RESEARCH PAPER
Erythropoietin in amyotrophic lateral sclerosis:
a multicentre, randomised, double blind, placebo
controlled, phase III study
Giuseppe Lauria,
1Eleonora Dalla Bella,
1Giovanni Antonini,
2Giuseppe Borghero,
3Margherita Capasso,
4Claudia Caponnetto,
5Adriano Chiò,
6Massimo Corbo,
7,8Roberto Eleopra,
9Raffaella Fazio,
10Massimiliano Filosto,
11Fabio Giannini,
12Enrico Granieri,
13Vincenzo La Bella,
14Giancarlo Logroscino,
15Jessica Mandrioli,
16Letizia Mazzini,
17Maria Rosaria Monsurrò,
18Gabriele Mora,
19Vladimiro Pietrini,
20Rocco Quatrale,
21Romana Rizzi,
22Fabrizio Salvi,
23Gabriele Siciliano,
24Gianni Sorarù,
25Paolo Volanti,
26Irene Tramacere,
27Graziella Filippini,
27on behalf of
the EPOS Trial Study Group
For numbered affiliations see end of article.
Correspondence to Dr Giuseppe Lauria, 3rd Neurology Unit, Department of Clinical Neurosciences, IRCCS Foundation,“Carlo Besta” Neurological Institute, Via Celoria, 11-20133 Milan, Italy; [email protected] Received 15 July 2014 Revised 26 August 2014 Accepted 14 September 2014 Published Online First 9 February 2015
To cite: Lauria G, Dalla Bella E, Antonini G, et al. J Neurol Neurosurg Psychiatry 2015;86:879–886.
ABSTRACT
Objective To assess the efficacy of recombinant human erythropoietin (rhEPO) in amyotrophic lateral sclerosis (ALS).
Methods Patients with probable laboratory-supported, probable or definite ALS were enrolled by 25 Italian centres and randomly assigned (1:1) to receive intravenous rhEPO 40 000 IU or placebo fortnightly as add-on treatment to riluzole 100 mg daily for 12 months. The primary composite outcome was survival, tracheotomy or >23 h non-invasive ventilation (NIV). Secondary outcomes were ALSFRS-R, slow vital capacity (sVC) and quality of life (ALSAQ-40) decline. Tolerability was evaluated analysing adverse events (AEs) causing withdrawal. The randomisation sequence was computer-generated by blocks, stratified by centre, disease severity (ALSFRS-R cut-off score of 33) and onset (spinal or bulbar). The main outcome analysis was performed in all randomised patients and by intention-to-treat for the entire population and patients stratified by severity and onset. The study is registered, EudraCT 2009-016066-91.
Results We randomly assigned 208 patients, of whom 5 (1 rhEPO and 4 placebo) withdrew consent and 3 ( placebo) became ineligible (retinal thrombosis, respiratory insufficiency, SOD1 mutation) before receiving treatment; 103 receiving rhEPO and 97 placebo were eligible for analysis. At 12 months, the annualised rate of death (rhEPO 0.11, 95% CI 0.06 to 0.20; placebo: 0.08, CI 0.04 to 0.17), tracheotomy or >23 h NIV (rhEPO 0.16, CI 0.10 to 0.27; placebo 0.18, CI 0.11 to 0.30) did not differ between groups, also after stratification by onset and ALSFRS-R at baseline. Withdrawal due to AE was 16.5% in rhEPO and 8.3% in placebo. No differences were found for secondary outcomes.
Conclusions RhEPO 40 000 IU fortnightly did not change the course of ALS.
INTRODUCTION
Several in vitro and in vivo models of degenerative, toxic and inflammatory peripheral and central nervous system diseases have shown that
erythropoietin (EPO) and its non-erythropoietic derivatives have neuroprotective properties.1 EPO is a circulating glycoprotein whose principal func-tion is the producfunc-tion of red blood cells through the inhibition of erythroid progenitor apoptosis and regulation of differentiation in the bone marrow. Endogenous EPO and the recombinant human EPO (rhEPO) form are identical regarding the sequence of amino acids, but have heterogeneous bioavailabil-ity and pharmacokinetics due to the different com-position of the sugar side chains, indicated by a Greek letter suffix (eg, α, β, γ and δ). EPO and its classical receptor (EPOR) are expressed in human neurons and astrocytes. Preclinical studies demon-strating that non-erythropoietic EPO derivatives could also be tissue protective2suggested the exist-ence of a further non-haematopoietic receptor (EPOR)2, shared by members of the interleukin-3
receptor family.3 Nevertheless, studies in rodent models demonstrated that the expression of the clas-sical EPOR was mandatory for EPO-induced neuroprotection.4
The hypothesis that EPO could specifically exert a protective effect on motor neurons arose from several lines of research. EPO was found to protect cultured motor neurons from serum-BDNF (brain-derived neurotrophic factor) deprivation or long-term kainate exposure.5 Treatment with EPO and non-erythropoietic derivatives improved motor per-formances and protected from motor neuron degeneration in the wobbler mouse model, although the effects were limited to women and survival was not prolonged.6In SOD1G93A mice, increased EPO and EPOR expression in the brain cortex was interpreted as a possible compensatory effect of altered neuronal function.7 In the same model, EPO treatment delayed the onset of motor deterioration and protected thoracic spinal cord motor neurons from degeneration.8However, this effect could not be replicated.9EPO was also found to reduce SOD1 aggregates in motor neurons.10In patients with amyotrophic lateral sclerosis (ALS), the cerebrospinalfluid EPO level was lower than in
Open Access Scan to access more
patients with other neurodegenerative diseases, and its concen-tration was suggested to correlate with the progression of the disease.11 Moreover, the cerebrospinal fluid concentration of EPO and vascular endothelial growth factor were found to be significantly higher and lower, respectively, in hypoxaemic patients with ALS, suggesting an intact common oxygen-sensor pathway.12 On the basis of the results of our previous pilot study,13 we designed a double-blind, randomised, placebo
con-trolled trial to assess the efficacy of rhEPO as an add-on treat-ment in patients with sporadic ALS.
