CHAPTER 2
ROSMARINIC ACID CONTENT IN BASIL PLANTS
GROWN IN VITRO AND IN HYDROPONICS
2.1 INTRODUCTION
Sweet basil (Ocimum basilicum L.) in the Lamiaceae is one of the most important herbs, as it is widely cultivated worldwide (Makri and Kintzios, 2007). Basil leaves are largely employed as a flavoring agent for food. For instance, sweet basil is the main ingredient of the well-known ‘pesto’ sauce and in Italy the cultivation of this species increased considerably in the last years due to the growing demand from the food industry (De Masi et al., 2006). Along with other species in the Ocimum genus, sweet basil is used for pharmaceutical and cosmetical preparations due to the high content of essential oils (Makri and Kintzios, 2007; Zheljazkov et al., 2008) and rosmarinic acid (Javanmardi et al., 2002; Juliani et al., 2008). Rosmarinic acid (RA) is a caffeic acid ester with several important biological properties, including antioxidantand, antibacterial, antiviral and anti-inflammatory activities (Petersen and Simmonds, 2003).
Medicinal and herbal plants, including basil, are typically cultivated in open fields, resulting in year-to-year variability in the biomass production as well as in the content of secondary metabolites, which are both affected by many factors such as weather, soil fertility, growing practices and the presence of pests and diseases (Orcutt and Nilsen, 2000). Therefore, there is an increasing interest for the artificial cultivation of these crops, either in vitro or in vivo (i.e. greenhouse hydroponic culture), where growing conditions can be strictly controlled to produce high-standard plant material all-year round (Canter et al., 2005; Karuppusamy, 2009). Sweet basil can be grown in hydroponics, which offers several advantages over traditional soil culture, such as higher yield per unit ground area and better quality standards of harvested biomass. Hydroponically grown plant material is clean and easy to process due to minimal contamination from pollutants, pests and pathogens
(Pardossi et al., 2006). Floating systems is an hydroponic technique that is increasingly used for greenhouse production of fresh-cut leafy vegetables, including basil (Miceli et al., 2003), and has also been utilized for the cultivation of medicinal plants (Dorais et al., 2001; Maggini et al., 2010).
Cell and tissue culture, or in vitro approaches, constitute an alternative to conventional agriculture for the production of high-value plant metabolites, because they provide an opportunity to regulate plant biosynthetic pathways in a controlled environment (Matkowski, 2008; Karuppusamy, 2009). The possible application of plant tissue culture for the production of bioactive compounds has been demonstrated in several species (Matkowski, 2008; Karuppusamy, 2009), although at present only very few substances are produced in vitro on a commercial scale (Weathers et al., 2010). Different basil species were found to accumulate larger quantities of RA in cell, callus, hairy roots and shoot cultures than in vivo (Bais et al., 2002; Kintzios et al., 2003, 2004; Rady and Nazif, 2005). The total phenolic and RA levels were higher in sweet basil grown hydroponically then in soil-grown plants (Sgherri et al., 2010).
Compared to in vitro culture, hydroponics offers the advantages of an higher rate of biomass production per unit area and less expensive growing structures (Ahloowalia, 2004; Montero et al., 2009), although the production cost per unit weight of the metabolites of interest is not necessarily lower in vivo than in vitro (Chaterjee et al., 2010).
In this work, the accumulation of caffeic acid derivatives (CADs), in particular RA, was studied in three cultivars of sweet basil, with green (Genovese and Superbo) or purple (Dark Opal) leaves, grown either in vitro or in hydroponic culture. To our knowledge, no paper has been published on the influence of micropropagation phase on the accumulation of these metabolites in basil.
2.2 MATERIALS AND METHODS
PLANT MATERIALThe plants were originated from seeds purchased from SAIS (Cesena, Italy) and grown at University of Pisa either in vitro or in hydroponics (floating system).
IN VITRO EXPERIMENTS
An original protocol for the micropropagation was developed previously.
Nodal segments were excised, at the time of flowering, from basil seedlings grown in floating system under greenhouse conditions. To prevent pathogen contamination, the mother plants were sprayed weekly with Benomyl (1.0 g L−1; Du Pont Agricultural products, Wilmington, Delaware, USA) in the last three weeks before the collection of explants.
The explants were reduced in size, washed in current water for 30 min, surface-sterilized by soaking them under agitation in a 15% aqueous solution of NaOCl (8% active chlorine) with a few drops of Tween 20TM (Sigma-Aldrich, Milan, Italy) for 15 min and rinsed three times with sterile deionized water. The explants were then cut in single 1-cm long nodal segments under laminar flow cabinet. Each explant was placed horizontally in 30-ml polycarbonate UryTM vials (PBI, Milano, Italy) with 5 ml of MS (Murashige and Skoog) (Murashige and Skook, 1962) with 30 g L-1 of sucrose, 300 mg L-1 of reduced gluthatione (GSH) and 7 g L-1 agar. 2-(N-morpholino) ethanesulfonic acid (MES) was added (500 mg L-1 ) to stabilize the pH, which was adjusted to 5.8 before autoclaving at 121°C for 15 min. MES concentration (2.3 mmol L-1) was much lower than the levels that were found to be toxic to callus culture (10 mmol L-1) (Parfitt et al., 1988) or to affect secondary metabolism in Catharanthus roseus hairy roots (50 mmol L-1) (Morgan et al., 2000). During shoot initiation, 0.25 mg L-1 6-benzylaminopurine (BA) was added to the basal medium and the explants were placed in a climatic chamber at 25 ±1°C with a 16 h photoperiod and an irradiance of 70 mol s-1 m-2 from cool fluorescent tubes. In
the multiplication phase, nodal explants were sub-cultured every four weeks using PCCV25TM boxes (TQPL Co., New Milton, United Kingdom) with 50 ml of solid growth medium (eight explants per box). Root formation in nodal explants (1 cm long, excised from shoots at the end of the multiplication phase) was induced in PCCV25TM boxes containing 100 ml of perlite soaked with 50 ml of half-strength MS nutrient solution without growth regulators (eight explants per box).
Finally, well rooted plantlets were acclimatized in 4-cm height LinfaboxTM boxes (Micropoli, Milano, Italy) containing 200 ml of sterilized peat-perlite mixture (1:1, v:v); five plantlets were transplanted in each box. The containers were wrapped with plastics and incubated in a growth chamber at 20 ±3°C at 16 h of photoperiod with 100 mol s-1 m-2 PAR (from HPS lamps). Plastic covers were partially opened after one week and completely removed the subsequent week. Acclimatization concluded after four weeks, when the survived (ex vitro) plants were transferred to a greenhouse.
