Volume 274, number 1,2, 193-198 FEBS 09102 November 1990
Intracellular i m m u n i z a t i o n
Cloning and intracellular expression of a monoclonal antibody to the p21
r a sprotein
T h o m a s M . W e r g e , Silvia B i o c c a a n d A n t o n i n o C a t t a n e o CNR Institute o f Neurobiology, Viale Carlo Marx 15, 00156 Roma, Italy
Received i August 1990; revised version received 26 September 1990
Following the demonstration that intracellular expression of antibodies ('intracellular immunization') may be utilized to engineer new traits in mam- malian cells [I], we undertook experiments to perturb the function of p21 ras proteins, by engineering the intracellular expression of the anti-p21 r~s antibody Y13-259 [2]. The variable regions of this antibody have been cloned and, after verifying their antigen binding activity, expressed in general purpose vectors for the intracellular expression of antibodies. The results confirmed that the cloned antibody has been efficiently expressed both in the secretory and the intracellular forms. Thus, intracellular immunization of mammalian cells against p21 ras, or any other antigen for which
a monoclonal antibody is available, can now be performed. Intracellular immunization; p2 lras protein; Antibody expression vector
1. I N T R O D U C T I O N
Ras genes e n c o d e f o r m e m b r a n e associated G T P - b i n d i n g proteins that are involved in the c o n t r o l o f cell p r o l i f e r a t i o n a n d d i f f e r e n t i a t i o n [3]. M e m b e r s o f this gene family are highly conserved t h r o u g h o u t e v o l u t i o n a n d are widely expressed in cells o f b o t h lower a n d higher e u k a r y o t e s . All the available evidence points to ras proteins as key r e g u l a t o r y molecules linking g r o w t h f a c t o r receptors to their signal t r a n s d u c t i o n pathway(s). In S a c c h a r o m y c e s cerevisiae a genetic a p p r o a c h has led to the identification o f f u n c t i o n s f o r the RAS1 a n d R A S 2 proteins as well as f u n c t i o n s u p s t r e a m a n d d o w n s t r e a m o f the R A S gene p r o d u c t s [4]. In m a m - m a l i a n cells, where such an a p p r o a c h is n o t possible, the f u n c t i o n o f ras gene p r o d u c t s remains unclear. H o w e v e r , m i c r o i n j e c t i o n o f the anti-p21 ras a n t i b o d y Y13-259 [2] was s h o w n to block the serum stimulated D N A synthesis in N I H 3T3 cells, the nerve g r o w t h fac- t o r i n d u c e d differentiation o f P C 1 2 cells, the t r a n s f o r - m a t i o n by a n u m b e r o f o n c o g e n e s as well as ras s t i m u l a t e d adenylate cyclase activity in yeast cells [3]. This a n t i b o d y , t h e r e f o r e represents a valuable reagent to interfere with the f u n c t i o n o f p21 ~as. H o w e v e r , the intrinsically transient n a t u r e o f the a n t i b o d y microin- j e c t i o n experiments limits the usefulness o f such an ap-
Correspondence address: A. Cattaneo, CNR Institute of Neurobio- logy, Viale Carlo Marx 15, 00156 Roma, Italy
p r o a c h to study ras gene functions in m a m m a l i a n cells. W e recently p r o p o s e d the stable intracellular expression o f m o n o c l o n a l antibodies as a means to inactivate cellular proteins by d e m o n s t r a t i n g that h e a v y a n d light chains redirected to an intracellular c o m p a r t m e n t in- deed associate to f o r m f u n c t i o n a l antibodies [1]. W e r e p o r t in this p a p e r the molecular cloning o f the variable regions o f the h e a v y a n d light chain o f the m o n o c l o n a l a n t i b o d y Y13-259, a n d their r e c o n s t i t u t i o n in general p u r p o s e vectors f o r extracellular a n d in- tracellular expression o f i m m u n o g l o b u l i n s in m a m - m a l i a n cells. These constructs were transfected into c u l t u r e d cells, c o n f i r m i n g the subcellular localization o f the expressed a n t i b o d y chains. Experiments o n in- tracellular i m m u n i z a t i o n o f cells against the p21 ras p r o - tein c a n t h e r e f o r e be p e r f o r m e d .
