Abstract
Discovery of a new pharmacologically active molecule able to determine pharmacological effects favoring the cure of diseases or the attenuation of the associated symptoms is only the first step in the drug developmental process. The delivery of a defined amount of active principle to the target site at a determined time or duration is as important as its discovery. In order to realize the optimal therapeutic outcomes, a delivery system should be designed to achieve the optimal drug concentration at a predetermined rate and at the desired site. Moreover, in the drug developmental process should be considered the influence of drug physicochemical properties on passive diffusion process through biological membranes.
Biological membranes are composed of small amphiphilic molecules, phospholipids with two hydrophobic chains and cholesterol or other related structures, which associate into lipid bilayers in aqueous media. Drug molecules must possess some lipophilicity to be able to permeate biological membranes as hydrophilic part of biological membranes are commonly thought to be less important for drug absorption.
In general, drug solubility and subsequently its ability to permeate biological membranes since only dissolved drug molecules are able to penetrate them, is among the main physicochemical properties to evaluate in formulation of new and known chemical entity. Poor aqueous solubility of drugs is an important factor essentially associated to their limited oral bioavailability but it is also able to hinder drug delivery via non-oral routes such as buccal, ocular, nasal, pulmonary, rectal, ungual and vaginal route. Numerous methods have been proposed to enhance aqueous solubility of poorly soluble drugs and to enhance the drug permeation through biological membranes often by the design of innovative systems for the controlled release of drugs.
The aim of this research work was the development of suitable semisolid formulations for transungual and transdermal drugs delivery and of tablets for oral and vaginal extended-release.
To develop a transungual drug delivery a combination of iontophoresis and chemical enhancers was used in order to enhance the solubility and the permeation of an antimycotic drug (nystatin) through hoof bovine membranes chosen as experimental model.
A polymeric monolithic hydrogel was prepared to control the transdermal release of diltiazem hydrochloride, an antihypertensive drug with high hydrophilicity.
As topical vaginal drug delivery by once daily bioadhesive vaginal tablets might be an important approach for the treatment of candidiasis, in the present study high viscosity polymers were used to control the release of clotrimazole from tablets, and both chemical enhancers and cyclodextrin were used to increase the apparent water solubility of this drug. Finally, a metformin extended-release dosage form by floating gastroretentive approach was designed. Gastroretentive dosage forms were prepared by using an hydrophilic polymer and a gas-forming agent to obtain a low density dosage form able to float above gastric fluid for a prolonged period of time. While the system is floating on gastric content, the drug will be released slowly at desiderate rate from the system.
Alla mia famiglia, a Fernanda
e a tutti i miei amici
Ringraziamenti
Durante questi tre anni ho avuto il piacere si collaborare con diverse persone che hanno contribuito alla mia formazione professionale, vorrei esprimergli tutta la mia gratitudine. Desidero ringraziare la mia tutor, la prof.ssa Patrizia Chetoni, per i suoi insegnamenti, la sua pazienza e la sua grande disponibilità. Per avermi sempre lasciato libero di “ricercare” e per avermi corretto quando sbagliavo.
Non da meno vorrei ringraziare le dott.sse Daniela Monti e Susi Burgalassi (per me come delle prof.), per il loro aiuto, la disponibilità, le discussioni costruttive e per aver sempre trovato delle risposte ai miei quesiti.
A Silvia e Nadia, a distanza di tre anni dai ringraziamenti della laurea questa volta ho tolto i titoli di dott.sse, colleghe e compagne di viaggio di questo dottorato. Per aver sempre trovato una persona con cui parlare, a cui chiedere, per avermi inizialmente istruito e successivamente sopportato. Grazie.
Non posso non ringraziare gli studenti, adesso dottoresse che mi hanno aiutato in questo lavoro: Maria Giulia Martellucci, Linda Viviani, Giulia Barbieri. E tutti gli studenti che mi hanno tenuto compagnia in questi anni.
Ed infine, non per importanza, vorrei ringraziare la Regione Toscana, a cui devo la borsa di dottorato messa a concorso e finanziata tramite i fondi europei.
I
Index
Section I: Transungual Drug Delivery
1
1. The Iontophoresis 3
1.1 Theoretical basis of iontophoressis 3 1.2 Factors affectsing iontophoretic delivery 5
1.3 Electro-osmotic flow 9
2. The nail structure, physiology and disorder 11
2.1 Anatomy and biology of the nail unit 11
2.2 Microscopic anatomy 13
2.3 Infection of the nail 16
2.4 The therapy 17
3. Transungual Iontophoresis 21
4. Influence of iontophoresis on in vitro transungual permeation of Nystatin 25
4.1 Introduction 25
4.2. Materials and Methods 26
4.3 NIST delivery/permeation experiments 28
4.4 Results and discussion 29
4.5 Conclusions 35
Bibliography 36
Section II: Oral Drug Delivery
395. Drug Release in the Gastrointestinal Tract 41
5.1 The importance of drug delivery system 42 5.2 The GI tract: anatomy and physiology 42 5.3 Stomach: role and General Description of the Stomach 45 5.4 Preformulation and Biopharmaceutical considerations for controlled release drugs 47 5.5 Formulation of Controlled Release Drugs 49
II
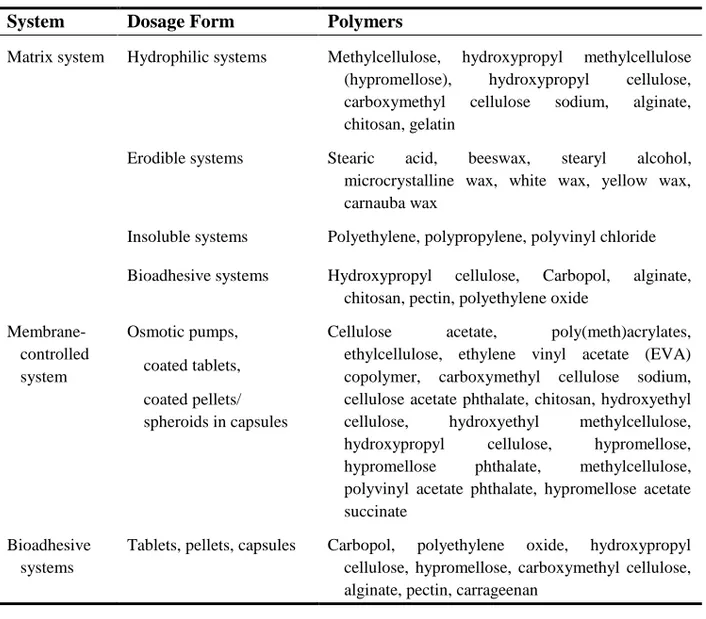
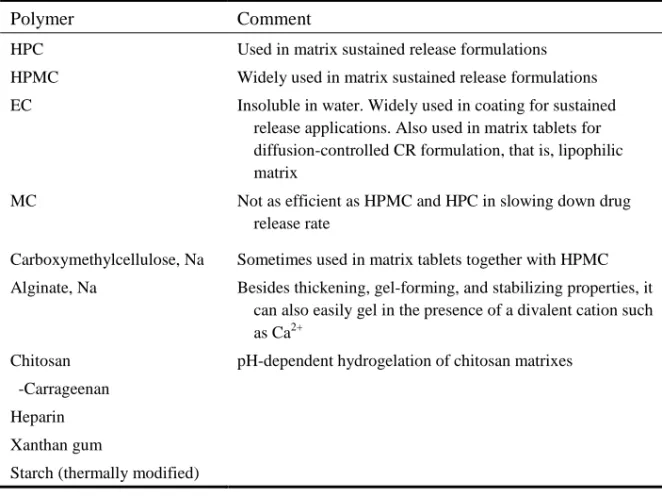
6.1 Polymers used for Controlled Release 54
6.2 Type of Polymer 57
7. Oral Extended Release Hydrophilic Matrices: formulation and design 63
7.1 Hydrophilic Matrices 64
8. Oral Targeted Drug Delivery Systems: Gastricretention Devices 69
8.1 Introduction 69
8.2 Gastrointestinal absorption 71
8.3 Drugs and gastric retention 72
8.4 Review of gastric retention tecnologies 72
9. A New Gastricretentive Tablet for Extend-Release of Metformin HCl 79
9.1 Introduction 79
9.2 Materials and Methods 80
9.3 Results and Discussion 83
9.4 Conclusions 94
Bibliography 95
Section III: Vaginal Drug Delivery
97
10. Vaginal Anatomy and Physiology on Drug Delivery 99
10.1 Introduction 99
10.2 Anatomy and histology 99
10.3 Vaginal fluid 101
10.4 Vaginal mucus 103
10.5 Vaginal flora 104
11. Vaginal Drug Absorption and Dosage Form 105
11.1 Intravaginal drug absorption 105
11.2 Development of an intravaginal drug delivery system 108
11.3 Vaginal drug delivery platforms 110
12. Formulation Vaginal Tablets 115
12.1 Mucoadhesive polymers in vaginal delivery 115
12.2 Enhancer drug solubility 116
13. Evaluation and Management of Vaginitis 121
13.1 Introduction 121
13.2 Yeast Vaginitis 122
14. Preparation and in vitro evaluation of extended-release of Clotrimazole
III
14.1 Introduction 127
14.2 Material and method 128
14.3 Results and Discussion 132
14.4 Conclusions 139
15. An clotrimazole β-cyclodextrin inclusion complex for extended-release
hydrophilic matrix 141
15.1 Introduction 141
15.2 Material and Methods 142
15.3 Results and Discussions 145
15.4 Conclusions 153
Bibliography 154
Section IV: Hydrogel for Transdermal Drug Delivery
157
16. Hydrogel: property and develop 159
