RevistaBrasileiradeFarmacognosia28(2018)374–377
w ww.e l s e v i e r . c o m / l o c a t e / b j p
Short
communication
Anti-angiogenic
activity
of
iridoids
from
Galium
tunetanum
César
Mu ˜
noz
Camero
a,
Maria
Paola
Germanò
b,
Antonio
Rapisarda
b,
Valeria
D’Angelo
b,
Smain
Amira
c,
Fatima
Benchikh
c,
Alessandra
Braca
a,∗,
Marinella
De
Leo
aaDipartimentodiFarmacia,UniversitàdiPisa,Pisa,Italy
bDipartimentodiScienzeChimiche,Biologiche,FarmaceuticheeAmbientali,UniversitàdegliStudidiMessina,PoloUniversitarioSS,Annunziata,Messina,Italy cDepartmentofAnimalBiologyandPhysiology,UniversityofSetif,Setif,Algeria
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:Received29January2018 Accepted29March2018 Availableonline25May2018 Keywords:
Anti-angiogenicactivity Asperuloside
Chickchorioallantoicmembrane Geniposidicacid
IridoidV1
a
b
s
t
r
a
c
t
ThephytochemicalstudyofGaliumtunetanumLam.,Rubiaceae,leavesledtotheisolationof13 com-poundsfromthechloroform–methanolandthemethanolextracts,including sixiridoidglycosides, onenon-glycosideiridoid,twop-coumaroyliridoidglycosides,twophenolicacids,andtwoflavonoid glycosides.Thestructuraldeterminationoftheisolatedcompoundswasperformedbymono-and bidi-mensionalNMRspectroscopicdata,aswellasESI-MSexperiments.Allcompoundswereisolatedfrom thisspeciesforthefirsttime.Theanti-angiogeniceffectsoftheisolatediridoidswerealsoreportedon newbloodvesselsformationusingthechickembryochorioallantoicmembraneasinvivomodel.Results showedthatamongtheisolatediridoidstestedatthedoseof2g/egg,asperuloside(1),geniposidicacid (2),andiridoidV1(3)reducedmicrovesselformationofthechorioallantoicmembraneonmorphological observationsusingastereomicroscope.Theanti-angiogeniceffectsoftheactivecompounds,expressed aspercentagesofinhibitionversuscontrol,were67%(1),59%(2),and54%(3),respectively.Inaddition, theactivecompoundswereabletoinhibitangiogenesisinthechorioallantoicmembraneassay,ina dose-dependentmanner(0.5–2g/egg)ascomparedtothestandardretinoicacid.
©2018SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Plants belonging to Galium genus, Rubiaceae, comprising
approximately1300species,areknowninethnobotanicalfieldfor
thetreatmentofavarietyofpathologicalconditions,suchas
psori-asis,skininfections(Oumeish,1999),hepatitis(Bolivaretal.,2011),
kidneydisorders,andassedative,diuretic,andtotreattheepilepsy
andhysteria(Shahetal.,2006).G.tunetanumLam.isaperennial
herb,nativetoTunisia,Algeria,Marocco,Spain,andSicily(Casimiro
etal.,2012).Tothebestofourknowledge,intheliteraturethere
isonlyonereportabouttheantioxidantactivityofthemethanol
extractofitsleaves(Gaamouneetal.,2014)butnophytochemical
studieshavebeencarriedoutsofar.
Galium genus is well-known for producing several classes
of secondary metabolites such as iridoid glycosides, saponins,
triterpenes, anthraquinones, and flavonoid glycosides (Mocan
et al., 2016). Iridoids are a large class of natural products,
exhibitingawiderangeofpharmacologicalactivitiessuchas
anti-inflammatory,anticancer,cardioprotective,andneuroprotective.
Interestingly,theiridoidglycosidegeniposidewasfoundtohave
∗ Correspondingauthor.
E-mail:[email protected](A.Braca).
apotentanti-angiogenicactivityinthechickembryo
chorioallan-toicmembrane(CAM)assay(Kooetal.,2004).Angiogenesisisthe
growthofnewbloodvesselstoensurewoundhealing,
reproduc-tion,anddevelopmentsofcells.Thisphysiologicalprocessplaysan
importantroleintheexpansionofveinsandbloodcapillariesand
inthenutritionoftumorcells.Thus,angiogenesisinhibitionmight
beapromisingapproachforanticancertherapies.
