UNIVERSITA’ POLITECNICA DELLE MARCHE
Biomolecular Sciences
XXXI Cycle - 2018
DOCTORAL THESIS
Natural and functionalized molecules with antioxidant
and scavenging activity as components of innovative
lipid-based artificial tears
PhD student:
Minnelli Cristina
Academic tutor:
Dott. Giovanna Mobbili
Aziendal tutor:
Abstract
List of Abbreviations
PART I
– State of art
CHAPTER I
ROS and Antioxidants: A continuous battle inside the cells
1.1. Properties and generation of Reactive Oxygen Species (ROS) 1.2. Endogenous and exogenous sources of ROS
1.3. Cellular damage induced by ROS 1.3.1. Nucleic acids
1.3.2. Lipids 1.3.3. Proteins
1.4. Essential role of antioxidants in protection against oxidative stress
CHAPTER II
Ocular Diseases Associated with Oxidative Stress
2.1. Introduction
2.2. Age-related Macular Degeneration (AMD)
2.5. – limitation and therapeutics
CHAPTER III
Lipid-based nanocarriers
3.1. Lyotropic Liquid Crystals (LLC) 3.2. Lipid/Water Polymorphysm
3.3. Lipid-based nanocarriers relevant to topical ocular drug delivery 3.3.1. Lamellar phase: liposomes
3.3.2. Reversed bicontinuous Cubic phase and Cubosomes
PART II – Natural and functionalized Epigallocatechin-3-gallate (EGCG) as
components of lipid-based eye drops
CHAPTER IV
Epigallocatechin-3-gallate (EGCG)
4.1. Antioxidant Properties
CHAPTER V
Liposomal formulations for an efficient delivery of Epigallocatechin-3-Gallate:
characterization and protective effect in ARPE19 cells
5.1. Introduction
5.2. Optimization of EGCG encapsulation efficiency inside liposomal formulations 5.2.1. Salts and Lipid Composition effects on EGCG Encapsulation Efficiency
inside Multilamellar Vesicles (MLVs)
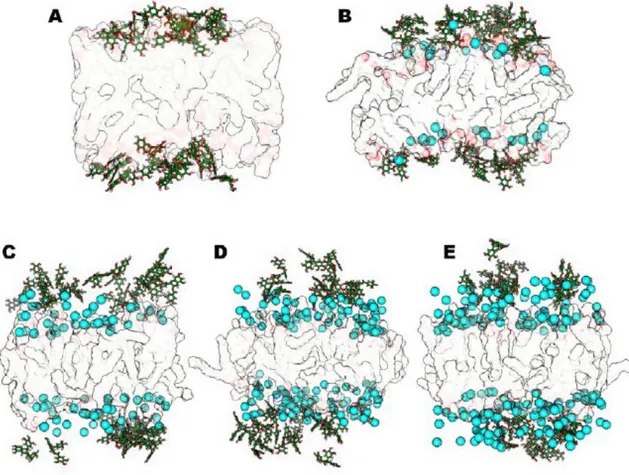
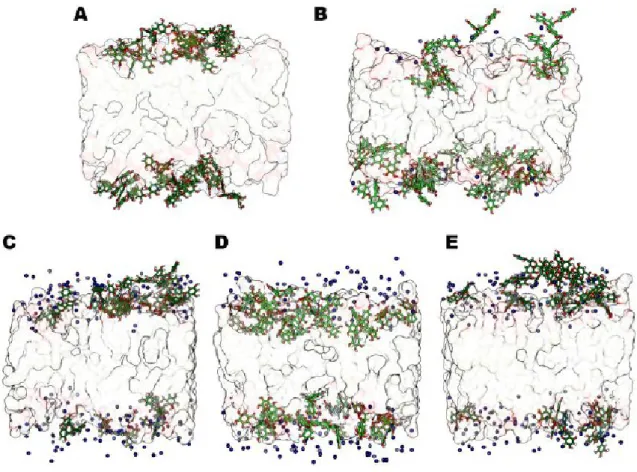
5.2.2. Interaction of EGCG with lipid bilayer: in silico results 5.3. Colloidal stability: optimization of the best liposome formulation
5.3.1. Magnesium chloride effect on the anionic liposome 5.3.2. Effect of Poloxamer-407 addition
5.4. Characterization of bulk and nanodispersed liposomal phase 5.4.1. X-ray diffraction measurements
5.4.2. Dynamic Light Scattering measurements
5.4.3. Encapsulation Efficiency studies in presence of Poloxamer-407 5.4.4. In vitro drug release
5.5. Cellular Experiments
5.5.1. Cytotoxicity of EGCG-loaded magnesium-poloxamer liposomes in Retinal Pigment Epithelial Cells
5.5.2. Enhancement of EGCG efficacy by magnesium-poloxamer liposome incorporation
CHAPTER VI
Epigallocatechin-3-gallate (EGCG)-loaded cubic or hexagonal phase gels and
its dispersed forms: pre-formulation studies
6.1. Introduction
6.2. Preparation and Encapsulation Effiency studies of bulk (Nondispersed) phase and its dispersed forms (Cubosomes and Hexasomes)
6.3. Nanostructural Characterization
6.3.1. Small Angle Neutrons Scattering (SANS) studies
6.3.2. Colloidal stability of dispersed phases: Dynamic Light Scattering (DLS) 6.4. Conclusion and future perspective
CHAPTER VII
A new lipophilic derivative of EGCG
7.1. Introduction
7.2. Synthesis and characterization of an ether derivative of EGCG 7.3. Antioxidant properties of new derivative
7.3.1. Scavenging Activity on the DPPH Radical 7.3.2. Protection of liposome oxidation: TBARS assay
7.3.3. Protective effect against oxidative stress induced-cell death 7.4. Conclusion and future perspective
PART III
– A new lipophilic derivative of Edaravone: antioxidant properties
and liposomal formulations
CHAPTER VIII
Edaravone derivative
8.1. Introduction
8.2. Synthesis and characterization of C18- Edaravone
8.3. Antioxidant activity of C18-Edaravone (C18-EdV)
8.3.1.
Scavenging Activity on the DPPH Radical
8.3.2.
Protection of liposome oxidation: TBARS assay
8.3.3.
Cellular experiments
8.4. C18-EdV loaded liposome: characterization
8.4.1.
Fluidity of lipid bilayer: in silico studies
8.4.2.
Colloidal stability of liposome formulations
8.4.3.
Encapsulation efficiency studies
8.5. Cellular Experiments: the effect of liposomal encapsulatin
8.6. Conclusion and perspective
Materials and Method
I, Cristina Minnelli, declare that this thesis titled “Natural and functionalized molecules with antioxidant and scavenging activity as components of innovative lipid-based artificial tears” and the work presented it are my own and has been generated by me as the result of my own original research. Where I have consulted the work of others, this is always clearly stated.
I confirm that parts of my PhD work have been published in the following papers:
Cristina Minnelli, Paolo Moretti, Gianluca Fulgenzi, Paolo Mariani, Emiliano Laudadio, Tatiana
Armeni, Roberta Galeazzi, Giovanna Mobbili. A Poloxamer-407 modified liposome
encapsulating epigallocatechin-3-gallate in the presence of magnesium: Characterization and protective effect against oxidative damage. International Journal of Pharmaceutics (2018),
552:225–234.
Emiliano Laudadio, Cristina Minnelli, Adolfo Amici, Luca Massaccesi, Giovanna Mobbili, Roberta Galeazzi. Design of proper liposomial vector for an efficient encapsulation
Epigallocatechin-3-gallate: an in silico/experimental approach. Molecules (2018), 23(2), 441.
Minnelli Cristina, Cianfruglia Laura, Laudadio Emiliano, Galeazzi Roberta, Pisani Michela,
Crucianelli Emanuela, Bizzaro Davide, Armeni Tatiana, Mobbili Giovanna. Selective induction
of apoptosis in MCF7 cancer-cell by targeted liposomes functionalised with mannose-6-phosphate.Journal of Drug Targeting (2018), 26(3):242-251.
Andrea Scirè, Laura Cianfrugia, Minnelli Cristina, Tatiana Ameni, Francesco Galli. Glutathione
compartmentalization and its role in glutathionylation and other regulatory processes of cellular pathways. Biofactors (2018).
protein interactions of human glyoxalase II: findings of a reliable docking protocol.Organic Biomolecular Chemistry (2018), 16(28):5167-5177.
Laudadio Emiliano, Galeazzi Roberta, Mobbili Giovanna, Minnelli Cristina, Barbon Antonio, Bortolus Marco, Stipa Pierluigi. Depth Distribution of Spin-Labeled Liponitroxides within
Lipid Bilayers: A combined EPR and Molecular Dynamics approach. ACS Omega. Accepted
Manuscript. 2019.