16.1 Crosslinked polymers 159
16.2 Hydrogels synthesis 160
16.3 Expansion of a hydrogels structure 161
16.4 Swelling forces in hydrogels 162
16.5 Swelling mechanism 165
16.6 Water in hydrogels 166
15.7 Hydrogels properties 167
16.8 Hydrogels characterization 167
17. The Skin: anatomy and physiology 171
17.1 Components of normal human skin 171
17.2 Functions of skin 174
17.3 Skin maintenance 174
17.4 Keratinocytes 175
18. Topical and Transdermal Drug Delivery 177
18.1 Topical drug delivery 177
18.2 Transdermal patch systems 178
18.3 The epidermis and stratum corneum as a physical barrier 178 18.4 Percutaneous absorption pathways 179 18.5 Transdermal diffusion-controlled systems 180
18.6 Enhancement of skin permeation 181
18.7 Current challenges and future trends 183
18.8 Modulation of skin reactions 184
IV
19.1 Introduction 185
19.2 Materials and Methods 186
19.3 Results and discussion 190
19.4 Conclusions 195
20. Preparation and evaluation of Diltiazem Hydrochloride patch 197
20.1 Introduction 197
20.2 Materials and Methods 198
20.3 Results and discussions 200
20.4 Conclusions 202
3
1.
The Iontophoresis
Drug delivery through or into the biological membranes can be assisted by electrical energy. The physical force involved can be electrorepulsion, electro-osmosis or electroporation. The electric current from a power source is delivered to the solution containing the drug where it is converted to an ionic flow taking place through the solutions and biological membranes. The terminals leading the current into and out of the solution are electrodes, the positive pole being the anode and the negative pole being the cathode. At the beginning, this technique was designed to increase percutaneous absorption, then it has been developed in ocular and ungual drug delivery.
1.1 Theoretical basis of iontophoressis
The techniques for electrical enhancement of percutaneous absorption, in realistic terms, constitute a rather complex area with a large number of operating variables and the results depend on the drug candidate being studied. Many of the theoretical equations for iontophoresis have been derived based on experimentation with simple ions such as sodium transport studies. These equations may not be applicable to many drugs, especially for those that bind to the skin or to macromolecules. The situation may be further complicated for peptide drugs which have the potential to undergo enzymatic degradation during transport through the skin and also undergo other losses such as by adsorption and self-aggregation. In general, the flux of an ionic species, i, is given as:
flux=concentration×mobility×driving force or,
(1.1)
Transungual Drug Delivery
4
(1.2)
The thermodynamic expression for the electrochemical potential, ui, is given as: (1.3)
where ui(o) is the standard chemical potential and zi is the valence of the species i, F is
Faraday’s constant, E is the electrostatic potential, R is the gas constant and T is the temperature in Kelvin. Assuming ui(o) is constant and substituting Eq. 1.3 into dui/dx in Eq. 1.2, we get: (1.4) The mobility, m, is related to diffusivity, D, as:
(1.5)
Substituting Eq. 1.5 form in Eq. 1.6, we get:
(1.6)
This is a fundamental relationship, called the Nernst-Planck equation, and is widely used to describe the membrane transport of ions.
Several more rigorous theoretical models have been developed for iontophoretic delivery, which have been reviewed and discussed for those interested in a more detailed treatment. A better appreciation of the meaning of this equation may be achieved by considering the case of a nonelectrolyte, in which the charge, z=0. In this case, the Nernst-Planck equation is reduced to:
(1.7)
which is Fick’s first law of diffusion. On the other hand, for an ion with a uniform concentration throughout the system (dCi/dx=0), the Nernst-Planck equation becomes:
(1.8)
which is the equation for electrophoresis. When both a concentration gradient and an electric field exist, the ionic flux is a linear sum of the fluxes that would arise from each effect alone. Though the cutaneous permeability of ionized drugs is low, it cannot be assumed to be negligible and thus the potential contribution of passive flux needs to be considered. An expression has been derived for macroscopic membranes which takes into account the lag time. This expression was derived for the effective lag time and enhancement ratio under the application of a uniform electric field to an uncharged
Transungual Drug Delivery
5 homogeneous membrane. The enhancement ratio (E.R.), which is the ratio of the steady-state flux with applied voltage divided by steady-steady-state flux by passive diffusion alone, was given as:
, where (1.9)
E.R. can also be expressed as a ratio of permeability coefficients by iontophoretic transport (PΔE) over passive diffusion (P0):
(1.10)
Again, this assumptions are not likely to be valid for a complex membrane like the skin which is known to be a charged heterogeneous structure with a pHiso of 3–4. This will lead
to some deviation from the theory. However, the model may be a useful tool for analysing the details of iontophoresis experiments.
1.2 Factors affectsing iontophoretic delivery
There is a complex multitude of factors operating during iontophoresis. In order to understand the delivery profiles and to be able to use this technology commercially, it is important to understand the various formulation, electrochemical and biological factors involved in the process.
1.2.1 Electric current
If the transport pathways across the skin are current dependent, then an increase in current density is expected to increase the amount of drug that will be delivered. According to Faraday’s laws of electrolysis, the transport of one molar concentration of a univalent ion requires the passage of 96485 coulombs of electricity, if the ion has a transport number of unity. Hence, the maximum rate of transport, Jmax, is:
(1.11) where (MW) is the molecular weight of the ion and I is the current. The more general case for a species i is:
(1.12)
where F is Faraday’s constant, z is the number of charges per drug molecule (valency) and ti is the transport number. The transport number (or transference number parameter), ti, of an ionic species is the fraction of total applied current carried by that ionic species and may be calculated as:
Transungual Drug Delivery
6
(1.13)
This suggests that the ti can have a fixed value for a drug ion under a set of experimental conditions even though this value could be considerably less than unity because of competition with Na+ or Cl- ions in the body. At low electric fields, the iontophoretic enhancement is in reasonable agreement with the prediction of the constant field model for electro diffusion. However, at higher power levels, the drug transference number in the membrane needs to be taken into account. If ti is known, then theoretical flux can be calculated since all other parameters are known. Thus, a good prediction for each drug can be made.
Alternatively, the iontophoretic skin permeation (mg/h) can be predicted as follows:
The steady-state plasma levels can then be calculated by dividing the steady-state iontophoretic skin permeation by plasma clearance. As expected from Eq 1.12, a linear dependence of the flux (J) on the total current density (IT) applied at steady state is expected.