2. M A T E R I A L S A N D M E T H O D S 2.1. Plasmids and bacterial strains
MI3-VnPCR1, MI3-VKPCR1, pSV-gpt-Huvl and pSV-hyg-HuCr were a gift from G. Winter (MRC Cambridge); plasmid p805 was received from M. Neuberger (MRC Cambridge) BW2029 bacteria (E. coli N6105 (Adhya Sankar, NIH) harbouring a temperature-sensitive ),-repressor controlling expression of p21 from pJCL-E30 [5]) were provided by B. Willumsen (University of Copenhagen).
2.2. Cloning of immunoglobulin variable regions by PCR
Total cytoplasmic RNA was prepared from Y13-259 hybridoma cells as descried [6]. First strand cDNA synthesis and PCR were per- formed as in [7], except that annealing was carried out at 60°C. Abbreviations: PCR, polymerase chain reaction; NLS, nuclear
localization sequence; FCS, fetal calf serum; BG, ~-globin-promoter; Vn, variable region of the heavy chain; VK, variable region of the light chain; HuC~:, constant regions of the human heavy ~-1 chain; HUCK, constant region of the human light x chain
2.3. Plasmid and mutant construction
The ~-globin promoter region was excised as a 850 bp Hin- dlll/Ncol fragment from p805 and cloned into MI3-VnPCRI and M13-VKPCRI [7], cut with HindlI1/NcoI, giving MI3-/3G-VHPCR and M 13-~G-VKPCR. The PCR amplified DNA was force cloned into
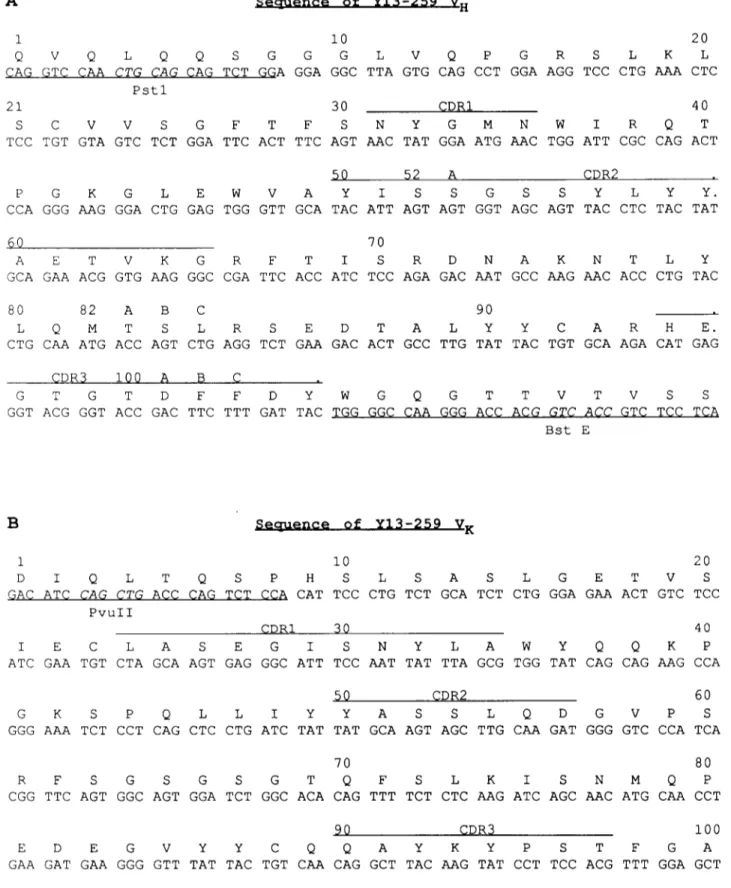
V o l u m e 274, number 1,2 A F E B S L E T T E R S S e c r u e n c e o f Y 1 3 - 2 5 9 V H N o v e m b e r 1990 1 i0 20 Q V Q L Q Q S G G G L V Q P G R S L K L C A G G T C C A A C T G C A G C A G T C T G G A G G A G G C T T A G T G C A G C C T G G A A G G T C C C T G A A A C T C P s t l 21 30 G D R I 40 S C V V S G F T F S N Y G M N W I R Q T T C C T G T G T A G T C T C T G G A T T C A C T T T C A G T A A C T A T G G A A T G A A C T G G A T T C G C C A G A C T 50 52 A C D R 2 P G K G L E W V A Y I S S G S S Y L Y Y. C C A G G G A A G G G A C T G G A G T G G G T T G C A T A C A T T A G T A G T G G T A G C A G T T A C C T C T A C T A T 60 70 A E T V K G R F T I S R