Stefano Suzzi
REGULATION OF NEUROGENESIS IN PRION DISEASE
Master of Science Thesis Dissertation
University of Pisa
July 2012
To Auntie Maria, whom I will always miss
To Grandpa Vincenzo, who has ever cared a lot about me
VII
Contents
Abstract ... - 1 -
Introduction ... - 2 -
1. Neurogenesis in the Adult Mammalian Brain ... - 4 -
1.1. Historical Perspective ... - 4 -
1.2. Methodologies to Assess Adult Neurogenesis In Vivo ... - 8 -
1.2.1. Incorporation of Nucleotide Analogues ... - 8 -
1.2.2. Retroviral Lineage Tracing ... - 9 -
1.2.3. Analysis of Specific Marker Expression ... - 10 -
1.3. Composition and Localisation of the Adult Neurogenic Pool ... - 13 -
1.3.1. Adult Neural Progenitors ... - 14 -
1.3.2. Adult Neurogenic Niches ... - 16 -
2. Role and Regulation of Neurogenesis in Health and Disease ... - 23 -
2.1. Role of Neurogenesis ... - 23 -
2.2. Molecular and Cellular Driving Forces ... - 26 -
2.2.1. Extracellular Determinants ... - 26 -
2.2.2. Intracellular Determinants ... - 28 -
2.3. Influence of Environmental Factors ... - 29 -
2.3.1. Effects of a Rich Environment ... - 30 -
2.3.2. Effects of Stress ... - 31 -
2.4. Response to Pathological Stimulation ... - 32 -
3. Changes in Neurogenesis during Neurodegeneration ... - 34 -
3.1. Leading Events in Neurodegeneration and Adaptive Responses ... - 34 -
3.2. Alzheimer’s Disease ... - 36 -
3.3. Parkinson’s Disease ... - 38 -
3.4. Huntington’s Disease ... - 39 -
4. Prion Disease as a Model of Chronic Progressive Neurodegeneration ... - 41 -
4.1. Neurodegeneration in Prion Disease ... - 41 -
4.2. Neurogenic Response during Prion Disease ... - 42 -
VIII
Materials and Methods ... - 45 -
Results ... - 54 -
Discussion ... - 65 -
Conclusions ... - 74 - References ... IX Acknowledgements ... XXVIII
- 1 -
Abstract
Neurogenesis is impaired during chronic progressive neurodegenerative diseases, with the potential to replace damaged neurons at lesion sites. These findings support the need for a better understanding of injury-induced neurogenesis in the adult brain undergoing neurodegeneration and suggest the potential of directing neural precursor regulation as a strategy for brain repair. Among the existing experimental models of neurodegeneration, prion disease models present many features in common with diseases such as Alzheimer’s or Parkinson’s and is an ideal tractable laboratory model to study chronic progressive neurodegenerative diseases.
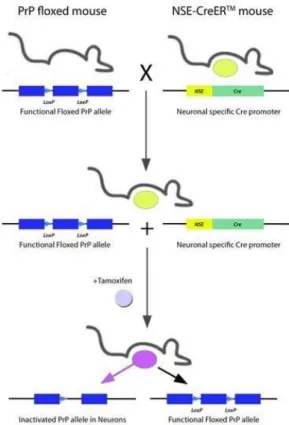
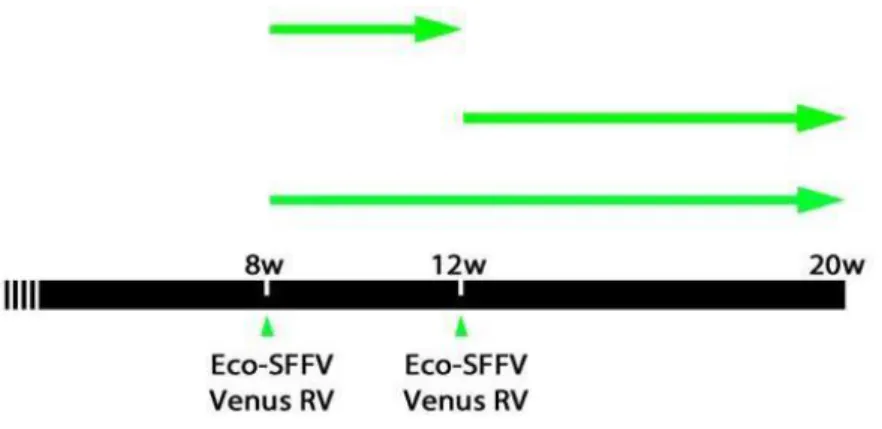
In the present work, using the ME7 murine model of prion disease, we studied the dynamics and regulation of neurogenesis since this would provide a basis for the therapeutical manipulation of neurogenesis which would impact both behaviour and pathology. Our results provide insights into the dynamics of hippocampal cell death and the generation of neural precursor cells and new neurons in the dentate gyrus (DG) region. Using retroviral vectors to label and trace proliferating cells, we found significant changes in the maturation and integration of newly generated neurons within the DG. In addition, we analysed the impact on the population of GABAergic interneurons and, by using conditional ablation of the prion protein (PrP) in neurons, we further investigated the role of differentiated neurons in the regulation of neurogenesis during the course of prion disease. Finally, we studied neurogenesis in hippocampi from variant Creutzfeldt-Jacob’s disease (vCJD) and Alzheimer’s disease (AD) patients, showing similarities with the results obtained in the murine model of prion disease.
The present results describe for the first time the regulation of neurogenesis during prion disease and open a window for the full understanding of the regulation and role of neurogenesis during chronic progressive neurodegenerative diseases.
- 2 -
Introduction
A prerequisite to the regenerative capacity of a tissue is the proliferative ability of its cells. Over many decades, the lack of proof for mitosis in the adult central nervous system (aCNS) seemed to reasonably explain why brain pathological insults may produce such devastating effects and long-term disabilities. In fact, in comparison with other organs such as skin or liver, the brain undergoes minimal healing after lesion. Once lost, neurons are not replaced and the injured area is permanently damaged. These considerations led the Italian histologist Giulio Bizzozero to classify the postnatal nervous tissue as “perennial”, meaning that any possibility of growth or regeneration is definitely precluded after birth (Bizzozero, 1894). Many prominent neuroscientists consolidated what became the “central dogma of neurology” – one for all, the Spanish Santiago Ramón y Cajal, who wrote that neuronal mitotic activity ceases “irrevocably” by the time embryonic development has ended (Ramón y Cajal, 1913). There was also another, ideological reason why the inability of nerve cells to proliferate in the aCNS has been maintained as an unquestionable notion over so many decades. The postnatal addition of new neurons could not happen without significantly modifying the unique repertoire of connections individuals build during their lifetime. If this surely represents an advantage for lower Vertebrates, it does not for more sophisticated animals, upon whom the evolution bestowed the ability to keep old neurons instead of turning them over. The dominating doctrine taught that a fully developed brain was too complex a structure for neurons to come and go and attempts to investigate the contrary were regarded as somewhat heretical (for review, see Colucci-D’Amato et al., 2006; Specter, 2001).
It was greatly to the credit of some of those heretics, who stubbornly withstood a wall of hostility, that it is finally manifest that the generation of new neurons, hereafter referred as “neurogenesis”, does continue after birth, although declining with age. Thenceforth, technical advances allowed extensively documenting adult neurogenesis in numerous mammalian species, including humans. However, neurogenesis in the adult does not take place everywhere in the brain. On the contrary, the neurogenic capacity appears restricted to anatomically and functionally distinct areas, called “neurogenic
- 3 -
niches”. Many brain districts have been proposed to host and/or constitutively produce adult neural progenitors but only two are at present definitely established: the
subventricular zone (SVZ), lining the lateral walls of the lateral ventricles; and the subgranular zone (SGZ) in the dentate gyrus (DG) of the hippocampus. Newly
generated cells from the SVZ migrate through the rostral migratory stream (RMS) to reach their final destination in the olfactory bulb (OB), where they differentiate into mature neurons (for review, see Ming & Song, 2005; Weinandy et al., 2011). In the SGZ, progenitor cells multiply and migrate for much a shorter distance through the granular layer (GL) before becoming synaptically integrated granule cells (Kempermann et al., 2004; Ming & Song, 2005). In addition, research has shown that neurogenesis in the adult may also occur in normally non-neurogenic regions in response to pathological stimuli of diverse nature, allowing newly generated cells to replace damaged neurons at lesion sites. These findings unleashed a flood of information which not only paved the way for a better understanding of brain ontology and physiology, but also opened a tantalizing route towards future therapeutics against several neuropathological conditions, including neurodegeneration.