Several published reports support this expected result. The release of the neuropeptide angiotensin by microiontophoresis was found to be proportional to the current density applied. The drug delivery rate for inorganic ions through various types of excised skin has been shown to be have a linear relationship with the current density. However, the current density cannot be indefinitely increased as it will irritate and/or damage the skin, and also produce an unpleasant electrical sensation. In general, 0.5 mA/cm2 is often stated to be the maximum current density that should be used on humans without specifying the surface area.
1.2.2 Physicochemical properties of the drug
The charge, size, structure, and lipophilicity of the drug will influence the efficacy of iontophoresis. Ideal candidates for iontophoresis should be water-soluble, exist in salt form with high charge density. Conductivity experiments can be done to identify the best candidates for iontophoresis and to select the optimum pH for maximum delivery. The commonly used local anaesthetic hydrochloride salts show excellent conductivity, with the best values around pH 5. The conductivity of a drug can also be used to estimate the competitive transport between the drug and other extraneous ions during iontophoretic
Transungual Drug Delivery
7 transport. In a study on the anodal transport of a model cation across excised human skin, the ratio of the specific conductance of the cation in deionized-distilled water to that of the solution applied in the donor compartment was used. A linear relationship between iontophoretic flux and specific conductance was observed, but only after a certain threshold conductivity value.
Structure-transport relationships are hard to predict because of a multitude of factors involved in iontophoretic delivery. The efficiency of delivery of carboxylate ions showed the following rank order: acetate > hexanoate > dodecanoate, suggesting that smaller and more hydrophilic ions are transported faster than larger ions.
Using a series of positive, negative or uncharged solutes, the data for their iontophoretic delivery across excised human skin were best described by a linear relationship between the logarithm of the iontophoretic permeability coefficient and the molal volume, as predicted by the ‘free volume’ model. In one study1, the mobility of seven medications suitable for
iontophoretic administration was investigated at pH 5, 7 and 9. Three positive basic drugs, acyclovir, lidocaine hydrochloride and minoxidil, were used. The other four drugs, methylprednisolone sodium succinate, dexamethasone sodium phosphate, adenine arabinoside monophosphate, and metronidazole, had a negative charge. Acyclovir was ionized by protonation of the imidazole nitrogens at positions 3 and 7 on the guanine rings. At its first pKa of 2.27, it was 50:50 di-cation:monocation, while at its second pKa of 9.25, it was 50:50 mono-cation:neutral species. Its mobility was observed to be best at pH 7, suggesting that mobility is best when the maximum amount of mono-cation and minimum amount of free base is available. The salt form of the drug can also play an important role in controlling delivery efficiency if a protonated drug is being used and is the primary current-carrying species in the formulation. At neutral pH, cationic drugs often exist as a mixture of protonated and unprotonated species. As the protonated drug migrates towards the skin under the electric field, an imbalance is created with more protonated drug in the boundary layer. This in turn lowers the pH in the boundary layer which results in higher proton transport. The pH may even drop below the pK of the acid. The problem will not occur with positively charged drugs which are not protonated, such as, quaternary ammonium salts. This problem can be avoided by using a weak acid salt of the drug, such as the acetate rather than the hydrochloride. For instance, the succinate salts of verapamil, gallopamil and nalbuphine all had a higher flux and current efficiency than the corresponding hydrochloride salts. Non-ionized molecules will also be delivered typically by
Transungual Drug Delivery
8
iontophoresis owing to electro-osmosis. However, if the molecule has a good passive permeability, there is not advantage to the use of iontophoresis.
1.2.3 Formulation factors
The drug concentration, pH, ionic strength and viscosity of the formulation will affect the iontophoretic delivery of the drug. An increase in the drug concentration in the formulation typically result in higher iontophoretic delivery, as seen with acetate ions or metoprolol. Increasing the concentration will increase iontophoretic delivery up to some point, where the flux may become independent of concentration. This could be because the boundary layer becomes saturated with the drug while the bulk donor solution is still unsaturated. In order to avoid pH changes, a buffer system is usually used. The iontophoretic delivery of a drug is reduced by the buffer ions as they will compete with the drug to carry the current. As buffer ions are usually small, mobile and highly charged, they will usually be more efficient at carrying the current.
HEPES buffer is often used in iontophoresis research as it has a high buffering capacity at pH 7.4 because it is zwitterionic at this pH and thus has reduced charge carrying ability. Addition of sodium chloride provides one primary cationic (Na+) and one primary anionic (Cl-) charge carrier. Chloride also participates in the electrochemical reaction to form silver chloride. The buffer should be optimized for the system. In literature is reported the use of 25 mM HEPES along with about 75 mM sodium chloride and 0.02 per cent sodium azide. An ethanolamine/ethanolamine HCl buffer has also been used for human studies with iontophoresis because it does not give additional chloride ions.
An increase in ionic strength will decrease delivery as the extraneous ions will compete with the drug. Since many drugs such as peptides are very potent, they are used in low concentrations so that a small amount of additives can have a rather large negative influence on delivery efficiency. Furthermore, peptides are macromolecules and have low mobility to start with. The co-ions from buffering agents are usually more mobile than the drug and will thus reduce the fraction of current carried by the drug ion, decreasing the iontophoretic delivery. Extraneous co-ions can also be introduced at the electrodes, such as the generation of hydronium and hydroxide ions when platinum electrodes are used. These ions are even more harmful to transport efficiency, their mobility being three to five times greater than small inorganic ions such as sodium, potassium and chloride. The ionic strength of the buffer should thus be a compromise to achieve just adequate buffer capacity to avoid pH drifts but not be too high to minimize the competition for current.
Transungual Drug Delivery
9 An increase in solution viscosity may also decrease the iontophoretic flux by hindering the mobility of the drug. The efficiency of drug delivery will be determined by the concentration of extraneous ions and the mobility of the drug ion in the skin relative to the mobility of these other ions.
Another important factor is the pH of the formulation. The pH can determine either the charge of drug ore the ratio of the charged and uncharged species. In the case of polypeptides, the type of charge is also controlled by the formulation pH relative to the isoelectric point of the polypeptide. Iontophoretic delivery of a drug may be hindered by the presence of high concentrations (> 15% v/v) of cosolvents (such as propylene glycol) in the formulation. This could be due to a decrease in the conductivity of the drug solution as well as a decrease in electro-osmotic flow.
1.3 Electro-osmotic flow
If a voltage difference is applied across a charged porous membrane, bulk fluid flow or volume flow, called electro-osmosis, occurs in the same direction as the flow of counterions. This flow involves a movement of the fluid without gradient of concentration. Electro-osmositic flow is a significant factor in iontophoresis and was found to be of the order of μl/h per cm2 through hairless mouse skin. Since skin is a permselective membrane
with negative charge at physiological pH, the counterions are usually cations and electro-osmotic flow occurs from anode to cathode, thus enhancing the flux of positively charged (cationic) drugs. The stratum corneum is considered a cation permselective membrane. The major cation transported through epidermis, Na+, has a transport number of 0.6, which is about twice the transport number of Cl-, so that cations are transported more readily across the epidermis. The transport number of ions in skin can be calculated from potentiometric measurements, that is, of the potential of a system at zero net current. The ion selectivity of the epidermis can be determined by measuring the potential difference generated when the epidermal membrane separates two chloride salts solutions of different concentrations. Owing to electro-osmotic flow, it usually is also possible to deliver neutral drugs can be delivered using anodic iontophoresis. The term ‘iontohydrokinesis’ was used in early literature to describe the water transport during iontophoresis. Electro-osmotic flow can also hinder drug flux in a situation where a negatively charged drug (anion) or a neutral drug undergo catodic iontophoresis. In such cases, the flux may increase after the current is stopped. For example, the cathodal mannitol flux through hairless mouse skin was retarded with respect to passive transport owing to net volume flow in the opposite direction. The
Transungual Drug Delivery
10
transport of mannitol increased significantly after the current stopped. If the skin reverses its charge, such as at a pH below its isoelectric point, the direction of electro-osmotic flow will also reverse. pI value of the skin surface is about 3-4, which is about the isoelectric point of keratin in the stratum corneum layer. As the solution pH is decreased towards 4, electroosmotic flow decreases and eventually will reverse direction. Some positively charged peptides may interact with the skin reducing or neutralizing its negative charge. In these cases, the cation permselectivity of the skin may be lost, resulting in cathode-to-anode flow.
