Chapter 1 General introduction on root architecture and low oxygen conditions
3-22
Chapter 2 Establishment of endogenous hypoxia is required for the inhibition of auxin sensitivity to control lateral root development in Arabidopsis primordia
23-66
Chapter 3 Jasmonates contribute to primary root inhibition upon oxygen deficiency in Arabidopsis thaliana
67-92
Chapter 4 Establishment of a chromatin-immunoprecipitation protocol to test RAP2.12 binding on gene promoters
93-112
Summarizing discussion 113-114
3
General introduction on root architecture and low
oxygen conditions
Vinay Shukla1
1 Institute of Life Science, Scuola Superiore Sant’Anna, Pisa, Italy
Molecular events in root development
In order to cope with the fluctuations in water and nutrient availability in soil, plant root development and growth require high plasticity. Root system architecture (RSA) is a vital trait to thrive in heterogeneous soil environments in addition to its functions of anchorage and resource storage. Impromptu growth determination is essential to define optimal RSA to explore the soil in the most efficient way. RSA refers to the three-dimensional structure of the roots in the soil (Lynch, 1995) and is mainly constituted by the primary root and postembryonic development of lateral roots and production of root hairs. The direction of primary root growth, deployment of lateral roots along the former and abundance of root hairs define the proficiency of the root system for water and nutrient uptake. Several intrinsic and extrinsic factors, such as genetic programs, hormonal control, abiotic stresses and nutrient availability, regulate the root growth patterns to achieve the most efficient structure in the actual environment.
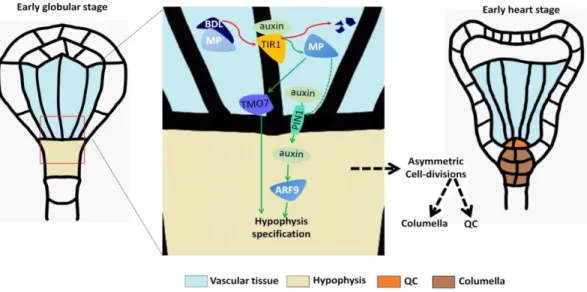
In Arabidopsis embryos, the origin of the primary root traces back to the formation of radicle (embryonic root) from the hypophysis. During embryogenesis, proembryo and basal cell result from the first asymmetric division of the zygote, with the large basal cell undergoing several anticlinal cell divisions to form the suspensor. The apical cell of the suspensor is specified to form the hypophysis, which gives rise to the quiescent center (QC) by asymmetric division (Mansfield and Briarty, 1991; Dolan et al., 1993; Scheres et al., 1994). This process is primarily under the control of the auxin hormone, through the interaction of the transcriptional regulators BODENLOS (BDL)/IAA12 and MONOPTEROS (MP)/AUXIN RESPONSIVE FACTOR 5 (ARF5). In fact, auxin-dependent degradation of IAA12 allows ARF5 to induce TARGET OF MONOPEROUS 7 (TMO7) and PIN-FORMED 1 (PIN1) expression, which are required to specify hypophysis identity (Hardtke and Berleth, 1998; Hamann et al., 2002; Weijers et al., 2006; Schlereth et al., 2010). Interestingly, this IAA12-ARF5 regulatory module acts in a non–cell-autonomous way, since it takes place in the neighboring provascular cells but affects the asymmetric division of the first suspensor cells (Hamann et al., 2002; Weijers et al., 2006). PIN1 expression and its basal localization in provascular cells allows auxin to flow into the basal one, where it activates a second ARF transcription factor, ARF9,
4
responsible for local hypophysis specification (Rademacher et al., 2012). The basic helix-loop helix transcription factor TMO7 is able to move via plasmodesmata from provascular cells into the hypophysis, to regulate transcription in its nucleus (Schlereth et al., 2010) (Fig. 1). Besides auxin, also cytokinins play a role in hypophysis development by activating the transcriptional repressors ARABIDOPSIS RESPONSE REGULATOR 7 (ARR7) and 15 (ARR15). After the asymmetric division of the hypophysis into lens shaped cell (LSC) and basal cell (BC), ARF7 and ARF15 dampen cytokinin signaling in the latter, enabling instead high auxin responsiveness in the BC, which will give rise to the QC (Müller and Sheen, 2008). Two rounds of asymmetric cell divisions in the hypophysis lead to the formation of the QC that controls the activity of root organizer cells (initial cells). The stem cell niche contains cortical/endodermal initials (CEI), stele initials, lateral root cap/epidermal initials (LRP/Epi) and columella stem cells (CSCs). These will later produce all the different root tissues corresponding to the vasculature, ground tissues, epidermis, lateral root cap and columella (Spradling et al., 2001; Scheres, 2007). Organizer cells are required to maintain their undifferentiated state, a task accomplished in a non-cell-autonomous way by the QC. Four Plethora (PLT1-4) transcription factors are known to promote cell division in the stem cell niche, thereby maintaining the meristematic activity (Galinha et al., 2007). Additionally, the SHORT-ROOT (SHR)/SCARECROW (SCR) module is also involved in the maintenance of stem cell niche. The GRAS-family protein SHR is produced in stele cells and moves into the QC and other initials to induce the expression of SCR, with which it forms a dimer (Helariutta et al., 2000; Levesque et al., 2006; Cui et al., 2007). This SHR/SCR dimer is responsible for maintaining QC and stem cell identities, by inducing the transcription factor WOX5 which, in turn, regulates the size of the stem cell pool (Fig. 2). Specifically, WOX5 represses the cell cycle gene CYCLIND3;3 (CYCD3;3) to restrict the division rate of the QC (Forzani et al., 2014). Instead, the organizer cells need to undergo continuous cell divisions, and hence several mechanisms have been identified that limit the expression of WOX5 to QC. For instance, REPRESSOR OF WUSCHEL1 (ROW1) is expressed in the cortical/endodermal initials (CEI) and stele to epigenetically suppress WOX5 transcription (Zhang et al., 2015).
5
Fig. 1. Hypophysis specification in the early globular stage of embryo development and subsequent formation of the QC. Auxin dependent degradation of BDL allows the induction of TMO7 and PIN1 by MP. TMO7 moves to the uppermost suspensor cell and PIN1 allows transport of auxin in the same cell. Here, auxin induced ARF9 and other ARFs, along with TMO7, lead to hypophysis specification. The hypophyseal cell divides once periclinally to generate the lens shape upper cell and the basal cell. The upper one then divides anticlinally to generate the two cells that compose the QC (adapted from ten Hove et al., 2015).
In embryos, at late globular stages, organizer cells undergo anticlinal cell division to form daughter cells while maintaining their regenerative status, a process that is assumed to be controlled differently in different initials. Two mechanisms are known to control asymmetric cell divisions in columella stem cells (CSC) (Fig. 2). First, the small signaling peptide CLE40 (CLAVATA3/EMBRYO SURROUNDING REGION) binds and activates the receptor-like kinases CLAVATA1 (CLV1) and ARABIDOPSIS CRINKLY4 (ACR4) in columella stem cells (CSC), to initiate a signaling cascade that represses WOX5 outside of the QC (Stahl et al., 2009; Stahl et al., 2013). Oppositely to this, WOX5 proteins synthesized in the root niche organizer move via plasmodesmata towards columella differentiated cells to repress CDF4 (CYCLING DOF FACTOR 4) through chromatin deacetylation, thereby creating opposite gradients of WOX5 and CDF4 and allowing distant daughter cells to escape the stem-cell state (Pi et al., 2015).
The lateral root cap/epidermal (LRC/Epi) initials produce LRC and epidermal daughter cells by a first periclinal and a second anticlinal division, followed by a series of cell divisions to form LRC and epidermis (Dolan et al., 1993; Fendrych et
6
al., 2014). A feedback loop between the NAC domain transcription factors FEZ and SOMBRERO (SMB) controls this process. FEZ promotes cell division in LRC/Epi initials and simultaneously induces SMB. SMB, in turn, represses FEZ establishing a feedback inhibition loop (Willemsen et al., 2008).