Inthecourseofourinvestigationonplantsbelongingtothe
African flora (Beladjila et al., 2017), the chemical study of G.
tunetanum leaves was performed, and the isolation and
struc-tural characterization of 13 compounds, including nine iridoid
glycosides(1–9),twophenolic acids(10–11),andtwo flavonoid
glycosides(12–13)washereinreported.Theanti-angiogeniceffect
ofiridoids1–8onnewbloodvesselsformation,usingtheCAMassay
asinvivomodel,wasalsoexplored.
Materialsandmethods
Oneandtwo-dimensionalNMRexperimentswereperformed
on a Bruker DRX-600 spectrometer at 300K (Bruker BioSpin,
Rheinstetten,Germany) equippedwitha Bruker5mm TCI
Cry-oProbe, acquiring the spectra in methanol-d4. Pulse sequences
and phase cycling wereused for DQF-COSY,TOCSY, HSQC, and
HMBC,experiments.NMRdatawereprocessedusingXWinNMR
https://doi.org/10.1016/j.bjp.2018.03.010
0102-695X/©2018SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
C.M.Cameroetal./RevistaBrasileiradeFarmacognosia28(2018)374–377 375
software (De Leo et al., 2017). ESI-MSwere obtained from an
LCQAdvantageThermoFinniganspectrometer(ThermoFinnigan,
USA), equipped with Xcalibur software. Column
chromatogra-phies (CC) were performed over Sephadex LH-20 (40–70m,
AmershamPharmaciaBiotechAB,Uppsala,Sweden)andIsolera®
Biotage® purificationsystem(flashSilicagel60SNAP340g
car-tridge,flowrate90ml/min)(Milellaetal.,2016).Reversephase–
highperformance liquidchromatography(RP-HPLC)separations
were conducted on a Shimadzu LC-8A series pumping system
equippedwithaShimadzuRID-10Arefractiveindexdetectorand
ShimadzuinjectoronaC18-Bondapakcolumn(30cm×7.8mm,
10mWaters,flowrate2ml/min,Milford,MA,USA).ThinLayer
Chromatography(TLC)analyseswerecarriedoutusingprecoated
Kieselgel60F254(0.20mmthickness)plates(Merck,Darmstadt,
Germany);compoundsweredetectedbyceriumdisulfate/sulfuric
acid(Sigma–Aldrich, Milan,Italy). Allthesolvents used forthe
extractionandseparationprocessesandretinoicacidusedforthe
CAMassayasantiangiogenicreferencecompoundwerepurchased
fromSigma-Aldrich(Milan,Italy).
GaliumtunetanumLam.,Rubiaceae,leaveswerecollectedand
identifiedbyauthorsSmainAmiraandFatimaBenchikhinDjilma,
45kmawayfromJijel,NortheastAlgeria,inJune2013.Avoucher
specimen has been deposited at the Herbarium Horti Botanici
Pisani,Pisa,Italy(n.8486Galiumtunetanum/1,NuoveAcquisizioni).
Briefly, dried leaves of the plant (1kg) were extracted
with solvents of increasing polarity: n-hexane, chloroform,
chloroform–methanol(9:1),andmethanolbyexhaustive
macera-tiontogive4.0,13.3,11.9,and48goftherespectiveresidues.The
methanolextractwaspartitionedbetweenn-butanolandwaterto
affordan-butanolresidue(10.8g),thatwassubmittedtoSephadex
LH-20 column chromatography (5×75cm, flow rate 1ml/min)
usingmethanolaseluentandcollectingninemajorfractions(A–I)
groupedbyTLC.PartofthefractionB(1.5g)wassubjectedto
RP-HPLCwithmethanol–water(3:7)aseluent,togivecompounds2
(0.7mg,tR7min)and7(1.4mg,tR14min).FractionsE(273.3mg),
F(707.3mg),G(724.0mg),andI(818.2mg)weresubmittedto
RP-HPLCusingmethanol–water(35:65)aseluent,togivecompounds
3(5.0mg, tR 14min) and 8(1.7mg, tR 55min) fromfractionE;
compounds10(1.5mg,tR9min)and 9(0.5mg,tR22min)from
fractionF;compounds 11 (6.0mg, tR 6min) and 12 (1.3mg, tR
32min) fromfractionG;compound13 (2.6mg,tR 39min)from
fractionI,respectively.TheremainingfractionsB(874.2mg)andC
(922.3mg)weresubjectedtoRP-HPLCwithmethanol–water(1:4)
aseluent,togivecompound6(1.3mg,tR5min)fromfractionBand
compound2(1.3mg,tR8min)fromfractionC,respectively.Partof
thechloroform–methanolresidue(5.6g)wassubjectedtoIsolera
Biotagecolumnchromatography(340gsilicaSNAPcartridge,flow
rate90ml/min),elutingwithchloroformfollowedbyincreasing
concentrationsofmethanolinchloroform(between1%and100%).
