ContentslistsavailableatScienceDirect
Health
Policy
and
Technology
journalhomepage:www.elsevier.com/locate/hlpt
Original
Article/Research
Effective
tools
to
manage
biosimilars
prescription:
The
Italian
experience
Elisa
Guidotti
a ,∗,
Bruna
Vinci
b,
Francesco
Attanasio
c,
Federico
Vola
aa Management and Health Laboratory, Institute of Management and Department EMbeDS, Scuola Superiore Sant’Anna, Pisa, Italy
b Management and Health Laboratory, Institute of Management and Department EMbeDS, Scuola Superiore Sant’Anna; SSFO, Scuola Specializzazione
Farmacia Ospedaliera, Università di Pisa, Pisa, Italy
c Drugs and Appropriateness Policy Sector, Tuscany Region, Florence, Italy
a
r
t
i
c
l
e
i
n
f
o
Article history: Available online xxx Keywords: Biosimilar Uptake Italy Policy Toola
b
s
t
r
a
c
t
Introduction: Biosimilarsrepresentbothaclinicaland afinancialopportunity.However,lowbiosimilar penetrationhasbeenobservedacrossEurope.Italyhasregisteredahighandincreasingbiosimilars up-take,butnotuniformacrossregions.
Objectives: Thepaperaimstodescribedifferentmanagerialtoolsthatcan beleveragedtoexploitthe biosimilars’potential.A focusonItalyand theTuscanyregionwillbe specificallycarried out. Further-more,this researchis intended to proposesomepreliminary considerations about the mosteffective measurestosupportbiosimilars’uptake.
Methods: Thisarticleisbasedonbothdeskresearchandonactionresearch.Adeskresearchwascarried outtoexploretheexistinginternational and nationalliterature onthe measuresimplementedto pro-motebiosimilars’penetration.Theactionresearchconcernedalongitudinalcooperationprojectbetween theManagementandHealthLaboratoryofScuolaSuperioreSant’AnnaofPisaandtheDrugsand Appro-priatenessPolicySectorofTuscanyRegion.TheactivitysawthedirectinvolvementoftheManagement and HealthLaboratory insupporting theregional pharmaceuticalgovernanceinmanaging biosimilars’ penetration.
Results: Severaldemandandsupplysidepoliciestofosterbiosimilarpenetrationhavebeenimplemented bothatnationaland regionallevels.InItaly,mostregions haveadopted specificbiosimilar policiesat differentmomentsandwithheterogeneouscontents.TheTuscanRegionalHealthSystemimplemented threemainmeasures:formaltop-down,bottom-upand“mixed” measures.Thestrategywaseffectivein increasingbiosimilaruptake.Thesuccessisprobablyassociatedwithitscomprehensiveapproachandto thecoherenceofthedifferentmeasuresitwascomposedof.
© 2020FellowshipofPostgraduateMedicine.PublishedbyElsevierLtd.Allrightsreserved.
1. Background
1.1. TheItaliannationalhealthsystemandpharmaceuticalcare The Italian National Health System(INHS) was established in 1978by following aBeveridge model,withprovision ofuniversal coveragelargelyfreeofchargeatthepointofservice.
TheINHSisinchargeofprovidingpharmaceuticalcareand ac-counts forthe largestpart oftotal pharmaceuticalspending,
dis-∗Corresponding author at: Scuola Superiore Sant’Anna, Piazza Martiri della Lib-
ertà 33, 56127 Pisa Italy.
E-mail address: [email protected] (E. Guidotti).
pensing drugs eitherthrough hospital orcommunitypharmacies. [1] .
Sincetheearly 1990s,legislativereformshavegradually trans-ferred political, administrative,fiscal andfinancial responsibilities regarding the provision of healthcare from the national govern-menttothetwenty Italianregions[2] .TheINHSistherefore cur-rentlyorganisedandgovernedaccordingtothreeinstitutional lev-els:national,regionalandlocal.Italianregionalhealthsystemsare progressively levelling out, but significant heterogeneity emerges inthequalityofcarethey provide,thelevelofhealthcare expen-ditureandtheirfinancialperformance[3] .
Italy has beenharshly hit by the globaleconomic crisis since 2008. Coherently with its three layer-institutional architecture, Italyrespondedtothecrisis through:a)plansandother
interven-https://doi.org/10.1016/j.hlpt.2020.10.011
2211-8837/© 2020 Fellowship of Postgraduate Medicine. Published by Elsevier Ltd. All rights reserved.
tions devised by the centralgovernment; b) actionsjointly taken by thenational and regionallevels of government; andc) initia-tivesautonomouslyendorsedbyregions.
The economic crisis ledtheINHS topayeven closerattention to the pharmaceuticalsector and contributed to acceleratingthe reformprocess.Inparticular,atthenationallevel,threemaintools havebeenenvisagedtosupportappropriateness:a)thefixationof expenditure caps;b) the introduction ofweb-based “clinical reg-istries”;c)theadoptionofmanagedentryagreements.
At regional level, policies have been differently devised and implemented across regions, but they have generally entailed: strengthening the direct distribution of pharmaceutical products; centralizing the procurement process; and leveraging managerial tools(suchasbudgetingandpayforperformance),inorderto ori-entprescriptionstowardsoff-patentand/orlowercostmedicines.
Inparticular, reformswereaimed ataddressingthe challenges broughtby a dynamiccontext, characterizedby theentryofnew drugs,theexpenseofbiologicalmedicines,andthesubsequent ar-rivalofmoreaffordablebiosimilardrugs.