METHODS Patients
The trial was coordinated by the ALS Centre at the IRCCS Foundation ‘Carlo Besta’ of Milan. Consecutive patients were screened for eligibility at all the 25 Italian ALS centres. Patients aged 18–75 years and diagnosed with probable laboratory-supported, probable or definite ALS according to El Escorial revised criteria were eligible. Inclusion criteria were onset of weakness ≤18 months and slow vital capacity (sVC) ≥70% of predicted in seated position at screening visit. Exclusion criteria were haematocrit >51% in men and >49% in women, haemo-globin value >17 g/dL; non-invasive ventilation (NIV) >6 h daily; known familial ALS or first-degree relative with ALS; diagnosis of frontotemporal dementia; history and/or instru-mental evidence of previous thrombotic vascular event or cardiac diseases; uncontrolled hypertension (systolic ≥160 mm Hg and diastolic ≥95 mm Hg irrespective of treat-ments at two consecutive evaluations); active solid or myelopro-liferative malignancy; known hypercoagulable disorders. All patients were asked to continue riluzole 100 mg daily or to be on a stable dose for at least 30 days prior to screening. Patients who did not take riluzole at the screening visit were considered eligible and could be randomized; in this case, they could not start riluzole during the entire study period. The protocol was approved by the ethics committee at each site. All patients pro-vided written informed consent before any study-related procedure.
Trial outcomes
The primary outcome was one single composite outcome of time from randomisation to death, tracheotomy or >23 h NIV daily for 14 consecutive days. Secondary outcomes were changed from baseline in the ALSFRS-R score, sVC in the seated position and patients’ quality of life measured by the ALSAQ-40 questionnaire. Tolerability was evaluated analysing adverse events (AEs) causing withdrawal.
Randomisation
Patients were randomly assigned to receive either intravenous rhEPO 40 000 IU or matching placebo (1:1 ratio) fortnightly for 12 months. Randomisation was stratified by centre, disease severity (ALSFRS-R≤33 vs >33) and onset (spinal or bulbar), with a block size of four within each centre. The Neuroepidemiology Unit at the coordinating centre, independ-ent of the study, generated the computer-based randomisation sequence known only to two staff persons and the drug dispen-ser. Treatment was allocated by a web-based randomisation system, available 24 h a day. The procedure incorporated eligi-bility checks according to protocol and was performed on request from the centres. The sequence was always available for emergency unmasking.
Masking
RhEPO (Eprex) was purchased from Janssen-Cilag SpA (Cologno Monzese, Italy) by the coordinating centre and dir-ectly shipped to the company (Pierrel Research IMP srl, Cantù, Italy) in charge of preparing the investigational drug (rhEPO or 1 cc of saline) in syringes of identical appearance, sealed in sequentially numbered identical containers according to the allocation sequence. Shipping was performed for each patient within 1 week after randomisation using a refrigerated express carrier. Each centre stored the investigational drug at−4°C.
Patients, neurologists, laboratory biologists/technicians (two at each centre) and a statistician were masked to treatment alloca-tion and did not have access to any data related to rhEPO haem-atopoietic activity. Briefly, all participating centres generated a specific access code for the blood withdrawals of patients with ALS enrolled in the EPOS trial. At each treatment visit, the local laboratory unit received the de-identified vial reporting the ran-domisation number before treatment administration. Blood parameters were emailed to the coordinating unit where a trained person readily alerted the treating neurologist on the decision to administer or delay the treatment based on safety protocol parameters (see below). Patients and treating neurolo-gists did not have access to any data on blood parameters, whereas laboratory personnel did not have access to any data allowing the identification of the patient. This procedure was elaborated to maintain the blindness of the study. Finally, when we designed the study, we reasoned that patients treated with rhEPO could have had a higher risk of treatment delay as defined by the safety protocol. Therefore, the randomisation centre generated a list of placebo patients randomly assigned to 1 week treatment delay balanced with the number of patients allocated on rhEPO treatment needing true delay at each centre. This procedure was elaborated to maintain the blindness of the study.
