I
>2 vt <I ....>:- >2.- *. . 24vt&ft, /i . *. .VKfft.*-<t... '.A.
remnant liverremoval. Bindingreactionswere
per-formedessentially asdescribed(3, 4) with nuclear
extractsfrommouseliver cells after hepatectomy.For
STAT binding, the probe usedwas apreviously
an-nealed high-performance liquid chromatography-pu-rified double-stranded oligonucleotidefrom the
sis-induciblefactor binding element in the c-fos promoter
(5'-GATCCTCCAGCATTTCCCGTAAATCCTCCAG-3') (22) and end-labeled with
[-y-32P]adenosine
triphosphate(ATP). Supershiftexperiments wereper-formed by incubating1,ulof primary antibodywith the nuclear extracts inbinding bufferfor 1 to 2 hours at
4°C before addition of the labeled oligonucleotide.
Antibody toSTAT3 (anti-STAT3)andanti-STAT5were
fromSanta Cruz Biotechnology.
13. S. Ruff-Jamison, K. Chen, S. Cohen, Proc. Natl. Acad.Sci. U.S.A. 92, 4215 (1995).
14. N.-O. Ku,S. Michie, R. G. Oshima,M. B.Omary, J. Cell Biol. 131, 1303(1995).
15. D. E.Cressmaneta/., unpublisheddata. 16. Hepatectomized animals were anesthetized and
ventral laparotomywasperformed. Normalliver was
prepared by subjecting animalsto laparotomy
fol-lowed by perfusionasdescribed(26). One hour be-fore theremnantliverwasharvestedandfixed, ani-mals wereinjected intraperitoneallywith BrdU (50 mg/kg) (0.2% solutioninPBS) [B. Schutte,M. M. J. Reynders, F. T. Bosman, G. H. Blijham, J. Histo-chem. Cytochem. 35, 1343 (1987)]. The portal vein
wascannulatedwith a22-gauge angiocatheter, the
liver wasflushed with PBS, and4% paraformalde-hyde (pH 7.2)(4°C)wasthen perfused for 10min at a rateof 6 ml/min. The fixed liverwasremovedand cutinto 5-mm slices with a razorblade and then fixed for1hourin4%paraformaldehydeat4°C. An auto-mated tissue processor wasused to embed liver
slices withparaffin. Tissuesections(5pm)were cut onamicrotomeandadheredto
poly-L-lysine-coat-edglass slides.Stainingof fixed tissuesampleswith anantibodytoBrdU (Boehringer Mannheim) allows
one todiscern proliferating cells(brown, stained
nu-clei) from quiescent ones(clear, unstained nuclei). The immunohistochemicalstudywasperformed
es-sentially as described [S. M. Hsu, L. Raine, H.
Fanger, Am. J. Clin. Pathol. 75, 734 (1981); L. E. Greenbaum,D. E.Cressman,B. A.Haber,R.Taub, J.Clin. Invest. 96(1995)]. StatWorks and Student's
ttest,respectively,wereused for statisticalanalyses
onanimal liverweights and DNA synthesis.
17. M. D.Dabeva andD. A.Shafritz,Am.J.Pathol. 143,
1606(1993).
18. H. M. Rabes, G. Iseler,S. Czichos, H. V.Tuczek, Cancer Res.37, 1105 (1977).
19. R.Taub,in LiverRegenerationandCarcinogenesis,
R. L.Jirtle, Ed. (Academic Press, San Diego, CA, 1995),p. 71.
20. ForRNApreparation, animalswerekilled atthe
indi-catedtimes, and total liverRNApreparation, North-ernblots, and hybridizationswereperformedas de-scribed [K.L. Mohnetal., Mol. Cell. Biol. 11,381
(1991)]. For immunoblots,20
pLg
of nuclear or whole-cell extract waselectrophoresedon10 to15% SDS-polyacrylamide gels, transferred to nitrocellulose,and detected by chemiluminescence (Amersham) accordingtotheinstructions ofthe manufacturer as
described(3,4). Primary antibodies usedinprotein immunoblots, electrophoretic gel-mobility
super-shift, and immunohistochemistry studieswere
anti-Fos andanti-cyclinDl (Santa Cruz Biotechnology), anti-p50-NF-KB1(2), anti-JunB,and anti-LRF[J.-C. Hsu, R. Bravo, R.Taub, Mol. Cell. Biol. 12, 4654
(1992)]. TheAP-1probewas adouble-stranded
oli-gonucleotide containing the consensus AP-1 site
(3'-CGCTTGATGAGTCAGCCGGAA-5')(Promega). TheNF-KBprobewas apreviouslyannealed high-performance liquid chromatography-purified dou-ble-strandedoligonucleotidefromtheclass major histocompatibility complexenhancer elementH2-KB (5'-TCGAGGGCTGGGGATTCCCCATCTC-3')(2).