An anticlinal division in CEI gives rise to CEI daughter cells, which generate cortex and endodermal precursors, a process under control of the SHR/SCR module (Benfey et al., 1993; Scheres et al., 1995). Additionally, the cell differentiation factor RBR (RETINOBLASTOMA-RELATED) promotes asymmetric cell division in CEI by interacting with SHR/SCR and thereby inducing the expression of the cell cycle regulator CYCD6;1 (Wildwater et al., 2005). Recently, a family of C2H2-type zinc finger transcription factors called BIRD (BLUEJAY, IMPERIAL EAGLE, MAGPIE and NUTCRACKER), along with the previously known JKD (JACKDOW) TF, were identified to positively regulate cell division in the CEI lineage by both a SHR dependent and independent mechanisms (Welch et al., 2007; Moreno-Risueno et al., 2015).
Fig. 2. Scheme depicting the regulation of WOX5 in the QC and neighboring cells by hormones and diffusible proteins and peptides in order to orchestrate proper stem cell maintenance and cell differentiation. Adapted from Drisch and Stahl, 2015.
7
The stele includes pericycle, xylem, phloem and procambial cells, which are essential for transport purposes and secondary growth. Xylem and phloem are further subdivided into protoxylem or metaxylem and protophloem or metaphloem, depending on their developmental stage. Unlike the radial symmetry of ground tissue and epidermis, the stele follows a bilateral symmetry which is primarily regulated by the transcription factor basic helix-loop-helix transcription factor LONESOME HIGHWAY (LHW) (Ohashi-Ito and Bergmann, 2007). The transcriptional regulators HD-III ZIP, VND6 and VND7 are characterized to control xylem development. High levels of HD-III ZIP and VND6 activity promote differentiation into metaxylem, whereas, low levels of HD-III ZIP and VND7 activity promote protoxylem formation (Kubo et al., 2005; Carlsbecker et al., 2010; Yamaguchi et al., 2010).
Cytokinin and auxin cross-talk defines the differentiation of phloem cells. The cytokinin receptor WOODEN LEG (WOL/CRE1)/ ARABIDOPSIS HISTIDINE KINASE4 (AHK4) induces periclinal division in phloem initials, whereas the auxin-induced ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN6 (AHP6) induces protoxylem cell-fate (Scheres et al., 1995; Mähönen et al., 2000; Inoue et al., 2001; Mahonen, 2006). Besides, local cytokinin production induced by auxin has been proposed using mathematical modelling. Here, auxin-induced dimerization of TARGET OF MONOPTEROS 5 (TMO5) and LHW would lead to activation of LONELY GUY 4, an essential enzyme for cytokinin biosynthesis (Manzano et al., 2014). Thus, during embryogenesis all different tissues of roots are established, and they gradually achieve maturity into their specific differentiated stage during post-ebryonic development.
At maturity, the root apex is traditionally subdivided into three distinct zones. These are defined as the meristematic zone (MZ), elongation zone (EZ) and differentiation zone (DZ). The MZ includes cells undergoing cell division: the EZ zone is located immediately above the MZ and comprises cells that ceased division and undergo rapid cell expansion; the DZ is formed when elongated cells acquire specialized morphology and functions. In addition, the zone in between MZ and EZ, where cells are exiting from the mitotic cell cycle, is called transition zone (TZ) (Beemster and Baskin, 1998).
In Arabidopsis, the exit from mitotic cell cycle is believed to be controlled by repression of the core component of anaphase-promoting complex/cyclosome (APC/C) CDC27B (HOBBIT/CELL DIVISION CYCLE 27 HOMOLOG B) (Blilou et al., 2002; Serralbo et al., 2006; Pérez-Pérez et al., 2008). The CCS52A1 (CELL CYCLE SWITCH PROTEIN 52A1) is also known to control the APC/C activity by promoting the differentiation in the TZ (Vanstraelen et al., 2009). The unidirectional growth of the cells in the EZ involves reorganization of the cytoskeleton, development of central vacuole and its expansion as consequence of water uptake (Cosgrove, 1993; Verbelen et al., 2006). The arrangement of microtubules plays a crucial role
8
during cell expansion as alterations in cell wall are necessary for polar expansion. In fact, mutants exhibiting misoriented cortical microtubules show defects in cell expansion (Bichet et al., 2001; Burk et al., 2001). Longitudinal localization of anchored protein COBRA (COB) is known to influence the unidirectional growth of the cell (Schindelman et al., 2001).
The mechanism by which elongated cells acquire their specialized functions is still somewhat unclear. The differentiation zone can be distinguished from other zones by two recognizable features – the Casparian strip and root hairs.
The Casparian strip is an impermeable barrier of lignin and suberin deposits on the transverse sides of endodermal cells. It prevents apoplastic water flow between cortex and stele, thereby forcing water and nutrients to be selectively taken up in the cytoplasm of endodermal cells via transporters. Formation of the Casparian strip requires the expression of CASP genes, which are specifically expressed in the endodermis (Birnbaum, 2003; Roppolo et al., 2011).
Moreover, some epidermal cells in the DZ differentiate into root hairs under the control of a developmental pathway that relies on the underlying cortical cells. Typically, only epidermal cells in contact with two cortical cells will become a root hair. In epidermal cells in contact with only one cortical cell, activation of the transcription factor GLABRA2 (GL2) by the complex of TRANSPARENT TESTA GLABRA1 (TTG1), GLABRA3 (GL3), ENHANCER OF GLABRA3 (EGL3), and WEREWOLF (WER) leads to non-hair cell fate. Instead, a signalling pathway led by JKD in adjacent cortical cells induces SCRAMBLED (SCM) expression in the above-lying epidermal cell. Here, SCM represses WER and thereby allows CAPRICE (CPC) to form the complex that allows the formation of root hair (Hassan et al., 2010). Different plant hormones are known to collectively regulate root growth with their synergistic or antagonistic interactions, depending on tissue type and relative cell position. The establishemnt of different zones of root apex is a result of complex hormonal cross-talk between the plant hormones auxin, cytokinin, gibberellic acid (GA), abscisic acid (ABA), jasmonic acid (JA), brassinosteroid (BA), and ethylene. Among other interactions, the antagonistic cross-talk of auxin and cytokinin is essential for establishment and maintenance of different zones of root apex. Auxin maximum in the MZ is achieved by basipetal transport of auxin from shoot through PIN proteins in the vasculature. PIN proteins are also responsible for acropetal auxin transport towards the shoot in external tissues. Additionally, PIN1, PIN3 and PIN7, in the TZ, direct auxin back in the vasculature, leading to downward transport in order to establish an auxin gradient in the apex with a maximum corresponding to the QC and stem cell initials (Gälweiler et al., 1998; Wildwater et al., 2005; Grieneisen et al., 2007). This auxin gradient results in a gradient of PLT protein expression, by activation of auxin-response TFs such as MP and NPH4 (NONPHOTOTROPIC HYPOCOTYL4)(Aida et al., 2004). In turn, PLT gradients define
9
whether a cell has to remain in its stem-cell state (high PLTs), divide (moderate PLTs) or differentiate (low PLTs). These regulatory steps explains the perspective of auxin being a key player in stem cell maintenance and cell proliferation (Aida et al., 2004; Galinha et al., 2007). Additionally, auxin also stimulates cytokinin biosynthesis via IPT5 to form a feedback inhibition loop (Dello Ioio et al., 2008). In fact, a cytokinin stimulus exploits ARR1 to repress auxin signaling via SHY2 (SHORT HYPOCOTYL2)/IAA3, a repressor that heterodimerizes with several ARFs. Cytokinin also represses PIN1, 3 and 7 protein expression in the TZ and thereby hinders cell division in favor of differentiation division (Dello Ioio et al., 2007; Dello Ioio et al., 2008).