Procedures
After giving informed written consent, eligible patients under-went haematological examinations (haemochromocytometry, renal and liver function, serum iron, ferritin, transferrin, reticu-locyte count, coagulation tests), blood pressure and body mass index (BMI) measurement and sVC, ALSFR-R and ALSQ40 assessment. Randomisation was performed within 15 days after the screening visit. At each fortnightly treatment visit, safety parameters (haematocrit >51% in men and >49% in women or haemoglobin value >17 g/dL and value raised >1 g/dL at the end of the interval between two subsequent doses), blood pres-sure and BMI were assessed. Symptoms of nocturnal hypoventi-lation (nocturnal arousals, morning headache, excessive daytime sleepiness, vivid dreams), medications and AE were actively monitored and recorded. ALSFR-R and sVC were assessed monthly. At the 6-month and study end or dropout visit, the patient also underwent complete haematological examinations and ALSAQ-40 assessment. At each treatment visit, treatment administration was allowed or delayed for 1 week by the trial coordinating office after assessment of safety parameters. If treatment was delayed, the following week safety parameters were repeated before drug administration. The delay of treat-ment administration for more than two times caused the dropout of the patient. After the study end at month 12, patients underwent monthly follow-up visits for a further 6 months to record primary outcome events. All centres were provided with a spirometer (Spirobank G multifunction, Medisan srl, Milano, Italy) and disposable tubes for sVC
assessment, and trained in its use. All data were recorded by an electronic case record form specifically developed (Nubilaria srl, Novara, Italy). Trial monitoring was performed by an independ-ent contract research organisation (CROM srl, Verona, Italy) that assured consistency in measuring outcomes across centres by scheduled site visits.
Co-treatments
Nutritional status and ventilation could affect survival and thus the primary outcome of the trial. During the first investigator meeting held in Milan on 6 June 2010, all participating centres agreed on the approach to cotreatments, sharing the opinion that the ultimate decision would be personal to each patient. We agreed that percutaneous endoscopic gastrostomy or an equiva-lent device should be proposed to all patients in the case of score 1 or 2 at item 3 of the ALSFRS-R, unintentional loss of body weight >10% in the past 3 months or choking during food, liquids or medication ingestion. NIV should be proposed to all patients in the case of score 0 or 1 at item 10 or 11 of the ALSFRS-R, sVC<50% or abnormal nocturnal oximetry (SaO2
<90% for 4% of the overnight recorded time).
Sample size estimation
On the basis of the results of our pilot study (ie, observed rates of 0.56 for death and 0.33 for tracheotomy at 18 months in the placebo group),13 we estimated that we would need a sample
size of 203 patients followed up for 12 months to give 97% power to detect a significant difference between rhEPO and placebo corresponding to a 67% relative reduction of risk of
death and 74% power to detect a 70% relative reduction of risk of respiratory events (tracheotomy or >23 h NIV), with a two-sided type 1 error of 5% and given an anticipated dropout rate of 10%.
Statistical analysis
The main analysis of primary and secondary outcomes included all randomised patients who took at least one dose of the investi-gational drug in their original assigned groups. All analyses were performed both for the entire population and for the subgroups of patients with ALSFRS-R ≥33 or <3314 and with spinal or bulbar onset at randomisation. A per-protocol analysis was carried out excluding non-compliers ( patients who took <80% therapy). Demographic characteristics and clinical features of randomised patients at baseline were reported by treatment arm and compared usingχ2test, student t test, Wilcoxon rank-sum
test or Fisher’s exact test as appropriate. Time from randomisa-tion to death, tracheotomy or >23 h NIV daily for 14 consecu-tive days was analysed in terms of the annualised rate with the corresponding 95% CI, and p value using a χ2 test with one
degree of freedom for rate comparison (based on Poisson regres-sion). The Kaplan-Meier method was used to obtain survival curves with the corresponding log-rank test. A Cox model was applied to estimate the treatment effect in terms of HR with 95% CI, adjusted for sex, age, ALSFRS-R score at baseline and disease duration. The number of patients experiencing an AE causing withdrawal were reported and compared between the two groups by Kaplan-Meier curves of the time to withdrawal and the corresponding log-rank test. Change from baseline in sVC,
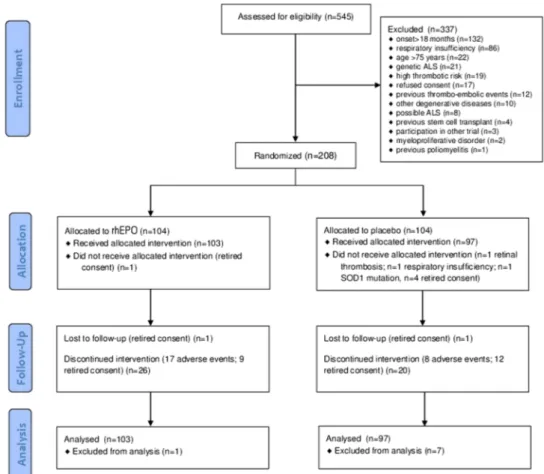
Figure 1 Flow chart of the EPOS trial. CONSORTflow diagram. Flow diagram showing the progress of patients throughout the EPOS trial. ALS, amyotrophic lateral sclerosis rhEPO; recombinant human erythropoietin.
ALSFRS-R and ALSAQ-40 was assayed by mixed effect models. All data were analysed using SAS V.9.3 (SAS Institute INC. Cary, North Carolina, USA). This study is registered with EudraCT, number 2009-016066-91 (EPOS trial).
