Chapter 3
Materials and Methods
3.1 Structural materials in tissue engineering (PLLA and
PLGA)
A variety of synthetic biodegradable polymers can be used to fabricate tissue engineering matrices. In general, these materials may be utilized as structural elements in the scaffolds, to deliver or immobilize the cells, or to achieve both purposes. Poly(glycolic acid) (PGA) and poly(lactic acid) (PLA) (figure 1) are the most commonly used synthetic polymers in tissue engineering. These polymers are also extensively used in other biomedical applications such as drug delivery.
However, in principle, any biodegradable polymer that produces nontoxic degradation products can be used in tissue engineering. The polymers listed in Table 1 are usually recognized as biodegradable.
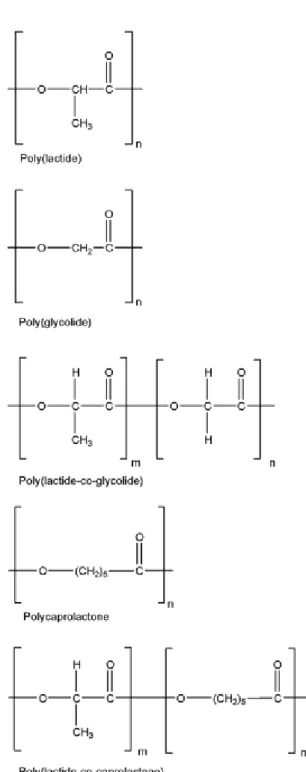
Figure 1: Chemical structures of poly(glycolic acid), poly(lactic acid), polycaprolactone and copolymers
In this chapter the chemistry and structure of a some of these polymers is reported, to give an idea of specific tissue engineering applications for which these polymers might be suitable.
Aliphatic polyesters of the poly(α-hydroxy acids) are perhaps the most common biodegradable synthetic polymers known. Their general formula is –[-O-CH(R)-CO-]- which derives from corresponding HO-CH(R)-COOH where R=H in the case of glycolic acid (GA) and R=CH₃ in the case of lactic acid (LA), the latter being chiral, i.e., D- or L-isomer is possible. These polymers have been used extensively in bone osteosynthesis and reconstruction [2], and later in drug delivery [3]. There are also extensive examples using PLA, PGA and their copolymers in tissue engineering of skin [4].
PLA and PGA synthesis
PLA and PGA can be prepared in two different ways, namely, polycondensation and ring opening polymerization (ROP). Generally, the simple polycondensation is less expensive, but the resulting polymers have low, uncontrolled molecular weight, and it is difficult to prepare copolymers. It is believed that the antimony trioxide catalyst typically used to effect polycondensation acts as both polymerizing and depolymerizing agent. Moreover, glycolic and lactic acids have a great tendency to cyclodimerize under these conditions, and this renders simple polycondensation an unsuitable method. The preferred method for producing high molecular weight polymers is ROP of cyclic dimer, glycolide (and/or lactide).
Depending on catalyst involved, three different mechanisms have been reported: cationic, anionic, and insertion. Among these, the insertion mechanism using metal alkoxides or carboxylates is the most desirable pathway and is the choice in commercial productions [5]. Typical examples of catalysts of this class are aluminum, zinc, titanium, zirconium, antimony, tin(IV), and tin(II) alkoxides or carboxylates. The insertion mechanism allows the preparation of high molecular weight polylactides without racemization up to temperatures above 150˚C [6]. The tin catalyst, tin(II) octoate, was used extensively because of its acceptance by the FDA as a food stabilizer. The polymerization of glycolide can be carried out in bulk at 220˚C for 4 h, at which time a 96% conversion and molecular weights from 10⁴ to 10⁶ have been reported. Copolymerization of glycolide with lactide has also been investigated. The reactivity ratios at 200˚C have been found to be 2.8 for glycolide and o.2 for lactide. This result indicates that copolymers of glycolic and lactic acids have broad compositional ranges, with glycolide always being preferentially polymerized at
low conversions and lactide being incorporated to an ever-increasing extent as the glycolide is depleted [7].
