalysed by two piridoxal 5’-phosphate (PLP)-dependent enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CGL). Cystathionine β-synthase catalyses the condensation of Hcy and Ser to form cystathionine in an irreversible reaction (Banerjee R., et al. 2003; Finkelstein JD. 2000). Cystathionine is hydrolysed by CGL to give cysteine, α-ketobutyrate and ammonia. The highest CBS activity was found in rat pancreas and liver (Mudd et al., 1965) CBS it is also detectable in kidney, small intestine, brain and adipose tissue (Mudd et al., 1965). High CGL activity was found in rat liver, kidney and pancreas (Mudd et al., 1965), while CGL is absent in fetal liver (fetal liver contained an higher level of cystathionine than adult liver) and brain (Gaull G., et al., 1972). Experimental evidences in favour of the presence of transsulfuration pathway in the lens are a quite recent achievement. Persa and colleagues evaluated the presence of transsulfuration pathway both in human lens epithelial cells (HLE) and in pig lenses, detecting the expression and activity of CBS, the first enzyme of transsulfuration pathway. Results demonstrated a decreasing gradient of CBS protein distribution in pig lenses moving from epithelial layer to the nucleus (Persa C., et al., 2004). Also from very recent data the controversial existence of transsulfuration pathway in brain seems to be untangled; in fact Diwkar and Ravindranath (2007) found that the use of propargylglycine (PPG) a specific inhibitor of CGL, caused a fall in GSH levels in brain.
Fig 5: Transsulfuration pathway and its connection with pathways involving methionine and glutathione . Modified from Mosharov E., et al., 2000.
1.5) Cystathionine γ-lyase in mammals.
The very first studies on CGL from rat and mouse liver revealed a homotetrameric structure with a molecular weight for the single subunit of 45 and 40 kDa, respectively, using density gradient centrifugation and molecular exclusion chromatography (Bikel et al., 1978). In 2004 Ishii and colleagues
cloned and sequenced the full-lenght mouse CGL cDNA and the nucleotide sequence is 92,5% identical to the rat sequence and 81,6% identical with the human sequence. Also Human CGL that has a subunit molecular mass of 44.5 kDa, purifies as an homotetramer (Steegborn C. Et al., 1999). One PLP cofactor is bound per subunit and is present as a Schiff base linkage to the active site Lys 212. The mouse ORF encodes a 43.6 kDa protein (Ishii I., et al., 2004), which shares high amino acid identity with those of rat and human CGL (93,5 and 85, 6% respectively)
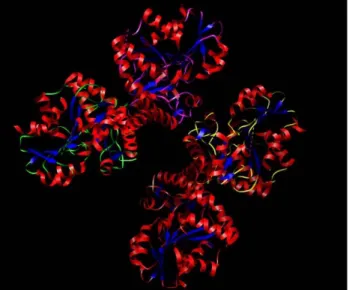
(Zhao W., et al., 2001; Lu Y., 1992). A putative PLP binding site with an active lysine (Lys 211) (Erickson PF. Et al., 1990) is located in the middle of ORF which is fully conserved with rat CGL. In mouse tissues the transcript of CGL gene is detected in brain, heart and lung. Concerning the protein, anti-CGL N-terminal antibody detected both in rats and mice the presence of a 44 kDa protein mainly in liver and kidney with much reduced level in small intestine and stomach, with a parallel distribution of enzyme activity. CGL expression in murine adult kidney was localized to the cortex rather than medulla especially to the renal tubule in the inner cortex or cortical tubule fraction (Ishii I., et al., 2004). Steegborn and colleagues in 1999 characterized recombinant human CGL both in term of substrate specificity and susceptibility to inhibition. For yeast CGL, specific activities toward L-cysteine and L-cystine amounting to 78% and 10% respectively, relative to the L-cystathionine-directed activity, were measured (Yamagata S., et al., 1993) and similar results were obtained in rat liver CGL (Uren J.R., et al., 1978; Matsuo Y., and Greenberg D.M., 1958). In contrast, human CGL displays a strong substrate specificity toward L-cystathionine, being L-cysteine and L-cystine converted orders of magnitude slowly than the natural substrate L-cystathionine. Regarding inhibition, among three possible inhibitors studied, PPG resulted to be the most effective one in term of selectivity and potency, since trifluoroalanine and aminoethoxivinylglycine inhibit also other correlated activities (Steegborn C. Et al., 1999). A great impulse to the inhibition studies was given by the work of Sun and colleagues in 2009. In fact the crystal structure of h-apo-CGL, h-CGL-PLP and h-CGL-PLP-PPG gave a precious insight into the structural basis for the inhibition mechanism of h-CGL. Human CGL exists entirely as a tetramer in the PLP-bound state, and predominantly as tetramers in apo state (Fig. 6). Structures of the individual monomers of apo-CGL , h-CGL-PLP and h.CGL-PLP-PPG, are very similar
Fig. 6: Crystal structure of human CGL. The figure was produced using UCSF Chimera (Pettersen EF., et al., 2004) and the PDB file 2NMP.
