UNIVERSITÁ DEGLI STUDI ROMA TRE
Facoltà di Scienze Matematiche, Fisiche e Naturali Dipartimento di Biologia
Scuola Dottorale in Biologia
Sezione di Biologia Applicata alla Salute dell’Uomo
– XXIV ciclo –
NEUROGLOBIN: MOLECULAR, CELLULAR AND
BIOMEDICAL ASPECTS
NEUROGLOBINA: ASPETTI MOLECOLARI,
CELLULARI E BIOMEDICI
PhD student: Dr. Elisabetta De Marinis
Tutors:
University of Roma Tre CSIC Instituto Cajal
Prof. Paolo Ascenzi Prof. Luis Miguel Garcia-Segura
Prof. Maria Marino Dr. Maria Angeles Arevalo
Coordinator of Biology applied to Human health section: Prof. Paolo Visca A.A. 2010/2011
INDEX
SUMMARY………...i
RIASSUNTO...iv
1. BACKGROUND …...………...1
1.1 Globins: an overview ……….……….….….….1
1.2 Neuroglobin, an hexa-coordinated globin ..……….………3
1.2.1 Neuroglobin localization ………..……….5
1.2.2 Neuroprotective functions of neuroglobin ……….………..…….6
1.2.3 Cellular mechanisms underlying neuroglobin neuroprotective effects……….…….8
2. AIM………..14
3. SEX STEROID HORMONES AS ENDOGENOUS MODULATORS OF NEUROGLOBIN LEVELS IN NEURONAL CELLS ………16
3.1 Introduction ……….16
3.2 Results ………..………...………...19
3.2.117β-estradiol effect on neuroglobin protein levels……….19
3.2.2 Androgen effect on neuroglobin levels ………..22
3.2.3 Estrogen receptor involvement in 17β-estradiol-induced neuroglobin levels …….………..23
3.2.4 Mechanisms involved in the 17β-estradiol-induced increase of neuroglobin levels ….………..26
3.3 Discussion ………...……..32
4. INVOLVEMENT OF NEUROGLOBIN IN 17β-ESTRADIOL-INDUCED PROTECTION AGAINST NEUROTOXICITY ...35
4.1 Introduction ……….35
4.2 Results ..……….36
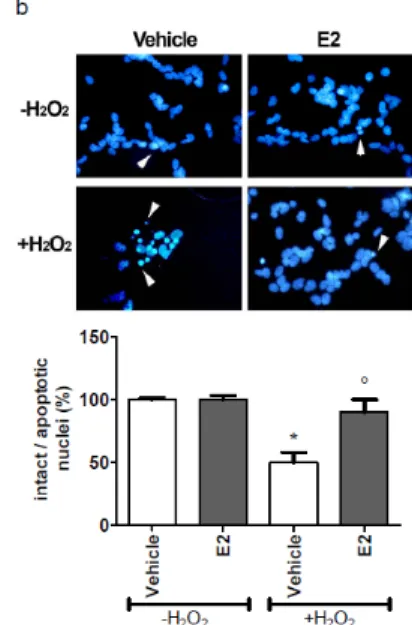
4.2.1 Neuroglobin is involved in 17β-estradiol-induced protection against H2O2-mediated apoptosis ...……….36
4.2.2 17β-estradiol changes neuroglobin intracellular localization …39 4.2.3 17β-estradiol promotes neuroglobin-cytochrome c association.42 4.3 Discussion .………47
5. INVOLVEMENT OF NEUROGLOBIN IN 17β-ESTRADIOL ANTI-INFLAMMATORY EFFECTS ....………50
5.2 Results ……….…………..51
5.2.1 17β-estradiol effect on neuroglobin protein levels in mouse primary cortical astrocytes ..………51
5.2.2 Effect of lipopolysaccharide on neuroglobin protein levels ...54
5.2.3 Neuroglobin involvement in 17β-estradiol-mediated anti-inflammatory effects against lipopolysaccharide ………...……58
5.3 Discussion .………60
6. CONCLUSION ………..………62
REFERENCES …………...……….66
ACKNOWLEDGMENTS ...84 APPENDIX A.
Materials and Methods ...(available on CD-ROM) APPENDIX B.
i
S
UMMARYAlthough globins are among the best-investigated vertebrate proteins, no other distinct types of globins have been identified so far in this taxon.
In 2000, it was identified a third globin type in humans and rodents. This protein was predominantly expressed in the brain, and therefore they have called it neuroglobin (Ngb).
The discovery of Ngb aroused a great interest among scientific community inducing to consider heme-globins not only as mere O2 storage/delivery proteins.
Ngb is a highly conserved protein, with an evolutionary rate that is about threefold slower than that of myoglobin and hemoglobin. Thus, Ngb has remained largely unchanged during evolution, pointing to an important role of this protein.
In particular, an important role in neuroprotection has been addressed to Ngb, especially against ischemia and oxidative stress-related neurodegenerative diseases, but many divergences between in vivo and in
vitro experimental approaches still render unclear the biological role of this
novel globin.
Several mechanisms underlying Ngb neuroprotective effects have been proposed. Indeed, Ngb has been hypothesized: (i) to act as an O2 buffer, (ii) to facilitate O2 diffusion to the mitochondria, (iii) to catalyze the formation and the decomposition of reactive nitrogen and/or oxygen species, and (iv) to be part of intracellular signaling pathways by inhibiting the dissociation of GDP from Gα proteins and triggering the release of the Gβγ complex, and by reducing cytochrome c. Although it is unlikely that Ngb has so many distinct roles, there is no doubt that Ngb displays a protective function(s) in the brain.
The emerging neuroprotective role of Ngb arises the challenge to investigate the mechanisms able to modulate its expression. Indeed, a significant contribution to highlight the role played by Ngb in neuroprotection could derive from the identification of Ngb endogenous modulator(s) (e.g., neuroactive hormones and neurotransmitters), but, as far as we know, no Ngb involvement in the hormone signal transduction pathways has been identified yet.
Thus, aim of this project is to approach to the knowledge of Ngb physiological role (i) identifying Ngb endogenous modulator(s), (ii) identifying the molecular mechanisms responsible of Ngb expression and induction, and (iii) the role played by Ngb in neuroprotective signaling pathways.
ii
In the first part of the project was evaluated if sex steroid hormones may act as endogenous modulators of Ngb levels in SK-N-BE human neuroblastoma cell line and in mouse primary hippocampal neurons. The reported results indicate that physiological concentration of the estrogen 17β-estradiol (E2), but not androgens, acts as endogenous modulator of Ngb in both cell models. This effect is mediated by estrogen receptor β (ERβ) via genomic and extranuclear signals involving p38/MAPK pathway.
In the second part, the involvement of Ngb in the neuroprotective effects of E2 against H2O2-induced toxicity has been investigated in SK-N-BE cells.
Indeed, E2 exerts a protective effect against the H2O2-induced injury, and requires ERβ. E2 pretreatment impairs H2O2-induced caspase-3 and PARP activation, enhancing cell viability. However, in Ngb-silenced SK-N-BE cells E2 was unable to counteract the H2O2-induced decrease in cell number and the activation of the pro-apoptotic cascade suggesting that Ngb can be regarded as part of signals activated by E2 to exert neuroprotective effects, definitely validating the role played by Ngb as an anti-apoptotic neuroprotective globin.
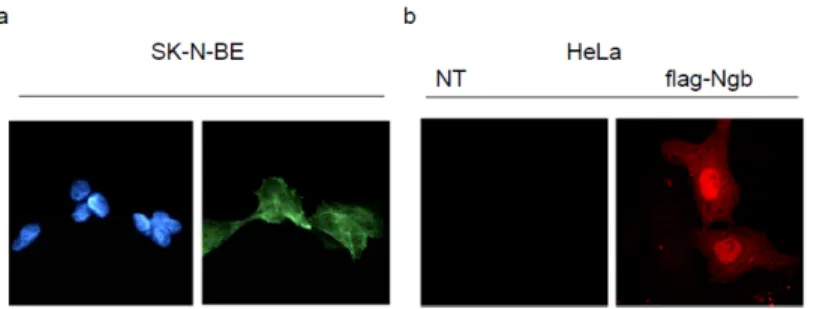
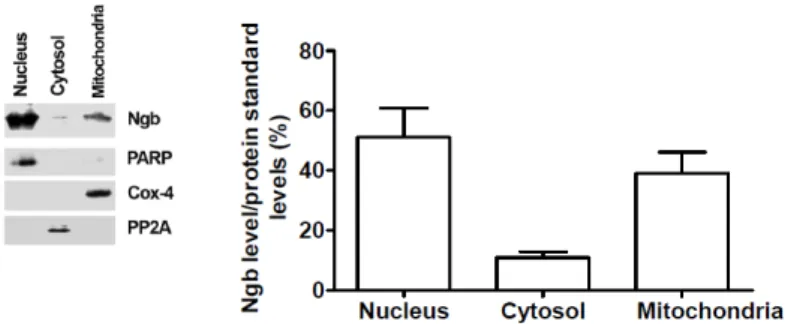
Thus, it has been clarified the Ngb sub-cellular localization to understand how this novel globin can intercept the apoptotic pathway.
