C
HAPTER1.
I
NTRODUCTION1.1 - Toxic heavy metals
1.1.1 - Sources, contamination, characteristics and specific effects
A wide range of contaminants affect the environment, but the main problems usually arise from a small number of elements among which toxic metals. Heavy metals are the major pollutants present in areas with road traffic and near smelters, heating systems and metallurgic industries. In addition, very high values of metals have recently been observed in many agricultural environments because of long-term use of phosphatic fertilisers and sewage sludge applications.
Although there is no clear definition of what a heavy metal is, in most cases, density is taken as a criterion. Heavy metals are generally considered to be those of which the density exceeds five grams per cubic centimetre (NCR, 1991). A large number of elements falls into this category, but only arsenic (As), cadmium (Cd), chromium (Cr), cobalt (Co), copper (Cu), lead (Pb), mercury (Hg), nickel (Ni), tin (Sn), vanadium (V) and zinc (Zn) are of relevance in the environmental context. In fact, the other heavy metals only rarely occur in concentrations high enough to cause harmful effects. Even though arsenic is actually a semi-metal or metalloid, it is usually regarded as a hazardous heavy metal.
Although some metals are naturally found in the body and are essential to human health, several adverse health effects due to exposure to heavy metals have been known for long time. Iron, for example, prevents anaemia and zinc is a cofactor in over one hundred enzyme reactions. Nickel, manganese and copper are other familiar metals that are necessary for proper metabolism. However, these essential metals are needed in very small amounts and excessive accumulation is detrimental to health. In addition, other heavy metals, such as arsenic, cadmium, lead, act, also at very low concentrations, as poisonous interferences to the metabolism. In general, heavy metals are systemic toxins with specific neurotoxic, nephrotoxic, fetotoxic, and teratogenic effects. They can
directly influence behaviour by impairing mental and neurological functions, influencing neurotransmitter production and utilization as well as altering numerous metabolic body processes (NCR, 1991; Järup, 2003). Systems in which toxic metal elements can induce impairment and dysfunction include the blood and cardiovascular, eliminative pathways (colon, liver, kidneys, skin), endocrine glands (hormonal), energy production pathways, enzymatic, gastrointestinal, immune, nervous (central and peripheral), reproductive and urinary systems. In addition, toxic metals can increase allergic reactions, cause genetic mutations, compete with essential trace metals for biochemical binding sites and act as antibiotics killing beneficial bacteria. Heavy metals can also increase the acidity of the blood and set up the conditions that lead to inflammation in arteries and tissues, causing more calcium to be drawn to the area as a buffer, contributing to hardening of the artery walls with progressive blockage of the arteries and osteoporosis. However, much of the damages produced by toxic metals stems from the proliferation of oxidative free radicals (NCR, 1991; Järup, 2003). High-concentration exposure is not necessary to produce a state of toxicity in the body tissues. In fact, the toxicity of heavy metals is often defined as a function of concentration and time of exposure (NCR, 1991). Heavy metals are stable elements, meaning that they cannot be broken down by the body, therefore, they could reach over time toxic concentration levels.
In spite of these adverse health effects, exposure to heavy metals continues and is even increasing in some parts of the world, in particular in less developed countries, over the last one hundred years. For example, mercury is still used in gold mining in many parts of Latin America. Arsenic is still common in wood preservatives and tetraethyl lead remains a common additive to petrol, although its use has decreased dramatically in the developed countries (Järup, 2003). Today, heavy metals are abundant in our drinking water, air and soil due to their increased use. Heavy metals are present in virtually every area of modern consumerism from construction materials to cosmetics, medicines, processed food, fuel sources and personal care products. For this reason it is very difficult to avoid exposure to any of the many harmful metals that are prevalent in our environment.
Emissions of heavy metals into the European environment occur via a wide range of processes and pathways and concern all physical spheres: the atmosphere (e.g. during combustion, extraction and processing), the surface waters (via runoff and releases from storage and transport), the soil and, therefore, the groundwater and crops (Table1.1).
Table1.1. Emission of heavy metals (tonnes/year) in European environment in
2002 (Pacyna, 1984 brought up to 2002)
Cd Cr Cu Mn Ni Pb Zn
Electric power stations 111 1315 1514 1112 5038 1251 1447
Heating systems 170 1738 2242 1516 8214 1817 2006 Fuels 33 - - 99 1463 81730 - Minings 1 - 211 302 1804 1199 506 Metal-working industries 2435 16940 11199 1847 528 46950 71687 Smelters 92 58 286 125 11 884 6468 Phosphatic fertilisers 30 - 85 - 84 7 253 Cement factories 17 729 - - - 820 - Total 2889 20780 15537 15001 17142 134658 82367
Atmospheric emissions tend to be the greatest concern in terms of human health, because of the quantities involved and the widespread dispersion (Järup, 2003). However, the concern about the contamination of many agricultural areas is increasing. The introduction of heavy metals in the soil may result in damage to or loss of some or several functions of soils and possible cross contamination of water. In addition, the occurrence of these contaminants in soils, especially above certain levels, entails multiple negative consequences for all types of ecosystems and natural resources, for the food chain and thus for human health. To assess the potential impact of soil contaminants, account needs to be taken not only of their concentration but also of their environmental behaviour and exposure mechanisms. A distinction is often made between soil contamination originating from clearly confined sources (local or point source contamination) and that caused by diffuse sources (Commission of the European Communities, 2002).
Diffuse pollution is generally associated with atmospheric depositions, certain farming practices and inadequate wastewater recycling and treatment. Atmospheric deposition of airborne pollutants, due to the emissions of industries and traffic, releases into soils acidifying contaminants (e.g. SO2, NOx), several organic compounds (e.g. dioxins,
PCBs, PAHs) and some heavy metals such as arsenic, cadmium, lead and mercury. However, some farming practices are considered to be the principal source of diffuse heavy metal soil contamination. In fact, these contaminants are present in fertilisers and animal feeds. An additional problem relates to heavy metals in sewage sludge, the final product of the treatment of wastewater. On average, 65% of municipal waste generated in the EU (190 million tonnes in 1995) is still landfilled. The application of this sludge to the agricultural soils can result in an increase of the soil concentration of toxic metals that are persistent, meaning they cannot be broken down to harmless molecules by soil microorganisms (Commission of the European Communities, 2002). Of particular concern are the waste landfills that have operated in the past, without complying with the minimum set of technical requirements proposed by the Landfill Directive (Council Directive 1999/31/EC).
Local heavy metal contamination is generally associated with mining, industrial facilities and other facilities both in operation and after closure. In mining, the risks are associated with the storage or disposal of tailings and use of certain chemical reagents. However, industrial facilities both in operation and after closure are the major source of local contamination (Commission of the European Communities, 2002).
Although the largest and most affected areas are concentrated around the heavily industrialised regions in Northwest Europe, contaminated sites exist everywhere throughout the continent. Estimates of the number of contaminated sites in the EU range from 300,000 to 1.5 million (European Environment Agency, 1999). This wide range in estimations is due to the lack of a common definition for contaminated sites and relates to different approaches to acceptable risk levels, protection targets and exposure parameters. Fortunately, within the EU there are no important areas contaminated with artificial radio nuclides (Commission of the European Communities, 2002).
1.1.2 - Legislation toward heavy metals contamination: the European and Italian situations
The presence of such massive amount of toxic metals and the concern about the risks associated with long-term exposure to them has recently led the European Union to pay much more attention to the land contamination and to fix some limit values for heavy metals in soils and sludges (Table 1.2).