We conducted two experiments using this previously-described protocol. In the first experiment, growth and tissue concentration of CADs and pigments (anthocyanins and chlorophyll) were determined in shoot explants of cv. Genovese at the end of each micropropagation phase. In the second experiment, we investigated the effect of BA concentration (0.1, 0.25, 0.5 and 1 mg L-1) on the rate of shoot proliferation and the content of CADs and pigments in all genotypes, at the end of the multiplication phase.
HYDROPONIC CULTURE
Seeds were germinated in rockwool tray plugs in a growth chamber (25 ± 1°C; 250 mol s-1 m-2 PAR; 12 h photoperiod) and seedlings were transferred at the second-leaf stage to a glasshouse under natural temperature and light conditions. Two weeks after sowing, the plants were transferred in 12 separate hydroponic systems, each consisting of a polystyrene plug tray floating in a plastic tank with fairly stagnant nutrient solution (300 L m-2), which was continuously aerated (oxygen content > 6.0 mg L-1). Crop density was 40 plants per tank, corresponding roughly to 160 plants
m-2 (ground area). The nutrient solution contained the following concentration of macro- and micro-nutrients: 4.0 mol m-3 N-NO3, 1.0 mol m-3 N-NH4, 0.5 mol m-3 P-H2PO4, 2.5 mol m-3 K, 3.0 mol m-3 Ca, 1.0 mol m-3 Mg, 0.5 mol m-3 S-SO4, 40.0 mmol m-3 Fe, 40.0 mmol m-3 B, 5.7 mmol m-3 Cu, 17.6 mmol m-3 Zn, 10.0 mmol m-3 Mn, 1.0 mmol m-3 Mo. Electrical conductivity (EC) of nutrient solution oscillated between 1.55-1.80 dS m-1 and pH was maintained between 5.5 and 7.0 by frequent adjustment with diluted sulphuric acid.
Climatic parameters were continuously monitored by means of a weather station located inside the greenhouse. The minimum and ventilation air temperature were 16 and 27 °C, respectively; maximum temperature reached up to 30–32 °C during sunny hours in early summer. Daily global radiation and mean air temperature averaged, respectively, 12.0 MJ m-2 and 25.1 °C.
Plants were sampled during the vegetative and flowering stage (two and four weeks after transplanting, respectively) for growth and chemical analysis. Plant height and the fresh and dry weight of leaves, stems and roots were measured in each sample consisting of one individual plant. All the roots and the leaves (apart from a few basal leaves, if senescent) were sampled and a representative aliquot was extracted for the determination of CADs and pigments.
PHYTOCHEMICAL ANALYSIS
Plant samples from in vitro or hydroponic cultures were rapidly washed in tap water, rinsed in deionised water and gently dried with a towel. A sub-sample of approximately 0.5 g FW was frozen in liquid nitrogen and stored at -80 °C before laboratory analysis, which was performed within a few weeks following sampling. The concentration of CADs in each sample was determined as reported by Maggini et al. (2010). Briefly, frozen tissue was ground in a mortar with 5 ml extraction solvent (MeOH:H2O:HCl 70:29:1 v/v) and sieved into plastic tubes. Samples were shaken for 4 h on a magnetic stirrer and then centrifuged for 8 min at 5000 rpm. Following centrifugation, the supernatant was collected in plastic tubes and stored
supernatants were combined for analysis. All extracts were filtered with Chromafil® 0.45 μm cellulose mixed esters membrane, 25 mm diameter syringe filters (Macherey-Nagel, Düren, Germany) prior to HPLC separations.
HPLC grade solvents and the following chemically pure standards were used: chlorogenic acid, caffeic acid, ferulic acid, t-cinnamic acid, p-coumaric acid (Sigma– Aldrich, Milano, Italy) and RA (Extrasynthese S.A., Genay, France). HPLC analytical equipment was composed of a Jasco (Tokyo, Japan) PU-2089 four-solvent low-pressure gradient pump and an UV-2077 UV/Vis multichannel detector. Analyses were performed using a Macherey–Nagel C18 250/4.6 Nucleosil® 100-5 column, at a flow rate 1 ml min-1, equipped with a guard column, using acetonitrile (solvent A) and aqueous 0.1% phosphoric acid (solvent B) as elution solvents. The gradient elution was programmed as follows: 0.0-0.4 min, B 95%; 0.4-0.5 min, B 95-85%; 0.5-10 min, B 85-80%; 10-20 min, B 80-60%; 20-21 min, B 60-5%; 21-25 min, B 5%; 25-26 min, B 5-95%; 26-30 min, B 95%. Detection was made at four wavelengths: 325 nm, 280 nm, 300 nm and 350 nm. The injection volume was 20 μL and the analyses where performed at room temperature (23–29 °C). The substances of interest were identified by comparing their retention times with those of reference standards and quantified on the basis of the integrated peak area, as compared with a standard curve.
The content of CADs was expressed per gram DW on the basis of the dry matter content determined in an aliquot of each samples after desiccation in ventilated oven at 85°C (to reach a given weight). The detection limit of the analytical protocol was on the order of 0.05 mg g-1 DW.
Peak identification was accomplished by LC-MS and LC-MS-MS using a PE Sciex API 365 triple quadrupole mass spectrometer LC-MS System (Concord, ON, Canada), equipped with a Turbospray source and coupled to a 200 Series HPLC system with quaternary pump and autosampler. The separations were carried out with the same column used for HPLC analysis. In order to improve the analytical sensitivity, phosphoric acid was replaced by formic acid because the former produces
a strong suppression effect under electrospray ionization conditions (Wolfender, 2009); this modification did not alter the elution pattern. The identification of selected CADs was confirmed by comparison with authentic standards showing the same retention times and MS-MS fragmentation patterns.
Total content of chlorophyll and anthocyanins was determined spectrophotometrically and expressed on a DW basis. Leaf samples (20 mg) were extracted with 2 ml ethanol 95% (v/v) overnight at 4°C in the dark. The extracts were centrifuged (3 min at 5000 rpm) and subsequently analyzed at 666.2 nm and 654.6 nm. Clorophyll concentrations were calculated according to Lichtenthaler’s (1987). For anthocyanin analysis, methanol 80% (v/v) containing HCl 1.2 M was used for extraction and the concentration of cyanidin-3-glucoside equivalents was determined by measuring the absorbance of the extracts at 535 nm.