During the advanced stages of most ROPs, additional reactions such as ester-ester interchange and chain unzipping may take place. The extents of these reactions are affected by reactivity of ester moieties. These events can have a significant effect on the composition of the final product. Due to ester exchange, cyclic dimer, trimer, and, to a less extent, cyclic oligomers can be found along with reformed monomer. In the case of copolyerization, additional randomization of the polymer chain will occur as a consequence of ester-ester exchange of different ester moieties [8]. The microstructures of PLGA copolymers can be determined by both proton and carbon NMR spectroscopy [9]. It has been reported that the block lengths increase and, at same time, extent of transesterification decreases with decreasing polymerization temperature.
Mechanical strength and morphology of PLA and PGA
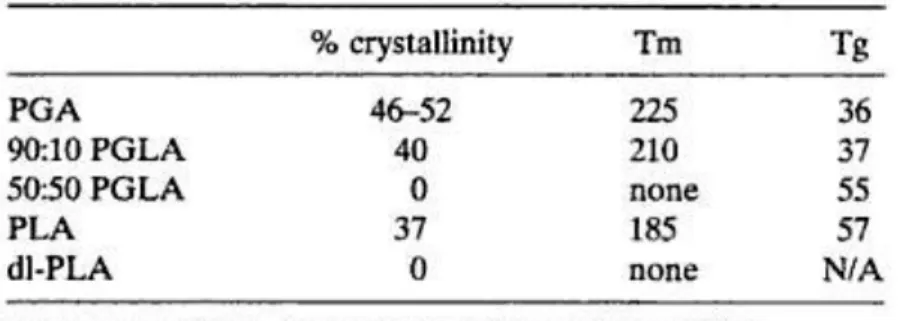
PGA was first developed as the synthetic absorbable suture, Dexon [10]. PGA has highly cristallinity, a high melting point, and low solubility in organic solvents (table 2).
Table 2: Crystallinity and thermal properties of PGA, PLA and copolymers
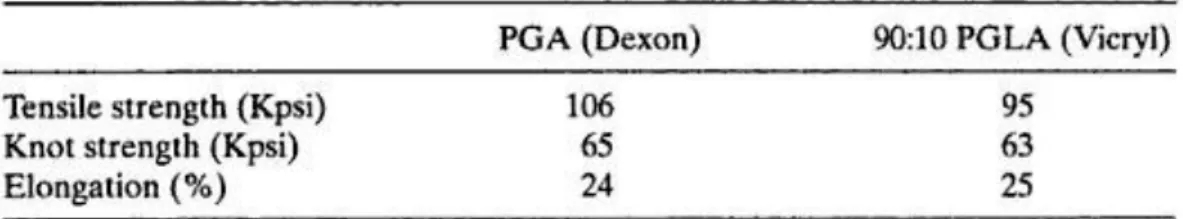
The polymer (fiber grade, inherent viscosity=1.2-1.6 dL/g in hexafluoro-isopropanol) can be spun into multifilament or monofilament yarns for the production of braided and monofilament sutures, respectively [5]. A typical suture braid has a tensile strength of 80-100 Kpsi (table 3).
Table 3: Mechanical properties of PGA and 90:10 PGLA
Owing to the hydrophilic nature of PGA, Dexon sutures tend to lose their mechanical strength rapidly (50%) over a period of two weeks and are absorbed in about 4 weeks after implantation [11].
