MedChemComm
CONCISE ARTICLE
Cite this: Med. Chem. Commun., 2015, 6, 823
Received 12th December 2014, Accepted 1st February 2015 DOI: 10.1039/c4md00556b www.rsc.org/medchemcomm
Evaluation of the anti-inflammatory/
chondroprotective activity of aldose reductase
inhibitors in human chondrocyte cultures
Annamaria Panico,
aRosanna Maccari,*
bVenera Cardile,
cSergio Avondo,
dLucia Crascì
aand Rosaria Ottanà
bIJ5-Arylidene-4-oxo-2-thioxothiazolidin-3-yl)acetic acids (6) and 5-arylidene-4-oxo-2-thioxothiazolidines (7), which we recently synthesised and assayed as aldose reductase inhibitors, were evaluated as anti-inflammatory/antidegenerative agents in cultures of human chondrocytes stimulated by IL-1β. In this screening, most of the tested compounds were able to control key components of the IL-1β-induced inflammatory signalling, by reducing the levels of NF-kB, ICAM-1, and NO as well as increasing the produc-tion of glycosaminoglycans by chondrocytes. Moreover, these 4-thiazolidinone derivatives exhibited anti-oxidant properties and were shown to inhibit MMP-3 and MMP-13 at micromolar concentrations, with a generally marked preference toward MMP-13 which plays a major role in cartilage degeneration. Thus, on the whole, compounds 6 and 7 were shown to be capable of both counteracting inflammatory events and contributing to restore normal levels of cartilage components. This anti-inflammatory/antidegenerative profile makes them interesting cell-permeable molecules that can be assumed as lead compounds in the search for novel anti-inflammatory agents.
1. Introduction
Inflammation is a complex pathophysiological process criti-cally implicated in the etiology of numerous degenerative human diseases, such as osteoarthritis, cancer, and diabetes mellitus, which are major causes of morbidity and mortality in worldwide populations.
Osteoarthritis (OA), the leading cause of joint disorder in elderly patients, is characterized by progressive loss of articu-lar cartilage as the result of complex inflammatory/oxidative processes responsible for degradation of various components of the cartilage matrix.
Articular cartilage is mainly composed of an extracellular matrix (ECM), which is largely formed by type II collagen and proteoglycans (PGs), with the latter being composed of sulphated glycosaminoglycans (GAGs) linked to a protein core. Chondrocytes, the only type of cells present in the carti-lage, produce and secrete PGs, collagen and enzymes
aDepartment of Drug Sciences, University of Catania, V.le A. Doria 6, 95125
Catania, Italy
bDepartment of Scienze del Farmaco e dei Prodotti per la Salute, University of
Messina, Polo Universitario dell'Annunziata, 98168 Messina, Italy. E-mail: [email protected]; Tel: +39 90 6766406
cDepartment of Biomedical Sciences, University of Catania, V.le A. Doria 6, 95125
Catania, Italy
dDepartment of Surgery, University of Catania, V.le A. Doria 6, 95125 Catania,
Italy
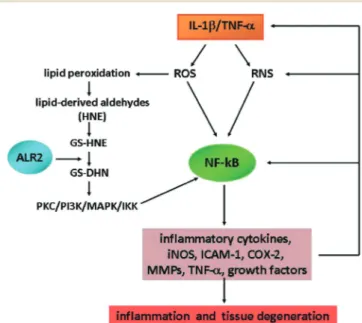
Fig. 1 Role of NF-kB and ALR2 in the IL-1β-induced oxidative/inflam-matory signalling pathways. ALR2: aldose reductase; COX: cyclooxy-genase; GS-DHN: glutathionyl-1,4-dihydroxynonene; GS-HNE: gluta-thione-4-hydroxy-trans-2-nonenal; HNE: 4-hydroxy-trans-2-nonenal; ICAM: intercellular adhesion molecule; IKK: inhibitor-kappa B kinase; iNOS: inducible nitric oxide synthase; MAPK: mitogen activated protein kinase; MMPs: matrix metalloproteinases; NF-kB: nuclear transcription factor-kappa B; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; RNS: reactive nitrogen species; ROS: reactive oxygen species; TNF-α: tumor necrosis factorα.
involved in cartilage metabolism.1,2 Under OA conditions, cartilage destruction arises from PG degradation and depriva-tion of cartilage-specific type II collagen, as well as from increased production of reactive oxygen species (ROS), reac-tive nitrogen species (RNS) such as nitric oxide (NO) and its free radical derivative peroxynitrite, adhesion molecules (e.g., ICAM-1) and matrix metalloproteinases (MMPs). ROS, RNS, cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α), and other inflammatory stimuli pro-mote a persistent activation of the nuclear transcription factor-kappa B (NF-kB) and, consequently, the NF-kB-dependent expression of inflammatory cytokines, growth fac-tors and MMPs.3–6 This creates a cascade of events that sus-tain inflammatory response and tissue damage (Fig. 1).
Proinflammatory cytokine IL-1β also leads to loss of GAGs, fundamental components of PGs, thus sustaining cartilage damage. The degree of GAG decrease is considered indicative of the severity of articular cartilage damage.7,8 Moreover, the NF-kB-dependent expression of ICAM-1, a transmembrane glycoprotein belonging to the immunoglobulin superfamily of adhesion molecules, was shown to be higher in the synovium of OA patients than in that of healthy individuals.9 ICAM-1 plays a predominant role in recruitment and traffick-ing of leucocytes to sites of inflammation and is expressed in a variety of inflammatory diseases.