STATISTICAL ANALYSIS
The experimental design was completely randomized. Data were subjected to a one-way or two-one-way analysis of variance (ANOVA) and the means were separated using the Tukey’s test. Each experiment was repeated two or three times with similar results and those from a representative are reported in this paper.
2.3. RESULTS
QUANTIFICATION OF CADS IN BASIL TISSUES
Preliminary experiments were conducted in order to validate the HPLC method adopted for this study and to identify the major CADs contained in frozen, not dried, or oven-dried (70°C) roots and leaves of sweet basil plants grown in hydroponic culture and/or in vitro.
Among the six CADs of interest, only RA was found in considerable concentration in all analyzed samples while the other metabolites were present in trace quantities (e.g. ferulic and caffeic acid) or below the detection limits (0.05 mg g-1 DW). The
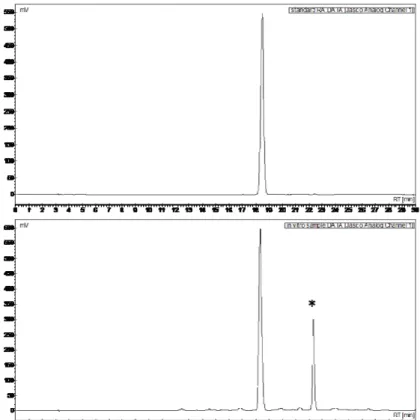
typical chromatogram of methanolic extract of basil sample showed two main peaks, one of which was identified as RA by means of LC-MS analysis while the other corresponded to a methylated derivative of this substance (Fig. 2.1). LC-MS-MS analysis suggested that methylation was present on the caffeic acid moiety.
Figure 2.1. Typical HPLC chromatogram of pure standard of rosmarinic acid (RA, top) and HCl-methanolic extract of in vitro shoots of sweet basil (O. basilicum L.) (bottom). The peak marked with asterisk in the bottom chromatogram was identified by LC-MS as a methylated form of RA.
Frozen, not dried samples of all types of tissues contained invariably much more RA and the methylated derivative compared to the dried ones (data not shown). Therefore, in the experiments with both in vitro and in vivo plants, only frozen, not dried samples were analyzed.
Only trace amounts of chlorogenic acid, caffeic acid, ferulic acid, t-cinnamic acid and p-coumaric acid were detected in the analyzed samples, while the methylated form of RA was detected at significant concentrations in all samples, although a quantitative determination of this compound was not performed.
IN VITRO CULTURE
In all tissue culture experiments, the plantlets developed normally and exhibited well-formed shoots without hyperhydric symptoms in each phase of micropropagation (Fig. 2.2). All ex vitro plants survived and adapted well to greenhouse cultivation.
Figure 2.2. Micropropagation of sweet basil (O. basilicum L., cv. Genovese): in vivo (hydroponic) cultivation of mother plants (A); shoot bud induction on MS + 0.25 mg L-1 BA (B); shoot proliferation on MS + 0.25 mg L-1BA (C); rooting of nodal segments on ½ MS (D); acclimatized plants (E).
Total dry mass of in vitro plantlets (on average, 50.34 mg each) and the length of newly-formed shoots (1.05 cm) were similar at the end of induction and multiplication phase, whereas shoot number was higher in the latter stage (Table 2.1). Dry mass and shoot formation were lower during rooting than in any other culture phase (Table 2.1). Acclimatized plantlets exhibited the highest dry biomass of all stages sampled.
Table 2.1. Growth parameters of sweet basil (O. basilicum L., cv. Genovese) in vitro plantlets at the end of each micropropagation phase. Each phase lasted four
weeks. Mean values of 15 replicates, each consisting of one plantlet. One-way ANOVA was performed. Values followed by different letter differs significantly (P ≤ 0.05).
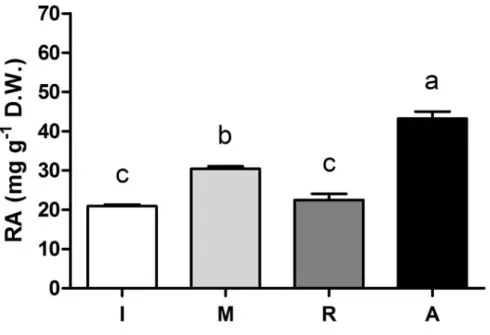
Rosmarinic acid was detected in in vitro plantlets during all phases of micropropagation (Fig. 2.3). The highest RA content (43.21 mg g-1 DW) was found in acclimatized plantlets, although the highest production of RA under genuine in
vitro conditions was detected in the explants at the multiplication phase.
In the second experiment, which was carried out with all three cultivars, we demonstrated that the proliferation rate of explants could be increased by modulating the BA concentration in the medium. No clear effects of the tested BA concentrations (0.10, 0.25, 0.50 and 1.00 mg L-1) were found on the number and the length of regenerated shoots, and on the total dry weight of individual plantlets (data
Phase Shoot number Shoot length (cm) Plant height(cm) Fresh weight (g) Dry weight (mg) Induction 1.88b 0.96bc 1.80c 0.598a 50.68b Multiplication 2.57a 1.14ab 2.52b 0.602a 50.00b Rooting 1.09c 1.41a 1.65c 0.169b 11.50c Acclimatization 1.20c 0.55c 3.80a 0.742a 82.77a
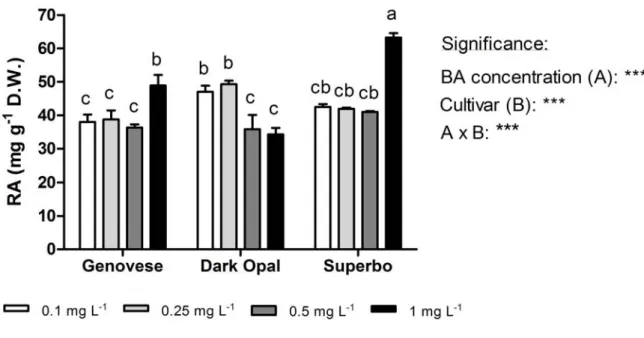
not shown), which averaged 2.14 shoot per explants, 1.35 cm and 48.16 mg, respectively. However, a strong genotype-dependent effects of BA was observed on RA production. In green-leaf cultivars, the highest RA contents were observed in the plantlets grown with 1.0 mg L-1 BA in the medium, while in purple-leaf Dark Opal RA concentration decreased with increasing BA level (Fig. 2.4).
