Academic Years 2019-2020
PhD Programme in Translational Medicine
STUDY
ON
THE
ROLE
OF
SEVOFLURANE
IN
PROMOTING
THE
RELEASE
AND
UPTAKE
OF
ENDOTHELIAL
EXOSOMES
PROTECTING
CARDIOMYOCYTES
AGAINST
PERIOPERATIVE
ISCHEMIA/REPERFUSION
INJURY.
PhD Candidate:
Nora Terrasini, MD
Supervisor:
Prof. Vincenzo Lionetti, MD, PhD
Tutor:
Ai miei genitori, al mio compagno e a miei figli G. & S.
Con amore.
“No! Try not. Do! Or do not. There is no try.”
Jedi Master Yoda
3 Contents SUMMARY ... 5 Background ... 5 Aim 1 ... 6 Aim 2 ... 7 Aim 3 ... 7 BACKGROUND ... 9
Frontiers in the management of critical illness ... 9
Biogenesis, characterization and function of circulating exosomes ... 13
The role of exosomes in acute myocardial injury ... 16
The role of exosomes in ALI/ARDS ... 17
The role of exosomes in acute kidney injury ... 18
The role of exosomes in sepsis ... 19
Limitations of exosomes isolation protocols ... 21
RATIONALE ... 23
PROJECT AIMS ... 29
AIM 1: CLINICAL STUDY ... 30
Ethical Approval ... 30
Trial Design ... 30
Inclusion and Exclusion Criteria ... 31
Surgical Technique ... 32
Outcomes ... 35
Sample Size ... 36
Randomization and Blinding ... 36
Statistical Analysis ... 37 Interventions ... 38 Results ... 39 Baseline Characteristics ... 39 Primary Outcome ... 41 Operative Data ... 44 Postoperative Outcomes ... 44
4
Postoperative Complications ... 45
Limitations ... 46
Conclusion ... 46
AIM 2: ISOLATION AND CHARACTERIZATION OF CIRCULATING EXOSOMES IN PATIENTS DURING THE CLINICAL STUDY ... 49
Methods ... 50
Sample collection (pilot study) ... 50
Exosomes Isolation from plasma samples ... 50
BCA assay ... 51
Western blot ... 53
Results ... 54
Conclusions ... 57
AIM 3: IN VITRO STUDY ON SEVOFLURANE CARDIOPROTECTION THROUGH EXOSOME RELEASE ... 59
Materials and Methods ... 59
Cell lines ... 59
Sevoflurane treatment of MCEC cells ... 60
Isolation of Exosomes Secreted by MCEC Cells ... 61
Treatment of HL-1 cardiomyocytes with exosome containing or exosome-depleted MCEC conditioned medium ... 63
MTT Assay ... 64
Tunel Assay ... 65
Dihydroethidium (DHE) assay ... 67
Results and Discussion ... 69
Characterization of MCEC viability under different culture conditions ... 69
Characterization of the exosomes secreted by the sevoflurane-conditioned MCEC ... 72
Effect of MCEC medium on HL1 cell viability under oxidative stress conditions ... 73
PERSPECTIVES AND CONCLUSIONS ... 75
5
SUMMARY
Background
To date, the perioperative myocardial infarction is the leading cause of morbidity and mortality after cardiac surgery. Hence, an improvement of the clinical outcome of patients exposed to myocardial ischemia/reperfusion (I/R) is still a desirable achievement. Several studies have demonstrated that volatile anesthetics prevent cardiac I/R injury. Moreover, it has been demonstrated that cardioprotection by sevoflurane is not mediated by inflammatory cytokines. To best of our knowledge, halogenated ethers including sevoflurane exert cardioprotection activating pro-survival kinases similar to coronary ischemic preconditioning. In 2014, it was demonstrated that remote ischemic preconditioning increases the release of cardiac exosomes and that exosomes recapitulate cardioprotection due to ischemic preconditioning. Exosomes are 40–100 nm-sized particles stored in endosomal compartments, which are secreted when these intracellular bodies fuse with the plasma membrane. Conventional surface markers of exosomes include the tetraspanin family members CD63 and CD81, among others. Exosomes carry proteins, lipids, and nucleic acids including DNA, mRNAs, long non-coding RNAs and microRNAs (miRNAs), which they can shuttle to recipient cells, even at a distance. The hypothesis of my study is that Sevoflurane induces the endothelial release of cardioprotective exosomes during cardiac surgery with extracorporeal cardiopulmonary bypass (CPB).
6
Aim 1
To evaluate the effects of Sevoflurane or Propofol in modulating the profile of circulating
biomarkers of myocardial injury in patients undergoing on-pump minimally invasive mitral valve
surgery.
For this purpose, I have performed a single-center randomized controlled trial named Trial MINI-SEVO (NCT02551328) at FTGM/OPA Hospital (Massa, Italy) in accordance with the Declaration of Helsinki. The study was approved by the Area Vasta Toscana Ethics Committee (CEAVNO no. 452/14).
Patients signing informed consent and undergoing to minimally invasive mitral valve repair or replacement (MIMV) via right mini thoracotomy were recruited to one of two groups: Sevo-group (31 patients) and Propofol group (31 patients). Between March 2015 and December 2016, 225 patients were assessed for study inclusion; of these, 160 did not meet the inclusion/ exclusion criteria and were excluded. During CPB, sevoflurane was administered in the oxygenator circuit through a calibrated vaporizer. In both groups, depth of anesthesia was monitored with bispectral index. The primary outcome was myocardial injury, assessed by measuring myocardial Troponin I from venous blood samples collected pre-operatively (baseline), before CPB, before cardiac reperfusion and 6, 12, 24, 48 and 72h after cardiac reperfusion. Secondary outcomes were systemic metabolic stress as assessed by: blood pH, lactate, serum creatinine level, mechanical ventilation time, length of ICU stay, left ventricular function.
Concentrations of cTnI were increased postoperatively and peaked at 6hours after surgery in both groups. Postoperative blood pH, lactate, and serum creatinine tended to be lower in the sevoflurane group compared to the propofol group. Mechanical ventilation time was lower in the
7
sevoflurane group compared to the propofol group (p = 0.05), whereas ICU stay and total length of stay were similar in both groups.
There were neither in-hospital deaths nor unexpected serious adverse events. All patients were discharged with no or negligible residual mitral regurgitation.
Aim 2
To evaluate the effects of Sevoflurane or Propofol in modulating the profile of circulating exosomes
in patients undergoing on-pump minimally invasive mitral valve surgery.
For this purpose, the exosome-rich fraction was isolated from blood samples of 24 patients using a standard protocol of serial, differential centrifugation and ultracentrifugation steps. I have measured levels of CD63+ and CD81+, established exosome biomarkers, by Western Blotting. CD81 is a marker of endothelial exosomes, whereas CD63+ is a generic exosomal surface marker. CD81+/CD63+ ratio, an index of endothelial exosomes, in patients treated with propofol was higher than patients receiving Sevoflurane. I found an inverse relationship between cardiac troponin I values and endothelial exosomes levels in the propofol group, but not in the Sevoflurane group.
Aim 3
To evaluate the cardioprotective effects of endothelial-derived exosomes in vitro.
Murine coronary endothelial cells were exposed to increasing dose of Sevoflurane or PBS for 6h. Then, cells were stressed with transient exposure to 1.5mM hydrogen peroxide for 1h in order to simulate I/R injury. Dihydroethidium staining was used for detection of superoxide anion. The pretreatment of endothelial cells with sevoflurane caused a statistical significant dose-dependent reduction of superoxide anion, apoptotic index revealed by TUNEL assay and higher release of
8
exosomes rich of HSP70. In order to verify if endothelial exosomes protect cardiomyocytes, murine cardiomyocytes HL1 were pre-treated for 6h with medium of Sevoflurane-treated or PBS-treated cells. Medium exosome depleted of Sevoflurane-PBS-treated cells was also used as control. Finally, HL-1 cells were exposed to 1.5mM hydrogen peroxide.
When cardiomyocytes were exposed to hydrogen peroxide 1mM for 1h, cell survival was reduced to 31.76%, (P <0.05). Never the less conditioned medium of Sevoflurane-treated cells increased cell survival (P <0.05). Conversely, exosome depleted conditioned medium failed in evoking cardioprotection (p <0.05).
9
BACKGROUND
Frontiers in the management of critical illness
The care of the critically ill patient is becoming increasingly complex. A major limitation in the bedside management of critical illness is the lack of reliable indicators of early evolution of organ failure as well as of individual patient’s response to treatment. Therefore, an intense translational research activity is recently prompted. The rapidly evolving sciences in nanotechnology, biotechnology and omics (genomics, proteomics, metabolomics) is offering the opportunity to tailor new reliable biomarkers (Table 1) and to develop sophisticated accurate tools even to target therapeutic agents in critically ill patients (Table 2).