Another consequence of NF-kB activation is the produc-tion of MMPs by chondrocytes. Specific members of the
MMP family play key roles in the loss of structural and func-tional integrity of the cartilage, particularly MMP-3 and MMP-13 which are considered attractive targets in the search for novel therapeutic agents.10,11 MMP-3, also known as stromelysin 1, acts directly on the cartilage, by degrading var-ious components of the matrix, such as PGs, fibronectin, col-lagen type IV, laminin and colcol-lagen type IX. MMP-13 (collage-nase-3) acts by degrading the collagen fibrils, especially those of type II that are the most abundant in the cartilage.12
Furthermore, recent studies have demonstrated that aldose reductase (AKR1B1, ALR2), an enzyme which is impli-cated in the onset and progression of diabetic complica-tions,13,14acts as a key component in the mediation of oxida-tive stress-induced inflammation as well as in the pathophysiology of inflammatory diseases, both under normoglycemic and hyperglycaemic conditions.15,16 This aldo-keto reductase is overexpressed under various oxidative conditions and catalyses the reduction of aldehydes derived from the ROS-mediated peroxidation of lipids. Certain resulting metabolites, such as GS-DHN, are responsible for the activation of NF-kB, via the PKC/PI3K/MAPK/IKK cascade, thus triggering inflammatory response and tissue damage (Fig. 1). Therefore, based on these findings, it has been pro-posed that ALR2 inhibitors (ARIs) could be developed as new anti-inflammatory agents for the management of a number of inflammatory disorders, including sepsis, asthma, cancer, diabetes and arthritis.14–16
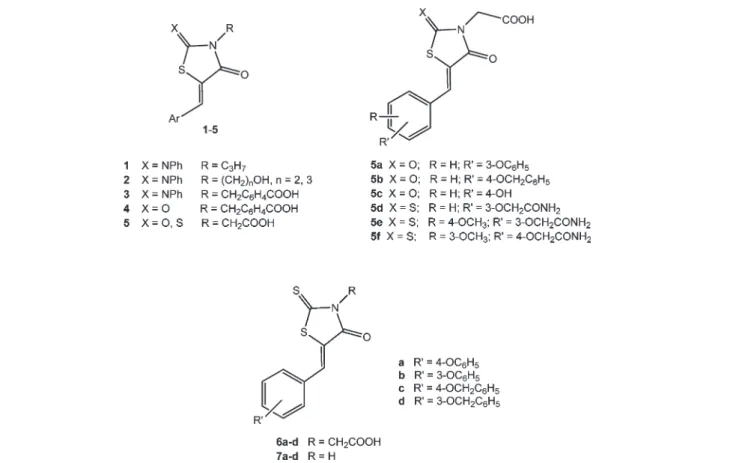
Fig. 2 General structure of previously reported anti-inflammatory 4-thiazolidinones 1–5 (ref. 17–20) and structures of IJ5-arylidene-4-oxo-2-thio-xothiazolidin-3-yl)acetic acids (6a–d) and 5-arylidene-4-oxo-2-thioxothiazolidines (7a–d).
In the past few years, we have reported 5-arylidene-2-phenylimino-3-propyl-4-thiazolidinones (1) (Fig. 2), which showed promising anti-inflammatory activity in animal models of acute inflammation,17 and their 3-hydroxyalkyl substituted analogues 2 (Fig. 2), which displayed anti-inflam-matory/chondroprotective effects by blocking degenerative cellular events induced by IL-1β in human chondrocyte cul-tures.18More recently, we have described the anti-inflamma-tory/antidegenerative activity of 4- ij(5-arylidene-4-oxo-2-phenyl-iminothiazolidin-3-yl)methyl]benzoic acids (3), 4- ij(5-arylidene-2,4-dioxothiazolidin-3-yl)methyl]benzoic acids (4) andIJ5-arylidene-4-oxothiazolidin-3-yl)acetic acids (5) (Fig. 2), which also produced interesting effects in chondrocyte cul-tures stimulated by IL-1β.19,20Among these, acetic acid deriv-atives 5a–c (Fig. 2) provided encouraging results, being endo-wed with greater potency than that of indomethacin in reducing the NO production induced by IL-1β and with a chondroprotective effect similar to that of indomethacin.19 Analogues 5d–f (Fig. 2) significantly reduced the NF-kB acti-vation induced by interferon-γ plus histamine in human keratinocytes, and moreover, 5e and 5f were able to reduce iNOS expression in the same cells.20 On the whole, our results indicated that the anti-inflammatory/antidegenerative effects of these derivatives could be mainly related to the 4-thiazolidinone core, whereas the 5-arylidene group appeared to be able to modulate their anti-inflammatory activity.17–20 The IJ4-oxothiazolidin-3-yl)acetic acid moiety of compounds 5 was shown to be particularly favourable.19,20
The promising activity of acetic acid derivatives 5 encour-aged us to evaluate the anti-inflammatory/anti-degenerative effects of selected analogous IJ5-arylidene-4-oxo-2-thio-xothiazolidin-3-yl)acetic acids (6) (Fig. 2). In addition, in order to further investigate the influence of the acetic acid chain on the activity, we tested the corresponding N-unsubstituted 5-arylidene-4-oxo-2-thioxothiazolidines (7) (Fig. 2).