RESULTS
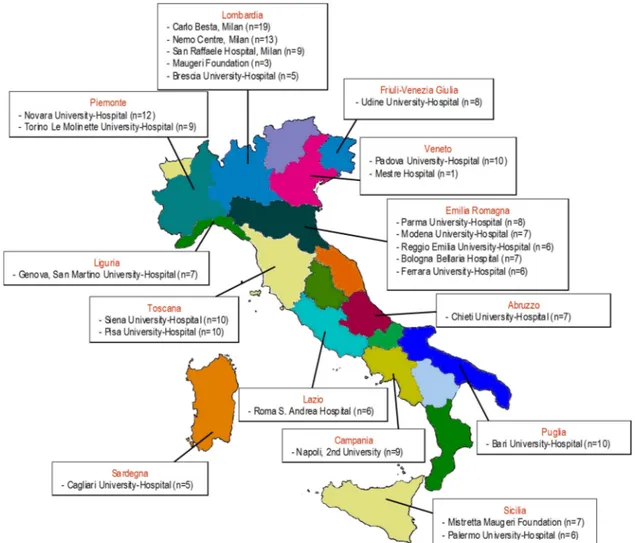
Between August 2010 and November 2012, 208 of 545 eligible patients (38%) were randomly allocated to the treatment arms (104 in the rhEPO arm and 104 in the placebo arm;figure 1). Patients were recruited at 25 Italian ALS centres from 13 regions (figure 2).Table 1shows the demographic and clinical character-istics of the treatment groups at baseline. In the analysis of the primary outcome performed on all patients who received at least one dose of the interventional treatment (eg, 103 patients in the rhEPO arm and 97 patients in the placebo arm), the rate of events (death, tracheotomy, >23 h NIV) at 12-month follow-up did not differ between the treatment groups, even after strati fica-tion by disease severity and onset (table 2). The Kaplan-Meier analysis did not disclose any difference in terms of the log-rank test ( p=0.99;figure 3) and Peto test ( p=0.89). The correspond-ing HR between rhEPO and placebo, adjusted for gender, age, ALSFRS-R score and disease duration, was 1.02 (95% CI 0.57 to 1.83). The analysis of efficacy by intention-to-treat performed on all randomised patients (eg, 104 patients in the rhEPO and placebo groups) yielded the same results.
The percentage of AE causing withdrawal was twice as high in the rhEPO (16.5%) group as in the placebo (8.3%) group,
although the difference was not significant in terms of the log-rank test ( p=0.16), most likely due to the small number of events (table 3;figure 4). In the rhEPO group, we recorded four cases of deep venous thrombosis complicated in two cases by pulmonary embolism, two cases of cardiac arrhythmia, four cases of treatment suspension for >8 weeks and seven cases of delay more than two times due to altered safety parameters. In the placebo group, we recorded one case of deep venous throm-bosis, one case of cardiac infarction, four cases of treatment sus-pension for >8 weeks and two cases of delay more than two times due to altered safety parameters (table 3).
The analysis of the secondary outcomes during the 12 months of follow-up did not show differences between groups either in ALSFRS-R ( p of mixed-effects models=0.31) and sVC ( p of mixed-effects models=0.47) decline (figure 5) or ALSAQ-40 score (+29 points in the rhEPO group and +37 points in the placebo group from baseline to 12 months; p of mixed-effects models=0.23).
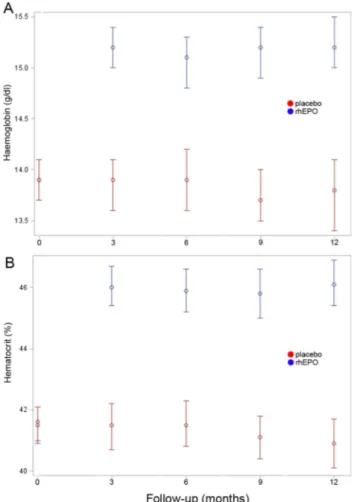
All the above analyses were also performed for the subgroups of patients with ALSFRS-R≥33 or <33 at randomisation and with spinal or bulbar onset, and per-protocol, and did not show any significant difference between the two treatment groups (data not shown). Haemoglobin and haematocrit values over-lapped in the rhEPO and placebo groups at baseline, whereas they were significantly (p<0.01) higher in the rhEPO group than the placebo group throughout the entire study period (figure 6).
At 18-month follow-up, 6 months after the treatment was stopped, 41 (41%; 3 lost to follow-up) of 100 patients in the rhEPO group and 31 (33.3%; 4 lost to follow-up) of 93 placebo patients reached the primary outcome (death or tracheotomy).
DISCUSSION
Several studies suggested that EPO can promote the homeostasis of cells under stress and exert protective actions on different
tissues. Peripheral administration allows EPO to penetrate through an intact blood-brain-barrier,15–17 and this has been exploited to test its neurotrophic effects in multiple sclerosis, Parkinson’s disease and schizophrenia.18 19Encouraging
preclin-ical studies with EPO and its non-erythropoietic derivatives in models of central and peripheral nervous system degenerative diseases,2 expression of the non-erythropoietic receptor (EPOR)2 in motor neurons6 and preliminary data from ALS
mouse models8and a pilot clinical trial13suggested that patients with ALS might benefit from rhEPO treatment. Conversely, our study demonstrated that rhEPO administered at the dose of 40 000 IU fortnightly did not change either survival or disability at 12 months. Patients’ demographic and clinical features were well balanced between the treatment arms, supporting the reli-ability of the results. The significant and stable increase of haemoglobin and haematocrit values in the rhEPO group throughout the entire treatment period demonstrated that rhEPO exerted its expected haematological effects, and there-fore it was not degraded in patients with ALS. This observation strengthens our negative findings, suggesting that the lack of neuroprotective effect could not be attributed to an altered bio-logical activity of rhEPO at the haematobio-logical level.