1.2. The“bio-opportunity”
1.2.1. Biologicalandbiosimilarmedicines
Italy wasthethirdmarket byvalueforbiologicalmedicines in Europe in2018 [4] .The consumption of biological medicines has beenconstantlygrowingacrossthecountryinthelastyears,thus, exertingsignificantpressureonnationalpharmaceuticalspending. Bythetimepatentportfoliosfororiginatorbiologicalmedicines were closetoexpiration, pharmaceuticalcompanieshavebrought severalbiosimilarmedicines– or“biosimilars” – tothemarket.
Biosimilarsrepresentbothaclinicalandafinancialopportunity. Evidenceacquiredovertenyearsofclinicalexperiencehas demon-strated thatbiosimilarmedicinesapprovedintheEuropeanUnion canbeusedforalltheirregisteredindicationsassafelyand effica-ciouslyastheirreferencebiologicalmedicines[5] .
In addition, asa result of patentexpiration and scaled-down market authorisation requirements, biosimilar medicines have been commercialised atlower prices than originators [6] . There-fore,biosimilarshavewidelybeenreckonedasasolidopportunity for healthcare systemsto jointly assure clinical effectiveness and financialsustainability[7] .
Despitethepotentialforincreasingpatientaccesstobiological medicinesandfinancialsustainability,todatelowbiosimilar pene-trationhasbeenobservedacrossEurope[8] andhighvariationsin biosimilars’uptakehasemergedamongdifferentcountries[9] .
Italy, compared to the EU5 countries, ranks among the coun-trieswiththehighestbiosimilarsuptake,withasteadilyincreasing trend [9] ,however biosimilars’penetration isnot uniformacross Italianregions[10] .
The present paper aims to shed some light on the different managerialtoolsthatcanbeleveragedtoexploitbiosimilars’ clini-calandeconomicpotentialanddrivetheirpenetration.Afocuson Italy andthe Tuscany regionwill be specificallycarried out. Tus-cany representsan interesting casestudyfor threemain reasons. First, Tuscany ranks amongthe best Italian regions in the provi-sion of healthcare services [11] . Second, in the last few years, a strong effortwasput inplace bythe regionalgovernment to re-formthegovernanceofdrugsandcontroltherelatedexpenditure [12] .Third,Tuscanycanbe consideredafirstmoverinthe imple-mentationofpoliciesorientedatfosteringbiosimilarspenetration. Thisresearchtherefore:(1)investigatesthepenetrationof biosim-ilarsintheTuscanRegionalHealthSystem(TRHS)in2017;(2) an-alyzesthe2018setofmanagerialtoolsadoptedbytheDrugs and Appropriateness Policy Sector in Tuscany to increase biosimilars’ penetration; (3) propose some preliminary considerations about themosteffectivemeasurestosupportbiosimilars’uptake.
2. Methods
This articleis based on both desk research andon action re-search.Concerningtheformer,an analysisoftheexisting interna-tionalandnationalliteratureonthemeasuresimplementedto pro-mote the penetration ofbiosimilars wasconducted. Furthermore, Italiannationalandregionalbiosimilarresolutionswere screened. Thedocumentsunderstudywerefoundthroughkeywordsearches onthemostpopularsearchengines.Thekeywordsusedwere, re-spectively:“biosimilar”,“policies”,“tools”,“penetration”,“uptake”.
The action research occurred within the multiannual cooper-ation project between the Management and Health Laboratory (MeS-Lab) of Scuola Superiore Sant’Anna of Pisa and the Drugs andAppropriateness PolicySectoroftheTRHS. MeS-Labhasbeen actively collaborating withthe regional healthcareadministration since2004.Thestudyistheresultofalongitudinalactionresearch process:theactivitycarried out withinthis collaborationin 2017 and2018 actually saw the direct involvement ofthe MeS-Lab in supporting the regional pharmaceutical governance in managing biosimilars’penetration.
Atleastthreekeyelementsdistinguishactionresearchfromthe otherempiricalapproaches:1)thecollectionofdataandresearch material that cannot be usually retrieved; 2) the active involve-ment of researchers in the design of the solution to an organi-sational problemfaced by the hostorganisation (typicallyjointly developed with the members of the organisation); 3) the evalu-ation of the jointly developed solutions, typically by teaming up withthemembersofthehostorganisationandbysupportingthe implementationofnewsolutions.Hence,organisationalchange(or atleastanattempttoaccomplishit)isanimportantoutputofthis kindofstudydesign[13] .
Our research was articulated accordingly in three steps. The first stepof the collaborationconsisted ofa quantitative analysis ofregionalpharmaceuticaladministrativeflowstodetectthemost relevant molecules in economic terms (total expenditure). Sec-ondly, thelevel ofbiosimilars’penetration forthesemoleculesin Tuscanyin2017wascomputed. Thiswasthepreconditionforthe TuscanDrugsandAppropriatenessPolicySectortodesignand im-plementa payforperformance system,addressedto theregional healthauthorities.MeS-Lab’sresearcherswere involvedin provid-ingbothdatatodefinethemostappropriatetargetsandexpertise forfine-tuningtheindividualgoals [14] .Inthefinal step,asetof indicatorsonthepenetrationoftheaforementionedmoleculeswas usedtobenchmark the2017-2018 trendofbiosimilarpenetration in Tuscany versus other regions (pre-, post- and cross-sectional comparison),so asto providea preliminary evaluationofthe ef-fectivenessofthedifferent2018Tuscangovernanceactionsandto suggestthemostappropriatemanagerialtoolstosupport biosimi-lars’penetration.DatawereprocessedusingSAS®,version9.4. 3. Results
3.1. Surfingthebio-wave:toolstofosterbiosimilars’prescriptions Europeangovernmentshavedesignedandimplementedspecific policiestoenhancetheuseofbiosimilars.Differencesexistinthe pricingandreimbursementprocedures,levelsofeducation, charac-teristicsofcoveredpopulation,andincentivisationofstakeholders (physicians,pharmacists orpatients), leadingto variationsin up-takeanddivergencesinsavingsfrombiosimilarsuseacrossEurope, andevenwithinthesamecountry[15 ,16] .