Water transference number, tw, defined as the number of moles of water transported per
equivalent of electricity represents electro-osmotic flux. Though both excised human skin and excised hairless mouse skin give significant electroosmotic flow, tw in human skin is
stated to be at least five times greater than this in hairless mouse skin at the same salt concentration. However, this may not correspond to higher flux for neutral species. The contribution of electro-osmotic flow depends on the sign of the membrane charge, the concentration of charges in the membrane, the pore radius, the Stokes radius of the drug species, and the ionic concentration in the membrane. For high molecular weight species such as proteins, the overall flux will be low even though the relative contribution of electro-osmotic flow will be large, as Stokes radius is large and diffusion is slow. For a protein with a small net negative charge, it is possible that delivery may be higher under the wrong polarity, that is, under the anode rather than the cathode because of the higher contribution of electro-osmotic flow. For example, the flux enhancement was found to be greater for anodic delivery than for cathodic delivery of the high molecular weight anionic species, carboxy inulin and bovine serum albumin. For a pore radius of about 2.5 nm, the ratio of electro-osmotic flow to ionic flow is greater than unity for species larger than about 1 nm. In another study2, the contribution of electro-osmosis for model permeants with a molecular weight range of 60–504 (urea, mannitol, sucrose and raffinose) was studied using a synthetic membrane. The enhancement factor was found to depend on molecular weight, being about four times greater for raffinose than for urea.
11
2.
The nail structure, physiology and disorder
2.1 Anatomy and biology of the nail unit
The nail plate acts as a protective covering for the fingertip. Fingernails typically cover approximately one-fifth of the dorsal surface, whereas on the great toe the nail may cover up to 50% of the dorsum of the digit.
The nail apparatus is schematically shown in Figure 2.1. The rectangular nail is firmly attached to the nail bed; it is less adherent proximally, apart from the postero-lateral corners. Approximately one-quarter of the nail is covered by the proximal nail fold, and a narrow margin of the sides of the nail plate is often occluded by the lateral nail folds. Underlying the proximal part of the nail is the white lunula (half-moon, lunula); this area represents the most distal region of the matrix. It is most prominent on the thumb and great toe and may be partly or completely concealed by the proximal nail fold in other digits, the reason for the white color is not known. The natural shape of the free margin of the nail is the same as the contour of the distal border of the lunula. The nail plate distal to the lunula usually appears pink, due to its translucency, which allows the redness of the vascular nail bed to be seen through it. The proximal nail fold has two epithelial surfaces, dorsal and ventral; at the junction of the two, the cuticle projects distally onto the nail surface. The lateral nail folds are in continuity with the skin on the sides of the digit laterally, and medially they are joined by the nail bed. The definition of nail matrix is controversial. There is a localized region beneath proximal nail which produces the major part of the normal nail plate, termed simply the matrix, or germinal matrix.
Transungual Drug Delivery
12
Figure 2.1 nail apparatus
The matrix can be subdivided into dorsal (ventral aspect of the proximal nail fold), intermediate (germinal matrix or matrix) and ventral (nail bed) sections. The nail bed is also termed the sterile matrix and its role in the production of nail is unclear. Although it appears that the nail plate may thicken by up to 30% as it passes from the distal margin of the lunula to the end of the nail bed, this is not associated with an increase in cell numbers and may represent compaction of the nail from distal tip trauma rather than nail bed or nail plate production.
The situation may change in disease, where the nail bed changes its histological appearance to gain a granular layer and may contribute a false nail of cornified epithelium to the undersurface of the nail. The gap beneath the free edge is known as the hyponychium. When the attached nail plate is viewed from above, two distinct areas may be visible: the proximal lunula and the larger pink zone. On close examination, two further distal zones can often be identified: the distal yellowish-white margin and immediately proximal to this the onychodermal band. Histologically, it is defined as the most distal attachment of cornified epithelium to the undersurface of the nail. As such, it is structurally significant for the adherence of nail plate to the nail bed. Once breached, as in conditions such as psoriasis, separation of the nail bed from the nail plate can be progressive.
Transungual Drug Delivery
13 Figure 2.2 Nail matrix
2.2 Microscopic anatomy
2.2.1 Nail foldsThe proximal nail folds are similar in structure to the adjacent skin but are normally devoid of dermatoglyphic markings and pilosebaceous glands. There is a normal granular layer. From the distal area of the proximal nail fold the cuticle adheres to the upper surface of the nail plate; it is composed of modified stratum corneum and serves to protect the structures at the base of the nail, particularly the germinal matrix, from environmental insults such as irritants, allergens, and bacterial and fungal pathogens.
2.2.2 Nail matrix
Nail matrix (intermediate matrix) produces the nail plate (Figure 2.2). The basal compartment of the matrix is broader than the same region in normal epithelium or in other parts of the nail unit, such as the nail bed. There is no granular layer, and cells differentiate with the expression of trichocyte “hard” keratin as they become incorporated into the nail plate, alongside normal epithelial keratins. During this process, they may retain their nuclei until more distal in the nail plate. These retained nuclei are called pertinax bodies. Apart from this, the detailed cytological changes seen in the matrix epithelium under the electron microscope are essentially the same as in the epidermis.
The nail matrix contains melanocytes in the lowest three cell layers and these donate pigment to the keratinocytes. The presence of 6.5 melanocytes per millimetre of matrix basement membrane can be used as a guide to a normal matrix melanocyte population. Matrix melanocytes are further distinguished from those elsewhere by their failure to produce melanin in normal circumstances in white people.
Transungual Drug Delivery
14
2.2.3 Nail bed
Nail bed consists of epidermis with underlying connective tissue closely apposed to the periosteum of the distal phalanx. There is no subcutaneous fat in the nail bed, although scattered dermal fat cells may be visible microscopically.
The nail bed epidermis is usually no more than two or three cells thick, although there may be tongues of epithelium that extend obliquely down. The transitional zone from living keratinocyte to dead ventral nail plate cell is abrupt, occurring in the space of one horizontal cell layer; in this regard it closely resembles the Henle layer of the internal root sheath of the epidermis. Nail bed cells do not have any independent movement, and it is yet to be clearly demonstrated whether they are incorporated into an overlying nail plate as it grows distally. The process of nail bed keratinization has been likened to that seen in rattail epidermis, possibly being affected by pressure changes. Within the connective tissue network lie blood vessels, lymphatics, a fine network of elastic fibres and scattered fat cells; at the distal margin, eccrine sweat glands have been seen.
2.2.4 Nail plate
The nail plate comprises three horizontal layers: a thin dorsal lamina, the thicker intermediate lamina and a ventral layer in contact with nail bed. This is not always apparent with normal light microscopy using routine stains, where the nail demonstrates a transition between flattened cells dorsally and thicker cells on the ventral aspect. Electron microscopy shows squamous cells with tortuous interlocking plasma membranes. At high magnification, the contents of each cell show a uniform fine granularity similar to the hair cuticle.
The nail plate contains significant amounts of phospholipid, mainly in the dorsal and intermediate layers, which contribute to its flexibility. The nail plate is rich in calcium, found as the phosphate in hydroxyapatite crystals; it is bound to phospholipids intracellularly. The relevance of other metals (copper, manganese, zinc, iron and others), which are present in smaller amounts, is not known. Calcium is present in a concentration of 0.1% by weight, 10 times greater than its concentration in hair.
2.2.4 Nail keratin
Nail keratin analysis shows essentially the same fractions as in hair, but aminoacid analysis shows higher amounts of cysteine, glutamic acid and serine, and less amont of tyrosine in nail compared to hair and wool.
Transungual Drug Delivery
15 The keratins can be divided in “soft” epithelial keratins and “hard” trichocyte keratins. The latter are characteristic of hair and nail, where the high sulphur content is responsible for the rugged physical qualities. This is matched by the resistance of trichocyte keratins to dissolution also in strong solvent.