except for two loops near the active site region and a disordered loop at the monomer interface of the dimer in apo-CGL. Each CGL monomer consists of two domains: a larger PLP binding domain (Ala 9- His 263) and a smaller domain. PLP is anchored by strong hydrogen bonding between the phosphate moiety and residues contributed by neighbouring subunits. Lys212 binds PLP and Tyr114 exhibits aromatic π-stacking interactions with the pyridine ring of PLP. The crystal structure of h-CGL-PLP-PPG revealed that the inhibitory effect is not exerted through an impairment of the CGL ability to bind PLP. PPG occupies the space of the side chain of the substrate and inhibits CGL by blocking accessibility of the substrate to the active site via steric hindrance (Sun Q., et al., 2009). Additionally PPG covalently binds and traps residues Tyr114, which is believed to facilitate the release of substrate (Clausen T., et al 1998). Such structural analysis highlighted the singular importance of Tyr114 in h-CGL and its unique specific covalent interaction with PPG, which forms the basis of the PPG dependent inhibition of CGL.
1.6) Disorders in transsulfuration pathway and related pathologies.
Patients with greatly elevated concentrations of Hcy in their urine were first reported in 1962 (Field CMB. et al., 1962; Carson NAJ. And Neill DW., 1962). Later was discovered that defects in CBS enzyme activity were at the base of such clinical manifestation (Mudd SH., et al., 1964). Since that time, several mutations of CBS gene were discovered in homocysteinuric patients (Mudd et al., 1985; Krauss et al., 1999); in particular most mutations are found in exon 3, that contains lysine PLP binding residues (Kery V., et al., 1999). High levels of Hcy was found to be associated to cardiovascular diseases (Aleman et al., 2001). CBS deficiency is inherited as autosomal recessive trait. The primary metabolic consequence of a deficient activity of CBS is a tendency for homocysteine to accumulate intracellularly. In such conditions, mammalian cells export it (Christensen B., et al., 1991), and, in the plasma, the tendency to intracellular accumulation is reflected by abnormal concentration of a variety of homocysteine derivatives, such as cysteine-homocysteine and homocysteine-cysteinylglycine mixed disulfides. The most common clinical features of CBS deficiency are: dislocation of optic lens, osteoporosis, mental retardation, and thromboembolism. Management of CBS-deficient patients emphasizes amelioration of the characteristic biochemical abnormalities. Treatment of early diagnosed patients, with low-methionine and cysteine-supplemented diets, obtained good results preventing mental retardation and dislocation of optic lens (Mudd SH., Levy HL., Kraus JP., 2001).
Regarding the latter enzyme of transsulfuration pathway, CGL deficiency leads to the persistent excretion of large amount of cystathionine (cystathioninuria) in the urine as well as to cystathionine accumulation in body tissues and fluids. Cystathioninuria is due to mutations in CGL gene and such a disorder has an estimated prevalence of about 1:14,000 live births (Wang J., and Hegele RA., 2003). Cystathioninuria can also be secondarily associated with a wide range of diseases including diabetes insipidus, Down’s syndrome, neuroblastoma, hepatoblastoma, and celiac disease (Wang J., and Hegele RA., 2003; Tadiboyina VT., et al., 2005). Sequence analysis of CGL gene in cystathioninuric patients has identified four rare non synonymous CGL mutations (Wang J., and Hegele RA., 2003). Two of these are nonsense mutations leading to premature termination, and two are
missense mutations T67I and Q240E. The first mutation falls at the monomer-monomer interface , while the second at the dimer-dimer interface. Despite the particular localization of both mutations could let infer some consequences in the oligomeric structure Zhu and colleagues verified that the oligomeric structure of the mutants was the same as the wilde type (Zhu W., Lin A., Banerjee R., 2008). T67I and Q240E display a lowering of Vmax of 3,5 and 70 fold respectively if compared to wild type. Moreover the two mutants showed a lowered content of PLP and a correspondingly a lower enzyme activity with respect to wild type. These data indicate that both T67 and Q240 play important role in PLP binding and also that cystathioninuric patients could be responsive to pyridoxine therapy (Zhu W., Lin A., Banerjee R., 2008).