It appeared to be of interest that, similarly to compounds 5, most selected derivatives 6 and 7 had been shown to be potent ARIs, with IC50values ranging from 0.11μM to 2.94 μM,
as we reported in a recent article.21 Moreover, their ALR2 inhibitory effectiveness had been shown to be generally higher than that of the corresponding 2,4-thiazolidinediones.21–23
Both compounds 6 and 7 were synthesised in high yields by the Knoevenagel condensation between IJ4-oxo-2-thio-xothiazolidin-3-yl)acetic acid or 4-oxo-2-thioxothiazolidine
and appropriate aldehydes (Scheme 1).21 Similarly to analogous 2,4-thiazolidinediones and 5-arylidene-2-phenylimino-4-thiazolidinones,22,23 only the preferred Z isomers were obtained. The chemical–physical data of com-pounds 6 and 7 and the source of starting materials were previously reported in a recent article by some of us.21
The anti-inflammatory/anti-degenerative effectiveness of 2-thioxo-4-thiazolidinones 6 and 7 was assessed in cultures of human chondrocytes stimulated by IL-1β. This is a useful experimental model to reproduce the mechanisms involved in arthritis and other inflammatory diseases, which we uti-lized to investigate previously reported analogues.1,18,19 The effects of the tested compounds on the production of NF-kB, ICAM-1, NO and GAGs were evaluated.
Considering the predominant role of oxidative stimuli in sustaining inflammatory signalling in IL-1β-activated chondrocytes,5,6 we also evaluated the antioxidant activity of derivatives 6 and 7 by means of the Oxygen Radical Absor-bance Capacity (ORAC) method. Finally, we assessed their in vitro inhibitory capability towards MMP-3 and MMP-13.
2. Results and discussion
2.1 Cellular assays
Compounds 6 and 7 were assayed at 2.5μM and 10 μM con-centrations in cultures of IL-1β-stimulated chondrocytes.
The cytotoxic effect of the tested compounds was evalu-ated by means of a cell viability test based on the cleavage of 3-IJ4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) by mitochondrial dehydrogenases of metabolically active cells.24The results showed that the compounds under study did not affect the ability of chondrocytes to metabolize tetrazolium salts, thus demonstrating that they do not inter-fere with cell viability (data not shown).
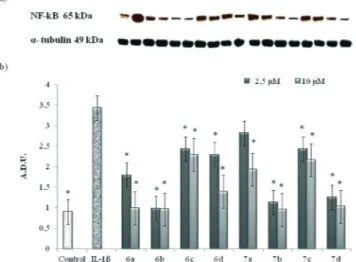
2.1.1 Determination of NF-kB levels. The treatment of human chondrocytes with IL-1β (10 ng ml−1) increased NF-kB levels (measured as NF-kB p65 subunit levels), compared to untreated cells. IL-1β-stimulated chondrocytes were then treated with compounds 6a–d and 7a–d and the levels of NF-kB were measured after 72 h. All compounds reduced NF-NF-kB levels at both 2.5μM and 10 μM concentrations, compared to cells treated only with IL-1β (Fig. 3). The best results were obtained with both 5-IJ3-phenoxybenzylidene) substituted derivatives 6b and 7b, which reduced NF-kB to the levels of the control, at both concentrations (Fig. 3). The correspond-ing 5-IJ4-phenoxybenzylidene) analogues (6a and 7a) displayed lower efficacy. In particular, 5- ij(4-phenoxybenzylidene)-4-oxo-2-thioxothiazolidin-3-yl]acetic acid (6a) restored the NF-kB levels of the untreated cells only at 10μM, whereas at lower concentration it provided about 50% reduction of NF-kB levels compared to IL-1β-treated cells (Fig. 3). N-Unsubstituted analogue 7a was not able to restore the NF-kB levels of the control, although it possessed moderate activ-ity compared to the IL-1β-treated cells at both concentrations. Benzyloxybenzylidene analogues (6c, 6d, and 7c) generally caused a more moderate decrease of NF-kB levels than Scheme 1
phenoxy substituted analogues (6a, 6b, 7a, and 7b), with the exception of compound 7d which provided effects similar to those of 3-phenoxy homologue 7b.
The significant ability of compounds 6b, 7b and 7d to reduce NF-kB levels in IL-1β-activated human chondrocytes appears to be of interest because NF-kB activation is an upstream event in the inflammatory cascade. In fact, the reduction of NF-kB-dependent gene transcription can lead to decreased levels of other proinflammatory mediators, such as cytokines and diverse enzymes (such as MMPs and iNOS), thus interfering with downstream signaling components cru-cial for tissue inflammatory response and subsequent degen-erative processes.
2.1.2 Determination of ICAM-1 levels. Resting chondrocytes did not express ICAM-1, whereas their incuba-tion with IL-1β induced marked ICAM-1 expression. The treatment of the IL-1β-stimulated chondrocytes with all com-pounds 6a–d and 7a–d, at both 2.5 μM and 10 μM concentra-tions, reduced ICAM-1 expression (Fig. 4). Compounds 6b, 7b and 7d exhibited the greatest potency, providing at least 50% reduction of ICAM-1 levels at both tested concentrations, and this finding may be correlated to their significant capability of counteracting NF-kB production. Analogues 6a and 6d pro-duced the same effect only at 10μM concentration, whereas 6c, 7a and 7c, which were only moderately capable of reduc-ing NF-kB levels, showed poor activity in this assay (Fig. 4).