Figure 2.3. The content of rosmarinic acid (RA) in vitro plantlets of sweet basil (O. basilicum L. cv. Genovese) sampled at the end of each micropropagation phase. Each
phase lasted four weeks: Induction (I); Multiplication (M); Rooting (R); Acclimatization (A). Mean values of four replicates, each consisting one single plantlet. One way ANOVA was performed. Values followed by different letters differ significantly (P ≤ 0.05).
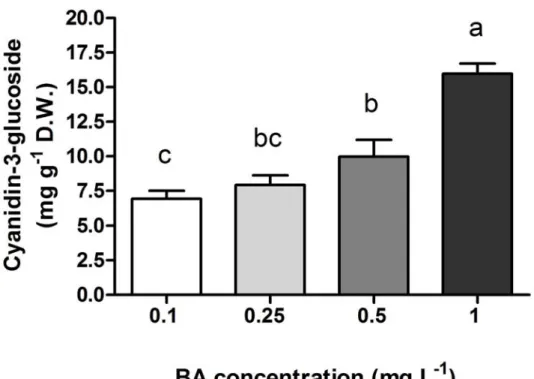
The content of anthocyanins was much lower in the green-leaf cultivars (0.61 mg g-1 DW, on average) than in Dark Opal (on average, 8.59 mg g-1 DW) and, in the latter cultivar, increased with BA concentration (Fig. 2.5). The chlorophyll content was not significantly affected by plant genotype and BA level, averaging 9.92 mg g-1 DW.
Figure 2.4. The effect of BA concentrations in the growing medium on the content of rosmarinic acid (RA) in different cultivars (Genovese, Dark Opal and Superbo) of sweet basil (O. basilicum L.) in vitro plantlets at the end of multiplication phase, which lasted four weeks. Mean values of four replicates, each consisting of one single plantlet. The effects of BA concentration (A) and genotype, and their interaction (A x B), were significant at P ≤ 0.001 according to ANOVA. Values followed by different letters differ significantly (P ≤ 0.05).
Figure 2.5. The effect of BA concentrations in the growing medium on the content of anthocyanins (Cy-3-Glc equivalents, measured spectrophotometrically) in the shoots of sweet basil (O. basilicum L. cv. Dark Opal) grown in vitro and sampled at the end of the multiplication phase, which lasted four weeks. Mean values of four replicates, each consisting of one single plantlet. One way ANOVA was performed. Values followed by different letters differ significantly (P ≤ 0.05).
HYDROPONIC CULTURE
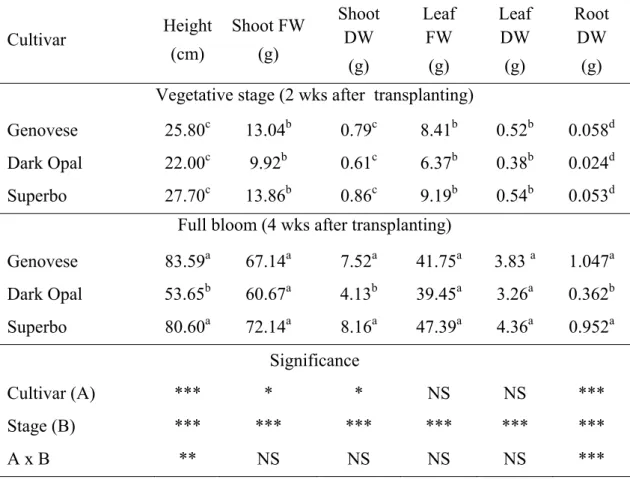
In the floating systems, plants grew healthy and vigorously and flowered abundantly within one month after planting (Fig. 2.6). Fresh and/or dry matter (Table 2.2) accumulation in shoots, leaves and roots of hydroponically-grown plants was much higher at the flowering stage compared to the vegetative stage. Results indicate that four to five crops per year could be performed with an estimated annual leaf fresh biomass production of about 30 kg m-2. The root to shoot DW ratio increased approximalely two-fold at flowering stage in all the cultivars.
At the vegetative stage, there were no significant differences among the cultivars in terms of plant height and total dry mass. However, the purple-leaf Dark Opal plants were much smaller than the green-leaf genotypes (Genovese and Superbo) when sampled at the flowering stage (Table 2.2).
Figure 2.6. Hydroponic cultivation (floating system) of different cultivars (Genovese, Dark Opal and Superbo) of sweet basil (O. basilicum L.): plants at full bloom, which typically occurred four weeks after planting (A); the typical leaves of cv. Genovese (B), cv. Dark Opal (C), cv. Superbo (D); the typical inflorescences of the cultivars with purple leaves (cv. Dark Opal; E) or green leaves (cv. Genovese and Superbo; F).
Table 2.2. Growth parameters of different cultivars (Genovese, Dark Opal and Superbo) of sweet basil (O. basilicum L.) plants grown hydroponically and sampled during vegetative stage and at full bloom. The shoots included inflorescences when sampled at flowering stage. Mean value of 15 replicates, each consisting of one individual plant. Values followed by different letters differ significantly (P ≤ 0.05). Two-way ANOVA was performed (* P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; NS = not significant).