Laudadio Emiliano, Mobbili Giovanna, Minnelli Cristina, Massaccesi Luca, and Galeazzi Roberta. Salts Influence Cathechins and Flavonoids Encapsulation in Liposomes: a
Molecular Dynamics Investigation. Molecular Informatics (2017), 36 (11).
Gianmarco Mangiaterra, Emiliano Laudadio, Marta Cometti, Giovanna Mobbili, Cristina
Minnelli, Luca Massaccesi, Barbara Citterio, Francesca Biavasco, Roberta Galeazzi. Inhibitors of multidrug efflux pumps of Pseudomonas aeruginosa from natural sources: An in silico high-throughput virtual screening and in vitro validation. Medicinal Chemistry Research
This thesis presents an extended work concerning the development of lipid-based drug delivery systems able to prevent and control the ocular diseases associated with oxidative stress.
The eye drops dosage forms account for nearly 90% of currently available marketed formulations thanks of their simplicity, safety and acceptance by patients. However, achievement of adequate bioavailability of topically applied drugs is challenging due to unique anatomical and physiological barriers at the ocular surface that determine a low drug absorption of about 5%. The lipid-based nanocarriers, which are highly biocompatible, can carry the antioxidant to a specific site, release it in a controlled manner and protect the encapsulated molecule from premature degradation. Among the antioxidants used for ophthalmic applications, the attention was focused on Epigallocatechin-3-gallate and Edaravone molecules, which are well-known for being bioactive in ocular diseases associated with oxidative stress.
Since the final aim was to create a stable and efficient delivery system, the design of lipid-based nanocarriers has been based on three critical targets:
a) High encapsulation efficiency in order to optimize the delivery of the active principle at the
target sites.
b) Colloidal stability and characterization of the lipid-based systems
c) Ability to protect the retinal cells from oxidative stress-induced cell death
Briefly, from the obtained results, we concluded that the encapsulation percentage of EGCG inside liposomes showed specific ion effects together with an influence of the lipid matrix composition. More in details, the combination of an anionic lipid matrix with MgCl2 and Poloxamer-407
allowed us to obtain a system highly stable with 100% EGCG encapsulation that was able to protect retinal cells from oxidative stress. Using increasing concentrations of EGCG we optimized the encapsulation efficiency of the catechin into different types of lipidic supramolecular structures, presenting cubic and hexagonal mesophases; these lyotropic liquid crystals are other
chain in EGCG and Edaravone molecules also increased their encapsulation efficiency inside a liposomal vector as well as their ability to protect the retinal cells from oxidative stress.
ROS: Reactive Oxygen Species
NADPH oxidase: Nicotinamide Adenine Dinucleotide Phosphate oxidase) 5-LOX: 5-Lipoxygenase
DNA: Deoxyribonucleic Acid
8-OHdG: 8-hydroxydeoxy guanosine LPO: Lipid Peroxidation
PUFA: Polyunsaturated Fatty Acid GPx: Glutathione Peroxidase SOD: Superoxide Dismutase GR: Glutathione Reductase CAT: Catalase GSH: Glutathione GSSG: Glutathione Disulfide MDA: Malonyldialdehyde 4-HNE: 4-hydroxy-2-nonenal AMD: Age-Macular Degeneration RPE: Retinal Pigment Epithelium DEA: Dry Eye Disease
DES: Dry Eye Syndrome
EGCG: Epigallocatechin- 3-gallate TF: Tear Film
LLC: Lyotropic Liquid Crystals CPP: Critical Packing Parameter EE: Encapsulation Efficiency DLC: Drug Loading Capacity X-RD: X-Ray Diffraction DLS: Dynamic Light Scattering MLV: Multilamellar Vesicles
TEM: Transmission Electron Microscopy TBARS: Thiobarbituric Reactive Species
PART I -
State of Art
“Success is the ability to go from one failure to another with no loss of enthusiasm” -Winston
Churchill-2
CHAPTER I
ROS and Antioxidants: A continuous battle inside the cell
1.1. Properties and generation of Reactive Oxygen Species (ROS)
A free radical can be defined as any species capable of independent existence that contains one or more unpaired electrons (Halliwell, 2007). Additionally, the term is also used to describe certain non-radicals that contain their full complement of electrons, but in an unstable or reactive state, like ozone (O3), hypochlorous acid (HClO), singlet oxygen (1O2), and hydrogen
peroxide (H2O2) and that can be converted easily to radicals (Halliwell, 2007). All these entities
are usually reactive, although the chemical reactivity of free radicals varies over a wide spectrum.
Radicals can be formed via homolytic cleavage of a covalent bond in a non-radical species, by breakage of a radical to give another one, and via redox reactions (Halliwell, 2007; Bahorun et al 2006). In order to achieve a stable state, they react with other molecules, which become unstable by this interaction, thereby causing an oxidative chain reaction.
Radicals derived from oxygen represent the most important class of radical species generated in living systems. They are collectively referred to as “Radical Oxygen Species” (ROS). The molecular oxygen (dioxygen; O2) has in its ground state two unpaired electrons and therefore
it is qualified as radical. Since the most stable organic molecules are in singlet state (electron spin quantum number equal to one), the molecular oxygen, in triplet state, is less reactive to cellular component respect to others ROS (Halliwell and Gutteridge, 1989). However, triplet oxygen may be converted to the much more reactive ROS forms either by energy transfer or by electron transfer reactions. The first mechanism leads to the formation of singlet oxygen, whereas the latter process results in the sequential reduction to superoxide, hydrogen peroxide, and others (Figure 1).
Figure 1. Generation of different ROS by energy transfer or sequential univalent reduction of
3
Some of them, such as superoxide anion (O2•-), hydroxyl radical (•OH) and hydrogen peroxide
(H2O2) are the most relevant ROS in physiological or pathophysiological conditions (see
below).They are generated from both endogenous and exogenous sources by reactions that can be mediated or not by enzymes.
The generation of O2•- occurs by iron-based oxidation reactions (Eq. 1) and by enzymatic and
non-enzymatic reactions in which an electron is transferred to molecular oxygen (Eq. 2) (Michelson et al, 1977). The non-enzymatic reaction represents the major source of O2•- and
occurs within the mitochondria. The enzymes involved in superoxide anion formation comprise several cellular oxidase systems such as NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase), xanthine oxidase, lipooxygenase and peroxidases (Babior, 1999;Vignais, 2002). Superoxide anion is a relatively unreactive intermediate with poor ability to cross biological membranes but can inactivate specific enzymes or initiate lipid peroxidation, also in its protonated form, hydroperoxyl HO2•. Under physiological pH the most occurring form is
superoxide. It can act as reducing agent and transform iron Fe+3 to Fe+2 thus stimulating the Fenton reaction (Eq. 6).
O2 + Fe2+ → Fe3+ + O2•- (1)
O2 + e- → O2•- (2)
H2O2 is mainly formed in a dismutation reaction catalyzed by a scavenging enzyme, the
superoxide dismutase (SOD) (Eq. 3) (Halliwell and Gutteridge, 1999). The production of H2O2
can occur also by the non-enzymatic dismutation of O2•- (Eq. 4). It is a non-radical potent
oxidizing agent, which can diffuse across cell membrane because it is electronically not charged.
2O2•- + 2H+ → O2 + H2O2 (3)
O2•- + e- → (O22- - + 2H+) → H2O2 (4)
OH• is the most reactive free radical in vivo (Bedwell et al, 1989). It is formed by the reaction between superoxide radical and H2O2 in a reaction called Haber–Weiss reaction (Eq. 5). It is
4
also produced by the Fenton reaction (Fenton, 1893), in which H2O2 reacts with metal ions
(Fe+2 or Cu+), often bound in complex with different proteins such as ferritin (an intracellular
protein that stores iron) and ceruloplasmin (plasma copper carrying protein) or other molecules (Haber and Weiss, 1934). This reaction represents the major non-enzimatically sources of OH• inside the cells. The Fe3+ ions react more slowly (if at all) with H
2O2 than Fe2+ complexes so
that reducing agents stimulate Fenton reactions. This can occur with ascorbate (AscH2)
(Walling, 1982) and even with superoxide radical (Fenton, 1893) (Eq. 6). The •OH radical could be also generated by photolytic decomposition of alkyl hydroperoxides (Valko et al, 2004). Finally, the exogenous sources, such as ionizing radiations, can produce two •OH radicals by radiolysis of water (homolytic scission) (Riley, 1994). The hydroxyl radical is a diffusible moleculeresulting in a very reactive entity, within and out of the cells (Al-Guborya et al, 2010), and it is the oxidant primarily responsible for oxidative damage of DNA bases.