2.1.3 Determination of nitrite levels. NO production was measured as the amount of nitrite released by the cells in the culture medium, determined by using the spectrophotomet-ric method based on Griess reaction.25 NO production by chondrocytes under basal conditions (untreated cells) was very low and mainly due to the activity of the constitutive
NOS. In human chondrocytes treated with IL-1β, a noticeably increased production of NO was observed (Fig. 5).
All compounds 6a–d and 7a–d exhibited a significant dose-dependent capability to reduce NO release, N-unsubstituted 4-oxo-2-thioxothiazolidines 7 being generally more effective than the corresponding acetic acid derivatives 6. Once again, compounds 6b, 7b and 7d showed the best activity, almost restoring the NO levels of the untreated cells at both tested concentrations. Analogously to the observed reduction of ICAM-1 expression, this effect could be corre-lated to the capability of reducing NF-kB levels, being a con-sequence of lower NF-kB-induced iNOS expression.
Fig. 3 Effects of 6a–d and 7a–d on NF-kB levels induced by IL-1β on human chondrocytes. a) Representative blot. b) Nuclear factor-kappa B (NF-kB) levels in cultures of IL-1β-stimulated human articular chondrocytes 72 h after the addition of compounds 6a–d and 7a–d at 2.5μM and 10 μM concentrations, determined by Western blot analysis. The data show the relative expression (mean ± SEM) of NF-kB, calculated as arbitrary densitometric units (A.D.U.), from three independent experi-ments.*P < 0.05, compared with respective IL-1β values.
Fig. 4 Effects of 6a–d and 7a–d on ICAM-1 expression. a) Representa-tive blot. b) ICAM-1 expression in IL-1β-stimulated human articular chondrocytes 72 h after the addition of compounds 6a–d and 7a–d at 2.5μM and 10 μM concentrations, determined by Western blot analysis. The data show the relative expression (mean ± SEM) of ICAM-1, calcu-lated as arbitrary densitometric units (A.D.U.), from three independent experiments.*P < 0.05, compared with respective IL-1β values.
Fig. 5 Determination of NO levels. NO production (measured as nitrite,μM) (means ± SEM) in the culture medium of IL-1β-stimulated articular chondrocytes 72 h after the addition of compounds 6a–d and 7a–d at 2.5 μM and 10 μM concentrations. *Significantly different from IL-1β-treated samples (P < 0.05).
However, the reduction of NO release provoked by the other tested analogues was also appreciable, especially at 10 μM (Fig. 5), thus suggesting that different factors other than NF-kB activation might be involved in determining NO production.
2.1.4 Determination of GAG levels. The treatment with IL-1β induced a marked reduction in the GAG amount pro-duced by chondrocytes. Conversely, the tested com-pounds prevented the depletion of GAGs induced by IL-1β in a dose-dependent manner. In particular, at 10μM concentra-tion, they restored GAG production to almost the levels of the control, with the exception of compound 7b which was not able to provide high GAG levels (Fig. 6). It may be hypothesised that the behaviour of compound 7b is related to its poor ability to inhibit MMP-13 and to its irrelevant anti-oxidant capability (see below). In fact, MMP-13 activity is strongly related to the baseline turnover of GAGs.
2.2 Radical scavenging activity
ORAC assay is an established in vitro method used to quan-tify the antioxidant effectiveness of compounds. The assay measures the reduction of fluorescence resulting from the oxidative degradation of fluorescein by the free radical-generator 2,2′-azobisIJ2-amidino-propane)dihydrochloride (AAPH), using Trolox as a standard. Both series of com-pounds 6a–d and 7a–d exhibited from moderate to marked antioxidant activity, being able to delay the reduction of fluo-rescence of a fluorescein–AAPH solution (Table 1). In particu-lar, compound 6c showed excellent antioxidant effectiveness, producing the same effect of the reference Trolox (Table 1).
2.3 Inhibition of matrix metalloproteinases MMP-3 and MMP-13
The tested compounds inhibited the activity of both MMP-3 and MMP-13 in vitro, with IC50 values generally in the low
micromolar range. MMP-13 was observed to be more
susceptible to the inhibitory effect of all compounds (except 7b) than MMP-3. In particular, 5-IJ4-benzyloxybenzylidene) substituted analogues 6c and 7c were shown to be the most selective MMP-13 inhibitors in the respective series of acetic acid derivatives 6 and N-unsubstituted 4-oxo-2-thio-xothiazolidines 7. Compound 7c was also the most effective MMP-13 inhibitor among all tested analogues 6 and 7, with a submicromolar IC50value (Table 2).
The replacement of the 4-benzyloxy group (6c, 7c) with a phenoxy one (6a, 7a) led to a significant increase in MMP-3 inhibitory activity and, consequently, a considerable reduc-tion in selectivity (Table 2). A similar effect was observed by displacing the benzyloxy substituent from the para to the meta position of the 5-benzylidene ring. In particular, 3-benzyloxy substituted derivative 6d was the most potent inhibitor of both MMP-3 and MMP-13 among the tested com-pounds, displaying scarce selectivity (Table 2). In addition, the removal of the terminal phenyl ring from 5- IJ3/4-benzyloxybenzylidene) substituted derivatives led to 5- IJ3/4-methoxybenzylidene) substituted analogues which showed a marked decrease in the inhibitory potency, especially towards MMP-13 (data not shown).