Trichocyte and epithelial keratins are intermediate filaments representing the major cytoskeletal protein of epithelial cells. They share the normal classification into type I or type II based on gene hybridization, which reflects segregation on two-dimensional electrophoresis into acidic and basic proteins. Each acidic keratin is expressed in a tissue with a corresponding basic keratin to form specifi cheterodimers, which are assembled into higher-order protofibrils and protofilaments.
Keratin distribution in the nail and associated epithelium has been studied in adult, infant and embryonic digits. Immunohistochemistry of the epithelial structures of normal nail demonstrates that the suprabasal keratin pair K1/K10 is found in the proximal nail fold and to a lesser degree in the matrix. However, it is absent from the nail bed. This is reversed when there is nail bed disease, such as onychomycosis or psoriasis, where a granular layer develops and K1/K10 becomes expressed at corresponding sites. The nail bed contains keratin synthesized in normal basal layer epithelium, K5/K14, which is also found in nail matrix. An antibody marking the epitope characteristically associated with keratin expressed in the basal layer is found throughout the thickness of the nail bed, but only basally in the matrix.
Recent examination of the nail bed using monospecific monoclonal antibodies to the keratin pair K6/K16 demonstrates these proteins in the nail bed but not the germinal matrix. This is paradoxical given our understanding that K6/K16 is characteristic of psoriasis and wound healing, where proliferation is a prominent feature. It has been shown that the nail bed has very low rates of proliferation, and it may be that K6/K16 more precisely illustrate a loss of differentiation, often associated with proliferation in skin but representing the resting state of nail bed epithelium.
In the nail, it demonstrates a well-demarcated suprabasal region corresponding to the matrix. Proximally, it does not extend onto the ventral aspect of the proximal nail fold, sometimes described as the dorsal matrix. Distally, the keratin expression is limited to a margin taken as corresponding to the lunula. According to its distribution it appears to define a matrix consistent with the classic description of the germinal matrix.
Transungual Drug Delivery
16
2.3 Infection of the nail
Onychomycosis, a fungal infection (Figure 2.3) of the fingernails and/or toenails, is the most common infection and represents up to half of all nail diseases. It affects 10–20% of adults, especially the elderly. Multiple factors are associated with an increased risk of onychomycosis including old age, male gender, underlying medical diseases (diabetes, peripheral arterial disease, and immunodeficiency), smoking, and predisposing genetic factors. More than 90% of onychomycosis cases are caused by dermatophytes, such as Trichophyton rubrum and T. mentagrophytes are the most common. Other less commonly encountered causative organisms include yeasts such as Candida and nondermatophyte molds such as Scopulariopsis brevicaulis and Scytalidium hyalinum.
Figure 2.3 Type of infection of the nail
Five clinical subtypes of onychomycosis are recognized. Distal and lateral subungual onychomycosis (DLSO), the most common subtype usually caused by T. rubrum, manifests as subungual hyperkeratosis, onycholysis, nail thickening, and discoloration secondary to fungal invasion which starts at the hyponychium and spreads proximally along the nail bed. Superficial onychomycosis (SO) usually presents as white (caused by T. mentagrophytes) or black (caused by dematiaceous fungi) patchy nail discoloration due to fungal invasion of the dorsal surface of the nail plate. Proximal subungual onychomycosis (PSO) commonly
Transungual Drug Delivery
17 affects immunocompromised individuals and presents clinically as a white spot under the lunula that progresses distally. It results from fungal invasion (usually T. rubrum), from the proximal nail fold to the nail plate. Endonyx onychomycosis (EO) is an uncommon form caused by T. soudanense; it resembles DLSO; however, the nail thickness is normal and there is no hyperkeratosis or onycholysis. Total dystrophic onychomycosis (TDO) is an advanced form, characterized by progressive nail plate destruction leaving an exposed abnormally thickened nail bed. TDO may be observed in immunodeficient patients such as those with chronic mucocutaneous candidiasis and is fairly acute or may be progressive representing an end stage of other forms of onychomycosis. Treatment of onychomycosis has to be preceded by accurate diagnosis identifying the causative pathogen. Several diagnostic methods including KOH-based microscopy, fungal cultures, and histopathology with PAS may be used alone or in combination; the latter being the most sensitive.
2.4 The therapy
Onychomycosis fungal infection of the nail unit, namely onychomycosis, is a common disorder that is currently treated with broad-spectrum antifungals delivered via systemic and ⁄or topical routes.3, 4 Topical agents penetrate and accumulate in the nail plate, with the
highest concentrations found near the surface; the keratinized structure of nails provides a high resistance to drug permeation so that topical monotherapy is currently recommended only for the earlier stages of the disease.5
Systemic antimycotics reach the infection site via the nail bed and the nail matrix compromising the entire nail. However, oral administration of terbinafine or azoles does not always result in cure, and failure may be expected in 25–50% of cases1; moreover, the risk
of serious adverse reactions and drug interactions cannot be excluded.6 Recently several
reports have described successful combinations of topical and oral antifungals in the treatment of severe onychomycosis. The rationale of combined therapies consists of exploiting synergistic drug activities and ⁄or complementary penetration routes into the nail unit, where each drug alone does not accumulate in sufficient concentrations: oral drugs rapidly reach the nail bed via blood circulation while topical agents penetrate the nail plate and may be effective in preventing reinfection.7
The major challenge encountered in the treatment of onychomycosis is to deliver and maintain an effective concentration of antimycotic agents [greater than the minimum inhibitory concentration (MIC)] in the inaccessible deeper nail stratums.8 Current treatment
Transungual Drug Delivery
18
Unfortunately, oral therapy is associated with severe systemic and GI side effects.9 For the
topical therapy to be successful, the drug is required to penetrate across the nail plate and distribute in the nail stratums at therapeutically effective amounts. Unfortunately, there are at least two factors that could limit the accumulation and activity of drugs in the nail on topical application. First, the physicochemical properties of the drug need to be favorable for absorption through nail matrix. The nail matrix is reported to be relatively more permeable to polar compounds than nonpolar compounds.10,11 Second, binding of the drug
to keratin reduces the availability of the free drug. Antifungal drugs are reported to possess high-binding affinity to keratin.12 Therefore, the total amount of drug present in the nail
matrix does not represent the actual active levels relative to MIC. This, most likely, is one of the reasons for prolonged durations of treatment of nail disorders. Moreover, the bound form of the drug does not contribute to the concentration gradient due to the lack of thermodynamic activity. This decreases the amount of drug penetrating into the deeper nail layers. In agreement with this, Hui et al.13 found that a large portion of topically applied
drugs were retained in the surface layers of the nail due to binding which reduced the depth of drug penetration in the nail.
Therefore, topical administration of antifungal drugs has been primarily used in combination with oral therapy.14 Because of these issues, the development of a rapid,
transungual iontophoretic drug delivery technique for treating nail infections was explored. Conventional topical dosage forms such as creams or ointments are inadequate for delivering drugs through the nail unit, as the textures of the stratum corneum and nail keratin are remarkably different from a physicochemical point of view; moreover, constant contact between the nail plate and adhering surfaces such as socks, shoes and sheets could result in loss of medicinal product from the application site. Therefore, the best approach to transungual delivery of drugs consists of applying, on the nail surface, medicated lacquers that shortly evaporate, leaving on the nail a drug-charged polymeric film acting as a drug reservoir. Nail lacquers represent the most suitable topical formulations, instead of creams and solutions, as permanence of the formulation at the site of action is a critical factor for treatment success.3
Monti et al.5 had recently reported a nail permeation study of Ciclopirox (CPX) with
watersoluble nail lacquer (CPX ⁄sol, 8% CPX) based on hydroxypropyl chitosan (HPCH; ONY-TEC; Polichem SA, Lugano, Switzerland), that forms an invisible nonirritating film, easily removed by washing with water and applicable to periungual skin; this new
Transungual Drug Delivery
19 formulation appeared more efficient in promoting in vitro penetration of CPX through keratin membranes, when compared with the water-insoluble lacquer Penlac®, chosen as
reference. Their results suggest that application of the CPX ⁄sol nail lacquer allows fast penetration of CPX through the nail plate and its release to the nail bed; moreover, CPX levels are sufficient to inhibit fungal replication and are maintained for a prolonged period of time (30 h) after application of lacquer dose. In vitro behaviour of the CPX ⁄sol nail lacquer appeared as superior to the market reference MRF nail lacquer (Loceryl®) both for
more permeable active ingredient and for superior vehicle (HPCH) in terms of active release to the nail, with a higher efficacy on all nail pathogens, predicting better in vivo performance of the new nail lacquer.