Chronic neurodegeneration is characterised by the progressive loss of structure and/or function of neurons, with consequent damage to neural circuits and cell death. The high prevalence worldwide of neurodegenerative diseases propels research in this field to come on in leaps and bounds, but still many questions remain unanswered. Scientific advances have shown that many neurodegenerative diseases share similar features on both cellular and sub-cellular level. The elucidation of these similarities may help to mitigate many diseases at the same time and to spare future generations from this scourge.
Unfortunately, despite the use of transgenic animal models of neurodegeneration allowed teasing out a huge collection of data, they usually mimic only some of the facets displayed in human diseases (for review, see Winner et al., 2011). Moreover, most neurodegenerative conditions in humans have a sporadic origin, far from the genetic nature of the most common models of neurodegeneration. Nevertheless, remarkable steps forward in the field of neurodegeneration have shown that both the intercellular and the intracellular transfer of inclusions made of misfolded specific proteins plays a pivotal role in the spread of the neuropathology. Interestingly, this
- 4 -
process is reminiscent of that by which prions propagate in transmissible spongiform encephalopathies, also known as prion diseases (for review, see Goedert et al., 2010). This observation made prion disease an attractive field for researchers looking for new insights into neurodegeneration. Because almost every pathological feature in both animals and humans has been reproduced in several murine strains, prion disease is considered a convincingly good laboratory model also for other protein misfolding neurodegenerative diseases (for review, see Prusiner, 2001).
Cumulative evidence has shown that neurogenesis is also impacted in the adult brain undergoing neurodegeneration. On the other hand, how and to what extent are neurogenic phenomena altered during the progression of disease is yet to be completely defined. Though still far from setting up successful treatments and neurorestorative strategies by exploiting adult neural progenitor biology, many encouraging results suggest the potential of manipulating the neurogenic capacity in the diseased brain as a promising target to promote brain endogenous repair. Therefore, the clarification of the regulation of neurogenesis during chronic progressive neurodegenerative disease would be instrumental for the exploration of its role and possible health relevance.
1. Neurogenesis in the Adult Mammalian Brain
1.1. Historical Perspective
A huge number of neurons and glia are produced during the embryonic stages of neural development. Early symmetric divisions increase the pool of neural precursors but at later time points asymmetric daughters are generated. Post-mitotic elements migrate and begin to populate the different districts of the emerging nervous system, where they finally complete differentiation. While gliogenesis persists throughout life, neurogenesis is terminated perinatally. Nonetheless, it was noticed that in some species brain is not completely formed by the time of delivery (Hamilton, 1901), but the first suggestion that new neurons are formed in the adult brain date back to the observations of Ezra Allen, who revealed the presence of mitotic figures in the wall of the lateral ventricles of albino rats as old as 120 days (Allen, 1912). However, by the primitive
- 5 -
techniques then used it was impossible to carry out fate-mapping studies and to discriminate between new neurons or glia. It was not until the introduction of tritiated thymidine (3H-TdR) incorporation assays that the stereotyped and precisely orchestrated events leading to brain histogenesis were clearly established. Briefly, if a pulse of thymidine molecules carrying a radioactive hydrogen atom is given, at least some of them will eventually serve for DNA synthesis in actively proliferating cells. By autoradiography it is thus possible to individuate cohorts of cells which underwent the S-phase of the cell cycle during label administration. Thanks to this technique, in the early 1960s Joseph Altman, while studying glial proliferation kinetics after experimental brain trauma in rats, incidentally found the presence of 3H-TdR-labelled neurons “in brain regions not necessarily associated with the lesion area” (Altman 1962a, b). One year later he suggested the possibility that new neurons may proliferate in several forebrain structures in both adult rats and cats (Altman, 1963). Given that rats and cats are altricial animals, i.e. they are born immature, it was speculated that their delayed neural development was related to the curtailed intrauterine development. Conversely, Altman was able to prove postnatal neurogenesis also in guinea pigs, which are precocial animals (Altman, 1967). Finally, Altman provided the first demonstration of adult neurogenesis in the hippocampus (Altman & Das, 1965) and the olfactory bulb (Altman, 1969). Despite the great interest these findings would raise, initially they were greeted with scepticism by the scientific community. First, there was no hint of evidence that postnatally generated neurons are eventually integrated in the pre-existing circuits. Second, no clear functional significance was attributable. Ostracised, Altman abandoned the field and his work became soon forgotten.
However, as people say, it is always darkest before the dawn. The deathblow to the long-held notion that no new neurons are originated into the adult brain was dealt by several studies on melody learning in songbirds. Songbirds, like canaries, zebra finches and chickadees, are able to learn new tunes every year of their life. This ability is acquired by males only during a critical period when the early rudimentary vocalisations, or subsong, turn into a more structured plastic song and hence into a
stable song with sexual maturity. Between the 1970s and the 1980s, the ethologist
Fernando Nottebohm was intrigued by sexual dimorphisms in avian vocal nuclei. These brain areas are much bigger in males but can increase their volume as much as twofold
- 6 -
in testosterone-treated females (Nottebohm & Arnold, 1976; Nottebohm, 1980). At the beginning the phenomenon was not understood and it was proposed that resident cells swelled and shrank periodically during the course of the year (Nottebohm, 1981). The controversial idea of bursts of neurogenesis balanced by periods of regression as a likely explanation came later on (Nottebohm, 1983) and laid the groundwork for further investigation. This time, the concept of neurogenesis met favour with the academic world for two reasons. First, 3H-TdR-autoradiography combined with electrophysiology demonstrated that newly generated neurons are functionally recruited (Paton & Nottebohm, 1984). Second, given the importance of vocal nuclei in melody learning, local neurogenesis in adult songbirds could explain the formation of new memories and tune repertoires through profound circuit-remodelling and cell-replacement mechanisms (Nottebohm, 1989). Another striking discovery emerged from comparisons between adult free-range and captivity-raised birds. In the former group of animals a constant production of neurons recruited at the level of the hippocampus occurred, with a peak in autumn, whereas in the latter the phenomenon was much more modest (Barnea & Nottebohm, 1994). These results suggested that significant cell renewal in the hippocampus would grant a greater capacity for learning and memory. In fact, animals living in a natural environment need to court a female in the spring, store food when the season turns cold, avoid predators and defend the territory over the whole year and all this solicits new memory formation (Barnea & Nottebohm, 1996).
Despite the credit Nottebohm’s work received, its extraordinary significance was not thoroughly understood. Indeed, it was welcomed so warmly just because it was birds. The idea that comparable neurogenic events happen in higher Vertebrates during adulthood, not to mention in humans, still appeared risible. As a result of this considerable conceptual obstacle, the field of adult neurogenesis was left behind for many years. A heavy toll was taken, as directly experienced by Michael Kaplan. Kindled by Atman’s findings, he was the first to conduct electron microscopic studies on light autoradiograph of 3H-TdR-treated rats and reported neurogenesis in several brain regions, included the visual cortex (Kaplan 1977, 1980). These findings met with great scepticism, if not fierce disbelief. In particular, Pasko Rakic, the foremost authority in the field of primate brain studies, brought into question whether Kaplan neurons were actually such. Commenting on the existence of adult neurogenesis at the
- 7 -
top of the evolutionary ladder, Rakic strenuously defended the old standpoint and clearly stated that “a stable population of neurons in primates, including humans, may be important for the continuity of learning and memory over a lifetime” (Rakic, 1985). Feeling bitter about all the difficulties met, Kaplan eventually quitted the field (for his personal recount, see Kaplan, 2001).