Adaptability of root system architecture towards heterogeneous soil is majorly assisted by root branching (Lavenus et al., 2013). Lateral root (LR) density and positioning along the length of primary root are essential for water and nutrient uptake from soil for appropriate plant growth and development. The development of lateral roots is extensively discussed in Chapter 3 of the thesis. Briefly, LR formation is a de novo process which has been subdivided into three steps – formation of lateral root primordia, the emergence of primordia and activation of meristem and elongation as LR. Initiation of LRP formation starts from specifically primed pericycle cells on the xylem pole, called lateral root founder cell (LRFC). The process of initiation and formation of lateral root primordia has been distinguished into seven discrete stages at the end of which LRP emerges out as LR. Auxin plays a central role in this process, and different auxin signalling modules control different stages of development (Malamy and Benfey, 1997; De Smet et al., 2007; De Rybel et al., 2010; Goh et al., 2012).
RSA responses to nutrient availability and abiotic stresses
Nutrient availability has a profound impact of RSA as it is known to alter primary root (PR) length, density of LRs, density of secondary LRs and density of root hairs. To sustain successful growth and development, plants need to acquire water and nutrients in soil characterized by fluctuating conditions. For instance, the effect of low or excessive availability of nutrients on Arabidopsis roots has been extensively studied, mostly in plants grown vertically on agar plates. Reduction in inorganic nutrients has an inhibitory effect on primary root length, whereas it exerts a positive effect on LR production and length although only up to a threshold concentration, after which it impacts negatively. Zinc and Sulphur represent an exception to this general assumption (Kellermeier et al., 2014). This has been observed for conditions of nitrogen starvation, where this developmental process is mediated by NITRATE TRANSPORTER 1.1 (NRT1.1), which acts as a sensor for low nitrate availability and is also involved in basipetal auxin transport in developing LRP (HO 2009). In fact, it has been observed that, under low nitrate conditions, NRT1.1 protein levels increase in LRPs with a concomitant decrease in auxin accumulation (Okamoto et al., 2003; Krouk et al., 2010; Bouguyon et al., 2015).
10
Also phosphate starvation has an inhibitory impact on primary root growth in Arabidopsis, while it induces LR development and root hair formation (Bouain et al., 2016). Instead, potassium deficiency reduces both primary root length and LR length, although it stimulates the formation of second order LRs (Kellermeier et al., 2014). Calcium or boron starvation enhances LR density with unchanged LR length, whereas magnesium or iron deficiency results in lower LR density and shorter LRs. In general, it can be assumed that plant RSA is always affected by nutrient availability, with both common and element specificity features (Gruber et al., 2013; Kellermeier et al., 2014).
Abiotic stresses can also modulate the root growth significantly. Cold-stress leads to loss of gravitropic nature of root by affecting the lateral localization of PIN2 and PIN3 proteins (Shibasaki et al., 2009). Under drought stress, ABA antagonistically interferes with auxin-mediated gravitropism and stimulates the growth of the root towards soil profile with higher water content (Taniguchi et al., 2010). Salinity stress exerts a dose-dependent impact on root growth. A milder salinity stress reduces primary and lateral root elongation while it enhances LR density. Stronger salt stress severely impacts root elongation (Zolla et al., 2010). In general, oxidative stress due to the production of reactive oxygen species under biotic or abiotic stress response also impairs auxin signalling and LR formation (Bashandy et al., 2010).
Low oxygen is one of the major environmental stress that can be faced by plant root systems. Molecular oxygen acts as substrate for many biochemical reactions, making it essential for normal plant growth and development. For aerobic respiration, oxygen serves as a terminal electron acceptor to generate chemical energy in the form of ATP at the end of mitochondrial electron transport chain. Besides, oxygen plays a crucial role in lipid metabolism, reactive oxygen species production and biosynthesis of several phytohormones such as jasmonic acid, ethylene, gibberellic acid, abscisic acid (Blokhina and Fagerstedt, 2010; van Dongen and Licausi, 2014). In plants, oxygen is supplied to tissues primarily by passive transport processes - diffusion and convection. Therefore, the resistance of diffusion across the tissues create the oxygen gradients with the lowest oxygen concentration in the core of the plant organs (Armstrong and Armstrong, 2014). Plants never developed an active oxygen distribution system such as blood vessels of animals and hence several factors such as environmental conditions, the rate of diffusion, metabolic activities define the oxygen levels in tissues.
Regardless of plants being the primary source of oxygen on Earth, sub-atmospheric oxygen levels or high metabolic rates may lead to hypoxic conditions, especially in non-photosynthesizing plant organs such as roots. In situ measurements of oxygen concentration using oxygen microsensors in maize (Zea mays) roots revealed the steep oxygen gradients in the root tissues. For instance, plants grown in aerobic environment exhibited an internal O2 concentration of 10% in the cortex and 5% in
11
the stele (ARMSTRONG et al., 1994). Also, environmental conditions that reduce the ambient oxygen concentration, such as flooding or waterlogging, can cause a severe decrease in oxygen levels in roots and impact the growth of a plant, including root development. Several species produce adventitious roots above the water level, from de novo or preformed primordia, a process that is known to be mediated by ethylene and auxin signalling in Solanaceae (WAMPLE and REID, 1978; Vidoz et al., 2010; Dawood et al., 2014). Additionally, aerenchyma formation to enable gas exchanges between the shoot and the root is another adaptation known to improve the gas diffusion under submergence (Evans, 2004). In Arabidopsis, inhibition of primary root growth has been observed when roots are exposed to a hypoxic atmosphere containing 8% oxygen, suggesting an association between oxygen availability and root elongation (van Dongen et al., 2009). Recently, root bending against gravitropism has been proposed as an escape mechanism from zones characterized by low oxygen availability in the soil (Eysholdt-Derzso and Sauter, 2017). From this study it was hypothesized that local oxygen deficiency may redirect primary root growth by influencing root bending via asymmetric auxin distribution. In fact, hypoxia was shown to cause asymmetric reduction of PIN2 abundance. Surprisingly, this response was suppressed in plants over expressing ERF-VII TFs suggesting the existence of a compensative mechanism by these transcription factors (Eysholdt-Derzso and Sauter, 2017).
Since mitochondrial respiration is impaired by reduced oxygen levels in tissues, substrate-level phosphorylation is activated to fulfil the energy demands (Gibbs and Greenway, 2003). Only two ATP units are produced through substrate-level phosphorylation when compared to 36 ATP units from oxidative phosphorylation, and hence, plants acquire several temporary metabolic adaptations to save energy. In Arabidopsis, the lack of oxygen is reported primarily by ethylene-responsive transcription factor group-VII (ERF-VII), which are responsible for preparing plants to endure anaerobic conditions. ERF-VIIs are oxygen-labile transcription factors, whose half-life is determined by a dedicated branch of the proteolytic pathway known as N-end rule (Fig. 3). Five members of ERF-VII TFs in Arabidopsis are known: RAP2.2 (RELATED TO APETELA 2), RAP2.3, RAP2.12, HRE1 (HYPOXIA-RESPONSIVE ERF 1) and HRE2. RAP2.2, RAP2.3 and RAP2.12 are constitutively expressed and sufficient to redundantly induce the anaerobic response (Bui et al., 2015), whereas HRE1 and HRE2 have been shown to be required to sustain the expression of the same set of genes when the hypoxic stress persists. RAP2.12, a member of ERF-VII TFs, has been shown to be capable of controlling the major anaerobic pathways and inducing fermentative enzymes under fully aerated conditions, when expressed in an oxygen-insensitive form (Paul et al., 2016). Under low oxygen conditions, plants adopt different tolerance strategies in order to save energy. For instance, preference of sucrose synthase over invertase for being energetically less expensive for sucrose breakdown and bifurcation of tricarboxylic acid (TCA) cycle have been described as metabolic
12
adaptations employed by plants (Vanlerberghe et al., 1989; Rocha et al., 2010; Sweetlove et al., 2010).