21
3.
Transungual Iontophoresis
Iontophoresis, very efficient in transdermal drug delivery, can be applied successfully go the nail for treating onychomycosis and other nail diseases.
In the last five years, many works had studied how iontophoresis can increased the transungual delivery of drugs.
Murthy et al.15 had studied the factors that influenced the passive and iontophoretic
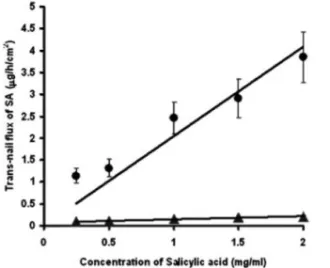
transport of salicylic acid (SA) through the nail. Cathodal iontophoresis was applied to enhance SA permeation from a 50 mM phosphate buffer solution (pH= 7.1) and SA flux increased by increasing drug concentration in the donor phase (Figure 3.1). Iontophoretic flux was surely greater than that passive and the mechanism of transport enhancement is probably a combination of electrorepulsion, electroosmosis, and by permeabilization of the barrier. Cathodal iontophoresis is not likely to be associated with electroosmosis under the present experimental conditions because, at pH 7.1, the nail (pI 5) is most likely to carry a high density of negative charge and is presumed to be cation selective.
The influence of current density (mA/cm2) on SA transungual flux is shown in Figure 3.2. Transungual flux increases by improving current density probably because the number of ions moving through the barrier rises.
Transungual Drug Delivery
22
Figure 3.1 Trans-nail flux of salicylic acid at
different concentrations of SA. The filled triangles represent thepa ssive transport and the filled circles represent iontophoretic transport.
Figure 3.2. Influence of current density
(mA/cm2) on the trans-nail iontophoretic transport flux of SA.
Besides the buffer ionic strength influences SA transungual flux: SA flux increases by increasing buffer ionic strenght up to 50 mM; at 100 mM it remains constant, then optimal ionic strength for effective current flow across the nail appears to be in the range of 50–100 mM (Figure 3.3).
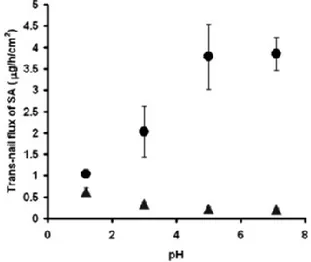
Finally, the influence of pH on SA iontophoretic transport is illustrated in Figure 3.4 the best condition was pH 5-7.1 where the drug was completely ionized and the driving force of the process was the electrorepulsion. In different condition of pH the electro-osmosis could be the prevalent mechanism.
Understanding the importance of the relative contributions of electrorepulsion and convective solvent flow to trans-nail iontophoretic transport is essential for the optimization of drug delivery,16,17 this phenomena can be used to enhancer the transport of neutral soluble molecule. Murthy et al.18 had reported the transungual flux of Grisefulvin (a sparingly water-soluble antifungal drug) and glucose. Passive flux of glucose did not differ significantly at different pH conditions. At pH 7, the total transport of glucose due to anodal iontophoresis was 12.42 ± 1.3 nmol/(cm2h); whereas at pH < 5, it was less than the passive flux of glucose. The pH dependent transport due to cathodal iontophoresis followed an opposite trend. In the case of cathodal iontophoresis, the transport was many folds higher at pH < 5 as compared to that at any pH > 5. The cathodal iontophoretic transport flux of glucose at physiological pH was 1.12 ± 0.21 nmol/(cm2h). At pH 5, the anodal and cathodal transports of glucose did not differ significantly from the passive flux. These
Transungual Drug Delivery
23 results clearly indicate that the nail plate exhibits iontophoretic permselectivity similar to human skin.16
Figure 3.3 Influence of ionic strength of the
buffer on the passive transport flux (filled triangles) and iontophoretic transport flux (filled circles) of SA.
Figure 3.4 Influence of pH on the passive
transport flux (filled triangles) and iontophoretic transport flux (filled circles) of SA
Nail at pH < pI carries a net positive charge and at pH > pI a net negative charge. At pH > 5, the anodal iontophoretic transport was high due to the net negative charge on the nail plate which attracts the cations. The cation transport is associated with the convective water flow in the anode to cathode direction, which in turn is responsible for the enhanced glucose transport observed in this case. The decrease in the anodal iontophoretic transport of glucose at pH < 5 is most likely due to the reversal of net charge present on the nail plate at lower pH. When the net charge on the nail plate turns positive, the convective water flow occurs in the opposite direction from cathode to anode. This hinders the transport of glucose from donor compartment to receiver compartment. In other words, the cathodal transport is facilitated by pH < pI and is affected at pH > pI. However, at pH = pI the effect of the net charge on the nail plate is relatively less apparent. Therefore the anodal and cathodal iontophoretic transport of glucose did not differ significantly at pH 5 in the present experiments (Figure 3.5).
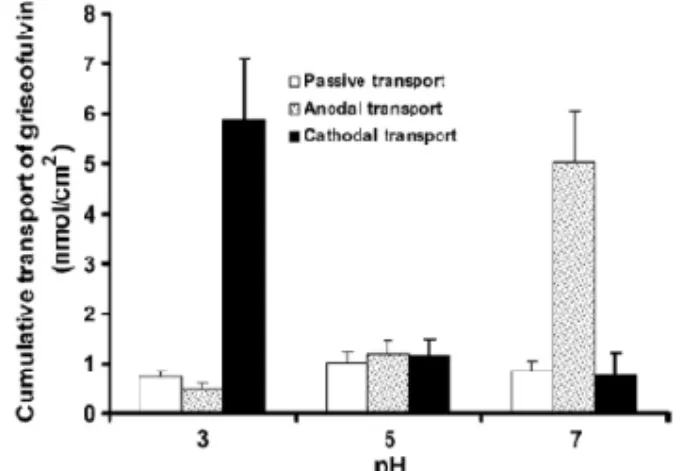
Griseofulvin is a sparingly water-soluble electroneutral antifungal drug with logP 2.0.19
Therefore its ability to permeate into hydrophilic keratinous nail plate is limited. The passive transport of the drug did not vary as a function of pH. The anodal iontophoretic transport was favored by pH 7 and the cathodal transport by pH 3 buffers (Figure 3.6). At pH 5, the iontophoretic transport was not significantly different from passive transport. The
Transungual Drug Delivery
24
transport of griseofulvin could be enhanced 8-fold by iontophoresis. Water solubility being one of the criteria for drug permeation across the nail, antifungal drugs that are poorly water-soluble does not appreciate significant penetration across nail plate. Provided a suitable iontophoretic device and formulations could be designed and the electrical protocols are optimized, the transport of not only the ionic drugs, but also uncharged drugs could be enhanced across the nail stratums.
Figure 3.5 pH dependent trans-nail transport
(flux) of glucose by anodal and cathodal iontophoresis at current density was 0.5 mA/cm2.
Figure 3.6 pH dependent trans-nail cumulative
transport of griseofulvin by anodal iontophoresis at current density of 0.5 mA/cm2.
25
4.