H2O2 + O2•- •OH + OH- + O2 (5)
1.2. Endogenous and exogenous sources of ROS
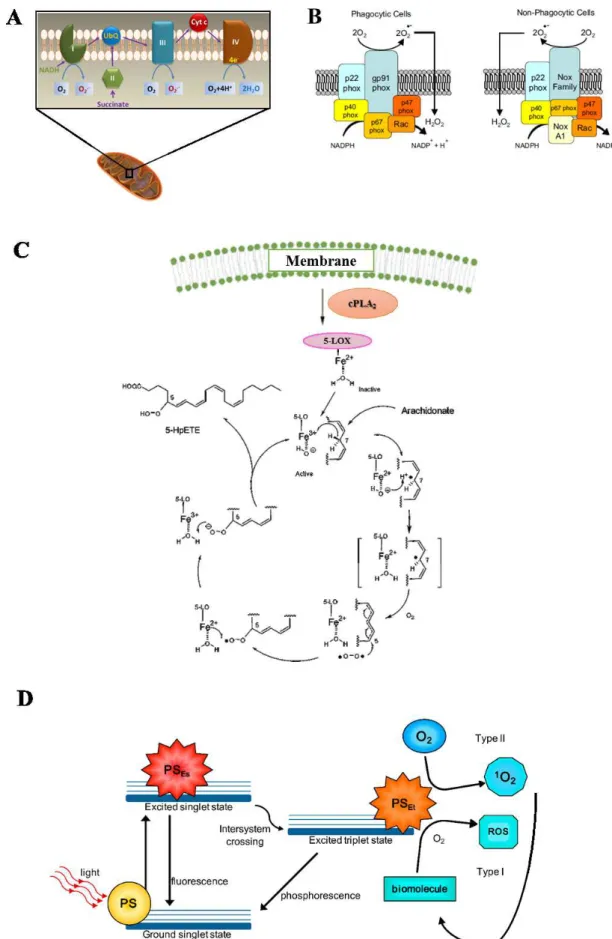
Mitochondria, NADPH oxidase (NOX) complexes and 5-Lipoxygenase represent the major endogenous sources of ROS in living non-photosynthetic cells in addition to photosensitizers that are present mainly at retina level (Figure 2).
Mitochondria provides energy from aerobic metabolism mainly with an oxidative phosphorylation process, which involves the transport of protons (hydrogen ions) across the inner mitochondrial membrane by the electron transport chain. The electrons are transferred through a series of proteins via oxidation-reduction reactions. The last destination is oxygen molecule, which accepts four electrons, thus forming two water molecules. Approximately 1-5% of electrons 'flowing' through the mitochondrial electron transport chain, in particular at complex I and complex II levels and O2•- is formed (Fig. 2A). Then, the SOD enzyme converts
it into H2O2 that can easily cross the mitochondrial membrane (Cadenas and Davies, 2000).
5
Figure 2. The most relevant source of ROS in living cells: (A) mitochondria, (B) NADPH oxidase, (C)
6
The NADPH oxidases enzyme of the NOX family are the major cellular sources of ROS (Reviewed in Bedard and Krause, 2007) (Fig. 2B). They catalyze the production of superoxide by the one-electron reduction of oxygen using NADPH as electron donor; then, the O2•- radical
generated is the starting material for the production of a large variety of reactive oxidants, including oxidized halogens and singlet oxygen. It is presents in both professional phagocytic cells (macrophages, neutrophils and eosinophils) and nonphagocytic cells. They have a similar structure composed by six subunits (Schalk et al. 1996). The enzyme is normally dormant in phagocytic cells but is rapidly activated upon several stimulation such as hormones, growth factors, cytokines, lipopolysaccharide and mechanical stress. Activation of NADPH oxidase occurs by relocation of the cytosolic components to the cell membrane with consequential O2•-
generation that plays a vital rolein killing intracellular pathogens (Babior et al, 2002). This can be illustrated by examining patients with chronic granulomatous diseasecaused by mutations in the genes encoding subunits of the NADPH oxidase system leading to poor formation of O2•.
Thus, the patient suffer severe and persistent multiple infections (Deffert et al, 2014). In the nonphagocytic cells the NOX is instead constitutively active, producing low level of O2•- that
may influence the redox signaling in numerous cellular processes such as cell growth, apoptosis, migration, and extracellular matrix remodeling (Nowicki et al, 2001).
5-Lipoxygenase (5-LOX) is a regulator of chronic inflammation and oxidative stress generation (Gubitosi et al, 2008). 5-LOX, a nonheme iron enzyme, catalyzes the dioxygenation of arachidonic acid (AA) in response to several stimuli, particularly growth factors and cytokines (Fig. 2C). The reaction cascade starts from activation of phospholipase A2 (PLA2) that induces
the release of AA from phospholipids. Then, 5-LOX catalyzes the stereoselective hydrogen abstraction (L•); the resulting radical migrates over the neighboring double bond and can react with molecular oxygen to form a peroxyl radical (LOO•). Oxidation by Fe2+, present in the
active site, delivers to LOO• one electron; the formation of a peroxyl anion (LOO-) followed by protonation gives a 5-hydroper-oxyeicosatetraenoic acids (5-HPETEs), which is a lipid hydroperoxide (LOOH). Finally, the 5-LOX catalyzes the dehydration reaction to form the epoxide moiety of leukotrienes-A4 (LT4, R-epoxide moiety) that is rapidly metabolized to leukotriene B4. This latter is linked to the ROS generation, cytokine activation, and apoptosis. Generally, the expression of 5-LOX is limited to leukocytes, such as granulocytes, mast cells, and monocytes; however, the detection of its expression in foam cells within atherosclerotic lesions, illustrates the potential importance of this enzyme in the initiation and/or development
7
of atherosclerosis (Mehrabian et al, 2002). In the same way, Gubitosi-Klug et colleagues have shown the involvement of 5-LOX in degeneration of retinal capillaries and therefore in the pathogenesis of diabetic retinopathies (Gubitosi-Klug et al, 2008).
A photosensitizer is a light absorbing substance involved in the initiation of photochemical reactions that are responsible of light damage, particularly in the retina (Mellerio, 1994).After initial photon absorption, a change in distribution of electrons in the photosensitizer molecules occurs, generating an excited triplet state. In the type I photosensitization, the ROS are produced via electron transfer from excited photosensitizer to biomolecules forming a radical which reacts with molecular oxygen to generate ROS; while, in the type II photosensitization, the excited sensitizer transfers its excess energy to ground-state molecular oxygen (3O2), producing
excited state singlet oxygen (1O2). This latter reacts with the biomolecules to generate oxidized
products (Kruft and Greer, 2011) (Fig. 2D). The photosensitizers included the lipofuscin, melanin, bilirubin, hemoglobin and other iron containing proteins (e.g. cytochrome C), flavins, flavoproteins, visual pigments in photoreceptor cells such as rhodopsin, and others (Boulton et al, 2001). An important photosensitizer is lipofuscin, a lipid-protein aggregate that has an autofluorescens activity when excited with short wavelength light. It is formed in the lysosomal compartment and found in active postmitotic cells such as cardiac myocytes, select neurons, and in RPE. Because it accumulates with age, lipofuscin is usually defined an “age pigment,” and it is considered a marker of cellular senescence. The pathological accumulation of lipofuscin can compromise the normal function of cells through distortion of cellular architecture (Young, 1988). Moreover, the lipofuscin acts as photosensitizers, inducing the generation of ROS and therefore the oxidative damage of surrounding tissue (Gaillard et al, 1995). For these reasons, its presence is correlated to a variety of age-related diseases such as ADM, Alzheimer's disease, certain lysosomal disorders, and myopathy (Jeffrey and Keller, 2006; Futerman and Van Meer, 2004).
Others endogenous sources comprise the peroxisomes, that are known to produce H2O2 (Valko
et al, 2004), and enzymes such as nitric oxide synthase (Vazquez-Vivar and Kalyanaramam, 2000), xanthine oxidase (Halliwell and Gutteridge, 1999), and lysyl oxidase (Rojkind et al, 2002), the enzyme catalyzing the formation of the aldehyde precursors of cross-links in collagen and elastin, can give rise to H2O2.
Exogenous ROS result from air and water pollution, cigarette smoke, alcohol, heavy or transition metals, certain drugs (cyclosporine and tacrolimus), industrial solvents, cooking
8
(smoked meat, used oil, fat), ultraviolet-A light (UVA) and radiation (Kielbassa et al, 1997; Riley, 1994; Halliwell and Cross, 1994). After penetration into the body by different routes, these exogenous compounds are decomposed or metabolized into free radicals. For example, the ionizing radiation, such as x-rays and neutrons, can produce OH• by radiolysis of water or ROS via secondary reactions (Riley, 1994); the cigarette smoke contains more than 7,000 chemical compounds and oxidative agents (Halliwell and Cross, 1994) that are responsible of ROS generation in the human body.
1.3. Cellular damage induced by ROS
Membrane lipids, protein and nucleic acids are the mainly molecular target of oxidative damage caused by ROS. This damage contributes to the pathogenesis of many diseases including ocular disorder.