On the whole, the 5-arylidene moiety was able to apprecia-bly modulate the MMP inhibitory activity, whereas the influ-ence exerted by the acetic acid residue on N-3 (compounds
Fig. 6 Determination of GAG release. GAG release (μg ml−1) in the culture medium of IL-1β-stimulated articular chondrocytes 72 h after the addition of compounds 6a–d and 7a–d at 2.5 μM and 10 μM con-centrations. *Significantly different from IL-1β-treated samples (P < 0.05).
Table 1 Antioxidant activity of compounds 6a–d and 7a–d
Compounds ORAC unit (TE/μM)a
6a 0.76 ± 0.06* 6b 0.61 ± 0.10* 6c 1.078 ± 0.30* 6d 0.65 ± 0.09* 7a 0.52 ± 0.07* 7b 0.39 ± 0.03* 7c 0.60 ± 0.10* 7d 0.63 ± 0.09*
aThe ORAC values are reported as ORAC unit, corresponding to
μmol of Trolox equivalents (TE) per μM of sample. Reported values are the means ± SEM (n = 3). *Significantly different at P < 0.05 compared to Trolox.
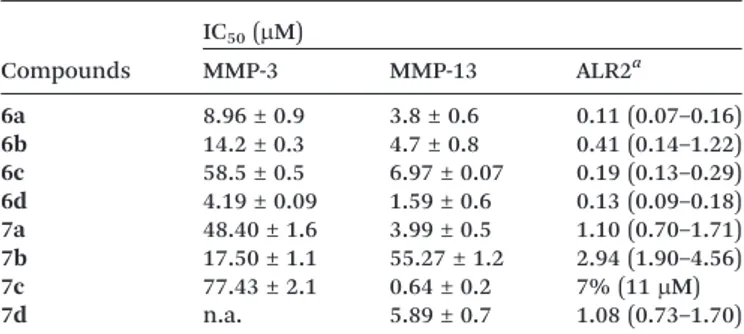
Table 2 In vitro inhibitory activity of compounds 6a–d and 7a–d towards MMP-3, MMP-13 and ALR2 Compounds IC50(μM) MMP-3 MMP-13 ALR2a 6a 8.96 ± 0.9 3.8 ± 0.6 0.11 (0.07–0.16) 6b 14.2 ± 0.3 4.7 ± 0.8 0.41 (0.14–1.22) 6c 58.5 ± 0.5 6.97 ± 0.07 0.19 (0.13–0.29) 6d 4.19 ± 0.09 1.59 ± 0.6 0.13 (0.09–0.18) 7a 48.40 ± 1.6 3.99 ± 0.5 1.10 (0.70–1.71) 7b 17.50 ± 1.1 55.27 ± 1.2 2.94 (1.90–4.56) 7c 77.43 ± 2.1 0.64 ± 0.2 7% (11μM) 7d n.a. 5.89 ± 0.7 1.08 (0.73–1.70) Representative results of at least 3 independent experiments are reported. IC50 (μM) (95% C.L.) or % inhibition at the given
6a–d) did not appear to be univocal. In fact, its removal (com-pounds 7a–d) led to generally less active MMP-3 inhibitors with different potencies against MMP-13 (Table 2).
Finally, comparing the MMP-13 inhibitory activity of 2-thioxo-4-thiazolidinone derivatives 6b and 6c (IC50= 4.7μM
and 6.97 μM, respectively) with those of the corresponding 2,4-thiazolidinedione counterparts (IC50= 11.1μM and 22.3 μM,
respectively)19 highlighted that the replacement of the car-bonyl group in position 2 of the thiazolidinedione ring with the bioisostere thiocarbonyl one provided a 2- to 3-fold gain in potency.
3. Conclusions
In the context of the search for new anti-inflammatory agents, it is desirable to identify agents which are able to block or reduce the actions of mediators involved in oxida-tive/inflammatory cascade and, at the same time, to protect tissues against degenerative damage.