Therefore, in SK-N-BE cells has been demonstrated that Ngb is expressed in the nucleus, mitochondria and is scattered in the cytoplasm.
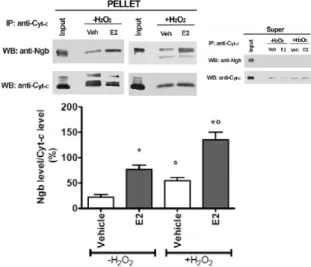
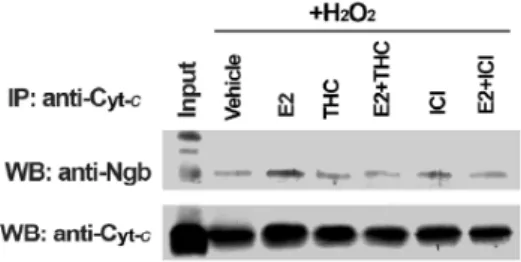
E2 reallocates Ngb mainly at mitochondria strengthened the hypothesis that Ngb directly interferes with the intrinsic pathway of apoptosis, being mitochondria just the starting site of this process. Indeed, it has been assessed that Ngb co-immunoprecipitates with cytochrome c in mitochondrial fraction and this association is enhanced pretreating SK-N-BE cells with E2, suggesting that E2-induced reallocation of Ngb facilitates Ngb-cytochrome c interaction. Remarkably, E2 pretreatment before the addition of H2O2 strongly enhances Ngb co-immunoprecipitation with cytochrome c. This E2 effect is stronger during oxidative stress condition rather than in basal condition and requires ERβ activity. Thus, the mechanism underlying Ngb protection against H2O2 stress is the interception of the intrinsic pathway of apoptosis interfering directly with cytochrome c release.
In the light of Ngb neuroprotective potential, linked with E2-mediated signals, the third part of the project was aimed to characterize the E2-mediated regulation of Ngb levels in astrocytes, where E2 exerts a well known anti-inflammatory effect. In mouse primary cortical astrocytes E2 affects Ngb expression at physiological concentration. The effect of E2 on Ngb levels specifically requires ERβ, confirming also in astrocytes the
iii
direct involvement of ERβ in Ngb modulation, as already reported for human neuroblastoma SK-N-BE and mouse hippocampal neurons. Although it has been established that Ngb is an E2-inducible protein and that, from a functional point of view, the E2-mediated Ngb upregulation allows to promote the E2-induced outcomes, also a putative role of Ngb as a compensatory protein responding to challenging stimuli, must be considered. The finding that lipopolysaccharide (LPS) is able to increase Ngb protein levels, although with a lesser degree compared with E2, further provides an additional contribution to understand the role of Ngb also as offsetting protein. Interestingly, although both E2 and LPS are able to increase Ngb protein levels, a negative cross-talk between ERs and LPS-induced signal (i.e., NFκB) seems to be present. In fact, ERα-activated signals (which are not involved in E2-mediated Ngb upregulation) block LPS-mediated Ngb increase, whereas on the other hand, LPS impairs the ERβ-induced upregulation of Ngb protein levels. Therefore, the co-activation of ERα and ERβ is pivotal to regulate Ngb expression in presence of LPS-activated signals (i.e., NFκB).Despite LPS, via NFκB, is able to increase Ngb levels, the role of this globin is not addressed to promote LPS effects, as observed for E2. Indeed, Ngb seems to be pivotal to mediate the E2 anti-inflammatory effects (i.e., inhibition of IL-6 and IP-10 synthesis), since Ngb knocking down prevents the protective effect of E2.
As a whole, the well known neuroprotective effects elicited by E2 may, at least in part, be explained by an enhanced Ngb expression in neurons and astrocytes. The principal role played by Ngb in the brain could be the reduction of neuronal death by resetting the trigger level of apoptosis and inhibition of pro-inflammatory molecules expression, leading to the onset of physiological response to stress. E2 acts to accelerate Ngb neuroprotective effect rapidly enhancing its protein levels in both neurons and astrocytes.
In addition, the possibility that other hormones and neurotransmitters may upregulate Ngb levels in brain a potential new opportunity for the development of neuroprotective strategies and drugs against stroke damage, inflammation, neurodegenerative diseases (e.g., Alzheimer’s and Parkinson’s disease), excitotoxicity, and injuries related to oxygen or glucose deprivation.
iv
R
IASSUNTOSebbene le globine siano le proteine più studiate tra i vertebrati e siano note molte varianti funzionali della emoglobina e della mioglobina, per molto tempo non sono state identificate altre tipologie di globine.
Nel 2000 è stata identificata una terza globina nell’uomo e nelle specie murine. Tale proteina è stata chiamata neuroglobina (Ngb) in quanto identificata inizialmente nel sistema nervoso.
La scoperta della Ngb ha fatto sorgere un forte interesse nella comunità scientifica, portando a considerare le eme-globine non soltanto come proteine atte al trasporto o al mantenimento dei livelli intracellulari di O2.
La Ngb è una proteina altamente conservata, presenta infatti un tasso di evoluzione circa tre volte più lento di quelle delle più note mioglobina ed emoglobina. Quindi la Ngb è rimasta largamente immutata durante l’evoluzione, indicando un ruolo importante di tale proteina.
In particolare, è stato evidenziato un importante ruolo della Ngb nella neuroprotezione, specialmente nei confronti dell’ischemia e delle patologie legate allo stress ossidativo. Tuttavia i meccanismi alla base di tale ruolo neuroprotettivo sono ancora poco chiari, a causa delle molte divergenze tra gli approcci sperimentali in vivo e in vitro ad oggi adottati.
Tra i meccanismi alla base della funzione neuroprotettiva è stato suggerito che la Ngb (i) possa agire come sensore dell’ O2; (ii) possa facilitare la diffusione di O2 ai mitocondri; (iii) possa catalizzare la sintesi e la detossificazione delle specie reattive dell’ossigeno e dell’azoto; e (iv) possa far parte delle vie di segnalazione intracellulari inibendo la dissociazione del GDP dalle proteine Gα, e quindi determinare rilascio del complesso Gβγ. Inoltre, è stato ipotizzato che la Ngb possa agire riducendo il citocromo c ossidato che è alla base dell’attivazione della via intrinseca dell’apoptosi. Sebbene sia improbabile che la Ngb possa avere così tanti e distinti meccanismi d’azione, non v’è dubbio sulle sue funzioni protettive nel cervello.
La funzione neuroprotettiva emergente della Ngb spinge a comprendere quali siano i meccanismi in grado di modularne l’espressione. Infatti, un significativo contributo a comprendere il ruolo della Ngb nella neuroprotezione potrebbe derivare dall’identificazione di possibili modulatori endogeni (ad esempio neurotrasmettitori o ormoni neuroattivi) che al momento non sono noti.
Pertanto, lo scopo di questo progetto è quello di approfondire il ruolo fisiologico della Ngb (i) ricercando possibili modulatori endogeni; (ii) identificando i meccanismi molecolari alla base dell’espressione e
v
induzione della Ngb, e (iii) identificando il ruolo della Ngb nelle vie di segnalazione alla base della neuroprotezione.Nella prima parte del progetto è stato valutato se gli ormoni sessuali steroidei potessero agire come modulatori endogeni della Ngb nella linea cellulare SK-N-BE (neuroblastoma umano) e in neuroni primari murini di ippocampo. I risultati indicano che concentrazioni fisiologiche di 17 β-estradiolo (E2), il più attivo fra gli estrogeni, inducono un aumento di Ngb in entrambi i modelli cellulari. Tale effetto però non è mediato dagli androgeni.
E2 aumenta i livelli di Ngb attraverso l’isoforma β del recettore degli estrogeni (ERβ) che agisce attraverso meccanismi genomici e non genomici, tra i quali risulta coinvolta la via di segnale p38/MAPK.
Nella seconda parte, è stato studiato il coinvolgimento della Ngb negli effetti neuroprotettivi di E2 verso la tossicità indotta da H2O2 nelle cellule SK-N-BE.
Infatti E2, attraverso ERβ, esercita un effetto protettivo rispetto a un danno da H2O2. Il pretrattamento con E2 inibisce l’attivazione delle proteine pro-apoptotiche caspasi-3 e della proteina PARP indotte da H2O2, aumentando così la sopravvivenza cellulare. Tuttavia, nelle cellule in cui la Ngb è silenziata l’effetto di E2 viene meno, indicando che la Ngb esercita un ruolo chiave nei meccanismi di protezione di E2, inibendo, in particolare, l’attivazione delle vie apoptotiche.
Per comprendere come la Ngb possa interferire nella via apoptotica è stata innanzitutto esaminata la sua localizzazione intracellulare.