The conventional performance measures are often poor predictors both of early disturbance of the organ tolerance threshold and of spread of single organ dysfunction to other distant organs. Since the impairment of organ function occurs before it would otherwise be clinically evident, new nanobiotechnology will allow monitoring of critical changes in the intercellular cross-talk of by-products of signal messaging within a stressed tissue. The mechanistic understanding of critical illness at the cellular level may allow for more effective stratification of patients and the design of personalized therapy. In fact, the complex cell-cell communication mediated by paracrine factors is essential to maintain physiologic tissue environment necessary for response to stressors in different distant organs (1, 2). As an emerging paradigm, the tracking of organ function will fast move from the conventional macroscopic approach to an earlier molecular analysis of evolving critical illness. In the last few years, the discovery of new molecules dedicated to the management of critical illness has been a primary goal since the conventional tools have clear limitations. Even if ideal biomarkers of critical illness with enough sensitivity and specificity have not yet been
10
discovered (3), the use of cell-derived membrane-bound nanovesicles (exosomes) opens new avenue in promoting the detection (diagnostics) and delivery (therapeutics) of endogenous molecules modulating pro-survival (4), immunomodulatory (5) or other clinically relevant biological pathways (6). Therefore, circulating exosomes represent a new theranostic tool of promising clinical applications in intensive care units (7).
Table 1: Exosomes as biomarkers Disease Associated Protein, mRNAs or
miRNA
Biofluid Correlated Parameters Reference
AMI miR-133a Blood Marker for cardiomyocyte
death
(8)
miR-1 Urine Marker for cell damage with a
positive correlation between serum TnI and urine miR-1 levels
(9)
AKI GPRC5B Urine Marker for tubulogenesis and
wound healing
(10)
ATF3 Urine Marker for severity of renal
tubular injury
(11)
Fetuin-A Urine Marker for structural renal
injury (proximal tubule cells) and not early renal response to injury
(12)
SEPSIS MiRNA-34a, 15a and
miR-27a Blood/ human endothelial progenitor cells conditioned medium
Marker for illness severity, development of endothelial dysfunction and progression to shock.
(13)
16, 17, 20a, 20b, 26a, 26b, miR-106a, miR-106b, miR-195, and miR-451
Blood Marker for presence of sepsis (14)
11
Table 2: Exosomes as therapeutic drug carriers and delivery vehicle
Disease
Associated Protein, mRNAs
or miRNA
Biofluid Effects Reference
ALI/ ARDS
Caspase-3 bronchoalveolar
lavage fluid
activation of macrophages and mediation of inflammatory lung responses involved in lung injury
(15)
KGF mRNA cell culture medium reduction in pulmonary edema, in lung
protein permeability and in inflammation (16) AMI miR210 and -132 conditioned medium of CPCs
inhibiting apoptosis in cardiomyocytes and enhancing tube formation in endothelial cells (4) miR15b, 17, -20a, -103, -199a, -210, and -292 Conditioned medium of CPC exposed to hypoxia
enhanced tube formation of endothelial cells and decreased pro-fibrotic gene expression fibroblasts
(17)
EMMPRIN conditioned medium
of CPCs
involved in angiogenesis and endothelial cell migration
(18) miR-144/451 cluster conditioned medium of CPCs protection of cardiomyocytes from oxidative stress in vitro and ischemia-reperfusion injury in vivo
(19)
miR-30a conditioned medium
of cardiomyocytes exposed to hypoxia
attenuated apoptosis of
cardiomyocytes induced by hypoxia
(20) miRNA-17, 19a, 19b, 20a, 30c and 126 conditioned medium of cardiomyocytes exposed to glucose deprivation
increased angiogenesis and induced cell cycle and proliferation of targeted HUVEC
(21)
(-) conditioned medium
of MSCs exposed to hypoxia
improved cardiac function and promoted angiogenesis in ischemic heart in AMI rat model
(22)
(-) conditioned medium
of MSCs
improvement in cardiac function through angiogenesis and anti-inflammation after myocardial infarction in rat model
(23)
Bcl-2 family conditioned medium
of MSCs
improved cardiac systolic function in AMI rats and reduced cardiac fibrosis, suppressed cell apoptosis, and promoted cell proliferation
(24) AKI GPRC5B conditioned medium of MDCK cells expressing either GFP or GPRC5B-GFP
augmented ERK1/2 activation in recipient cells through exosomal transfer and improved response to HGF for invasive growth
(10)
TGF-b1 mRNA conditioned medium
of mouse and human TEC lines,
to engage fibroblasts in the repair/fibrotic response
through a rapid production of TGF-b1
12
(Terrasini N. and Lionetti V. Crit Care Med. 2017 Jun;45(6):1054-1060).
MCT and HK2, (tubular epithelial cells, TECs) protein Specific subset of mRNA conditioned medium of MSCs
accelerated morphologic and functional recovery of glycerol-induced AKI in SCID mice by inducing proliferation of tubular cells; in vitro, stimulated proliferation and apoptosis resistance of TECs (26) miR-126 and miR-296 conditioned medium of EPCs
improved recovery from
ischemia/reperfusion AKI in mice; anti-apoptotic and pro-angiogenic effects on hypoxic peritubular endothelial cells and tubular epithelial cells in vitro
(27)
- conditioned medium
of ECFCs
(endothelial colony-forming cells)
attenuated renal injury I mice, inhibition of endothelial cell apoptosis after hypoxia/ reoxygenation injury (H/R)
(28)
ATF3 RNA Urine Attenuated MCP-1 expression in the
epithelial cells and macrophage infiltration after H/R
(29)
- conditioned medium
of MSCs
inhibited apoptosis and stimulated tubular epithelial cell proliferation in rats after renal ischemia reperfusion injury (30) (ERK)1/2 pathway conditioned medium of human umbilical cord mesenchymal stem cells (hucMSC)
promoted cell proliferation in cisplatin-induced nephrotoxicity in vitro,
attenuated renal injury in cisplatin-induced AKI rats
(31)
RNA conditioned medium
of bone marrow-derived
mesenchymal stem cells (BMSCs)
enhanced recovery of renal function in an aminoglycoside AKI rat model
(32)
SEPSIS
SOCS1 conditioned medium
of alveolar macrophages
inhibition of STAT-1 activation in alveolar epithelial cells
(33)
NADPH oxidase blood samples of 16
patients with early (< 24h) diagnosis of septic shock
induced apoptotic death of endothelial and smooth muscle
cells in culture (34) - blood samples of 55 patients with diagnosis of septic shock
decreased myocardial contractility in isolated heart and papillary muscle preparations
13
Biogenesis, characterization and function of circulating exosomes
In the 1980s, Harding et al. have detected the ability of mammalian cells to release nanovesicles (size 30-100 nm) that were enclosed by a plasmalemma-derived membrane (36) and termed
exosomes (37). However, the recent development of large-scale protein analysis techniques and
imaging approaches has allowed researchers to clarify the type of cargo transported by exosomes and their functions. The growing amount of data on the changes induced by exosomes on target cells has recently prompted explorations into their potential clinical applications.
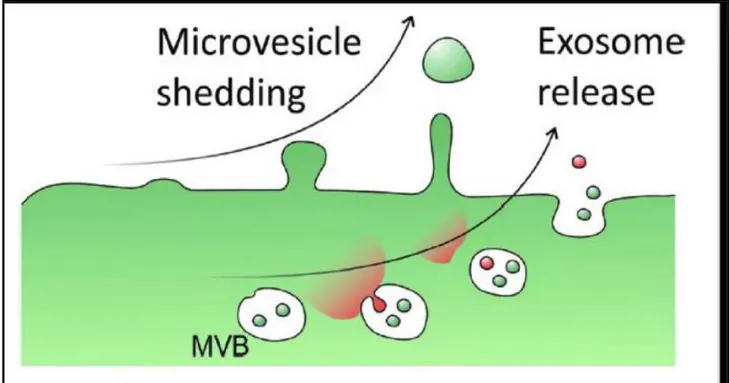
Exosomes are generated within intracellular multivesicular bodies and released into the extracellular milieu through fusion with the plasma membrane (Fig. 1), whereby they act as molecular Trojan horse for effective delivery of key mediators of cell-cell communication (38).
Fig 1: Exosome release from multivesicular bodies (MVB). (Yellon et al. Circ Res. 2014, 14, 315-24).