Our negativefindings appear to be in keeping with those dis-appointing from previous clinical studies investigating rhEPO neuroprotection in critical illness and patients with stroke.1 RhEPO may remain a promising treatment in schizophrenia19
and in the prevention of cognitive impairment after cardiac surgery and cardiopulmonary bypass,20 though these
prelimin-ary data need larger confirmatory studies. Therefore, despite the bulk of preclinicalfindings in favour of a substantial protective activity of EPO outside the bone marrow, no evidence is cur-rently available to support the hypothesis that rhEPO can rescue injured neurons in patients with acute or chronic progressive neurological diseases like stroke and ALS.
In most previous clinical studies of neuroprotection, cardio-protection and renal cardio-protection, rhEPO was acutely administered at doses ranging from 40 000 IU daily for 3 days to 50 000 IU 24 and 48 h after the event, whereas in critically ill patients the schedule was 40 000 IU weekly for 3 weeks.1 Ours is the first trial in which rhEPO was chronically administered to
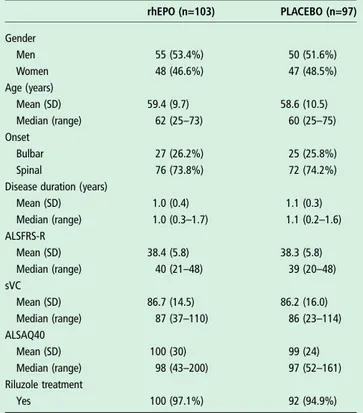
non-Table 1 Demographic characteristics and clinical features of randomised patients at baseline in the two treatment groups
rhEPO (n=103) PLACEBO (n=97) Gender Men 55 (53.4%) 50 (51.6%) Women 48 (46.6%) 47 (48.5%) Age (years) Mean (SD) 59.4 (9.7) 58.6 (10.5) Median (range) 62 (25–73) 60 (25–75) Onset Bulbar 27 (26.2%) 25 (25.8%) Spinal 76 (73.8%) 72 (74.2%)
Disease duration (years)
Mean (SD) 1.0 (0.4) 1.1 (0.3) Median (range) 1.0 (0.3–1.7) 1.1 (0.2–1.6) ALSFRS-R Mean (SD) 38.4 (5.8) 38.3 (5.8) Median (range) 40 (21–48) 39 (20–48) sVC Mean (SD) 86.7 (14.5) 86.2 (16.0) Median (range) 87 (37–110) 86 (23–114) ALSAQ40 Mean (SD) 100 (30) 99 (24) Median (range) 98 (43–200) 97 (52–161) Riluzole treatment Yes 100 (97.1%) 92 (94.9%)
Svc, slow vital capacity; rhEPO, recombinant human erythropoietin.
Table 2 Analysis of efficacy for the primary outcome at 12-month follow-up
rhEPO (n=103; PY=92) Placebo (n=97; PY=88) p Value
Overall events (death, tracheotomy, >23 h NIV)
N (annualised rate; 95% CI) 25 (0.27; 0.18 to 0.40) 23 (0.26; 0.17 to 0.39) 0.88
Death
N (annualised rate; 95% CI) 10 (0.11; 0.06 to 0.20) 7 (0.08; 0.04 to 0.17) 0.52
Tracheotomy or >23 h NIV
N (annualised rate; 95% CI) 15 (0.16; 0.10 to 0.27) 16 (0.18; 0.11 to 0.30) 0.77
Overall events stratified by disease onset and severity
Spinal onset n=76; PY=68 n=72; PY=65 0.73
N (annualised rate; 95% CI) 21 (0.31; 0.20 to 0.47) 18 (0.28; 0.17 to 0.44)
Bulbar onset n=27; PY=24 n=25; PY=23 0.71
N (annualised rate; 95% CI) 4 (0.17; 0.06 to 0.45) 5 (0.22; 0.09 to 0.52)
ALSFRS-R≥33 n=86; PY=79 n=84; PY=77 0.69
N (annualised rate; 95% CI) 17 (0.22;0.13 to 0.35) 19 (0.25; 0.16 to 0.39)
ALSFRS-R <33 n=17; PY=13 n=13; PY=12 0.37
N (annualised rate; 95% CI) 8 (0.60; 0.30 to 1.20) 4 (0.35; 0.13 to 0.93)
The analysis was performed in terms of the annualised rate with the corresponding 95% CI and p value using aχ2test with one degree of freedom for rate comparison (based on
Poisson regression).
anaemic patients with a degenerative disease for a 12-month period. One limitation may appear to be the lack of a previous dose-finding study. However, we have chosen the dosing schedule of rhEPO 40 000 IU fortnightly based on the pharmacokinetic profile, the known linear relationship between a single dose administered and erythropoietic response, and the turnover of reticulocytes,21with the aim of reducing the thrombotic risk in patients. Moreover, acute higher doses used in previous clinical studies increased the rate of thrombotic vascular events.1 40 000 IU approximately equals one-third of the maximally effective single dose in a 70 kg participant (eg, 1800 IU/kg). In our study, it did not significantly increase the rate of thrombotic complications compared with the placebo group, most likely due to the small number of events, being the overall number of AEs twice as high as in the rhEPO treated group. In rodent models,22 the dose of 2500 IU/kg/day was reported to be the most effective for neuroprotection but caused an increase of haematocrit value that would not be acceptable in humans.
Figure 3 Primary outcome analysis. Survival probability in terms of death, tracheotomy and 23 h non-invasive ventilation for 14 consecutive days during the 12 months of the EPOS trial, with the corresponding p value of the log-rank test.