21. P. Cofferetal., Oncogene 10, 985(1995); L. M.
Robertsonetal.,Neuron14,241(1995).
22. B.J.Wagneretal., EMBOJ.9,4477(1990).
23. X.Oian,U. Samadani,A.Porcella,R. H.Costa, Mol.
Cell. Biol. 15,1364(1995).
24. R. H.Diamond, D. E.Cressman,T. M.Laz, C. S.
Abrams, R.Taub, ibid. 14, 3752(1994); F. Hilberg, A.Aguzzi, N.Howells, E. F. Wagner, Nature365,
179(1993); C. Schmidtetal., ibid.373, 699 (1995). 25. B.A.Haberetal., J. Clin. Invest. 95,832(1995).
26. J. Lee etal., Hepatology19,656(1994).
27. H.Baumann,K.K. Morella, S.P.Campos,Z.Cao, G.P.Jahreis, J. Biol. Chem.267,19744(1992).
28. J. I.Daksis,R. Y.Lu,L. M.Facchini,W. W.Marhin,L.
J.Z.Penn,Oncogene 9, 3635 (1994); J. Phuchareon andT.Tokuhisa,CancerLett.92,203(1995). 29. T. Hunter and J. Pines, Cell 79, 573 (1994); D.
Resnitzky,M. Gossen,H. Bujard, S. I. Reed, Mol. Cell. Biol.14,1669 (1994);J. H.Albrecht,M. Y.Hu, F.B.Cerra,Biochem.Biophys. Res. Commun. 209,
648(1995).
30. J. Deviere et al., Clin. Exp. Immunol. 77, 221
(1989);A. M.Gressner, KidneyInt.49, S-39 (1996); C.McClain,D.Hill, J.Schmidt,A. M.Diehl,Semin. Liver Dis. 13,170(1993);H.Tilgetal., Gastroen-terology 103,264(1992);P.Greenwel,J.Rubin,M.
Schwartz,E. L.Hertzberg,M.Rojkind,Lab. Invest.
69,210
(1993).
31. J.Baueret
al.,
Blood72,
1134(1988);
T.Kishimoto, S. Akira, T.Taga,
Science 258, 593(1992);
Y.Yamada, I.
Kirillova,
J. J.Peschor,
N.Fausto,
inpreparation.
32. Wethank J.Darnell for the STAT3cDNA,U. Muller
Eberhard and S. Maeda for the
hemopexin
(HPX)
and serumamyloid
P-component (SAP) probes,
andC. Steerfor thegift
of thecyclin
Dl cDNA andhelpful
discussions. We also thankC.Deutsch-mann, R.Diamond,D.Tewari,P.Traber,M.Lazar, andF.Rauscherfor
helpful discussions;
S.Hwang
andV. Miles for technicalassistance;and J. Mat-thewsfor assistancewith
manuscript
preparation.
Thiswork was in part
supported by
NIHgrants
DK44237,
DK49210,
andDK49629(to R.T.);
NIHgrant K08
(to
L.E.G.);
theUniversity
of Pennsylva-nia GeneticsTraining
Grant(to
R.A.D.);
andtheHoward
Hughes
Medical Institute.17July 1996;
accepted
1October1996Neuroprotection by Aspirin
and
Sodium
Salicylate Through
Blockade of NF-KB Activation
Mariagrazia
Grilli,*
Marina
Pizzi,
Maurizio
Memo,
PierFranco Spano
Aspirin
(acetylsalicylic acid)
is
acommonly prescribed drug
with
awide
pharmacological
spectrum.