Byconsideringoneframework[17] ,policiesconcerning biosim-ilars can be divided into “demand-side” and “supply-side” ones. Demand-sidemeasures aim tosteer the prescription of biological drugstowardsthebiosimilaralternative,whilesupply-sidepolicies focusontheconditionsassociatedwithprovisionoftheproduct.
Among thedemand-sidepolicies,agroup ofmeasures deserve particular attention. Several countries have introduced prescrip-tion budgetsorquotas forbiosimilars.Astill littlediffusedpolicy concerning biosimilars is “automatic substitution”, that only few countries permit (Estonia, France,Latvia, Poland, Russia) [18] . Fi-nally,educationalpolicieshavespreadacrosscountries,suchasthe drafting of prescribing and clinical guidelines for physicians, the organisationofscientificconferences,seminarsandlecturesonthe theme ofbiosimilars to informdifferentstakeholders andtrigger biosimilarsadoption[15] .
Supply-sidepoliciesaremultipleandrangefromreference pric-ing – both internal and external (Italy adopts external reference pricing)– totendering.
Coherently with its double-layered institutional governance, Italy hasdevisedtwodifferentsets ofpoliciesatnational and re-gionallevels.Forthenationallevel,boththedemandsideand sup-plysidepolicieshavebeenrecentlyimplemented.
Regarding theformer, theItalian Medicines Agency(AIFA) has recently published two position papers on biosimilars, to inform healthcareprofessionalsandcitizensaboutbiologicandbiosimilar medicinesandtosupportinterchangeability[19–21]
As for national supply side policies, national pricing and re-imbursement policies provide that biosimilars are automatically placedinthesamereimbursementclassasthereferencemedicine when the price proposed by the company is of ‘obvious conve-nience’(withnopricenegotiationbetweentheCompanyandAIFA) andthat,whenthenegotiationoccurs,thepriceofabiosimilarhas tobesetatavalueatleast20%lowerthantheoriginator’s[22] .
Regions do not differ inprescription or indicationrestrictions concerning biologicalmedicines:they operateuniformly,by com-plyingwiththenationalguidelinesandtheindicationsofthe Ital-ian Medicines Agency(AIFA).Nevertheless,mostregions have re-centlyimplementedarangeofmanagerialtoolsspecificallyaimed atsteeringbiosimilars’uptake.Theywereadoptedatdifferent mo-ments and withheterogeneous content, both leveraging demand side and supply side measures. Campania Region, in November 2009, legislatedon theuseof biosimilars,pushing forthechoice ofbiosimilarsasfirsttreatmentfornewpatients.In2010, Veneto published theGuidelines forthe useand purchaseofbiosimilars [23] .Inline,theregionreleasedthreedecreesnumbered331/2015 [24] ,90/2017 [25] and112/2018 [26] aimed at improving knowl-edge on biosimilars andproviding informationabouttheir safety andefficacy.Since2011,FriuliVeneziaGiuliastartedimplementing multipleincentivesforbiosimilarsprescriptions.Specificprotocols were adoptedattheArea Vastalevel(i.e.theentityappointedto coordinateLHAs andTHs actionsinageographical area); further-more,prescriptionquotasfornewlydiagnosedpatientswerefixed ("drug naive") [27 ,28] . In2014, Basilicata,Calabria,Puglia and Si-ciliaRegionlegislatedontheimportance ofpreferringbiosimilars totheoriginatorsinnaïvepatientswheneconomicallyviable[29– 32] .
Coherently withthepreviously outlined institutionalstructure, theItaliannationallevelhasthereforedefinedtheframework con-cerningdemandside(leveraginginformativepolicies)andthe sup-ply side approaches(bysettingthelegislative framework).Regions haveheterogeneouslyinflectedinthenationalframeworkbybetter specifyingandcontextualizingthenationalindications(seefor in-stanceCampania’sfocusonnaïvepatients,ortheVeneto informa-tive decrees),or by complementing the national framework with managerialtools,such asincentives’schemesorprescription quo-tas(e.g.,FriuliVeneziaGiulia).
Assessingthecorrelationbetweentheadoptionofspecific poli-ciesandthepenetration levelsstillseemsprematureandrequires some caution.Some studies attemptto correlate the adoption of different nationalpolicies withdifferentlevels ofbiosimilars’ up-take. Several factors have been reported as potential drivers of
biosimilaruptakeandmightexplain thedifferencesobserved be-tween EU memberstates: i.e., physician andpatient adoption of biosimilars, national healthcare systems specificities in terms of pricing, reimbursement, and procurement policies [33] . Policies aimedatsolelygrantingpricereductionsdonotseemtostrongly favor biosimilars’ uptake[16 ,34] . Substitution was indicated as a key potential driver of biosimilar uptake, though this could be prevented if there are limited outcomes. Thus, the suggestion is to adoptandevaluatesuch a policy when strongbiosimilar real-world evidence is available [35] . Education initiatives (addressed tophysiciansandpatients),competition-drivenpricingpolicies,the introductionofincentivesorquotaswasrecommendedtoimprove biosimilars’uptake,althoughbasedonqualitativejudgmentsby se-lectedstakeholders[15] .