14
(39) and their circulating levels remain stable for prolonged periods even after the parental cells die. This feature is unique among other soluble mediators of cellular response to stressors. (i.e.: cytokines). Exosomes have a typical antigenic profile due to the combination of different membrane tetraspanins (CD9, 81, 63, 82, 37) (40). To date, multiple subpopulations of circulating exosomes have been identified in different human pathophysiological scenario, such as Parkinson’s disease (41), sepsis (35) colorectal cancer (42), focal segmental glomerulosclerosis (43), acute rejection following lung transplantation (44) and mitral valve repair (45). Interestingly, exosomes may also regulate immune response (46) and allow antigen presentation (47) essential for the adaptive immune response.
Even if all factors mediating exosome packaging, cellular release and uptake (i.e.: endosomal-sorting complex responsible for transport, ESCART; Alix; vacuolar protein endosomal-sorting 4) are still under investigation, emerging data indicate that exosomes can exert multiple functions (48). Exosomes alter gene expression and mediate functional effects in recipient cells, but their functional effectiveness depends on the cargo, the type of recipient cells (49) and the tissue microenvironment (50).
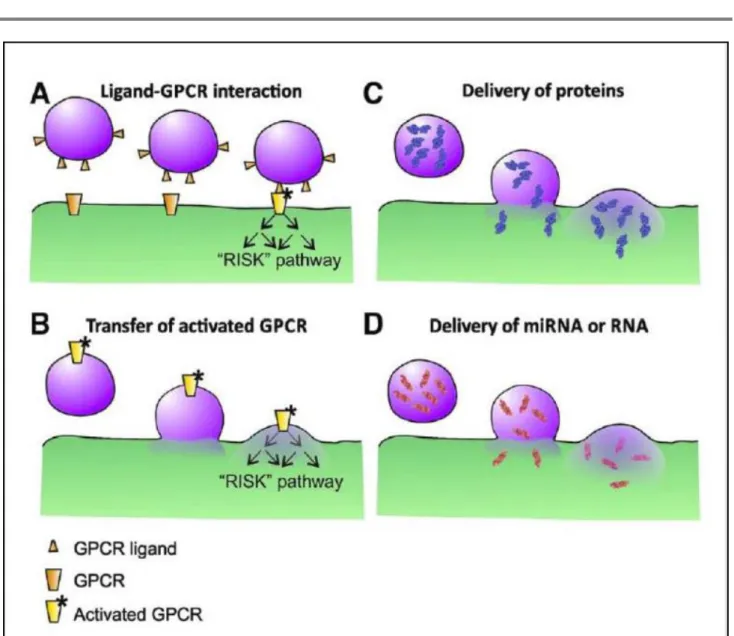
Exosomes carry proteins (transcriptional factors (50, 51), enzymes (52), heat shock proteins (53), tumoral antigens (54)), virulence factors (55), oligonucleotides (DNA, mRNA, microRNAs and non-coding RNA) (56, 57), metabolites (lipids, lactic acid and glutamic acid) (58) or virus (59) into different recipient cells (Fig. 2).
15
Fig. 2: Pathways of exosomes action. A, protein ligand in the exosome membrane activate receptors and downstream signaling pathways. B, exosomes deliver activated receptors for RISK pathway. C, exosomes deliver protein. D, exosomes deliver miRNA or mRNA to recipient cells. G-protein-coupled receptors (GPCR), microRNA (miRNA), RiboNucleic Acid (RNA). (Yellon et al. Circ Res. 2014, 14, 315-24).
In addition, protein ligands in the exosomal membrane may activate pro-survival signaling pathways as RISK (reperfusion injury signaling kinase) after binding G-protein coupled receptors (GPCR) of recipient cells. In addition, exosomes may deliver activated GPCR to regulate gene expression by phosphorylation cascade (60).
16
The potential clinical application of exosomes is also due to their high stability in biological fluids and culture medium. In fact, exosomes can be isolated from small sample volume of bioptic tissue culture medium (4), plasma (61), urine (12), saliva (62), amniotic fluid (63), and ascites (64). Finally, latest technologies have allowed to set isolation protocols much faster, easier, and more reliable for clinical use, such as in critical care units.
The role of exosomes in acute myocardial injury
Plasma concentrations of N- terminal pro-B-type natriuretic peptide and high-sensitivity cardiac troponin T (HSCT-T) are elevated following acute myocardial injury (AMI) in ICUs, mostly in patients with severe sepsis or septic shock (65) or in vascular surgery patients (66). Nonetheless, the dosage of HSCT-T may not be diagnostic if the onset of symptoms occurs less than three hours before AMI and this method should be part of a comprehensive triage. (67). In order to solve this limitation, recent investigators have focused on the new relationship between plasma exosomal microRNAs (miRs) and early myocardial injury (68, 69). In fact, serum levels of cardiac exosomal miRNAs (miR-1 and miR-133a) peaked before 3 hours after chest pain and decreased gradually thereafter (8). Thus, cardiac exosomal miRNAs are able to detect AMI earlier than conventional cardiac troponin assays (70). Even if HSCT-T levels are normally not detectable in the urine after AMI, exosomal miRNAs of cardiac origin are still measurable in the urine. Therefore, circulating exosomes may provide a very versatile clinical biomarker in intensive monitoring for early detection of ischemic cardiac injury (9). A number of studies are currently underway to define the major cellular source of cardiac exosomes.
Exosomes secreted by resident adult cardiac progenitor cells (CPCs) (CD9+, CD63+, CD81+,HSP70+, Alix+ and Tsg101+) (71) are helpful in cardioprotection (72) and repair of infarcted hearts (4, 71). It
17
is conceivable that CPCs may regulate the magnitude of the adaptive response to stressors in a dose- and time-dependent manner. In fact, exosomes released by chronically hypoxic CPCs deliver a pool of miRNAs (miR-15b, -17, -20a, -103, -199a, -210, and -292) that enhances angiogenesis, reduces pro-fibrotic genes expression and increases myocardial contractile function, respectively. These effects are not induced by exosomes derived from CPCs exposed to normoxia or acute hypoxia (17). However, other studies have demonstrated that exosomes from normoxic CPCs may improve cardiac function (4, 18, 19).
Recent studies have observed that ischemic cardiomyocytes even release prosurvival exosomes (20, 21). Yang et al. (20) have shown that cardiomyocytes exposed to anaerobic low-glucose culture medium, an ischemic-like environment, release exosomes delivering miR-30a, which have anti-apoptotic effects. In fact, exosomal miR-30a levels increase in the serum of AMI patients (20).
The role of exosomes in ALI/ARDS
In critically ill patients, the acute lung injury (ALI) and its worst form, the acute respiratory distress syndrome (ARDS) represent the principal cause of acute respiratory failure, a major clinical problem in ICUs. Even if cytokines have been proposed as important mediators of tissue injury (73), their inhibition does not protect against cell death in patients affected by ALI/ARDS (74, 75). Recently, Moon et al. (15) have demonstrated that lung epithelial cell-derived and caspase-3 enriched exosomes activate the alveolar and the systemic macrophages via Rho-associated Coiled-Coil kinase I (ROCK I) in a murine model of hyperoxia-induced ALI/ARDS. On the contrary, the depletion of circulating exosomes containing caspase-3, a pro-apototic factor, attenuates lung injury in vivo (15). Similarly, lung macrophages-derived exosomes delivering IL-36γ, a pro-inflammatory cytokine, are released in response to both Gram-negative and Gram-positive
18
bacteria and may induce pathogen-associated lung injury (76). Thus, more detailed knowledge on exosomal trafficking may open new avenues to treat pneumonia through modifications of innate host immune responses. Furthermore, Zhu et al. have demonstrated that the intratracheal instillation of extracellular vesicles (EVs) derived from human mesenchymal stem cells (MSCs) reduces lung inflammation and edema through the alveolar expression of keratinocyte growth factor in a murine model of ALI (16).
These findings define a subclass of exosomes as novel potential therapeutics in the treatment of ALI/ARDS. In addition, the use of MSC-derived EVs will draw on the immunomodulatory properties of the MSCs without the risk of iatrogenic tumor formation (77). Finally, exosomes may have therapeutic applications also in other pulmonary diseases, such as asthma, pulmonary artery hypertension and cystic fibrosis (78).