Nelle cellule SK-N-BE la Ngb è espressa nel nucleo, nei mitocondri e nel citosol. Il trattamento con E2 induce una rilocalizzazione della Ngb soprattutto a livello mitocondriale, rafforzando l’idea che la Ngb possa interferire direttamente con la via intrinseca dell’apoptosi, essendo il mitocondrio proprio il sito di innesco di tale processo.
Infatti la Ngb co-immunoprecipita con il citocromo c nella frazione mitocondriale; tale associazione aumenta in presenza di E2, e in maniera ancor più significativa quando E2 è somministrato prima di un trattamento pro-apoptotico, come H2O2. Anche questo effetto dell’E2 è mediato da ERβ. Quindi, il meccanismo alla base della protezione della Ngb dallo stress indotto da H2O2 è l’inibizione della via intrinseca dell’apoptosi interferendo direttamente con il rilascio al citosol del citocromo c che attiverebbe la via intrinseca dell’apoptosi.
Alla luce del potenziale neuroprotettivo della Ngb, associato con le vie di segnale attivate da E2, la terza parte del progetto è stata indirizzata a caratterizzare la regolazione della Ngb, mediata da E2, negli astrociti, dove E2 ha un ruolo ben noto nella protezione dall’infiammazione.
vi
Negli astrociti corticali primari murini E2, a concentrazioni fisiologiche, aumenta i livelli proteici di Ngb. Anche in questo tipo cellulare l’effetto di E2 è mediato specificatamente da ERβ, come visto anche nelle cellule SK-N-BE e nei neuroni primari di ippocampo.Sebbene sia stato stabilito che la Ngb sia una proteina inducibile e che, da un punto di vista funzionale, l’aumento di Ngb promuova gli effetti protettivi di E2, anche un possibile ruolo della Ngb come proteina regolatoria, che risponda a segnali di stress, deve essere considerato. Per contribuire alla comprensione di tale ruolo della Ngb, gli astrociti primari sono stati trattati con la molecola pro-infiammatoria lipopolisaccaride (LPS). I risultati indicano che LPS è in grado di aumentare i livelli proteici di Ngb.
Sebbene sia E2 che LPS siano in grado di aumentare i livelli di Ngb, è interessante notare la presenza di una interazione contrastante tra gli ERs e i segnali attivati da LPS (NFκB). Infatti l’attivazione di ERα blocca l’aumento di Ngb indotto da LPS; d’altra parte, LPS inibisce l’induzione di Ngb mediata da ERβ. Quindi, la co-attivazione di ERα e ERβ risulta essere fondamentale per regolare l’espressione di Ngb in presenza delle vie di segnale attivate da LPS.
Nonostate LPS, attraverso NFκB, sia in grado di aumentare i livelli di Ngb, la funzione di tale globina non risulta essere quella di promuovere gli effetti di LPS, come osservato nel caso di E2. Infatti il silenziamento della Ngb negli astrociti previene gli effetti anti-infiammatori di E2 (inibizione della sintesi delle molecole pro-infiammatorie IL-6 e IP-10).
In conclusione, i ben noti effetti neuroprotettivi esercitati da E2 possono dipendere, almeno in parte, dall’aumentata espressione di Ngb nei neuroni e negli astrociti. Il principale meccanismo protettivo della Ngb nel cervello può essere ascritto alla riduzione della morte neuronale, attraverso l’inibizione della via intrinseca dell’apoptosi, e all’inibizione dell’espressione delle principali molecole pro-infiammatorie, garantendo l’attivazione dei meccanismi fisiologici di risposta allo stress. E2 agisce accelerando la comparsa degli effetti neuroprotettivi della Ngb aumentandone i livelli proteici sia nei neuroni che negli astrociti.
Inoltre, la possibilità che anche altri ormoni e neurotrasmettitori possano aumentare i livelli proteici di Ngb nel cervello costituisce una nuova opportunità per lo sviluppo di interventi terapeutici e farmacologici in grado di contrastare l’ischemia, la neuroinfiammazione, e le malattie neurodegenerative correlate allo stress ossidativo o alla deprivazione di ossigeno o glucosio.
1
1.
B
ACKGROUND1.1 Globins: an overview
Globins are small globular metalloproteins typically consisting of about 150 amino acids. They are phylogenetically ancient molecules whose intricate adaptive evolution is demonstrated by their widespread occurrence in bacteria, fungi, plants, invertebrate and vertebrate animals. Most known globins fulfill respiratory functions, supplying the cell with adequate amounts of O2 for aerobic energy production via the respiratory chain. Together with O2 transport and storage, these proteins display also well documented (pseudo-)enzymatic properties such as cytoprotection against reactive oxygen species and NO scavenging (Bolognesi et al., 1997; Ascenzi et al., 2007; Hoy and Hargrove, 2008; Vinogradov and Moens 2008).
Globins contain a heme prosthetic group (Fe-protoporphyrin IX) which is a chemically highly active group that has been involved in biological processes as soon as life appeared. To remain soluble and active heme needs to be surrounded by a hydrophobic environment that is obtained by a few structural 3D protein arrangements.
Heme participates in many biochemical functions among which are the binding and transport of gaseous ligands (O2, NO, CO), scavenging of free oxidant species, oxido-reduction, or oxygen-sensing. The specificity of these functions is directed by the structure of the protein to which it is associated, and also by the hexa- or penta-coordination of iron atom. The most recognized is the penta-coordinated ligation (e.g., in hemoglobin; Hb, and in myoglobin; Mb), but also iron hexa-coordination is widespread, occurring in some plant and bacterial Hbs, and also in invertebrate and vertebrate nerve globins (Trent and Hargrove, 2002, Dewilde et al., 2001).
Despite the enormous diversity in their primary and quaternary structures (amino acid sequences and aggregation states) globin proteins exhibit a characteristic tertiary structure (the “globin fold”) suggesting a common ancestry (Weber and Fago, 2004). The ancestral globin gene appears to have evolved 18,000 million years ago, when O2 started to accumulate in the atmosphere suggesting that the protein’s original function may have been to scavenge toxic O2, CO and NO gases (Hardison, 1999). The evolutionary, proteome-related implication is that globins provide opportunity to trace structure-function relations in a single protein family throughout the five Kingdoms of living organisms.
Indeed, the classical globin molecule is characterized by the canonical three-on-three α-helical Mb fold. This molecule, ca 150 residues long, is
2
characterized by a heme group surrounded by 8 helices designated A through H from the N to the C terminal. Helices A, B, C and E are on distal side of the heme and helices F, G and H on the proximal side. Moreover, an important feature of this structure is represented also by a pattern of 37 hydrophobic residues at conserved and solvent-inaccessible positions (Wajcman et al., 2009, and literature therein).
The tetrameric (e.g., Hb) and monomeric (e.g., Mb) vertebrate globins stand in contrast with the enormous variation in structure and function encountered in non-vertebrate globins. Microorganism globins form three families: (a) chimeric flavoproteins, where heme-carrying globin domains are linked to oxido-reductive FAD-dependent domains, (b) truncated Hbs with short polypeptide chains, and (c) bacterial Hbs (Weber and Vinogradov, 2001; Wajcman and Kiger, 2002). Plant Hbs comprise symbiotic Hbs (“legHbs”) from root nodules of leguminous plants that harbor symbiotic nitrogen-fixing bacteria, as well as non-symbiotic Hbs, that may be involved in several metabolic pathways (Arredondo-Peter et al., 1998).
Despite the large variation in structure and sequence, the tertiary structures of truncated Hbs and mini-Hb (from nemertean) are subeditings of the three-on-three a-helical sandwich, highlighting the striking structural plasticity of the globin fold.
Invertebrate Hbs illustrate phenomenal structural and functional diversity varying from single-chain monomers with molecular masses of 11.2 kDa, to multisubunit and multidomain crustacean Hbs, extracellular (3600 kDa) annelid Hbs where each molecule consists of 144 O2 binding globin chains and a number of heme-free “linker” chains, and include even larger (12,000 kDa) complexes found in some bivalve mollusks.
These proteins serve a wide range of functions apart from transporting and storing O2, such as controlling in vivo O2 levels, protection against sulphide, and enzymatic (oxidase and peroxidase-like and superoxide dismutase) activities (Weber and Vinogradov, 2001).
Notably, several intracellular globins have been found in the invertebrate and vertebrate nervous system possibly sustaining the consume of large amounts of metabolic energy, which requires a continuous supply of O2 (Geuens et al., 2004; Burmester and Hankeln, 2008). The role of globins in the brain seems to be pivotal to support nervous system functions during temporary periods of hypoxia, which may follow environmental or pathologic insults, therefore avoiding serious damages to the nervous system (Burmester et al., 2007; Williams et al., 2008; Cheng et al., 2009; Mitz et al., 2009; Avivi et al., 2010).