1.3.1. Nucleic acids
Oxidative damage of deoxyribonucleic acid (DNA)leads to the formation of different oxidative DNA lesions including single and double stranded breaks in DNA. In particular, the OH• radical directly reacts with all components of DNA such as bases (purine and pyrimidine) and deoxyribose sugar backbone (Halliwell and Gutteridge, 1999) thus modifying their structures. One of the resultant purine adducts, 8-hydroxydeoxy guanosine (8-OHdG),is the major oxidative DNA-damage product that can produce mutations because of its base pairing with adenine as well as cytosine. The body has several mechanisms to defend itself against these attacks, for example by using DNA-repair enzymes (base excision repair or mismatch repair) but only the 86% of 8-Oxo-dG is restored to G (Yasui et al, 2014). The levels of 8-OHdG are higher in mitochondrial DNA than in nuclear DNA because it is located in close proximity to the electron transport chain (Barja, 2000). It is not a surprise that increased levels of 8-oxo-dG are frequently associated with several diseases including cancer, Alzheimer's and systemic lupus erythematosus (Valavanidis et al, 2013; Cooke et al, 2003).
The ribonucleic acid (RNA) is more vulnerable to oxidative attack than DNA, in humans, due to its single stranded nature and due to the lack of an active repair mechanism for oxidized RNA (Hofer et al, 2005). Even here, the oxidized guanosine
9
nucleotide is the main damage product and its levels increase in various pathological conditions like Alzheimer’s disease (Abe et al, 2002), Parkinson’s disease (Kikuchi et al, 2002), atherosclerosis (Martinet et al, 2004) and myopathies (Tateyama et al, 2003).
1.3.2. Lipid
Lipid peroxidation is a complex and dynamic process that produces numerous peroxidation compounds over time. The membrane lipids, especially the polyunsaturated fatty acid residues (PUFA) of phospholipids are particularly susceptible to oxidation induced by free radicals. In response to membrane lipid peroxidation (LPO), and according to specific cellular repair capacities, the cells may promote cell survival or induce cell death. The cellular response is dependent on the lipid peroxidation amount. At low peroxidation levels, antioxidant defense systems or signaling pathways are activated, resulting in an adaptive stress response. At high level, instead, the lipid peroxidation results in the loss of membrane functioning, for example, alteration of fluidity, permeability, inactivation of membrane bound enzymes and receptors. Moreover, LPO products have been shown to be mutagenic and carcinogenic and are implicated in numerous pathologic conditions such as cardiovascular diseases, cancer, neurological disorders, and aging.
The autoxidation mechanism is a radical-chain process involving three sequences: initiation, propagation, and termination. The Figure 3 shows a schematic process of the lipid peroxidation.
Figure 3. Lipid peroxidation process.
In the initiation process, prooxidants abstract an allylic hydrogen from a polyunsaturated fatty acid and form the carbon-centered lipid radical; the carbon radical tends to stabilize by a molecular rearrangement to form a conjugated diene (step 1). In the propagation phase, lipid radical rapidly reacts with oxygen to form a lipid peroxyl radical (step 2) which abstracts a hydrogen from another lipid molecule generating a new lipid radical and lipid hydroperoxide (step 3). In the termination
10
In the initiation step, the key event is the formation of a lipid radical (L•): the free radical (R•) subtracts an allylic hydrogen atom from a reactive methylene group of polyunsaturated fatty acid side chains (Eq. 7).
LH + R• → L• + HR (Eq. 7)
The free radical initiator can be a superoxide anion, a hydroxyl radical or a hydrogen peroxide. Metal ions contribute to these radical formations by Fenton reaction (Eq. 6) and therefore it is a detrimental participant in lipid peroxidation. Alternatively, thermal or photochemical homolytic cleavage of LH bound may occur. In all cases, the resulting carbon-centered lipid radical can have several fates but the most likely one in aerobic cells is to undergo bond rearrangement that results in stabilization by diene-conjugate formation.
In the propagation step, the oxygen reacts with a carbon centered radical to give a peroxyl lipid (LOO•) (Eq. 8). The addition of molecular oxygen to a lipid radical is the faster than other radical reactions: therefore, the peroxyl species is the principal chain-carrying species. LOO• propagates a free radical chain by hydrogen atom abstraction to give a conjugated diene lipid hydroperoxide (LOOH) and a new lipid radical, which continues another chain reaction (Eq. 9). The hydrogen atom transfer represents the rate limiting reaction. Furthermore, the peroxyl free radical undergoes addition reactions to carbon-carbon double bonds but this process has not been studied extensively in the context of lipid peroxidation.
L• + O2→ LOO• (Eq. 8)
LOO• + LH → LOOH + L• (Eq. 9)
Lipid hydroperoxides, also called lipid peroxide, is the first stable product of the lipid peroxidation reactions. However, in the presence of transition metal ions, LOOH gives raise to the generation of alkoxyl radicals, LO• (Eq. 10) and peroxyl radicals (Eq. 11), thus perpetuating the chain reaction of lipid peroxidation (Eq. 12, 9) (Halliwell and Gutteridge, 1984).
11
Fe3+ + LOOH LOO• + H+ + Fe2+ (Eq. 11) LO• + LOOH → LOH + LOO• (Eq.12)
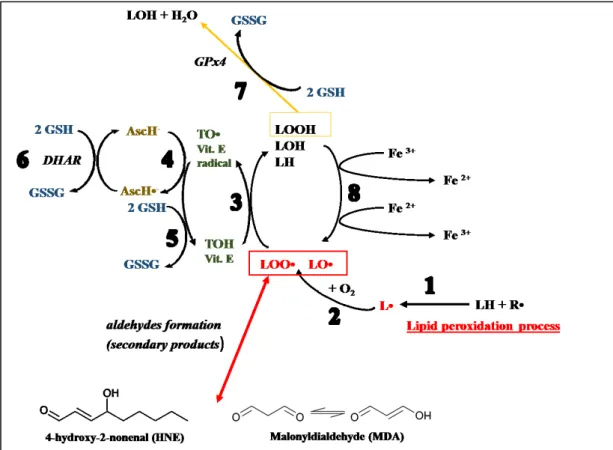
The termination stage is mainly characterized by the formation of non-radical secondary oxidation products through the radical–radical combination (radical quenching) (Eq.13-15). The reaction can also terminate in the presence of a chain-breaking antioxidant like vitamin E (α-tocopherol, α-TOH) (Halliwell and Gutteridge, 1984), which donates a hydrogen atom to the LOO•, LO• and L• species, thus forming the corresponding protonated forms (Eq. 16-18) and vitamin E radical (α-TO•). Glutathione (GSH) and ascorbic acid (Vitamin C) may complement the antioxidant function of α-tocopherol by providing reducing equivalents necessary for its recycling/regeneration. Moreover GSH is also able to reduce the tocopherol radical of Vitamin E indirectly, via reduction of semidehydroascorbate (AscH-) to ascorbate (AscH•-), reaction catalyzed by dehydroascorbate reductase (DHAR). In the Figure 4, I want to give a summary of these linked reactions. Another important chain-branching antioxidant is an isoform of Glutathione peroxidase (GPx4) that converts glutathione (GSH) into oxidized glutathione (also called glutathione disulfide, GSSG) and, during this process, reduces lipid hydroperoxides (LOOH) to corresponding stable alcohols (Liang et al, 2009) (Eq. 19). More in details, the GPx4-mediated reduction of lipid peroxides may depend on vitamin E, another key redox player. This fat-soluble vitamin can be defined as a pre-conditioning cofactor that prepares the substrates for the reaction. Vitamin E is in fact responsible for the 1-e- step (proton-donation reaction) that reduces a peroxyl radical to
the peroxide substrate of the GPx reaction (Eq. 18, Fig. 4) (Galli et al., 2017).
LOO• + LOO• → LOO-OOL (Eq. 13) LOO• + LOO• → LOH + LO + 1O
2 (Eq. 14)
L• + L• → L-L (Eq. 15)
L• + α-TOH → LH + α-TO• (Eq. 16) LO• + α-TOH → LOH + α-TO• (Eq. 17) LOO• + α-TOH → LOOH + α-TO• (Eq. 18)
2 GSH + LOOH → GSSG+ LOH + H2O (Eq. 19) GPx4
12
Figure 4. Role of antioxidants in the management of lipid peroxidation process. Reaction 1: The
generic radical (R•) can abstract an electron from a polyunsaturated fatty acid (LH) and give rise to a carbon-centered lipid radical (L•). Reaction 2: The lipid radical (L•) can further interact with O2 to give
a peroxyl radical (LOO•). Reaction 3: The LOO• is reduced within the membrane by the reduced form
of Vitamin E (TOH) resulting in the formation of a lipid hydroperoxide (LOOH) and a radical of Vitamin E (TO•). Reaction 4: The regeneration of Vitamin E by Vitamin C: the Vitamin E radical (TO•) is reduced back to Vitamin E (TOH) by ascorbic acid (ascorbate monoanion, AscH−) leaving behind the ascorbyl radical (Asc•−). Reaction 5: The regeneration of Vitamin E by GSH: the oxidised Vitamin E radical (TO•) is reduced by GSH and GSSG is formed. Reaction 6: The ascorbyl radical (Asc•−) is reduced back to ascorbate monoanion, AscH−, by GSH. Reaction 7: Lipid hydroperoxides are reduced to alcohols and water by GPx4 using GSH as the electron donor. Reaction 8: Lipid hydroperoxides can react with Fe2+
to form lipid alkoxyl radicals (LO•), or with Fe3+to form lipid peroxyl radicals (LOO•). Reaction 9:The
peroxidation reactions leads to the production of several aldehydes such as malonyldialdehyde (MDA) and 4-hydroxy-2-nonenal (HNE).