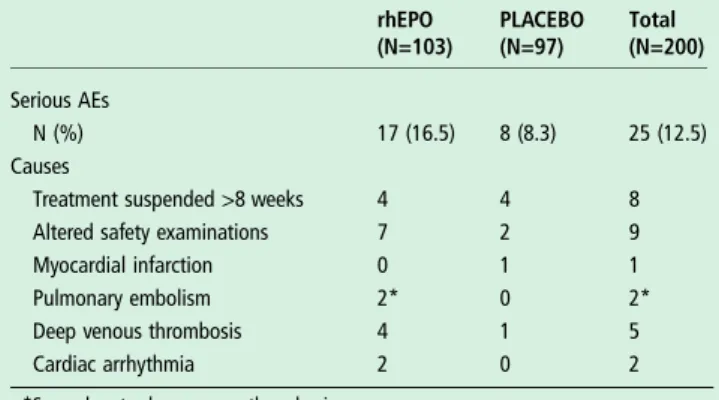
Table 3 Number and percentage of AEs causing withdrawal in the two treatment groups
rhEPO (N=103) PLACEBO (N=97) Total (N=200) Serious AEs N (%) 17 (16.5) 8 (8.3) 25 (12.5) Causes
Treatment suspended >8 weeks 4 4 8
Altered safety examinations 7 2 9
Myocardial infarction 0 1 1
Pulmonary embolism 2* 0 2*
Deep venous thrombosis 4 1 5
Cardiac arrhythmia 2 0 2
*Secondary to deep venous thrombosis.
AEs, adverse events; rhEPO, recombinant human erythropoietin.
Figure 4 Adverse event analysis. Survival probability in terms of adverse events causing withdrawal during the 12 months of the EPOS trial, with the corresponding p value of the log-rank test.
A recent reappraisal of patients’ clinical features included in ALS trials suggested that the intrinsic limitations imposed by a classical randomised clinical trial can lead to the exclusion of patients representing the ALS population in clinical practice, reducing the reliability of the results.23It has been suggested that the enrolment of patients in the earliest phases of ALS could increase the prob-ability of identifying successful disease-modifying treatments. In our study, the percentage of patients excluded due to respiratory insufficiency (15%) and of those who did not reach the diagnostic certainty level of probable ALS according to the revised El Escorial criteria (1.5%) at randomisation was small compared with previ-ous trials. Similarly, gender distribution was well balanced between arms, thus avoiding the underrepresentation of women observed in other trials.23However, the mean age of ALS onset was slightly
lower than that recorded in epidemiological studies,24–27possibly accounting for the lower 1-year death rates.
Like previous trials in ALS, results from our pilot study did not replicate in the larger phase III trial. In particular, in the pilot study, we had observed a higher prevalence of primary outcome events (death and tracheotomy) in the placebo group at 18 months follow-up, most likely a chance result due to the small sample size. In ALS, the most important goal of new treat-ments which can protect motor neurons and axons from pro-gressive degeneration in a time frame that is useful to patients remains far from be achievement. Our phase III randomised trial demonstrated that rhEPO does not have any positive effect
on the course of ALS, lengthening the list of disappointing results from all the previous studies.28–37
Author affiliations
1Neuromuscular Disease, IRCCS Foundation,“Carlo Besta” Neurological Institute,
Milan, Italy
2NESMOS Department, Neuromuscular Disease Unit, Sant’Andrea Hospital and
University of Rome“Sapienza”, Rome, Italy
3Neurologic Unit, Monserrato University Hospital, Cagliari, Italy 4
Neurologic Clinic, SS. Annunziata Hospital, Chieti, Italy
5Departments of Neurosciences, Rehabilitation, Ophtalmology, Genetics, Mother and
Child Disease, IRCCS University Hospital San Martino IST, Genova, Italy
6Department of Neurosciences, ALS Centre,“Rita Levi Montalcini” Azienda
Ospedaliero Universitaria Città della Salute e della Scienza, Turin, Italy
7NEMO Clinical Centre, Milan, Italy 8
Department of Neurorehabilitaton, Casa Cura Policlinico, Milan, Italy
9Neurology Unit, S. Maria della Misericordia University Hospital, Udine, Italy 10
Department of Neurology, IRCCS“San Raffaele” Hospital, Milan, Italy
11Neurologic Clinic, University of Brescia, Brescia, Italy 12
Department of Medical and Surgery Sciences and Neurosciences, University of Siena, Siena, Italy
13
Neurologic Clinic, University of Ferrara, Italy
14ALS Research Centre, BioNeC, University of Palermo, Palermo, Italy 15
Department of Neurology and Psychiatry, University of Bari, Bari, Italy
16Department of Neurosciences, S. Agostino-Estense Hospital, Modena, Italy 17
ALS Centre, Neurologic Clinic, Maggiore della Carità University Hospital, Novara, Italy
18
2nd Neurologic Clinic, 2nd University of Naples, Naples, Italy
19IRCCS“Salvatore Maugeri” Foundation, Milan, Italy Figure 5 Secondary outcome analysis. Progression of ALSFRS-R
(A) and slow vital capacity (sVC) (B) in the two treatment groups since the baseline visit through the 12-month trial period, with the corresponding p value of the repeated measure analyses.