Influence of iontophoresis on in vitro
transungual permeation of Nystatin
Experimental chapter
4.1 Introduction
Delivery of drug through the nail continues to receive significant attention due to the need for efficacious topical therapies for onychomycosis, common fungal infection of the nail. Currently, it is treated by oral administration of antifungal drugs. The major task in treatment of the drug is to accumulate and maintain effective concentrations of the drug (above the minimum inhibitory concentrations) in the deeper nail stratum during the treatment period. This requires high doses and frequent administration of drug. Oral therapy is inherently associated with GI and systemic side effects. Topic therapy eliminates this such adverse effects and improve patient compliance. The low permeability of nail plate, factor limiting the efficacy of topical therapy, can be overcame by using different methods: a number of chemical enhancers, such as glycolic acid, urea, thioglycolic acid, N-acetyl-L – cysteine and mercaptan compounds, have been investigated to facilitate transungual drug penetration.20,21,22
Physical enhancement methods such as iontophoresis were investigated to increase transungual permeation of hydrophilic and macromolecular agents, both ionic and neutral permeants.23,24
Iontophoresis involves delivery of a compound across a membrane using an electric field (elettromotive force). This technique was successfully applied to increase transungual permeation of antimychotic agents for treating topically onychomycosis and other nail diseases such as ciclopirox22 and terbinafine using normal or onycomycotic nails.25 The enhanced permeation of charged drug molecules by iontophoresis was principally due to electrorepulsion with a lesser contribution by electroosmosis. The influence of current
Transungual Drug Delivery
26
density, vehicle pH, type of electrode, co-ions, drug concentration, and charge on drug transungual permeation is known.23, 26
In the present study nystatin (NIST) was chosen as model drug, an antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi, including Candida albicans, C. parapsilosis, C. tropicalis, C. guilliermondi, C. pseudotropicalis, C. krusei, Torulopsis glabrata, Tricophyton rubrum, T. mentagrophytes and the effect of chemical enhancers (cetylpyridinium chloride and polyoxyethylene (20) sorbitan monooleate) on passive and iontophoretic transport of NIST across fully hydrated bovine hoof membranes was investigated. Finally a suitable, pharmaceutically acceptable gel-type vehicle topical formulation containing NIST to treat onycomicosis with the help of iontophoresis at low density current (0.2 mA/cm2) was developed.
4.2. Materials and Methods
4.2.1 MaterialsThe following materials were used: nystatin (NIST, Sigma, Germany), cetylpyridinium chloride (CPC, Sigma, Italy), polyoxyethylene (20) sorbitan monooleate (Tween 80, TW80, Riedel-de Haen), hydroxypropylcellulose(HPC, Klucel JF, Hercules), HEPES buffer (AppliChem Gmbh, Germany). methanol (MeOH, Carlo Erba, Italy), ethanol (Et, Carlo Erba, Italy) and dimethylformamide (DMF, Fluka, Germany) were all HPLC-grade. All other chemical and reagents used (sodium chloride, sodium hydroxide, sodium phosphate monobase monohydrate, di-sodium hydrogen phosphate dried) were analytical grade. All the solutions were prepared using high-purity deionized water(18MΩ).
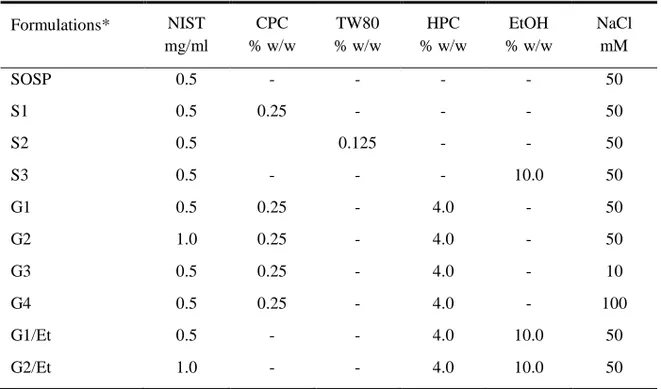
4.2.2 Formulations
The formulations under study (Table 4.1) were prepared in pH 5.6, 25mM Hepes buffer and adjusted to desired total ionic strength with sodium chloride providing aprimary cationic (Na+) and anionic (Cl-) charge carrier. The suspension of NIST (SOSP) was prepared introducing directly the drug in buffer solution; S1 and S2 formulations were prepared dissolving surfactants in buffer solution and then adding NIST. A cationic, CPC, and a non-ionic surfactant, TW80, were used as solubilizing agents of NIST.
G1-G4 gels were prepared by dissolving at the first CPC in Hepes buffer, then appropriate amounts of HPC was added at room temperature under continuous stirring for 12h; finally, NIST was introduced in this dispersion. To prepare hydroalcoholic gels (G1/Et, G2/Et), HPC was dispersed in Hepes buffer containing 10% w/w of ethanol at room temperature under continuous stirring for 12h, and at the end NIST was added.
Transungual Drug Delivery
27 Measurement of viscosity was carried out on the semisolid formulations using Haake Rheostress viscometer RS150 and sensor system C60/4 (Thermo Electron Corporation, Waltham MA, USA). Tests were performed in triplicate at 32 °C by applying increasing and decreasing values of the shear rate to detect possible thixotropy of the systems. The rheological profiles were determined with RheoWin Pro software. All gels exhibited a newtonian behaviour, with linear relationship between shear rate (D) and shear stress (tau).
Table 4.1 Composition of the formulations under study
Formulations* NIST mg/ml CPC % w/w TW80 % w/w HPC % w/w EtOH % w/w NaCl mM SOSP 0.5 - - - - 50 S1 0.5 0.25 - - - 50 S2 0.5 0.125 - - 50 S3 0.5 - - - 10.0 50 G1 0.5 0.25 - 4.0 - 50 G2 1.0 0.25 - 4.0 - 50 G3 0.5 0.25 - 4.0 - 10 G4 0.5 0.25 - 4.0 - 100 G1/Et 0.5 - - 4.0 10.0 50 G2/Et 1.0 - - 4.0 10.0 50
*all formulations were prepared in pH 5.6, 25 mM HEPES buffer solution
4.2.3 Analytical Method
Quantitative determination of NIST in the samples was carried out by HPLC. The apparatus consisted of a Shimadzu LC-10AD system with an UV SPD-10AV detection and a software Cromatoplus analyser. The injection valve was a Rheodyne with a capacity of 20µl. A Phenomenex C18 analytical column (4.60 × 100 mm, Luna, 3µ) was employed. The mobile phase consisted of MeOH:Water:DMF (60:30:10) mixture; the flux and the detection wavelength were 1ml/min and 305nm, respectively. The limit of determination and quantification were 6.9 ng/ml and 2.0 ng/ml, respectively. The amount of drug in each sample was determined from standard curves, obtained by plotting the concentration of known solutions vs. the corresponding peak areas of HPLC chromatograms.
Transungual Drug Delivery
28
4.3 NIST delivery/permeation experiments
4.3.1 NIST in vitro permeation through bovine hoof membranes
In vitro permeation experiments were carried out on bovine hoof slices (thickness 90-120 mm) as previously described.27
Bovine hoof membranes (diffusion area = 1.32 cm2) were introduced in modified Gummer vertical diffusion cells, consisted of receptor and donor chambers having capacities of 5.8 and 2 mL, respectively.
To evaluate the influence of iontophoresis on transungual permeation of NIST, Ag/AgCl electrodes were fixed in the donor and receptor chambers at a distance of 3 mm from the hoof membranes. Iomed Phoresor II dose controller was used to apply a constant direct current (DC). The anode was introduced into donor chamber and catode into receptor chamber (anodal iontophoresis). Current density of 0.2mA/cm2 was applied to stimulate NIST penetration in the experiments. The formulations (SOSP, S1, S2, G1-4, G1/Et and G2/Et) were placed in the donor compartment. The receptor solution (catodal chamber) consisted of 25 mM HEPES buffer pH7.4 plus 154mM NaCl, stirred at 600 rpm. At predetermined intervals of time receiving phase were completely withdrawn and replaced with equal volume of fresh buffer. The amount of NIST permeated was determined by HPLC analysis.
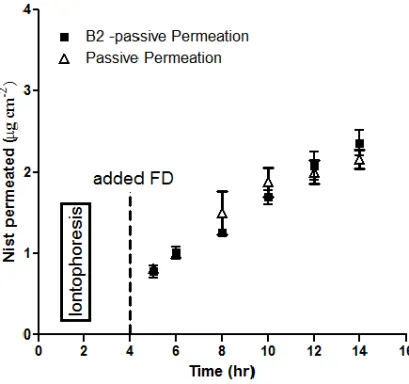
Two experimental protocols were used: Protocol A consisted of passive permeation experiments for 6 h (control); Protocol B consisted of iontophoretic treatment for 4 h applying constant direct current of 0.2 mA/cm2 (B1- 1° period), followed by 2h of passive permeation (B2- 2° period). Each test was replicated at least five times. Linear regression analysis of pseudo steady-state diffusion data allowed calculation of J, the steady-state flux (given by Q / A t-1, where Q is the amount of permeant diffusing across the area A in time t), amount of drug permeated after 4 hours of experiment. The enhancement factor (EF) is expressed as the ratio of the iontophoresis flux over the passive flux.