The presence of an extra methyl group poly(L-lactic acid) (PLA) or poly(D-lactic acid) (d-PLA) makes them more hydrophobic than PGA. For instance, films of PLA only take up approximately 2% in water [7]. In addition, the ester bond in PLA is less labile to hydrolysis due to steric hindrance of the methyl group. Therefore PLA degrades much more slowly than PGA [7] and has higher solubility in organic solvents. PLA is employed much more often than d-PLA, since the hydrolysis of PLA yields L-lactic acid and which is the natural occurring stereoisomer of lactic acid. Whereas PLA possesses about 37% crystallinity, the optically inactive poly(DL-lactic acid) (dl-PLA) is amorphous. The difference in crystallinity of dl-PLA has important practical consequences. For instance, amorphous dl-PLA is usually considered in drug delivery application in which a homogeneous dispersion of active species within a monophasic matrix is desired [12]. However, semicrystalline PLA is preferred in cases where high mechanical strength and toughness, for example, in orthopedic devices [13]. It is permanent to note that γ-irradiation of PLA causes chain scission, cross-linking, and a decrease in crystallinity. Therefore, caution must be taken when sterilizing polymer matrices by γ-irradiation.
To widen the range of materials properties exhibited by PGA, copolymers of GA and LA (PLGA) have been studied. Whereas PGA is highly crystalline, PLGAs usually exhibit lower crystallinity and Tm. For example, while PGA and PLA are partially crystalline, 50:50 PLGA is entirely amorphous (table 2). These morphological changes result in an increase in the rates of hydration and hydrolysis. Thus, copolymers tend to degrade more rapidly than PGA and PLA.
Biodegradation
The degradation mechanism of PLA, PGA and copolymers in vitro is usually regarded as bulk erosion [3]. This is evident from fact that a significant molecular weight decrease usually precedes monomer release from polymer samples (figure 2).
Figure 2: Change of molecular weight of samples in aqueous medium at 37˚C in vitro
This mechanism of degradation may be undesirable in certain applications. The relatively rapid release of large quantities of acid (glycolic and/or lactic acid) may lead to a local acidosis if a large mass of these polymers is present in a concentrated form. However, highly porous scaffolds are typically utilized in tissue engineering applications and contain a relatively low mass of polymer per unit volume. The highly porous structure of scaffolds assists cell penetration as well as polymer degradation. The rate of degradation is affected by morphology of the scaffold, and large surface areas speed up the diffusion of water molecules into the bulk of polymers when they are placed in an aqueous environment (in vivo). The polymers undergo random chain scission by simple hydrolysis of ester bond linkage, and the monomer diffuses out of polymer bulk into water [14]. It is important to note that loss of mechanical strength of PGA is faster when the polymer is incubated at a temperature higher than its Tg. This indicates that the glassy state protects PGA from hydrolysis since all short term chain motions are frozen. Water diffusion, and therefore hydrolysis, is more facile at temperature above Tg. It is also relevant to mention that the Tgs of PLA, PGA and some copolymers are very close to physiological temperature. Polymeric materials may undergo significant structural change after implantation due to water penetration and loss of
mechanical strength. It is also speculated that enzymatic action may partially contribute to biodegradation of PLA and PGA in vivo. The chemical compositions and the ratio of monomers used in polymerization reaction strongly influence the degradation characteristics of copolymer. The degradation rates for copolymers of LA and GA have been shown to be influenced by factors that affect polymer chain packing, crystallinity, and hydrophobicity. Since degradation is induced by hydrolysis, a crystalline structure of hydrophobic polymer composition disfavors dissolution and degradation.