D N A K N T L Y G C A G A A A C G G T G A A G G G C C G A T T C A C C A T C T C C A G A G A C A A T G C C A A G A A C A C C C T G T A C 80 82 A B C 90 L Q M T S L R S E D T A L Y Y C A R H E. C T G C A A A T G A C C A G T C T G A G G T C T G A A G A C A C T G C C T T G T A T T A C T G T G C A A G A C A T G A G G D R $ 1 O O A B G G T G T D F F D Y W G Q G T T V T V S S G G T A C G G G T A C C G A C T T C T T T G A T T A C T G G G G C C A A G G G A C C A C G G T C A C C G T C T C C T C A B s t E B S e u u e n c e o f Y 1 3 - 2 5 9 V K 1 i0 20 D I Q L T Q S P H S L S A S L G E T V S G A C A T C C A G C T G A C C C A G T C T C C A C A T T C C C T G T C T G C A T C T C T G G G A G A A A C T G T C T C C P v u I I C D R I 30 40 I E C L A S E G I S N Y L A W Y Q Q K P A T C G A A T G T C T A G C A A G T G A G G G C A T T T C C A A T T A T T T A G C G T G G T A T C A G C A G A A G C C A $0 G D R 2 60 G K S P Q L L I Y Y A S S L Q D G V P S G G G A A A T C T C C T C A G C T C C T G A T C T A T T A T G C A A G T A G C T T G C A A G A T G G G G T C C C A T C A 70 80 R F S G S G S G T Q F S L K I S N M Q P C G G T T C A G T G G C A G T G G A T C T G G C A C A C A G T T T T C T C T C A A G A T C A G C A A C A T G C A A C C T 90 C D R 3 i 0 0 E D E G V Y Y C Q Q A Y K Y P S T F G A G A A G A T G A A G G G G T T T A T T A C T G T C A A C A G G C T T A C A A G T A T C C T T C C A C G T T T G G A G C T G T K L E I K G G G A C C A A G C T G G A G A T C A A A < B g l I I / B c l I >
Fig. 1. Nucleotide and amino acid sequence of Y 13-259 heavy (A) and light (B) variable regions. The PCR primers and the adjacent flanking regions are underlined.
Volume 274, number 1,2 FEBS LETTERS November 1990 these M13 vectors [7], giving M13-/3G-VH259 and M13-/3G-VK259
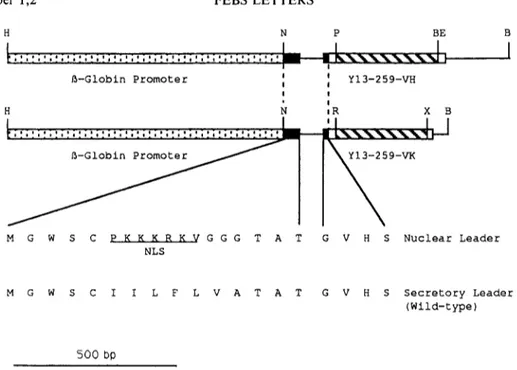
(Fig. 4), and subsequently sequenced [8]. The HindlIl/BamHl fragments from these vectors were recloned in the modified pSV-gpt and pSV-hyg vectors [7], giving p/3G-VH259 and p/3G-VK259.