The role of exosomes in acute kidney injury
Even though acute kidney injury (AKI) has higher prevalence in ICUs (79), reliable early theranostics are still lacking. Exosomes delivering GPRC5B, an orphan G protein coupled receptor with tubulogenic properties, significantly enrich the urine of patients at the onset of AKI (10). In addition, urinary exosomal activating transcription factor 3 (ATF3), an inflammatory transcription factor, is isolated from patients with AKI but not from healthy subjects. Exosomal ATF3 is detected in murine urine from 2 to 24h after renal ischemia/reperfusion injury prior than the rise of serum creatinine levels (11). This finding suggests that exosomal ATF3 is an indicator of AKI more sensitive than conventional assays. Similarly, exosomal Fetuin-A, a multifunctional protein (80), has been also suggested as specific biomarker for early AKI induced by cisplatin or ischemia/ reperfusion. In fact, its urinary levels increase only in critical patients with AKI (12). In order to
19
support the use of exosomes in treating AKI, recent experimental studies have demonstrated that the systemic administration of MSCs-derived exosomes (CD9+, CD81+, CD29+, CD44+ and CD73+) contributes to the repair of kidney injured by glycerol or ischemia or cisplatin (26, 28, 30, 31). MSCs-derived exosomes contain specific miRNAs or mRNA (TGF-1 or ATF3) (25, 29) preventing cell apoptosis, proliferation and angiogenesis in tubular cells (27, 32), which are hallmarks of kidney injury. In particular, the exosomal ATF3mRNA attenuates the ischemia/reperfusion injury in murine kidney by preventing the monocyte chemoattractant protein-1 (MCP-1) expression in the epithelial cells and macrophage migration. Hence, strategies to induce the release of exosomes delivering ATF3mRNA may open new avenues to treat AKI in critical ill patients (29). Currently, the monitoring of urinary levels of exosomes delivering aquaporin-1 (AQP-1) proteins is being utilized in nephrology as indicators of renal ischemia and reperfusion injury (81). Further studies are required to determine specificity of the abovementioned exosomes as effective tools for early diagnosis/therapy of AKI.
The role of exosomes in sepsis
Sepsis is a clinical syndrome characterized by systemic post-infectious inflammation and widespread organ insult. To date, there are no molecular biomarkers to indicate vulnerability to sepsis or to the related organ dysfunction. Clinically relevant sepsis biomarkers may be helpful to establish the organ dysfunction severity and to evaluate the patient's clinical course, but not to escalate antibiotic therapy (82). Ultimately, it is not possible to use a single conventional biomarker to conduct a personalized assessment of patient with sepsis since the current indicators do not directly reflect the inflammatory cellular response and do not predict the magnitude of early immune cellular activation. In fact, positive blood culture, one of the most used assay in
20
sepsis diagnosis, is limited by time delay for results and it is not specific in the majority of cases (83). Hence, there is necessity to identify more accurate and reproducible cellular-specific mediators within the body fluids of septic patients. Bourdonnay et al. (33) have discovered that SOCS-1 (suppressor of cytokine signaling 1), a member of STAT-induced STAT-inhibitor proteins, is delivered into distinct exosomes which are taken up by alveolar endothelial cells to prevent the toxicity of STAT activation at early stage of sepsis. This first evidence of exosomal SOCS proteins opens new avenues for the simultaneous early diagnosis and treatment of dysregulated inflammatory response in sepsis. However, the theranostic use of exosomes in septic patients is still controversial. Some exosome subpopulations, as platelet-derived exosomes (high levels of CD9, CD63, CD81; low levels of protein binding annexin V), may mediate the multiple organ injury in sepsis. It has been demonstrated that platelet-derived exosomes isolated from septic patients display an increased NADPH oxidase activity, which induces oxidative stress and triggers apoptosis of vascular cells (34, 84). In fact, platelet-derived exosomes of septic patients cause contractile dysfunction of isolated rabbit hearts through peroxynitrite generation (35). Interestingly, the inhibition of both exosomes biogenesis and release with specific inhibitor (GW4869), at 12h after the experimental induction of sepsis, has prevented cardiac injury and promoted the survival of treated mice (85). It is conceivable that GW4869 inhibits the release of platelet-derived exosomes, yet further investigations are required.
On the contrary, MSCs-derived exosomes delivering miR-223 protect cardiomyocytes against lipopolysaccharide (LPS) through inhibition of release of pro-inflammatory cytokines from macrophages and improve the survival in a murine model of polymicrobial sepsis induced by cecal ligation and puncture (86). In addition, specific exosomal miRNAs represent suggestive potential
21
biomarkers of sepsis. Serum high levels of exosomal miR-16, miR-17, miR-20a, miR-20b, miR-26a, or miR-26b increase in a time dependent manner in experimental sepsis (14). In the light of the above scientific evidences, it would be clinically relevant the future development of exosome removal during continuous hemofiltration in septic patients in order to minimize the exposure of the patients to toxic exosomes and to concentrate circulating protective exosomes in order to prevent multiple organ dysfunction. Actually, the up-regulation of miRNA-34a and the down-regulation of miR-15a, and -27a in plasma exosomes are helpful to identify which patients will develop septic shock with realistic accuracy (13).
Exosomes might be also effective to deliver antiseptic drugs or biological factors without affecting their bioavability (87). Interestingly, dendritic cells (DCs)- derived exosomes (CD80+, CD86+) may be filled with milk fat globule EGF factor 8 (MFGE8), a secretory protein necessary to opsonize apoptotic cells for phagocytosis, usually downregulated in experimental sepsis (88) and in patients with coronary heart disease (89). In vivo, the administration of DCs-derived exosomes enriched with MFGE8 determines a reduction of inflammatory response, and improves survival (89).
Limitations of exosomes isolation protocols
The rapid development of molecular diagnostic platforms is contributing to diagnostic and therapeutic research on exosomes in critical illness but also to clinical service in ICUs. Since exosomes represent a new type of circulating biomarker and an effective vehicle of therapeutics, the methods used to isolate them from various body fluids is a critical issue nowadays. Differential centrifugation coupled with ultracentrifugation (UC) is the technique most frequently used in clinical laboratories (10, 17). However, this technique has some limitations, such as the time-consuming protocol, the risk of contamination of exosome pellet with non-exosomal proteins and
22
the operator-dependent yield (90, 91). In order to avoid these limitations, novel approaches have been proposed so far, such as the exosome isolation by the density gradient (87, 91) or the immune-affinity (33, 34, 92, 93) or the size-dependent method (94–97).
Kalra et al. (91) have demonstrated that the density gradient method is more effective than UC and immune-affinity purification. Unfortunately, the density gradient protocol is complex and time-consuming, so it cannot be used routinely for diagnosis in clinical setting. Conversely, this method is effective in the concentration of purified exosomes for therapeutics use since they may be stably stored over 90 days under different conditions before to be reinjected in the same patient (91). Ashcroft et al. (93) have developed a timeless method of exosomes isolation by adding specific antibodies directly in the plasma samples. However, this method is still not accurate for clinical use (93). On the contrary, the ultrafiltration of blood samples with subsequent liquid chromatography (UF-LC) is best suitable for clinical applications on a large amount of samples. In fact, the sample processing by UF-LC is less traumatic than UC in isolating theranostics exosomes, which remain intact and maintain their biophysical properties (97).
23
RATIONALE
Even though the cardiovascular diseases affect the outcome of surgical patients getting older, the leading cause of morbidity and mortality after cardiac surgery remains the perioperative myocardial infarction (PMI) (98). To date, the development of novel approach to improve the clinical outcome of anesthetized patients exposed to myocardial ischemia/reperfusion (I/R) insult due to prolonged cardiopulmonary bypass (CPB) is still a desirable achievement.
The anesthesiologists play a critical role in the design of new strategies to prevent the loss of cardiomyocytes, contractile dysfunction and poorer prognosis even after minimally invasive cardiac surgery (99). Several studies have demonstrated that volatile anesthetics prevent cardiac I/R injury in various experimental settings, yet the heart is already arrested by cardioplegic solution (100, 101). Interestingly, the cardioprotection by halogenated ether (i.e.: sevoflurane) is independent of the type of blood cardioplegia used (102). Halogenated ethers maintain the depth of anesthesia and exert negative inotropism in a dose dependent manner. In the 1997s, it has been shown in rabbits that the cardioprotection of halothane, toxic halogenated ether, is independent of its hemodynamic effects (103). The volatile anesthetics attenuate the cardiac oxygen consumption (104–106) and the surgical activation of inflammation as well (107, 108), but it is conceivable that they promote the dose-dependent release of circulating factors protecting the heart at cellular level. Although minimally invasive cardiac surgery leads to less expression of inflammatory cytokines, some studies demonstrated that the inhalation of anesthetic dose of sevoflurane, safer and broadly used halogenated ether, reduces the systemic inflammatory response without reducing postoperative markers of myocardial injury after CPB (109, 110). Conversely, a few clinical studies have demonstrated that sevoflurane exert cardioprotection
24
higher than intravenous anesthetics (i.e.: propofol) (111, 112). Even though the dose-dependent benefits of sevoflurane during cardiac surgery are still debated, the abovementioned studies have demonstrated that they are not mediated by inflammatory cytokines.