The LPO alters the functions of the cells and their survival, since membranes form the basis of many cellular organelles like mitochondria, plasma membranes, lysosomes,
13
peroxisomes, reticulum endoplasmic, etc. Moreover, the damage associated to LPO may also impair the efficiency of liposomial dispersion containing PUFAs as drug delivery systems.Thus, prevention of LPO represents an interesting therapy in many diseases and pathologies involving free radicals and is an important aspect in the preparation and preservation of liposomes used as carriers for several agents in medicinal, pharmaceutical, cosmetic and food industry applications.
In addition to the changed characteristics of cellular membranes, the peroxidation reactions lead to the production of several aldehydes that, as well as ROS, exhibit high reactivity with biomolecules, such as proteins, nucleic acids and phospholipids. Compared with free radicals, aldehydes are highly stable and spread out from the cell attacking targets far from the site of their production. About 32 saturated and unsaturated aldehydes are identified as products of lipid peroxidation. Among them, unsaturated 4-hydroxy-2-nonenal (HNE) is the most important aldehydes causing cellular damage (Fig. 4) (Uchida, 2003; Uchida, 1992). The hydroxyl-group close to a carbonyl group present in HNE chemical structure is related to its high reactivity. In particular, this lipidic product reacts with proteins and phospholipids thus modifying their structure and therefore their functions (Uchida, 2003; Uchida, 1992). Instead, the malonyldialdehyde (MDA) (Fig. 4), considered for a long time the most important lipid peroxidation metabolite, is practically no toxic (Uchida, 2003; Uchida, 1992).
1.3.3. Proteins
The ROS attack on proteins, results in specific amino acid modifications, fragmentation of the peptide chain, aggregation of cross-linked reaction product, alteration of electrical charge and increased susceptibility to proteolysis (Butterfield et al, 1998). The mainly amino acids susceptible to oxidation by ROS is the sulphur containing amino acids such as methionine and cysteine thatare converted to disulphides and methionine sulphoxide (Pryor et al, 1994;Brodie and Reed, 1990) respectively. The ROS oxidize also others amino acid residues such as lysine, proline, threonine and arginine and yields carbonyl derivatives that have been considered as the marker of ROS mediated protein oxidation
14
(Chevion et al, 2000).Oxidative degradation of proteins is enhanced by the presence of metal cofactors that are capable of redox cycling.
1.4. Essential role of antioxidants in protection against oxidative stress
The cellular antioxidant defense system comprises both enzymatic and non-enzymatic mechanisms that are usually distributed within the cytoplasm and various cell organelles. Moreover, the protection against ROS is also supplied by non-enzymatic exogenous antioxidants, which cannot be synthetizes endogenously by humans.
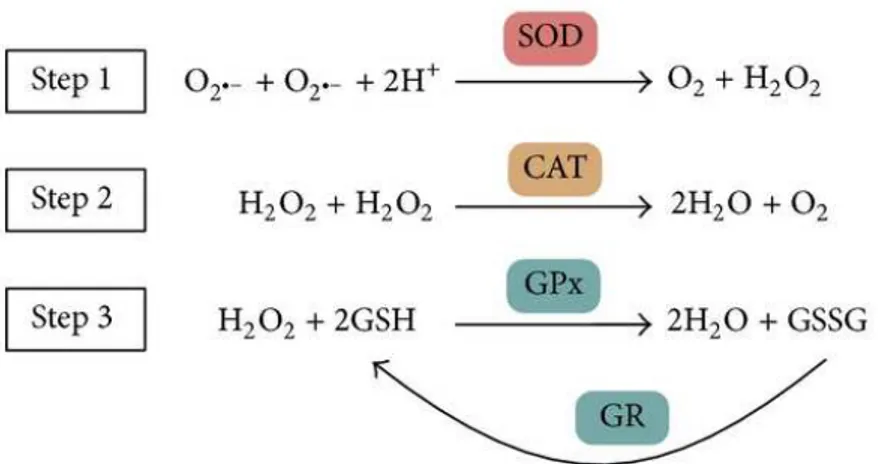
The major endogenous antioxidant enzymes directly involved in the neutralization of ROS and other free radicals are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) andglutathione reductase (GR) (Figure 5).
Figure 5. Main enzymatic antioxidant defense system in vivo and their reactions on
scavenging free radicals and hydrogen oxide. SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; and GR, glutathione reductase.
These enzymes are the main defense system against ROS in vivo. The Superoxide dismutase (SOD) is the most powerful antioxidant in the cell. It acts as a component of first line defense system against reactive oxygen species (ROS) catalyzing the dismutation of two molecules of superoxide anion radical (O2•ˉ), a precursor of several other ROS, to hydrogen peroxide (H2O2)
and molecular oxygen (O2) (Fig. 5, Step 1). The SOD category comprises metalloenzymes and
although they differ in respect to their metal cofactor, all isoenzymes catalyze the same reaction. The three isoforms of SOD present in humans are CuZnSOD (SOD1), which mainly exist in
15
the cytoplasm, MnSOD (SOD2), located in the mitochondrial matrix, with manganese being present in the active site, and an extracellular Cu-Zn containing SOD (EC-SOD or SOD3) (Nozik-Grayck et al, 2005). Compartmentalization of different forms of SOD provides an important mechanism for fine spatial control of ROS homeostasis. The importance of SOD isoforms is manifested by severe pathologies associated with the lack of this enzyme in mouse models (Lee et al, 2013; Kliment et al, 2009). In addition, the levels of all SOD isoforms decline with age, whereas free radical formation increases. It has been suggested that proper daily SOD supplementation could protect the immune system, thus reducing the development of age-related diseases, and ultimately slows down aging process (Krishnamurthy and Wadhwani, 2012). The human catalase (CAT) is a heme enzyme using iron as cofactor. It catalyzes the degradation or reduction of two molecules of hydrogen peroxide (H2O2) to two water molecules
and molecular oxygen, consequently completing the detoxification process started by SOD (Fig. 5, Step 2). It is found mainly in peroxisomes but it is absent in mitochondria of mammalian cells (Schrader and Fahimi, 2006). This implies that another enzyme known as glutathione peroxidase carries out the scavenging of hydrogen peroxide. The deficiency or mutation of CAT enzyme have been linked with various disease conditions and abnormalities (Góth et al, 2004) such as colorectal cancer, type 2 diabetes mellitus and retinal disorders. The glutathione peroxidase (GPx) enzyme converts GSH into GSSG and, during this process, reduces H2O2 to
H2O (Fig. 5, Step 3) and lipid hydroperoxides (LOOH) to the corresponding alcohols (Fig. 4;
Eq. 18). Generally, its activity depends on a micronutrient cofactor known as selenium. For this reason, GPx is often referred to as a selenocysteine peroxidase. The GPx reaction couples to glutathione reductase (GR), which maintains reduced GSH levels (Fig. 5). There are eight GPx enzymes in humans and the GPx4 is the only isoform of GPx that breaks down phospholipid hydroperoxides (Ighodaro and Akinloye, 2017). Therefore, GPx4 enzyme is essential in inhibition of lipid peroxidation process (Gill and Tuteja, 2010), lipid signaling functions, such as that of the 12/15-lipoxygenase-induced apoptosis and regulation of inflammatory responses (Conrad and Friedmann, 2015; Toppo et al, 2009).
The non-enzymatic antioxidants involved in the protection against ROS and oxidants comprise endogenous and exogenous antioxidants, also called metabolic antioxidants and nutrient antioxidants respectively. Examples are GSH, metal-chelating proteins, antioxidants vitamin such as vitamin E and C, flavonoids, trace metals (selenium, manganese, zinc), etc.