The secretory leader sequence from the mouse V47 Via gene [9] utilized in the MI3-PCR vectors was substituted by the nuclear localization sequence (NLS) as described [3] giving the M 13-/3G/NLS- VH259 and M13-/3G/NLS-VK259 (Fig. 4). HindlII/BamHI fragments from these plasmids were then inserted into the pSV-gpt and pSV-hyg expression vectors, giving p/3G/NLS-VH259 and p/3G/NLS-Vk259. 2.4. Cells, transfections, immunofluorescence and Western blot
Rat pituitary GH3 [10] and simian COS cells were cultured in DMEM with 10070 FCS and transfected by DEAE-dextrane/chloro- quine [11]. Indirect immunofluorescence was performed as described in [1]. Expression of the p21 ras protein in BW2029 exponentially growing at 30°C was induced overnight at 42°C and harvested as described [5]. The p21 ras protein was revealed by Western blotting [5].
3. R E S U L T S
T h e variable regions o f the heavy a n d light chain o f the rat m o n o c l o n a l a n t i b o d y Y13-259 [2] were cloned b y the p o l y m e r a s e chain reaction ( P C R ) m e t h o d , as recently described [7]. T h e sequences were c o n f i r m e d
o n different clones o b t a i n e d f r o m i n d e p e n d e n t a m p l i f i c a t i o n reactions. Fig. 1 shows the nucleotide se- q u e n c e o f the heavy a n d light chain variable regions, between the t w o a m p l i f i c a t i o n primers (underlined), t o g e t h e r with the d e d u c e d a m i n o acid sequence. T h e a m i n o acid sequences c o r r e s p o n d i n g to the regions o f the P C R primers are likely to differ in Y13-259. T h e c o m p l e m e n t a r i t y determining regions ( C D R ) are overlined. Inspection o f the Kabat d a t a base s h o w e d t h a t the sequences o b t a i n e d are different f r o m all other i m m u n o g l o b u l i n variable regions.
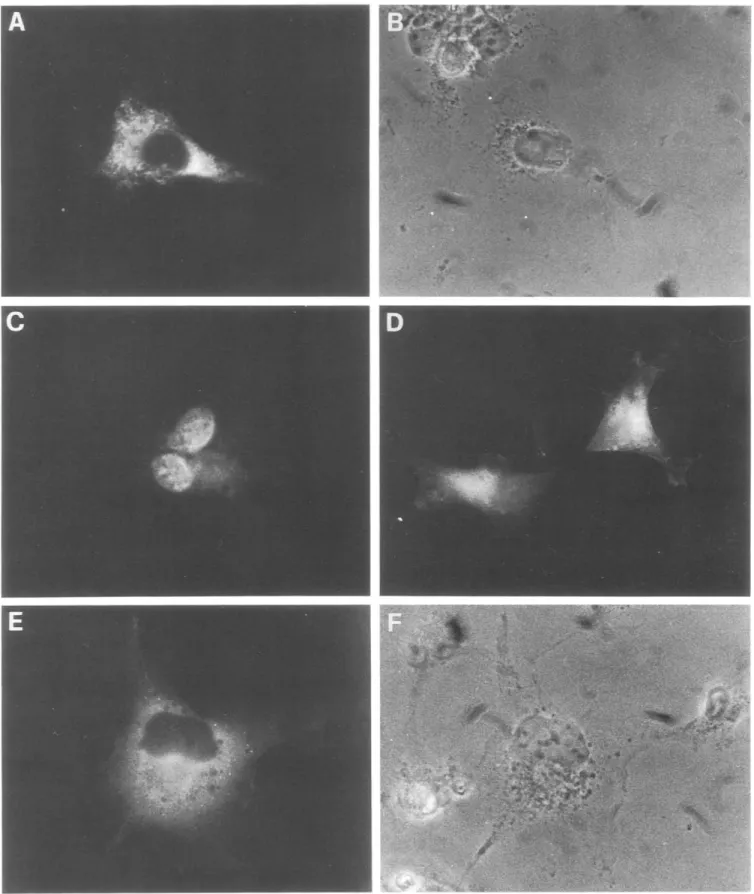
T h e VH a n d VK gene regions o f Y13-259 a n t i b o d y were assembled together with h u m a n C 7 and CK genes respectively, in vectors f o r expression o f secreted r a t / h u m a n chimaeric antibodies, pI3G-Vn259 and p/3G- V r 2 5 9 . C o t r a n s f e c t i o n o f these constructs into the cell lines C O S and G H 3 gave rise to high expression o f the c o r r e s p o n d i n g a n t i b o d y chains. Fig. 2 shows c o r r e s p o n - ding fields o f C O S cell t r a n s f e c t a n t s stained by d o u b l e indirect i m m u n o f l u o r e s c e n c e f o r light (Fig. 2A) a n d h e a v y (Fig. 2B) chain. B o t h d o u b l e positive a n d single positive cells are present in the cell p o p u l a t i o n . T h e staining p a t t e r n is typical o f a secretory protein.