Fractionsof27mlwerecollected,analyzedbyTLCandgroupedinto
fivemajorfractions(A–E).FractionsB(331.4mg)andC(1481.8mg)
weresubjectedtoRP-HPLCwithmethanol–water(3:7)aseluent,
togivecompounds5(1.3mg, tR 6min)and 7(3mg, tR 15min)
from fractionB; compound 1 (23.6mg, tR 8min) from fraction
C,respectively.FractionE(509.7mg)wassubmittedtoRP-HPLC
withmethanol–water(1:4)aseluent,togivecompound4(6.6mg,
tR7min).
The CAM assay was performed following the method of
Germanòetal.(2015)modified(Certoetal.,2017).Fertilizedeggs
ofGallusgalluswerepreviouslymaintainedinahumidified
incu-batorat37◦Cand,afterfourdaysofincubation,asmallwindow
wascreatedonthebroadsideoftheeggstoapplydifferentdoses
ofpurecompounds(0.5–2g/egg) directlyontheCAMsurface,
previously suspended in albumen. Retinoicacid(2g/egg) was
used as antiangiogenic reference compound. After treatment,
theeggswerereincubatedfor24h,thentheywereobservedby
meansofasteromicroscope(ZeissStemi2000-c)equippedwith
a digitalcamera(Axiocam MRc5 Zeiss)andphotographed.The
antiangiogeniceffectsontheCAMwerequantifiedbycountingthe
numberofbloodvesselbranchpointsinastandardizedareausing
aZeisssoftwareformicromorphometricanalysisandexpressedas
%ofinhibitionrespecttocontrol.Eachexperimentwasrepeated
threetimes.Thesignificanceof thedifferenceswasassessedon
thebasisofthet-test,consideringthedifferencesforp<0.05,and
finallycalculatedwithrespecttothelotofcontroleggstreatedonly
withalbumen.
Resultsanddiscussion
The phytochemical study of chloroform–methanol and
methanol extracts of G. tunetanum leaves afforded the
isola-tionofthirteencompounds1–13.Theirstructuraldetermination
was performed by 1D and 2D NMR spectroscopic techniques,
massspectrometryanalyses, andcomparisonofthesedatawith
those reported in the literature. Isolated compounds included
six iridoid glycosides identified as asperuloside (1) (Otsuka
etal.,1991),geniposidicacid(2)(Güvenalpetal.,2006),iridoid
V1 (3) (Mitova et al., 1999), deacetylasperuloside (4) (Otsuka
etal.,1991),monotropein(6)(Tzakouetal.,2007),and
daphyl-loside (7) (Demirezer et al., 2006); one non-glycoside iridoid
macedonine (5) (Mitova et al., 1996); two p-coumaroyl
iri-doid derivatives, 10-O-p-coumaroyl-10-deacetyldaphylloside
(8) (Ahn and Kim, 2012) and
10-O-p-coumaroyl-10-deacetylasperuloside (9) (Bai and Hu, 2006); two phenolic
acids characterized as p-hydroxyhydrocinnamic acid (10) and
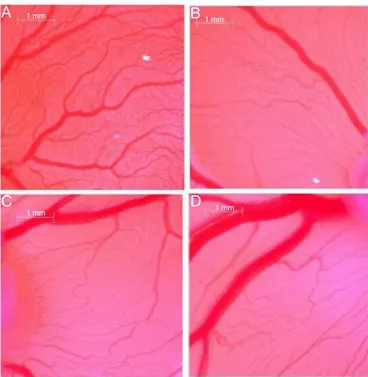
376 C.M.Cameroetal./RevistaBrasileiradeFarmacognosia28(2018)374–377 1 mm 1 mm 1 mm 1 mm
A
B
C
D
Fig.1. Chickembryochorioallantoicmembrane(CAM)treatedwithatadoseof2g/egg.(A)Control;(B)asperuloside(1);(C)geniposidicacid(2);(D)iridoidV1(3).