Rèmuzat and colleagues used a quantitative approach to demonstratethat:
• biosimilarpricediscount overoriginalbiologicprice, the num-berofanalogues,andthedistributionchannel werenot corre-latedwiththebiosimilaruptake;
• averagegenericpricediscount overoriginatorandthenumber ofbiosimilars show a trend toward statistical significance for correlationwithbiosimilaruptake,butdonotreachthe signif-icancethreshold;
• incentivepolicies and the date of first biosimilar market en-trywerecorrelatedwithbiosimilaruptake[17] ,althoughother studiessuggestedthatincentivesmightdifferentlyimpact prod-uctclasses[36] .
ConcerningItaly,ithasbeensuggestedthat thoseregionsthat firsthaveestablished policiesto promotetheentryofbiosimilars intothe therapeuticplanshaverecorded ahighpenetration rate; on the contrary, those regions that have drawn up late and un-focused policies seem to have experienced a low penetration of biosimilars[4] .
However, these findings refer to policies implemented at na-tional/regional level andrelated outcomes, disregarding the local context.Theydonotinquireabouttheeffectivenessofmanagerial strategies designedatlocal level; they miss local policy environ-ments;andtheydonotappropriately accountfortheinteractions amongthedifferentstakeholdersattheorganisationallevel[15] .
GiventhemultilevelorganisationoftheINHS[37] andthe rel-evanceofpoliciesadoptedatregionallevel,it isimportantto fo-cusontheanalysisofthosemanagerialtoolsregionshaveadopted tofosterbiosimilarpenetrationandtoconduct preliminary evalu-ationsoftheresultsreached.
3.2. FocusontheTuscanyregion
3.2.1. Analysisofregionalpharmaceuticaladministrativeflows The analysis considered patent-expired medicines whose biosimilar first marketing date occurred before December 2017. Specifically, Epoetin, Etanercept,Filgrastim, Follitropin alfa, Inflix-imab, Insulin Glargine, Rituximab, Somatropin were considered. Rituximab,Etanercept,SomatropinandEpoetinwerechosenas ob-jectsofthestudysincetheyemergedasthemostexpensiveforthe TuscanRegionalHealthSystem(TRHS)in2017.
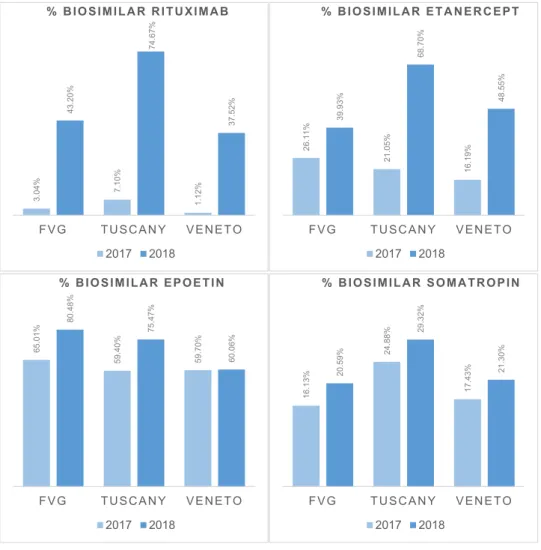
Highheterogeneityinbiosimilarpenetrationwasidentifiedfor these medicines in Tuscany in 2017. The percentage of biosim-ilar Rituximab was 7,10%, calculated as milligrams of biosimilar Rituximab administered in 2017 divided by total milligrams of Rituximab-baseddrugsadministeredthatyear.Inthespecificcase ofRituximab, the calculationwas carriedout in milligrams since someoftheadministrativeflowsdidnotpresentthelinkbetween thedrugadministeredandtheanonymised userID. Fortheother medicines, thechosen unit of calculationwasthe numberof pa-tients. The percentage of biosimilar Etanercept was 21,05%. The
Fig. 1. Inter-regional geographic variation - Friuli Venezia Giulia (FVG), Tuscany, Veneto
% BIOSIMILAR RITUXIMAB, calculated as milligrams of biosimilar Rituximab administered divided by total milligrams of Rituximab-based drugs administered. % BIOSIMILAR ETANERCEPT, calculated as the number of patients treated with biosimilar Etanercept over the total number of patients treated with Etanercept-based drugs. % BIOSIMILAR SOMATROPIN, calculated as the number of patients treated with biosimilar Somatropin over the total number of patients treated with Somatropin-based drugs. % BIOSIMILAR EPOETIN, calculated as the number of patients treated with biosimilar Epoetin over the total number of patients treated with Epoetin-based drugs.
Source: http://performance.sssup.it/network .
percentage ofbiosimilar Somatropin was24,88%.Greater biosim-ilarpenetrationwasrecordedforEpoetin(59,40%)(seeFig. 1 ). 3.2.2. Analysisofregionalresolutionsandothergovernancetools
Biosimilars penetration hasbeen the main cornerstone ofthe 2018 drug governance policy of the TRHS. For this purpose, the MeS-Lab researcherssupported theTRHS in designing a compre-hensivestrategythatincludedbothtop-downandbottom-up man-agerialtools.
Concerning the former, a regional resolution specifically aimed at fostering the biosimilars’ uptake was issued. Resolu-tion n°194/2018, dated 26th February 2018, provided that Tuscan
healthcareorganizationscouldnotask foranduseproducts other thanthoseawardedthroughatenderbytheregionalprocurement body (ESTAR).Furthermore,thoserequests ofdrugsnotsubjected to ESTARtendering procedureshadtobetreatedatregionallevel through a careful evaluation run by the Drugs and Appropriate-ness Policy Sector [38] .In the caseof the moleculesanalyzed, if a biosimilarwasawarded the tender, doctorswere asked to pre-scribethebiosimilar.Theuseofan originator,insuch asituation, becamesubjectedtotheevaluationandapprovaloftheTRHS.