Table 1.2. Limit values (mg Kg-1 d.w.) for heavy metals in soil and sludge
according to EU Directive 86/278/EEC*
Soil Sludge
Directive 86/278/EEC Future Directive 86/278/EEC Future
As 50 ~ n.i. - Cd 1-3 0.5-1.5 20-40 10 Cr n.i. 30-100 n.i. 1000 Cu 50-140 20-100 1000-1750 1000 Mo 4 ~ n.i. - Ni 30-75 15-70 300-400 300 Pb 50-300 70-100 750-1200 750 Zn 150-300 60-200 2500-4000 2500
*Limits depend on soil pH n.i. = no information available
However, the problems posed by contaminated sites are generally treated as national problems. For this reason, in the last two decades most EU countries have developed national strategies to face up to the situation. The approaches vary considerably and can hardly be compared among each other. In some countries the management of contaminated sites is regulated by specific soil clean-up legislation, whereas other countries, such as Italy, address the issue by generic legislation, for example on water protection or protection of the environment. In most cases the appropriate legislation is issued at national level and only in very few EU countries at the sub-national level, namely in Belgium, United Kingdom and Germany (Commission of the European Communities, 2002).
The clean up of contaminated sites is a problem that started only recently to be assessed and tackled in Italy. In 1989 the government made the first attempt by starting a regional plan on contaminated sites and obliging the Italian regions to check their territories. Major objective of the plan was to quantify the problems posed by polluted soils. The regions complied hesitantly; it took almost eight years until compiled data were suitable to be published. In 1994 a National Environment Agency (ANPA, Agenzia Nazionale per la Protezione dell’Ambiente) was established with the responsibility to proactively support environmental legislation. Legislation toward contaminated sites has been charged recently: by the beginning of 1997 a new law was promulgated regarding waste and contaminated sites assessment and management, demanding the development of use-oriented limit values for soil, groundwater and surface waters and obliging local authorities to compile registers on contaminated sites (Ministero dell’Ambiente, Art. 17, Decreto Legislativo n. 22, 1997). However, this legislation was insufficient and there was a great need to manage the problems posed by contaminated sites more efficiently. Cabinet Decree n. 471 issued in December 1999 was promulgated (Ministero dell’Ambiente, Decreto Ministeriale n. 471, 1999). In this piece of legislation limit values for heavy metals in soils (two land uses) were defined (Table 1.3). According to the Decree 471/1999, a site should be defined as polluted when contamination levels or chemical, physical, biological degradation of soil, ground or surface water determine hazards to public health, or the natural or built environment. For legal purpose a site was defined polluted when even a single substance in soil or water exceeded concentration limit values established in the same Decree (Table 1.3) (Ministero dell’Ambiente, Decreto Ministeriale n. 471, 1999). Following the rules of the Cabinet Decree 471/1999, 8873 contaminated sites were identified in Italy that included waste and industrial sites, abandoned as well still operating. However, it is noteworthy to mention that the total number of potentially contaminated sites is likely to exceed 10,000. In fact, the regions which had not provided any data cover approximately one third of the Italian population (European Environment Agency, 1999).
Recently, the D.L.vo n.152 was promulgated (Ministero dell’Ambiente e della Tutela del Territorio, Decreto Legislativo n. 152, 2006). This Decree, accomplishing the law 308/2004 that gave the Cabinet a proxy for reorganizing and integrating the
environmental legislation, has the improvement of the quality of the human life as primary purpose. This purpose must be realized by the protection and improvement of the environmental conditions and the proper use of natural resources.
Regarding the remediation of contaminated soils, the D.L.vo 152/2006 regulates the clean-up measures and establishes the procedures for the elimination of pollution sources and for the reduction of hazard substances.
According to the D.L.vo 152/2006, the first step for defining a site as polluted is the comparison of the levels of hazard substances found in the site with the threshold
concentrations of contamination fixed in the Decree itself. These values are exactly
the same of those reported in the Cabinet Decree 471/1999 (Table 1.3). However, the D.L.vo 152/2006 is much more innovative than the Decree 471/1999 because the attention is focused not only on the limit values of contamination but also on the real
Table 1.3. Limit values (mg kg-1 d.w.) for heavy metals in soils (two land
uses) according to Cabinet Decree 471/1999
Residential and private uses Commercial and industrial uses
As 20 50 Be 2 10 Cd 2 15 Co 20 250 Cr 150 800 Cr (VI) 2 15 Cu 120 600 Hg 1 5 Ni 120 500 Pb 100 1000 Se 3 15 Sb 10 30 Sn 1 350 Ta 1 10 Va 90 250 Zn 150 1500
characteristics of the contamination itself. Therefore, if a substance exceeds the threshold concentrations of contamination the site is defined potentially polluted and a characterization of the soil and a site-specific risk analysis become necessary (D.L.vo 152/2006). During the risk analysis some parameters such as the type and the source of contamination, the receptors of the contamination (inhabitants or workers, directly or indirectly in contact with the contamination) and the ways of their exposition must be considered (D.L.vo 152/2006). Applying the site-specific risk analysis, the site-specific
threshold concentrations of risk can be defined. A site is polluted if even a single
substance exceeds the site-specific threshold concentrations of risk (D.L.vo 152/2006). The idea of analysing the risks linked to the contamination is very interesting and innovative although it could be very difficult to apply and it could need long procedural time increasing the inefficiency of the system.
In the D.L.vo 152/2006 as well as in the Decree 471/1999 no informations are available about the limit values for heavy metals in agricultural soils. Until today, this problem is tackled at national level only by the D.L.vo 99/1992 regarding the utilization of sludges in agriculture (Ministero dell’Ambiente, Decreto Legislativo n. 99, 1992, attuazione della Direttiva 86/278/CEE). Some limit values for heavy metals in sewage sludge as well as in soil have been defined in that Decree (Table 1.4).
Table 1.4. Limit values (mg kg-1 d.w.) for heavy metal in soil
and sewage sludge according to D.L.vo 99/1992
Soil Sewage sludge
Cd 1 20 Cu 100 1000 Hg 1 10 Ni 75 300 Pb 100 750 Zn 300 2500
However, in default of an updated national law regarding agricultural soils, some Italian regions have attempted a task for managing the problems linked to the contamination of
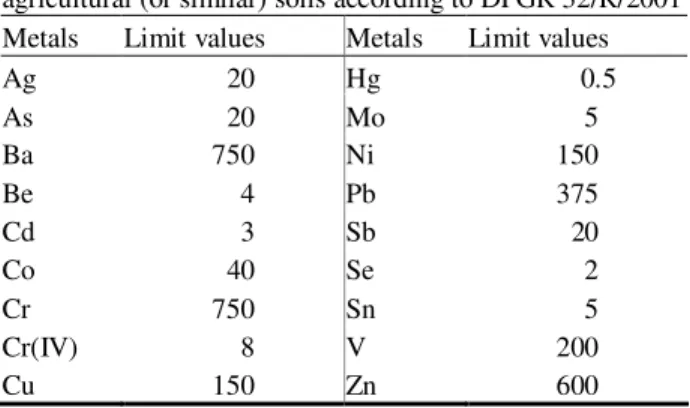
the agricultural soils more efficiently. For example, in 2001 the Tuscany region promulgated a law regarding agricultural contaminated soils (Regione Toscana, DPGR n. 32/R, allegato n. 8, 2001) in which limit values for heavy metals were defined (Table 1.5).