80% 0.5 µg 1 µg 2 µg 70% 60% 40% 50% 30% 20% 10% 0% INHIBITION Geniposidic acid Asperuloside Iridoid V1 Retinoic acid
Fig.2.Dose-dependentanti-angiogenicactivityofasperuloside(1),geniposidicacid(2),andiridoidV1(3)inthechickembryochorioallantoicmembrane(CAM)assay. Retinoicacidwasusedasapositivecontrol.CAMsweretreatedwithcompoundsatdosesof0.5–2g/egg.Eachgroupcontainedatleast10eggs.Eachvaluerepresentsthe mean±SDofthreeexperiments.
glycosidesrutin (12)andapigenin-7-O-glucoside(13)(Agrawal,
1989).
Isolatediridoids,except9thatwasobtainedintoosmall
quan-tity, were subjected to CAM assay in order to evaluate their
anti-angiogeniceffects.
Theanti-angiogeniceffectsofisolatediridoids(2g/egg)inthe
CAMassayshowedthatcompounds1,2,and3wereabletoreduce
CAMmicrovesselformationwithinhibitionsof67%,59%,and54%,
respectively.Besides,compounds4–8demonstratedthefollowing
inhibitionvalues:43%,31%,23%,19%,and16%.Noteworthy,1has
ahigheranti-angiogenicactivityinrespecttothestandardretinoic
acid(62%).RepresentativemicroscopicimagesoftheCAMafter
treatmentwiththeactivecompounds1–3arereportedinFig.1.
Controleggsshowedthepresenceofaclearvascularnet-workwith
largevesselsconvergingtowardtheembryo(Fig.1A).Conversely,
avisiblereductionofbloodvesselbranchpointsisevidencedin
theCAM treatedwith1,2,and 3(Fig.1B–D).In addition,these
activecompoundsdemonstratedtoinhibitCAMangiogenesisina
dose-dependentmanner(0.5–2g/egg)(Fig.2).
Itisknownthatinhibitionofangiogenesishasbeenrecognized
tobeadvantageousforthepreventionofinflammationand
neo-plasticgrowth.Forthisreasonnowadaysthereisagrowinginterest
todiscovernewinhibitorsofangiogenesisfromnaturalsources.
TheCAMmodeloffersadvantagesthat includethecomparative
easeofculture,lowcost,andeasyobservationofthe
neovasculari-sation(Koutsavitietal.,2017).Amongtheisolatediridoidstested,
asperuloside(1),geniposidicacid(2),andiridoidV1(3)exhibited
highinhibitoryactivityonCAMangiogenesis.Theseresultsarein
accordancewiththestudyofKooetal.(2004)wheretheiridoid
C.M.Cameroetal./RevistaBrasileiradeFarmacognosia28(2018)374–377 377
activityofGardeniajasminoidesfruitsethanolextract.Insummary,
theresultsobtainedmaybethestartingpointforconsideringG.
tunetanumanewsourceofanti-angiogeniccompounds.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thatnoexperimentswereperformedonhumansoranimalsfor
thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Authors’contributions
ABplannedtheexperiments.CMCcarriedouttheextractionand
purificationofcompounds.MDLperformedtheNMRandESI-MS
experiments.SAandFBcollected,identifiedtheplantmaterialand
contributedtotheinterpretationofresults.MPG,AP,VDperformed
thebiologicalassays.Allauthorscontributedtothecriticalrevision
ofthemanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
References
Agrawal,P.K.,1989.Carbon-13NMRofFlavonoids.Elsevier,NewYork.
Ahn,D.,Kim,D.K.,2012.IridoidglycosidesfromtheaerialpartsofGaliumspurium L.Nat.Prod.Sci.18,195–199.
Bai,H.,Hu,L.,2006.StudyonthechemicalconstituentsofDaphniphyllum angusti-folium.Helv.Chim.Acta89,884–894.
Beladjila,K.A.,Cotugno,R.,Berrehal,D.,Kabouche,Z.,DeTommasi,N.,Braca,A.,De Leo,M.,2017.CytotoxictriterpenesfromSalviabuchananiiroots.Nat.Prod.Res. 20,1–6.
Bolivar,P.,Cruz-Paredes,C.,Hernandez,L.R.,Juárez,Z.N.,Sánchez-Arreola,E., Av-Gay,Y.,Bach, H., 2011.Antimicrobial, anti-inflammatory,antiparasitic, and cytotoxicactivitiesofGaliummexicanum.J.Ethnopharmacol.137,141–147.
Casimiro,F.,Pérez,A.V.,Cabezudo,B.,2012.SobrelapresenciadeGaliumtunetanum Lam.enlaSierradelasNieves(MálagaEspa ˜na).Acta.Bot.Malac.37,238–240. Certo,G.,Costa,R.,D’Angelo,V.,Russo,M.,Albergamo,A.,Dugo,G.,Germanò,M.P.,
2017.Anti-angiogenicactivityandphytochemicalscreeningoffruitfractions fromVitexagnuscastus.Nat.Prod.Res.31,2850–2856.