Notwithstanding, Rakic’s monolithic views started creaking. Several decisive technical advances produced an outburst of research that pushed the field further than ever. The biggest revolution perhaps consisted in the introduction of 5-bromo-2’-deoxyuridine (BrdU), a synthetic thymidine analogue, as another S-phase marker. Unlike the 3H-TdR, BrdU can be detected by immunohistochemistry (IHC). Co-localisation of BrdU with cell-specific markers can be detected by confocal microscopy, thus allowing for unambiguous detection of DNA synthesis in a given cell population. Through such new molecular-labelling methods, Elizabeth Gould succeeded into definitely demolishing the residual reluctance against the retention of neurogenic potential even in the most evolved brains. While investigating the relationship between low levels of adrenal hormones and normally occurring hippocampal cell death during the early postnatal period in rats, Gould observed that the removal of adrenal glands resulted in an increased number of dying granular cells without significant changes in the density of healthy cells (Gould et al., 1991b). These results were consistent with the concomitant addition of new cellular elements in place of the lost ones. Further work led Gould to shift her attention from rodents to higher mammals and this right intuition led to the discovery of adult hippocampal neurogenesis first in tree shrews, animals phylogenetically between insectivores and primates, and then in New World (Gould et al., 1998) and Old World monkeys (Gould et al., 1999a).
In the same years, Rakic partly recanted his opinions and finally admitted adult neurogenesis to occur also in advanced animals, though playing a diminished role (Rakic, 1998; Kornack & Rakic, 1999). However, a ground-breaking study carried out by Peter Eriksson and Fred Gage in the end of the 1990s was the final straw, when for the first time the neurogenic hypothesis was tested in humans (Eriksson et al., 1998). Five terminal cancer patients, aged between fifty-seven and seventy-two, underwent intravenous delivery of BrdU and, following their death, brain specimens were collected. Co-labelling for BrdU and one among selected neuronal markers proved that new
- 8 -
neurons were generated in the adult human hippocampus. The creed that we as humans are born with the same nerve cells we will ever have was overturned.
1.2. Methodologies to Assess Adult Neurogenesis In Vivo
Great insights into adult neural progenitor biology have been permitted so far by the extraordinary development of in vitro techniques, whose primary advantage is to get rid of the biological complexity of a living organism by reproducing single, isolated processes. However, culture conditions often affect how cells behave in physiological conditions. For this reason, extrapolation back to a whole biological system may prove challenging and considerable debate exists as to whether proposed adult neural progenitor populations are indeed such. On the other hand, despite being more respectful towards the factual reality, in vivo studies present many methodological issues, which can be reduced to mainly three: one is the detection of proliferating adult neural progenitor and their progeny; another is the identification of their phenotype and differentiation; the last one is the assessment of effective functional integration.
1.2.1. Incorporation of Nucleotide Analogues
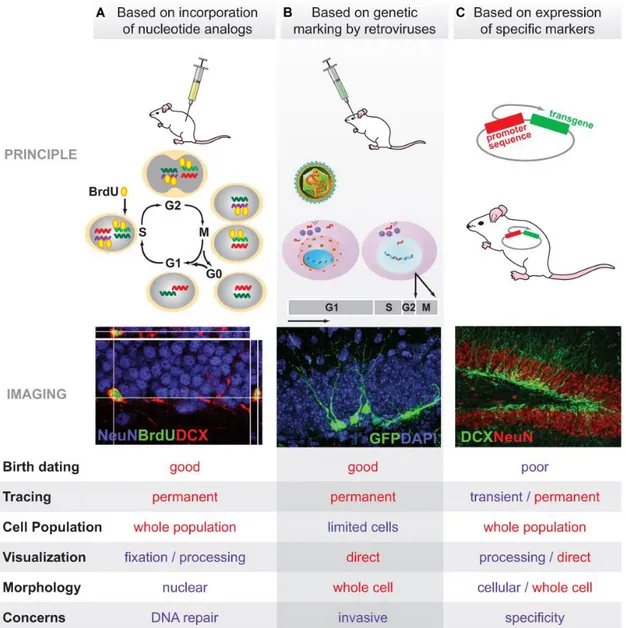
Proliferative events in the adult brain were first identified through autoradiographic analysis of 3H-TdR-labelled samples, now largely superseded by thymidine analogue incorporation assays combined with IHC and stereological quantification. The most commonly used thymidine analogue is 5-bromo-2’-deoxyuridine (BrdU), but other compounds have been synthetically obtained by attaching a thymidine molecule to different chemical groups: iodine or chlorine in IdU or CldU; ethynyl in EdU (for review, see Cavanagh et al., 2011). The possibility to inject animals with different thymidine analogues has made it feasible to track and compare cell populations undergoing DNA synthesis at different time points (Figure 1A). By modifying experimental conditions such as the lag between label administration and animal’s sacrifice, one may estimate how many cells proliferated, eventually differentiated or survived in a given timeframe (Ming & Song, 2005).
- 9 -
Even if this labelling method is generally considered the gold standard to assess adult neurogenesis, several pitfalls exist (Arias-Carrión et al., 2009). First, live cell analysis is not viable, for IHC requires tissue fixation and sometimes DNA denaturing for label detection (Gould & Gross, 2002). Second, because the label is available only for a short time after administration, the thymidine analogue undergoes progressive dilution at each cell division cycle (Ming & Song, 2005). Third, because thymidine analogues stain the nuclei, co-localisation with at least one cell-specific marker and careful confocal 3D-reconstruction are required for unequivocal phenotypic identification (Sanai et al., 2007). Possible sources of false negativity and false positivity need to be excluded as well. In fact, it must be considered that not all the DNA-synthesising cells might incorporate the label, nor do all the cells with proliferative potential enter lineage production at the same time (Sangiorgi & Capecchi, 2008). Moreover, DNA manufacturing is not exclusive to dividing cells, but it has also been reported in other biological phenomena such as repair, DNA-endoreduplication, mitosis-like apoptosis, fusion between neurons and proliferating glia (Arias-Carrión et al., 2009). Additional confounding factors may arise from potential toxic effects of the thymidine analogues or influences upon label accessibility, stability and diffusion caused by experimental manipulations (Ming & Song, 2005). These drawbacks have led to the adoption of alternative proliferation markers like proliferating-cell nuclear antigen (PCNA), phospho-histone H3 (pHH3), mini-chromosome maintenance protein 2 (Mcm2) and nuclear antigen Ki-67 (van Strien et al., 2011). However, the expression of these markers is limited mostly to the active phases of the cell division cycle and phenotypic differentiation of post-mitotic daughter cells is therefore precluded (Arias-Carrión et al., 2009).
1.2.2. Retroviral Lineage Tracing
Recent insights in neurogenesis benefitted enormously from retrovirus biology. Retroviruses are RNA viruses which use the reverse transcriptase enzyme to convert their RNA genome into a DNA molecule, which is then integrated into the DNA of the host genome (Kempermann et al., 2008). Retroviral genome is typically modified so that: 1) viral replication competence is abolished; and 2) alien genes coding for a variety
- 10 -
of molecules, like fluorescent reporters, are inserted (Sanes et al., 2006). These modifications make it so that: 1) only originally infected cells can endow their progeny with the viral information; and 2) all the daughter cells will express the viral information, without dilution over successive cell division cycles (Sanes et al., 2006). Lentiviruses and oncoretroviruses have been widely used for retroviral vector engineering, but the latter have proved particularly suited for cell-lineage tracing studies (van Praag et al., 2002). In fact, while lentiviruses label both dividing and non-dividing cells, oncoretroviruses successfully transduce only mitotically active cells, where the nuclear envelope breaks down and the host genome is readily accessible for integration (Kempermann et al., 2008).
For these reasons, retroviruses represent a useful strategy to study many aspects of neurogenesis (Figure 1B). For example, the expression of a fluorescent reporter which localises in the cytoplasm permits the experimenter not only to track birthdates, but also to consider some stereological and morphometric features of interest, such as spatial localisation, anatomical relationships (Jessberger et al., 2007), dendritic arborisation complexity, dendritic spine density and morphology (Jessberger et al., 2008) or axon terminal size (Toni et al., 2008). By using different vectors at different time points, it is possible to compare temporally-distant labelled-cell populations. Furthermore, it is possible to determine the phenotypic identity of retrovirus-labelled cells by IHC for a specific marker. The powerful approach of retroviral-tracing presents two important limitations (Ming & Song, 2005). First, invasive stereotactic surgery is required. Second, only a limited number of cells get infected.