Figure 6 Haematological effects of recombinant human
erythropoietin (rhEPO) mean values of haemoglobin (A) and hematocrit (B) in the two treatment groups since baseline visit through the 12-month trial period. At baseline, haemoglobin and haematocrit values overlapped, whereas they were significantly higher (p<0.01) in the rhEPO group than in the placebo group at 3, 6, 9 and 12-month follow-up.
20Department of Neurosciences, Neurology Unit, University of Parma, Parma, Italy 21Neurology Unit, dell’Angelo Hospital, Mestre, Italy
22Neurology Unit, Department of Neuro-Motor Diseases, IRCCS Arcispedale Santa
Maria Nuova, Reggio Emilia, Italy
23IRCCS Neurological Sciences Institute, Bologna, Italy 24
Department of Clinical and Experimental Medicine, Neurology Unit, University of Pisa, Pisa, Italy
25
Department of Neurosciences, University of Padua, Padua, Italy
26Intensive Neurorehabilitation Unit, IRCCS“Salvatore Maugeri” Foundation,
Mistretta, Italy
27Neuroepidemiology Units, IRCCS Foundation,“Carlo Besta” Neurological Institute,
Milan, Italy
Acknowledgements The authors thank all the patients participating in the trial and their families. The authors thank all the collaborating nurses. The authors thank Laura Mastrosimone, Domenico Arenella and Laura Ferradini of the EPOS trial coordinating centre and Ilaria Riela for administrative and legal assistance. The authors also acknowledge the Fondazione Italiana di per la SLA—Sclerosi Laterale Amiotrofica.
Collaborators EPOS trial study group: Daniele Cazzato (‘Carlo Besta’ Neurological Institute, Milan); Edward Cesnik, Elisabetta Groppo, Elisabetta Sette (Ferrara); Carla Pani, Emanuela Costantino, Francesco Orlandini, Daniela Boi (Cagliari); Giorgia Querin, Carla D’Ascenzo (Padua); Anna Sagnelli, Giovanni Piccirillo (Naples); Marina Aiello, Alfredo Chetta, Andrea Grassi (Parma); Christian Lunetta, Eleonora Maestri (NEMO Centre, Milan); Alessandro Padovani, Mariasofia Cotelli, Alice Todeschini (Brescia); Stefania Morino, Antonella Di Pasquale, Pamela Latino (Rome); Stefania Casali, Stefania Battistini, Marilena Pirrelli (Siena); Roberto Cantello, Nicola Nasuelli, Serena Servo (Novara); Riccardo De Gennaro, Ernesto Gastaldo (Mestre); Eleni Georgoulopoulou, Nicola Fini (Modena); Alfonsa C. Taiello, Tiziana Colletti (Palermo); Andrea Calvo, Cristina Moglia, Giuseppe Fuda (Torino); Kalliopi Marinou (‘S.Maugeri’, Milan); Nilo Riva, Federica Cerri, Ignazio D. Lopez (S. Raffaele Hospital, Milan); Domenico De Cicco, Gianluca Battaglia (‘S.Maugeri’, Mistretta); Norina Marcello, Manuela Rinaldi (Reggio Emilia); Carlo Scialò, Vittorio Mantero, Maria Mascolo (Genova); Cecilia Carlesi, Elena Caldarazzo Ienco (Pisa); Antonio Di Muzio (Chieti); Lorenzo Verriello, Delia D’Amico (Udine); Isabella L. Simone, Rosanna Tortelli, Rosa Cortese (Bari), Ilaria Bartolomei (Bologna).
Contributors GL and GF have designed the study; GL was the principal investigator and wrote the manuscript; GF was responsible for the randomisation unit; IT has performed the statistical analyses; GA, GB, MC, CC, AC, MC, RE, RF, MF, FG, EG, VLB, GL, JM, LM, MRM, GM, VP, RQ, RR, FS, GS, GS, PV acted as site principal investigators, participated at the investigator meetings and definition of the final protocol, and approved thefinal manuscript; EDB and all the neurologists listed in the EPOS trial study group have actively participated in the enrolment of patients and assessment of the outcomes, and have read and approved thefinal manuscript. Funding The study was funded by the IRCCS Foundation‘Carlo Besta’ Neurological Institute, Milan, Italy, an institution of research and care of public body. The study database is held by the Neuroepidemiology Unit.
Competing interests None. Patient consent Obtained.
Ethics approval Each of the 25 participating centres obtained approval from the local ethics committee.
Provenance and peer review Not commissioned; externally peer reviewed. Open Access This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/ licenses/by-nc/4.0/
REFERENCES
1 Solling C. Organ-protective and immunomodulatory effects of erythropoietin—an update on recent clinical trials. Basic Clin Pharmacol Toxicol 2012;110:113–21. 2 Leist M, Ghezzi P, Grasso G, et al. Derivatives of erythropoietin that are tissue
protective but not erythropoietic. Science 2004;305:239–42.
3 Brines M, Grasso G, Fiordaliso F, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA 2004;101:14907–12.
4 Sanchez PE, Fares RP, Risso JJ, et al. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc Natl Acad Sci USA 2009;106:9848–53.
5 Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA 2001;98:4044–9.
6 Mennini T, De Paola M, Bigini P, et al. Nonhematopoietic erythropoietin derivatives prevent motoneuron degeneration in vitro and in vivo. Mol Med 2006;12:153–60.
7 Chung YH, Joo KM, Kim YS, et al. Enhanced expression of erythropoietin in the central nervous system of SOD1(G93A) transgenic mice. Brain Res 2004;1016:272–80. 8 Grunfeld JF, Barhum Y, Blondheim N, et al. Erythropoietin delays disease onset in
an amyotrophic lateral sclerosis model. Exp Neurol 2007;204:260–3.