16
The GSH is a tripeptide constituted from glutamic acid, cysteine and glycine. The sulfhydryl group (SH) of cysteine is the functional group of the molecule, responsible for its biological activity. The thiol group of GSH is a potent reducing agent, rendering GSH able to scavenge many dangerous endobiotic and xenobiotic electrophiles, both through direct or indirect enzymatic reactions (Birben et al, 2012; Galano and Alvarez-Idaboy, 2011). This ubiquitous molecule is the main low-molecular-weight thiol found in living cells, also considered one of the major non-protein defenders against oxidative stress and the most important thiol-disulfide redox buffer of the cell. As described above, the GSH acts as a substrate for H2O2-removing
enzymes and it is a cofactor of other enzymes such as glutathione transferase. The glutathione is also able to regenerate the most important antioxidants, Vitamins C and E, back to their active forms; glutathione can reduce the tocopherol radical of Vitamin E directly, or indirectly, via reduction of semidehydroascorbate to ascorbate (Fig. 4).
The metal-chelating proteins as ceruloplasmin, ferritin, transferrin and albumin, inhibit the formation of new reactive species by binding transition metal ions (e.g. iron and copper). Vitamin E (the most biologically active form is -tocopherol) is a key essential fat-soluble vitamin with high antioxidant potency. Because of its fat-solubility, α-tocopherol safeguards cell membranes from damage by free radicals. In vivo and in vitro studies have shown that vitamin E acts as a chain-breaking antioxidant protecting unsaturated lipids from peroxidation by scavenging peroxyl radicals (Fig. 4). Animals and humans with a low vitamin E status present several problems at muscles and at neurological level that have been related to the protective effects of vitamin E against damage to PUFA in cell membranes and confirm its role as an essential nutrient. Vitamin E has shown to prevent several diseases development such as colon and breast cancer, arthritis and certain neurological disorders (Traber and Stevens, 2011; Traber, 2014). Moreover, the high dietary intake of vitamin E appears to decrease the risk of cataracts (Tavani et al, 1996; Theodoropoulou et al, 2014) and age-macular degeneration (AMD) (van Leeuwen et al, 2005;Bibiloni et al, 2014). However, despite its reputation of being healthy, vitamin E has a dark side: Mitchel et al. (Mitchel and McCann, 1993) have shown that vitamin E owns tumor-promoting activity in the skin of mice; in one in vitro model, in the presence of Cu2+, α-tocopherol shows an oxidative DNA-damaging effect (Yamashita et al, 1998). Finally, the α-Tocopherol can also reduce iron or copper, as a pro-oxidant (Yamamoto and Niki, 1988). Therefore, the benefit or the dangerous effect of vitamin E appears to be associated with its concentration.
17
Zinc (Zn), copper (Cu), manganese (Mn), and selenium (Se) ions are the key components of enzymes with antioxidant function, and are designated as antioxidant micronutrients. For examples, Zn, Mn, and Cu ions are cofactors of superoxide dismutase (Cu/Zn-SOD) while the selenium is essential for GPx activity. Vitamin C or ascorbic acid, which is the primary antioxidant in plasma and cells, must be provided from fresh fruits and vegetables since the humans are not able to synthesize it.
18
CHAPTER II
Ocular Diseases Associated with Oxidative Stress
2.1. Introduction
The eye is one of the highest oxygen-consuming tissue in the human body and the mainly part of the body normally exposed to light. Thus, oxidative and particularly photo-oxidative damage occurs in the eye causing from ocular to retinal diseases. Most of them are linked with aging that has been defined as “the progressive accumulation of changes with time that are associated with or responsible for the ever-increasing susceptibility to disease and death which accompanies advancing age” (Harman, 1981). The eye is one of the organs subjected to the processes of aging in which antioxidant level declines and ROS level increases, ensuring oxidative stress. By aging, glutathione S-transferase-1 expression level (Hammond et al, 1997), and vitamin E level (Hammond et al, 1998) decrease while lipofuscin (Hammond et al, 1997), mitochondrial DNA damage, and lipid peroxidation (Hammond et al, 1997) increase. These processes can lead to functional and morphological impairments in retinal pigment epithelium (RPE), endothelial cells, lens epithelium cells (LECs) and retinal ganglion cells (RGCs). There are mainly ocular disorder linked with oxidative stress including the age-related macular degeneration (AMD), cataracts and dry eye disease (DEA). I will briefly discuss the pathologies in which the oxidative stress play pivotal roles in their development and in which the uses of antioxidants may be an effective therapeutic strategy.
2.2. Age-related Macular Degeneration (AMD)
Age-related macular degeneration (AMD) is the leading cause of permanent and irreversible blindness in elderly individuals throughout the world, and approximately 50 million people suffers of AMD (Gordois et al, 2012). The disease affects the macula at the center of the eye, which may lead to a partial or complete vision loss in one or both eyes in older people (age > 55 years). The rise in life expectancy has determined the increase in the absolute number of affected people globally (Williams et al, 1998; Gordois et al, 2012).
19
AMD is classified into two types: “dry” and “wet” that account for about 85% and 15% of cases respectively (Bhutto and Lutty, 2012; De Jong, 2006). However, dry-type AMD pathology may progress to the wet-type AMD,which contributes to rapid loss of vision (Fine et al 2000). The wet-type AMD also called “exudative-AMD” is the most severe form of AMD and is generally associated with subretinal (i.e. between the retina and choroid) neovascularization because of pathological angiogenesis: the use of antiangiogenic agents (e.g. Lucentis and Avastin) can ameliorate the patients conditions (e.g. Lucentis and Avastin) (Andreoli and Miller, 2007). In the dry-type AMD the visual loss is caused mainly by the “geographic atrophic” death of photoreceptors and retinal pigment epithelial (RPE) cells. It is typifies over that by cell loss, also by oxidative stress, drusen (yellow deposits located under the retina), and the lipofuscin accumulation. All these characteristic represent the diagnostic features of dry-type AMD (Bhutto and Lutty, 2012). Apart from positive correlation of the disease with age, other risk factors are cigarette smoking, white race, female sex, blue iris colour, obesity, nutritional factors and insufficient antioxidants in the diet (Kaarniranta et al. 2011).
Retinal cells are in fact highly metabolically active and are constantly exposed to light thus representing an ideal environment for the generation of reactive oxygen species, especially with increasing of age. As mentioned above, the retina is one of the highest oxygen consuming tissues in the human body and consequently a large oxygen gradient is created from the choroid and its associated vasculature towards the inner retina and residing photoreceptors. Instead, the continuous exposition to light generates the photon flux across the retina in which photosensitizer are most abundant, leading to light-induced reactive oxygen species. More in details, radiation reaching the eye is partially absorbed by the cornea and the lens, but the rest of it (400–760 nm) penetrates the eye reaching the retina. The sensitivity of the retina to light damage is dependent both to wavelength light, which is greater with shorter wavelength light, and duration of exposure (Wiegand et al, 1983). At a retinal level, the visible light stimulates RPE cells to phagocytosis (ingestion) of rod outer segments thus inducing the formation of superoxide anion (Figure 6).
20
Epidemiological evidences suggest a direct relationship between phototoxicity (cumulative light exposure) and the development of AMD (Hunter et al, 2012). Since the photoreceptors and retinal synapses are rich in polyunsaturated fatty acids, the retina is also particularly vulnerable to auto-oxidation. In fact, the polyunsaturated fatty acid docosahexanoic acid (DHA) (22:6ώ-3) represents the 30-50% of the lipid bilayer of rod outer segment membranes, and proteins make up the remaining percentage (Stone et al, 1979).This very high proportion of long-chain PUFAs is a feature unique of the retinal lipids. Their oxidation leads to the development of high levels of peroxides and organic radicals, as well as other secondary products such as 4-HNE, which form adducts withproteins thus accumulating in the retina. The results of lipid peroxidation is the functional and structural impairment of cell membranes and finally leads to the degeneration of photoreceptors. Furthermore, oxidized PUFAs are not efficiently digested in the lysosomes of aged RPE cells and become deposited in the form of lipofuscin that represents the main component of drusen (Figure 6). As described above, the lipofuscin is a RPE photosensitizer that, after having absorbed a high-energy photon especially that of blue light, undergoes a variety of photochemical reactions. One of the formed reactive photoproducts is the N-retinylidene-N-retinylethanolamine A2E that represents the main fluorophore of RPE lipofuscin and it is responsible of singlet oxygen and superoxide generation (Sparrow et al. 2000). Indeed, the elevated level of lipofuscin may induce apoptosis. Since RPE cells are postmitotic, their death result in the reduction of RPE cell density in the RPE layer (Del Priore et al. 2002). Thus, the oxidative stress increases and the antioxidant molecules level declines, resulting in pathogenic process. Therefore, effective ROS scavenging may be essential for preventing and/or treatment AMD.
Figure 6. Diagram of the eye. The macula is the
central portion of the retina, which contains photoreceptor cells (rods and cones) adjacent to a monolayer of RPE. In this cartoon, the outer segment to RPE ratio is simplified for clarity. RPE, retinal pigment epithelium. From Ben-Shabat et al, 2001.