The resolution wasaccompanied by bottom-up measures. The Drugs and Appropriateness Policy Sector, supported by MeS-Lab, activated an engagement process with managers and specialists
of Tuscan LHAs and Teaching Hospitals (THs) to open a debate on the use of biosimilars and to define shared targets for in-creasing their uptake. More specifically, two monthly meetings with chief medical officers of all the Tuscan LHAs with a fo-cus on pharmaceuticals,one monthly gatheringwith directorsof pharmaceuticalservices and one monthly focus on pharmaceuti-cal expenditure during budget revisions with chief executive of-ficers were organised throughout 2018, to discuss the level of biosimilarpenetrationindifferentstructures,monitorthe achieve-ments, benchmark providers against each other, and revise the targets.
Third,thiscomprehensivestrategyincludeda“mixed-side” ap-proach,that combined some top-down elementswithbottom-up ones:thelevelofbiosimilars’penetrationinTuscanyin2017was computedandthiswasthepreconditionfortheTuscanDrugsand Appropriateness PolicySector todesign andimplementa payfor performance system, addressedtotheregional healthauthorities. MeS-Lab’sresearcherswereinvolvedinprovidingbothdatato de-finethemostappropriatetargets,andexpertiseinfine-tuningthe individual goals.In thefinal step, aset ofindicatorson the pen-etrationoftheaforementioned moleculeswasdesignedand com-puted,soastobenchmarkthe2017-2018trendofbiosimilar pen-etration in Tuscany versus other regions (pre-, post- and cross-sectionalcomparison).
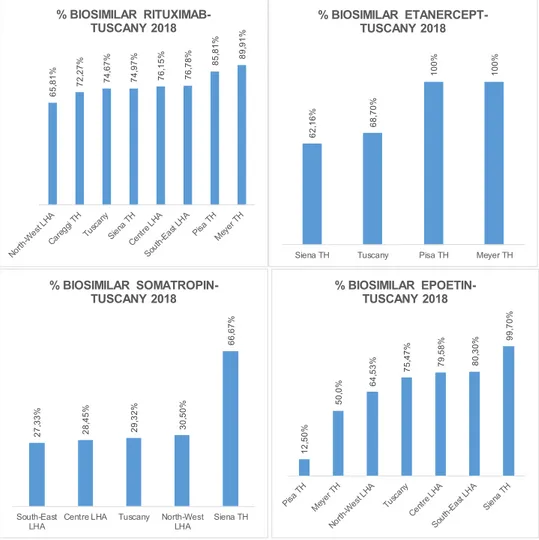
Fig. 2. Intra-regional geographic variation, Tuscany 2018. Source: http://performance.sssup.it/network .
3.2.3. Preliminaryevaluation– 2017-2018penetrationtrends The high heterogeneity in biosimilars’ penetration we iden-tified in Tuscany in 2017 clearly relates to timing in their avail-ability. However, biosimilars’ market entry is equally granted at national scale and comparisonsamong regions offer nevertheless interesting insights concerning the effects ofthe implementation ofdifferentmanagerialtools,atregionalscale.Biosimilar penetra-tion increased for all four molecules over the 2017-2018 period in Tuscany. The percentage of biosimilarRituximab grew tenfold from7,10% in2017 to74,67% in2018. Thepercentageof biosimi-larEtanerceptreached68,70%,thepercentageofbiosimilar Soma-tropin29,32%andthepercentageofbiosimilarEpoetin75,47%.
Furthermore,whencomparingwithotherregionsthat arepart of the Italian Regional Performance Evaluation System(IRPES), a PerformanceMeasurementSystemdevelopedin2004bythe MeS-Lab jointlywith the TRHS and eleven other Italian Regions [39] , showed that Tuscany has registered an increase either higher or inlinewithotherregions.ThepercentageofbiosimilarRituximab had a remarkable rate of 67,57%, greater Veneto’s of 36,4% and Friuli Venezia Giulia’s (FVG) 40,16%. Biosimilar Etanercept regis-teredasignificantincreasewithagrowthinpenetrationof47,65%, far higher than observed for Venetoand FVG. The percentage of biosimilar Somatropin and Epoetin registered a growth in line with that of the other regions. The graph below reports 2017-2018 penetrationtrendsfortheIRPESregionsthat decidedto up-load their data according to a common calculation methodology (seeFig. 1 ).
Asignificant intra-regional geographicvariation wasobserved. Thepenetrationofthesamebiosimilarvariedgreatlyacross differ-entTuscanLHAsandTHs.ThepercentageofBiosimilarRituximab in2018variedfrom65,81%inNorth-WestLHAto89,91%inMeyer TH;the percentageofbiosimilarEtanercept from62,16% inSiena TH to 100% in PisaTH; the percentage of biosimilar Somatropin from27,33%inSouth-EastLHAto66,67%inSienaTH;the percent-ageofbiosimilarEpoetinin2018 Epoetin from12,50% inPisaTH to99,70%inSienaTH(see Fig. 2 ).Data areshownforthoseLHAs andTHsthatusedbiosimilarsin2018.