1.7) The role of transsulfuration pathway in maintaining GSH pool.
Mosharov and colleagues in 2000 evaluated the fraction of Cys supplied by transsulfuration pathway for GSH synthesis. In liver cells, there are two sources of intracellular Cys that can support GSH synthesis. The first is through import of extracellular Cys, and the second is via transsulfuration pathway. To examine the relative importance of transsulfuration pathway in maintaining intracellular levels of GSH pool in human hepatic cells line, the concentrations of Cys and GSH were evaluated in the absence or in the presence of PPG. CGL inhibition leads to a fall in cysteine concentration from 160 ± 20 µmol (L of cells)-1 to a value of 65 ± 13 (L of cells)-1. Diminution of cysteine pool was paralleled by depletion of glutathione whose concentration decreased from 6.2 to 2.6 mM. These data indicate that approximately the 50% of GSH is derived via transsulfuration pathway (Mosharov E., et al., 2000).
In a later work of Kim and Kim (Kim S.K., Kim Y.C.; 2001), the effect of PPG was also evaluated on GSH synthesis in ICR mice. The PPG treatment induced significant alterations in the tissue and plasma levels of GSH due to the almost complete blockage of Cys synthesis through transsulfuration pathway, thus confirming the transsulfuration pathway plays a critical role in Cys supply for GSH synthesis.
It is well known that GSH depletion occurs in lenses from elderly humans and old animals particularly in the nucleus of the lens (Ferrer J., et al., 1990; Giblin F., et al., 2000; Lou MF., et al., 2000). CGL activity seems to play a key role in maintaining an appropriate GSH pool in the aging lens, since Cys uptake by lens markedly declines with aging (Rathbun WB., and Murray DL:, 1991). Indeed CGL inhibition by PPG causes GSH depletion in eye lens. In rat lenses both CGL activity and mRNA levels of CGL were markedly reduced in old lenses with respect to young ones, explaining the age-associated GSH deficiency. In particular regarding old rats two populations were identified, one whose lenses lack CGL activity (56%), and the rest displaying a low but detectable enzyme activity (Sastre J., et al., 2005). Furthermore CGL protein was absent in those lenses lacking enzyme activity. Since these results also indicate that oxidative stress targets CGL in the eye lens upon aging, the authors supposed the existence of a post-translational regulation phenomenon that lead to proteolytic degradation. Targets of such modification could be represented by CGL free amino groups present in its active site, particularly lysine that binds to PLP (Martel et al. 1987). Finally Diwkar and
loss of GSH, indicating that the availability of adequate amount of Cys through the action of transsulfuration pathway is important for maintenance of GSH levels in central nervous system. In that study the loss of GSH, caused by PPG administration, induces inhibition of complex I of the electron transport chain; the connection with cellular redox state is highlighted by the recovery of complex I using reducing agents such as dithiothreitol (DTT) (Diwakar L., and Ravindranath V., 2007). The loss of GSH in CNS results in extensive glutathionylation of proteins and in particular of critical thiol groups in complex I subunits; this probably underlies the complex I dysfunction seen after PPG exposure.
1.8) The
redox regulation of transsulfuration pathway.
Since the established role of transsulfuration pathway in maintaining the appropriate GSH pool and the importance of GSH as antioxidant buffer, an interesting point is represented by the redox regulation of transsulfuration.