Soil clean up is a difficult operation with very high costs. Expenditure for soil decontamination of contaminated sites greatly varies among European States. In 2000 the Netherlands invested EUR 550 millions in decontamination, Austria 67 and Spain 14. Such disparities reflect different perceptions of the severity of the contamination, different remediation policies and targets and different ways of estimating expenditure. The European Environmental Agency (EEA) has estimated the total costs for the clean up of contaminated sites in Europe to be between EUR 59 and 109 billion (Council Directive 1999/31/EC).
As regard Italy, procedures for assessment and remediation project design and approval protocols by local authorities, together with monitoring tasks, are established in the Cabinet Decree 471/1999 as well as in the D.L.vo 152/2006. Different remediation solutions are outlined:
• clean-up
• clean-up with safety measures
Table 1.5. Limit values (mg kg-1 d.w.) for heavy metals in
agricultural (or similar) soils according to DPGR 32/R/2001
Metals Limit values Metals Limit values
Ag 20 Hg 0.5 As 20 Mo 5 Ba 750 Ni 150 Be 4 Pb 375 Cd 3 Sb 20 Co 40 Se 2 Cr 750 Sn 5 Cr(IV) 8 V 200 Cu 150 Zn 600
• emergency and permanent safety actions
Clean-up with safety measures is defined as the different actions needed to reduce site contamination to concentration levels exceeding acceptable legal limits when these cannot be reached, according to EU principles (EU Directive 96/61/CE), by the best available technologies as affordable costs. In these cases, reuse of the site implies safety measures, monitoring, control and use limitations. All the remediation solutions outlined in the above-mentioned laws can be applied in situ or ex situ. In situ can refer to where a clean up or remediation of a polluted site is performed using and stimulating the natural processes in the soil, contrary to ex situ where contaminated soil is excavate and cleaned elsewhere. Remediation costs for clean-up measures deemed necessary on a short-term basis and on a mid-term basis were calculated as well. The individual costs provided by the regions were very heterogeneous and were hence only a first estimate of the future costs. The compiled calculations estimated costs on a short-term basis at approximately EUR 340 millions and on a mid-term basis at more than EUR 200 millions (Ministero dell’Ambiente, 1997). The polluter-pays-principle is applied as far as possible (D.L.vo 152/2006). The new waste regulation defines the liability for those sites where a polluter cannot be identified. In those cases the Environment Agency, communities and regions are jointly held liable and are responsible for the implementation of appropriate safety measures.
Even if national laws regulate the decontamination of polluted sites, the clean up is always treated as local problem. In the D.L.vo 152/2006 the clean up of contaminated areas is entrusted to the administration of Provinces and Regions that have the freedom to establish appropriate funds with the purpose to finance the clean-up procedures.
1.1.3 - Remediation technologies: an evaluation
Metals can cause significant damages to the environment and human health as a result of their mobilities and solubilities in soils directly correlated with the heavy metals concentration in plants. The selection of the most appropriate soil remediation method depends on the site characteristics, concentrations and types of pollutants to be removed
and on the end use of the contaminated medium (Mulligan et al., 2001). The approaches include isolation, immobilisation, toxicity reduction, physical separation and extraction. Many of these technologies have been used full-scale (Mulligan et al., 2001).
• Isolation: contaminants are isolated and contained to minimize further movements. Physical barriers made by steel, cement, bentonite and grout walls can be used for isolation and minimization of metal mobility (Mulligan et al., 2001).
• Solidification/stabilization also known as in situ immobilization: contaminants are contained in an area by mixing or injecting agents. Solidification encapsulates contaminants in a solid matrix while stabilization involves formation of chemical bonds to reduce contaminants mobility. An excellent understanding of the hydrogeologic regime is essential and, generally, these technologies could be applied with success to moderate to high permeability soils (Mulligan et al., 2001).
• Size selection process: the larger, cleaner particles are removed from the smaller more polluted ones. Characterization in terms of particle size and contaminant level in each fraction is the most important parameter in determining the suitability of this process. To accomplish the size selection process, several methods are used. They include hydrocyclones which separate the particles by centrifugal force, fluidised bed separation which removes smaller particles at the top in the countercurrent overflow in a vertical column by gravimetric settlings, and flotation which is based on the different surface characteristics of contaminated particles. Addition of special chemicals such as frothers or flotation agents and aeration causes these contaminated particles to float (Mulligan et al., 2001).
• Pyrometallurgical processes: high temperatures (200 – 700 °C) are used to volatilise metals in contaminated soil. Because the high costs of this type of treatment, it is most applicable to highly contaminated soils were metal recovery is profitable (Mulligan et al., 2001).
• Electrokinetic processes: a low intensity electric current between a cathode and an anode imbedded in the contaminated soil is used. Ions and small charged
particles, in addition to water, are transported between the electrodes. The metals can be removed by electroplating or precipitation/coprecipitation at the electrodes. This technology, that has been full-scale applied in the USA and in a limited manner also in Europe, is used for copper, zinc, lead, arsenic, cadmium, chromium and nickel. The duration of time that the electrode remains in the soil, and spacing are site-specific (Mulligan et al., 2001). Techniques for the extraction of metals by biological means have been not extensively applied up to this point. The main methods include bioleaching, oxidation/reduction reactions and phytoremediation.
• Bioleaching: Thiobacillus sp. bacteria are used under aerobic and acidic conditions (pH = 4) at temperature between 15 and 55°C, depending on the strain. Leaching can be performed by direct means, oxidation of metal sulphides to produce sulphuric acid, which then can desorb the metals on the soil by substitution of protons (Mulligan et al., 2001). Another leaching technique that has potential for remediation of metal contaminated soil, is through the production of citric and gluconic acids by the fungus Aspergillus niger. They can act as acids (pH = 3.5) and chelating agents for the removal of metals such as copper from oxide mining residues (Mulligan et al., 2001). • Oxidation/reduction reactions: microorganisms are able to oxidize and reduce
metal contaminants. Mercury can be oxidized while arsenic, chromium (VI) and iron can be reduced (Mulligan et al., 2001). Biomethylation involves the addition of a methyl group to a metal such as arsenic, mercury, cadmium or lead. This technique is currently under development and is not commercially available (Mulligan et al., 2001). Volatilisation of selenium from contaminated agricultural soils has shown some promises (Thompson-Eagle and Frankenberger, 1990).
• Phytoremediation: an emerging technology that aims to extract or inactivate heavy metals present in different environmental substrates by using plants (Salt et al., 1998).
1.2 - Phytoremediation
We often think of plants primarily as a source of wood, food and fibre. Secondarily, we may also appreciate their presence for aesthetic reasons as well as for “altruistically” providing habitat for other species. Interestingly, their values as environmental counterbalances to industrialization processes are being appreciated. Plants have long been recognized for their consumption of CO2 and, more recently, of other gaseous
industrial products (Simonich and Hites, 1994). Recently, their role in slowing the rate of global warming has been further appreciated in both the scientific and popular press. The basic idea that plants can be used for environmental remediation is very old and cannot be traced to any particular source. However, a series of fascinating scientific discoveries combined with an interdisciplinary research approach have allowed the development of a new promising, cost-effective and environmentally friendly technology called phytoremediation. Phytoremediation is defined as the use of green plants to remove pollutants from the environment or to render them harmless, and it could be applied to both organic and inorganic pollutants, present in solid substrates (e.g. soil), liquid substrates (e.g. water) and air. Because of the several uses, phytoremediation is currently divided into the following areas (Salt et al., 1998):
• phytodegradation: the use of plants and associated microorganisms to degrade organic pollutants;
• rhizofiltration: the use of plant roots to absorb or adsorb pollutants, mainly metals, from water and aqueous waste streams;
• phytostabilisation: the use of plants to reduce the bioavailability of pollutants in the environment;
• phytovolatilisation: the use of plants to volatilise pollutants among which some heavy metals such as arsenic, mercury and selenium;
• phytoextraction: the use of pollutant-accumulating plants to remove metals or organics from soil by concentrating them in the harvestable parts.