4. Discussion
Italian health authorities are increasingly focusing on imple-menting pharmaceuticalcost-containmentpolicies,since pharma-ceutical expenditure has increased at a rapid pace over the last yearsandhassignificantlyimpactedcountries’totalhealthcare ex-penditures [8] .Italy has progressivelymoved towards the reform ofthepharmaceuticalsector[7] .Thereformprocesshasrequired newtoolstomanage innovationandguaranteeeconomic sustain-ability. Most Italian regions have implemented ad-hoc biosimilar policies,though these are differentboth in termsof contentand time of adoption. Tuscany,Veneto and FVG are among those re-gionsthat havefirstmoved topromotebiosimilars’uptake. While Venetofocusedontheprogressiveadoptionofguidelinestofoster populationknowledge on biosimilars[24 ,25] ,FVG adoptedmixed
tools, such as prescription quotas and shared protocols [27 ,28] . TheDrugs andAppropriatenessPolicySectoroftheTRHSadopted biosimilar policies designed as elements of a coherent strategy, groundedonthreepillars:a)top-downformal measures(regional resolutions); b) bottom-up measures (meetings to foster profes-sionalengagement); c)“mixed” measures (benchmarkingandpay forperformancemechanisms).
Since the strategy is intrinsically intertwined, it has proved challengingtodisentangletheeffectsofeachsingleapproachand their interactions. Nevertheless, the strategy’s effectiveness could be associated with its comprehensive approach in the following ways:
1) the“three-pillar” strategythatinvolvedtwoofthemain stake-holdersinsteeringtheprescribingchoices:notonlymanagers, butprofessionals themselves were targeted, to aligntheir ob-jectives with theregion’s. This approach iscoherent withthe determinants associated with biosimilars’ penetration, where theprescribers’role ispivotal.Theappropriate combinationof demand-sideandsupply-sidepoliciesmightbethepreeminent reasonexplainingtheTuscansuccess;
2) by combining top-down and bottom-up measures, the Tus-canstrategyjointlyleveragedextrinsicandintrinsicmotivation [40] .Itmightbesuggestedthat theformal resolutionsoffered anextrinsiclegitimisation,whilesystematicbenchmarking and informal meetings fostered professionalengagement and peer pressure,byleveraginginternalmotivation;
3) the institutional cohesion between regional and national ac-tions. The AIFAposition papers offeredthe legitimisation that the Italianregions neededto fullyexploit thebiosimilars’ op-portunity andTuscany wasone ofthe firstregionstoseize an allianceatthenationallevel.
Two final considerations may deserve some attention. Firstly, the Tuscan strategy entailed an overarching approach that indis-criminately targetedall biosimilars, regardless of their therapeu-tic area. Since they refer to a wide range of clinical treatments, prescribers refer to different disciplines (e.g.,hematology, oncol-ogy,immunology,nephrology).Itinteresting tounderlinethatthe biosimilars’penetrationinTuscanyoutpacedtheotherregions, re-gardless of the clinical specialty. A transversal alliance occurred thattranscendedclinicalboundaries.
Ontheotherside,intra-regionalvariationissignificantandmay suggestsome considerationsconcerningtheprescribingprocesses. Asanexample,whileSienaTHregisterslowpenetrationregarding biosimilarEtanercept,itrepresentsabestpracticewithregardsto Epoetin andSomatropin (seeFig. 2 ). Thisfluctuatingperformance maysuggestthatfinalresultsdonotsimplyoriginatefromthe im-plementationofexternalmeasures,butratherderivefromthe pro-fessional responsetoit.Intra-regional variationmayrelateto dif-ferent areasofexpertiseof differentcentersexamined (i.e.,there are areasof expertisesuch asrheumatologywere biosimilar pre-scriptionconstitutesamajortopicalissue[41] )anddifferent case-mix.Ontheotherhand,inter-regionalvariationmaybereasonably associatedtodifferentpoliciesimplemented.
Prescribing behaviors seem to result from a triadic interac-tion between the regional regulator (that sets the prescribing framework bymediatingbetweenthegeneralcontextandthe re-gional characteristics), clinicians (who mediate between the re-gional framework and the singularity of each patient) and local managers(whoareinchargeofmediatingbetweenthepotentially diverging interestsof theother two stakeholders).The success of anygovernancemeasureseemstodependonitsabilitytobalance theinteractionsbetweenthesethreestakeholders.
5. Conclusions
ThepreliminarylessonthatcanbedrawnfromtheTuscancase seemstosuggestthatthedesignofspecificpoliciesaimedat steer-ing thecurrentprescribing behaviorsby overcomingthe classical approachesneedstogranta“triplecoherence”:
-aninstitutionalcoherence,betweentheregulators(national, re-gionalandlocalones),
-amotivationalcoherence,betweenthemainstakeholders(the regionaladministrators,themanagersandtheclinicians), -aninstrumentalcoherence,betweenthemeasuresthatcan be
leveraged(top-down,bottomup,mixedones),soasto care-fullybalancinginternalandexternalmotivation.
Asalimitation,ourresearchmainlyfocusedononeregionand data benchmarking was carried out within a limited number of Italian regions. A quantitative approach to assess the correlation betweenpolicyadoptionandbiosimilars’penetrationseemed pre-mature.Furthermore,theMeS-Labdoesnot accessthesamedata foralltheregions,thusacompleteevaluationoftheinter-regional avoidablevariationandoftheNorth-Southgradientwasnot possi-ble.Third,ourpaperwasspecificallyaimedatinferringsome con-siderationsconcerning potential effects of differentregional poli-ciesonbiosimilars’uptake:itdidnotanalyzeanypotentialeffects onappropriateness.Awiderprescribingofbiosimilarsmightbe as-sociatedtopotentialinappropriatebehaviors,suchasfirst-line pre-scriptionof medicines rather recommendedas second-line treat-ments.However,thisgoesbeyondthescopeofourstudyandwill beexaminedinfurtherresearch.