1.2.3. Analysis of Specific Marker Expression
Phenotypic analysis of newly generated cells can be conducted at both morphological and molecular level (Ming & Song, 2005). Reporter gene expression or retrograde tracing allow for direct visualisation of cell structure, whereas electron microscopy unveils ultrastructural details like synaptic connections and axon projections (Arenkiel, 2011). Expression of stage-specific neurogenic markers allows for monitoring not only the fate of newborn neurons but also the time-course of adult neurogenesis (von Bohlen & Halbach, 2007; Figure 1C). Over the past two decades,
- 11 -
great effort has been made to identify a plethora of genes that are turned on and off during the different stages of adult neurogenesis (Table 1). However, the main problem lies in the spatial and temporal specificity of the markers used (von Bohlen & Halbach, 2007). In fact, no marker exclusively expressed in the neurogenic niches has been so far identified (Arias-Carrión et al., 2009). Moreover, the expression timeframes of certain markers overlap and caution must be used to draw definitive conclusions.
Functional integration of newly generated neurons can be assessed by means of several methods. Structural and ultrastructural characteristics, trans-synaptic tracing, expression of synapse-specific or activity-related markers after stimulation provides useful cues on synapse maturation in the context of a single cell or an entire neuronal circuit. Electrophysiology and non-invasive behavioural assays are carried out to examine synaptic efficacy up to the whole-organism level (for review, see Ming & Song, 2005). Combinations of multiple strategies allow for investigation of the regulation and role of adult neurogenesis under a range of experimental conditions. Despite the mammoth progress made in this direction, the game is still open for play.
Proliferation markers Description
PCNA
Proliferating cell nuclear antigen
Protein acting as a processivity factor for DNA polymerase δ and involved not only in DNA replication but also repair and thus a less sensitive and less specific proliferation marker (Kordek et al., 1996)
pHH3
Phospho-histone H3
Phosphorylation of chromatin-associated protein histone H3 is required for chromosome condensation and entry into mitosis (van Hooser et al., 1998)
Mcm2
Mini-chromosome maintenance protein 2
DNA replication licensing factor expressed during the G1-phase and required for entry into S-G1-phase (Stoeber et al., 2001)
Ki-67 Localised within the nucleus during the interphase and in association with chromosomes during the M-phase in proliferating cells but absent from resting cells and thus a highly reliable proliferation marker (Kordek et al., 1996)
Precursor cell markers
Sox2
SRY (Sex-determining Region Y)-box 2
Transcription factor highly expressed during embryonic development and later involved in adult neural progenitor proliferation and/or maintenance (Episkopou, 2005)
Nestin Intermediate filament protein expressed by early neural precursors (Reynolds & Weiss, 1992) but also under several pathological conditions (von Bohlen und Albach, 2007)
- 12 - GFAP
Glial fibrillary acidic protein
Intermediate filament protein, characteristically expressed by mature astrocytes but also found in neural stem cells (Garcia et al., 2004)
Pax6
Paired box protein 6
Homeodomain transcription factor widely expressed during embryonic development and important for neural precursors proliferation and fate determination (Götz et al., 1998), but also present in certain mature hilar neurons and astrocytes in the adult hippocampus (Nacher et al., 2005)
Dlx2
Distal-less homologue gene 2 (Dlx2)
Homeodomain transcription factor expressed by early neural precursors within the SVZ (Ming & Song, 2005)
Maturation markers
DCX
Doublecortin
Brain-specific microtubule-associated protein whose function is still not known, though apparently linked to migration and process outgrowth (Knoth et al., 2011), but not uniquely restricted to adult neurogenesis (Klempin et al., 2011)
CR
Calretinin
Vitamin D-dependent intracellular calcium-binding protein transiently expressed during late phases of SGZ neurogenesis but also present in a subpopulation of hippocampal GABAergic interneurons (von Bohlen und Albach, 2007) and hilar glutamatergic mossy cells (Fujise et al., 1998)
Calb
Calbindin
Family of intracellular calcium-binding proteins highly expressed by mature hippocampal granule cells but also present in cerebellar Purkinje cells (Sequier et al., 1988) PSA-NCAM
Polysialylated-neuronal cell adhesion molecule
Cell surface protein involved in several aspects of adult neurogenesis such as migration, survival and neurite development (Gascon et al., 2010), but also expressed by normally non-neurogenic brain regions (Nacher et al., 2002) TUC-4
TOAD (Turned On After Division)/Ulip (Unc-33-like phosphorprotein)/CRMP (Collapsin Response-Mediated Protein)
Family of proteins expressed by late mitotic precursors/early post-mitotic neurons (von Bohlen und Albach, 2007)
Tuj1
Neuron-specific class III beta-tubulin
Microtubule component in some still mitotically active precursors and immature post-mitotic neurons (von Bohlen und Albach, 2007)
NeuN
Neuronal Nuclei
Broadly but non-ubiquitously-expressed mature neuron-specific soluble nuclear protein (von Bohlen und Albach, 2007)
NeuroD
Neurogenic Differentiation
Basic helix-loop-helix transcription factor expressed by later stages of neuron development (von Bohlen und Albach, 2007)
Table 1. List of the most commonly used markers to study adult neurogenesis. Markers are grouped
in proliferation, precursor cell and maturation markers. No terminal differentiation markers are included. Each marker is accompanied by a brief description of what they label for and at what time during adult neurogenesis are they expressed.
- 13 -
Figure 1. Methodologies for analysis of adult neurogenesis in vivo. Three different approaches are
illustrated, each accompanied by a sample picture and a list of the main advantages/disadvantages. (A) Analysis based on nucleotide analogue incorporation. (B) Analysis based on retroviral lineage tracing. (C) Analysis based on the expression of specific markers (from Ming & Song, 2005).
1.3. Composition and Localisation of the Adult Neurogenic Pool
Once the belief in a mitotically-inactive postnatal brain had been gradually abandoned, the biggest issue concerned the origin of newly generated neurons. In fact, postnatal neurons are highly specialised cells and engage in intricate connections over long distances with specific synaptic partners (Hall & Miller, 2006). Therefore, the hypothesis that mature neurons de-differentiate and replicate seemed implausible. Instead, the current opinion is that special populations of cells are preserved in the
- 14 -
aCNS within particular niches that resemble the embryonic environment (Johansson et al., 2010; Alvarez-Buylla & Lim, 2004).
According to this view, while the neural tube and brain vesicles foster continuous and ubiquitous neurogenesis in the embryo, in the adult the phenomenon occurs at a much lesser extent and is restricted to specific anatomic locations. Thus, the embryonic neurogenic pool is not exhausted during organogenesis but is instead maintained, though after dramatic regression. A dedicated milieu for new neuron production is required not only for housing but also for dynamically regulating adult neurogenesis at different stages: proliferation, fate specification, migration, integration and survival (Ming and Song, 2005).
1.3.1. Adult Neural Progenitors
The main functional difference between embryonic and adult neurogenesis is that the former supplies constitutive elements to the presumptive nervous system whereas the latter is supposed to take part in tissue maintenance and repair. In both cases the presence of neural progenitors is required. Neural progenitors can be defined as proliferative cells with the capacity to give rise to all (in case of embryonic neurogenesis) or at least some (in case of adult neurogenesis) of the many types of nerve cells forming the aCNS. Proliferation and differentiation are tightly controlled by intrinsic specification and positive/negative signals provided by the environment (Sanes et al., 2006). Thus, progressive lineage decisions are key events regulated by a myriad of factors. Progenitor cells comprise both stem cells and precursor cells.
Stem cells are by definition characterised by two distinctive properties: self-renewal and multipotency (Ming & Song, 2011). Self-self-renewal is the ability to generate more stem cells; multipotency is the ability to give birth to multiple, but limited cell lineages. Precursor cells are more committed than stem cells, can still divide symmetrically but differentiate into generally fewer, more differentiated cell types (Scheffler et al., 1999). Unlike their embryonic counterparts, adult neural progenitors are typically dormant (Johansson et al., 2010; Wang et al., 2011). This feature is of extreme importance as a protective measure against depletion and grants an available reservoir of new cells when needed. As a consequence, different progenitor populations
- 15 -
co-exist within the adult neurogenic niches: quiescent neural stem cells, which maintain a low metabolic rate and divide rather slowly; active neural stem cells, which derive from quiescent stem cells, are highly proliferative and more responsive to external stimuli; transient amplifying cells, which derive from active stem cells, are lineage-restricted and incapable to self-renew and give rise to neuroblasts, or immature neurons (Wang et al., 2011). It is plausible that the balance between quiescent versus active states requires specific niche signals; to unravel them is one of the primary goals of current research.