If the reconfiguration of plant metabolism to oxygen deficiencies has been investigated extensively, the information about the regulatory mechanisms actuated to adapt developmental processes during low oxygen conditions is instead still scarce. In the present study, I explore the possibility of the existence of a crosstalk between reduced oxygen availability and hormonal control of development in root tissues, focusing on the involvement of the oxygen-responsive ERF-VII transcription factors.
Fig. 3. Schematic representation of oxygen signaling in plants. Constitutively expressed ERF-VII RAP2.12 and possibly RAP2.2 are bound to the membrane localized acyl-CoA binding protein 1 and 2 (ACBP1/2). N-terminal methionine is removed from ERF-VII proteins by methionine aminopeptidase (MAP) leaving a cysteine residue exposed. Under aerobic conditions (blue arrows), ERF-VII are degraded following the N-end rule pathway. Upon hypoxia (red arrow), reduction of oxygen levels prevents the degradation of ERF-VII, which are translocated into the nucleus where they induce anaerobic genes. Upon reoxygenation, ERF-VII proteins are rapidly degraded via the some proteolytic pathway (adapted from Licausi et al. 2011).
13
AIM OF THIS THESIS
As discussed above, the plant root architecture is defined by a plethora of factors that include biotic and abiotic stresses as well as mineral and water availability. A severe impact of oxygen limitations on root growth has been reported in past studies. However, the question that remains unanswered is whether a proactive mechanism is involved or rather root growth inhibition is an inevitable consequence of energy limitation. Therefore, my work in the previous three years has aimed at studying the potential interaction between molecular mechanisms that are involved in oxygen sensing those dedicated to the control of root growth and developmental using Arabidopsis thaliana as a model species. Since oxygen availability may vary within tissues and organs, we decided to investigate conditions of reduced oxygen availability that occur as a part of the regular growth and developmental patterns as well as exogenous stress. Since in the past several studies discovered and analyzed in details the involvement of hormonal pathways in the definition of root architecture, the present work focused on the signaling pathway downstream of the main growth regulators and their possible cross-talk with hypoxia inducible ERF-VII TFs. This involved the extensive genetic and phenotypic manipulation and evaluation of the anaerobic response pathway by exploiting existing and novel gain of- and loss of-function transgenics. With this strategy, we aimed at identifying novel ERF-VII interaction proteins and direct or indirect target genes, in order to link these transcriptional regulators to root development, in addition to the previously described stress and shoot-growth related pathways.
14
OUTLINE OF THIS THESIS
In Chapter 2 (‘Establishment of endogenous hypoxia is required for the inhibition of auxin sensitivity to control lateral root development in Arabidopsis primordia’), we identify hypoxic niches formed during lateral root primordia development in Arabidopsis plants, which prompt hypoxia responsive ERF-VII TFs into action. We assess the cross-talk between ERF-VII proteins and auxin signalling modules involved in regulation of lateral root development. Finally, we elucidate the role of ERF-VII activity during primordia development in defining the plant root architecture.
We also investigated the mechanism of growth inhibition of primary root under low-oxygen stress. In Chapter 3 (‘Jasmonates contribute to primary root inhibition upon oxygen deficiency in Arabidopsis thaliana’) we provide first evidence that root growth inhibition in Arabidopsis seedlings under exogenous hypoxia involves prompt response to jasmonic acid signalling. We also study the role of ERF-VII TFs and test their potential to interfere with jasmonate signalling in roots upon exogenous hypoxia.
Chapter 4 (‘Establishment of a Chromatin-immunoprecipitation protocol to test RAP2.12 binding on gene promoters’) describes the establishment and optimization of a ChIP protocol in our lab, which has been used to assess ERF-VII direct association with discrete genomic regions. This protocol has been used in the two former chapters and has been published as part of the article ‘Age-dependent regulation of ERF-VII transcription factor activity in Arabidopsis thaliana’, by Giuntoli et al. (2017).
15
LITERATURE CITED
Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119: 119–120
Armstrong W, Armstrong J (2014) Plant internal oxygen transport (Diffusion and convection) and measuring and modelling oxygen gradients. Plant Cell Monogr 21: 267–297
ARMSTRONG W, STRANGE ME, CRINGLE S, BECKETT PM (1994) Microelectrode and Modelling Study of Oxygen Distribution in Roots. Ann Bot 74: 287–299 Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y,
Reichheld J-P (2010) Interplay between the NADP-Linked Thioredoxin and Glutathione Systems in Arabidopsis Auxin Signaling. Plant Cell 22: 376–391 Beemster GTS, Baskin TI (1998) Analysis of Cell Division and Elongation Underlying
the Developmental Acceleration of Root Growth in Arabidopsis thaliana. Plant Physiol 116: 1515–1526
Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser M-T, Aeschbacher RA (1993) Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development 119: 57–70
Bichet A, Desnos T, Turner S, Grandjean O, Höfte H (2001) BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J 25: 137–148
Birnbaum K (2003) A Gene Expression Map of the Arabidopsis Root. Science (80- ) 302: 1956–1960
Blilou I, Frugier F, Folmer S, Serralbo O, Willemsen V, Wolkenfelt H, Eloy NB, Ferreira PCG, Weisbeek P, Scheres B (2002) The Arabidopsis HOBBIT gene encodes a CDC27 homolog that links the plant cell cycle to progression of cell differentiation. Genes Dev 16: 2566–2575
Blokhina O, Fagerstedt K V. (2010) Oxidative metabolism, ROS and NO under oxygen deprivation. Plant Physiol Biochem 48: 359–373
Bouain N, Doumas P, Rouached H (2016) Recent Advances in Understanding the Molecular Mechanisms Regulating the Root System Response to Phosphate Deficiency in Arabidopsis. Curr Genomics 17: 308–314
Bouguyon E, Brun F, Meynard D, Kubeš M, Pervent M, Leran S, Lacombe B, Krouk G, Guiderdoni E, Zažímalová E, et al (2015) Multiple mechanisms of nitrate
16
sensing by Arabidopsis nitrate transceptor NRT1.1. Nat Plants 1: 15015 Bui LT, Giuntoli B, Kosmacz M, Parlanti S, Licausi F (2015) Constitutively expressed
ERF-VII transcription factors redundantly activate the core anaerobic response in Arabidopsis thaliana. Plant Sci 236: 37–43
Burk DH, Liu B, Zhong R, Morrison WH, Ye ZH (2001) A katanin-like protein
regulates normal cell wall biosynthesis and cell elongation. Plant Cell 13: 807– 27
Carlsbecker A, Lee J-Y, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vatén A, Thitamadee S, et al (2010) Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465: 316– 321
Cosgrove DJ (1993) How do plant cell walls extend? Plant Physiol 102: 1–6 Cui H, Levesque MP, Vernoux T, Jung JW, Paquette AJ, Gallagher KL, Wang JY,