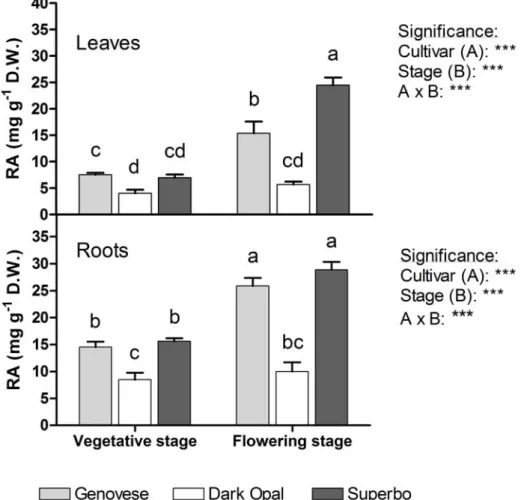
Rosmarinic acid content increased at the flowering stage in both leaves and roots of Genovese and Superbo plants compared to the vegetative stage, with levels reaching 28.88 mg g-1 DW (Fig. 2.7). In contrast, no differences in RA levels between developmental stages were observed in Dark Opal plants, which on average contained much less RA than the other genotypes (4.87 vs 13.60 mg g-1 DW in the leaves and 9.29 vs 21.23 mg g-1 DW in the roots). A significant difference in RA levels between Genovese and Superbo was found only in the leaves of blooming
Cultivar Height (cm) Shoot FW (g) Shoot DW (g) Leaf FW (g) Leaf DW (g) Root DW (g) Vegetative stage (2 wks after transplanting)
Genovese 25.80c 13.04b 0.79c 8.41b 0.52b 0.058d Dark Opal 22.00c 9.92b 0.61c 6.37b 0.38b 0.024d Superbo 27.70c 13.86b 0.86c 9.19b 0.54b 0.053d
Full bloom (4 wks after transplanting)
Genovese 83.59a 67.14a 7.52a 41.75a 3.83 a 1.047a Dark Opal 53.65b 60.67a 4.13b 39.45a 3.26a 0.362b Superbo 80.60a 72.14a 8.16a 47.39a 4.36a 0.952a Significance Cultivar (A) *** * * NS NS *** Stage (B) *** *** *** *** *** *** A x B ** NS NS NS NS ***
plants, which was considerably higher in the latter cultivar (Fig. 2.7). The level of RA was much higher in the roots (on average, 17.64 mg g-1 DW ) than in the leaves (10.69 mg g-1 DW) regardless of the cultivar and the physiological stage, with the exception of flowering Superbo (Fig. 2.7). There were no differences among the cultivars in chlorophyll content, which averaged 16.65 μg g-1 DW. However, the leaves of Dark Opal seedlings contained much more anthocyanins than those of other cultivars (on average, 13.15 mg g-1 DW against 0.89 mg g-1 DW).
Figure 2.7. The content of rosmarinic acid (RA) in leaves and roots of different cultivars (Genovese, Dark Opal and Superbo) of sweet basil (O. basilicum L.) plants grown hydroponically and sampled during vegetative stage and at full bloom. Vegetative and flowering plants were sampled two and four weeks after transplanting, respectively. Mean values of four replicates, each consisting of one single plant. The effects of cultivar (A) and physiological stage (B), and their interaction (A x B), were significant at P ≤ 0.001 according to ANOVA. Values followed by different letters differ significantly (P ≤ 0.05).
2.4. DISCUSSION
CADs QUANTIFICATION
Many authors found several CADs in basil tissues although, in many cases, RA was the most abundant one (Javanmardi et al., 2002; Juliani et al., 2008). In contrast, in our samples only RA was present at concentration well above the detection limit of the analytical method used in this study; all other CADs were found in trace quantities regardless of growing system and plant tissue. In addition, we observed a peak in all samples that LC-MS analysis attributed to a methylated form of RA. In many samples, the peak area corresponding to this compound was even higher than the one of RA (data not shown).
Methyl rosmarinate (MeRA) has been detected in several plants species, particularly within the Lamiaceae (Jiang et al., 2005; Fecka and Turek, 2007), however, to our knowledge no paper has been published reporting the presence of MeRA in basil tissues. In the present study, LC-MS-MS analysis excluded the correspondence of the unknown peak with MeRA because the methylation was present on the caffeic acid moiety. Similarly to our work, a later-eluting RA methylated derivative was found in basil cell suspensions by Strazzer et al. (2011). Since RA methylation can occur both
in vivo and during extraction with methanol, Strazzer et al. (2011) quantified RA in
samples by considering both the area of the RA peak and that of its methylated form.
In vitro studies showed that the antioxidant capacity of RA alkyl derivatives could be
higher than RA (Laguerre et al., 2010) and further work is in progress to more thoroughly characterize the methylated form of RA and to verify whether its production is a result of genuine biosynthesis or artifactual methylation during methanol extraction.
The high levels of RA detected in our study (approximately, from 4 to 60 mg g-1 DW), were within those reported in the literature for sweet basil tissues, which range from less than 0.1 mg g-1 DW (Kintzios et al., 2004; Sgherri er al., 2010) to nearly 100 mg g-1 DW (Javanmardi et al., 2002). This big variability is probably a
consequence of differences of plant genotype, growing conditions as well as of the method used for the quantification.
In a preliminary work, RA content was much lower in samples that had been desiccated at 70°C. Sample heating was found to reduce the root content of echinacoside in Echinacea angustifolia (Kabganian et al., 2003) and Echinacea
pallida (Li and Wardle, 2001) due to volatilization or thermal decomposition.
Consequently, only frozen, not dried, samples were processed and analyzed in our reported experiments.
IN VITRO CULTURE
In experiment 1, the proliferation aptitude of explants, as indicated by the number and the length of the shoots at the end of multiplication stage, was lower compared with those found in similar studies with sweet basil (Sahoo et al., 1997; Begum et al., 2002). Nevertheless, our protocol resulted in biomass production on the order of 50 mg DW per plantlet (Table 2.1), which was nearly 50-fold higher than that observed by Kintzios et al. (2004) in nodal segments cultured in a bioreactor. The lowest shoot dry weight was found in rooted plantlets (Table 2.1). Root formation may have occurred at the expense of shoot development as found in other plant species grown
in vitro (e.g. Lucchesini and Mensuali-Sodi, 2004).
Ex vitro plantlets acclimatized successfully, with considerable growth (1.56 mg per
plantlet per day) measured in the four weeks following the transfer out of in vitro vessels, although newly-formed lateral shoots were shorter compared to those developed in the in vitro phases (Table 2.1). Ex vitro conditions in combination with the absence of cytokines application likely restored apical dominance, enhancing plant elongation and reducing the development of lateral shoots.
The highest RA content (43.21 mg g-1 DW) was found at the end of acclimatization (Fig. 2.3). Increased RA accumulation at this stage was likely a response to the stress induced by ex vitro conditions (Hazarika, 2006) which stimulated secondary metabolism. It is well known that the synthesis of CADs is enhanced by both biotic and abiotic stress (Dae et al., 2009). The accumulation of considerable amounts of
biomass (Table 2.1) and the high RA content (30.45 mg g-1DW; Fig. 2.3) indicates the feasibility of in vitro shoot culture for the production of RA in sweet basil.
In experiment 2, we did not observe any significant effect of BA on shoot proliferation. In contrast, previous studies testing BA concentrations similar to those used in our study have demonstrated a strict correlation between shoot proliferation and the level of BA in the medium for O. basilicum (Begum et al., 2002; Siddique and Anis, 2008). The influence of cytokines on shoot initiation in in vitro culture is affected by many factors, including the growing protocol and plant genotype (Van Staden et al., 2008), which may explain the differences between our findings and those reported by other authors.