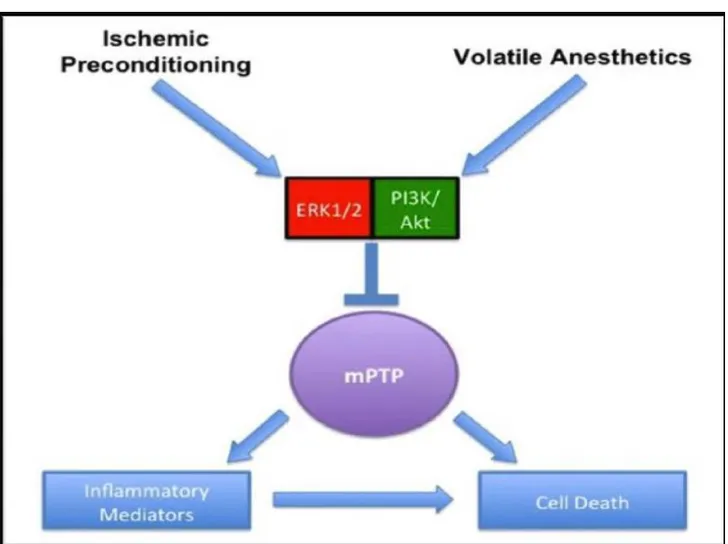
Despite the lacking of mechanisms and a few underpowered clinical evidences (113, 114), 340 cardiac anesthesiologists, cardiac surgeons and cardiologists from 65 countries, attending the first international consensus conference on cardiac anesthesia, recommended the use of general volatile anesthetics to preserve adult heart during cardiac surgery (115, 116). However, further mechanistic studies are mandatory to better assess the dose, the route and the timing of sevoflurane administration during cardiac surgery, and to better manage post-operatory recovery. The reduction of lethal cardiac events in patients undergoing CPB is related to myocardial expression of protective genes, as detected in atrial biopsies after sevoflurane administration (i.e.: low PECAM-1, high catalase) (117). Nevertheless, the mechanisms modulating sevoflurane-induced gene expression are still unknown. To best of our knowledge, halogenated ethers activate pro-survival kinases similar to coronary ischemic preconditioning (Fig. 3).
25
Fig. 3: Common protective pathways elicited by ischemic preconditioning and volatile anesthetics.
Extracellular-signal kinases (ERK), phosphatidyl-inositide 3-kinases/Akt (PI3K/Akt), mitochondrial permeability transition pore (mPTP) (ALLEN et al. Medical Gas research 2012, 2-22).
Sevoflurane prevents cell death through the opening of the mitochondrial KATP channels following the activation by phosphorylation of different kinases (118, 119). However, the sarcolemmal, mitochondrial, and nuclear KATP channels of cardiomyocytes, are also the main targets of other anti-remodeling drugs (120), which protect the heart without increasing oxygen consumption (121). Recently, Olson et al. (122) have demonstrated that isoflurane, a halogenated ether, induces an acute up-regulation of microRNA(miR)-21, a short noncoding nucleotide sequence, which protects rodent cardiomyocytes through down-regulation of programmed cell death protein 4. This study supports the hypothesis that sevoflurane post-transcriptionally modulates the
26
expression of protective genes in cardiomyocytes of arrested and non-perfused heart through systemic signals.
It is still unknown whether paracrine factors released by other cells, such as endothelial cells, mediate remote conditioning on cardiomyocytes during CPB by intravascular sevoflurane.
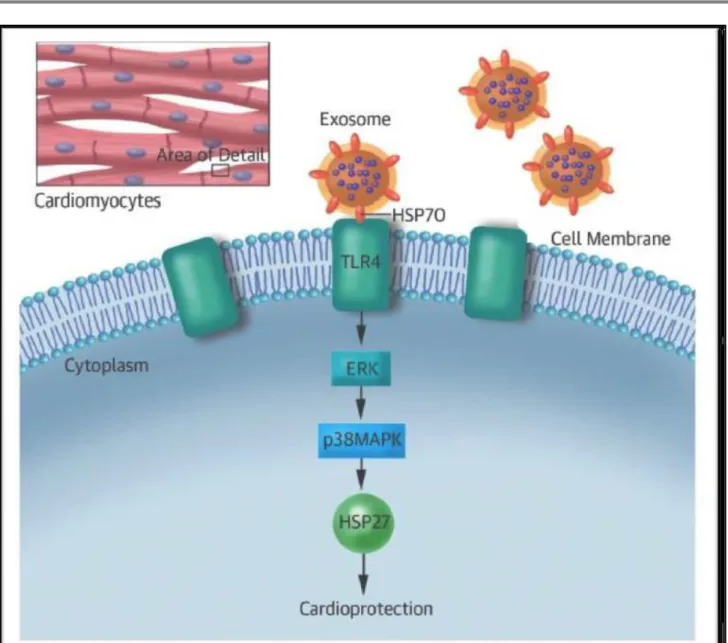
In 2014, Giricz et al. (123) have demonstrated that remote ischemic preconditioning increases the release of cardiac exosomes, very small cup-shaped extracellular microvesicles, which prevent cardiac ischemic injury. Exosomes are stored in the endosomal compartments of cells and released when their lipid bilayer merges with the plasma membrane acting as remote paracrine mediators of intercellular communication (38, 124). Exosomes show a typical antigenic profile and can simultaneously act different pathways. Protein ligands in the membrane of the exosomes may activate G-protein coupled receptors (GPCR) and downstream pro-survival signaling pathways (RISK, reperfusion injury signaling kinase) in recipient cells; although, circulating exosomes may also deliver activated GPCR into the recipient cells to trigger phosphorylation and transcriptional factors or miRNAs to regulate gene expression (60). Circulating exosomes may deliver also endogenous anti-ischemic signals to the cardiomyocytes, such as heat shock proteins (HSPs) (125). As shown in Figure 4, HSP70 stimulates toll-like receptor 4 (TLR4) leading to activation of ERK1/2 - p38MAPK axis and HSP27 by phosphorylation. Interestingly, kinase pathways activated by exosomes are also involved in ischemic preconditioning (125–127). It is conceivable that exosomes are the key paracrine mediators of cardioprotection induced by sevoflurane.
27
Fig. 4: Pathways of exosome-dependent cardioprotective signals mediated by surface heat shock protein (HSP) 70. Extracellular-signal kinases (ERK), P38 mitogen-activated protein kinases (p38MAPK), heat shock protein 27 (HSP27). (Vicencio et al. J Am Coll Cardiol 2015, 65 1525-36).
Recently, Barile et al. (4) have shown that exosomes released from cardiac progenitor cells contain anti-apoptotic (miR-210) and pro-angiogenic (miR-132) microRNAs, which are taken up by cardiomyocytes. The intramyocardial delivery of high dose of exosomes containing miR-210 prevented ischemic heart failure. In addition, exosomes released by fibroblasts deliver miR-21 into cardiomyocytes (128). To date, however, it is still unknown the role of exosomes released by
28
endothelial cells in response to potential cardioprotective drugs, such as sevoflurane. We cannot exclude that endothelial cells exposed to higher dose of intravascular sevoflurane release large amount of exosomes, which can deliver miR-210 and miR-21 into cardiomyocytes exposed to I/R insult and target pro-survival pathways.
29
PROJECT AIMS
1. To evaluate the effects of Sevoflurane or Propofol in modulating the profile of circulating biomarkers of myocardial injury in patients undergoing on-pump minimally invasive mitral valve surgery.
2. To evaluate the effects of Sevoflurane or Propofol in modulating the profile of circulating exosomes in patients undergoing on-pump minimally invasive mitral valve surgery.
30
AIM 1: CLINICAL STUDY
Ethical Approval
The trial MINI-SEVO was registered with Clinical Trials.gov (NCT02551328). The study protocol was approved by the Area Vasta Toscana Ethics Committee (reference CEAVNO no. 452/14) and
conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent prior to study participation.
Trial Design
The MINI-SEVO was a single-center, single-blind, randomized controlled trial. Participants were allocated randomly to propofol or sevoflurane anesthetic regimens using a 1:1 ratio.