At
concentrations
compatible
with amounts in
plasma during
chronic
anti-inflammatory
therapy, acetylsalicylic
acid and its metabolite sodium
salicylate
werefound to be
protective against neurotoxicity
elicited
by
the
excitatory
amino acid
glu-tamate in rat
primary neuronal cultures
and
hippocampal
slices. The site of action of the
drugs
appeared
to be downstream of
glutamate receptors
and to involve
specific
inhi-bition of
glutamate-mediated
induction of nuclear factor
kappa
B.
These results
maycontribute to
the
emerging
theme of
anti-inflammatory drugs
and
neurodegeneration.
Glutamate
is
the most abundant excitatory
neurotransmitter in
the
brain; however,
un-der
certain
conditions,
it
may
become
a
potent
excitotoxin
and contribute
to
neu-rodegeneration (1).
On
the other
hand,
an
accumulation
of
clinical and
experimental
evidence suggests that
neurodegeneration
is
often associated with
inflammation
(2).
We
tested
the
possibility that the
anti-inflam-matory
drugs aspirin
[acetylsalicylic acid
(ASA)]
and sodium
salicylate (NaSal),
be-cause
of
their wide spectrum of
pharmaco-logical
activities
and
multiple
sites of action
(3), may confer neuroprotective properties.
Several models
of neurons in
culture
have been
used to
unravel the
molecular
events
triggered by glutamate that lead
to
cell
death
as
well
as to
develop
pharmaco-logical compounds able
to counteract
exci-totoxicity.
Here we
used
primary cultures of
rat
cerebellar
granule cells, where
a brief
pulse of glutamate, through
activation
of
DivisionofPharmacology,DepartmentofBiomedical
Sci-encesandBiotechnologies, Universityof Brescia Medical
School,Brescia,1-25123Italy.
*To whomcorrespondenceshould be addressed. E-mail:
the
N-methyl-D-aspartate
(NMDA)
type
of
glutamate
receptor,
induces cell death
(4).
ASA
and NaSal
were
added
to
the culture
medium
5
min
before
and
during
a
15-min
application
of
50
pM
glutamate
(5),
a
con-centration
that reduced cell survival
by
70
to
80%.
The
range
of
concentrations
for
both
drugs
was
correlated with the
amounts
in
plasma (1
to
3
mM)
for
optimal
anti-inflammatory
effects
in
patients
with
rheu-matic
diseases
(3).
A
concentration-depen-dent
protection against
glutamate-induced
neurotoxicity
was
observed
in
the
presence
of
both
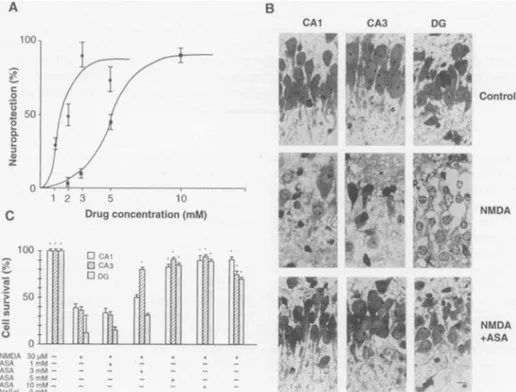
drugs (Fig. 1A).
For
ASA,
the
calculated median effective
concentration
(EC50)
was
1.7
mM, with maximal
effect
(83%
protection)
at
3 mM.
The
concentra-tion
of
NaSal
giving 50%
protection
was
5
mM,
and maximal
response
(87%
protec-tion)
was
observed
at
10 mM.
Unlike
sa-licylates,
at concentrations
compatible
with
the
plasma
levels
during
chronic
drug
treat-ment
(1
to
20
,uM) (3),
the
anti-inflamma-tory
drug
indomethacin
wasunable
to
pre-vent
glutamate-induced
cell
death
(6).
Neuroprotection
was
also
evaluated
in
hippocampal
slices of
8-day-old
rat
brain
SCIENCE * VOL.
274
* 22 NOVEMBER 1996 1383on October 29, 2012
www.sciencemag.org
(7),
a system
that
more
closely
represents in
vivo
conditions.
In
the
hippocampal slices,
most
pyramidal
neurons
of
CAI
and
CA3
and granule cells of dentate
gyrus
(DG)
be-came
acutely
necrotic,
exhibiting swollen
cytoplasm with
large
vacuoles,
nuclear
shrinkage, and focal
clumping
of chromatin
(Fig.