Authorstatement
ElisaGuidottiwrotethepapertogheterwithFedericoVola. Fed-erico Vola had a foundamental role in defining the methology and supervising the entire work. Elisa Guidotti and Bruna Vinci were responsibleforconceptualization,datacollection,data cura-tion and formal analysis. Francesco Attanasio, Bruna Vinci, Elisa Guidotti andFedericoVola havebeen partofthe actionresearch processthatoccurredbetweentheDrugandAppropriateness Sec-toroftheTuscanRegionalHealthSystemandtheManagementand HealthLaboratoryofScuolaSuperioreSant’AnnaofPisa.
Ethicalapproval Notrequired Funding
None.
DeclarationofCompetingInterest Nonedeclared
Acknowledgements
Thisstudywassupported by the ItalianRegional Performance Evaluation System (IRPES) steered by Laboratorio Management e Sanità, Institute of Management, ScuolaSuperiore Sant’Anna Pisa (Italy).TheauthorsacknowledgeProfessorSabinaNutiandallthe researchers from Laboratorio Management e Sanità, Institute of Management, ScuolaSuperiore Sant’Anna, fortheir constant sup-portandusefulcomments.
References
[1] Ferre F , de Belvis AGI , Valerio L , Longhi S , Lazzari A , Fattore G , et al. Italy: health system review. Health Syst Transit 2014;16(4):1–168 .
[2] Ferré F , Noto G , Vola F . O sistema de saúde Italiano e a crise: uma visão geral das políticas e sua implementação. An Inst Hig Med Trop (Lisb) 2018;17(1):47–58 .
[3] France G , Taroni F , Donatini A . The Italian health-care system. Health Econ 2005;14(SUPPL. 1):187–202 .
[4] IQVIA. Farmaci biologici e biosimilari - Scenari terapeutici e stima del risparmio per il Sistema Sanitario Italiano [internet]. [cited 2020 May 12]. Available from: http://magazine.imshealth.it/report/(IQVIA) _ Farmaci _ biologici _ e _ biosimilari.pdf .
[5] European Commission & European Medicines Agency (EMA). Biosimilars in the EU - information guide for healthcare professionals [internet]. [cited 2020 May 12]. p. 395–411. Available from: https://www.ema.europa.eu/en/documents/ leaflet/biosimilars- eu- information- guide- healthcare- professionals _ en.pdf . [6] Farfan-Portet MI , Gerkens S , Lepage-Nefkens I , Vinck I , Hulstaert F . Are biosim-
ilars the next tool to guarantee cost-containment for pharmaceutical expendi- tures? Eur J Heal Econ 2014;15(3):223–8 .
[7] Bria E , Conte P . Biosimilars as a strategy to improve sustainability. ESMO Open 2017;2(May(2)):e0 0 0192 .
[8] European Commission Organisation for Economic Co-operation and Develop- ment (OECD). Health at a Glance: Europe 2018. 2018.
[9] IQVIA. Advancing biosimilar sustainability in Europe - a multi-stakeholder as- sessment. 2018;(September):40.
[10] Agenzia Italiana del Farmaco (AIFA). Monitoraggio consumi e spesa biosim- ilari [Internet]. [cited 2020 May 12]. Available from: https://www.aifa.gov.it/ monitoraggio- consumi- e- spesa- biosimilari .
[11] Ministero della SaluteMonitoraggio dei LEA attraverso la cd. Griglia LEA 2017 . [12] Fantini MP , Nuti S , Vola F . Il Governo Dell’innovazione Farmaceutica in Italia.
Dallo Stato Dell’arte a Un Modello Di Gestione Regionale Equa e Sostenibile Dei Farmaci Innovativi e Ad Alto Costo. Del Gallo Editore 2016 .
[13] Kasanen E , Lukka K . The constructive approach in management accounting re- search. J Manag Account Res 1993(5):243–64 .
[14] Vainieri M, Vola F, Gomez Soriano G, Nuti S. How to set challeng- ing goals and conduct fair evaluation in regional public health systems. Insights from Valencia and Tuscany regions. Health Policy (New York) [Internet]. 2016;120(11):1270–8. Available from http://dx.doi.org/10.1016/j. healthpol.2016.09.011 .
[15] Moorkens E , Vulto AG , Huys I , Dylst P , Godman B , Keuerleber S , et al. Policies for biosimilar uptake in Europe: an overview. PLoS One 2017;12(12):1–17 . [16] IMS Institute for Healthcare InformaticsThe impact of biosimilar
competition [internet]. IMS Inst Healthc Inf 2016. [cited 2020 May 12]. p. 1–29Available from http://www.medicinesforeurope.com/docs/ IMS- Impact- of- Biosimilar- Competition- 2016.pdf .
[17] Rémuzat C , Kapu ´sniak A , Caban A , Ionescu D , Radière G , Mendoza C , et al. Sup- ply-side and demand-side policies for biosimilars: an overview in 10 European member states. J Mark Access Heal Policy 2017;5(1):1307315 .
[18] European Medicines Agency (EMA). Biosimilar medicines: Overview [Inter- net]. [cited 2020 May 12]. Available from: https://www.ema.europa.eu/en/ human-regulatory/overview/biosimilar-medicines-overview .
[19] Agenzia Italiana del Farmaco (AIFA). Position paper : i farmaci biosim- ilari [Internet]. 2013 [cited 2020 May 12]. p. 1–15. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/AIFA _ POSITION _ PAPER _ FARMACI _ BIOSIMILARI.pdf .
[20] Agenzia Italiana del Farmaco (AIFA)Secondo position paper AIFA sui Farmaci biosimilari [Internet]. Aifa 2018. [cited 2020 May 12]. p. 1– 28. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/ 2 _ Position- Paper- AIFA- Farmaci- Biosimilari.pdf .