An important breakthrough in the study of adult neurogenesis was the development of the neurosphere assay to isolate and propagate neural progenitors in
vitro (Reynolds & Weiss, 1992). In an attempt to identify epidermal growth factor
(EGF)-responsive cells in the adult mouse brain, Brent A. Reynolds and Samuel Weiss observed that plating dissociated striatal and SVZ cells on a non-adhesive substrate in the presence of EGF yielded clusters of proliferating elements that soon detached and formed floating spheroidal aggregates or neurospheres. These neurospheres contained Nestin+-cells that expanded and subsequently developed the morphology and antigenic properties of neurons and astrocytes. This finding provided the first demonstration for the existence of progenitor-like cells within the adult mammalian brain that can be induced to multiply and undertake different fates in vitro. Nearly two decades later, Bettina Neumeister and colleagues showed that spherogenic cells harvested from postnatal/adult mouse brains and expanded in vitro could be successfully transplanted in embryos, where they contributed to the generation of neurons and glia. In addition, these cells retained their stem potential over many months, replenishing the resident neurogenic pool (Neumeister et al, 2009). This result was striking, because it indicated that adult/postnatal neural progenitors not only retain their potential in vitro, but also in
vivo and beyond transplantation, throughout the life of the organism.
The implications of such findings for regenerative medicine are obvious. If neural progenitors could be obtained, expanded in vitro, inducted towards particular fate decisions and successfully transplanted, their potential for brain repair would be enormous. Alternatively, endogenous progenitors could be instructed to migrate towards target brain areas, differentiate into specific cell types and functionally integrate into
- 16 -
pre-existing circuits. These avenues spur us on to shed light on the mechanisms regulating adult neural stem cell biology.
1.3.2. Adult Neurogenic Niches
The adult neurogenic niches are often referred as a “displaced neuroepithelium, pockets of cells and local signals that preserve enough embryonic character to maintain neurogenesis for life” (Alvarez-Buylla & Lim, 2004). However, the presence of neural progenitors within a particular anatomical site is per se not sufficient to define a neurogenic niche. In fact, neurogenic niches closely interact with neural progenitors controlling their self-renewal, differentiation and activation (Doetsch, 2003). Two main germinative regions have been so far well documented and are nowadays referred as the
classical neurogenic zones: the subventricular zone (SVZ) of the lateral ventricle; and
the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG).
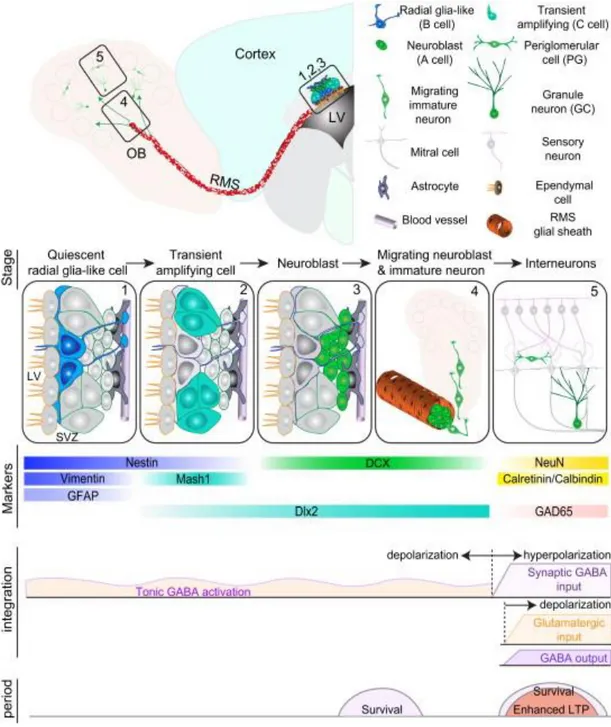
The SVZ is a paired forebrain structure situated at the lateral ependymal walls of the lateral ventricles (Figure 2). The cell density and spatial organisation vary greatly among mammals as a consequence of the different anatomy of the lateral ventricles (Bonfanti & Peretto, 2011). In rodents, adult neural progenitors and their progeny are stuck together and engage in a characteristic pattern of migration (chain migration) by forming tangentially oriented chains which coalesce in the rostral migratory stream (RMS), directed towards the olfactory bulb (OB). Conversely, in humans, proliferating and migrating neurons sharply decline within the first 18 months of life and leave room for a hypocellular gap separating the outer ependymal layer from the inner ribbon of astrocytes (Sanai et al., 2004; Sanai et al., 2011). As a consequence, and in spite of previous controversial claims (Curtis et al., 2007; criticised by Sanai et al., 2007), apart from sporadic immature-like cells, no significant neurogenesis occurs in adult humans at the SVZ. Another noteworthy feature of the limited postnatal human SVZ neurogenesis is that not all newborn neurons are destined to the OB, but some are hijacked towards an alternative medial migratory stream (MMS), targeting the prefrontal cortex (Sanai et al., 2011). The cellular components of the rodent neurogenic SVZ niche comprise five cell types (Doetsch, 1997):
- 17 -
1) SVZ astrocytes (type B cells): ultrastructurally distinguishable into subtype B1 (neural stem cells, with lighter cytoplasm and dispersed chromatin, often extending processes between ependymal cells terminating with a short, single cilium protruding in the ventricular lumen) and B2 (mostly at the interface with the striatal parenchyma), express GFAP, Nestin, Vimentin but not PSA-NCAM or Tuj1 (Doetsch et al., 1997);
2) Actively proliferating cells (type C cells): present throughout the SVZ but not in the RMS, express Nestin (Doetsch et al., 1997);
3) Proliferating migrating precursors (type A cells): ensheathed in glial tunnels formed by SVZ astrocyte processes, give rise to the RMS and express PSA-NCAM, Tuj1, Nestin, Dlx2, but not GFAP or Vimentin (Doetsch et al., 1997; Ming & Song, 2011);
4) Tanycytes (type D cells): quite infrequent, intermingled with the ependymal cells and deputed to the production of the cerebrospinal fluid (CSF) filling the ventricular cavities (Doetsch et al., 1997);
5) Multi-ciliated ependymal cells (type E cells): facing the ventricular lumen, bear microvilli and cilia on their free apical surface, facilitating the circulation of the CSF (Doetsch et al., 1997).
Both type A and type B2 cells have been found to incorporate 3H-TdR, making them unlikely to represent quiescent neural stem cells (Doetsch et al., 1997). On the other hand, type B1 and type D cells, which were not observed to replicate, show highly differentiated phenotypes (Doetsch et al., 1997). Nevertheless, despite robust data supporting type E cells to be the SVZ adult neural stem cells (Johansson et al., 1999), their interpretation remains debatable. At present, the most accepted opinion sponsors type B1 cells as promising candidates (Luque & Giménez y Ribotta, 2004). Type C cells display immature-like features and, given their high mitotic activity, have been proposed to be the transient amplifying cells (Doetsch et al., 1997). This interpretation receives support from the fact that the glial tunnel is open where focal type C cell clusters are found, making them likely ‘hotspots’ where neuroblasts are generated and canalised to the migratory chains (Doetsch et al., 1997). To sum up, the current model posits that type B quiescent stem cells become activated in response to specific niche signals and generate localised clusters of type C transient amplifying cells, which in
- 18 -
turn give rise to type A immature neuroblast precursors, constituting the migratory chains of the RMS (Figure2).
Chain migration is an extensive phenomenon involving the tangential displacement along the ventricle walls, then the channelling through the RMS and finally the radial dispersion in the OB. Many molecular factors are involved in the control of the motility and directionality of chain migration (PSA-NCAM, EphB2/ephrin-B2, Netrin/DCC, Slits/Robos) and neuroblast detaching once in the OB (Reelin, Tenascin-R) (Ming & Song, 2005).
Synaptic integration requires the tonic activation of radially migrating neuroblasts, which depolarise in response to GABAergic inputs provided by local circuits (Figure 2).