Blilou I, Scheres B, Benfey PN (2007) An Evolutionarily Conserved Mechanism Delimiting SHR Movement Defines a Single Layer of Endodermis in Plants. Science (80- ) 316: 421–425
Dawood T, Rieu I, Wolters-Arts M, Derksen EB, Mariani C, Visser EJW (2014) Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB Plants. doi: 10.1093/aobpla/plt058 Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B
(1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84
van Dongen JT, Fröhlich A, Ramírez-Aguilar SJ, Schauer N, Fernie AR, Erban A, Kopka J, Clark J, Langer A, Geigenberger P (2009) Transcript and metabolite profiling of the adaptive response to mild decreases in oxygen concentration in the roots of arabidopsis plants. Ann Bot 103: 269–80
van Dongen JT, Licausi F (2014) Oxygen Sensing and Signaling. Annu Rev Plant Biol 66: 150112150216002
Drisch RC, Stahl Y (2015) Function and regulation of transcription factors involved in root apical meristem and stem cell maintenance. Front Plant Sci. doi: 10.3389/fpls.2015.00505
Evans DE (2004) Aerenchyma formation. New Phytol 161: 35–49
Eysholdt-Derzso E, Sauter M (2017) Root bending is antagonistically affected by hypoxia and ERF-mediated transcription via auxin signaling. Plant Physiol
17 pp.00555.2017
Fendrych M, Van Hautegem T, Van Durme M, Olvera-Carrillo Y, Huysmans M, Karimi M, Lippens S, Guérin CJ, Krebs M, Schumacher K, et al (2014)
Programmed cell death controlled by ANAC033/SOMBRERO determines root cap organ size in arabidopsis. Curr Biol 24: 931–940
Forzani C, Aichinger E, Sornay E, Willemsen V, Laux T, Dewitte W, Murray JAH (2014) WOX5 suppresses CYCLIN D activity to establish quiescence at the Center of the root stem cell niche. Curr Biol 24: 1939–1944
Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B (2007) PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449: 1053–1057
Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K, Went FW, Weij GH van der, Goldsmith MHM, et al (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–30
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30: 1–47
Goh T, Joi S, Mimura T, Fukaki H (2012) The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139: 883–893
Grieneisen VA, Xu J, Marée AFM, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449: 1008–1013
Gruber BD, Giehl RFH, Friedel S, von Wiren N (2013) Plasticity of the Arabidopsis Root System under Nutrient Deficiencies. PLANT Physiol 163: 161–179 Hamann T, Benkova E, Bäurle I, Kientz M, Jürgens G (2002) The Arabidopsis
BODENLOS gene encodes an auxin response protein inhibiting
MONOPTEROS-mediated embryo patterning. Genes Dev 16: 1610–1615 Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a
transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411
Hassan H, Scheres B, Blilou I (2010) JACKDAW controls epidermal patterning in the Arabidopsis root meristem through a non-cell-autonomous mechanism. Development 137: 1523–1529
18
Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser M-T, Benfey PN (2000) The SHORT-ROOT Gene Controls Radial Patterning of the Arabidopsis Root through Radial Signaling. Cell 101: 555–567
ten Hove CA, Lu K-J, Weijers D (2015) Building a plant: cell fate specification in the early Arabidopsis embryo. Development 142: 420–430
Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S,
Shinozaki K, Kakimoto T (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409: 1060–1063
Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S (2007) Cytokinins Determine Arabidopsis Root-Meristem Size by Controlling Cell Differentiation. Curr Biol 17: 678–682 Dello Ioio R, Nakamura K, Moubayindin L, Perilli S, Taniguchi M, T. Morita M,
Aoyama T, Costantino P, Sabatini S (2008) A genetic framework for genetic control of cell division and differentiation in the root meristem. Science (80- ) 1543–1547
Kellermeier F, Armengaud P, Seditas TJ, Danku J, Salt DE, Amtmann A (2014) Analysis of the Root System Architecture of Arabidopsis Provides a
Quantitative Readout of Crosstalk between Nutritional Signals. Plant Cell 26: 1480–1496
Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, et al (2010) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18: 927–937
Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 19: 1855–1860
Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L (2013) Lateral root development in Arabidopsis: Fifty shades of auxin. Trends Plant Sci 18: 1360–1385
Levesque MP, Vernoux T, Busch W, Cui H, Wang JY, Blilou I, Hassan H, Nakajima K, Matsumoto N, Lohmann JU, et al (2006) Whole-genome analysis of the short-root developmental pathway in Arabidopsis. PLoS Biol 4: 739–752 Lynch J (1995) Root Architecture and Plant Productivity. Plant Physiol 109: 7–13 Mahonen AP (2006) Cytokinin Signaling and Its Inhibitor AHP6 Regulate Cell Fate
19
Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y (2000) A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev 14: 2938–2943
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44
Mansfield SG, Briarty LG (1991) Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can J Bot 69: 461–476
Manzano C, Pallero-Baena M, Casimiro I, De Rybel B, Orman-Ligeza B, Van Isterdael G, Beeckman T, Draye X, Casero P, Del Pozo JC (2014) The Emerging Role of Reactive Oxygen Species Signaling during Lateral Root Development. Plant Physiol 165: 1105–1119
Moreno-Risueno M a., Sozzani R, Yardımcı GG, Petricka JJ, Vernoux T, Blilou I, Alonso J, Winter CM, Ohler U, Scheres B, et al (2015) Transcriptional control of tissue formation throughout root development. Science (80- ) 6: 1–20 Müller B, Sheen J (2008) Cytokinin and auxin interaction in root stem-cell
specification during early embryogenesis. Nature 453: 1094–1097 Ohashi-Ito K, Bergmann DC (2007) Regulation of the Arabidopsis root vascular
initial population by LONESOME HIGHWAY. Development 134: 2959–2968 Okamoto M, Vidmar JJ, Glass ADM (2003) Regulation of NRT1 and NRT2 gene
families of Arabidopsis thaliana: Responses to nitrate provision. Plant Cell Physiol 44: 304–317
Paul MV, Iyer S, Amerhauser C, Lehmann M, van Dongen JT, Geigenberger P (2016) RAP2.12 oxygen sensing regulates plant metabolism and performance under both normoxia and hypoxia. Plant Physiol 172: pp.00460.2016
Pérez-Pérez JM, Serralbo O, Vanstraelen M, González C, Criqui MC, Genschik P, Kondorosi E, Scheres B (2008) Specialization of CDC27 function in the
Arabidopsis thaliana anaphase-promoting complex (APC/C). Plant J 53: 78–89 Pi L, Aichinger E, van der Graaff E, Llavata-Peris CI, Weijers D, Hennig L, Groot E,
Laux T (2015) Organizer-Derived WOX5 Signal Maintains Root Columella Stem Cells through Chromatin-Mediated Repression of CDF4 Expression. Dev Cell 33: 576–588
Rademacher EH, Lokerse AS, Schlereth A, Llavata-Peris CI, Bayer M, Kientz M, FreireRios A, Borst JW, Lukowitz W, Jürgens G, et al (2012) Different Auxin Response Machineries Control Distinct Cell Fates in the Early Plant Embryo. Dev Cell 22: 211–222
20
Rocha M, Licausi F, Araujo WL, Nunes-Nesi A, Sodek L, Fernie AR, van Dongen JT (2010) Glycolysis and the Tricarboxylic Acid Cycle Are Linked by Alanine Aminotransferase during Hypoxia Induced by Waterlogging of Lotus japonicus. PLANT Physiol 152: 1501–1513