The specific factors affecting copolymer crystallinity and hydrophobicity are summarized as follows:
The ratio of lactide or/to glycolide monomer in polymer and/or copolymer
The stereoregularity of the monomer units in polymer affects polymer chain packing
Randomness of lactide and/or glycolide decrease the ability of chains to crystallize
Low molecular weight polymers degrade faster than high molecular weight polymers, especially when the end groups are free acid rather than capped with ester or other groups
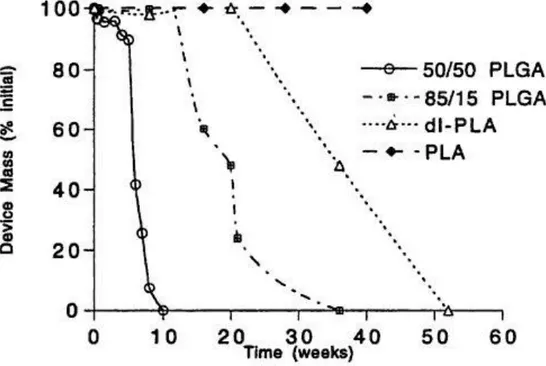
Some of these structural effects are demonstrated in Figure 3. Mass loss from polymer samples comprised of PLA is significant in the experimental time period (50 weeks). However, those comprised of copolymers of GA and LA or dl-PLA degrade much faster. The presence of monomers and low molecular weight cyclic oligomers in absorbable polymers should be avoided, for they degrade much more rapidly than the polymers and can lead to undesirable chemical and biological effects [8]. It has been shown that polylactide with increased monomer content exhibits a higher rate of bioabsorption and a more drastic decrease of molecular weight.
Figure 3: Mass loss of devices fabricated from different polymers
3.2 Carbon Nanotubes (CNTs)
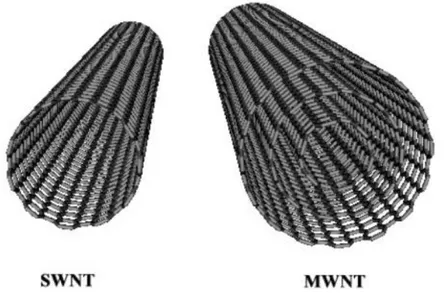
Carbon nanotubes are tubular forms of carbon that can be envisaged as graphitic sheets rolled into cylindrical form. These nanotubes have diameters in the range of few nanometers and their lengths are up to several micrometers. Each nanotube is a single molecule made up of a hexagonal network of covalently bonded carbon atoms. Carbon nanotubes are of two types: single-walled and multi-walled. A single-walled carbon nanotube (SWNT) consists of a single graphene cylinder, whereas a multi-walled carbon nanotube (MWNT) comprises several concentric graphene cylinders. A schematic representation of a SWNT and MWNT structure is shown in the Figure 4. Strong covalent bonding, unique one-dimensional structure and nanometer size, together impart unusual properties to the nanotubes. These properties include exceptionally high tensile strength, high resilience, electronic properties ranging from metallic to semi-conducting, the ability to sustain high current densities and high thermal conductivity. Thus carbon nanotubes could be used as fillers in super-strong composite materials, as wires and components in
nano-electronic devices, as tips of scanning probe microscopes and in flat panel displays and gas sensors. Iijima reported the preparation of MWNTs by the arc-discharge of graphite electrodes in 1991. In 1993, Ijima and Ichihashi at NEC and Bethune et al. at IBM independently reported the preparation of SWNTs. Today, MWNTs are prepared in large quantities by the chemical vapor deposition process. SWNTs can be prepared in reasonably high yields by three techniques: arc-discharge of Ni-Y catalyzed graphite electrodes, laser ablation of Ni-Co catalyzed graphite targets and vapor phase pyrolysis of CO and Fe(CO)5 (HiPCO process). Carbon nanotube samples are always contaminated with impurities including amorphous carbon, residual metal catalyst and graphitic nanoparticles. Thus the purification and chemical processing of carbon nanotubes remains as a key step in any application.