IB).
Application
of
ASA
preserved
hippocampal cell viability
from
the
NMDA-mediated
injury
(Fig.
1, B
and
C).
ASA
did
not
modify cell viability
at
1
mM,
but
at
3
mM
it
specifically produced
significant
neu-roprotection in
the
CA3
region
(Fig. IC).
Higher
concentrations
of
ASA
completely
inhibited
the
NMDA
effect
in
CAI
and
DG
as
well
as
in
CA3
cells (Fig.
1, B
and
C).
Compared with
primary
cultures of
rat
cere-bellar
granule
cells,
2 mM
NaSal
efficiently
counteracted NMDA-mediated
toxicity
in
hippocampal slices (Fig. IC).
To
dissect the molecular mechanisms
by
which
salicylates preserved cell
viability
against
excitoxicity,
we
tested
whether these
drugs diminished
glutamate-mediated
calci-um
entry
(8).
In rat
cerebellar
granule cells,
application
of
glutamate
in
the
absence
of
external
Mg2+
caused
a
rapid
increase in
the
A
C .o 0 00.
0. zC
intracellular Ca2+ concentration
([Ca2+]i)
followed by
a
sustained
plateau (Fig. 2A),
principally
because of the
NMDA
receptor
subtype
activation
(9). ASA, applied
at
neu-roprotective concentrations
(1 to 3
mM),
induced
a
very
low
and
short-lasting
[Ca2+]i
increase
and
did not
modify
glutamate-me-diated calcium
entry
(Fig. 2B). Similar
re-sults
were
obtained with
NaSal (9). Thus,
it
was
likely that
salicylates
were
acting on
intracellular
molecular
targets
further
down-stream
of
glutamate
receptor
activation, a
property
that makes them
distinguishable
from
most
neuroprotective
drugs.
It
also
ap-pears
that neuroprotection occurred
inde-pendently of mechanisms controlling
[Ca
21
homeostasis.
At
plasma
concentrations
maintained
dur-ing treatment
of
chronic
inflammatory
diseas-es,
ASA
and
NaSal, but
not
indomethacin,
inhibit the
activation
of nuclear factor
kappa
B
(NF-KB)/Rel
transcription
factors
in T
and
pre-B
lymphocytes (10). The NF-KB/Rel
family
is
implicated
in
controlling
expression
of
several
genes
crucially involved
in
immune
and
inflammatory function (11). NF-KB/Rel
proteins are present in primary neurons
and
in
B
CAl
several
brain areas
(12). Administration of
glutamate to primary cultures of rat cerebellar
granule cells also results
in
up-regulation
of
NF-KB
nuclear
activity
(13)
and of the
tran-scriptional complex AP-1 (14). Cells were
exposed
to
50
F.M glutamate
in
the absence
or
presence
of ASA (1 or 3
mM)
and NaSal
(3
or
10
mM),
and nuclear extracts
(15)
were
prepared 1 hour after
stimulation. Both
drugs
10C 8C _ 6C -I + 4C 2C o0
o0-)O
0* 10-0 II10001
800-2 c 6C -T + 4C 2C 10-JU CA3 DGA
Glutamate 1 2 3 4 5I6 1 2 3 4 5 6B
ASA Glutamate 1 2 3 4 Time(min) 5 6Fig.
2.Original recording showing the
glutamate-induced
[Ca2-]j
increase
in ratcerebellar granule
cells.
(A) Effect of 50 ,uM glutamate (n
=95). (B)
Control Effect of 50
F.M glutamate
inneuronspretreated
with 3 mM
ASA (n
=98). Traces
arefrom
repre-sentative cellrecordings.
Drug concentration(mM) l... 0 | CAI I0- A NMDA ASA(mM) - - 3 NaSal(mM) - 3 10 Glutamate I_ I_
_I_
(50 pM) C NMDA +ASA NMDA 30pM -ASA 1 mM-ASA 3mM-ASA 5mM ASA 10 mM-NaSal 2 mMFig.
1.Neuroprotection
bysalicylates. (A)Concentration-dependent
effectelicited by ASA and NaSal in ratcerebellargranule cells. A glutamate(50F.M)
pulse was applied in the absence or presence of ASA(-)
andNaSal
(m).