Another electrophysiological property which is instrumental to synaptic integration is the enhanced synaptic plasticity. Taken together, these features provide adult neuroblasts with an advantage in the competition with mature neurons for synapse formation and perhaps also an exclusive role in information processing during that same period (for review, see Ming & Song, 2011). In the end, two main types of OB interneurons are contributed by SVZ adult neurogenesis: 1) granule cells, all GABAergic; and 2) periglomerular neurons, 40% of which are GABAergic (of those, 65% are also dopaminergic) and the remaining express either calretinin or calbindin (Hack et al., 2005). Critical periods regulating survival (neuroblast and synaptic integration stage) and synaptic plasticity (synaptic integration stage) exist (Ming & Song, 2001) (Figure 2).
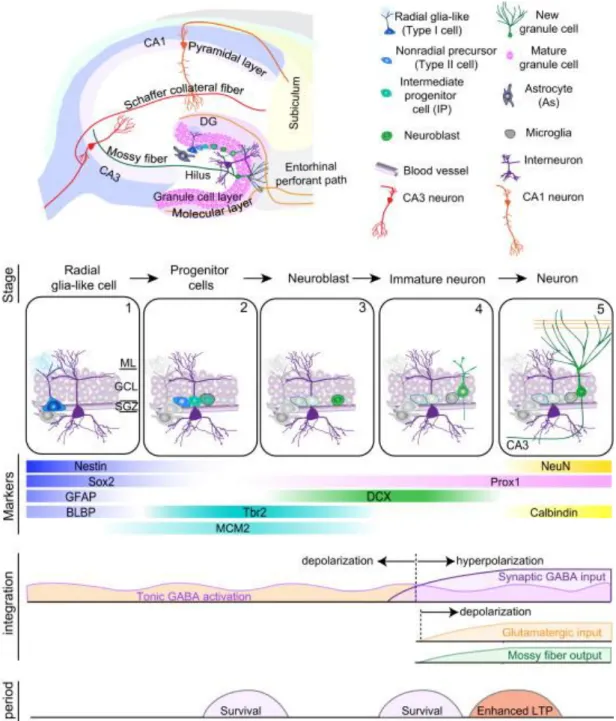
The SGZ is a narrow layer of cells between the granular layer (GL) and the hilus of the hippocampal dentate gyrus (DG). Because it is a ‘parenchymally-embedded’ structure with no connections to the lateral ventricles, unlike the SVZ, the DG shows little anatomical variation across mammals. However, qualitative and quantitative differences in SGZ adult neurogenesis may be more pronounced as regards the functional adaptation to environmental pressure across different species (Bonfanti & Peretto 2011). The most striking example is provided by certain bat species, where adult neuron production is very low or even absent (Amrein et al, 2007). Distinguishing feature of the SGZ neurogenic niche is the importance and pervasiveness of blood vessels, which are closely associated with neural progenitors, suggesting mutual
co-- 19 co--
regulation (Doetsch, 2003). Many cellular components of the SGZ niche have been described, but a uniform nomenclature is still lacking, mainly because of their elusive identity. From the structural and ultrastructural point of view, a former classification has been proposed (Seri et al., 2001). However, a more realistic distinction takes into account proliferative, morphological, immunohistochemical and electrophysiological properties (Kempermann et al., 2004):
1) Type 1 cells: bipotent radial glia-like stem cells, express GFAP and Nestin (Kempermann et al., 2004);
2) Type 2 cells: transient amplifying cells, derive from type 1 cells, keep expressing Nestin but not GFAP and can be further distinguished into type 2a and b based on the expression of DCX (Kempermann et al., 2004);
3) Type 3 cells: transient amplifying cells, keep expressing doublecortin (DCX), but stop expressing Nestin (Kempermann et al., 2004);
4) Early post-mitotic neurons: keep expressing DCX but are defined by the expression of NeuN and a transient expression of calretinin (CR) (Kempermann et al., 2004; von Bohlen und Halbach, 2007);
5) Late post-mitotic neurons: keep expressing NeuN but switch the expression of CR to calbindin (Calb) (Kempermann et al., 2004).
Type 1 cells coincide with radial glia-like cells (Kempermann et al, 2004). No proliferative activity was detected in astrocytes expressing the calcium-binding protein S100, known to label horizontal astrocytes (Kempermann et al., 2004). Type 2 and 3 are intermediate progenitors and might represent temporally distinct phases during the course of one single developmental stage (Kempermann et al., 2004). Putting it altogether, Type 1 radial progenitors produce Type 2/3 transient amplifying cells which in turn give rise to post-mitotic elements, which undergo synaptic integration and maturation (Figure 3).
The molecular mechanisms responsible for later stages of SGZ neurogenesis are still poorly understood. In general, like in the SVZ, immature neurons exhibit hyperexcitability and enhanced synaptic plasticity and get tonically activated initially by GABA molecules released in the environment by local interneurons, next by GABAergic and lastly glutamatergic inputs (Ming & Song, 2011). Critical periods for
- 20 -
survival (intermediate progenitor and neuroblast stage) and synaptic plasticity (synaptic integration stage) have been determined (Ming & Song, 2011) (Figure 3).
The two classical neurogenic zones differ profoundly in: 1) the number; and 2) the identity of the new neurons they contribute. Genetic fate-mapping analysis in mice revealed at least two distinct modes of neuron addition: conspicuous and constant within the glomerular layer of the OB, up a third of the total neuronal population over nine months; much more limited within the GL of both OB and DG (Ninkovic et al., 2007). Moreover, while the granular neurons are largely considered to be the unique cell type to be born throughout adulthood within the DG, many different neuronal subtypes are contributed by the SVZ to the OB and their specification is subject to region and time restrictions (Weinandy et al., 2011). The relative contribution of adult-generated neurons to the composition and function of pre-existing networks is matter of intense investigation. Notwithstanding the significant discrepancies, molecular and cellular aspects of adult neurogenesis within the two classical neurogenic zones are quite similar and different comprehensive models have been proposed to explain the potential adult neural stem cell lineage-relationships (Ming & Song, 2011).
As a final remark, the possibility of new neuron formation has been widely alleged in numerous other brain areas, although there is still lack of unanimous consent (Gould et al., 1999b; Bernier et al., 2002; Emsley et al., 2005; Bauer et al., 2005; Pekcec, 2006; Arias-Carrión, 2009; Migaud et al, 2010; Bonfante & Peretto, 2011; Lee et al., 2012). Provided that cell populations with neurogenic potential actually exist within the normally non-neurogenic areas, the current view is that new neuron formation would be completely/partially prevented or maintained at a low level by a local non-permissive microenvironment, at least under physiological conditions. On the other hand, since most data are inferred from studies mainly conducted on rodents, a comparative perspective would turn useful to clarify possible species or, in some cases, even strain-specific variations in time-course, spatial localisation, anatomical organisation and regulation of adult neurogenesis in mammals (Bonfanti & Peretto, 2011).
- 21 -
Figure 2. Adult neurogenesis in the SVZ. Summary of the different stages of SVZ adult neurogenesis.
Quiescent radial glia-like cells within the SVZ become activated (1) and undergo asymmetric divisions to yield transient amplifying cells (2), which in turn generate neuroblasts or immature neurons (3). Immature neurons coalesce and migrate through the RMS and, once in the OB, delaminate and radially slither towards their final destination (4), where eventually are functionally integrated and complete their maturation (5). During specific timeframes of adult neurogenesis, various markers are expressed. Synaptic integration is achieved through sequential changes of the electrophysiological properties of the cell membrane. At least two critical periods regulating survival and plasticity have been individuated (Ming & Song, 2011).
- 22 -
Figure 3. Adult neurogenesis within the SGZ. Summary of the different stages of SGZ adult
neurogenesis. Quiescent radial-glia like cells within the SGZ become activated (1) and undergo asymmetric divisions to yield transient amplifying cells (2) and later neuroblasts or immature neurons (3). Immature neurons migrate for a short distance within the GL (4), become functionally integrated and then definitively mature into granule cells (5). During specific timeframes of adult neurogenesis, various markers are expressed. Synaptic integration is achieved through sequential changes of the electrophysiological properties of the cell membrane. At least three critical periods regulating survival and plasticity have been described (Ming & Song, 2011).