T h e s u p e r n a t a n t o f the C O S d o u b l e transfectants was assayed by Western blotting f o r the p r o d u c t i o n o f anti- p21 *as antibodies (Fig. 3). T h e experiment d e m o n s t r a t e s t h a t these cells indeed secrete, albeit in low a m o u n t s , antibodies that recognize p21 *as protein (Fig. 3, lane 3) as d o the parental Y13-259 antibodies (Fig. 3, lane 1).
T h e secretory f o r m o f the cloned Y13-259 a n t i b o d y is unlikely to interact with its c o r r e s p o n d i n g antigen p21 ras inside the cell, due to their different intracellular localization. In order to redirect the a n t i b o d y a w a y f r o m the secretory p a t h w a y , the h y d r o p h o b i c core o f the secretory leader was substituted with the nuclear localization signal P K K K R K V f r o m the SV40 large T
Fig. 2. Coexpression of secretory heavy and light chains from Y 13-259 in COS cells transfected with pbG-Vn259 and pbG-VK259.48 h after transfection cells were stained by double indirect immunofluorescence with anti-light chain (A) and anti-heavy chain (B) antibodies. The
same field is shown for the two fluorochromes used.
I 2 3 4 5 6
30.0 "
-
! 4
iii:
21.0 ,~W
....
Fig. 3. Western blot analysis of COS cell transfectants. Cell extracts from heat shock induced (lanes 1, 3, 5) or uninduced (lanes 2, 4, 6) BW2029 bacteria were probed after SDS-PAGE with Y13-259 an- tibodies (lanes 1, 2) and with supernatant from COS cells transiently transfected with p~G-VH259 and p/3G-VK259 (lanes 3, 4) or with cor- responding plasmids encoding a non-relevant antibody (lanes 5, 6). The molecular size markers are indicated with arrows as well as the
Volume 274, number 1,2 FEBS LETTERS November 1990 H N P B E ! ~ - G l o b i n P r o m o t e r J l I H N M G W S C P K K K R K V G G G T A N L S I t Y I 3 - 2 5 9 - V H J ! I iR X B 5 9-VK G V H S N u c l e a r L e a d e r M G W S C I I L F L V A T A T G V H S S e c r e t o r y L e a d e r ( W i l d - t y p e ) 500 bO
Fig. 4. Shuttle vectors for expression of immunoglobulin genes. The amino acid sequences of the wild-type secretory leader and of the nuclear localization leader are shown. The Y13-259 sequences (hatched boxes) and the adjacent flanking regions (unfilled boxes) correspond to the se- quences shown in Fig. 1. The lines represent the leader intron and part of the V-C intron. Any variable region amplified with the primers described in [11] can be cloned into these vectors. H = H i n d l I I ; N = N c o l ; P = P s t l ; BE = B s t E I I ; B = B a m H 1 ; R = P v u l I ; X = B c l I / B g l l I (the BclI
site in the parental vector is lost upon cloning).