The hormone composition of the growth medium may affect the synthesis of secondary metabolites in vitro (Matkowsky, 2008; Lucchesini et al., 2009). For instance, addiction of cytokines to the culture medium enhanced the accumulation of alkamide and CADs in Echinacea angustifolia (Lucchesini et al., 2009). In this work, we observed the most significant differences in RA content among the three cultivars at the highest BA concentration (Fig. 2.4). Previous research has also shown a large variability in RA accumulation of different basil cultivars in culture (Nguyen and Niemeyer, 2008). Increasing BA concentrations resulted in an increase of RA content in green-leaf cultivars, which coincides with previous findings (Fig. 2.4). Rady and Nazif (2005) observed that RA content increased in in vitro shoots of Ocimum
americanum L. with BA concentration up to of 1 mg L-1. In contrast to green-leaf
basil genotypes, RA content decreased in purple-leaf Dark Opal at BA concentrations above 0.5 mg L-1. Anthocyanin accumulation increased in response to BA addition in Dark Opal plantlets (Fig. 2.5), which agrees with previous findings in other species such as Catharanthus roseus, Celosia argentea and Cordyline
terminalis (Taha et al., 2008).
Regardless of genotype, the concentration of RA in O. basilicum shoot tissues (40 to 60 mg g-1 DW) exceeded the levels previously reported by Kintzios et al. (2003, 2004) and by Moschopoulou and Kintzios (2011), who used different in vitro
systems (e.g. callus, cell suspension and immobilized cells) and growth regulators (e.g. NAA, 2,4D). These findings suggest that the pattern of RA accumulation in
vitro is not predictable. On the other hand, increased synthesis of secondary
metabolites is often associated with cell or tissue differentiation, such as aggregation of cell suspensions or the formation of more complex structures such as adventitious roots or shoots (Collin, 2001) which may explain the accumulation of high amount of RA in cultured shoots in our study.
HYDROPONIC CULTURE
The floating system was found to be an efficient approach for producing large amount of plant material that can be easily processed on an industrial scale (Table 2.2) in agreements with previous findings (Miceli et al., 2003; Pardossi et al., 2006). One of the advantages of hydroponic growth system is that it facilitates harvesting of root tissue, which accounted for 10% of total dry mass (Table 2.2) and had significantly higher RA concentrations than shoot tissues (Fig. 2.7). Previous studies on members of Lamiaceae also reported higher RA content in the roots compared to the leaves (Bais et al., 2002; Karam et al., 2003). The antimicrobial properties of RA may represent a constitutive defense of the plants against microbial infection, playing an important role in the root-pathogen interaction (Bais et al., 2002).
Shoot and root concentrations of RA ranged between 4 and 29 mg g-1 DW, and were within those reported in previous studies (Javanmardi et at., 2002; Nguyen and Niemeyer, 2008). The highest levels of RA were detected at full bloom in both leaves and roots (Fig. 2.7). Juliani et al. (2008) reported that leaf concentration of RA in basil increased during flowering with respect to the vegetative stage, although to a lesser extent in Dark Opal plants than in the green-leaf genotypes under investigation. Similarly, the highest content and the best composition of essential oils are generally found in basil plants at full bloom (Zheljazkov et al., 2008). In contrast, in other Lamiaceae, such as rosemary (Papageorgiou et al., 2008), spearmint and peppermint (Fletcher et al., 2010), leaf RA content was lower at the flowering stage than at the vegetative stage.
Contrasting results are reported in the literature regarding RA content in basil cultivars with different leaf color. In our study, leaf and root concentrations of RA in the purple-leaf cultivar Dark Opal were lower than in the green-leaf cultivars (Fig. 2.7), in agreement with Javanmardi et al. (2002). Opposite results were reported by Juliani et al. (2008) and Nguyen and Niemeyer (2008), who found higher RA levels in Dark Opal plants than in other cultivars with green leaves.
IN VITRO VS HYDROPONIC CULTURE
In vitro grown shoots, especially those of the Dark Opal cultivar, contained much
higher levels of RA (Fig.s 2.3, 2.4) than did the plants in hydroponic culture (Fig. 2.7). In experiments with various Ocimum species, tissues or cells cultured in vitro accumulated RA to levels that were 3 to 11-fold higher than in donor plants (Kintzios, 2003; Hakkim et al., 2007). Secondary metabolism may be altered by artificial environmental conditions provided by in vitro culture and by the composition of growing medium (Van Staden, 2004; Matkowski, 2008).
The lower content of both chlorophylls and anthocyanins in basil tissues grown in
vitro compared to hydroponically-grown plants was likely a result of the presence of
sugars in the medium as well as of the reduced irradiance inside the cultivation vessels. Exogenous supply of sucrose reduced chlorophyll content in Solanum
tuberosum (Kovač and Ravnikar, 1998). Anthocyanins are able to reduce photo-oxidative injury in leaves and their concentration is generally reduced when the plants are grown under low irradiance (Neill and Gould, 2003). Moreover, the reduction of gas exchanges in cultivation vessels could lead to an accumulation of ethylene and CO2, which stimulates the degradation of photosynthetic pigments (Lucchesini et al., 2006).
When in vivo and in vitro plants, green and purple-leaf genotypes or the Dark Opal plantlets cultured in vitro at different BA concentrations in the basal medium are compared in terms of RA and anthocyanins content, it emerges a negative relation between the accumulation of these metabolites. A common substrate of RA and anthocyanins pathways is 4-coumaroyl-CoA (Petersen et al., 2010) and a competition
between their individual biosynthesis could be hypothesized, as also suggested by the higher RA content in green- vs red- varieties of Perilla frutescens (Yamazaki et al., 2003). However, in cell suspensions of Dark Opal basil Strazzer et al. (2011) found a positive correlation between the content of RA and anthocyanins.
In conclusion, the highest RA content were observed during the in vitro multiplication, in the acclimatized plants and in the roots of hydroponically-grown seedlings at full bloom. In vitro, BA reduced the accumulation of RA in purple-leaf Dark Opal cultivar, but increased RA accumulation in the green-leaf genotypes. Our findings suggest that RA production can be improved by utilizing in vitro or hydroponic culture of sweet basil.