Neuronal survival was expressed as percent of neuroprotection, with glutamate inducing 78 +3%
ofcell loss. Thexaxis represents drug concentrations. Points represent the means+SEM
of sixexperiments,
runintriplicate,
ondifferentculture preparations. (B) Prevention of excitotoxic effect of NMDA in rathippocampal
slicesbyASA. Sections
wereexposed to vehicle (control), 30 ,uM NMDA(NMDA),
or30p.M
NMDA and 5 mMASA (NMDA +ASA). Cell viability was evaluated in CA1,CA3,
andDG.
Scalebar,
10pLm.
(C)
Effect of ASA and NaSal on NMDA-induced cell loss in rathippocampal
slices. Test drugs were added to the slices at the indicated concentrations and cell viability inCA1,
CA3,
andDG
wasanalyzed.Columns
represent the means ± SEM of three experiments run on four slices each.Differences
compared
with NMDAaloneweresignificant at P<0.01 asindicatedby anasterisk.Fig.
3. Effect ofneuroprotective
concentrations ofASA
andNaSalonglutamate-induced
NF-KB and AP-1 DNAbinding
activities. Nuclear extracts fromratcerebellargranule
cellsweresubjected
to anelectrophoretic mobility-shift
assay with-y_32p-labeled
oligonucleotide probes containing
theim-munoglobulin
KB(lanes
1 to6)
and the AP-1 DNAbinding
sites(lanes
7 to12) (15).
Cellswereeither unstimulated(lanes
1and7)
orstimulated with 50,uM glutamate
(1
5-minpulse)
in the absence(lanes
2and8)
orpresence(lanes
3to6and 9to12)
of thedrugs
asindicated. SCIENCE * VOL. 274 * 22 NOVEMBER 1996rsn 1384 3 3 0 . . I I . A. I A kA 71 I I
on October 29, 2012
www.sciencemag.org
Downloaded from
inhibited the glutamate-induced
increase
of
NF-KB
activity in a
concentration-dependent
manner
(Fig.
3),
with calculated EC50 values
of 1.3
and
6 mM
for ASA
and NaSal,
respec-tively. Parallel
experiments
in
which cell
vi-ability
was
measured
24
hours
later revealed
a
strict
correlation between
neuroprotective
concentrations of
anti-inflammatory
drugs
and
blockade of
NF-KB
induction
(EC50
val-ues
of 1.5 mM for ASA
and
5.8 mM
for
NaSal). The
salicylate effect
on
NF-KB/Rel
proteins was
specific.
In
fact,
ASA
and NaSal
failed
to
modify the
glutamate-mediated
nu-clear induction of
the
transcriptional complex
AP-1
(Fig.
3).
Thus,
at concentrations
compatible with
amounts
in
plasma during
treatment
of
chronic inflammatory
states,
salicylates
pre-vented
glutamate-induced
neurotoxicity.
The neuroprotective
effect
correlated
nei-ther
with the anti-inflammatory
properties
of
these
compounds
nor
with
cyclooxygen-ase
inhibition.
In
fact, indomethacin
exert-ed anti-inflammatory but
not
neuroprotec-tive
properties,
and NaSal
was
neuroprotec-tive
but did
not
interfere
with
cyclooxygen-ase
activity
(3). The
common
molecular
target
for
ASA
and NaSal but
not
for
in-domethacin
(10, 16)
was
the blockade
of
NF-KB
induction,
suggesting a
link between
neuroprotection
and
the nuclear
event.
Here
we
provide evidence
for
an
unusual
pharmacological
effect of ASA
and
its
me-tabolite NaSal.
In view
of
their distinct
ability
to
act
not
merely
as
anti-inflamma-tory
compounds but also
as
neuroprotective
agents against excitotoxicity,
these
drugs
appear to
possess
a
wider
pharmacological
spectrum
than other nonsteroidal
anti-in-flammatory
drugs.
REFERENCES AND NOTES
1. S. A. LiptonandP. A.Rosenberg,N. Engl. J. Med.
330,613(1995);M.Memo, M.Pizzi,A.Valerio,M.
Grilli,P.F.Spano,Int.Rev.Psychiatry 7,339(1995).