The necessity to develop new agents able to target both inflammatory and degenerative events is also emphasized by considering that the effects of classical non-steroidal anti-inflammatory drugs (NSAIDs) on cartilage metabolism are still debated. Beneficial actions on the cartilage are not established for most NSAIDs, whereas some findings suggested that a certain number of these drugs might accel-erate joint damage.26,27
In the present work, IJ5-arylidene-4-oxo-2-thioxothiazolidin-3-yl)acetic acids (6a–d) and 5-arylidene-4-oxo-2-thio-xothiazolidines (7a–d) were shown to control certain key inflammatory events induced by IL-1β in human chondrocytes. Among them, ij5-IJ3-phenoxybenzylidene)] substituted derivatives 6b and 7b and ij5-IJ3-benzyloxybenzylidene)] analogue 7d exhibited the most prom-ising anti-inflammatory profile, being able to reduce both NF-kB and NO levels to those of the IL-1β-untreated control and to lower ICAM-1 expression significantly. The observed capability of reducing the levels of NF-kB is an attractive aspect, because this ubiquitously expressed nuclear transcrip-tion factor is critically involved in inflammatranscrip-tion and can play a crucial role in the development and progression of many human diseases, such as atherosclerosis, diabetes, cancer, and arthritis. The comparable activity trend of compounds 6 and 7 in reducing NF-kB levels, ICAM-1 expression and NO production highlights that these events are closely correlated in IL-1β-stimulated chondrocytes. In particular, it is likely that the appreciable ability of derivatives 6b, 7b and 7d to reduce ICAM-1 and NO amounts is a consequence of the reduced NF-kB levels. As described above, NF-kB activation can be promoted by both ROS and ALR2, through lipid perox-idation, the subsequent production of GS-DHN and the downstream PKC/PI3K/IKK cascade. Compounds 6b, 7b and 7d are potent ARIs (Table 2); therefore, they may reduce ALR2-mediated NF-kB activation. However, we found that compounds with more marked ALR2 inhibitory and antioxi-dant properties, such as 6a, 6c and 6d, showed lower
capability of reducing NF-kB levels. This suggested that 6b, 7b and 7d might also inhibit NF-kB activation through differ-ent mechanisms involved in the complex IL-1β-induced NF-kB activation pathways, besides via ALR2 inhibition.
Compounds 6 and 7, with the exception of 7b, were also able to oppose or mitigate catabolic events triggered by IL-1β stimulation, in particular by improving GAG production by chondrocytes and contributing to restore a normal PG syn-thesis. In addition, they were shown to inhibit MMP-3 and MMP-13, two enzymes critically involved in the loss of carti-lage integrity in OA.
These findings contribute to extend our knowledge on the biological profile of the 4-thiazolidinone class of ARIs and to delineate their potential as anti-inflammatory agents. On the whole, the multiple anti-inflammatory/antidegenerative pro-file of 2-thioxo-4-thiazolidinones 6 and 7 along with their marked ALR2 inhibitory effectiveness make them interesting cell-permeable molecules that can be assumed as lead com-pounds in the search for novel agents targeting inflammatory diseases.
4. Experimental section
4.1 Human articular chondrocyte culture
Normal human articular cartilage was obtained at replace-ment surgery from some patients with femoral neck acciden-tal fractures whose informed consent was obtained under an approved Institutional Review Board protocol.28
The isolation procedure was conducted under antiseptic conditions. The cartilage was cut into small fragments and carefully washed with Dulbecco's modified Eagle's medium (DMEM) culture medium containing NaHCO3, 25 mM Hepes,
1 mM sodium pyruvate, 50 mg ml−1gentamycin, 100 U ml−1 penicillin, 100 mg ml−1 streptomycin and 2.5 mg ml−1 amphotericin B. Chondrocytes were isolated through three sequential passages of enzymatic digestion of the extracellu-lar matrix: incubation with 0.1% hyaluronidase type III (1 mg ml−1for 100 mg of cartilage), for 30 min at 37°C; incubation with 0.5% pronase type XIV (5 mg ml−1for 100 mg of carti-lage), for 60 min at 37°C; finally incubation with 0.2% colla-genase type IA (2 mg ml−1for 100 mg of cartilage), for 45 min at 37°C. The obtained cellular suspension was filtered (filters from 100 and 70 mm) to eliminate the residues of the diges-tion, cellular aggregates. Freshly isolated chondrocytes were seeded into the monolayer culture at a cell density of 2× 105 cells and cultured in 1 ml of DMEM supplemented with 10% foetal bovine serum (FCS) at 37°C in 5% CO2/95% air.
Con-fluent chondrocytes of primary culture were used for all the experiments: the tested compounds were assayed and dissolved in DMSO, appropriately diluted in DMEM and dispensed to the wells treated as follows: control, IL-1β (10 ng ml−1), 6, 7a–d (10, 2.5 μM) + IL-1β (10 ng ml−1). After 72 h, the supernatants of the cartilage culture were collected for different assays.
4.1.1 Cell viability assay. The cytotoxic effect of the tested substances was evaluated by a cell viability test based on the
cleavage of 3-IJ4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) by mitochondrial dehydrogenases of metabol-ically active cells.24
4.1.2 Western blot analysis: NF-kB and ICAM-1. Chondrocytes were washed twice with ice-cold PBS and col-lected with lysis buffer (10 mM tris-HCl plus 10 mM KCl, 2 mM MgCl2, 0.6 mM PMSF and 1% SDS, pH 7.4). After cooling
for 10 min at 0 °C, cells were sonicated. Equal amounts of total protein (60μg per lane) were separated by 4–12% Novex bis–tris gel electrophoresis (NuPAGE, Invitrogen, Italy) and transferred into nitrocellulose membranes (Invitrogen, Italy) in a wet system. Protein transfer was verified by staining the nitrocellulose membranes with Ponceau S. Membranes were blocked with Tris buffered saline containing 0.01% Tween 20 (TBST) and 5% nonfat milk, washed briefly, incubated with primary antibodies at 4 °C overnight, and then incubated with corresponding HRP-conjugated secondary antibodies for 1 h at room temperature. Mouse monoclonal anti-ICAM-1 (1H4: sc-51632, Santa Cruz Biotechnology, Milan, Italy), anti-NF-kB p65 (ab95020, Abcam, Prodotti Gianni, Milan, Italy) and anti-α-tubulin (Sigma, Milan, Italy) antibodies were diluted in TBST at 1 : 200, 1 : 150 and 1 : 5000, respectively. Protein bands were visualized by incubating membranes with the enhanced chemiluminescence detection Supersignal West Pico Chemiluminescent Substrate (Pierce Chemical Co., Rockford, IL) before exposure to an X-ray film. Chemilumi-nescence signals were densitometrically analyzed using Image-Pro Plus version 6.0 software (Media Cybernetics Inc., Bethesda, MD) and their relative density was calculated based on the density of theα-tubulin bands in each sample. Values were expressed as arbitrary densitometric units (A.D.U.) cor-responding to signal intensity.