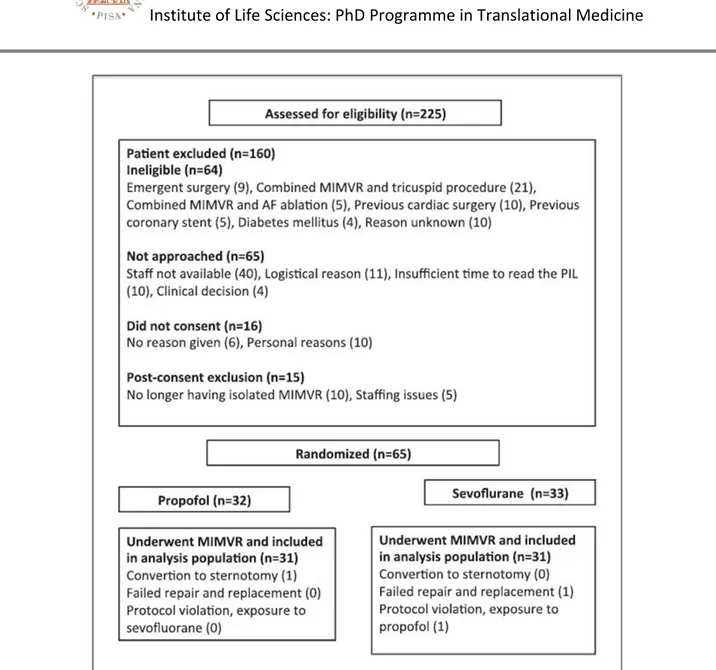
Between March 2015 and December 2016, 225 patients were assessed for study inclusion; of these, 160 did not meet the inclusion/ exclusion criteria and were excluded. After randomization, 3 patients were excluded due to: concomitant tricuspid surgery and AF ablation, protocol violations (1 patient allocated to the sevoflurane group received propofol), conversion to sternotomy. So, 62 patients undergoing to minimally invasive mitral valve repair or replacement (MIMV) via right mini thoracotomy were recruited and prospectively followed at Fondazione Toscana Gabriele Monasterio (FTGM)/Ospedale del Cuore (OC) (Fig. 5).
31
Fig. 5: Flow of patient enrollment. Abbreviations: AF, atrial fibrillation; MIMVR, minimally invasive mitral valve repair; PIL patient information leaflet.
Inclusion and Exclusion Criteria
Inclusion criteria were:
- Age > 18 years.
- Patients undergoing elective first-time MIMV. - Informed consent.
32 Exclusion criteria were:
- Cardiogenic shock or cardiac arrest.
- Previously treated coronary artery disease (i.e. history of coronary stenting or coronary artery bypass grafting).
- Renal failure (with a GFR <30 ml/min/1.73m2). - Surgical complications (i.e. sternotomy).
- Documented allergy to propofol or sevoflurane.
- Inability or refusal to provide written informed consent.
Surgical Technique
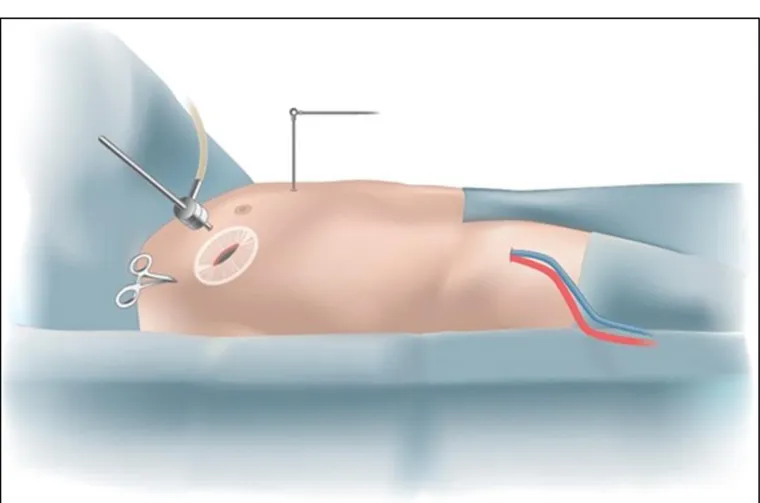
Minimally invasive mitral valve repair or replacement (MIMV) via right mini thoracotomy consists of a 10 cm incision made through the right side of the chest between the ribs. A central aortic cannulation was performed, and venous drainage was achieved by percutaneous cannulation of the right femoral vein (Fig. 6). A small, 5-cm incision was made at the level of the fourth intercostal space (Fig. 7). Two ports were used for camera insertion, cardiotomy venting, CO2 insufflation, and other pericardial stay sutures. After CPB, the aorta was clamped under direct supervision and cold crystalloid cardioplegia (Custodiol HTK Solution, Dr.Franz köhler chemie, Bensheim, Germany) with single antegrade delivery (25 mL/kg) was used and repeated if ischemic time exceeded 60 minutes; CPB was carried out with roller pump in all cases with normo-or slightly hypothermic (35°C) temperature with an average flow of2.4L/min/m2. The mitral valve then was exposed through a left paraseptal atriotomy. Mitral repair was performed in accordance with the mechanism of regurgitation.
33
Fig. 6: Heart-lung bypass representation. Heart-lung bypass is instituted with small tubes placed in the femoral artery and vein of the right leg. The heart is then stopped and the left atrium is opened to expose the mitral valve (internet image).
34
Fig. 7: Minimally invasive mitral valve repair or replacement (MIMV) plus/minus tricuspid valve repair via right mini thoracotomy. MIMV consists of a 10 cm incision made through the right side of the chest between the ribs (internet image).
35
Outcomes
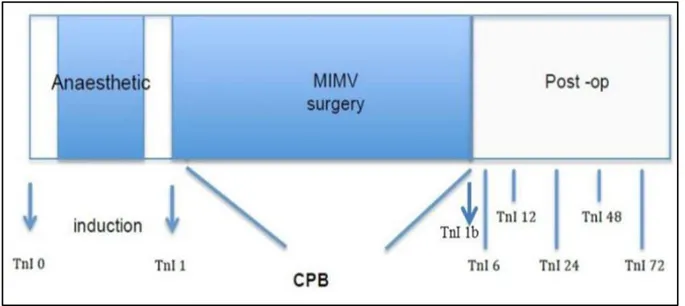
The primary outcome is myocardial injury, assessed by measuring myocardial Troponin I and circulating exosomes from venous blood samples which I collected pre-operatively (baseline), before CPB, before cardiac reperfusion and 6, 12, 24, 48 and 72h after cardiac reperfusion. In total 8 blood samples will be collected from each patient (Fig. 8). The institutional laboratory quantified cTnI by immune assay (Cobas 6000 analyzer series, Roche Diagnostics).
Fig. 8: Flow of blood samples. Troponin I (TnI)
Secondary outcomes were systemic metabolic stress assessed by:
blood pH,
lactate,
serum creatinine level,
mechanical ventilation time,
length of ICU stay,
36
Blood samples for measuring lactate and pH were taken before CPB, during CPB (at 20 and 40 min), and at the end of surgery. Blood samples for measuring serum creatinine were taken at 6, 24, 48, and 72 hours after surgery. Left ventricular function was assessed preoperatively and between days 4 and 6 post-operatively. Examinations were performed using the Philips Health-care iE33 echocardiography to quantify left ventricular function.
Sample Size
Cardiac troponin was measured the day before surgery (baseline) and 6 times after exposure to the anesthetic regimen; considering an average pre-post correlation of 0.3 and an average post-correlation of 0.5, I calculated a sample size of 60 (30 patients in each group) (129). With analyses of variance and covariance, this sample size provided 90% power to detect a standardized difference in serum markers of 0.43 between groups providing there was no interaction between group and pathology. If there was an interaction, a sample size of 60 had 80% power to detect a standardized difference in serum markers of 0.55 between groups.
Randomization and Blinding
Random allocations were generated by computer software. Treatment allocations were blocked with (a) varying block sizes to ensure approximate balance in the number of participants allocated to each group; (b) generated prior tothe study; and (c) accessed using a secure, Internet-based randomization system to guarantee concealment until each participant’s identity and eligibility was confirmed and documented securely. A designated research nurse who was not involved in data collection performed patient randomization after acquiring written consent and as close as possible to the operation date. Participants were blinded to the intervention, given that the
37
intervention was performed in the operatory theatre and there were no visible signs of the anesthetic regimens. Operating staff were not blinded to treatment, given the nature of the study.