[21] European Commission. What you need to know about biosimilar medicinal products [Internet]. [cited 2020 May 12]. Available from: file://10.90.0.9/laboratorio/PhD/Guidotti/Articolo Biosimilari/Letteratura/What you Need to Know about Biosimilar Medicinal Products .pdf.
[22] Gazzetta Ufficiale della Repubblica Italiana. DELIBERAZIONE 1 febbraio 20 01 [Internet]. 20 01 [cited 2020 May 12]. Available from: https://www. gazzettaufficiale.it/eli/id/20 01/03/28/0 01A3188/sg .
[23] Ingrasciotta Y , Giorgianni F , Bolcato J , Chinellato A , Pirolo R , Tari DU , et al. How much are biosimilars used in clinical practice? a retrospective italian popula- tion-based study of erythropoiesis-stimulating agents in the years 2009-2013. BioDrugs 2015;29(4):275–84 .
[24] Regione del VenetoAllegato A al Decreto n. 16 del 29/06/2010 2015;17:1–17 . [25] Regione del Veneto. Regione del Veneto Coordinamento Regionale
Unico sul Farmaco - CRUF: Linee di indirizzo regionali per l’impiego dei farmaci intravitreali per la cura della degenerazione macu- lare senile [internet]. 2017 [cited 2020 May 12]. p. 1–17. Available from: https://www.regione.veneto.it/c/document _ library/get _ file?uuid= dd94d7e8- 230f- 46a7- 8937- 5d8f35c4eb7e&groupId=10793 .
[26] Regione del Veneto. Decreto n.112 del 13 settembre 2018_Biosimilari.pdf. [27] Regione Friuli Venezia Giulia. Programma preventivo consolidato 2012 del
Servizio Sanitario Regionale. 2012 (May).
[28] Regione Friuli Venezia Giulia. Programma preventivo consolidato 2016 del servizio sanitario regionale. 2016.
[29] IRCSS CROB - Regione Basilicata. Farmaci Biosimilari - Direttiva Vincolante [internet]. [cited 2020 May 12]. Available from: http://www.crob.it/crob/files/ docs/11/20/41/DOCUMENT _ FILE _ 112041.pdf .
[30] Regione Calabria. DPGR - CA n. 37 del 21 Marzo 2014 [internet]. 2014 [cited 2020 May 12]. Available from: http://old.regione.calabria.it/sanita/allegati/ dpgr _ 2014/d.p.g.r. _ n. _ 37 _ del _ 21.03.2014.pdf .
[31] Regione Puglia. DELIBERAZIONE DELLA GIUNTA REGIONALE 26 febbraio 2014, n. 216 [Internet]. 2014 [cited 2020 May 12]. Available from: http://www. quotidianosanita.it/allegati/allegato2190575.pdf .
[32] Regione Sicilia. Misure volte a promuovere l’utilizzo dei Farmaci Orig- inatori o Biosimilari a minor costo di terapia [Internet]. 2014 [cited 2020 May 12]. Available from: http://pti.regione.sicilia.it/portal/page/ portal/PIR _ PORTALE/PIR _ LaStrutturaRegionale/PIR _ AssessoratoSalute/ PIR _ Infoedocumenti/PIR _ DecretiAssessratoSalute/PIR _ Decreti/PIR _ Decreti2014/ PIR _ Provvedimentiorganiindirizzopolitico/Decreto sull’utilizzo dei farma. [33] Renwick MJ, Smolina K, Gladstone EJ, Weymann D, Morgan SG. Postmarket
policy considerations for biosimilar oncology drugs. Lancet Oncol [Internet] 2016;17(1):e31–8 Available from:. doi: 10.1016/S1470-2045(15)00381-2 . [34] Bocquet F , Paubel P , Fusier I , Cordonnier AL , Sinègre M , Le Pen C . Biosimi-
lar versus patented erythropoietins: learning from 5 years of European and Japanese experience. Appl Health Econ Health Policy 2015;13(1):47–59 . [35] Mestre-Ferrandiz J , Towse A , Berdud M . Biosimilars: how can payers get
long-term savings? Pharmacoeconomics 2016;34(6):609–16 .
[36] Bocquet F , Paubel P , Fusier I , Cordonnier AL , Le Pen C , Sinègre M . Biosimilar granulocyte colony-stimulating factor uptakes in the EU-5 markets: a descrip- tive analysis. Appl Health Econ Health Policy 2014;12(3):315–26 .
[37] De Rosis S, Guidotti E, Zuccarino S, Venturi G, Ferre’ F. Waiting time in- formation in the Italian NHS: A citizen perspective. HEALTH POLICY 2020. doi: 10.1016/j.healthpol.2020.05.012 .
[38] Regione Toscana - Uffici Regionali Giunta Regionale. Delibera n °194/2018 [internet]. Vol. 2016. 2016 [cited 2020 May 12]. p. 1–5. Available from: https://www.regione.toscana.it/bancadati/atti/Contenuto.xml?id= 5173900&nomeFile=Delibera _ n.194 _ del _ 26- 02- 2018 .
[39] Nuti S , Noto G , Vola F , Vainieri M . Let’s play the patients music: a new generation of performance measurement systems in healthcare. Manag Decis 2018;56(10):2252–72 .
[40] Benabou R , Tirole J . Intrinsic and extrinsic motivation. Rev Econ Stud 2003;70:489–520 .
[41] Beck M , Michel B , Rybarczyk-Vigouret MC , Levêque D , Sordet C , Sibilia J , et al. Rheumatologists’ perceptions of biosimilar medicines prescription: find- ings from a French web-based survey. BioDrugs 2016;30(6):585–92 .