With regard to transsulfuration pathway the treatment of HLE with H2O2 induced CBS up-regulation, furthermore, the up-regulation and activation of CBS in cultured lenses is correlated to oxidative stress induced cataract formation (Persa C., et al., 2004). Interestingly Mosharov and Vitvitsky found the same result in hepatic human cells where oxidative stress stimulated CBS, and concurrently inhibited the remethylation enzyme methionine synthase (Mosharov E., et al., 2000). In contrast antioxidants such as catalase, SOD, and vitamin E elicited the opposite effect and resulted in diminished flux of Hcy through the transsulfuration pathway (Vitvitsky V.et al., 2003). This probably constitutes an adaptative response to GSH need in oxidative stress to divert homocysteine pool from Met synthesis, so that more Cys molecules are available for GSH synthesis.
The up -regulation of transulfuration pathway in conditions of oxidative stress was already observed in the fetal to neonatal transition in rats. In particular CGL activity resulted to be higher in newborn than in fetus both in humans and rats, but while in human CGL mRNA levels do not change significantly in at term fetus compared with newborn, in rat liver both mRNA levels and enzyme activity increased at birth. The increase of CGL beside being mediated by hormonal control, seems to be sensitive to a moderate oxidative stress as that occurring in the fetal to neonatal transition; in fact the same effect was induced by a low tert-butylhydroperoxide concentration (Martin JA:, et al., 2007). In summary the above mentioned studies provide the first evidence for reciprocal sensitivity of transsulfuration pathway to pro- and antioxidants and demonstrate that transsulfuration pathway is redox sensitive. All these works lead to the conclusion that transsulfuration pathway can be considered as an alternative source for supplying redox potential to the cells under oxidative stress, or as a pathway for antioxidant generation (Persa C., et al., 2004). The effect of redox regulation of transsulfuration pathway could be enhanced by redox regulation of enzymes involved in GSH metabolism. In fact the activity of γ- GC synthase has been reported to be affected by the presence of a disulphide bond. In particular, Huang and colleagues (Huang et al., 1993) suggested that reduction of the disulfide bond relaxes the γ-GC synthase substrate binding site making it accessible for dipeptides and tripeptides and thereby susceptible to competitive inhibition. Moreover, γ-GC synthase is up regulated under oxidizing conditions (Griffith OW. 1999; Iwata-Ichiwata E., et al. 1999; Shukla GS. Et al., 2000; Stover SK. Et al., 2000) thus increasing GSH production. Also transcription of
GSH synthase gene is induced by the oxidants buthionine-sulfoxime or tert-butylhydroquinone, which also up-regulated the expression of both subunit of γ-GC synthase (Huang ZA., et al., 2000).
1.9) CGL and the production of H
2S: physiological role and possible
pathological implications.
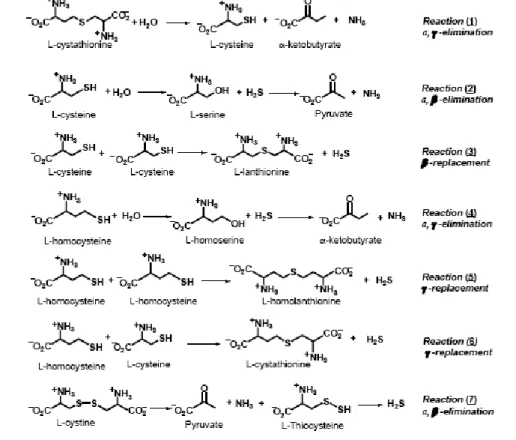
H2S, which is well known as a toxic gas, has been proven to be produced endogenously by three enzymes: CBS, CGL and 3-mercapto-sulfurtransferase (Moore PK., et al., 2003; Hosoki R., et al., 1997; Kimura H., et al., 2005; Tang CS., 2006). H2S has been shown to be the third gaseous transmitter, beside NO and CO, and plays important roles, both in physiological conditions and in the process/progress of several diseases. Concentration of H2S ranging from 50-160 µM have been reported in brain (Goodwin LR., et al., 1989) where it appears to function as a neuromodulator by potentiating the activity of the N- methyl-D-aspartate receptor and by altering induction of long-term potentiation in the hippocampus, which is important for memory and learning (Abe K., Kimura H., 1996). Another important function is the vasorelaxant action that from in vitro studies seems to be mediated by opening potassium ATP dependent channels in vascular smooth muscle cells. It is widely assumed, based on the reported absence of H2S production in brain of CBS knock-out mice (Eto K., et al., 2002), that CBS is the primary source of H2S in this organ, whereas CGL plays the equivalent role in the peripheral vascular tissue (Li LR., et al., 2006). However recent studies have demonstrated that H2S is detected in brain of transgenic mice lacking CBS (Eto K., et al., 2004). In 2008, Yang and colleagues convincingly demonstrated the major role of CGL in H2S biogenesis in the peripheral system. In fact CGL knock-out mice exhibit significantly reduced H2S levels in the serum and lower H2S production rates in aorta and heart (Yang G., et al., 2008). Moreover knock-out mice display hypertension and reduced endothelium-dependent vasorelaxation, the latter data are consistent with a previous finding about the PPG ability to increase blood pressure (Zhao W., et al., 2003). Just for the role of CGL in producing H2S, it is important to investigate the biogenesis of such a molecule and its regulation, the understanding of both aspects could disclose pharmacological approach to increase H2S production to treat hypertension. Recently Chiku and colleagues performed a detailed analysis of kinetic parameters of the CGL- catalyzed reactions to yield H2S. Product analysis provided the evidence for five of six possible CGL dependent H2S-generating reactions (i.e. reactions of 2-6) (Fig.7). Through the analysis of kinetic parameters they simulated the relative contribution of each reaction to H2S formation: it resulted that under normal conditions, ,β-elimination of cysteine (reaction 2) is predicted to be the major source of CGL-derived H2S accounting for ~70% of the total. The ,γ-elimination of homocysteine (reaction 4) is the second significant contributor with the 29%, while β- and γ-replacement reactions (reactions 3, 5-6) are of negligible importance. Increasing levels of Hcy, determined an higher contribute of the ,γ-elimination (reaction 4) reaction of Hcy, that becomes a significant source of H2S at moderate, and the principal source at severely elevated Hcy concentrations. γ-replacement (reaction 5) has a squared dependence on the concentration of homocysteine. In this light homolanthionine production could be a useful biomarker for
H2S production (Chiku T., et al., 2009). Indeed homolanthionine was reported in urine sample of homocystyinuric patients (Perry TL., et al., 1966). Therefore under hyperhomocystenemic condition H2S production may be enhanced and could contribute to cardiovascular pathology (Chiku T., et al., 2009). In an in vivo model for myocardial ischemia-reperfusion, when H2S donor was administrated at the time of reperfusion a cardioprotective effect of H2S was highlighted, in particular the most effective cardioprotection was exerted with decreasing concentrations of H2S (Elrod J., et al., 2007). In rat model of stroke, administration of high NaHS levels increased infarct volume (Qu K., et al., 2006). Mutation in CBS are the most common cause of severe hyperhomocysteinemia, so in such pathology CGL may be the major source of H2S, and for this reason the inhibition of CGL could be a useful strategy for attenuating the attendant of cardiovascular pathology associated with this disease.
Fig. 7: Cystathionine cleavage and H2S generating reactions catalyzed by CGL, Scheme from (Chiku T., et
1.10) The role of CysteinylGlycine in GSH homeostasis in cultured bovine
lens.
Experimental evidences obtained in our research unit (De Donatis GM. PhD thesis, 2005) suggested for CysGly, an intermediate of GSH catabolism trough the action of γ-GT (see Fig. 4), a role in modulation of GSH metabolism in cultured bovine lenses. In fact incubation of bovine lenses in the presence of exogenous GSH and serine-borate, a competitive inhibitor of γ-GT, appears to induce an extrasynthesis of GSH, which is released in culture medium without any change in intralenticular GSH levels. The supplementation of culture medium with CysGly impaired the observed GSH extrasynthesis; these data suggest a possible regulatory activity of CysGly in GSH homeostasis. The same result on impairment of GSH extrasynthesis was obtained using PPG a specific inhibitor of CGL.