Statistical Analysis
The assumption of normality of each variable distribution was tested with the Shapiro–Wilk test. Normally distributed variables are reported as the mean ± standard deviation or median and interquartile range (IQR). Categorical variables are reported as the percentage. Continuous outcomes are summarized and presented graphically as geometric means and standard errors; a natural logarithmic transformation was applied to the data to normalized distributions. Categorical data are summarized as the number (percentage). Differences between continuous variables were explored with Student test (unpaired t-test); differences between frequencies were compared with the χ2 test. The cTnI and other markers were analyzed by fitting multilevel mixed effect linear regression models (continuous variable measured at different time points). Model validity was checked and poor fit was addressed by exploring transformations. Outcomes analyzed on a logarithmic scale were transformed back to the original scale after the analysis, and results were presented as geometric mean ratios (GMRs). Likelihood ratio tests were used to determine statistical significance. The effect of cross-clamp time on cTnI release was explored with a Pearson’s correlation analysis. No subgroup analyses were planned. The trial is not powered to detect differences in clinical outcomes; therefore, frequencies are tabulated descriptively. The threshold for statistical significance was p < 0.05. All analyses were performed using R-project (RCoreTeam, Vienna, Austria; http://www.R-project.org/).
38
Interventions
Beta-adrenergic antagonists and other relevant medications were continued until the morning of surgery in all patients. In the operating room, patients were monitored with 5-lead electrocardiography, left radial artery catheter, capnography, pulse oximetry, blood findings, and rectal/urine bladder temperatures. Transesophageal echocardiography was used in all patients. In the group randomized to sevoflurane (Sevoflurane, Abbott), anesthesia was induced with intravenous sufentanil (0.5-1 mg/kg; Hameln Pharmaceuticals, Hameln, Germany) and midazolam (0.08-0.2mg/kg; Mayrhofer Pharmazeutika, Linz, Austria). Tracheal intubation was facilitated with intravenous rocuronium (0.6-1mg/kg; Pharmadox Healthcare, Malta). Anesthesia was maintained with sevoflurane at a minimum end-tidal concentration of at least 1 minimal alveolar concentration (MAC) throughout the entire procedure, including cardiopulmonarybypass (CPB) and endovenous sufentanil (0.01-0.02 mg/kg/min). During CPB, sevoflurane was administered in the oxygenator circuit through a calibrated vaporizer, and MAC was measured at the outlet of the oxygenator of the extracorporeal circulation. Patients of this group received no propofol during surgery or during intensive care unit (ICU) stay. In the group randomized to propofol (Diprivan, Astra-Zeneca, Stockholm, Sweden), anesthesia was induced with intravenous sufentanil (0.5-1 mg/kg) and propofol (2mg/kg). Tracheal intubation was facilitated with intravenous rocuroium (0.6-1mg/kg). Anesthesia was maintained with propofol (0.1-0.5 mg/kg/min) and sufentanil (0.01-0.02 mg/kg/min). No volatile anesthetic was used at any time during the procedure. In both groups, depth of anesthesia was monitored with bispectral index (BISXP,Aspect Medical System, Newton, MA); the dosage of propofol and sevoflurane (within the above ranges) was titrated to maintain BIS values from 40 to 60. Postoperatively, the inspired oxygen fraction was set at 0.5 and
39
the positive end-expiratory pressure at 5 cm H2O. Sedation in the ICU was continued for 3 to 4 hours, with propofol in the propofol group and boluses of midazolam if required in the sevoflurane group. After 3 to 4 hours, if partial pressure of oxygen/fraction of inspired oxygen was above 200, PaCO2 between 35 and45mmHg, and bleeding from chest tubes o1 mL/kg, sedation was stopped. Then, extubation was performed if patients’ cardio-circulatory, respiratory (partial pressure of oxygen /FiO2 < 250, respiratory rate 425/min, FiO2 40%-50%), and neurologic criteria were within range. Criteria for intensive care discharge were hemodynamic stability with no inotropic support, spontaneous breathing, and clear neurologic status.
Results
Baseline Characteristics
The mean age of the population was 64.7 ± 11.9 years, and 28 of 62 patients (45.1%) were male. Patients allocated to the propofol group were older than those allocated to the sevoflurane group (68.3 ± 9.5 years vs 60.7 ± 12.5 years, respectively, p = 0.03) and had a higher creatinine level (0.89 ± 0.2 mg/dL vs 0.78 ± 0.2 mg/dL, p = 0.03).
The median logistic EuroSCORE was 3.7 (IQR, 5), with no significant differences between groups (p = 0.07). Other base line characteristics and mitral valve regurgitation values are summarized in Table below (Table 3).
40
Table 3: Patient characterization and clinical parameters
Values are presented as median (interquartile range), mean ± standard deviation, or n(%). There were no missing data.
Abbreviations: CV, cardiovascular; LV, leftventricle; NYHA, New York Heart Association classification; TIA, transient ischemic attack. (Moscarelli M., Terrasini N. J Cardiothorac Vasc Anesth. 2018 Dec;32(6):2562-2569).
41 Primary Outcome
Time courses of cTn I values are shown in Figure 9A. Preoperative concentrations of cTnI were undetectable in both treatment groups (< 0.01 ng/mL). Concentrations of cTnI were increased postoperatively and peaked at 6hours after surgery; these values were, on average, 8% lower in the propofol group compared to the sevoflurane group, but this difference was not statistically significant (GMR 0.92; 95% confidence interval 0.63-1.27, p = 0.62). Given the difference in age and baseline creatinine in the two groups, the multilevel mixed effect linear regression model was adjusted considering separately the two variables age and creatinine.
There were no statistically significant effects of the two variables in the variation of TnI (primary outcome) between the 2 groups: age-adjusted P value is 0.73 and creatinine-adjusted P value is 0.43. Besides, the multilevel mixed effect linear regression model was adjusted considering also the variable gender and there is no statistically significant effects in the variation of TnI; Gender-adjusted Pvalue 0.44.
Moreover, Peak cTnI release occurring at 6 and 12 hours after surgery was correlated positively with cross-clamp time in both groups (p = 0.001) (Fig. 10 and 11).
42
Figure 9: Primary Outcomes. Geometric means and 95% confidence intervals (CIs) are shown for different postoperative time points by group. Geometric mean ratios (GMRs) and 95% Cis for propofol versus
sevoflurane are also shown. Data are expressed as the mean and standard deviation (SD) at each study time point by group and the mean difference (MD) and 95% CI for propofol vs sevo. (Moscarelli M., Terrasini N. J Cardiothorac Vasc Anesth. 2018 Dec;32(6):2562-2569).
Fig 10: Correlation between cross-clamp time and troponin I concentration at 6 hours post-surgery. Propofol (blue), adjusted R2 value 0.43, correlation 0.67, p = 0.001; sevoflurane (red), adjusted R2 value 0.34,
correlation 0.6, p = 0.001. (Moscarelli M., Terrasini N. J Cardiothorac Vasc Anesth. 2018 Dec;32(6):2562-2569).
43
Fig 11: Correlation between cross-clamp time and troponin I concentration at 12 hours post-surgery. Propofol (blue), adjusted R2 value 0.35, correlation 0.61, p = 0.001; sevoflurane (red), adjusted R2 value 0.34, correlation 0.61, p = 0.001. (Moscarelli M., Terrasini N. J Cardiothorac Vasc Anesth. 2018
44 Operative Data
Total CPB times and cross-clamp times were similar between the propofol and sevoflurane groups (CPB: 139.7 ± 40.5 min vs 141.2 ± 38.7 min, p = 0.55, cross clamp, p = 0.33, respectively). All patients received cold crystalloid antegrade cardioplegia. The average sevoflurane MAC was 1.2 ± 0.2. The average propofol infusion was 2.6 ± 0.7 mg/kg. Except for 1 patient, mitral valve repair was successful in all cases with no residual regurgitation on intraoperative transesophageal echocardiography. The type of rings implanted and repair techniques used are summarized in Table 4.
Table 4: Intra-operative parameters of patients
Values are presented as median (interquartile range), mean ± standard deviation, or n (%). There were no missing data.
* Patients may have more than 1 technique. ♮ One patient had mitral valve replacement with mechanical
prosthesis.