21
Natural and synthetic antioxidants as ascorbic acid (Organisciak et al, 1985) and ginkgo biloba extract (Ranchon et al., 1999) prevent retinal light damage and photoreceptor cell loss; they probably act as antioxidants during light exposure. The same effect has epigallocatechin- 3-gallate (EGCG), a major polyphenol in green tea, which is able to protect human retinal pigment epithelium (ARPE-19) cells from viable blue light-induced disorders (Li et al, 2014). Others, including saffron (Maccarone et al., 2008) and sulforaphane (Tanito et al, 2005), induce the synthesis of antioxidant enzymes. Synthetic antioxidants that have also protective and curative effect on ADM are free radical spin trap such as phenyl-N-tert-butylnitrone (PBN) that catalyzes the degradation of superoxide (Ranchon et al, 2001). The synthetic Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a free radical scavenger which will be widely described in the followed chapter, has shown to be effective against retinal degeneration both in vivo and
in vitro (Imai et al, 2010; Masuda et al, 2016).
2.3. The Role of ROS and the Oxidative Stress in Cataractogenesis
Cataract, as well as AMD, is one of the leading causes of blindness in the worldaffecting about 25 million people (World Health Organization, 2009). The pathology is characterized by progressive loss of transparency of the eye lens that results in visual impairment. The transparency of the eye lens depends on maintaining the native tertiary structures and solubility of the lens crystallin proteins over a lifetime.
The cataract formation is caused by several factors (genetic factors, diabetes, aging, smoking, drugs, malnutrition, and radiation) but the free radical induced oxidative stress is considered as one of the major underlying mechanism of cataract disorder (Beebe et al, 2010). The UV radiation represents the mainly contributory factor to oxidative damage of the lens tissue. As widely discussed, the UV light induces the photochemical generation of ROS resulting in oxidation of proteins, nucleic acid and lipid. However, the opacity of lens is mainly caused by protein oxidation and altered redox balance. Over 90% of crystalline protein sulfhydryl (protein-SH) groups are lost and as a consequence the cross link by non-disulfide bonds may occur (Berthoud and Beyer, 2009). The result is the insolubilization of the lens crystallins and this is the major cause of the yellow color residing in the lens. The toxic product such as HNE, produced during lipid peroxidation processes, induces the fragmentation of lens proteins contributing towards the opacity of the lens. The light absorbed by cornea may activate the
22
tryptophan amino acids, forming several photoproducts (Boettner and Walter, 1962). These photoproducts gradually accumulate in the center of the lens and successively bind to the crystalline proteins thus altering their electrical and solubility properties. Such a process may be involved in human lens browning and brunescent cataracts (Zigman et al, 1973). The loss of glutathione is a crucial feature that precedes cataract formation (Giblin, 2000). GSH is the essential and primary lenticular antioxidant (Reddy, 1990) and provides the maintenance of the lens transparency by scavenging ROS and protecting protein thiols.
Several in vitro and in vivo studies have shown how the antioxidant molecules can prevent or delay thelight-induced protein oxidation as well as photoperoxidation of lens lipids. The key player in the protection of the lens from oxidation is the vitamin C, which plays an important part in lens biology, both as an antioxidant and as a UV filter when present in aqueous (Hegde and Varma, 2004). The supplemental of Vitamin E decreases the lens opacity in human (Seth and Kharb, 1999). Even here, the EGCG molecules are able to prevent the tryptophan oxidation thus protecting the γB-crystallin and lens epithelial cells in humans from UV-induced damage (Chaudhury et al, 2015). The lycopene carotenoid as well as thecurcumin, the active principle of turmeric, protect against oxidative stress-induced experimental cataract (Gupta et al, 2003; Padmaja and Raju, 2004).
2.4. Tear Film and its dysfunction in Dry Eye Disease (DEA)
The International Dry Eye Workshop (DEWS) defines dry eye as a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolality of the tear film and inflammation of the ocular surface(Lemp, 2007).
The tear film (TF) is a liquid layer covering the cornea and acting as a barrier between the eye and environment. Acting as a shield of our ocular surfaces, its damage results in several symptoms, most of which have been associated with Dry Eye Syndrome (DES).
23
The tear film serves to remove foreign materials from the cornea and conjunctiva; to supply the cornea with nutrients by transporting oxygen and a limited number of other nutrients; to maintain a smooth surface for light refraction. Moreover, it has a lubricating power and contains immunoglobulins, lysozymes, lactoferrin which are involved in infections protection (Lakshmi, 2014).
The tear film is divided into three layers:the innermost mucin-rich layer, an aqueous layer in the middle, and the outermost tear fluid lipid layer (TFLL) (Figure 7). The mucin (mucous) layer at the bottom of the tear film provides a “sticky” foundation and acts as a barrier in the eye surface.The mucus helps the overlying watery layer to spread evenly over the eye. The aqueous layer is the “juicy” center and contains essentially a very dilute saltwater solution (Lakshmi, 2014). It is comprised of tears produced by the lacrimal glands. This layer keeps the eye moist and helps in the removal of any dust, debris, or foreign particles. Defects of this layer cause DES in most cases (Lemp, 1995). Finally, the top “oily” lipid layer of the tear film is made up of lipids or oils produced from the meibomian glands. This layer helps to decrease evaporation of the watery layer and it is very important for stability of tear film. Causes for DES include decreased tear production, excessive tear evaporation, and abnormality in the production of mucus or lipids of tear layer by meibomian gland. As a consequence, the concentration of solutes in the aqueous layer increases leading to hyperosmolarity of the tear film. This condition causes dryness and irritation and is associated with symptoms of discomfort (Lemp, 2007).
Epidemiological studies have shown that the incidence of dry eyes increases with age (Viso et al. 2009) and therefore with increased oxidative stress. Transgenic animal’s model with compromised antioxidant defenses have shown decreased production of tears, increased meibomian gland dysfunction (Ibrahim et al. 2014) and increasing ROS formation resulting in dry eye. Moreover, the hyperosmolarity of tears contributes to increase oxidative stress (Deng et al, 2015).
Many clinical trials have in fact suggested that oxidative stress can be a potential therapeutic strategy in dry eyes (Seen and Tong, 2018). Supplementation with oral antioxidants may
24
improve both tear stability and quantity (Drouault-Holowacz et al, 2009). Topical application of herbal extracts also improved clinical signs, decreased inflammation, and ameliorated oxidative stress marker as well as the ROS production on the ocular surface of the DES in model mice (Choi et al, 2016). In addition, lipids are helpful in attempting to supplement abnormal lipid production. In fact, the oily component can merges with the natural lipid layer reducing evaporative fluid loss.
2.5. Management of ocular diseases – limitation and therapeutic interventions
Oxidative stress can be reduced with systemic interventions in the form of dietary supplements, vitamins, omega-3 fatty acids, exercise, etc. However, these have antioxidant effects elsewhere in the body apart from the lacrimal glands and the ocular surface. Therefore, the ocular drug delivery requires different routes of administration including intravitreal and topical administrations. Although the intravitreal route has high therapeutically efficacy, it is associated with potential risks of complications (Gaudana et al, 2010): as a consequence, the topical application of drugs, typically in the form of eye drop formulations, represents the most common method of drug delivery for the treatment of ophthalmic ailments. This conventional dosage forms account for nearly 90% of currently available marketed formulations thanks of their simplicity, safety and acceptance by patients. However, achievement of adequate bioavailability of topically applied drugs is challenging due to unique anatomical and physiological barriers at the ocular surface that determines a low drug adsorption of about 5% (Urtti and Salminen, 1993).
Two major barriers to topical ocular drug delivery are present (Figure 8).
Precorneal tear film. As described above, an intact tear film is essential for a healthy ocular surface and its alteration has been associated with DES. However, for the penetration of drugs applied topically to ocular surface, tear film is a significant barrier.First, the tear film is subjected to a high turnover rate: reflex tearing, which may occur after drug instillation, results in an accelerated washout of the drug after application. Moreover, blinking creates a pumping mechanism to facilitate the flow of tears into the nasal cavity thus resulting in drug loss (Peters and Colby, 2006). The presence of gel-like mucus layer, which has a protective role for the ocular surface, alsoacts as a barrier to drug delivery systems.
25
Corneal epithelium. The epithelium of the cornea is in continuation with the conjunctiva and consists of 5–6 layers of cells packed closely and connected by tight junctions. These layers form a lipophilic barrier against water-soluble drugs. In particular, in order to penetrate these layers the optimal drug lipophilicity corresponds to log D values of 2–3 (Mannermaa et al, 2006). The presence of conjunctival blood capillaries and lymphatics can also cause significant drug loss into the systemic circulation, thereby lowering ocular bioavailability (Peters and Colby, 2006).