The metal phytoextraction is the area of major scientific and technological progress in the past years. This can be partially explained by the relative ease of detecting metals in various materials and by the prohibitively high cost of the available soil remediation
methods. Remediation of soil contamination by conventional engineering techniques often costs between $ 50 and $ 500 per ton. Certain specialised techniques can exceed costs of $ 1000 per ton. With an acre of soil 3 to 8-foot depth weighting approximately 4500 tons, this translates to a minimum cost of about a quarter of million dollars per acre (Cunningham et al., 1995). It is not surprising that the clean up of contaminated sites has not been proceeding at a rapid pace. In addition, the potential of the phytoextraction is clear in comparison with the existing chemical-physical technologies which may have drastic and undesirable effects on soil properties, removing all biological activities and fertility (Mulligan et al., 2001; Navari-Izzo and Quartacci, 2001; Quartacci et al., 2001a). Indeed, it has long been known that the life cycle of plants has profound effects on the chemical, physical and biological processes that occur in its immediate vicinity. In the processes of shoot and root growth, water and mineral acquisition, senescence and eventual decay, plants can profoundly alter the surrounding soil. The plant-driven processes also occur in areas heavily impacted by industrial, mining and urban activities. Moreover, phytoextraction is likely to be more acceptable to the public than the other traditional methods (Quartacci et al., 2001a; Navari-Izzo and Quartacci, 2001).
Two approaches have been proposed for phytoextraction of heavy metals, namely long-term continuous or natural phytoextraction and chemically-enhanced phytoextraction (Salt et al., 1998).
1.2.1 - Long-term continuous phytoextraction
An approach to metal accumulation is the reliance on the specialised physiological processes that allow plants to accumulate metals over the complete growth cycle (Fig. 1.1). This type of metal uptake is epitomized by hyperaccumulating plants that grow on soils rich in heavy metals and are naturally able to accumulate elevated levels of metals (Baker and Brooks, 1989). The authors define hyperaccumulating plants those in which the concentrations of cadmium, copper, lead and zinc in the dry matter of the leaves exceed the thresholds of 100, 1000, 1000 and 10.000 mg Kg-1dw, respectively.
The first hyperaccumulators characterised were member of the Brassicaceae and Fabaceae families. Presently, at least 45 plant families are known to contain metal-accumulating species (Salt et al., 1998). The ecological role of metal hyperaccumulation is still not entirely clear. It has been suggested that metal accumulation provides protection against fungal and insect attack. Some evidences have confirmed the protective function of nickel hyperaccumulation against fungal and bacterial pathogens and insects (Martens and Boyd, 1994). The anti-herbivores effect of zinc has been also demonstrated in zinc hyperaccumulator T. caerulescens (Pollard and Baker, 1997). The unique capacity of hyperaccumulators to accumulate high foliar heavy metal concentrations makes these plants suitable for the development of continuous phytoextraction. Most known hyperaccumulator plants have low biomass and/or slow growth rates, whereas rapidly growing high-biomass crop plants are sensitive to metals and accumulate only low concentrations in shoots. To overcome these limitations, a two-component long-term strategy needs to be developed for continuous phytoextraction to succeed. First, attempts to improve existing lines of phytoextracting plants should be continued as well as the search for new high-biomass metal hyperaccumulators. The usefulness of this search was demonstrated by the identification of Berkheya codii (Asteraceae), a tall, high-biomass plant from the north-eastern Transvaal, South Africa, capable of accumulating up to 3.7% nickel in its shoot dry biomass (Morrey et al., 1992). Biotechnological approaches to the production of high-biomass metal hyperaccumulators should also be considered. Modern genetic
Fig. 1.1. Schematic representation of continuous phytoextraction. Solid line represents metal concentration in shoot biomass; dashed line represents shoot biomass.
could be used to transfer hyperaccumulating genes to non-accumulating plants. Second, it is necessary to understand and exploit the biological processes involved in metal acquisition, transport and shoot accumulation in both hyperaccumulating and non-accumulating plants (Navari-Izzo and Quartacci, 2001).
1.2.2 - Chelated-assisted phytoextraction
Metal uptake is related to the availability of metals in soils and addition of synthetic chelators has been used to increase uptake and translocation of metals and to achieve high removal rates (Fig. 1.2).
This purpose could be achieved by adding both organic and inorganic agents to the soil (Schmidt, 2003). The two kinds of chelating agents must be clearly separated because their mechanisms of solubilization differ considerably.
The solubilization of heavy metals through inorganic agents relies mainly on desorption. Heavy metal solubility in soils is mainly controlled by the soil pH, the amount and kind of sorption sites and the total amount of heavy metals in soil (Schmidt, 2003).
Most inorganic agents used for phytoextraction reduce soil pH because the proportion of the soluble content of metals usually increases when the soil pH decreases. The
Fig. 1.2. Schematic
representation of chelate-assisted phytoextraction. Solid line represents metal concentration in the shoot biomass; dashed line represents shoot biomass.
mobility of arsenic, however, decreases with decreasing pH. Soil pH can be lowered by adding reduced sulphur with subsequent oxidation by soil microbes or by physiological acidification of the rhizosphere after ammonium fertilization, due to a surplus of cation exchange relative to anion uptake (Marschner, 1995). However, the growth of most crops is hampered if the soil pH is < 4 because of the toxicity of soluble ammonium and/or calcium and magnesium deficiency (Marschner, 1995). Once heavy metals are transferred from their sorption sites to the soil solution and eventually removed from the soil, further proton attack will dissolve several soil minerals. The extent of this process is dependent on soil buffer capacity, as determined by the nature of soil minerals. During continuous proton attack, however, the solubilization rate decreases with time. Several studies have also examined the effects of salt addition to contaminated soils using chlorine salts (Maxted et al., 2001). In fact, when salts are added to soils, they dissociate in the soil solution to positively and negatively charged ions. Depending on their concentration and type, the cationic components can exchange heavy metals from unspecific sorption sites in the soil. As an additional effect, soluble metal-chloride salts, especially the relatively stable CdCl2, followed by PbCl2, can be formed.
However, organic agents are usually more efficient than inorganic ones in increasing the solubility of metallic contaminants in soils and subsequently in enhancing their uptake by several crops. Because of the wide variety of organic agents, the choice of the appropriate one for extracting certain heavy metals is the first issue that must be raised. The solubilization of heavy metals is the first step in order to increase plant extraction efficiency. In the case of organic agents, the solubilization is mainly based on the formation of water-soluble metal-organic complexes. By complexation, metals are extracted or desorbed from different soil components or from the surface of these components. The extent of heavy metal solubilization by chelation with organic complexing agents follows, usually, the order of the stability constants, which were determined in aqueous solution using the ratio of metal to chelating agent (as their protonated forms) of 1:1 (Schmidt, 2003). Because acid functional groups are deprotonated with increasing pH, the solubility of organic substances increases, as does the stability of some metal-organic complexes. Thus, especially at higher pH, organic substances can contribute to heavy metal mobilization and accumulation.