Figure 4: SWNT and MWNT structure
Physical, Thermal, Electrical, Optical and Mechanical properties of CNTs
Is not simply the construction of the physical properties of carbon nanotubes that makes them so unique and such a hotly debated topic. Carbon nanotubes have been known to change depending on the situation they are placed into. They are capable of adapting and changing to meet the needs of electronic, thermal and structural properties. Additionally, the physical properties carbon
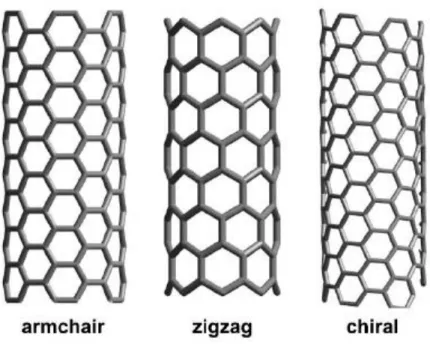
nanotubes change based on the type of nanotube being used—the type being defined by the length and diameter of the nanotube as well as the twist (also known as the chirality) (Figure 5).
Figure 5: Armchair, Zigzag and Chiral SWNT
The type of carbon nanotube as defined has a great deal to do with determining the electronic properties of a carbon nanotube. The chirality itself determines whether the carbon nanotube is a metal, semimetal or semiconductor and its implications for science and electronics will be determined by its makeup. Carbon nanotubes have been known for some time to be excellent conductors of electricity. This conductivity allows for the use of bundled carbon nanotubes as microscopic tweezers.
Carbon nanotubes have been described as being able to exist as a Single Walled Nanotube (SWNT) or as a Multiple Walled Nanotube (MWNT) (Figure 4). In the Multiple Walled Nanotube, one cylinder (or rolled sheet of carbon nanotubes) is inside another cylinder, like nesting dolls. Each of these types of carbon nanotubes have their own physical properties in addition to the standard physical property sets for carbon nanotubes and due to the complex nature of Multiple Walled Nanotubes, they often have many defects and are unusable for several of their major physical properties.
Some of the major properties of carbon nanotubes are optical. If a macromolecule has optical properties that means that it is has properties relating to the principles of photoluminescence, light absorption and that it is able to register light on the Raman spectroscopy. The abilities associated with the optical physical properties of carbon nanotubes are as yet unclear, but it is possible that carbon nanotube could have implications in the development of optics, photonics, LEDs (or Light Emitting Diodes) and even photo-detectors, among other optical devices.
But the properties of carbon nanotubes are not limited to optical properties. Thermal properties of carbon nanotubes have great implications for science as well. In some experiments, carbon nanotubes have been added to epoxy resin in a successful attempt to double the thermal conductivity in the resin. With this being achieved at only a 1% loading, the experiment proved that carbon nanotubes can be used successfully for thermal management applications when used as part of a composite material.
Additionally, nanotubes are said to have elastic properties as well. While these elastic properties are hotly debated in many scientific circles, it is agreed that carbon nanotubes are one of the most flexible macromolecules in existence today. While there are no defined uses for the extreme flexibility of carbon nanotubes, this elasticity could have implications for the develop of a wide variety of products, including bullet proof vests—though the strength of carbon nanotubes alone could merit this segue—and other safety devices.
This macromolecule is anisotropic which means that it is directionally dependent. On the other hand, isotropic molecules are not directionally dependent and do the same thing no matter which direction they are going or being pulled. This property is precisely how carbon nanotubes are able to fulfill the needs of many different physical properties without being deficient in any one area. The anisotropic properties of carbon nanotubes could have implications for a wide variety of fields, including but not limited to chemistry, computer graphics, wood and woodworking, real world imagery, geophysics, physics, medical acoustics, material science, engineering, microfabrication and a host of other fields of discovery and invention that are seeking perfect solution to every problems.
Biocompatibility of CNTs in Biomedical Applications
The growing use of carbon nanomaterials in several areas has needed to establish a paradigm for accurately predicting toxicity of nanomaterials. In order to create this paradigm, researchers must compile information about the specific physicochemical properties unique to nanomaterials, which drive toxicity [15]. These physicochemical properties include shape, surface chemistry and dimension, all of which have been found to be of great importance in nanotoxicity.