Roppolo D, De Rybel B, Tendon VD, Pfister A, Alassimone J, Vermeer JEM, Yamazaki M, Stierhof Y-D, Beeckman T, Geldner N (2011) A novel protein family mediates Casparian strip formation in the endodermis. Nature 473: 380–383
De Rybel B, Vassileva V, Parizot B, Demeulenaere M, Grunewald W, Audenaert D, Van Campenhout J, Overvoorde P, Jansen L, Vanneste S, et al (2010) A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol 20: 1697–706
Scheres B (2007) Stem-cell niches: nursery rhymes across kingdoms. Nat Rev Mol Cell Biol 8: 345–354
Scheres B, Dilaurenzio L, Willemsen V, Hauser MT, Janmaat K, Weisbeek P, Benfey PN (1995) Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development 121: 53–62
Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P (1994) Embryonic origin of the Arabidopsis primary root and root meristem initials. Development 2487: 2475–2487
Schindelman G, Morikami A, Jung J, Baskin TI, Carpita NC, Derbyshire P, McCann MC, Benfey PN (2001) COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in arabidopsis. Genes Dev 15: 1115–1127
Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D (2010) MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916
Serralbo O, Pérez-Pérez JM, Heidstra R, Scheres B (2006) Non-cell-autonomous rescue of anaphase-promoting complex function revealed by mosaic analysis of HOBBIT, an Arabidopsis CDC27 homolog. Proc Natl Acad Sci U S A 103: 13250–13255
Shibasaki K, Uemura M, Tsurumi S, Rahman A (2009) Auxin response in
Arabidopsis under cold stress: underlying molecular mechanisms. Plant Cell 21: 3823–38
21
Naudts M, Vanneste S, Audenaert D, et al (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690
Spradling A, Drummond-Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414: 98–104
Stahl Y, Grabowski S, Bleckmann A, Kühnemuth R, Weidtkamp-Peters S, Pinto KG, Kirschner GK, Schmid JB, Wink RH, Hülsewede A, et al (2013)
Moderation of arabidopsis root stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 receptor kinase complexes. Curr Biol 23: 362–371
Stahl Y, Wink RH, Ingram GC, Simon R (2009) A Signaling Module Controlling the Stem Cell Niche in Arabidopsis Root Meristems. Curr Biol 19: 909–914 Sweetlove LJ, Beard KFM, Nunes-Nesi A, Fernie AR, Ratcliffe RG (2010) Not just a
circle: Flux modes in the plant TCA cycle. Trends Plant Sci 15: 462–470 Taniguchi YY, Taniguchi M, Tsuge T, Oka A, Aoyama T (2010) Involvement of
Arabidopsis thaliana phospholipase Dζ2 in root hydrotropism through the suppression of root gravitropism. Planta 231: 491–497
Vanlerberghe GC, Horsey AK, Weger HG, Turpin DH (1989) Anaerobic Carbon Metabolism by the Tricarboxylic Acid Cycle : Evidence for Partial Oxidative and Reductive Pathways during Dark Ammonium Assimilation. Plant Physiol 91: 1551–1557
Vanstraelen M, Baloban M, Da Ines O, Cultrone A, Lammens T, Boudolf V, Brown SC, De Veylder L, Mergaert P, Kondorosi E (2009) APC/C CCS52A complexes control meristem maintenance in the Arabidopsis root. Proc Natl Acad Sci 106: 11806–11811
Verbelen J-P, De Cnodder T, Le J, Vissenberg K, Baluska F (2006) The Root Apex of Arabidopsis thaliana Consists of Four Distinct Zones of Growth Activities: Meristematic Zone, Transition Zone, Fast Elongation Zone and Growth Terminating Zone. Plant Signal Behav 1: 296–304
Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P (2010) Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J 63: 551–562 WAMPLE RL, REID DM (1978) Control of Adventitious Root Production and
Hypocotyl Hypertrophy of Sunflower (Helianthus annuus) in Response to Flooding. Physiol Plant 44: 351–358
Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, J??rgens G (2006) Auxin triggers transient local signaling for cell specification in Arabidopsis
22 embryogenesis. Dev Cell 10: 265–270
Welch D, Hassan H, Blilou I, Immink R, Heidstra R, Scheres B (2007) Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes Dev 21: 2196–2204
Wildwater M, Campilho A, Perez-Perez JM, Heidstra R, Blilou I, Korthout H, Chatterjee J, Mariconti L, Gruissem W, Scheres B (2005) The
RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell 123: 1337–1349
Willemsen V, Bauch M, Bennett T, Campilho A, Wolkenfelt H, Xu J, Haseloff J, Scheres B (2008) The NAC Domain Transcription Factors FEZ and SOMBRERO Control the Orientation of Cell Division Plane in Arabidopsis Root Stem Cells. Dev Cell 15: 913–922
Yamaguchi M, Goue N, Igarashi H, Ohtani M, Nakano Y, Mortimer JC, Nishikubo N, Kubo M, Katayama Y, Kakegawa K, et al (2010) VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 Effectively Induce Transdifferentiation into Xylem Vessel Elements under Control of an Induction System. PLANT Physiol 153: 906–914
Zhang Y, Jiao Y, Liu Z, Zhu Y-X (2015) ROW1 maintains quiescent centre identity by confining WOX5 expression to specific cells. Nat Commun 6: 6003
Zolla G, Heimer YM, Barak S (2010) Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J Exp Bot 61: 211–224
23
Chapter 2
Establishment of endogenous hypoxia is required for
the inhibition of auxin sensitivity to control lateral root
development in Arabidopsis primordia
Vinay Shukla1, Lara Lombardi2, Ales Pencik3, Ondrej Novak3, Pierdomenico Perata1, Beatrice Giuntoli1, Francesco Licausi1,2
1 Institute of Life Sciences, Scuola Superiore Sant’Anna, Pisa, Italy 2
Biology Department, Università di Pisa, Pisa, Italy
3
Palacky University, Olomouc, The Czech Republic
ABSTRACT
The existence of steep oxygen gradients has been revealed in plant organs irrespectively of the ambient oxygen concentration. Formation of hypoxic niches in the developing tissues of plants is not unexpected, due to limited diffusion of oxygen to satisfy the metabolic demands throughout development. As non-photosynthesizing organs, roots are dependent on diffusion of oxygen from the shoot and the external environment for their aerobic metabolism. We observed that specific stages of lateral root primordia development are characterized by hypoxic microniches in Arabidopsis roots grown under aerobic conditions. Therefore we studied the relationship of the oxygen sensing machinery with the hormonal regulation of lateral root (LR) development. To this end, we exploited gain and loss-of-function mutants to investigate the role of ERF-VII transcription factors controlling root development and discovered that these proteins control auxin sensitivity in lateral root primordia, possibly via directly binding to the promoters of the regulatory genes LBD16, LBD18 and PUCHI. In conclusion, this study sets the bases to understand the role of oxygen gradients in higher plant to control developmental patterns.
INTRODUCTION
Deployment of lateral roots along the length of primary root and development of root hairs defines the three-dimensional structure of the root system, termed as root system architecture (RSA) (Lynch, 1995). RSA is one of the highly plastic features of plant growth, adapting to extremely heterogeneous soil environments in order to optimize water and nutrient uptake and thereby ensuring adequate plant growth and development (Forde and Lorenzo, 2001; Bao et al., 2014).