9 Grignaschi G, Zennaro E, Tortarolo M, et al. Erythropoietin does not preserve motor neurons in a mouse model of familial ALS. Amyotroph Lateral Scler 2007;8:31–5. 10 Cho GW, Kim GY, Baek S, et al. Recombinant human erythropoietin reduces
aggregation of mutant Cu/Zn-binding superoxide dismutase (SOD1) in NSC-34 cells. Neurosci Lett 2011;504:107–11.
11 Janik P, Kwiecinski H, Sokolowska B, et al. Erythropoietin concentration in serum and cerebrospinalfluid of patients with amyotrophic lateral sclerosis. J Neural Transm 2010;117:343–7.
12 Just N, Moreau C, Lassalle P, et al. High erythropoietin and low vascular endothelial growth factor levels in cerebrospinalfluid from hypoxemic ALS patients suggest an abnormal response to hypoxia. Neuromuscul Disord 2007;17:169–73.
13 Lauria G, Campanella A, Filippini G, et al. Erythropoietin in amyotrophic lateral sclerosis: a pilot, randomized, double-blind, placebo-controlled study of safety and tolerability. Amyotroph Lateral Scler 2009;10:410–15.
14 Kaufmann P, Levy G, Thompson JL, et al. The ALSFRSr predicts survival time in an ALS clinic population. Neurology 2005;64:38–43.
15 Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 2000;97:10526–31. 16 Banks WA, Jumbe NL, Farrell CL, et al. Passage of erythropoietic agents across the
blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol 2004;505:93–101.
17 Xenocostas A, Cheung WK, Farrell F, et al. The pharmacokinetics of erythropoietin in the cerebrospinalfluid after intravenous administration of recombinant human erythropoietin. Eur J Clin Pharmacol 2005;61:189–95.
18 Ehrenreich H, Fischer B, Norra C, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain 2007;130(Pt 10):2577–88. 19 Fond G, Macgregor A, Attal J, et al. Treating patients with schizophrenia deficit
with erythropoietin? Psychiatry Clin Neurosci 2012;66:375–82.
20 Haljan G, Maitland A, Buchan A, et al. The erythropoietin neuroprotective effect: assessment in CABG surgery (TENPEAKS): a randomized, double-blind, placebo controlled, proof-of-concept clinical trial. Stroke 2009;40:2769–75. 21 Cheung WK, Goon BL, Guilfoyle MC, et al. Pharmacokinetics and
pharmacodynamics of recombinant human erythropoietin after single and multiple subcutaneous doses to healthy subjects. Clin Pharmacol Ther 1998;64:412–23. 22 Bianchi R, Buyukakilli B, Brines M, et al. Erythropoietin both protects from and reverses
experimental diabetic neuropathy. Proc Natl Acad Sci U S A 2004;101:823–8. 23 Chio A, Canosa A, Gallo S, et al. ALS clinical trials: do enrolled patients accurately
represent the ALS population? Neurology 2011;77:1432–7.
24 Logroscino G, Traynor BJ, Hardiman O, et al. Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry 2008;79:6–11. 25 Millul A, Beghi E, Logroscino G, et al. Survival of patients with amyotrophic lateral
sclerosis in a population-based registry. Neuroepidemiology 2005;25:114–19. 26 del Aguila MA, Longstreth WT Jr, McGuire V, et al. Prognosis in amyotrophic lateral
sclerosis: a population-based study. Neurology 2003;60:813–19.
27 Pupillo E, Messina P, Logroscino G, et al. Long-term survival in amyotrophic lateral sclerosis: a population-based study. Ann Neurol 2014;75:287–97.
28 Cudkowicz ME, van den Berg LH, Shefner JM, et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol 2013;12:1059–67. 29 Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with
amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 2007;6:1045–53. 30 Miller R, Bradley W, Cudkowicz M, et al. Phase II/III randomized trial of TCH346 in
patients with ALS. Neurology 2007;69:776–84.
31 Meininger V, Drory VE, Leigh PN, et al. Glatiramer acetate has no impact on disease progression in ALS at 40 mg/day: a double- blind, randomized, multicentre, placebo-controlled trial. Amyotroph Lateral Scler 2009;10:378–83. 32 Aggarwal SP, Zinman L, Simpson E, et al. Safety and efficacy of lithium in
combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:481–8. 33 Beghi E, Pupillo E, Bonito V, et al. Randomized double-blind placebo-controlled trial
of acetyl-L-carnitine for ALS. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:397–405.
34 Chio A, Mora G, La Bella V, et al. Repeated courses of granulocyte colony-stimulating factor in amyotrophic lateral sclerosis: clinical and biological results from a prospective multicenter study. Muscle Nerve 2011;43:189–95. 35 Dupuis L, Dengler R, Heneka MT, et al. A randomized, double blind,
placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PLoS ONE 2012;7:e37885.
36 de Carvalho M, Pinto S, Costa J, et al. A randomized, placebo-controlled trial of memantine for functional disability in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2010;11:456–60.
37 Meininger V, Bensimon G, Bradley WR, et al. Efficacy and safety of xaliproden in amyotrophic lateral sclerosis: results of two phase III trials. Amyotroph Lateral Scler Other Motor Neuron Disord 2004;5:107–17.