3
Invertebrate nerve globins have been observed in several Phyla such as Annelida, Arthropoda, Echiura, Mollusca, Nemertea, and Nematoda (Weber and Vinogradov, 2001; Burmester et al., 2002). The first to observe a globin in nerves was Lankester in 1872, when he recorded the brilliant red color of the ganglia of the polychaetous annelid Aphrodite aculeata (Wittemberg et al., 2002). Since then, globins have been found in or associated with nervous tissues of several other invertebrates and are now referred to as nerve globins. They are, however, not common and can be present or absent in closely related species (Yonetani et al., 2002; Wittemberg and Wittemberg, 2003).
Although the coordination of the heme iron atom differs being penta- (e.g., nerve globin of Aplysia spp.) or hexa-coordinated (e.g., nerve globin of Tellina alternata), the oxygen affinities of the invertebrate nerve globins are all quite moderate and similar to those of vertebrate Mb (Wittemberg et al., 1965; Wittemberg, 1992; Geuens et al., 2004).
In some species, nerve globins reach a millimolar local concentration, which is likely sufficient to facilitate O2 diffusion and storage (Vandergon and Riggs, 2008; Geuens et al., 2004; Hundahl et al., 2006a; Burmester and Hankeln, 2008).
Only in 2000, the first vertebrate nerve globin, named neuroglobin (Ngb), has been identified in neuronal tissues of mice and humans (Burmester et al., 2000). More recently, cytoglobin (Cygb), globin E, globin X, and α- and β-chains of Hb have been reported to be expressed in the vertebrate nervous system (Burmester et al., 2002; Kugelstadt et al., 2004; Roesner et al., 2005; Fuchs et al., 2006; Biagioli et al., 2009).
1.2 Neuroglobin, a hexa-coordinated globin
Although globins are among the best-investigated vertebrate proteins and several functional variants of the Hb subunits are known, no other distinct types of globins have been identified so far in this taxon.
In 2000, it was identified a third globin type in humans and rodents. This protein was predominantly expressed in the brain, and therefore they have called it neuroglobin (Ngb) (Burmester et al., 2000).
The human Ngb gene (NGB), located on chromosome 14q24, has a unique exon-intron structure.
In the databases of anonymous mouse and human complementary DNAs, Burmester and coworkers found partial globin-like sequences that do not correspond to any known Hb or Mb. They cloned and sequenced the coding regions of the human and mouse cDNAs and the genomic region of
4
the human gene. The mouse and human gene each code for proteins of 151 amino acids (17kDa) that are 94% identical; this homology is higher than the conservation between the orthologous Hbs or Mbs of these species (77±85% identity) and within the uppermost range of more than 1100 proteins compared between man and mouse (Makalowski et al., 1996). Although the proteins clearly belong to the globin superfamily, they share little amino-acid sequence similarity with vertebrate Mbs (ca 21% identity) and Hbs (ca 25% identity), suggesting a distinct evolution and function. Moreover, Hbs and Mbs of vertebrates are thought to have diverged about 550 million years ago (Goodman et al., 1975), but the lineage leading to the vertebrate Ngb must be older. A distinct position of Ngb is also suggested by its unique exon-intron structure. There is weak but consistent support for an association of Ngb with the intracellular globins of the annelids
Aphrodite aculeata (Dewilde et al., 1996) and Glycera dibranchiata (Zafar
et al., 1990). The Aphrodite globin displays the highest similarity with Ngb (30% amino-acid identity). Taking into account the nerve-based expression of both the Aphrodite globin and the vertebrate Ngb, both may be functionally and Ngb represents a distinct protein family that diverged early in metazoan evolution, probably before the Protostomia/Deuterostomia split.
Reliable sequences of Ngb from 11 mammals, 1 bird and 4 teleost fish species have been determined (Burmester et al., 2004); in all species apart from the trout, Ngb seems to be present as a single-copy gene. Structural analysis showed that Ngb sequences are consistent with the globin fold template, given the conservation of aminoacids involved in heme binding and ligand interactions, i.e., PheB10, PheCD1, TyrCD3, ValE11, LeuF4, ValFg1, ValFG3 and PheG5 (Pesce et al., 2003; Vallone et al., 2004).
Ngb promoter region contains several putative Sp1-binding sites and at least three transcription starting points, but lacks a TATA box motif (Burmester et al., 2000).
The NGB gene has three introns in correspondence of nucleotides encoding the helix B (position B12-2), of helix E (E11-0) and of helix G (G7-0). The introns at helix B and G are conserved in Hbs and Mbs of vertebrates and many other taxa (Dixon and Pohajdak, 1992; Hardison, 1996), but the central intron at helix E is unprecedented. On the basis of protein structural considerations, however, the presence of a central intron in ancient globin genes was previously proposed to be exactly at this position (Go, 1981). Therefore, the vertebrate globin gene ancestor might have displayed a 3 intron / 4 exon structure. Alternatively, the central E11-0 intron of neuroglobins may represent a case of independent intron acquisition (Hankeln et al., 1997; Logsdon et al., 1998).
5
Figure 1.1 Structure of human Ngb. His E7 and His F8 coordinating the Fe atom are highlighted (Pesce et al., 2003; PDB code 1OJ6). Molecular graphic image was produced using the UCSF chimera package (Pettersen et al., 2004)1.2.1 Neuroglobin localization
As described above, Ngb was so-named due to its first identification in neuronal cells (Burmester et al, 2000). Indeed Ngb expression is widespread among brain areas and in peripheral nervous system.
The expression pattern of Ngb mRNA and protein in mammal brain and the intracellular location have been debated, and the lack of consistency in the observations has been attributed to technical differences.
Ngb is found at relatively high concentrations in highly metabolically active cells and certain specialized cells, such as neurons of the hypothalamus, and particularly in retinal cells where its concentration has been estimated to be up to 100 μM (Schmidt et al., 2003; Bentmann et al., 2005; Fago et al., 2008; Hundahl et al., 2010).
In the central nervous system (CNS) Ngb is ubiquitously distributed in the olfactory bulb, the cerebral cortex (layers II-VI), subcortical regions (e.g., hippocampus, thalamus, hypothalamus, amygdala), the brain stem and the cerebellum, revealing that the expression of Ngb is a general feature of nerve cells (Reuss et al., 2002; Hankeln et al., 2004; Hundal et al., 2010).
6
The spinal cord also expresses Ngb in its gray substance and Ngb mRNA is also found in the peripheral nervous system, in sensory and autonomic ganglia (Reuss et al., 2002).
However, Ngb mRNA and/or protein expression was recognized also in non-neuronal tissues such as the gastrointestinal tract and some normal and tumoral tissues (e.g., breast, lung, kidney, lymphocytes) and endocrine tissues (Burmester et al., 2000; Moens and Dewilde, 2000; Reuss et al., 2002; Wystub et al., 2003; Burmester and Hankeln, 2004; Hankeln et al., 2004; Hankeln et al., 2005; Ostojić et al., 2006; Fordel et al., 2007a; Emara et al., 2010; Oleksiewicz et al., 2011).
In all CNS areas Ngb is expressed in neurons, but in contrast with previously reported data (Sun et al., 2001; Laufs et al., 2004; Hankeln et al., 2004) some groups demonstrated the presence of Ngb also in astrocytes and glioblastoma cells (Chen et al., 2005; Emara et al., 2009; Mitz et al., 2009; Dong et al., 2010; DellaValle et al., 2010; Emara et al., 2010). Intriguingly, Ngb expression increases in reactive astrocytes, within regions associated with the most severe pathology and the astroglial scar, in murine models of traumatic brain injury, cerebral malaria, and autoimmune encephalitis (DellaValle et al., 2010).
Among authors, a debate about the subcellular localization of Ngb has arisen. Until few years ago Ngb was recognized only in cytoplasmatic regions: in neurons Ngb mRNA and protein were consistently detected in perikarya and axonal processes (Zhang et al., 2002; Reuss et al., 2002; Wystub et al., 2003; Geuens et al., 2003) as well as perimitochondrially in axonal varicosities and terminal synapses (Schmidt et al., 2003; Hankeln et al., 2004). However, using immunolocalizion and electron microscopy, recent studies demonstrate that in neurons Ngb is also expressed in the inner wall of mitochondria and in the cell nucleus (Hundahl et al., 2010).
1.2.2 Neuroprotective functions of neuroglobin
Emerging experimental works suggest that Ngb expression is protective against hypoxic/ischemic injury in brain. Alteration of gene expression approaches were applied to address whether Ngb is neuroprotective. The first report showed that antisense-mediated knock-down of Ngb rendered cortical neurons more vulnerable to hypoxia, whereas overexpression of Ngb conferred protection of cultured neurons against hypoxia (Sun et al., 2001). Similar effects were observed in human neuroblastoma cell lines SH-SY5Y: Ngb overexpression enhanced cell
7
survival under condition of anoxia or glucose deprivation (Fordel et al., 2007b; Shao et al., 2009).