24
Postembryonic development of lateral roots (LRs) in plants, a major determinant of root architecture, is controlled by genetic and environmental factors and it affects the plant’s capacity to explore the soil and resist to abiotic stresses (Malamy, 2005; Desnos, 2008; Lavenus et al., 2013). Development of LR from the primary root is a case of de novo organ formation (Parizot et al., 2007). Primed pericycle cells located on xylem poles (XPPs), called LR founder cells (LRFCs), have been shown to initiate the formation of LR primordia in Arabidopsis with asymmetric anticlinal division (Malamy and Benfey, 1997; De Smet et al., 2007a; De Rybel et al., 2010b; Goh et al., 2012). Among all XPP cells, competency of LRFCs to develop into LRs is established early, when they are still in the so-called “auxin oscillation zone”, a region marked by regular fluctuations in auxin responsiveness that could only be visualized by means of a DR5::LUC reporter. (Moreno-Risueno et al., 2010; Van Norman et al., 2013). Here, in the basal meristem, auxin-mediated degradation of IAA28 initiate the specification of founder cells as pre-branch site by activating the transcriptional regulators ARF5, ARF8 and ARF19 that, in turn, control GATA23 expression (De Rybel et al., 2010b). LR primordia development initiates in response to an auxin stimulus with converging nuclear migration and asymmetric cell division of neighboring LRFCs, processes that are known to be controlled by the SOLITARY-ROOT(SLR)/IAA14-ARF7-ARF19 auxin signaling module (Fukaki et al., 2002; Fukaki et al., 2005; Okushima et al., 2005; Okushima et al., 2007). ARF7 and ARF19 have been demonstrated to co-regulate the expression of LBD16, LBD18, LBD29, LBD33 and PUCHI transcription factors involved in controlling cell division and patterning in the LRP (Okushima et al., 2005; Hirota et al., 2007; Okushima et al., 2007). A third auxin signalling module, BODENLOS (BDL)/IAA12-MONOPTEROS (MP)/ARF5 module, has also been reported to participate in the regulation of LRP initiation (De Smet et al., 2010; Smet, 2010). Subsequently, a series of periclinal and anticlinal divisions form multilayered lateral root primordia (LRP) and this chain of events have been defined as stage – I to VII (Malamy and Benfey, 1997; Lucas et al., 2013). Both the IAA12-ARF5 auxin module and the IAA14-ARF7/ARF19 module are known to control this process. Upregulation of cell wall remodeling enzymes by the IAA3-ARF7 auxin module in endodermis and induction of LAX3 by the IAA14-ARF7-ARF19 module in adjacent cortex cells allow LRP bulging and emergence (Bhalerao et al., 2002; Swarup et al., 2008; Goh et al., 2012; Kumpf et al., 2013). Developed LRPs physically break through the epidermis of the primary root, their apical cells acquire complete apical meristem identity and activity, and the resulting LR starts elongating (Lavenus et al., 2013). Auxin is known as the primary inducer of these signaling modules, although the mechanism behind the termination of these modules, once their function is completed, has yet to be studied in depth.
Sub-atmospheric oxygen concentrations inside plant tissues are rather common phenomena. The absence of an active circulation system limits the amount of oxygen to the tissues through diffusion and convection (van Dongen and Licausi,
25
2014). Low oxygen levels in the environment, a sudden rise in respiratory demands and endogenous oxygen diffusion barriers are additional factors leading to the establishment of internal hypoxic microenvironments (Bailey-Serres et al., 2012). Steep oxygen concentrations in different plant organs such as maize anther, castor bean stem, apple fruit, soybean seed have been thoroughly reviewed (van Dongen and Licausi, 2014).
Measurements of endogenous oxygen levels in primary roots of Arabidopsis plants revealed that the levels of available oxygen in the roots, characterized by an O2 partial pressure of 5kPa, is significantly lower than leaf petiole (O2 partial pressure 16.5kPa) in normoxic conditions (Lee et al., 2011). Accordingly, radial oxygen profile of maize primary root developed using polarographic microelectrode reports sharp oxygen gradients across the root tissues from cortex to stele (ARMSTRONG et al., 1994). Therefore, a sudden increase in oxygen demand can readily cause hypoxic environment inside the tissues in the root, taking into account that there are no photosynthesizing cells to supply oxygen.
Establishment of a hypoxic microenvironment in order to maintain the stem cells in an undifferentiated state has been well studied in animals. Adult mammalian epicardium and subepicardium, that host cardiac progenitor cells, have been identified as one of hypoxic niches, where hypoxia-inducible factor 1α (HIF-1α) helps them maintain the undifferentiated state (Kimura and Sadek, 2012). Possible involvement of endogenous hypoxia in growth and development in plants has yet to be explored in depth. We identified several hypoxic niches in Arabidopsis plants such as shoot apical meristem, shoot-root junction, stele and early stages of lateral root (LR) development using hypoxia responsive reporters grown in an anaerobic environment. We also observed a significant reduction in the number of LR in the plants treated with milder low oxygen treatment for a longer period. This directed our interest towards the investigation of the role of endogenous hypoxia in LR development.
In the current study, we are interested in understanding the role of endogenous hypoxia that occurs during LRP establishment in the regulation of these developmental pathways. We present evidence that group ERF-VII transcription factors, which are primarily responsible for hypoxia response in plants (Giuntoli et al., 2017), participate in the regulation of LRP developmental genes by hindering their auxin sensitivity and hence affect root architecture.
26
MATERIAL AND METHODS
Plant Material and Growth Conditions
The Columbia-0 (Col-0) ecotype of Arabidopsis thaliana was used as wild-type background in all experiments. Genotype promPCO1::GFP-GUS have been previously described in Weits et al., (2014). Transgenic line with stabilized RAP2.12 (35S:∆13RAP2.12 #10) used for experiments has been previously described in Licausi et al., (2011). The prt6 single mutant (N684039) seeds were obtained from The European Arabidopsis Stock Centre (NASC). The pentuple mutant erf-VII (rap2.12 rap2.2 rap2.3 hre1 hre2) have been previously described in Gibbs et al., (2014) and provided by Michael Holdsworth (University of Nottingham). Seeds were grown on vertical plates on agarized medium composed of half-strength Murashige and Skoog (Duchefa) basal salt mixture, 0.8% plant agar, supplemented with 1% sucrose (Sigma-Aldrich). Seeds were stratified at 4°C in the dark for 48 h and germinated at 22°C day/18°C night with a photoperiod of 12 h light and 12 h dark. For root development experiments, low- or high- oxygen treatments were applied as described in van Dongen et al., (2009) to plants grown in air containing 21% oxygen (normoxia) or 1% O2 as hypoxia and 80% O2 for hyperoxia, for the time indicated in the figure legends and keeping plants in the same growth photoperiod.
Cloning of the various genetic constructs
Coding and promoter sequences were amplified from complementary DNA templates or expression vectors using Phusion High Fidelity DNA-polymerase (New England Biolabs). Chimeric genes (mCherry-NLS, DII-RAP-VENUS, RAP-VENUS) were produced by overlapping PCR (Higuchi et al., 1988). All open reading frames were cloned into pENTR/D-TOPO (Life Technologies). The resulting entry vectors were recombined into destination vectors using the LR reaction mix II (Life Technologies) to obtain novel expression vectors. Vector pH7WGF2 was used to produce the promPCO1::GW vector by replacing the prom35S with SpeI and SacI restriction enzymes and ligating the 1143bp long PCO1 promoter with Anza T4 DNA ligase (Thermo-fisher) (Karimi et al., 2002). A complete list and description of the destination vectors and primers used is provided in supplementary tables.
Measurement root length and number of lateral roots
Transparent square plates (10 cm side) containing the Arabidopsis plants were scanned using Perfection V700 Photo scanner (Seiko Epson) with 300DPI at the end of treatment. The software EZ – Rhizo (Armengaud, 2009) was used for root length measurement, while the number of emerged lateral roots was calculated manually.
27 Gus staining
GUS staining was performed as described in Jefferson, (1989). Different stages of lateral root primordia were observed using SMZ-2T stereomicroscope (Nikon) with the 20x objective lens and images were taken using Digital Sight DS-U2 digital camera (Nikon).
Confocal Imaging
For GFP visualizations in lateral roots and lateral root primordia, plants were grown for eleven days on vertical square plates and stained with 10 µgml-1 propidium iodide (PI) (Sigma-Aldrich) cell-wall stain. The roots were observed with the 20x objective lens, under a FluoView1000 (Olympus) inverted confocal laser scanning microscope and ZEISS Axio Observer-7 equipped with LSM 800 laser. GFP fluorescence was excited with 488 nm laser light and collected with a 497-554 nm long-pass emission filter; RFP fluorescence was excited with 543 nm laser light and collected at 590-650 nm, PI fluorescence was excited with 488 nm laser light and collected at 650-700 nm. YFP fluorescence was excited with 488 nm laser light and collected with a 520-560 nm. Scanner and detector settings were optimized and kept unchanged for all the experiments. Images were analysed with the FluoView FV1000 software (Olympus) and ZEN 2012(Zeiss).