antigen [12] (Fig. 4), which we have shown directs im- munoglobulin chains away from the secretory pathway and to the cytoplasm, from where, in some cell types, they reach the nucleus [1]. The NLS was incorporated into M 13-~G-V,-I259 and M 13-~G-VK259 (Fig. 4). After further cloning the activity o f the final 'intracellular' expression vectors, p/~G/NLS-V,-I259 and p ~ G / N S L - VK259, was determined by immunofluorescence after transient transfection into COS cells. Results for the heavy chain transfectants are shown in Fig. 5. Many cells in the transfected population show a bright signal for this antibody chain, demonstrating that this polypeptide chain are correctly and efficiently syn- thesized. The intracellular staining pattern (Fig. 5C,D,E) was remarkably different from that obtained for the secretory antibody chains (Figs. 2 and 5A), in keeping with the engineered direction o f the antibody chain away from the secretory compartment. Different staining patterns can be found in the cell population, ranging from a predominant nuclear staining (Fig. 5C) to stainings in which both the nucleus and the cytoplasm are labelled (Fig. 5D). Cells with only the cytoplasm labelled are also found (Fig. 5E). Qualitatively similar results are obtained for the light chain (not shown), although the intensity of the signal is lower.
4. DISCUSSION
We have recently demonstrated the feasibility o f ex- pressing monoclonal antibodies in different in-
tracellular compartments to interfere with the function o f selected intracellular antigens [1], as one possible strategy to achieve 'intracellular immunization' [13] in mammalian cells. As a first application o f this ex- perimental strategy, we undertook experiments to per- turb the function o f the protooncogene c-ras. The monoclonal antibody Y13-259 is able to neutralize c- and v-ras activity following microinjection into living cells. We have therefore cloned the variable regions o f this antibody and confirmed the antigen binding activi- ty o f the reconstituted secretory antibody by assaying the supernatants o f transiently transfected COS ceils. The cloned antibody does indeed recognize specifically p21 ras protein, as the parental antibody (Fig. 3). The levels of antibodies secreted by COS cell transfectants appear to be quite low, possibly due to (i) the small percentage o f the cells in the transfected population ex- pressing the antibodies, and (ii) the reported low effi- ciency o f antibody secretion by COS cells and other fibroblast cell lines [14]. We are presently deriving stable cell lines (NSO myeloma and G H 3 pituitary cells) secreting the recombinant Y13-259 antibody (work in progress). We then engineered general purpose vectors for the intracellular expression o f any antibody of in- terest. The results obtained confirm that the cloned an- tibody has been successfully expressed both in the secretory and the intracellular versions. It is noteworthy that the levels o f expression o f the intracellular heavy chain are comparable to its secretory counterpart (Fig. 5). We further show that the nuclear localization se- quence (NLS) has proven efficient also for the heavy
V o l u m e 274, number 1,2 FEBS L E T T E R S N o v e m b e r 1990
C
E
D
!! ~ i ¸
Fig. 5. Cellular distribution of the secretory (A) and intracellular (C, D and E) Y13-259 recombinant heavy chain. COS cells were transfected with p~G-VH259 and p~G/NLS-Vn259 and stained 48 h later with anti-heavy chain antibodies. B and F show phase contrast pictures of the fields in
V o l u m e 274, n u m b e r 1,2 FEBS L E T T E R S N o v e m b e r 1990
chain, as previously reported for the light chain [1],
both in terms of preventing secretion, expression levels
and targeting to the nucleus (Fig. 5). The fluorescence
signal obtained for the light chain is somewhat lower,
possibly due to cleavage of the constant domain or
masking by some intracellular protein. This is consis-
tent with our previous finding [1] that probing for in-
tracellular antibodies with anti-idiotypic antibodies
gives a higher proportion of nuclear labelling than pro-
bing with anti-isotype antibodies. The presence of the
NLS targeting signal on the anti-p21 ras antibody might
divert the p21 ras protein from its normal localization,
thus contributing to inhibit its function.