In animal stroke models, intracerebral administration of a Ngb-overexpressing adeno-associated virus vector, significantly reduced infarct size in rats after medial cerebral artery occlusion (MCAO), and the outcome was reversed when Ngb antisense oligonucleotide was applied (Sun et al., 2003).
Using Ngb-overexpressing transgenic (Ngb-Tg) mice, it was shown that the cerebral infarct size after MCAO was reduced by 30% compared with wild type, and the same protective effect was observed also in transient focal cerebral ischemia where hypoxia-induced mitochondrial aggregation and neuronal death were abolished (Khan et al., 2006; Khan et al., 2008). Reduction of brain infarction in Ngb-Tg mice can be sustained up to 14 days after ischemia compared with wild type controls (Wang et al., 2008), suggesting that Ngb overexpression is neuroprotective against transient focal cerebral ischemia, although the possible mechanisms need to be further characterized (Yu et al., 2009).
Moreover, the neuroprotective role of endogenous Ngb has been supported by its knockdown in cell cultures, which renders cortical neuronal cultures more susceptible to hypoxia (Jin et al., 2008) and decreases viability of neuroblastoma cells under oxidative stress (Ye et al., 2009).
However, some questions have been raised concerning the capacity of Ngb to provide general protection to neurons in vivo (Hundahl et al., 2006b). Contradictions in data derived from in vivo experiments almost certainly arise, at least in major part, from differences in the nature, severity, and duration of challenge used in the various studies. Any proposed mechanism of action of Ngb, in neurons, must thus consider not only its capacity to provide a level of protection to many cell types in the brain but also account for its very nonuniform distribution in the brain.
In addition, using progeny of crosses between Ngb-Tg mice and transgenic mice expressing a mutant form of human amyloid precursor protein (APPSw,Ind) associated with familial Alzheimer’s Disease, it has been shown that Ngb overexpression inhibits Alzheimer’s disease-related raft aggregation and membrane polarization, and associated neuronal death. Moreover, these double-transgenic mice produce reduced amounts of amyloid-β peptides Aβ(1-40) and Aβ(1-42), and amyloid plaques in the brain (Khan et al., 2007). These findings indicate also an important protective role of Ngb in neurodegenerative processes.
It has been well documented that Ngb expression in the brain is confined to metabolically active, oxygen-consuming cell types and mitochondria comprise a central locus for energetic perturbations and
8
oxidative stress (Burmester et al., 2004). Experimental works have shown that overexpression of Ngb promotes cell survival against Aβ toxicity, Aβ- and hypoxia-induced mitochondrial dysfunction and aggregation, and neuron death (Khan et al., 2008; Liu et al., 2009).
Moreover, the influence of Ngb on cell death after oxidative stress was also evaluated, indicating that Ngb overexpression protects against hydrogen peroxide (H2O2)-induced cell death, attenuating H2O2-induced reactive oxygen species (ROS) / reactive nitrogen species (RNS) accumulation and lipid peroxidation, decreasing H2O2-induced mitochondrial dysfunction and apoptosis, and promoting overall cell survival (Fordel et al., 2006; Li et al., 2008).
All these processes are at least in part associated with the prevention of apoptotic cell death and are closely related to recent hypothesis and evidences that indicate a possible interaction between Ngb and cytochrome
c and thus a direct involvement of this globin in the apoptotic pathway
(Fago et al., 2008; Brittain et al., 2010a; Brittain et al., 2010b; Raychaudhuri et al., 2010).
The emerging neuroprotective role of Ngb issues the challenge to investigate the physiological factors and mechanisms able to modulate its expression. Indeed, a significant contribution to highlight the role played by Ngb in neuroprotection could derive from the identification of Ngb endogenous modulator(s) (e.g., neuroactive hormones and neurotransmitters), but, as far as we know, no Ngb involvement in the hormone signal transduction pathways has been identified yet.
1.2.3 Cellular mechanisms underlying neuroglobin neuroprotective effects
Although Ngb is the best-investigated ‘novel’ globin type, its exact physiological role is still uncertain. As reported above, Ngb has remained largely unchanged during evolution, pointing to an important role of this protein. Several functions of Ngb have been proposed: (i) Ngb may exert a Mb-like role, enhancing O2 supply to the mitochondria of the metabolically active neurons; (ii) Ngb may scavenge damaging ROS or RNS, which are generated for example by the respiratory chain; (iii) Ngb may detoxify harmful excess of NO to nitrate at normoxia or produce NO for signaling functions from nitrite at hypoxia for the control of blood pressure; (iv) Ngb may be involved in a signal transduction pathway, e.g. by inhibiting the dissociation of GDP from G protein α; (v) Ngb may be part of a redox
9
process that is instrumental in preventing apoptosis via reduction of cytochrome c (Burmester and Hankeln, 2009).
All these functions have been supported by some experimental data or based on analogy with other globins. However, it is unlikely that Ngb could fulfill so many distinct roles.
Initially, Ngb was suggested to be an O2 storage and transport protein, performing a function in neural tissue similar to that of Mb in muscle (Burmester et al., 2000). Indeed, Ngb expression is upregulated under hypoxic conditions (Sun et al., 2001; Sun et al., 2003; Schmidt-Kastner et al., 2006; Li et al., 2006), as is also observed for Mb and Hb.
Many in vivo studies performed in Ngb-overexpressing transgenic mice, in primary neurons, and in cultured cell lines sustain the neuroprotective role played by Ngb, although the cellular mechanisms remain poorly defined and still controversial.
Until recently, it has generally been accepted that any intracellular globin has a similar function to that of Mb, either storing O2 for hypoxic phases (e.g. in diving mammals) or facilitating O2 diffusion from the capillaries to the respiratory chain in the mitochondria. However, within the past years it has become evident that Ngb may carry out various other functions (Burmester and Hankeln, 2009).
The heme iron in both the ferrous and the ferric forms is directly bound not only to the proximal HisF8 (as canonical in all globins), but also to the distal HisE7 (figure 1.1). This internal coordination on the distal side implies that only upon rupture of the bond with HisE7 an externally added ligand (e.g., O2) can be bound; and therefore competition with the internal ligand is an additional component involved in determining the overall affinity for the gaseous ligands.
Therefore, ligand binding to the heme-Fe atom of Ngb needs the formation of the transient penta-coordinated HisF8-Fe species; the reversible intramolecular hexa-to-penta-coordination transition of the heme-Fe atom modulates exogenous ligand-binding properties of Ngb (Kiger et al., 2004; Pesce et al., 2004; Brunori and Vallone, 2006).
The O2 affinity of Ngb depends on several factors, including the relative binding rates of O2 with the heme sixth position and the competing distal histidine residue (Trent et al., 2001; Dewilde et al., 2001), and the redox state of the cell which controls the formation or cleavage of an internal disulfide bond between Cys46 CD7 (seventh residue on the inter-helix region between helices C and D) and Cys55 D5 (Hamdane et al., 2003; Hamdane et al., 2004). The presence of the S–S bond could perturb the three-dimensional structure of the CD-D (inter-helix region between helices C and D, extending to the end of helix D) region and affect the
10
location of the neighboring E-helix, thus modulating the binding of the endogenous HisE7 ligand to the heme group (Hamdane et al., 2003; Pesce et al., 2003). As a consequence, in ferrous Ngb, the distal histidine residue dissociation rate increases by a factor of 10 with respect to the protein form without the disulfide bond (Hamdane et al., 2003; Smagghe et al., 2006), leading to an effective increase in O2 affinity by the same factor (Fago et al., 2004). Moreover, determination of the O2 affinity of Ngb is particularly difficult due to its tendency to autooxidize with a half time, in air, that varies from 20 to 3 min depending on pH (Trent et al., 2001; Dewilde et al., 2001; Fago et al 2004).
However, the O2 half saturation pressure (P50) was estimated from kinetics and also by direct measurements to range between 2 and 20 torr depending on pH, temperature, and the redox state of the cell (Nienhaus and Nienhaus, 2007; Burmester and Hankeln, 2009, and literature therein).
The low average concentration of Ngb in the brain (ca 1 μM), its tendency to autooxidize and its relatively low O2 affinity under physiological conditions seem to suggest a primary role other than O2 storage and/or facilitated diffusion (Brunori and Vallone, 2007).
Moreover, O2 storage or diffusion to or from the heme group of Ngb may be assisted by the presence of a large protein matrix cavity/tunnel (approx. 120Å3), located between the heme distal site and the EF interhelical hinge and connected to the protein surface (Pesce et al., 2003; Vallone et al., 2004). The cavity may be reshaped after ligand binding, which may allow trapping of harmful ROS or RNS (Nicolis et al., 2007).