The first experiments designed to test the effect of applying organic agents on the accumulation of heavy metals by plants focused on lead and were conducted with chelating agents of high metal binding capacity like EDTA (ethylenediamine tetraacetic
acid), HEDTA (hydroxyethylenediamine triacetic acid), CDTA
(1,2-cyclohexylenedinitrilo tetraacetic acid) and DTPA (diethylenetriamine pentaacetic acid) (Blaylock et al., 1997; Huang et al., 1997; Cooper et al., 1999; Wu et al., 1999). These chelating agents have a chelate binding constant (log K) with Pb of more than 15 and consequently they are the most effective in increasing the metal mobility. Among these synthetic chelating agents, EDTA often resulted in superior Pb extraction under condition encountered in most soil environments. EDTA binds the free Pb+2 cation to form an anionic complex that becomes less prone to cation exchange and sorption phenomena, thereby maintaining higher concentrations of soluble and potentially plant available lead. Many authors reported that Pb concentrations in shoots of various test plants were directly proportional to the amount of EDTA or related agents added to the soil (Blaylock et al., 1997; Huang et al., 1997; Cooper et al., 1999; Wu et al., 1999; Shen et al., 2002). Once the databases are enlarged to include other crops, agents and metals, plant metal concentrations could probably be predicted based on the concentration of soluble metals in soils. However, differences have been reported in metal accumulation depending on a wide range of factors. Wu et al. (1999) found that Pb concentrations in corn plants differed if the seedlings were transplanted to or directly germinated in the contaminated soil. Grčman et al. (2001) studied the effect of agent application frequency on Pb accumulation efficiency. Finally, Kayser et al. (2000) showed that in pots filled with the same soil as later used for field studies, three times more Cd was found in tobacco and sunflower and even seven times more in Indian mustard than in the field. On average, in the field, heavy metal removal was only 20% of what was originally expected from the pot results. Therefore, further studies are necessary to get a better understanding and to provide more detailed informations for using the above-mentioned synthetic chelating agents in real phytoremediation programs.
Generally, plants extract about 1% of the soluble metals after the addition of EDTA or related substances; in the vast majority of all studies, this value was lower than 0.2%
(Schmidt, 2003). The conclusion of this relationship is that the risk of heavy metals leaching definitely increases with chelate applications, especially when EDTA or its derivatives, poorly biodegradable in soils, are used. EDTA was found recalcitrant to biological breakdown in soils by some groups (Allard et al., 1996; Bucheli-Witschel and Egli, 2001), and others observed a slow microbial EDTA decomposition under aerobic conditions (Tiedje, 1975, 1977; Means et al., 1980; Bolton, 1996). No EDTA mineralization was found under anaerobic conditions (Tiedje, 1977; Bolton, 1996). The recalcitrance of EDTA towards biodegradation has directed much attention to other mechanisms of elimination. It has been demonstrated that the most important elimination process in rivers is photolysis, which is, however, restricted to sunny days and to only one EDTA species, i.e. Fe(III)EDTA (Bucheli-Witschel and Egli, 2001). To what extent abiotic processes are important for EDTA breakdown in soils, especially in comparison to biodegradation, remains to be investigated. Furthermore, one should not forget that abiotic processes usually do not lead to a complete mineralization of the complexing agents, but merely to a transformation into compounds that still have metal-complexing properties (Bucheli-Witschel and Egli, 2001). The high persistence of metal-EDTA complexes (Bucheli-Witschel and Egli, 2001), together with the recalcitrance of the free chelator (Bucheli-Witschel and Egli, 2001), caution against the use of this organic agent in contaminated fields. As a precautionary measure at certain sites using EDTA-assisted phytoextraction, excavation of soil and the use of water impermeable liners to capture the leachate may be necessary. However, in order to reduce the potential risk connected to the uncontrolled metal migration, it is important to identify soil amendments that efficiently solubilize metals for plant uptake but are protective of the environment.
It is generally known that root exudates released into the rhizosphere have been implicated in several mechanisms for altering the level of soluble ions and molecules in the soil (Cataldo et al., 1988). Included among the various root exudates are organic acids which are negatively charged molecules under a wide range of soil conditions and can react strongly with metal ions in both the soil aqueous and solid phases (Jones and Darrah, 1994; Jones et al., 1996). Cieśliński et al. (1998) found that the high Cd accumulating wheat cultivar Kyle had more total LMWOAs (Low Molecular Weight
Organic Acids) in the rhizosphere soil than the low Cd accumulating cultivar Arcola
showing that the interactions of organic acids with heavy metals in the soil-plant system are important for solubilizing the metals from highly insoluble mineral phases in the soil. Blay et al. (1997) explained the heavy metal mobilization of natural organic acids by several mechanisms including surface complexation with subsequent complex dissociation, soil acidification, cation exchange (through ammonium) and reductive solution of soil metal (hydr)oxides. In addition, low molecular organic acids seem to have an indirect effect on microbial activity (Wu et al., 2003), rhizosphere physical properties (Wu et al., 2003) and toxicity of metals in the roots because of their participation into the conversion of metals into bound forms (Chen et al., 2003). These positive properties of LMWOAs allowed their use as ligands to assist phytoextraction of metals. Organic acids such as citric, malic, oxalic, aspartic and glutamic acids have been reported as being potential metal chelators (Naidu and Harter, 1998). However, the metal-solubilizing ability of the carboxylic organic acids (citric, malic, oxalic) is higher than that of the amino organic acids (aspartic, glutamic), in fact carboxylic acids, particularly citric and malic acids, can strongly bind divalent cations forming very stable complexes (Senden and Wolterbeek, 1990; Cieśliński et al. 1998). For the above mentioned reasons, only the carboxylic organic acids were usually used in the phytoextraction experiments (Argilla, 2003; Quartacci et al., 2005, 2006; Irtelli and Navari-Izzo, 2006) and little informations are available about heavy metal accumulation following the application of amino organic acids to contaminated soil (Nigam et al., 2001).
Several studies have demonstrated that organic acids and especially citric acid, were generally more effective in extracting uranium from the soil than synthetic chelating agents such as EDTA and HEDTA (Ebbs et al., 1998; Huang et al., 1998). The authors suggested that the strong mobilization of uranium by citric acid is due to the formation of citrate-uranyl complex rather than to the decreased pH (Ebbs et al., 1998; Huang et al., 1998). However, organic acids resulted to be less efficient than synthetic organic chelates in mobilizing other heavy metals. In cadmium-contaminated soils, for example, EDTA performed about 10-fold better than citric acid in terms of cadmium extraction (Argilla, 2003; Quartacci et al., 2005). Anyway, the organic acids application may be
advantageous in highly polluted soils where phytotoxic effects due to the enhanced soluble metals concentrations can reduce plant growth and impair plant detoxification mechanisms.
Other highly biodegradable chelators with a very high chelating ability have been recently discovered. These chelating agents have been called Transient Phytoextraction
Agents (TPAs) thanks to rapid degradation or inactivation of soluble metal-TPAs
complexes (Elless et al., 2005). Following degradation or dissociation of the metal-agent complexes, the metals precipitate from the solution or resorb onto soil particles, thereby reverting to a low-solubility state. Examples of TPAs include sodium nitrilotriacetate (NTA) and [S,S]-ethylenediaminedisuccinic acid (EDDS).