The differences in RA and anthocyanins content observed in vivo and in vitro suggest a negative relationship between the accumulation of these compounds. Work is in progress to elucidate the possible interaction between the synthesis of these metabolites in basil tissues as well as the nature and the origin (genuine in vivo biosynthesis or artifact during the methanol extraction) of the methylated form of RA detected in our samples.
2.5. LITERATURE
Ahloowalia, B.S. (2010). Integration of technology from lab to land. In: Low cost options for tissue culture technology in developing countries. Proceedings of a Technical Meeting organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Vienna, 26–30 August 2002. Printed by IAEA, 2004; pp. 87-89. ISBN 92–0–115903–X. Available at: http://www-pub.iaea.org/MTCD/publications/PDF/te_1384_web.pdf (11 Aug 2010).
Bais, H.P., Walker, T.S., Schweizer, H.P., Vivanco, J.M. (2002). Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of
Ocimum basilicum. Plant Physiol. Biochem. 40: 983-995.
Begum, F., Amin, M.N., Azad, M.A.K. (2002). In vitro rapid clonal propagation of
Ocimum basilicum L., Plant Tissue Cult. 12: 27-35.
Canter, P.H., Thomas, H., Ernst, E. (2005). Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends in Biotech.
23: 180-185.
Chaterjee, A., Shukla, S., Mishra, P., Rastogi, A., Singh, S.P. (2010). Prospects of in vitro production of thebaine in opium poppy (Papaver somniferum L.). Ind. Crops Prod. 32: 668-670.
Collin, H.A. (2001). Secondary product formation in plant tissue cultures. Plant Growth Regul. 34: 19–134.
De Masi, L., Silviero, P., Esposito, C., Castaldo, D., Siano, F., Laratta, B. (2006). Assessment of agronomic, chemical and genetic variability in common basil (Ocimum basilicum L.). Eur. Food Res. Technol. 223: 273-281.
Dorais, M., Papadopoulos, A.P., Luo, X., Leonhart, S., Gosselin, A., Pedneault, K. (2001). Angers P., Gaudreau L., Soilless greenhouse production of medicinal plants in North Eastern Canada. In Bar-Tal A and Plaut Z. (Eds.), Acta Horticulturae., vol. 1, Ma'ale Hachamisha, Israel. Pp. 297-303.
Fecka, I., Turek, S. (2007). Determination of Water-Soluble Polyphenolic Compounds in Commercial Herbal Teas from Lamiaceae: Peppermint, Melissa, and Sage. J. Agric. Food Chem. 55: 10908–10917.
Fletcher, R.S., Slimmon, T., Kott, L.S. (2010). Environmental factors affecting the accumulation of rosmarinic acid in Spearmint (Mentha spicata L.) and Peppermint (Mentha piperita L.). The Open Agric. J. 4: 10-16.
Hakkim, F.L., Shankar, C.G., Girua, S. (2007). Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J. Agric. Food Chem. 55: 9109-9117.
Hazarika, B.N. (2006). Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 108: 105-120.
Javanmardi, J., Khalighi, A., Kashi, A., Bais, H.P., Vivanco, J.M. (2002). Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J. Agric. Food Chem. 50: 5878-5883. Jiang, R.W., Lau, K.M., Hon, P.M., Mak, T.C.W., Woo, K.S., Fung, K.P. (2005).
Chemistry and Biological Activities of Caffeic Acid Derivatives from Salvia
miltiorrhiza. Curr. Med. Chem. 12: 237-246.
Juliani, H.R., Koroch, A.R., Simon, J.E. (2008). Basil: A new source of rosmarinic acid. In: Ho C.T., Simon J.E., Shahidi F., Shao Y. (Eds). Dietary Supplements, American Chemical Society Symposium Series 987, Publisher: American Chemical Society, Washington, D.C. USA. Pp. 129-143.
Kabganian, R., Carrier, D. J., Soskansanj, S. (2003). Drying of Echinacea
angustifolia roots. J. Herbs Spices Med. Plants. 10: 11-18.
Karam, N.S., Jawad, F.M., Arikat, N.A., Shibli, R.A. (2003). Growth and rosmarinic acid accumulation in callus, cell suspension, and root cultures of wild Salvia
Karuppusamy, S. (2009). A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 3: 1222-1239.
Kim, H.J., Kwon, D.Y., Yoon, S.H. (2009). Induction of Phenolics and Terpenoids in Edible Plants Using Plant Stress Responses, In: Hou C.T., Shaw J.F. (Eds), Biocatalysis and Agricultural Biotechnology, CRC Press. Pp. 249-258. DOI: 10.1201/9781420077070.ch17 H.J.
Kintzios, S., Kollias, H., Straitouris, E., Makri, O. (2004). Scale-up micropropagation of sweet basil (Ocimum basilicum L.) in an airlift bioreactor and accumulation of rosmarinic acid. Biotechnol. Lett. 26: 521-523.
Kintzios, S., Makri, O., Panagiotopoulos, E., Scapeti, M. (2003). In vitro rosmarinic acid accumulation in sweet basil (Ocimum basilicum L.). Biotechnol. Lett. 25: 405-408.
Kovač, M., Ravnikar, M. (1998). Sucrose and jasmonic acid interact in photosynthetic pigment metabolism and development of potato (Solanum
tuberosum L. cv. Sante) grown in vitro. Plant Growth Regul. 24: 101-107.
Laguerre, M.L., Lopez Giraldo, L.J., Lecomte, J., Figueroa-Espinoza, M.C., Barea, B., Weiss, J., Decker, E.A., Villeneuve, P. (2010). Relationship between hydrophobicity and antioxidant ability of “phenolipids” in emulsion: a parabolic effect of the chain length of rosmarinate esters. J. Agric. Food Chem. 58: 2869– 2876.
Li, T.S.C., Wardle, D.A. (2001). Effects of root drying temperature and moisture content on the levels of active ingredients in Echinacea roots. J. Herbs Spices Med. Plants. 8: 15-22.
Lichtenthaler, H.K. (1987). Chlorophylls and carotenoids: pigments of photosynthetic membranes. Meth. Enzymol. 148: 350-382.
Lucchesini, M., Bertoli, A., Mensuali-Sodi, A., Pistelli, L. (2009). Establishment of in vitro tissue cultures from Echinacea angustifolia D.C. adult plants for the production of phytochemical compounds. Sci. Hort. 122: 484–490.
Lucchesini, M., Mensuali-Sodi, A. (2004). Influence of medium composition and vessel ventilation on in vitro propagation of Phillyrea latifoglia L.. Sci. Hort. 100: 117-125.