In summary, three observations are in conflict with the Ngb role in O2 storage and transport: (i) The high autooxidation rate of ferrous Ngb argues against a function as an O2 storage protein; (ii) The reported P50 values in the range of 2-20 torr also shed doubt on this conjecture; under normal conditions, the oxygen pressure in the brain varies between 8 and 40 torr, and normal intracellular oxygen pressure of the neurons is maintained as long as the tissue pressure is kept above 10 torr (Nienhaus and Nienhaus, 2007), thus to supply O2 to the tissues, the protein should have a P50 below normal tissue pressures; (iii) Finally, the average Ngb concentration in the brain is in the micromolar range which is way too low to contribute to the oxygen supply. For comparison, Mb concentrations are much higher in heart and red muscle tissue, about 200 μM and 100-400 μM, respectively. However, locally high Ngb concentrations of up to 100 mM have been found in the retina, where they could contribute significantly to the O2 supply (Schmidt et al., 2003).
Ngb binds also other gaseous ligands (e.g., NO, and CO), and displays (pseudo-)enzymatic properties (e.g., O2-mediated NO detoxification). In
11
particular, the increase in the concentration of NO up to the low micromolar range as observed under ischemic conditions (Lipton, 1999; Nicolis et al., 2007), may be contrasted with its reaction with ferrous oxyNgb (oxygenated Ngb, NgbFeIIO2), yielding metNgb (ferric-Ngb, NgbFeIII) andNO3− through a hame-bound ONOO− (peroxynitrite) intermediate (Brunori et al., 2005):
NgbFeIIO2 + NO → NgbFe III
OONO−→NgbFeIII + NO3− (1)
Also metNgb reacts with NO (Herold et al., 2004) to generate the stable NgbFeIINO species by reductive nitrosylation. This form of Ngb is a good scavenger of the ONOO−, which is produced in significant amounts under ischemic conditions by the reaction of NO with O2 (Lipton, 1999), as follows (Herold et al., 2004):
NgbFeIII + NO → NgbFeIINO (2) NgbFeIINO + ONOO−→ NgbFeIIINO (3) NgbFeIIINO → NgbFeIII + NO (4)
The association rate of HbNO and ONOO− is almost two orders of magnitude smaller, which suggests that NgbNO may function in vivo as an efficient scavenger of this harmful compound (Herold et al., 2004).
Most importantly, it has been reported that metNgb, unlike metMb and metHb, does not react with hydrogen peroxide (H2O2) or ONOO− to the ferryl form (Herold et al., 2004; Nicolis et al., 2007), which is extremely oxidizing. This property is obviously beneficial under conditions of oxidative stress.
However, at low O2 conditions ferrous Ngb may react with NO2– which is normally present at fairly high concentrations within mammalian tissues (0.1-10 M) (Bryan et al., 2005) where it is a major end-product of the cellular messenger NO. This reaction results in the formation of NO and metNgb (Petersen et al., 2008):
NgbFeII + NO2− + H+→ NgbFeIIINO + OH−→ NgbFeIII + NO + OH− (5)
Therefore, the protective role of Ngb against NO in vivo is controversial (Giuffrè et al., 2008; Jin et al., 2008; Kakar et al., 2010). Although in vitro Ngb does not react with H2O2 (Herold et al., 2004; Nicolis et al., 2007) and the Ngb-NO2– adduct reacts with H2O2 facilitating the nitration of aromatic substrates (Nicolis et al., 2007), the correlation
12
between ROS and RNS formation/decomposition and Ngb expression in
vivo is debated (Fordel et al., 2007a; Burmester and Hankeln, 2009).
The intermediate O2 affinity, which is in the range of the partial pressures measured in the brain, suggests yet another function for Ngb, namely that of an oxygen sensor that reports hypoxic conditions to signal transduction chains, which trigger processes that protect the cell against the detrimental effects of low oxygen pressure. The Morishima group reported that human Ngb acts as a redox-coupled regulator of signal transduction (Wakasugi et al., 2003). Ferric human Ngb (but not NgbO2) was found to associate with the GDP-bound Gα subunit of heterotrimeric G proteins. Ferric Ngb was proposed to act as a guanine-nucleotide dissociation inhibitor (GDI) for Gα (Wakasugi et al., 2003), preventing exchange of GDP by GTP, which is required for Gβγ subunit reassociation with the Gα subunit. The free Gβγ subunit then activates signal transduction pathways that protect against oxidative stress. In particular, it has been demonstrated that Ngb binds two members of the Rho GTPase family, Rac1 and Rho A, as well as the Pak1 kinase, a key regulatory assembly and Rho-GDI-GTPase signaling complex activity under hypoxia. Thus, it has been hypothesized that Ngb may play a protecting role in neuronal cells by inhibiting the dissociation of the GTPase Rac-1 from its endogenous GDI, thus preventing the hypoxia-induced actin polymerization and microdomain aggregation (Khan et al., 2008).
Although intriguing, the Ngb-Gα interaction was questioned by Burmester and coworkers (Burmester and Hankeln, 2004) who failed to find sequence similarities between Ngb and other regulators of G proteins. On the other hand, Wakasugi and Morishima (Wakasugi and Morishima, 2005) found that zebra fish ferric Ngb is inactive toward G proteins, which argues against the general relevance of a GDI function. Ngb was also reported to interact with flotillin-1, a lipid raft microdomain-associated protein, and with the cysteine protease inhibitor cystatin C, suggesting that Ngb modulates the intracellular transport of cystatin C to protect against cell death caused by oxidative stress (Wakasugi et al., 2005). For these interactions, the in vivo relevance was disputed because of limited overlap of expression between these proteins (Hankeln et al., 2004; Nienhaus and Nienhaus, 2007).
Another possible involvement in cellular signaling has been proposed by Fago and coworkers (Fago et al., 2006). During ischemic episodes, cells can enter an apoptotic pathway via the partial release of ferric mitochondrial cytochrome c into the cytoplasm. Ferric cytochrome c is a required component of the caspase-cascade activating apoptosome (Nienhaus and Nienhaus 2007, and literature therein). Ngb may be able to reduce small
13
amounts of cytochrome c leaking from damaged mitochondria, thereby suppressing apoptosis (Fago et al., 2008; Brittain et al., 2010a; Brittain et al., 2010b; Raychaudhuri et al., 2010).
At present the physical association between Ngb and cytochrome c has been only suggested by computer modeling approach (Brittain et al., 2010a; Brittain et al., 2010b).
Although it is unlikely that Ngb has so many distinct roles, there is no doubt that Ngb displays a protective function(s) in the brain (Burmester and Hankeln, 2009).
14
2.
A
IMHb and Mb are without doubt the best-known proteins, and most intensively studied in biology. They have the ability to bind O2 for the purpose of transport or storage, thereby enhancing the O2 carrying capacity of the blood (Hb) or the availability of O2 inside muscle cells (Mb). For a long time, Hb and Mb were considered as the only globins found in vertebrates.
The discovery of Ngb in 2000 by Burmester and colleagues (Burmester et al., 2000) aroused a great interest among scientific community inducing to consider heme-globins not only as mere O2 storage/delivery proteins.
Until recently, it has generally been accepted that any intracellular globin has a similar function to that of Mb, either storing for hypoxic phases (e.g. in diving mammals) or facilitating O2 diffusion from the capillaries to the respiratory chain in the mitochondria. However, within the past ten years it has become evident that certain globins may carry out various other functions. For example, maize globin regenerates NAD+, which is used to promote glycolysis in low oxygen environments (Sowa et al., 1998). Some prokaryotic globin and globin domains are involved in O2 sensing (Hou et al., 2001). Escherichia coli flavoHb decomposes NO, conferring resistance to NO poisoning and aconitase inactivation (Gardner et al., 1998). A similar function has been demonstrated for mammalian Mb, which scavenges NO and thus protects cellular respiration (Flögel et al., 2001). Mb may also be involved in decomposition of harmful ROS (Flögel et al., 2004).
Ngb is a highly conserved protein, with an evolutionary rate that is about threefold slower than that of Mb and Hb (Burmester et al., 2004). Thus Ngb has remained largely unchanged during evolution, pointing to an important role of this protein.
In particular, an important role in neuroprotection has been addressed to Ngb, especially against ischemia and oxidative stress-related neurodegenerative diseases, but many divergences between in vivo and in
vitro experimental approaches still render unclear the biological role of this
novel globin (Hundahl et al., 2006b).
The emerging neuroprotective role of Ngb arises the challenge to investigate the mechanisms able to modulate its expression. Indeed, a significant contribution to highlight the role played by Ngb in neuroprotection could derive from the identification of Ngb endogenous modulator(s) (e.g., neuroactive hormones and neurotransmitters), but, as far as we know, no Ngb involvement in the hormone signal transduction pathways has been identified yet.
15
Thus, aim of this project is to approach to the knowledge of Ngb physiological role by i) identifying Ngb endogenous modulator(s); ii) identifying the molecular mechanisms responsible of Ngb expression and induction and iii) assessing the role played by Ngb in neuroprotective signaling pathways.
16
3.