4.1.3 Determination of nitrite. Nitrite was determined by adding 100 μl of Griess reagent (1% sulphanylamide and 0.1% naphthylethylenediamine dihydrochloride in 5% of hydrochloric acid) to 100μl of samples.25The optical density atλ = 540 nm was measured using a microtiter plate reader. Nitrite concentrations were calculated by comparison with respective optical densities of standard solutions of sodium nitrite standard curve (0–120 μM).
4.1.4 Determination of GAGs. The level of GAGs, an index of cartilage damage, was measured by spectrophotometry with a solution of 1,9-dimethylmethylene blue atλ = 535 nm using the method of Farndale et al.29The amount of GAGs was calculated from a standard curve (100–500 μg ml−1) obtained for shark chondroitin sulphate C, derived from shark cartilage.
4.2 Oxygen radical absorbance capacity (ORAC) assays The ORAC assay was performed as previously reported by Cao et al.30The measurements were carried out using a VICTOR Wallac 1420 Multilabel Counters fluorimeter (Perkin Elmer, USA) with fluorescence filter (excitation 540 nm, emission 570 nm). Fluorescein (10 nM) was the fluorescence probe and target molecule for free radical attack from AAPH (100 mM) as the peroxyl radical generator. The reaction was conducted
at 37°C at pH 7.0 with Trolox (12.5 μM) as control standard and phosphate buffer as blank. Compounds 6, 7a–d were appropriately diluted with DMSO/buffer (1 : 10) prior to analy-sis. The fluorescein fluorescence was recorded every 2 min after addition of AAPH. All measurements were expressed rel-ative to the initial reading. One blank, one standard, and a maximum of 10 samples were analyzed at the same time. Each measure was repeated at least three times. The ORAC value refers to the net protection area under the quenching curve of fluorescein in the presence of an antioxidant. The final results (ORAC value) were calculated and expressed using Trolox equivalents (TE) forμM of sample (TE/μM):
ORAC units (TE/μM) = K(Ssample– Sblank)/(STrolox− Sblank)
where K is a sample dilution factor and S is the area under the fluorescence decay curve of the sample, Trolox or blank. This area was calculated with Origin®7 (OriginLab Corpora-tion, Northampton, USA).
4.3 MMP-13/3 fluorimetric assay
The compounds were evaluated for their ability to inhibit the hydrolysis of fluorescence-quenched peptide substrate Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 (Vinci Biochem s.r.l.). The
MMP-13 assays were performed in 50 mM HEPES buffer containing 5 mM CaCl2, 0.1 mM ZnCl2, and 0.05% Brij-35, at
pH 7, using 10 nM proteolytic enzyme (catalytic domains of MMP-13) (Vinci Biochem s.r.l.) and 350 nM peptide. To pre-pare MMP-3 buffer, HEPES was replaced with 50 mM MES, at pH 6.
The enzyme was incubated at 25 °C with increasing con-centration of the inhibitor and the fluorescence (excitationmax
328 nm; emissionmax393 nm) was measured for 3 min after
the addition of the substrate using a VICTOR Wallac 1420 Multilabel Counters fluorimeter (Perkin Elmer, USA). Fitting of rates as a function of inhibitor concentration provided IC50values.31
4.4 Statistical analysis
All data are presented as mean ± SEM of at least three sepa-rate experiments. The statistical analysis was performed by using one-way ANOVA followed by Dunnett's post-hoc test for multiple comparisons with control. All statistical analyses were performed using the statistical software package SYSTAT, version 9 (Systat Inc., Evanston, IL, USA). The unpaired Student's t-test was used to compare two different groups. A P value of <0.05 was considered to be statistically significant.
Abbreviation list
ALR2 Aldose reductase
ARIs Aldose reductase inhibitors COX Cyclooxygenase
GS-DHN Glutathionyl-1,4-dihydroxynonene GS-HNE Glutathione-4-hydroxy-trans-2-nonenal HNE 4-Hydroxy-trans-2-nonenal
ICAM Intercellular adhesion molecule IKK Inhibitor-kappa B kinase iNOS Inducible nitric oxide synthase MAPK Mitogen activated protein kinase MMPs Matrix metalloproteinases
NF-kB Nuclear transcription factor-kappa B PI3K Phosphoinositide 3-kinase
PG Proteoglycan PKC Protein kinase C
RNS Reactive nitrogen species ROS Reactive oxygen species TNF-α Tumor necrosis factorα
References
1 S. B. Abramson and M. Attur, Arthritis Res. Ther., 2009, 11, 227–235.
2 A. M. Panico, P. Vicini, A. Geronikaki, M. Incerti, V. Cardile, L. Crascì, R. Messina and S. Ronsisvalle, Bioorg. Chem., 2011, 39, 48–52.