- 23 -
2. Role and Regulation of Neurogenesis in Health and Disease
2.1. Role of Neurogenesis
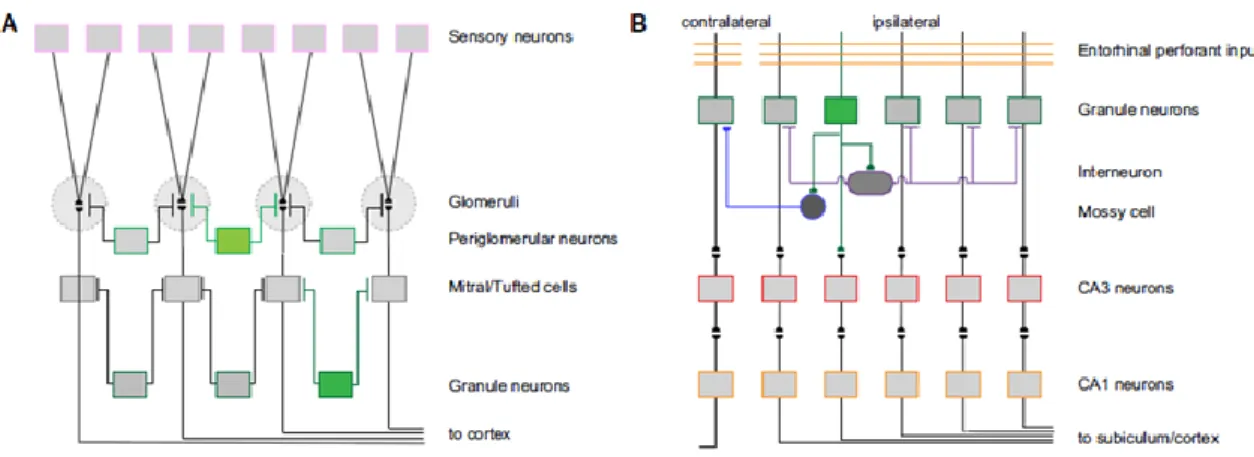
Recent advances in the field of adult neurogenesis have witnessed greater and greater efforts towards the understanding of its regulatory mechanisms and functions. Although the implications for a continuous generation of neurons are not yet definitive, a wave of publications points it out as a form of brain plasticity. Brain plasticity can be defined as the capacity of the nervous system to be modified by the environmental diversity in such a way as to increase its efficiency in dealing with it (Waddington, 1957). To accomplish this, the nervous system adopts transient and/or permanent changes in its structure and function in response to external factors. The modalities by which such changes are produced are topics of active neuroscience research given their involvement in healthy development, learning, memory and recovery from injury. In the past, two main plastic processes were identified: the rearrangement of connections and the alteration of their strength. The demonstration of the existence of adult neurogenesis provided evidence that not only the connections between cells, but also the number of constitutive elements in a neural network are subject to profound modifications over the lifetime. Moreover, the unique, transient physiological properties of newborn neurons at each stage of their development may be responsible for exclusive and unrepeatable tasks – for example, during the acquisition of new memories in a temporally correct manner (Nakashiba et al., 2012). In short, adult born neurons may function as both local circuit modifiers and autonomous encoding units (Figure 4).
Figure 4. Contribution of newly generated neurons to local circuit plasticity within the two classical adult neurogenic zones. (A) In the OB, newborn olfactory interneurons (green) provide lateral inhibition
- 24 -
intervening in two different levels during information processing. (B) In the hippocampus, newborn granule cells (green) receive inputs from the enthorinal cortex and relay to CA3 neurons, but at the same time they synapse onto hilar inhibitory interneurons and excitatory mossy cells, which in turn innervate and modulate mature DG granule cells (from Ming & Song, 2011).
If a flexible brain is required for information storage and regeneration, an enough rigid brain is necessary for the maintenance of the individual identity. For this reason, brain plasticity is not the sum of subsequent changes, but rather the result of a balance between rigidity, which means stability, and flexibility, which means adaptability. This clarification is really important because for an animal the adaptation to its environment is akin to the adoption of a new behaviour, which can prove decisive in a sink-or-swim world. In other words, a rejuvenating brain is a brain more suited to the ever-changing real world.
Ultimately, it is the level and function of specific molecules in the brain which up or down-regulate new neuron generation, both in health and disease (Grote & Hannan, 2007). A host of studies on the potential role of adult neurogenesis showed that, albeit the basal extent of the phenomenon is limited, several physiological and pathological stimuli wield sustained positive or negative effects (Table 2).
Regulatory factors Proliferation Survival Differentiation Potential mechanisms SVZ SGZ SVZ SGZ SVZ SGZ Mice strain +/− +/− +/− Gender n.c. +/− n.c. n.c. n.c. n.c. Aging − EGFR signaling − − n.c. − Corticosteroids levels + Hormones Corticosterone − Oestrogen n.c. + n.c. n.c. Serotonin? Pregnancy + n.c. Prolactin Afferents, neurotransmitters Dopamine − − D2L receptors Serotonin + + Acetylcholine − − −
- 25 - Glutamate − n.c. mGluR, NMDAR Norepinephrine n.c. + n.c. n.c. PAC1 and PKC PACAP + + Nitric oxide − n.c./− n.c. +/n.c. n.c. Growth factors FGF-2 + n.c. EGF + n.c. − IGF1 + + + Behaviour
Enriched environment n.c. +/n.c. n.c. +/n.c. n.c. n.c./+ VEGF Enriched odor exposure n.c. n.c. + n.c.
Physical activity n.c. + n.c. + VEGF Learning n.c. +/n.c. n.c. Dietary restriction n.c. + BDNF, NT-3 Stress − n.c. Glucocorticoids + Drugs Antidepressants + Serotonin Opiates − Methamphetamine − Lithium + n.c. Pathological stimulations Ischemia + +/− + + + NMDAR, CREB Seizures + +/− +/n.c. +/n.c. Inflammation +/− − − − IL6, TNFα Degenerative diseases (AD/HD/PD) + + Diabetes −
Table 2. Regulation of adult neurogenesis under physiological and pathological conditions. The table
- 26 -
increase; “−”, decrease; “n.c.”, no change; unmarked indicates “not examined” (adapted from Ming & Song, 2005; see original review for acronyms or further details).
2.2. Molecular and Cellular Driving Forces
The most salient feature of adult neurogenesis is that many of the mechanisms underlying embryonic development are recapitulated in the adult, although in specialised milieux. Both in the embryo and in the adult, the purpose of neurogenesis consists of the generation of different types of neurons from an undifferentiated proliferative pool and to place them in the appropriate circuitry. The number of cells and that of the connections are subject to numerous variables involving constructive and destructive events. The final outcome depends on a complex interaction between genetic specification and experience. The individual genetic background as well as epigenetic phenomena play a pivotal role in determining the expression of intrinsic neurogenesis-regulating factors and the vulnerability/resilience towards extrinsic factors (Ming & Song, 2011). Experience, on the other hand, evokes electrical activity-dependent molecular events responsible for neural structure maturation (Ming & Song, 2005).
Experimental conditions to investigate adult neurogenesis shall take into consideration variables like animal sex, age and, possibly, genetic predisposition, drug dose, route, interval and duration of administration and, in general, any uncontrollable experimental consequence on physiology and spontaneous behaviour.
2.2.1. Extracellular Determinants
A myriad of diverse signals are provided by the neurogenic niches and tightly control maintenance, activation, self-renewal and differentiation as well as survival and integration of adult neural progenitors. The major components of the niche environment are the proliferative neuroepithelium, a specialised extracellular matrix and a cocktail of humoral factors (Kazanis & ffrench-Constant, 2011).
The proliferative neuroepithelium consists of a patchwork of progenitors at different developmental stages and mature neurons. Apart from neurons, many other cellular elements are present and take part in complex cell-cell interactions. Astroglia
- 27 -
plays a dual role as: 1) physical, metabolic, trophic support for mature and developing neurons; and 2) pool of neural stem cells (Ming & Song, 2005). Ependymal cells are anatomical prerogative of the SVZ and, together with astrocytes, whose meandering processes occasionally reach the ventricular lumen, have immediate access to the CSF and the soluble determinants therein contained (Buddensiek et al., 2010). Vasculature plays a prominent role in both the SVZ and the SGZ, where crosstalk between angiogenesis and neurogenesis exists (Doetsch, 2003; Shen et al., 2008). Activated microglial cells secrete pro-inflammatory and anti-inflammatory molecules, whose net contribution may have opposing influences on the neurogenic potential (Battista et al., 2006).