S
EX STEROID HORMONES AS ENDOGENOUS MODULATORS OFNEUEROGLOBIN LEVELS IN NEURONAL CELLS 3.1 Introduction
The last decade has seen an exponential increase in evidence for structural, cellular, and molecular sex differences in the brain that can be described as true dimorphisms, defined as the occurrence of two forms in the same species. These include regions of human and animal brains that are important for cognition, memory, and affect, such as the hippocampus, amygdala, and cortex, and for regions controlling sensorimotor and reward systems (Gillies and McArthur, 2010, and literature therein). Although these biological sex differences are clearly important from a physiological point of view to maintain homeostasis, if the system is challenged by external factors, such as stress and disease, different organizations in circuitries in male and female brains will respond differently to environmental challenges (endogenous or exogenous) and emerge as different vulnerabilities to behavioral and neurological disorders. Several neurodegenerative and mental diseases differ markedly in their prevalence, symptomatology, progression, and/or severity between the sexes. Indeed, beneficial effects of estrogens are reported in several mental, namely schizophrenia and depression (Cyr et al., 2000; Cyr et al., 2002; Kulkarni et al., 2002; Osterlund et al., 2005; Morisette et al., 2008) and neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, multiple sclerosis and ischemic stroke (Henderson, 1997; Cyr et al., 2000; Kompoliti, 2003; Wooten et al., 2004; Czlonkowska et al., 2006; Shulman and Bhat, 2006). In contrast, in the onset and progress of these diseases postmenopausal women fare worse than men (Swaab, 2004; Morisette et al., 2008; Gillies and McArthur, 2010).
Without doubt, 17β-estradiol (E2), the most active and relevant estrogen, has been pinpointed as a critical protective factor in females that gives them the advantage in diseases prevalent in men, whereas its rapid decline after menopause may forfeit this advantage.
E2 exerts its effect through two cognate estrogen receptors (ERs), referred to as ERα and ERβ. Both ER subtypes are found in the brain with a differential distribution, in neurons and glia (Laflamme et al., 1998; Behl, 2002). In addition 20 ERα and 10 ERβ splice variants have been reported (Zhao et al., 2005; Morisette et al., 2008). ERs are members of a large family of nuclear receptors acting as ligand-activated transcription factors (Ascenzi et al., 2006; Acconcia and Marino, 2011, and literature therein).
17
Although there are contradictory reports on the relative contributions of ERα and ERβ to the neuroprotective effects of estrogens in most disease models (Brann et al., 2007; Suzuki et al., 2009), evidence is emerging that both ERs have protective capacity, but they operate via different mechanisms and possibly in different time frames. For example, in ischemic brain injury and experimental autoimmune encephalomyelitis, ERα expression is induced early, whereas ERβ is induced later (Suzuki et al., 2002; Tiwari-Woodruff and Voskuhl, 2009).
In summary, E2 might directly control the transcription of genes that code for proteins that modulate neuronal survival. These proteins might enhance neurotrophic support, suppress apoptosis and affect neuronal structure (McEwen, 2002; Marin et al., 2008; Morisette et al., 2008; Vasudevan and Pfaff, 2008; Marin et al., 2009; Gillies and McArthur, 2010).
An important role in neuroprotection is also played by the membrane-bound fraction of ERs. E2 can induce cellular events that are not mediated by a direct or indirect interaction with DNA. These non-genomic actions induced by E2 are often very rapid (within minutes or even seconds) and could be associated with activation of second messenger pathways via a direct interaction of E2 with palmitoylated and plasma-associated ERs existing in a variety of neural and extraneural targets to mediate steroid action (Levin, 2002; Acconcia et al., 2004; Acconcia et al., 2005a; Marino et al., 2005; Ascenzi et al., 2006; Marino and Ascenzi, 2006; Morisette et al., 2008, Acconcia and Marino, 2011). Association between E2 and membrane-bound ER involves the rapid stimulation of Src-protein tyrosine kinase, mitogen-activated protein kinase (MAPK) pathways, and phosphatidylinositol-3 kinase (PI3K)/AKT pathways (Singer et al., 1999; Dhandapani and Brann, 2002). Moreover, genomic and non-genomic actions of ER signaling may not act independently but are shown to converge on target genes (Bjornstrom and Sjoberg, 2005).
By modulating intracellular signaling processes, E2 could indirectly and rapidly affect the transcription of genes that are controlled by various downstream effectors. Some of the signal transduction pathways that are targets of E2 and ERs are directly involved in the control of neuronal survival.
The predominant circulating sex hormone in male after puberty is testosterone, which is common precursor, in both male and female, of E2 and the most active androgen dihydrotestosterone (DHT) that derive from aromatization (catalyzed from CYP19A1 aromatase) and reduction (catalyzed from 5α-reductase) of testosterone, respectively (Nguyen et al., 2010, and literature therein).
18
Both testosterone and DHT bind to androgen receptor (AR), which exists in several splicing isoforms, and belongs to the nuclear receptor superfamily. Beyond genomic effects, AR, as well as ERs, can have actions that are independent of their interactions with DNA. ARs interacts with signal transduction proteins (e.g., MAPKs) in the cytoplasm causing rapid changes in cell function independent of changes in gene transcription, such as changes in ion transport. Regulation of signal transduction pathways by AR can indirectly lead to changes in gene transcription, for example, by leading to phosphorylation of other transcription factors (Nguyen et al., 2010, and literature therein).
Epidemiological studies indicate that men experience a significant decrease in levels of testosterone in blood and brain due to normal aging (Nguyen et al., 2010, and literature therein). Age-related androgen loss in men adversely affects muscle and bone mass, sexual arousal, sperm production, and brain functions such as mood, memory, and cognition (Nguyen et al., 2010, and literature therein). Recent data also suggest that low levels of testosterone in aging men may be one of several risk factors for the development of Alzheimer’s disease (AD) (Rosario et al., 2004; Pike et al., 2009). Androgens have many beneficial actions in the CNS and the decrease in their levels may contribute to age-related neurological deficits and Alzheimer’s disease. For example, testosterone decreases levels of Aβ (Gouras et al., 2000; Ramsden et al., 2003a). In addition, androgens are positive regulators of neuronal plasticity in the spinal nucleus of the bulbocavernosus, excitability in the CA1 region of hippocampus, and spine density in hippocampus (Nguyen et al., 2010, and literature therein).
Androgens also prevent retraction or increase the length and size of neurites from motor neurons. Other neurotrophic effects of testosterone include cell differentiation, neurogenesis, and development of neurons in the hippocampus, and motor and autonomic systems (Nguyen et al., 2010, and literature therein).
In addition, androgens increase the speed of regeneration of injured axons of motor neurons in young and adult rats (Nguyen et al., 2010, and literature therein). Androgens are also endogenous regulators of viability in neurons challenged with toxic insults in adult animals. Adult male rats and mice depleted of endogenous androgens by orchidectomy exhibit increased vulnerability to hippocampal neuron loss induced by excitotoxins, an effect that can be reversed by treatment with DHT (Ramsden et al., 2003b). In primary neuron culture paradigms, testosterone and related androgens attenuate cell death induced by serum deprivation, Aβ, and H2O2 (Nguyen et al., 2010, and literature therein). However, androgens can fail to protect neurons and even exacerbate injury in response to some forms of injury
19
such as ischemia, mitochondrial toxin 3-nitropropionic acid, and muscimol-induced excitotoxicity (Nguyen et al., 2010, and literature therein). Why androgens are neuroprotective against some insults, but not others is unclear. Indeed, androgen neuroprotection is not well characterized in terms of either specificity or mechanism (Nguyen et al., 2010, and literature therein).
Moreover, often the protective effect of androgens were ascribed to testosterone, without the evaluation of which testosterone metabolite (i.e., E2 or DHT) were the effective protective molecule (Nguyen et al., 2010, and literature therein). Thus, it must be taken into account that many of the effects of androgen actually could be exerted by E2.
As a whole, these results sustain a neuroprotective role of sex steroid hormones.
Aim of this part of the project is to evaluate if sex steroid hormones may act as endogenous modulators of Ngb levels. Two different cell models have been utilized: human neuroblastoma cell line SK-N-BE and mouse primary hippocampal neurons. Indeed, these cells are sex-hormone sensitive since both models express ERs and AR. Moreover, hippocampus is one of the most dimorphic brain areas, and primary hippocampal neurons could represent a model more physiologically and metabolically related to the in
vivo condition.
3.2 Results
3.2.1 17β-estradiol effect on neuroglobin protein levels
To assess the responsiveness of the selected cell models, the expression of ERs has been evaluated. In both SK-N-BE cells and in primary hippocampal neurons ERα and ERβ were expressed. (figure 3.1). In SK-N-BE, ERβ subtype was more expressed compared with ERα, whereas in primary neurons, ERs were equally expressed.