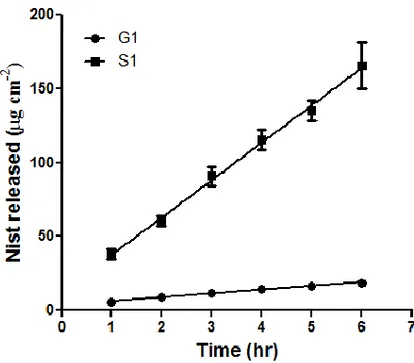
4.3.2 NIST in vitro release through cellulose acetate membranes
In vitro release of NIST from the S1-G1-G1/Et formulations through cellulose acetate membranes (SpectraPore3, MWCO3500, Spectrum®, NL) was investigated using vertical Gummer cells28 with an effective diffusion area of 1.23 cm2. Five millilitres of 25 mM HEPES buffer (pH 7.4) plus 154 mM NaCl, thermostated at 32 °C and stirred at 600 rpm, were used as the receptor medium. Formulations were put in contact with the membrane and represented donor phase. The donor compartment was hermetically sealed to avoid
Transungual Drug Delivery
29 evaporation. At predetermined time intervals, samples of the receiving phase were withdrawn for analysis and replaced with an equal volume of fresh buffer to maintain sink conditions. The amount of NIST released was determined by HPLC. Each release test was replicated at least three times.
4.4 Results and discussion
4.4.1 NIST in vitro permeation through bovine hoof membranes
Current density of 0.2 mA/cm2, 25 mM HEPES buffer pH 7.4 plus 154mM NaCl as receiving phase are commonly used to evaluate the effect of iontophoresis in cutaneous and ungual field.29 In this conditions, at physiological pH of 7.4, keratin membrane was net negatively charged since the keratin in the nail plate has an isoelectric point (IP) of 4.9-5.4. All formulations were prepared at the same pH value of 5.6, which NIST was partially positively charged in, to minimize the pH effect.
Table 4.2 summarizes steady-state flux (μg cm-2 h-1) of NIST for the different treatments and the
different formulations. The flux was calculated during passive permeation (protocol A), iontophoretic treatment (Protocol B1), and post iontophoretic treatment (Protocol B2).
Formulations Flux (μg h-2 cm-2 ± S.E., n=4)
Protocol A Protocol B
Passive Permeation B1
Iontophoretic transport
B2
Post- iontophoretic transport
SOSP 0.14 ± 0.011 0.63 ± 0.024 0.40 ± 0.056 S1 0.76 ± 0.027 2.12 ± 0.079 1.30 ± 0.057 S2 0.25 ± 0.024 0.45 ± 0.010 0.41 ± 0.003 G1 0.34 ± 0.020 2.08 ± 0.107 1.26 ± 0.137 G2 0.45 ± 0.021 1.97 ± 0.491 1.31 ± 0.379 G3 0.33 ± 0.022 1.63 ± 0.120 1.06 ± 0.097 G4 0.36 ± 0.035 3.29 ± 0.180 2.06 ± 0.135 G1/Et 0.31 ± 0.024 1.63 ± 0.116 1.08 ± 0.089 G2/Et 0.33 ± 0.025 1.89 ± 0.054 1.07 ± 0.082
At first this work was directed to understand the transungual kinetic of NIST from a suspension: SOSP produced a flux of 0.14 ± 0.011 μg cm-2 h-1, during the passive
Transungual Drug Delivery
30
permeation; applying iontophoresis raised NIST transungual permeation up to ~4.5-fold (0.63 ± 0.024 μg cm-2 h-1).
The addition of CPC to SOSP (S1) produced an improvement (~5-fold) on the permeability of bovine hoof membrane compared to the NIST suspension, passive diffusion was positively influenced by CPC; in fact CPC, used at a concentration above CMC (0.04% w/w), allowed a solubilization of NIST in pH 5.6 Hepes buffer; it is known that the enhancement in solubility of drug increases thermodynamic activity improving the permeation of drug across biological membranes.25
The application of iontophoresis (Protocol B1) when S1 formulation was used improved NIST flux from 0.76 ± 0.027 to 2.12 ± 0.079 μg cm-2 h-1 during 4 hours of treatment (Table 4.2) indicating that the drug and the cationic surfactant did not have a competitive mechanism.
On the contrary NIST transungual permeation was not influenced by the presence of TW80: there are no significant statistically differences in the passive diffusion with respect to SOSP and the flux during iontophoresis was not very different to that of the passive diffusion (0.45 vs 0.25 μg cm-2 h-1). The drug was not completely solubilized in donor phase and then the surfactant appeared to hinder the trasport of drug.
The amount of NIST permeated during iontophoresis with cationic surfactant was remarkably higher than non ionic surfactant. This suggests that the CPC with the formation of cationic micelles can promote iontophoretic transport, while TW80 doesn’t influence the iontophoretic transport. The combination CPC/iontophoresis appears suitable to transport NIST through the ungual barrier.
At this point NIST was introduced in a hydrogel that can provide an electroconductive base and will be more desirable for clinical use than an aqueous formulation. One advantage of hydrogel formulation would be the ease of application as a hydrogel pad can adapt to the application site. A hydrogel formulation might also be helpful in reducing skin hydration during the period of medication and might minimize the convective flow that often accompanies iontophoresis delivery.
NIST passive diffusion from a hydrogel containing HPC and CPC as chemical enhancer (G1) through the bovine hoof membrane was decreased to 0.34 ± 0.020 μg cm-2 h-1 with respect to 0.76 ± 0.027 μg cm-2 h-1 for S1. This can depend on the increase of vehicle viscosity (500 Pa s) that reduced the diffusion rate of drug through the gel matrix as demonstrated to decrease of diffusion rate of drug from viscous vehicles.
Transungual Drug Delivery
31 The application of current (protocol B1) improved the flux of NIST from G1 (2.08 ± 0.107 μg cm-2
h-1) to reach that of S1 (2.12 ± 0.079 μg cm-2 h-1, protocol B1).
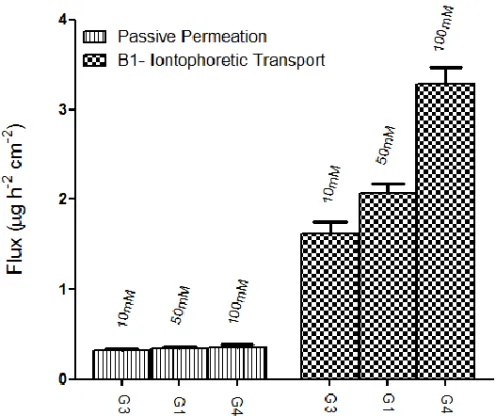
The effect of ionic strength on penetration was investigated by modifying NaCl concentration in the donor solution (10, 50 or 100 mM) since the concentration of electrolytes may compete with the drug ion. Increasing NaCl concentration from 10 mM to 100 mM in the donor phase improved the drug permeation under effect of the application of the current from 1.63 ± 0.12 to 3.29 ± 0.18 μg cm-2 h-1; otherwise no change of the passive diffusion was observed (Figure 4.1).
Figure 4.1 Flux (µh h-1 cm-2)of the formulations when ionic strength of the donor solution was increased from 10 to 100 mM.
In these experiments there is correlation between ionic strength and permeation enhancement with significant statistically differences, highlighting main role of electrosmosis with respect to electrorepulsion in contrast with that reported in literature.30 May be electroosmotic transport facilitates the passage of neutral fraction of drug. In fact it is known that the nail under normal circumstances, is a negatively charged, cation-permselective membrane. Under the influence of an electric field, therefore, the transport of cationic substances across the hydrated nail should be enhanced by anodal iontophoresis. Moreover a convective, electroosmotic flux proceeds in the anode-to-cathode direction,