The extracellular matrix (ECM) is not merely a scaffold of interstitial gel and molecules holding cells together and helping them to stay in shape. On the contrary, the interaction between several classes of cell surface receptors and ECM components triggers important feedback mechanisms (Balu & Lucki, 2009; Kazanis & ffrench-Constant, 2011). In addition, ECM stiffness is a determinant for cell motility and migration. The ECM can also influence indirectly cell behaviour by sequestering secreted ligands. By doing so, the extracellular environment tethers together critical signalling molecules at high concentrations in discrete foci (Doetsch, 2003). These bound stores can be mobilised by cells at the right moment following the release of various cleaving enzymes.
Several soluble extracellular factors are involved in the regulation of specific aspects of neurogenesis. A critical issue is the appropriate balance between active neural stem cells, fated to differentiation, and quiescent stem cells, tightly controlled to sustain self-renewal (Wang et al., 2011). Bone morphogenetic proteins (BMPs) promote glial versus neural differentiation and are endogenously antagonised by Neurogenesin-1 and Noggin, both produced in the SVZ and the SGZ (Ming & Song, 2011). In particular, BMPs have been demonstrated to diminish hippocampal neural stem cell proliferation and maintain their undifferentiated state in culture (Mira et al., 2010). Furthermore, intracerebral infusion of Noggin promotes the switch from the quiescent to the activated state leading to an initial increase in neurogenesis, then the depletion of the neurogenic pool (Mira et al., 2010). Similar effects have been obtained by downstream blockade of Notch signalling in both the SVZ and the SGZ (Ming & Song, 2011). Reportedly, Notch
- 28 -
canonical and non-canonical pathways play also a crucial role in preventing quiescent neural stem cells from activating and subsequent committing to neuronal fate (Ables et al., 2011). Interestingly, multiple neural stem cell populations showing Notch-dependence but selective responses to different external stimuli and reversible quiescence have been described (Lugert et al., 2010). In contrast, wingless-type MMTV integration site family members (Wnts) promote proliferation and neural fate commitment (Ming & Song, 2011). In mice, astrocytes release Wnt7a in the SVZ and Wnt3 in the SGZ (Wang et al., 2011). In addition, in both regions they release sonic hedgehog (Shh), an important morphogen required for the establishment and maintenance of radial glia-like cells in the two classical neurogenic zones (Wang et al., 2011; Ming & Song, 2011). Mitogens and growth factors drive proliferative events of activated progenitors and survival of maturing neurons. Basic fibroblast growth factor (bFGF or FGF-2) is expressed by radial glia-like cells within the SGZ in different species, including humans (Weickert et al., 2005). Despite being directly implicated in the promotion of hippocampal neurogenesis, its infusion in adult rats does not increase cell proliferation (Wald et al., 2011). However, bFGF appears to potentiate the mitogenic effects of the insulin-like growth factor 1 (IGF1) by intervening on the expression of its receptors and binding proteins (Wald et al., 2011). Contrary to bFGF, infusion of IGF1 in adult rats does increase cell proliferation, but has no effect on neuronal differentiation (Wald et al., 2011). Vascular endothelial growth factor (VEGF) is another important regulator playing a dual role in both neurogenesis and angiogenesis in the SVZ and SGZ, promoting survival and reducing cell death (Suh et al., 2009; Wald et al., 2011). Other growth factors, neurotrophins, cytokines, hormones, retinoids, cannabinoids and neurotransmitters are major contributors to adult neurogenesis (Jacobs et al., 2006; Grote & Hannan, 2007; Suh et al., 2009; Ming & Song, 2011).
2.2.2. Intracellular Determinants
The action of any factor with the potential to influence neurogenesis is ultimately conditioned by the genetic makeup of the organism. A huge number of genes have been found to be directly or indirectly implicated in the dynamics and the extent of the process in the adult or its susceptibility to the environmental pressure. Divergence in
- 29 -
cell proliferation and survival of hippocampal granule cells has been reported in different mouse strains (Grote & Hannan, 2007). Intracellular players such as cell-cycle regulators, transcription factors and epigenetic factors regulate gene expression at transcriptional and translational level (Ming & Song, 2011).
Cell proliferation is mainly controlled during the G1 phase. Cyclins and cyclin-dependent kinases (Cdks) combine to form regulatory complexes responsible for the progression of the cell division cycle. Cyclins D and E are present in the adult SGZ and Cdk1 is expressed in proliferating hippocampal neural stem cells (Balu & Lucki, 2009). Conversely, cell-cycle inhibitors like p16, p21 and p53 are involved in the maintenance of the quiescent state (Ming & Song, 2011).
An important transcription factor is Sox2, a major mediator of Notch and Shh signalling. Sox2 is expressed in self-renewing, multipotent neural progenitors (Suh et al., 2011). For this reason, it is commonly used as a marker for neural stem cells (Table 1). Deletion of Sox2 in the mouse embryonic or adult brain results in loss of neurogenesis and DG hypoplasia (Favaro et al., 2008). Neural stem cells isolated from Sox2 hypomorphic mutant mice, which show reduced levels of Sox2, fail to undertake the neuronal, but not the astroglial fate (Cavallaro et al., 2008). However, over-expression of Sox2 at early, but not later stages of differentiation, rescued the neuronal versus astroglial differentiation (Cavallaro et al., 2008). Together, these findings suggest that Sox2 is required for neural stem cell maintenance and priming towards the neuronal versus alternative lineages.
Epigenetic regulators act in cooperation with transcription factors and bear significant changes in the actuation of the intrinsic programmes without altering the genetic code. Such mechanisms include chromatin remodelling through histone modification and DNA methylation and non-coding RNAs, as well as crosstalk among these mechanisms (for review, see Sun et al., 2011).
2.3. Influence of Environmental Factors
In general, the environmental context plays not just an instructive, but also a permissive role towards neurogenesis. The possibility to explore a spacious environment and socially interact prompts the innate behaviour of the animal. As a
- 30 -
result, multi-sensory experience and spontaneous activity are enhanced and both correlate with an improvement of cognitive functions, particularly learning and memory. At the same time, the natural environment is a potential source of noxious, neurogenesis-inhibiting stimuli such as infectious agents, toxic chemicals and stressful experiences. While the former two are overtly heterogeneous in both the nature and the aetiopathology to allow for generalisation, the latter have been largely demonstrated to act on common, interwoven biochemical pathways which yield direct consequences on adult neurogenesis.
2.3.1. Effects of a Rich Environment
The conditions of a natural, rich environment can be partly recreated in an experimentally enriched environment (EE), which provides “a combination of complex inanimate and social stimulation” (Rosenzweig et at., 1978). In general, well-cared and environmentally enriched animals have got the edge over impoverished ones because they are more able to draw information out of a complex environment, find and exploit its resources and survive its constraints and a potentiated hippocampal neurogenesis definitely contributes to that. However, the complex stimulation provided by a naturalistic environment influence aspects other than neurogenesis like neuronal metabolism and vascular function, complementary to adult neurogenesis and altogether beneficial to brain plasticity. Conversely, progressive cognitive deterioration with aging can be ascribed to a continuous depletion of the neurogenic pool owing to defective neural progenitor proliferation, survival or maturation (for review, see Encinas & Sierra, 2012).
From the anatomical point of view, enriched living has been shown to promote new granule cell survival, but not proliferation, in the hippocampus (van Praag & Gage, 2000; Ming & Song, 2005 and 2011). Since an EE is a combination of a plurality of determining factors, attempts have been carried out in order to assess their influence on neurogenesis independently (van Praag et al., 1999). Voluntary exercise is sufficient alone to specifically increase granule cell proliferation, net survival and differentiation (van Praag et al., 1999; van Praag & Gage, 2000; Ming & Song, 2005 and 2011). Taken together, the two separate experimental paradigms of enriched housing and voluntary