DeLeo,M.,Peruzzi,L.,Granchi,C.,Tuccinardi,T.,Minutolo,F.,DeTommasi,N.,Braca, A.,2017.ConstituentsofPolygalaflavescensssp.flavescensandtheiractivityas inhibitorsofhumanlactatedehydrogenase.J.Nat.Prod.80,2077–2087. Demirezer, L.O., Gurbuz,F.,Güvenalp, Z., Stroch,K.,Zeeck, A.,2006. Iridoids,
flavonoidsandmonoterpeneglycosidesfromGaliumverumsubsp.verum.Turk. J.Chem.30,525–534.
Gaamoune,S.,Harzallah,D.,Kada,S.,Dahamna,S.,2014.Evaluationofantioxidant activityofflavonoidsextractedfromGaliumtunetanumPoiret.Res.J.Pharm. Biol.Chem.Sci.5,341–348.
Germanò,M.P.,Certo,G.,D’Angelo,V.,Sanogo,R.,Malafronte,N.,DeTommasi,N., Rapisarda,A.,2015.Anti-angiogenicactivityofEntadaafricanaroot.Nat.Prod. Res.29,1551–1556.
Güvenalp,Z.,Kilic¸,N.,Kazaz,C.,Kaya,Y.,Demirezer,L.O.,2006.Chemicalconstituents ofGaliumtortumense.Turk.J.Chem.30,515–523.
Koo,H.-J.,Lee,S.,Shin,K.-H.,Kim,B.-C.,Lim,C.-J.,Park,E.-H.,2004.Geniposide,an anti-angiogeniccompoundfromthefruitsofGardeniajasminoides.PlantaMed. 70,467–469.
Koutsaviti,A.,Tzakou,O.,Galati,E.M.,Certo,G.,Germanò,M.P.,2017.Chemical com-positionofJuniperusphoeniceaandJ.drupaceaessentialoilandtheirbiological effectsinthechoriallantoicmembrane(CAM)assay.Nat.Prod.Commun.12, 449–452.
Milella,L.,Milazzo,S.,DeLeo,M.,VeraSaltos,M.B.,Faraone,I.,Tuccinardi,T.,Lapillo, M.,DeTommasi,N.,Braca,A.,2016.␣-Glucosidaseand␣-amylaseinhibitors fromArcytophyllumthymifolium.J.Nat.Prod.79,2104–2112.
Mitova,M.,Handjieva,N.,Spassov,S.,Popov,S.,1996.Macedonine,anon-glycosidic iridoidfromGaliummacedonicum.Phytochemistry42,1227–1229.
Mitova,M.,Handjieva,N.,Anchev,M.,Popov,S.J.,1999.Iridoidglucosidesfrom Galiumhumifusum.J.Biosci.54,488–491.
Mocan,A.,Crisan,G.,Vlase,L.,Ivanescu,B.,Badarau,A.S.,Arsene,A.L.,2016. Phy-tochemicalinvestigationsonfourGaliumspecies(Rubiaceae)fromRomania. Farmacia64,95–99.
Otsuka,H.,Yoshimur,K.,Yamasaki,K.,Cantoria,M.C.,1991.Isolationof10-O-acyl iridoidglucosidesfromaPhilippinemedicinalplantOldenlandiacorymbosaL. (Rubiaceae).Chem.Pharm.Bull.39,2049–2052.
Oumeish,Y.,1999.TraditionalArabicmedicineindermatology.Clin.Dermatol.17, 13–20.
Owen,R.W.,Haubner,R.,Mier,W.,Giacosa,A.,Hull,W.E.,Spiegelhalder,B.,Bartsch, H.,2003.Isolation,structureelucidationandantioxidantpotentialofthemajor phenolicandflavonoidcompoundsinbrinedolivedrupes.FoodChem.Toxicol. 41,703–717.
Shah,S.R.U.,Quasim,M.,Khan,I.A.,Shah,S.A.U.,2006.Studyofmedicinalplants amongweedsofwheatandmaizeinPeshawarregion.Pak.J.Weed.Sci.Res.12, 191–197.
Tzakou,O.,Mylonas,P.,Vagias,C.,Petrakis,P.V.,2007.Iridoidglucosideswith insec-ticidalactivityfromGaliummelanantherum.J.Biosci.62,597–602.