In spite of its expected positive properties, few studies have been performed with NTA as ligand to assist phytoextraction of metals. The interest on NTA started some years ago when it was considered a suitable substitute for phosphates in detergents. Several studies showed that NTA is biodegraded in activated sludge (Shumate et al., 1970), sewage lagoons (Rudd and Hamilton, 1972), river water (Warren and Malec, 1972) and sea water (Erickson et al., 1970). In soils, NTA degrades as fast as citric acid and its degradation doesn’t correlate with pH, drainage, texture, plant cover and temperature (Tiedje and Mason, 1974). Although the rate of degradation in subsoil is always smaller than in surface soil of the same site, it was showed that NTA is also degraded in anaerobic conditions (Ward, 1986). NTA is a weaker chelator than EDTA but it is a very strong chelator compared with LMWOAs such as citrate (Wenger et al., 2003; Chiu et al., 2005; Quartacci et al., 2005, 2006; Irtelli and Navari-Izzo, 2006; Ruley et al., 2006). The effects of NTA and citric acid applications on cadmium extractability from soil as well as on its uptake and accumulation by Indian mustard were investigated (Quartacci et al., 2005; Irtelli and Navari-Izzo, 2006). Even though, NTA resulted more effective than citrate in removing Cd at lowest chelate application (10 mol kg-1 soil), the use of citric acid became advantageous when high chelate concentrations were used (Quartacci et al., 2005). However, the results of a study on a multiple metal-contaminated soil (Cd, Cr, Cu, Pb and Zn) showed that NTA treatment increased metal concentration in shoot of Indian mustard by a factor 2-3, whereas citric acid did not induce any difference compared to the control, confirming that NTA is a better
complexing agent than LMWOAs (Quartacci et al., 2006). In a pot experiment with a calcareous, mainly Cu contaminated soil, even low dosages of NTA increased the concentrations of Cd, Zn and Cu from 1.4 to 1.9-fold in lettuce and perennial ryegrass (Kulli et al., 1999). After adding higher NTA applications, the Cd, Zn and Cu concentrations in the aboveground plant biomass were 4-24 times greater than in untreated plants (Kulli et al., 1999). However, the increased plant uptake of metals had not the same behaviour for all three metals studied. Cadmium and zinc concentrations in shoots increased rather gradually with increasing NTA dose, while Cu concentrations were much more amplified at the highest NTA application level (Kulli et al., 1999). However, the reduction in growth following relatively high NTA applications resulted in total uptakes of metals that never exceeded 2.5-fold that of control (Kulli et al., 1999). Values of the same magnitude were found in a pot experiment conducted by Kaiser et al. (2000) on the same soil previously used by Kully et al. (1999). The experimental set-up differed because NTA was injected as a solution into the rooting zone of the plants. In that study very high values of extractable soil concentrations of Cd, Zn and Cu were observed after NTA amendments. However, they didn’t translate into an equivalent increase in metal uptake by plants. At 9.2 g NTA m-2, the Cd, Zn and Cu concentrations in the crops analysed (tobacco, corn, willow, sunflower and Indian mustard) were only doubled (Kaiser et al., 2000). The different effects of NTA on metal solubility in soil and plant uptake may partially be attributed to a short duration of the mobilizing effect. Uptake values of the same magnitude were also observed in pot and growth chamber experiments for Indian mustard and tobacco treated with NTA at different concentrations (Wenger et al., 2003; Quartacci et al., 2005; Irtelli and Navari-Izzo, 2006).
A new promising alternative to NTA and LMWOAs is the ethylenediaminedisuccinic acid (EDDS) (Fig. 1.3), a structural isomer of EDTA. The EDDS structure contains two chiral carbon atoms and, therefore, has three stereoisomers ([R,R]; [R,S]/[S,R]; [S,S]). Biodegradation screening of the 14C-labelled EDDS isomers mixture in a Batch Activated Sludge (BAS) test with various inocula revealed incomplete mineralization up to ca. 65% after 28 days. N-(2-aminoethyl)-aspartic acid (AEAA), probably the d-isomer was identified as the major portion of the 14C-material remaining in solution.
Further tests revealed that the stereisomer [R,R]-EDDS is non-biodegradable and the stereoisomers [R,S]- and [S,R]-EDDS are only partially degradable (Schowanek et al., 1997). By the contrast, the [S,S]-isomer is rapidly and completely mineralised using the criteria stipulated by the Organization for Economic Cooperation and Development (OECD) “60% of the compound must biodegrade within 28 days and within 10 days of achieving 10%”. The final CO2 yield for [S,S]-EDDS, assessed by the modified Sturm
test OECD 301B, exceeds 80% after 20 days (Jaworska et al., 1999; Schowanek et al., 1997). Though the readily biodegradability of [S,S]-EDDS has been amply demonstrated, the biodegradability of metal-EDDS has to be taken in consideration as well, because numerous studies, conducted on other chelating agents, have already shown that the biodegradability of metal-chelator complexes can differ drastically from the corresponding free chelating agent one (Satroutdinov et al., 2000 and papers reported therein). Activated sludge fed with [S,S]-EDDS as sole C and N source was shown to readily biodegrade 1 mM pulses of Ca-, Cr (III)-, Fe (III)-, Pb-, Al-, Cd-, Mg- and Na-EDDS at an average rate of 0.3 mM d-1. Zn-EDDS was rapidly degraded after an 11-day long lag phase (Vandevivere et al., 2001a). The biological mediation of metal-EDDS disappearance was demonstrated at the hand of sterile control showing that no significant EDDS disappearance occurred in the absence of biological activity (Vandevivere et al., 2001a). The Cu-, Ni-, Co- and Hg-EDDS complexes remained unchanged after 15 days incubation. At that time, 1 mM NaEDDS was added in order to see whether the lack of degradation was due to toxicity or recalcitrance. The pulse of EDDS was degraded in all cases except for the Hg-EDDS complex, indicating that only in the case of this metal the lack of biodegradation was a consequence of metal toxicity (Vandevivere et al., 2001a). The general lack of metal toxicity observed in that paper is
*
*
Fig. 1.3. Ethylenediamine disuccinate (EDDS) structure. The asterisks indicate the chiral carbon atom.