Chapter 2: MATERIALS AND METHODS
2.1) Materials
The following reagents were commercially available:
- Amphotericin B, anti-mouse IgG peroxidase conjugate, Bovine serum albumin, β-chloro-L-alanine, Bestatin, cystathionine, cysteinylglycine, gel filtration chromatography standards (alcohol dehydrogenase, β-amylase, ovoalbumin) peroxidase, PIPES disodium salt, propargylglycine, pyridoxal-5’-phosphate, Kodak® processing chemicals for autoradiography film fixer and developer, protease inhibitors cocktail, silver nitrate, thiamine pyrophosphate, (Sigma Chemical)
- Acetic acid 99-100%, disodium hydrogen phosphate, ethanol 95%, hydrochloric acid
36-38%, methanol, potassium chlorure, sodium chloride , trichloroacetic acid, (J.T. Baker)
- Pyruvate oxidase (Ashai kasei pharma)
- Acrylamide 99.9%, N, N’-methylene-bis- acrylammide, BIO-RAD Protein assay,
SDS-Page molecular weight standard, (BioRad Laboratories)
- Immobilion transfer membrane, concentration membranes Amicon YM10, chemiluminescent HRP substrate, non fat dry milk (Millipore)
- RPMI-1640 medium, L-glutamine, penicillin/streptomycin, trypsin /EDTA, fetal bovine
serum (FBS) (Lonza)
- Manganese chloride, Magnesium chloride, Photassium phosphate monobasic (Carlo Erba)
- Citric Acid, polyoxyethilene(20)sorbitanmonolaurate (Tween) (BDH) - Sephacryl S-300, Phenyl Sepharose 6 fast flow (GE Healthcare) - Ninhydrin (Merck)
- Sodium dodecyl sulphate (Beckman)
- Skimmed milk (Fluka)
- Formaldehyde solution 36.5% (Riedel de Haën)
-.N-(Carboxymethylaminocarbonyl)-4,4’- bis (dimethylamino)-diphenylamine Sodium Salt (Wako Chemicals GmbH).
- Cystathionine–γ-lyase monoclonal antibody (Ab-nova)
- EDTA (Juro supply Gmbh)
- Dialysis tubing (sulphur free EDTA treated cellulose cut-off 10 kDa) (Spectrum) - Dithiothreitol (DTT) (Inalco)
- Benchmark pre-stained protein ladder, (Invitrogen) - CL-XPosure™ Film (Thermoscientific)
- Diethylaminoethyl cellulose (DE-52) (Wathman)
The pH of buffers were measured with a glass electrode and the basic pHMeter (Denver
instrument company).
Two spectrophotometers, model DU-7, and DU-6 (Beckman) , were used for all spectrophotometric determinations.
Two refrigerated centrifuge model J2-21, and Allegra 21 (Beckman), were used for samples centrifugation.
Thermostatic bath (Bio-Instruments), was used during samples incubation, that required constant temperature.
Water jacketed CO2 incubator (Bio-Instruments), was used for cell cultures. SNAP system id™ instrument for western blot analysis (Millipore).
Blender (Thermozeta).
Statistical analysis were performed using GraphPad Prism version 4 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com.
METHODS
2.2) Calf kidney crude extract preparation.
Calf kidney of freshly slaughtered animals was frozen at -80°C. 150-160 g of frozen kidney were thawed at room temperature and blended with 250-260 ml of 50 mM sodium phosphate (NaPi) buffer pH 8, supplemented with 1 mM EDTA and 1mM DTT. The homogenized tissue was then centrifuged at 10000 xg for 30 minutes, the supernatant constituted the crude extract.
2.3) Calf lens crude extract preparation.
Calf capsule was cut and then the whole lens was dissolved (5 ml of buffer per lens) in 50 mM NaPi buffer pH 8 supplemented with 50 µM PLP, 1 mM EDTA, 2 mM DTT for 45 min at 4°C using a magnetic stirrer. The homogenate was then centrifuged at 10000 xg in a refrigerated centrifuge for 40 minutes; the supernatant constituted the crude extract.
2.4) Primary culture of bovine lens epithelial cells.
Primary cultures of bovine lens epithelial cells (BLEc) were obtained by explanting lens capsule from the intact eye of freshly slaughtered animals. The capsule was incubated in cell culture medium (RPMI-1640 with 2mM glutamine, 10%FBS, 10U/ml penicillin, 10 µg/ml streptomycin and 6µg/ml amphotericin B) at 37°C, 5% CO2 and 95 % humidity. After three weeks from the explant the first subculture was obtained in culture flask (75 cm2) with a density of 2x105 cell/flask; after additional ten days the second subculture was obtained in petri plates (10 cm diameter) with a density of 2,5x105 cells/plate, using a culture medium without amphotericin B. Cells at pre-confluent state with a density of 1 million cells/plate were used for the experiments.
2.5) BLEc crude extract preparation.