Shape was shown to play a role in toxicity in a study where MWCNT were found to be more toxic than multi-walled nano-onions to human skin fibroblasts [16]. Furthermore, studies examining the effect of surface chemistry on toxicity have shown that sidewall functionalized SWCNT were less toxic to human embryonic kidney cells than those without functionalization [17], highly derivatized water-soluble fullerene species were less toxic than those which were not derivatized [18] and functionalized carbon nanotubes could cross cell membrane without being toxic for up to 10 lM [19]. In addition, the effect of dimension on toxicity was shown in a study where CNF were less detrimental to osteoblast viability compared to larger diameter CF [20] and in another study where carbon nanomaterial toxicity was found to follow a mass basis with SWCNT being the most cytotoxic, followed by MWCNT, and then C60 [21]. Nanomaterial exposure is likely to occur through dermal contact and inhalation because skin and lungs are in direct contact with the environment [22]. Due to their strong chemical stability, CF, CNF, MWCNT, and SWCNT may not be broken down when inhaled, which could be damaging to tissues [23]. Carbon nanotubes have been shown to cause lung inflammation, fibrosis, and epithelioid granulomas, as well as biochemical and toxicological changes to the lung [24–25]. Furthermore, it has been reported that SWCNTs impair respiratory functions and slow down bacterial clearance from the lungs [25]. In comparison, MWCNTs also elicit changes in lung pathology, and therefore, it is speculated that they will have a similar impact on cardiopulmonary diseases [26]. Aside from inhalation, there is potential for dermal exposure to carbon nanotubes. Skin is the first line of defense against the outside environment and provides significant surface area for potential contamination. Unmodified MWCNT have been found to cause toxicity to human epidermal keratinocytes [27], and unmodified SWCNT exposed to human embryonic kidney cells were found to play a role in the mechanisms that cause G1 phase arrest and cell apoptosis [28]. Contrary to these findings, carbon nanotubes synthesized by catalytic chemical vapor deposition were found to be nontoxic to human umbilical vein endothelial cells [29]. In an in vivo test, carbon nanotubes were
administered to human volunteers with allergy susceptibility via skin patch and found to be nonirritant [30].
3.3 Realization of 3-D PLLA/CNTs Composite Scaffolds
Step by Step
The bio-polymer chosen for creating scaffolds studied during this thesis is PLLA (Poly-Left-Lactide-Acid) prepared in 20% (w/v) solution in Chloroform (www.sigmaaldrich.com). PLLA has been chosen because its degrading time perfectly matches to the bone regeneration process [31]. Then, a 0.1:1 (w/v) mixture of MWCNT in Benzene (www.sigmaaldrich.com) sonicated for 1 minute has been prepared. Finally, the PLLA and MWCNT solutions were mixed together to achieve a final concentration of 1.25% (w/v) MWCNT. Before using the PLLA/MWCNT solution there has been the necessity to sonicate (figure 6) for other 3 minutes to better disperse the MWCNT inside.
PAM Microfabrication Technique
The microfabrication technique used for scaffolds realization has been the P.A.M (Pressure Activated Microsyringe) system (figure 7). P.A.M is a CAD/CAM (Computer-Aided Design/Computer-Aided Manufacturing) system that uses compressed air to eject a solution of polymer in volatile solvent through a narrow capillary needle, which has a diameter between 10-200 μm. For this work a diameter of 150 μm has been chosen. The placement of solution in the x-y plane has been controlled by a micropositioning system, which can achieve lateral precisions of 0.1 μm. The physical dimensions of the structures can range from 5 μm to 600 μm, depending on different processing parameters [32]. P.A.M system has been used to fabricate three-dimensional scaffolds from PLLA (Poly-Left-Lactide-Acid), but it could theoretically be expanded to any polymer, synthetic or natural, that is soluble in a volatile solvent like PLGA (Poly-Lactic-co-Glycolic-Acid) or PCL (Polycaprolactone) [32].