not surprising since it is well known that complexed metals are less toxic than acquo- metals. However, when 5 mM phosphate (K2HPO4) was added to the sludge suspension,
the biodegradation of the recalcitrant Zn- and Cu-EDDS were faster being Zn-EDDS completely degraded in about 5 days and Cu-EDDS ca. 80% degraded in 1-week adaptation period (Vandevivere et al., 2001a). The addition of metal ligand such as phosphate to metal-EDDS solutions is expected to scavenge aquo-metals Mn+, which may decrease their concentration forming M-phosphate complexes. A smaller Mn+ concentration would translate into higher HEDDS3- concentration that should yield a higher rate of metal-EDDS biodegradation. Phosphate was not expected to affect Ni- and Co-EDDS degradation since these metals do not form stable phosphate derivatives (Vandevivere et al., 2001a). Other ligands, such as FeSO4 (rapidly oxidized to FeOOH
colloids), had a good effect on disappearance of Cu- and Co-EDDS; both complexes were fully degraded in about 10 days (Vandevivere et al., 2001a). Therefore, when metal-EDDS complexes were in equilibrium or pseudo-equilibrium with other metals, compounds and anions, that is the situation occurring in a real soil, the recalcitrance of above-cited complexes is usually overcome and EDDS is promptly degraded. However, the recalcitrance of metal-EDDS complexes needs to be well investigated because of the disagreement of the scientists about this argument. For example, Tandy et al. (2006a) have recently demonstrated that low Ni-EDDS concentrations are not recalcitrant at all. The limiting step to complex biodegradation has generally been identified as complex dissociation because it is thought that predominantly uncomplexed, or sometimes Ca-bound, EDTA or NTA are taken up by bacteria (Witschel et al., 1999). It is therefore expected that complexes with very high stability constants tend to be much less amenable to biodegradation than complexes with relatively low stability constants. Although a rough correlation was found between complex stability (Kst) and
biodegradability for metal-[S,S]-EDDS complexes (Vandevivere et al., 2001a), the sole consideration of the stability constant is, in this case, insufficient to predict metal complexes degradability. For example Pb- and Zn-EDDS complexes have actually the same Kst (1012.7 and 1013.5, respectively) but very different biodegradability. Ca- and
Cd-EDDS complexes are both readily biodegraded though Cd-Cd-EDDS has a much greater stability constant. In addition, the presence of phosphate may greatly stimulate the
biodegradation of certain metal-EDDS complexes, obviously without affecting their Kst
(Vandevivere et al., 2001a). On the contrary, a good correlation was found between metal-EDDS biodegradability and the concentration of free EDDS species (Vandevivere et al., 2001a). This observation suggests that metal-EDDS complexes first need to undergo dissociation before biodegradation can occur. EDDS is also photodegraded within the natural UV radiation range being its photodegradation independent of the initial speciation (Metsärinne et al., 2001).
The awareness of the biodegradability of EDDS and metal-EDDS complexes allows the use of this new chelator as an environmentally compatible metal decontamination agent. The interest for EDDS started some years ago when Procter & Gamble identified and commercially developed a new, biodegradable, strong transition metal chelator to use in laundry detergent applications. Subsequently, [S,S]-EDDS was indicated as the best chelating agent for pulp processing in terms of efficiency for transition metal ions sequestration, magnesium retention in pulp and avoidance of ligand distraction by calcium ions (Jones and Williams, 2002). Only recently, EDDS started to be applied as agent for environmental metal decontamination. Firstly, the biodegradable strong transition metal chelator [S,S]-stereoisomer of ethylenediamine disuccinate (EDDS) was investigated for its applicability in ex situ washing extraction of heavy metals from soils, sewage sludges and sediments (Vandevivere et al., 2001b). In these experiments, EDDS showed to have an extraction efficiency equal or superior to those obtained with the benchmark chelants, ethylenediamine tetraacetic acid (EDTA) and nitrilotriacetate (NTA), achieving 70-90% extraction of Zn, Pb and Cu from all the three solids tested (Vandevivere et al., 2001b). However, ex situ chelator-assisted soil washing involves stringent physical treatments, is harsh for the soil structure and flora and has, above all, very high costs due to the necessary removal of the soil. Therefore, in order to reduce clean-up costs, the possibility of an in situ soil washing treatment is very interesting even if several side effects such as metal mobilization and leaching must be taken into consideration. The discovery of the strong, highly biodegradable chelator EDDS allowed to develop an environmentally friendly in situ soil washing technique in which the use of permeable reactive barriers prevents heavy metal leaching (Kos and Leštan, 2003; Finžgar et al., 2004). The barriers, placed below the layer of contaminated soil,
were composed of substrates for enhanced microbial degradation activity and sorbents for heavy metal ions immobilization (Kos and Leštan, 2003; Finžgar et al., 2004). Firstly, the heavy metals were mobilized by application of EDDS solution into the soil. Water soluble, biodegradable heavy metal-EDDS complexes were then washed by irrigation from the layer of contaminated soil into the barrier where they were microbially degraded. Released metal ions chemically reacted with the sorbents in the barrier to form insoluble products and, consequently, accumulated (Kos and Leštan, 2003; Finžgar et al., 2004). By simulating in situ EDDS-assisted soil washing in soil column experiments, the removal of 11.6% of total initial Pb from the soil into the active barrier was achieved in a single cycle of 10 mmol Kg-1 [S,S]-EDDS application, while leaching of Pb through the barrier was efficiently prevented (Kos and Leštan, 2003).
A technique of combined [S,S]-EDDS induced phytoextraction and in situ soil washing was also evaluated for a multi-contaminated soil with Cannabis sativa as extracting plant (Kos and Leštan, 2004). The addition of [S,S]-EDDS (10 mmol Kg-1 dry soil) yielded Pb, Zn and Cd concentrations in aboveground plant biomass 1926, 7.5 and 11 times higher, respectively, if compared to treatments with no chelator addition (Kos and Leštan, 2004). Although this kind of treatment was less effective than ex situ soil washing (Kos and Leštan, 2004), the EDDS-assisted phytoextraction can be considered a gentle remediation technique preserving soil quality. As soil is a non-renewable natural source, the use of this new biodegradable chelator is getting more and more studied in order to fully evaluate its applicability as a soil-friendly remediation technology.
In 2003, Grčman and colleagues have compared for the first time the effect of EDTA and [S,S]-isomer of ethylene diamine disuccinate (EDDS) on the uptake of Pb, Zn and Cd by Chinese cabbage (Brassica rapa var. pekinensis) and on metal leaching through the soil profile. For Pb phytoextraction, single and weekly additions of all concentrations of EDTA (3 and 5 mmol Kg-1 soil) were more effective than comparable EDDS treatments, except at the highest single dose of chelate (10 mmol Kg-1 soil) where the effects of EDTA and EDDS were similar (Grčman et al., 2003). Weekly additions of EDDS on Pb plant uptake were less effective probably because of the rapid
Pb-EDDS complex biodegradation (Vandevivere et al. 2001a) and the consequent shorter-term availability of mobilised Pb species. Both EDTA and EDDS were similarly effective in enhancing Cd and Zn plant uptake (Grčman et al., 2003). In soil columns treated with weekly additions of 5 and 10 mmol Kg-1 soil EDTA, on average 22.7%, 7.0% and 39.8% of total Pb, Zn and Cd were leached through the soil profile. The same amounts of EDDS caused much lower leaching of Pb and Cd (only 0.8 and 1.5% of initial total concentrations) and comparable leaching of Zn (6.2% of the total concentration) (Grčman et al., 2003). The biotest with red clover (Trifolium pratense L.) indicated a greater phytotoxic effect of EDTA than EDDS addition. EDDS was also less toxic to soil fungi, as determined by phospholipid fatty acid (PLFA) analysis and caused less stress to soil microorganisms as indicated by the trans to cis PLFA ratio (Grčman et al., 2003). Starting from that study, other works have been done comparing EDDS and EDTA effects on metal mobilisation, uptake and translocation. EDDS and EDTA persistence and toxicity have been evaluated as well. Strong mobilization of heavy metals and, consequently, strong effect on plant uptake capacity were observed in both cases. However, distinct differences were found comparing the two amendants. Usually, Zn mobilization was comparable following application of both chelates (Meers et al., 2005a, 2005b), Cu was mobilised more by EDDS than by EDTA (Meers et al., 2005a, 2005b; Luo et al., 2005, 2006), Cd and Pb were mobilised more by EDTA than by EDDS (Meers et al., 2005a, 2005b; Luo et al., 2005, 2006) and initial mobilisation of Ni was highest for EDDS (Meers et al., 2005a, 2005b) but was overtaken by EDTA as time progressed (Meers et al., 2005a). However, all authors agreed about the stronger persistence of EDTA in soils. While the mobilisation effect decreased rapidly for the soil treated with EDDS, with calculated half-life of 3.8-7.5 days depending on applied doses, experimental conditions and soil type, the half-life observed in EDTA-treated soils ranged between 36 days and infinity (Meers et al., 2005a; Luo et al., 2005, 2006). However, the solubilization of metals from the soil and their uptake into the roots are not sufficient for improving phytoextraction efficiency. An ideal chelator should enhance also the translocation to the aboveground biomass of plants.