The medium was discharged from petri plates, then plates were washed with 20 ml of PBS, cells were collected mechanically using a scraper and supplemented with EDTA and PLP at final concentration of 1 mM and 5 µM respectively. Cells were frozen at -80°C and the crude extract was obtained through 3 cycles of freezing and thawing. Each cycle was made up of freezing for 30 min at -80°C and thawing at 30°C for five minutes.
The suspension was centrifuged at 9168 x g in a refrigerated centrifuge for 45 minutes, supernatant constituted the crude extract. Crude extract was dialysed, overnight at 4° C, against 10 mM NaPi buffer pH 8 supplemented with 5 µM PLP.
2.6) βB2 crystallin purification.
βB2 crystallin were purified according the method of Mostafapour MK. and Shwartz CA.(1981). Briefly 2 calf lenses were homogenized by a Potter Elvejehm in 20 ml of 10 mM NaPi buffer pH 7 (standard buffer). The homogenized sample was centrifuged in a refrigerated centrifuge at 10000 xg for 30 minutes; the supernatant constituted the crude
extract. To purify βB2 crystallin the crude extract was boiled for 5 minutes, then resuspended in standard buffer, and centrifuged at 10000 xg for 10 minutes; the resulting supernatant was subjected to the thermal treatment 2 more times. The purified sample was electrophoretically homogeneous and had a protein content of 0,44 mg/ml.
2.7) Determination of protein content.
Protein determination was performed according Bradford method (Bradford 1976), calibration curve was set up using BSA as standard protein.
2.8) Determination of Cystathionine -γ-lyase activity.
-2.8a) Ninhydrin assay
CGL activity, was determined using Gaitonde colorimetric method with minor modifications (Gaitonde MK, 1967). An enzymatic unit was defined as the amount of enzyme that catalyses the conversion of 1µmole/min of substrate in standard assay conditions. The assay mixture for the enzyme, containing 50µM PLP, 1 mM DTT 2mM Cystathionine in 10 mM NaPi buffer pH 8, was incubated for 40 minutes at 37°C. The reaction was stopped using TCA 5% (w/v).
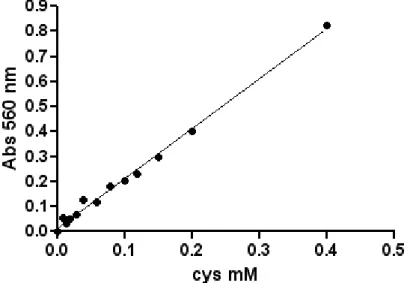
The sample was then centrifuged at 14000 rpm for 8 minutes, and 100 µl of supernatant was mixed with equal volumes of a 25 mg/ml ninhydrin solution (dissolved in 3:2 of acetic acid 95% : hydrochloric acid 12N) and Acetic acid. The specific reaction between Cys (the product of reaction) and ninhydrin occurred, boiling samples for 4 minutes. The reaction was stopped cooling the mixture. The mixture was supplemented with 1,7 volumes of 90% ethanol to stabilize the stained complex. The absorbance at 560 nm was read. In the assay conditions extinction coefficient of the complex is 2 mM-1cm-1.
2.8b) Bindschedler’s Green assay
CGL activity was determined using Ogasawara method (Ogasawara et al., 2001), with minor modifications. An enzymatic unit was defined as the amount of enzyme that catalyses the conversion of 1µmole/min of substrate in standard assay conditions. The assay mixture for the enzyme containing 5µM PLP, 13,6 mM β-chloro-L-alanine in 10 mM NaPi buffer pH 8 supplemented with 2,35 mM EDTA was incubated for 10 minutes at 37°C. The reaction was stopped adding 0,9 volumes of 63 mM PPG. The product of reaction pyruvate is determined adding to the sample 700 µl of a mixture containing 0,823 mM TPP, 6,5 mM MgCl2, 10,97 U/ml pyruvate oxidase, 0,274 peroxidase, 0,274 mM DA-64, in 0,1 M PIPES pH 6,4, and then incubating it for 10 minutes at 37°C. The final result of reaction is the oxidation of DA-64 to Bindschedler’s Green. The absorbance at 727 nm was read and pyruvate concentration was determined, εmM(727nm)= 87.
2.9) Determination of γ-GC and GSH synthase activities.