Postoperative Outcomes
Postoperative blood pH, lactate, and serum creatinine tended to be lower in the sevoflurane group compared to the propofol group (blood pH: mean difference 0.02, 95%CI -0.02 to 0.04, p = 0.19; lactate: GMR 1.12, 95%CI 0.92-1.23, p = 0.68; serum creatinine: GMR 1.13, 95% CI 1-1.19, p = 0.33) as shown in Figure 8 B, C e D. Mechanical ventilation time was lower in the sevoflurane group compared to the propofol group (median 497 minutes [IQR,460] v 589 minutes[285],
45
respectively; p = 0.05), whereas ICU stay and total length of stay were similar between the propofol and sevoflurane groups (ICU stay: mean1186 ± 176 v 1215 ± 122 minutes, p = 0.19); total length of stay: median 8days [IQR,3] v 8 days [IQR,2], p = 1) (Table 5). Mean pre-and postoperative left ventricle ejection fractions were 58.2% ± 6.8% and 50% ± 5.7% (p = 0.72) in the propofol group and 59% ± 7.9% and 50% ± 10.3% (p = 0.32) in the sevoflurane group, respectively.
Table 5: Post-operative parameters of patients
Values are presented as median (interquartile range), mean ± standard deviation, o rn (%).
Abbreviations: AV, atrioventricular; ICU, intensive care unit; LOS, length of stay; MI, myocardial infarction; TIA, transient ischemic attack.
Postoperative Complications
There were no in-hospital deaths. One patient in the sevoflurane group underwent reoperation for bleeding, but conversion to sternotomy was not necessary. Other event rates were similar between the 2 treatment groups (Table 5). All patients were discharged with no or negligible residual mitral regurgitation. No unexpected serious adverse events occurred.
46
Limitations
This study had some limitations. First, this research was a proof-of-concept analysis that primarily was based on troponin release. For practical reasons, participants, but not physicians and medical staff, were blinded to treatment group assignments. Second, this trial did not include a third arm to examine synergy between sevoflurane and propofol, nor were the dose-dependent effects of propofol and sevoflurane investigated (130); instead, the study design corresponded with routine anesthetic practice and evaluated potential differences between 2 standard regimens. Third, this trial included relatively low-risk patients, which may limit its generalizability to larger patient populations; there was also a certain level of heterogeneity between groups because patients randomized to propofol were, by chance, older, which might have influenced the results. It was observed a general low troponin release after mitral repair in both groups; such limited biomarker release might have affected the possibility of detecting differences in myocardial injury between the 2 different treatments.
Conclusion
The present study found that both anesthetic regimens were associated with similar degrees of myocardial injury. Bignami et al. compared propofol and sevoflurane in terms of cardiac troponin release in patients with coronary disease undergoing mitral surgery, (131) and Landoni et al. investigated the effects of desflurane versus propofol in patients undergoing mitral surgery (132); however, neither study clarified whether volatile anesthesia was superior to intravenous anesthesia for mitral valve repair. In the latter study, subjects with concomitant coronary disease were not excluded, and more over desflurane was used only as a preconditioning agent for 30 minutes. The vast majority of previous studies on anesthetic preconditioning were conducted in
47
the context of coronary bypass grafting, and very few investigations have focused on isolated valve repair (133). Additionally, investigations performed in patients with coronary disease failed to control for several confounding factors, including diabetes and related medication use, previous angina, hibernating myocardium, coronary microembolization, no-reflow, and hypercholesterolemia (134). Accordingly, these studies have yielded inconsistent results. I believed that patients with isolated valve disease such as degenerative mitral valve disease provide an ideal scenario for testing the abilities of potential cardioprotective agents to prevent myocardial damage. Furthermore, organic mitral valve disease has a lower association with coronary disease than aortic valve disease, which is associated with hypertension, diabetes, and dyslipidemia (135). It can be argued that different forms of anesthetic preconditioning (remote ischemic, volatile) do not add additional protection against cardioplegic arrest in the context of bypass grafting because patients with coronary disease may be naturally preconditioned already. In the present study, the authors were unable to demonstrate superiority of one anesthetic regimen over another for decreasing cTnI release in patients who underwent isolated mitral valve repair; patients in the propofol group tended to have lower cTnI release, but this difference was not statistically significant. These findings are in contrast with the results reported by a recent trial on anesthetics preconditioning in valve surgery, where the group randomized to sevoflurane exhibited lower troponin release than the propofol group (136). However, in the aforementioned study, a heterogeneous population was compared (eg, aortic, mitral, tricuspid disease), with different surgical techniques (valve replacement, tricuspid reshaping) with no mitral valve repair procedures. An important strength of this study was that patients with combined coronary disease were not included, ensuring no previous exposure to angina or other natural ischemic
48
preconditioning. Another important consideration is that in the propofol group, propofol infusion was continued in the ICU and may have provided postconditioning, whereas sevoflurane was not. There is some evidence that the preconditioning effect of sevoflurane is enhanced when treatment is maintained during the first 6hours after surgery (137),yet the most uniform cardioprotective effects are seen when sevoflurane is administered for the duration of the procedure (114). Finally, sufentanil was used in both groups; opioids produce cardioprotection against ischemia-reperfusion injury in rodents in a manner potentially related to adenosine receptor cross-talk (138).
49
AIM 2: ISOLATION AND CHARACTERIZATION OF CIRCULATING EXOSOMES
IN PATIENTS DURING THE CLINICAL STUDY
As a second aim of my study, I also purified the exosome-rich fraction from the plasma of 24 patients undergoing on-pump minimally invasive mitral valve surgery, using a standard protocol consisting of serial, differential centrifugation and ultracentrifugation steps. The amount of exosomes was inferred by protein quantification using a standard bicinchoninic acid assay (BCA) and the expression of the exosomal markers CD63 and CD81 on the isolated vesicles was validated by Western blotting (Fig. 12).
Fig 12: Exosomes isolation and characterization. Kilodalton (KDa), CD63 antigen (CD63).
50
Methods
Sample collection (pilot study)
This is a pilot study conducted in order to evaluate feasibility, time, cost, adverse events, and to improve the study design prior to perform of a full-scale research project. According to Connelly (139), extant literature suggests that a pilot study sample should be 10% of the sample projected for the larger study. For this reason, for the aims of this pilot study I employed blood samples obtained from 10% of the patients recruited in the MINI-SEVO clinical study. I used the blood samples of the first 12 patients in each group (for a total of 24 patients). Blood samples were collected pre-operatively (baseline), before CPB, before cardiac reperfusion and 6, 12, 24, 48 and 72h after cardiac reperfusion (Fig. 8).
Exosomes Isolation from plasma samples
An optimal method for the isolation and purification of exosomes has not yet been defined although a standardization of transport, isolation and analysis protocols is necessary to facilitate the comparison of the results obtained in different laboratories (140). It is very important to be sure to isolate contaminant-free exosomes because contamination with extracellular vesicles with different properties can alter the results of the experiments (141). The main approaches for the isolation of exosomes are differential centrifugation, sucrose gradient, microfiltration, antibody-coated magnetic beads, ExoQuick (142). Differential centrifugation is considered the gold standard, and is the most used method to isolate exosomes from biological fluids and from conditioned culture medium. Although several protocols exist, it generally consists of multiple steps: it starts with a low spin centrifugation (300g for 10 min) which eliminates dead cells and larger apoptotic bodies, followed by higher spin centrifugations, which varies from laboratory to
51
laboratory, from 1,000g to 20,000g and eliminates larger extracellular vesicles and cell fragments. A final 100.000g ultracentrifugation precipitates the exosomes (142).
In the current study, the blood samples were centrifuged at 2,600 g for 10 min at 4°C to obtain plasma, followed by centrifugation at 10,000 g for 30 min at 4°C to remove remnant cells and platelets as well as the larger extracellular vesicles. The exosomes were then isolated by ultracentrifugation at 100,000 g for 3 h at 4°C, washed in PBS and lysed in RIPA buffer to obtain a total protein extract. The RIPA buffer (radioimmunoprecipitation assay buffer) was prepared with150 mM sodium chloride, 1.0% NP-40 or Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS (sodium dodecyl sulfate), 50 mM Tris, pH 8.0, protease inhibitors.
BCA assay
The bicinchoninic acid assay (BCA assay), also known as the Smith assay, after its inventor, Paul K. Smith at the Pierce Chemical Company (143) is a biochemical assay for determining the total concentration of protein in a solution (0.5 μg/mL to 1.5 mg/mL), similar to Lowry protein assay, Bradford protein assay or biuret reagent. The total protein concentration is exhibited by a color change of the sample solution from green to purple in proportion to protein concentration, which can then be measured using colorimetric techniques (Fig. 13A and 13B).In the current study, BCA was performed using the Pierce BCA Protein Assay Kit (Thermo Scientific) and following the manufacturer instructions.
52
Fig 13A: BCA assay computing. It is performed in order to determine the total concentration of protein in a solution.
Fig 13B: BCA assay. The total protein concentration is exhibited by a color change of the sample solution from green to purple in proportion to protein concentration.