EDTA was shown to have a great influence in rapid shoot lead uptake. An increase in Pb shoot uptake of 400-fold in comparison with the control was found in B. juncea
amended with 0.75 mM EDTA (Vassil et al., 1998). Increases in shoot Pb concentrations in presence of EDTA were observed in other studies and mostly fall in the range of 2-10 times the control (Wu et al., 1999; Hernandez-Allica et al., 2003; Piechalak et al., 2003; Quartacci et al., 2005). Direct methods have been used to measure EDTA inside plants either as a complex (Collins et al., 2001, 2002; Sarret et al., 2001) or as total EDTA (Vassil et al., 1998; Epstein et al., 1999; Bell et al., 2003). These experiments have proved that EDTA is taken up from solutions containing high concentrations of chelator and that the formation of EDTA-Pb complex is responsible of improving Pb translocation. Also EDDS was shown to increase metal translocation from roots to shoots rapidly and dramatically. When 5 mmol Kg-1 EDDS were applied to the soil, the percentage of absorbed Cu translocated from roots to shoots increased from 8.5% to 83% and from 10.2% to 93% in corn and beans, respectively (Luo et al., 2005). The shoot uptake of Pb was enhanced in presence of EDDS as well (Tandy et al., 2006b). Direct investigations, used to measure EDDS inside the plants, confirmed that also this chelator, as seen for EDTA, is taken up by plants (Tandy et al., 2006b). In conclusion, EDDS shows the same effectiveness of its structural isomer EDTA regarding metal solubilization in soil and metal uptake and translocation in plants but it has a much shorter persistence being promptly biodegraded. The above-mentioned reasons make [S,S]-EDDS an optimal biodegradable alternative to EDTA in environmental uses and real field phytoextraction programs.
1.2.3 – Exudates role in metal exclusion and hyperaccumulation phenomena
Some compounds commonly exuded from the roots are implicated in several mechanisms including the mobilisation and uptake of nutrients by plants and microorganisms (e.g. P and Fe) (Rabotti and Zocchi, 1994; Johnson et al., 1996; Jones et al., 1996 and papers reported therein), the detoxification of metals by plants (e.g. Al) (Ma et al., 1997a,b; Ishikawa et al., 2000; Matsumoto, 2000; Watanabe and Osaki, 2002 and papers reported therein), microbial proliferation in the rhizosphere, and the
dissolution of soil minerals leading to pedogenesis (Cataldo et al., 1988; Marschner, 1995).
Higher plants employ two basic strategies to tolerate heavy metals in their environment: (1) exclusion, whereby uptake and/or root to shoot transport of metals are restricted; and (2) accumulation, whereby metals are accumulated and detoxified in the shoots (Baker, 1981). Metal exclusion is by far the most common strategy in metal tolerant species. On the other hand, metal accumulation is a rare phenomenon on terrestrial higher plants. To date, about 400 plant species have been identified as metal hyperaccumulators, representing less than 0.2% of all angiosperms. Although some progresses have been made towards an understanding of plant-internal mechanisms associated with metal hyperaccumulation, it is still not clear whether metal hyperaccumulators employ rhizosphere related processes to enhance metal accumulation. An understanding of the mechanisms by which hyperaccumulator plants solubilize and absorb heavy metals is important and it may help to pinpoint ways of improving metals removal from soils. Factors of major importance to the solubilization of metallic elements by plants could be: 1) root-induced changes in pH of the rhizosphere, 2) increased reducing capacity of the roots, and 3) amount and composition of root exudates (Marschner, 1995). Changes in pH and redox potential were studied in the rhizospheres of a nickel hyperaccumulator plant (Alyssum murale) and of a crop plant (Raphanus sativus) grown in heavy metal-contaminated substrates (Bernal et al., 1994). Differences in pH and reducing activity were found between the lateral and the main roots of both species, but the acidification and reducing capacity of the roots of A. murale were always smaller than those of R. sativus (Bernal et al., 1994) suggesting that the mechanism of metal solubilization by the hyperaccumulator plant does not involve either the reduction of pH in the rhizosphere or the release of reductants from roots. These results were confirmed by other studies that, using Thlaspi caerulescens, have definitively ruled out the role of rhizosphere acidification in metal hyperaccumulation (Knight et al., 1997; McGrath et al., 1997; Luo et al., 2000).
The other possibility is that the hyperaccumulators may release chelating compounds to the rhizosphere to mobilise heavy metals. Wenzel et al. (2003) found a significant increase of organic compounds in the Ni hyperaccumulator Thlaspi goesingese
rhizosphere in comparison with the bulk soil and the rhizospheres of the excluders Silene vulgaris L. and Rumex acetosella L. grown natively on the same serpentine site. Although these data suggest that exudation of organic ligands may contribute to enhance solubility and replenishment of metals in the rhizosphere of hyperaccumulating species, no other confirmations have been obtained to date. In contrast with Wenzel and colleagues are Salt et al. (2000) who did not find any high-affinity Ni-chelating compounds in the root exudates of the same Ni hyperaccumulator T. goesingese. Also for T. caerulescens no evidence was found that root exudates are involved in active mobilisation of Zn and Cd (Zhao et al., 2001). Two different ecotypes of the Zn/Cd hyperaccumulator T. caerulescens were compared with the non-accumulators wheat (Triticum aestivum) and canola (Brassica napus). The two ecotypes of T. caerulescens did not release significantly greater quantities of exudates than the other two species. In addition, the mobilisation capacity of root exudates of T. caerulescens was similar to that of canola but significantly smaller than that of wheat (Zhao et al., 2001). The results indicate that the root exudates of the Zn/Cd hyperaccumulator T. caerulescens contained no significant amounts of chelating compounds with high affinity for metals and therefore they are likely not involved in Zn and Cd hyperaccumulation. If so, other mechanisms can explain the phenomenon of hyperaccumulation better than the excretion of root exudates. First, roots of hyperaccumulator plants appear to sense and actively forage metals by proliferating in metal-rich patches in soil (Schwartz et al., 1999; Whiting et al., 2000). Second, the plasma membrane of root cells of hyperaccumulator plants seems to have a higher density of metals transporters (Lasat et al., 1996; Pence et al., 2000; Lombi et al., 2001).
The exudation of organic compounds seems to be linked more to the exclusion mechanism than to the hyperaccumulation phenomenon. For example, the exudation of organic acids from the roots is considered one of the most important strategies by which plants can exclude aluminium (Matsumoto, 2000; Watanabe and Osaki, 2002). De la Fuente and colleagues (1997) introduced a Pseudomonas aureginosa citrate synthase (CS) gene into tobacco and papaya, causing the CS activity and citrate exudation to increase and the Al tolerance to be enhanced in transgenic plants. High citrate exudation from roots was found also in other Al-excluder plants such as Al-resistant cultivars of