2. P. L.McGeerandE. G.McGeer, BrainRes. Rev. 21, 195(1995).
3. P.Insel,inGoodman and Gilman's The Pharmaco-logicalBasisof TherapeuticsJ.G.Hardman, P. B.
Molinoff,R. W. Rudden,A.Goodman Gilman, Eds. (McGraw-Hill,NewYork, 1996),pp.617-657;K.D.
Rainsford, in Aspirin and the Salicylates (Butter-worths,London, 1984); G. Weismann,Sci.Am. 84,
264(January 1991); J.P.Famaey andH.E.Paulus, Eds., Therapeutic Applications ofNSAIDS
Subpopu-lations and NewFormulations (Dekker, NewYork, 1992).
4. V. Gallo, M. T.Ciotti, F. Coletti, F.Aloisi, G. Levi, Proc. Natl. Acad.Sci. U.S.A. 79,7919(1982); M.
Favaronetal., ibid. 85, 7351 (1988).
5. Primaryculturesof cerebellargranulecells were
pre-pared from cerebella of8-day-old rats (Sprague-Dawley)killedaccordingtothePolicyon theUseof Animals in Neuroscience Research. The cultures wereused at 10 to 12daysinvitro and contained morethan95%glutamatergicneurons.
Neurotoxic-itywasinduced asdescribed[M. Pizzi, C.Fallacara, V.Arrighi,M.Memo,P.F.Spano,J.Neurochem.61, 683(1993)]. Drugstested forneuroprotectionwere added 5 min before andduringtheglutamate pulse. Cellsurvival was evaluated 24 hoursafterthe
gluta-mate pulse asdescribed by K. H. Jonesand J. A. Senft [J. Histochem. Cytochem. 33,77(1985)].ASA, NaSal, and indomethacindid notaffectneuronal
vi-abilityper se.
6. M. Grilli,M. Pizzi, M.Memo,P. F.Spano, datanot
shown.
7. Hippocampal slices wereobtained from brains of
8-day-oldratsandpreparedasdescribed[G. Garth-waite and J. Garthwaite, Neurosci. Lett. 97, 316
(1989)].Sliceswerepreincubatedwith either the test drugs,ASAandNaSal, orvehiclefor 30min, and
then NMDA (30pM)wasadded foranadditional30 min.Sliceswerewashedandincubated in fresh
buff-erfor 90minandthen fixedand embeddedinepoxy
resin. Semithin (1 ,um) sectionswere cut, stained
withmethylene blueand azur11, andexamined by
lightmicroscopy. For quantitation ofcell loss,cells werecounted in cell layer fields takenfrom CA1, CA3,andthe dorsalbladeoftheDGineachslice.
The fields measured1.5x 104 mm2. The
percent-ageof cell survivalwascalculated bythe ratio ofliving
cells to thetotal number of cells.
8. D. W.Choi,J.Neurosci.7,369(1987).
9. The[Ca2
2]i
wasmeasuredbyFura-2 microfluorim-etry insinglecellsasdescribed byM. Pizzi et al.[Mol. Pharmacol. 49, 586(1996)]. Cellswereexposedtoglutamatefor 2 min inMg2+-free Krebs-Ringer
solu-tion.ASAorNaSalwasadded2 minbefore
gluta-mateexposure. Fluorescenceimageacquisitionand
analysiswereperformed by usingtheMIRAcal (mul-tiple image ratioing and analysiswithcalibration) sys-temfromApplied Imaging(England).LikeASA,
Na-Sal (2 to 10 mM) produced a low and transient [Ca2
]i
increasewithoutaffecting glutamate-mediat-ed calciumentry.10. E. KoppandS. Ghosh,Science265, 956(1994).
11. H.C.Liou and D.Baltimore,Curr. Opin.CellBiol. 5,
477(1993); M. Grilli,J.-S.Chiu,M. J.Lenardo,Int. Rev.Cytol. 143,1 (1993).
12.C. Kaltschmidt, B. Kaltschmidt, P. A. Baeuerle,
Mech. Dev. 43, 135 (1993); C. Kaltschmidt, B.
Kaltschmidt,H. Neumann,H.Wekerle, P.A.