Figure 8. Two major barriers to topical ocular drug delivery. (a) Tear film barrier: a high turnover rate and the gel-like mucus layer make tear film a barrier in topical ocular drug delivery. (b) Corneal barrier: tight junctions exist in the corneal epithelial cells. Moreover, five layers of alternating polarity make the
cornea an important barrier to most lipophilic and hydrophilic drugs. From Gan et al, 2013.
Finally, the enzymatic degradation in tears and cornea reduces the ocular bioavailability of drugs (Lee et al, 1982). Therefore, eye drops are normally administered multiple times daily in order to achieve therapeutic efficacy.
In order to circumvent the permeation barriers for topically applied drugs, several ophthalmic drug delivery strategies have been emerged. These include hydrogels, polymeric micelles, nanosuspensions and lipid based-nanocarriers. Among them, the lipid-based nanocarriers are the most biocompatible and offer potential advantages as delivery systems for ocular
26
administration (Gan et al, 2013). As illustrated in Figure 9, the interactions between oil-in-water (o/w) emulsions and the tear film increase the bioavailability of the drug since:
- The lipids that make up these transport systems interact more effectively with the lipid layer of the tear film, facilitating the transport of the drug through the tear film barrier;
- Furthermore, the lipids released in aqueous solution contribute to the thickening of the superficial lipid layer, reducing the aqueous losses by evaporation resulting in enhanced tear film stability.
This intrinsic bionic tear film property of lipid-based nanocarriers makes them particularly suitable for the treatment of dry eye syndrome.Moreover, the lipid-based nanocarriers can be modified with mucoadhesive materials such as polymers, thus improving pre-corneal retention time and permeation (Mishra et al, 2011).
Figure 9. Interactions between o/w emulsions and the tear film. (a) After instillation, the water phase of the
emulsion can enhance the aqueous layer of tear film and moisten the cornea. (b) As the oil droplets break down, they release encapsulated emulsion components. The oil phase then merges with the natural lipid layer and enhances it, reducing fluid loss caused by evaporation. (c) Emulsifiers have been found to increase the depth of the mucus layer and improve the ‘wettability’ of tear film.
27
CHAPTER III
Lipid-based nanocarriers
3.1. Lyotropic Liquid Crystals (LLC)
Lyotropic Liquid Crystals (LLC) are interesting systems to be explored in the development of lipid-based nanocarriers; they can target the anterior and the posterior ocular structure and most of them have been clinically approved (Gan et al, 2013). They are often termed “mesophases,” representing intermediate states of matter between an isotropic liquid and a solid crystal
(Friberg, 1977). The characteristic of the molecular structures that commonly generate LLC phases is the amphiphilicity. Molecules with a hydrophilic head and a lipophilic tail form aggregates through a self-assembly process that is driven by the “hydrophobic effect” when they are mixed with water. Simply varying composition (concentration of amphiphiles, water or additives) and temperature, a wide range of mesophases can be obtained.In the next section, I want to give a brief description of different structures observable in lipid-water systems, focusing the attention on the lipid carriers used in my PhD work.
3.2. Lipid/Water Polymorphism
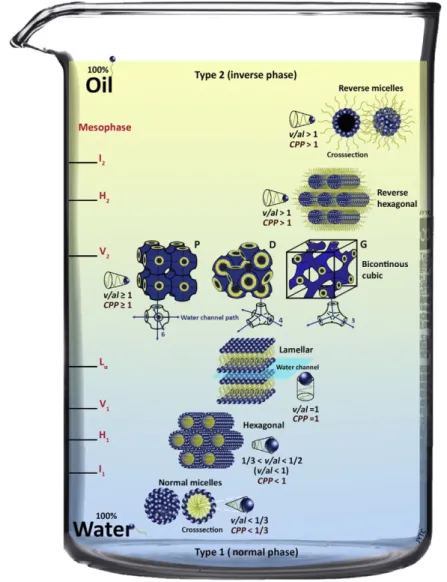
Amphiphilic molecule such as polar lipids, through a self-assembly process, can form different ordered structures in an aqueous environment which can be classified into lamellar (Lα), hexagonal and cubic phases. The architecture is mainly influenced by the water concentration as well as the molecular structure of the amphiphiles. It is possible to predict the phase structure by using the critical packing parameter (CPP) (Eq. 20),
CPP = v / a l (Eq. 20)
where (v) is the volume of hydrophobic liquid tail, (a) is the cross-sectional area of the head group, and (l) is the length of the lipid chain (Israelachvili, 1992). This critical packing parameter (CPP) enables the determination of the preferred structures formed because it estimates the lipid layer curvature. Lipids with CPP = 1 usually self-assemble into planar or
28
bilayer structures with zero mean curvature andbilayer lamellar structures are formed. When CPP < 1 the polar heads of the lipids form a convex interface and “normal oil in water” morphologies (Type 1) with positive curvature are formed including spherical micelles. On the contrary, if the CPP > 1, the curvature is towards the aqueous environment, resulting in negative curvature with “inverse water in oil” (Type 1) structures such as reversed bicontinuous cubic (QII) phase and the reversed hexagonal (HII) phase (Figure 10). By dispersion of these bulk structures in aqueous media, the main typically obtained nanostructures are liposomes, as result of the dispersion of a lamellar phase; cubosomes, formed by reversed bicontinuous cubic phase; and hexosomes, from the reversed hexagonal phase (Chang et al, 2015).
Figure 10. An illustration of the main types of lyotropic liquid crystal phases depending on the interface
curvature (molecular shape or concentration in water). The mesophases are denoted as I1, I2 (discrete micellar cubic phase), H1, H2 (hexagonal phase), V1, V2 (bicontinuous cubic phase), and Lα (lamellar phase). From
29
In addition to lipid molecular geometry, many factors including temperature, pressure, light, ions, water content and additives can alter the architecture of lipid system (Venugopal et al, 2011).
3.3. Lipid-based nanocarriers relevant to topical ocular drug delivery 3.3.1. Lamellar phase: liposomes
In nanoparticle technology, liposomes are the most widely studied lamellar phases, which are formed when the CPP is equal to one (Figure 10). They are defined as nanosized vesicular structures consisting of an aqueous core surrounded by one or more phospholipid layers; therefore, their structure is very similar to cell membrane (Figure
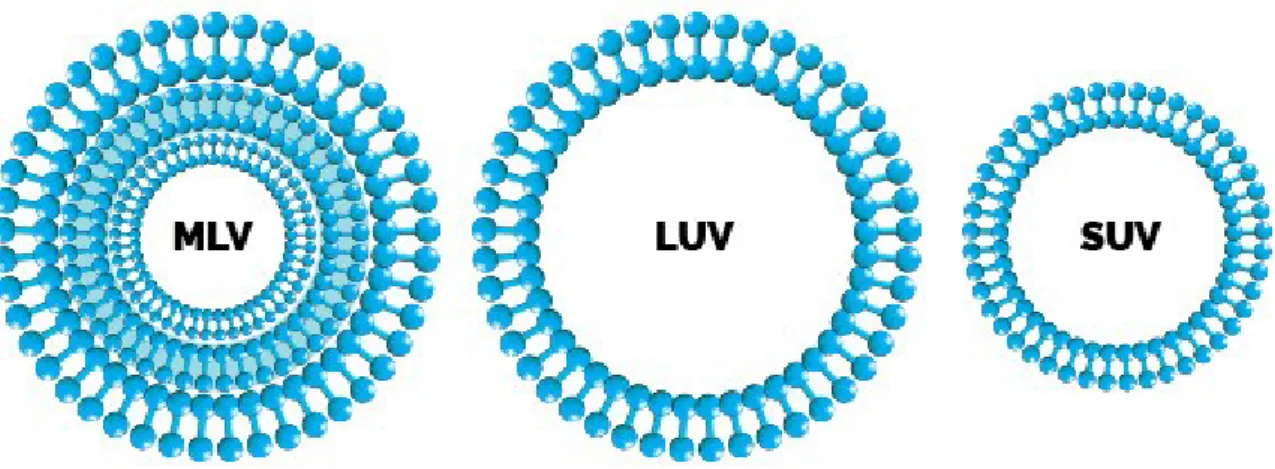
11). Liposomes can be classified into Multilamellar Vesicle (MLV), Large Unilamellar
Vesicle (LUV) and Small Unilamellar Vesicle (SUV) according to their particle size and number of lamellae. MLVs are formed by more lamellae with size ranging from 400 to 3500 nm in diameter. LUVs generally range from 200 to 1000 nm with the largest entrapment volume and the highest encapsulation efficiency. SUVs are in the range of 25 to 100 nm with the most uniform size distribution, which can be obtained from MLVs and LUVs by size reduction process (such as sonication and size extrusion).
Figure 11. Schematic representation of basic structures and different types of liposome.
These supramolecular aggregates can encapsulate enzymatic antioxidants but also hydrophilic and lipophilic chemical antioxidants, shielding and protecting them from