3 J. A. Roman-Blas and S. A. Jimenez, Osteoarthr. Cartil., 2006, 14, 839–848.
4 S. C. Gupta, C. Sundaram, S. Reuter and B. B. Aggarwal, Biochim. Biophys. Acta, 2010, 1799, 775–787.
5 A. F. Mendes, M. M. Caramona, A. P. Carvalho and M. C. Lopes, J. Cell. Biochem., 2003, 88, 783–793.
6 R. M. Clancy, P. F. Gomez and S. B. Abramson, Osteoarthr. Cartil., 2004, 12, 552–558.
7 A. M. Panico, V. Cardile, F. Garufi, C. Puglia, F. Bonina and G. Ronsisvalle, Life Sci., 2005, 77, 2479–2488.
8 L. A. Flugge, L. A. Miller-Deist and P. A. Petillo, Chem. Biol., 1999, 6, 157–166.
9 P. Lavigne, M. Benderdour, D. Lajeunesse, Q. Shi and J. C. Fernandes, Bone, 2004, 35, 463–470.
10 F. Iannone and G. Lapadula, Aging: Clin. Exp. Res., 2003, 15, 364–372.
11 C. B. Forsyth, A. Cole, G. Murphy, J. L. Bienias and R. F. Loeser, J. Gerontol., Ser. A, 2005, 60, 1118–1124.
12 W. B. Van Den Berg, Curr. Rheumatol. Rep., 2008, 10, 26–29. 13 M. Brownlee, Nature, 2001, 414, 813–820.
14 R. Maccari and R. Ottanà, in Advances in Molecular Mechanisms and Pharmacology of Diabetic Complications, ed.
M. Stefek, Transworld Research Network, Kerala, India, 2010, pp. 219–245.
15 S. K. Srivastava, U. C. S. Yadav, A. M. Reddy, A. Saxena, R. Tammali, M. Shoeb, N. H. Ansari, A. Bhatnagar, M. J. Petrash, S. Srivastava and K. V. Ramana, Chem.-Biol. Interact., 2011, 191, 330–338.
16 S. Pandey, S. K. Srivastava and K. V. Ramana, Expert Opin. Invest. Drugs, 2012, 21, 329–339.
17 R. Ottanà, R. Maccari, M. L. Barreca, G. Bruno, A. Rotondo, A. Rossi, G. Chiricosta, R. Di Paola, L. Sautebin, S. Cuzzocrea and M. G. Vigorita, Bioorg. Med. Chem., 2005, 13, 4243–4252. 18 R. Ottanà, R. Maccari, R. Ciurleo, M. G. Vigorita, A. M.
Panico, V. Cardile, F. Garufi and S. Ronsisvalle, Bioorg. Med. Chem., 2007, 15, 7618–7625.
19 A. M. Panico, R. Maccari, V. Cardile, L. Crascì, S. Ronsisvalle and R. Ottanà, Med. Chem., 2013, 9, 84–90.
20 R. Maccari, R. M. Vitale, R. Ottanà, M. Rocchiccioli, A. Marrazzo, V. Cardile, A. C. E. Graziano, P. Amodeo, U. Mura and A. Del Corso, Eur. J. Med. Chem., 2014, 81, 1–14. 21 R. Maccari, A. Del Corso, M. Giglio, R. Moschini, R.
Mura and R. Ottanà, Bioorg. Med. Chem. Lett., 2011, 21, 200–203.
22 G. Bruno, L. Costantino, C. Curinga, R. Maccari, F. Monforte, F. Nicolò, R. Ottanà and M. G. Vigorita, Bioorg. Med. Chem., 2002, 10, 1077–1084.
23 R. Maccari, R. Ottanà, C. Curinga, M. G. Vigorita, D. Rakowitz, T. Steindl and T. Langer, Bioorg. Med. Chem., 2005, 13, 2809–2823.
24 T. J. Mossmann, J. Immunol. Methods, 1983, 65, 55–63. 25 L. C. Green, D. A. Wagner, J. Glogowski, P. L. Skipper, J. S.
Wishnok and S. R. Tannenbaum, Anal. Biochem., 1982, 126, 131–138.
26 J. T. Z. Dingle, Z. Rheumatol., 1999, 58, 125–129.
27 E. Gineyts, J. A. Mo, A. Ko, D. B. Henriksen, S. P. Curtis, B. J. Gertz, P. Garnero and P. D. Delmas, Ann. Rheum. Dis., 2004, 63, 857–861.
28 A. M. Panico, P. Vicini, G. Massimo, V. Cardile, B. Gentile, S. Avondo, F. Vittorio and G. Ronsisvalle, Inflammation, 2004, 28, 231–235.
29 R. W. Farndale, C. A. Sayers and A. J. Barrett, Connect. Tissue Res., 1989, 2, 247–248.
30 G. Cao and R. Prior, Methods Enzymol., 1999, 299, 50–62. 31 I. Bertini, V. Calderone, M. Fragai, A. Giachetti, M. Loconte,
C. Luchinat, M. Maletta, C. Nativi and K. J. Yeo, J. Am. Chem. Soc., 2007, 129, 2466–2475.