Bae-uerle,Mol. Cell. Biol. 14, 3981(1994);M.Grillietal.,
J.Biol.Chem. 270,26774(1995); S.W.Barger and
M. P.Mattson,Mol. Brain Res. 40, 116(1996).
13. C.Kaltschmidt,B.Kaltschmidt,P. A.Baeuerle,Proc.
Natl.Acad.Sci. U.S.A. 92,9618(1995);L.Guerrini,
F.Blasi,S. Denis-Donini, ibid.,p.9077;M.Grilli,F.
Goffi,M. Memo, P. F.Spano, J. Biol. Chem. 271,
15002(1996).
14. T.Curran and B. R.Franza, Cell55,395(1988).
15. Nuclear extracts were prepared according to a
small-scale protocol described by N. C.Andrews
andD. V.Faller[NucleicAcids Res.19, 2499(199
1)1.
DNA bindingreactionswereperformedasdescribed [S.-M. Kang, A.-C. Tran, M. Grilli, M. J. Lenardo, Science 256,1452(1992)]withthefollowingmodifi-cations: 4
pg
of nuclearextracts wascombinedwith50,000cpm(0.2ng) ofy-32P-labeled oligonucleo-tidesin atotal volumeof 12
Rl.
Oligonucleotidese-quences were as follows: KB oligonucleotide
se-quencefrom theimmunoglobulinKlight-chain gene, 5'-CAGAGGGGACTTTCCGAGAGGC-3'; AP-1
oli-gonucleotide sequence, 5'-CGCTTGATGAGTCA-GCCGGAA-3'.
16. Indomethacin(1to20
pLM)
wastestedfor theabilitytointerfere withglutamate-induced NF-KB activation
in cerebellar granule cells. No inhibition was
ob-served.M. Grilli,M. Pizzi, M.Memo, P. F. Spano, unpublished material.
17. Thiswork waspartiallysupported byConsiglio Na-zionaledelleRicerche, Italy.
26 June1996;accepted25September1996
Tricorn
Protease-The
Core
of
a
Modular
Proteolytic System
Tomohiro Tamura, Noriko Tamura, Zdenka
Cejka,
Reiner Hegerl,
Friedrich
Lottspeich,
Wolfgang Baumeister*
Large macromolecular assemblies have evolved
as a meansof
compartmentalizing
reactions in
organisms lacking
membrane-bounded compartments. A tricorn-shaped
protease
wasisolated from the archaeon Thermoplasma and
wasshown
to
form
amultisubunit
proteolytic complex. The 120-kilodalton
monomerassembled
to
form
ahexameric toroid that
could
assemble further into
acapsid
structure.
Tricorn
protease
appeared
to act
asthe
coreof
aproteolytic system; when it interacted with several
smaller
proteins,
it
displayed multicatalytic activities.
In
vivo
proteolysis
is an
essential
element
of
many
regulatory
processes. It must
be
subject
to
spatial and temporal control
in
order
to
prevent
damage
to
the
cell.
Pro-karyotic cells, which lack
membrane-bounded
compartments,
have developed
large macromolecular assemblies
or
"molec-ular
organelles"
so as to
confine
proteolysis
to an
inner
cavity to
which
only
proteins
targeted
for
degradation
have access.
The
paradigm
of
such
a
proteolytic
complex
is
the
proteasome
(1),
which
is
ubiquitous
across
the three
urkingdoms
archaea
(2),
bacteria
(3, 4)
and
eukarya (5).
In
the
Max-Planck-lnstitute
forBiochemistry,D-82152Martins-ried,Germany.
*To whomcorrespondenceshould beaddressed.
course
of
searching
for
regulatory
compo-nents of
the
proteasome (6)
in
Thenro-plasma acidophilum,
we
discovered
a
proteo-lytic complex
of
high molecular
mass
that
is
not
related
to
the
proteasome.
This
com-plex
seems
to
be the
core
of
a
modular
proteolytic
system
generating
multicatalytic
activities.
We
purified
the
high-molecular-weight
(HMW)
protein
to
homogeneity by
a
se-quence of
chromatography
steps
(7).
The
purified
protein migrates at 720
kD in
gel
filtration
chromatography (versus
migration
at 680
kD
by
the 20S
proteasome), and
it
turned
out to
be composed
of
a
single
polypeptide
of 120 kD
when
subjected to
SDS-polyacrylamide
gel
electrophoresis
(SDS-PAGE) (Fig. 1).
The
purified
protein
SCIENCE * VOL.