Lucchesini, M., Monteforti, G., Mensuali-Sodi, A., Serra, G. (2006). Leaf ultrastructure, photosynthetic rate and growth of myrtle plantlets under different in vitro culture conditions. Biol. Plant. 50: 161-168.
Maggini, R., Raffaelli, A., Annaheim, K.A., Tozzini, L., Pacifici, S., Guidi, L., Paradossi, A. (2010). Effect of post-harvest handling and extraction on the content of echinacoside and cynarin in the root tissues of Echinacea angustifolia DC.. J. Food Agric. Environ. 8: 266-271.
pharmaceutical properties, and biotechnology. J. Herbs. Spices Med. Plants 13: 123-150.
Matkowski, A. (2008). Plant in vitro for the production of antioxidants – A review. Biotech. Advances. 26: 548-560.
Miceli, A., Moncada, A., Vetrano, F., D'Anna, F. (2003). First results on yield and quality response of basil (Ocimum basilicum L.) grown in a floating system. Acta Hort. 609: 377-381.
Montero, J.I., Stanghellini, C., Castilla, N. (2009). Greenhouse technology for sustainable production in mild winter climate areas: Trends and needs. Acta Hort. 80: 33- 44.
Morgan, J.A., Barney, C.S., Penn, A.H., Shanks, J.V. (2000). Effects of buffered media upon growth and alkaloid production of Catharanthus roseus hairy roots. Appl. Microbiol. Biotechnol. 53: 262-265.
Moschopoulou, G., Kintzios, S. (2011). Achievement of thousand-fold accumulation of rosmarinic acid in immobilized cells of sweet basil (Ocimum basilicum L.) by ten-fold increase of the volume of the immobilization matrix. J. Biol. Res. 15: 59-65.
Murashige, T., Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–493.
Neill, S.O., Gould, K.S. (2003). Anthocyanins in leaves: light attenuators or antioxidants. Functional Plant Biol. 30: 865-873.
Nguyen, P., Niemeyer, E. (2008). Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.). J. Agric. Food Chem. 56: 8685–8691.
Orcutt, D.M., Nilsen, E.T. (2000). The physiology of plants under stress soil and biotic factors, JohnWiley and Sons Inc., New York , 605 3rd Avenue, New York, NY 10158-0012. Hardback, 683 pp., ISBN 0-471-17008-9.
Papageorgiou, V., Mallouchos, A., Komaitis, M. (2008). Investigation of the Antioxidant Behavior of Air- and Freeze-Dried Aromatic Plant Materials in Relation to Their Phenolic Content and Vegetative Cycle. J. Agric. Food Chem. 56: 5743–5752.
Pardossi, A., Malorgio, F., Incrocci, L., Tognoni, F. (2006). Hydroponic technologies for greenhouse crops, In: Dris R. (Ed) Crops: Quality, Growth and Biotechnology, WFL Publisher, Helsinky. 23: Pp. 360-378.
Parfitt, D.E., Almehdi, A.A., Bloksberg, L.N. (1988). Use of organic buffers in plant tissue-culture systems. Sci. Hortic. 36: 157–163.
Petersen, M., Hans, J., Matern, U. (2010). Biosynthesis of Phenylpropanoids and Related Compounds, In: Wink M. (Ed), Annual Plant Reviews: Biochemistry of Plant Secondary Metabolism, Vol. 40, 2nd Edition, Wiley-Blackwell, Oxford, UK. Pp.182-230. doi: 10.1002/9781444320503.ch4.
Petersen, M., Simmonds, M.S.J. (2003). Molecules of interest Rosmarinic acid. Pytochem. 62: 121–125.
Rady, M.R., Nazif, N.M. (2005). Rosmarinic acid content and RAPD analysis of in vitro regenerated basil (Ocimum basilicum L.) plants. Fitoterapia. 76: 525-533. Sahoo, Y., Patfnaik, S.K., Chand, P.K. (1997). In vitro propagation of an aromatic
medicinal herb Ocimum basilicum L. (Sweet basil) by axillary shoot proliferation. In Vitro Cell. Dev. Biol. Plant. 33: 293-296.
Sgherri, C., Cecconami, S., Pinzino, C., Navari-Izzo, F., Izzo, R. (2010). Levels of antioxidants and nutraceuticals in basil grown in hydroponics and soil. Food Chem. 123: 416-422.
Siddique, I., Anis, M. (2008). An improved plant regeneration system and ex vitro acclimatization of Ocimum basilicum L.. Acta Physiol. Plant. 30: 493–499. Strazzer, P., Guzzo, F., Levi, M. (2011). Correlated accumulation of anthocyanins
and rosmarinic acid in mechanically stressed red cell suspension of basil (Ocimum basilicum). J. Plant Physiol. 168: 288-293.
Taha, H.S., Abd El-Rahman, R.A., Fathalla Abd-El-Kareem, M., Aly, U.E. (2008). Successful application for enhancement and production of anthocyanin pigment from calli cultures of some ornamental plants. Aust. J. Basic Appl. Sci. 2: 1148-1156.
Van Staden, J., Fennell, C.W., Taylor, N.J. (2004). Plant stress in vitro: the role of phytohormones. Acta Hort. 725: 55–61.
Van Staden, J., Zazimalova, E., George, E.F. (2008). Plant growth regulators II: Cytokinins, their analogues and antagonists, In: George E.F., Hall M.A., De Klerk G.J. (Eds), Plant Propagation by Tissue Culture, 3rd Edition, Springer, Dordrecht, The Netherlands. Pp. 205-226.
Weathers, P.S., Towler, M.J., Xu, J. (2010). Bench to batch: advances in plant cell culture for producing useful products. Appl. Microbiol. Biotechnol. 85: 1339– 1351.
Wolfender, J.L. (2009). HPLC in natural product analysis: The detection issue. Planta Med. 75: 719-734.
Yamazaki, M., Nakajima, J., Yamanashi, M., Sugiyama, M., Makita, Y., Springob, K., Awazuhara, M., Saito, K. (2003). Metabolomics and differential gene expression in anthocyanins chemo-varietal forms of Perilla frutescens. Phytochem. 62: 987–95.
Zheljazkov, V.D., Cantrell, C.L., Tekwani, B., Khan, S.I. (2008). Content, Composition, and Bioactivity of the Essential Oils of Three Basil Genotypes as a Function of Harvesting. J. Agric. Food Chem. 56: 380–385.