The results presented show that the general purpose
vectors engineered for intracellular expression of an-
tibodies perform very efficiently and can therefore be
utilized to study the phenotype resulting from the ex-
pression of any antibody of interest, in different cell
types. In particular, it is now possible to derive cell lines
in which the function of the p21 ras protein is inhibited,
thereby allowing the study of its role(s) in different
physiological and pathological contexts.
Acknowledgements: We thank A. Bradbury and P. Piccioli for helpful discussions and suggestions, G. Winter, M. Neuberger and B. Willumsen for the kind gifts of DNA and bacterial strains, and A. Di Luzio for excellent assistance. We are grateful to P. Calissano for constant support and laboratory space. The work was supported through grants from the Associazione Italiana per la Ricerca sul Can-
cro, CNR (Prog. Fin. Biotecnologia e Biostrumentazione), The Preuss Foundation to A.C.T.M.W. acknowledges fellowships from Sigma-Tau and Grosserer L.F. Fogth Foundation, Copenhagen. S.B. is on leave of absence from the Dipartimento di Scienze Biochimiche, Universitfi 'La Sapienza', Roma. A.C. is also at the Dipartimento di Fisiologia Generale, Universitfi di Napoli.
REFERENCES
[1] Biocca, S., Neuberger, M.S. and Cattaneo, A. (1990) EMBO J. 9, 101-108.
[2] Furth, M.E., Davis, L.J., Fleurdelys, B. and Scolnick, E.M. (1982) J. Virol. 43, 294-304.
[3] Barbacid, M. (1987) Annu. Rev. Biochem. 56, 779-828. [4] Hall, A. (1990) Cell 61,921-923.
[5] Lacal, J.C., Santos, E., Notario, V., Barbacid, M., Yamazaki, S., Hsiang-Fu Kung, Seamans, C., McAndrew, S. and Crowl, R. (1984) Proc. Natl. Acad. Sci. USA 81, 5305-5309.
[6] Bradbury, A., Belt, K.T., Neri, T.M., Milstein, C. and Calabi, F. (1988) EMBO J. 7, 3081-3086.
[7] Orlandi, R., Gussow, D.H., Jones, P.T. and Winter, G. (1989) Proc. Natl. Acad. Sci. USA 86, 3833-3837.
[8] Sanger, F., Nicklen, S. and Coulson, A.R. (1977) Proc. Natl. Acad. Sci. USA 74, 5463-5467.
[9] Neuberger, M.S. (1983) EMBO J. 2, 1373-1378.
[10] Bancroft, F.C. (1981) in: Functionally Differentiated Cell Lines (Sato, G. ed.) pp. 47-59, Alan R. Liss, New York.
[11] Pelham, H.R.B. (1984) EMBO J. 3, 3095-3100.
[12] Kalderon, D., Roberts, B.L., Richardson, W. and Smith, A. (1984) Cell 39, 499-509.
[13] Baltimore, D. (1988) Nature 335, 395-396.
[14] Cattaneo, A. and Neuberger, M.S. (1987) EMBO J. 6, 2753-2758.