Hormone quantification
Plants were grown and subjected to hypoxia as described for phenotypic experiments. Plants were subjected to 1% O2 for two time points – 6h and 4d. 20mg root tissue were sliced away from shoots using a scalpel and frozen in liquid nitrogen. The LC-MS analysis for quantification of absolute levels of IAA and other hormones was performed by Aleš Pěnčík and Ondrej Novak of Laboratory of Growth Regulators, Palacký University.
Luciferase transactivation assay in protoplast
Rosette leaves of Col-0 and ∆13RAP2.12 were used to isolate the protoplasts from Arabidopsis plants and transfected according to (Yoo et al., 2007). The effector plasmid with RAP2.12 and the RrLuc-overexpressing vector (35S::RrLuc26) used as normalization control were previously described in Weits et al., (2014). The reporter plasmid with LBD16 (1321bp), LBD18 (2070bp) and PUCHI (2571bp) promoter region controlling PpLuc expression was generated by recombining the entry vector to pGWL7 destination vector (Karimi et al., 2002). Four micrograms of p35S::RAP2.12 with four micrograms of pGWL7::promLBD16, pGWL7::promLBD18 and pGWL7::promPUCHI were used for each transformation. The treatment of protoplasts with auxin was done after 16h of transformation with 1µM IAA for 6h. Luciferase signal was measured using the Dual Luciferase Reporter Assay kit (Promega) with a Lumat luminometer (Bechtold Technologies).
28
RNA extraction and gene expression analysis by real-time qRT-PCR
Col-0 and erf-VII plants grown as described for previous experiments. The root apical meristems and shoots were sliced away using a sterile scalpel. RNA extraction was performed using a Spectrum Total RNA isolation kit (Sigma- Aldrich) and processed to cDNA according to Bui et al. (2015). Real-time PCR amplification was carried out with the ABI Prism 7300 sequence detection system (Applied Biosystems), using iQSYBR Green Supermix (Biorad). Ubiquitin10 (At4g05320) was used as the housekeeping gene. Relative expression of each gene was calculated using the 2-∆∆Ct method (Livak and Schmittgen, 2001).
Chromatin immunoprecipitation assay
The ChIP assay was performed using a modified version of Yamaguchi et al.,(2014) and have been previously described in Giuntoli et al. 2017. In detail, 15 days-old Arabidopsis seedlings, grown in vertical plates on agarized medium, were used for the experiment. Seedlings were sprayed with 1µM IAA (Sigma-Aldrich) incubated for two hours before the cross-linking. Five hundred mg of root tissue was harvested after slicing away the shoot with scalpel, cross-linked by dipping in 1% formaldehyde for 10 min and quenched with 0.125 M glycine under vacuum infiltration for 5 min. Seedlings were blotted on paper tissue to dry them and immediately frozen in liquid nitrogen. The ChIP assay was performed as described in chapter 4.
29
RESULTS
Low oxygen responsive reporter indicates the occurrence of hypoxic micro-niches during LRP development
Lateral root production is a complex developmental process that requires de novo establishment of a meristematic zone, the lateral root primordium (LRP). This continuous process has been separated into seven discrete stages, on cytological bases (Malamy and Benfey, 1997). Previously, a time-resolved transcript profile of each LRP stage has been reported, which allowed the identification of regulatory models that mediate this program, most of which depend on auxin signalling (Moreno-Risueno et al., 2010; Voß et al., 2015). This was achieved by collecting at different times the portion of a primary root of Arabidopsis thaliana where an auxin maximum is artificially established by mechanical bending. The RNA extracted from these samples was analysed by Affymetrix microarray to evaluate the expression level of each transcript (Voß et al., 2015). For instance, it has been observed that the auxin-directed degradation of the repressor IAA14 set the positive regulator ARF7 free to induce the expression of downstream players LBD29, IAA28, PUCHI, GATA23, which are involved in LRP progress (Fig.1 a). All four genes, as well as many others involved in LRP development (Voß et al., 2015), showed a bi-phasic behaviour mirroring their involvement in a specific stage of the LRP progression (Fig.1 b). Remarkably, we could observe in this dataset the up-regulation of several hypoxia-inducible genes in the late developmental stages. These genes have been shown to be controlled by oxygen availability through ERF-VII factors (Fig. d). Considering the core set of hypoxia-inducible genes (Mustroph et al., 2009), one third of them were upregulated. An equal number of genes was either downregulated or unchanged (Fig.1 c). This suggested the possible occurrence of low oxygen conditions during LRP development.
To test whether anaerobic genes are indeed induced during LRP development, we exploited a chimeric GFP-GUS gene placed under the control of the Plant Cysteine Oxidase 1 (PCO1) promoter, which has been shown to be very specifically induced by hypoxia (Weits et al., 2014). Arabidopsis plants bearing this promPCO1::GFP-GUS construct were super-transformed with a second one consisting in a nuclear localized mCherry RFP under control of the auxin-responsive DR5 promoter (Ulmasov et al., 1997). In these plants, green fluorescence is expected to report a cellular oxygen concentration below 5% (Kosmacz et al.2015), while nuclear localized red fluorescence acts as a proxy for auxin activity and thereby defines the developmental stages observed (Fig.1 f-m). In agreement with the microarray analysis by (Voß et al., 2015), the appearance of a green fluorescent signal was observed after stage IV and increased in intensity afterwards (Fig.1 i & q), when auxin-stimulated reached its peak in the central zone of the three-layered LRP. At this stage, GFP was produced especially in the vasculature underneath the LRP and
30
progressively accumulated in the dividing cells during the following developmental steps (Fig.1 n-u).
31
Fig.1 Genetic regulation of LR primordia development and hypoxia response. (a) Brief scheme of the auxin signalling module involved in LRP development. (b) Expression profiles of auxin-responsive LRP developmental genes in different stages of development. (c) Scheme of oxygen sensing in Arabidopsis plants and response of core anaerobic genes during stages of primordia development. (d) Graphical representation of the response of four of the core anaerobic genes during primordia development. (e) Stages of LRP development. (f-m) Dual reporter for auxin (Dr5::mcherry-NLS) and low oxygen response (pPCO1::GFP-GUS) displaying auxin activity and hypoxia response during seven stages and emergence of LRP. (n-u) Confocal images of GFP channel of the corresponding stages. Green channel, GFP; red channel, RFP and propidium iodide (Size bar -12.5µm).
Developing LRPs experience oxygen crisis at the late stages
Therefore, we decided to assess whether induction of the hypoxia-responsive genes, including PCO1, is actually due to reduced oxygen availability in the LRP or rather to a developmental program. Unfortunately, direct detection of cellular oxygen concentrations is not feasible in LRP considering the size of the sensors commonly used to measure oxygen in biological systems, which exceed that of these meristematic zones. Thus, we opted for observing the activity of our output transcriptional reporter (PCO1::GFP-GUS) in LRPs exposed to different oxygen concentrations. Transgenic plants were grown vertically on agarized MS plates in normoxia or treated for four days with hypoxia (1% O2 V/V) or hyperoxia (80% O2 V/V). Also in case of this reporter, GUS staining was observed after stage IV under
32
aerobic conditions but could be moved up to earlier stages by hypoxic conditions or almost entirely abolished when plants were treated with hyperoxia for 48h (Fig. 2, 3). Since in these last conditions, LRPs at all stages could be observed, we excluded that altering oxygen availability could entirely prevent LRP development. In light of these observations, we concluded that a low oxygen niche is indeed established in the late stages of primordia development.
Fig.2. The onset of hypoxic conditions in the developing LRP. (a) Scheme of the experimental design (b-g) Primordia of stage III to emergence displaying promPCO1 response in aerobic conditions in the form of GFP signal. (h-m) GFP signal showing promPCO1 activity under hypoxic environment (1%O2) in primordia from stage III to
emergence. Roots of promPCO1::GFP-GUS plants were imaged for GFP signal after 7 days of growth and a subsequent treatment with 21% O2 (air) and 1% O2