Frozen lens was rapidly divided with a cork borer (1cm. inner diameter) to obtain a longitudinal section, which was dissected in six transverse sections of about 1mm. Each of them was weighed, homogenized in a Potter Elvejehm homogenizer in 1 ml of 20 mM Tris sulphate buffer pH 7,6 and centrifuged at 10000 x g for 45 minutes. The supernatant was extensively dialyzed overnight against the same buffer for the assay activity of γ-GC synthase and GSH synthase. γ-GC synthase activity and GSH synthase activity were assayed by the inorganic phosphate assay (Rathbun and Betlach, 1969). The γ-GC synthase assay contained 15 mM L-glutamic acid, 5 mM L-cysteine, 2 mM ATP, 50 mM MgSO4 and 100 mM Tris sulphate buffer pH 7,6. The GSH synthase assay contained 2,5 mM γ-glutamylcysteine, 30 mM glycine, 2mM ATP, 5 mM MgSO4, 100 mM KCl and 100 mM Tris sulphate buffer pH 7,6. Phosphate concentration was evaluated according a reference curve obtained using phosphate inorganic as the standard in the same conditions of the assays. An enzyme unit for both these systems was defined as the amount of the enzyme that cleaved one µmole of ATP/min at 37°C.
2.10) SDS–PAGE.
Electrophoresis was performed on 12% polyacrilammyde gel according to Laemmli method (Laemmli U.K. 1970). Mini protean kit was used as support for gel preparation. Gels were run to a constant strength current of 20 mA per gel, with a running time of 55 minutes. Samples were prepared diluting them to a protein content ranging from 2 µg to 200 µg in sample buffer and boiling them at 100°for 10 minutes. Bio Rad low molecular weight standards were used when the gel was subjected to silver staining, while benchmark pre-stained molecular weight standard were used, when western blot analysis followed electrophoretic run.
2.11) Silver staining.
Silver staining was performed according to Wray method (Wray et al., 1981). Gel after run has been incubated for one hour in de staining solution of 50% (v/v) aqueous methanol, after extensively washing with milli-Q water, after it has been incubated for 15 minutes with a solution of silver diamine, after solution discharging, and then extensively washed with mill-Q water. The final developing of the staining was obtained soaking the gel in a aqueous solution of 238 µM citric acid and 0,018% formaldehyde until the complete appearance of protein bands. The developing was blocked using of 50% (v/v) aqueous methanol solution.
2.12) Western blot: transfer.
Western blot analysis was performed after protein separation on SDS-PAGE. Then proteins were transferred to a PVDF membrane, transfer was performed at 90 V for 80 minutes. Bench™ Mark pre-stained protein ladder was used as molecular weight standard.
-2.12a) Classical immunostaining: PVDF membrane was blocked, using a 5% (w/v)
skimmed milk solution in PBS + 0,05% (w/v) Tween-20, upon incubation time of 1 hour. The membrane was washed with PBS+ 0,05% (w/v) Tween-20, then probed with Abnova anti CGL monoclonal antibody (1 µg /ml in PBS+ 0,05% (w/v) Tween-20 + 1% skimmed milk.). The detection was performed using an IgG anti-mouse secondary antibody
peroxidise conjugate (protein content 7 mg/ml diluted 1:2000), incubation time 1 hour . Membrane was developed with ECL system. Detection of protein band was performed using autoradiographic film.
-2.12b) Immunostaining with Snap system device (Millipore): after transfer PVDF
membrane was placed on a peculiar support called SNAP system that was connected to a vacuum pump. Then the membrane was blocked using a 0,5% not fat milk solution in PBS 1X+ 0,1% Tween 20. Without intermediary washing the membrane was probed with Ab nova anti CGL monoclonal antibody (0,7 µg /ml in PBS+ 0,1% (w/v) Tween-20 + 0,5 % not fat milk) with an incubation time of 10 minutes. Membrane was washed with 3 volumes of PBS+ 0,1% (w/v) Tween-20 (washing buffer). The detection was performed using an IgG anti-mouse secondary antibody peroxidase conjugate (protein content 7 mg/ml diluted 1:2000), time of incubation 10 minutes. Finally before developing, membrane was washed with washing buffer 3 volumes. Both in case of antibodies and wash buffer the volume used depends on the size of blot holder used. Membrane was developed with ECL system, detection of protein band was performed by using autoradiographic film.