References
1) Huang SJ: Biodegradable Polymers. In: Polymers-Biomaterials and Medical Applications 2) Vert M, Christel P, Chalot F, Leray J: Bioresorbable plastic materials for bone surgery. In:
Macromolecular Biomaterials.
3) Gombotz WR, Pettit DK: Biodegradable polymers for protein and peptide drug delivery. Bioconjugate Chem 6: 332-351.
4) Cooper ML, Hansbrough JF, Speilvogel RL, Cohen R, Bartel RL, Naughton G: In vivo optimization of a living dermal substitute employing cultured human fibroblasts on a biodegradable polyglicolic acid or polyglactin mesh. Biomaterials 12: 243-248.
5) Frazza EJ, Schmitt EE: A new absorbable suture. J Biomed Mater Res Symp 1: 43-58.
6) Kricheldorf HR, Kreiser-Saunders I: Polylactides-synthesis, characterization and medical applications. Macromol Symp 103: 85-102.
7) Gilding DK, Reed AM: Biodegradable polymers for use in surgery-polyglycolic/poly(lactic acid) homo- and copolymers. Polymer 20: 1459-1464.
8) Shalaby SW, Johnson RA: Synthetic absorbable polyesters. Biomedical Polymers.
9) Kasperczky J: Microstructural analysis of poly (L,L-lactide-co-glycolide) by n.m.r. spectroscopy. Polymer 37: 201-203.
10) Schmitt EE: A new absorbable suture II. J Biomed Mater.
11) Katz AR, Turner R: Evaluation of tensile and absorption properties of polyglycolic acid sutures. Surg Gynecol Obstet 131: 701.706.
12) Engelberg I, Kohn J: Physico-mechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 12: 292-304.
13) Hay DL, von Fraunhofe JA, Chegini N, Masterson BJ: Locking mechanism strength of absorbable ligating devices. J Biomed Mater Res 22: 179-190.
14) Reed AM, Gilding DK: Biodegradable polymers for use in surgery-poly(glycolic)/poly(lactic acid) homo and copolymers: 2. In vitro degradation. Polymer 22: 494-498.
15) Oberdoster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol 2005;2(1):8.
16) Ding L, Stilwell J, Zhang T, Elboudwarej O, Jiang H, Selegue JP, et al. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett 2005;5(12):2448–64.
17) Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett 2005;161(2):135–42.
18) Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, et al. The differential cytotoxicity of water-soluble fullerenes. Nano Lett 2004;4(10):1881–7.
19) Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun 2003;2004(1):16–7.
20) Price RL, Haberstoh KM, Webster TJ. Improved osteoblast viability in the presence of smaller nanometer dimensioned carbon fibres. Nanotechnology 2004;15(8):892–900.
21) Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol 2005;39(5):1378–83.
22) Hoet P, Briusle-Hohlfeld I, Salata O. Nanoparticles-known and unknown health risks. J Nanobiotechnol 2004;2(1):12.
23) Gogotsi Y. How safe are nanotubes and other nanofilaments? Mat Res Innovat 2003;7(4):192–4.
24) Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Resp Toxicol 2004;77:126– 34.
25) Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci 2004;77:117–25.
26) Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol 2005;207:221–31.
27) Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicol Lett 2005;155(3):377– 84.
28) Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett 2005;155(1): 73–85.
29) Flahaut E, Durrieu MC, Remy-Zolghadr M, Bareille R, Baquey C. Investigation of the cytotoxicity of CCVD carbon nanotubes towards human umbilical vein endothelial cells. Carbon 2006;44(6): 1093–9.
30) Huczko A, Lange H, Calko E, Grubek-Jaworska H, Droszc P. Physiological testing of carbon nanotubes: are they asbestos-like? Fullerene Sci Technol 2001;9:251–4.
31) H. Pihlajamaki, J. Kinnunen, O. Bostman: In vivo monitoring of the degradation process of bioresorbable polymeric implants using magnetic resonance imaging.