20
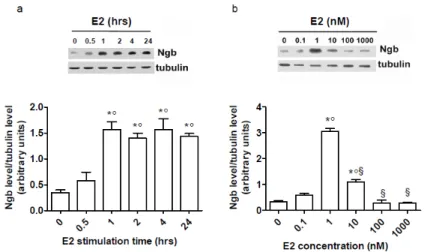
Figure 3.1 Characterization of ERs in SK-N-BE human neuroblastoma cell line and mouse primary hippocampal neurons. ER subtype (ERα and ERβ) levels in non-stimulated cells compared to recombinant proteins (5 ng) in SK-N-BE (a) and in primary hippocampal neurons (b). The amount of protein was normalized to tubulin levels. The data are typical Western blots of five independent experiments.E2 stimulation induced a time- and dose-dependent increase in Ngb levels in human neuroblastoma SK-N-BE cells (figure 3.2). E2 (10 nM) effect on Ngb levels started 30 minutes after stimulation, being significant after 1 hour, and remained constant 24 hours after hormone stimulation. The E2 dose-response curve was bell-shaped with a maximum effect at physiological E2 concentrations (i.e., 1-10 nM; 24 hours after stimulation). These data were confirmed in mouse primary hippocampal neurons (figure 3.3). Indeed, in these primary neurons, the E2 effect was rapid (1 hour) and persistent (24 hours) (figure 3.3a). In addition, also in mouse hippocampal neurons, 10 nM E2 increased Ngb levels, which remained significantly elevated in comparison to control cells even at higher E2 concentrations (figure 3.3b).
In line with the slight differences found in the E2 concentration to obtain the maximum effect in both cell types, 1 and 10 nM were used in the consecutive experiments to stimulate SK-N-BE cell line and hippocampal neurons, respectively.
21
Figure 3.2 Effect of E2 on Ngb protein levels in SK-N-BE cell line. a, Time-course analysis of E2 treatment (10 nM) on Ngb levels. b, E2 dose-dependent (0.1-1000 nM) effect on Ngb levels (24 hours of stimulation). The amount of protein was normalized to tubulin levels. Top panels are typical Western blots of five independent experiments. Bottom panels represent the results of the densitometric analysis. Data are means ± SD of five different experiments. Significant differences (p<0.001) were determined with ANOVA followed by Tukey-Kramer post-test. a, (*) significant difference vs. 0 hour and (°) vs. 0.5 hour; b, (*) significant difference vs. 0, (°) vs. 0.1, and (§) vs. 1 nM E2.Figure 3.3 Effect of E2 on Ngb protein levels in mouse primary hippocampal neurons. a,Time-course analysis of E2 treatment (10 nM) on Ngb levels. b, E2 dose-dependent (0.1-1000 nM) effect on Ngb levels (24 hours of stimulation). The amount of protein was normalized to with tubulin levels. The data are typical Western blots of five independent experiments.
22
3.2.2 Androgen effect on neuroglobin levels
Also AR was expressed in non-stimulated SK-N-BE cells (figure 3.4). DU145 human prostate cancer cell line was used as a positive control of AR expression (Alimirah et al., 2006). AR expression was previously reported in primary hippocampal neurons (Nguyen et al., 2009, and literature therein). However, the E2 effect on Ngb expression was specific since neither dihydrotestosterone (DHT) nor the common precursor of E2 and DHT, testosterone, were able to increase Ngb levels at any tested concentration in both SK-N-BE neuroblastoma cells and primary hippocampal neurons (figure 3.5). Thus, since only E2 was able to modify Ngb expression, no further studies on DHT and testosterone were performed.
Figure 3.4 Characterization of AR in SK-N-BE human neuroblastoma cell line. Analysis of AR expression levels in non-stimulated cells compared to AR-positive DU145 human prostate cancer cell line. The amount of protein was normalized to tubulin levels. The data are typical Western blots of five independent experiments.
Figure 3.5 Effect of DHT and testosterone on Ngb protein levels in SK-N-BE cell line (a, b) and in primary hippocampal neurons (c, d). a, c, Effect of different doses of DHT (0.1-1000 nM) on Ngb levels (24 hours of stimulation). b, d, Effect of different doses of testosterone (0.1-1000 nM) on Ngb levels (24 hours of stimulation). The amount of protein was normalized to tubulin levels. The data are typical Western blots of five independent experiments.
23
3.2.3 Estrogen receptor involvement in 17β-estradiol-induced neuroglobin levels
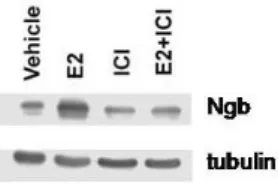
The pretreatment of SK-N-BE cells with the pure E2 antagonist, ICI 182,870 (ICI), completely prevented the E2 effect on Ngb levels (figure3.6), suggesting an ER-mediated mechanism.
Figure 3.6 Impact of ERs on Ngb protein levels in SK-N-BE human neuroblastoma cell line. Western blot analysis of Ngb levels in cells stimulated for 24 hours with either vehicle or E2 (1 nM) and/or the ER inhibitor ICI (1 μM). ICI was administrated 30 min before E2. The amount of protein was normalized to tubulin levels. The figure represents a typical Western blot of five independent experiments.
As SK-N-BE cells contain both ERβ and ERα (figure 3.1a), cells were stimulated with either the specific ERα agonist 4,4’,4’’-(4-propyl [1 H]-pyrazole-1,3,5-triyl)trisphenol (PPT) or the specific ERβ agonist 2,3-bis(4-hydroxyphenyl)propionitrile (DPN) to discriminate the role of each ER isoform in the E2-induced Ngb level increase. Only 1 and 10 nM DPN mimicked the E2 effect on Ngb levels (figure 3.7b), whereas PPT was unable to increase Ngb levels, at any concentration investigated (figure 3.7a). This result was confirmed by cell pretreatment with the specific ERβ inhibitor (R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol (THC), which completely prevented the E2 effect (figure 3.7b).
24
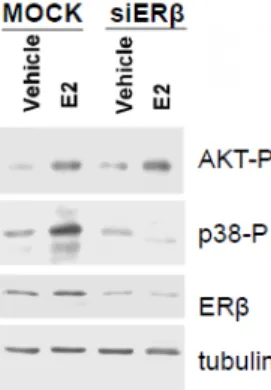
Figure 3.7 Impact of ERα and ERβ on Ngb protein levels in SK-N-BE human neuroblastoma cell line. a, Analysis of Ngb levels in cells stimulated for 24 hours with either vehicle, E2 (1 nM), or the ERα agonist PPT (1-100 nM). b, Analysis of Ngb levels in cells stimulated for 24 hours with either vehicle, E2 (1 nM), the ERβ agonist DPN (1-100 nM), or ERβ selective antagonist (THC 1μM). The amount of proteins was normalized to tubulin levels. Top panels are typical Western blots of five independent experiments. Bottom panels represent the results of the densitometric analysis. Data are means ± SD of five different experiments. Significant differences (p<0.001) were determined with ANOVA followed by Tukey-Kramer post-test. a, (*) significant difference vs. vehicle; b, (*) significant difference vs. vehicle, (°) vs. E2 , (§) vs. 1 nM, and (#) vs. 10 nM DPN.Furthermore, the decrease of ERβ protein level by ERβ short interfering RNA (siERβ) transfection caused an impairment of the E2 ability to increase Ngb levels (figure 3.8a).
A further confirmation of the ERβ involvement in the effect of E2 derives from the results obtained using the flavonoid naringenin (Nar) (figure 3.8b). Indeed, it has been previously reported that this flavonoid is a partial antagonist of E2 in the presence of ERα (Galluzzo et al., 2008) and an E2 mimetic in the presence of ERβ (Totta et al., 2004). Like E2, 0.1 μM Nar was sufficient to increase Ngb levels (figure 3.8b). This effect persisted at Nar high concentrations (i.e., 1 and 10 μM).
25
Figure 3.8 Impact of ERβ silencing and of ERβ agonist naringenin on Ngb levels in SK-N-BE human neuroblastoma cell line. a, Analysis of Ngb and ERβ levels in cells transfected with either MOCK (control) or ERβ small interference RNA (siERβ) in the absence or presence of E2 (1 nM). b, Analysis of Ngb levels in cells stimulated for 24 hours with either vehicle, E2 (1 nM) or naringenin (Nar; 0.01–10 μM). The amount of proteins was normalized to tubulin levels. Left panel is a typical Western blot of five independent experiments. Right panel represents the results of the densitometric analysis. Data are means ± SD of five different experiments. Significant differences (p<0.001) were determined with ANOVA followed by Tukey-Kramer post-test. (*) significant difference vs. vehicle, (°) vs. E2, (§) vs. 0.01, and (#) vs. 0.1 μM Nar.ERβ was also necessary for the E2-induced Ngb increase in mouse primary neurons. In these cells, containing a similar amount of ERα and ERβ (figure 3.1b), ICI prevented the E2 effect (figure 3.9), DPN mimicked the E2 effect (figure 3.10b), whereas PPT was unable to increase Ngb levels (figure 3.10a). Cell pretreatment with the specific ERβ inhibitor, THC, further confirmed this specificity (figure 3.10c).