1. INTRODUCTION
In the early 1950s crucial discoveries on endocrine autoimmune diseases were made.
Early on Voisin and Barber first described an experimental model of autoimmune orchitis in guinea pigs in 19511, Rose and Whitebsky, a model of thyroiditis in rabbits in 19562, and Colover and Glynn a model of adrenalitis in guinea pigs in 19583. The fourth endocrine gland to be recognized as a target of autoimmunity was the pituitary.
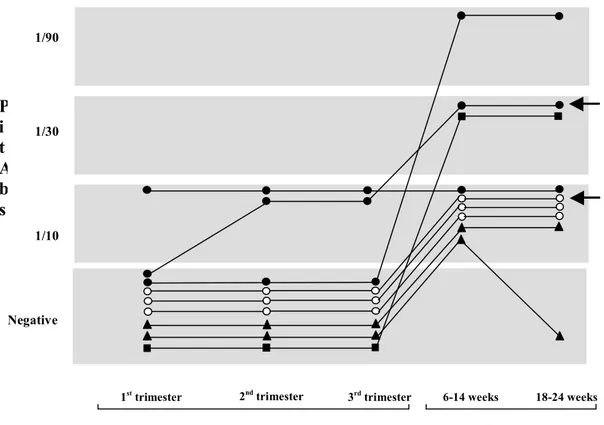
In 1962 Goudie and Pinkerton reported the case of a 22-year old woman who died 14 months after delivery, of cardiovascular shock having experienced severe vomiting, diarrhea and abdominal pain. The autopsy showed a firm, enlarged thyroid gland infiltrated with lymphocytes, atrophic adrenal glands and a undersized pituitary gland with extensive infiltration of the anterior lobe. Noting for the first time that lymphocytic infiltration involved both the thyroid and the pituitary gland, the authors hypothesized that not only thyroiditis but also hypophysitis could have an autoimmune origin. In addition, they linked pituitary autoimmunity to pregnancy, postulating that both diseases could be explained by “the onset of autoimmune reaction to thyroid and pituitary antigens released during the puerperal involution of these glands”4.
The very first review on pituitary autoimmunity was published in 1978: it comprised 8 patients5. As of March 2008, a total of 573 articles,
describing the features of about 500 patients with primary autoimmune hypophysitis, have been published.
2. SPECTRUM OF PITUITARY AUTOIMMUNITY
“Pituitary autoimmunity” can be considered as a complex spectrum of conditions that still needs to be fully clarified. In the attempt of such a classification, we will consider within the spectrum of pituitary autoimmunity the following conditions:
1. histologically proven or clinically suspected autoimmune hypophysitis 2. a variety of conditions in which humoral evidence of pituitary
autoimmunity has been demonstrated, as in: a. isolated pituitary hormone deficiencies,
b. pituitary diseases not typically considered autoimmune in nature (as Sheean’s syndrome, Primary empty sella, pituitary adenomas),
c. non-pituitary autoimmune diseases 3. Pituitary autoimmunity in healthy subjects
2.1 AUTOIMMUNE HYPOPHYSITIS
Definition Autoimmune hypophysitis (AH) is a primary inflammation, caused by mononuclear infiltration of the pituitary gland. It may involve the anterior lobe (adeno-hypophysitis), the posterior lobe, and/or the infundibulum (neuro-hypophysitis and/or infundibulo-neurohypophysitis) or both lobes (panhypophysitis). It is unknown whether these entities are
different diseases or purely different aspects of the same disease.
Epidemiology AH is more common and tends to present at a younger age in women than in men (F:M ratio is 4:1, mean age at presentation is 35±13 in women and 45±14 in men). In a significant percentage of women a clear association between pregnancy and adenohypophysitis, but not neurohypophysitis, is demonstrated6.
Pathology In AH the pituitary gland is infiltrated with mononuclear cells. Lymphocytes are the most common of these cell types; they are a mixture of B and T cells7 and tend to aggregate to form lymphoid follicles, and at times even demonstrating germinal centers. Not only plasma cells are found in the infiltrate (53% of the cases) but also eosinophils (12%), macrophages, hystiocytes and neutrophils (6%)8. Mononuclear cells infiltration ultimately disrupts the normal acinar architecture and damages the hormone secreting cells. As a final point, fibrotic and atrophic changes occur in the late stage of AH.
Other forms of primary hypophysitis Granulomatous and xanthomatous hypophysitis are the other two forms of primary hypophysitis. Granulomatous hypophysitis is very rare, affects females and males in equal proportion, and does not show an association with pregnancy or other autoimmune diseases. The pituitary shows A diffused collection of giant multinucleated cells and hystiocytes with surrounding lymphocytes and plasma cells. Interestingly, granulomatous hypophysitis can occur together with AH9. For this reason it has been suggested that AH and
granulomatous hypophysitis may represent two stages of the same disease, with granulomatous infiltration representing a later stage of lymphocytic infiltration10.
Xanthomatous hypophysitis was described for the first time in 199811. In this case the pituitary shows cystic lesions and infiltration with foamy hystiocytes and lymphocytes. To date only very few cases have been reported; therefore the epidemiology, natural history and clinical aspects of this disease have not yet been clarified.
Secondary hypophysitis is an inflammation of the pituitary gland caused by adjacent lesions or systemic diseases12. Germinomas, Rathke’s cleft cysts and pituitary adenomas are all lesions that may cause a secondary lymphocytic infiltration of the pituitary gland13, 14.
Systemic diseases that may cause secondary hypophysitis are: sarcoidosis, Wegener’s granulomatosis, Langerhans cell hystiocytes, syphilis and tuberculosis.
Clinical presentation of AH is variable, non-specific and no pathognomonic signs or symptoms are recognized. Because AH ultimately behaves as a sellar mass, the symptoms are those common to other lesions arising in the sella turcica. In particular, four categories of symptoms may be recognized: sellar compression, hypopituitarism, diabetes insipidus and hyperprolactinemia6. Symptoms of local compression are usually the first to appear. They include headache and visual disturbances, in particular defects in the visual field, or decreased
acuity. Compression symptoms are caused by an enlargement of the inflamed pituitary that usually expands upward to distend the dura mater and the optic chiasm. The next most common are symptoms due to a partial or complete deficit of the anterior pituitary function. These defects are considered to be as the direct result of the autoimmune attack against the pituitary acinar cells. Following are symptoms due to deficit of the posterior pituitary (diabetes insipidus), which can be attributed either to direct immune destruction or to compression of the posterior lobe and infundibular stem. Lastly are the symptoms due to hyperprolactinemia (mainly amenorrhea/oligomenorrhea and galactorrhea), which occur in about a quarter of patients. Therefore, these clinical features of AH are indistinguishable from those of any other expanding mass located in the sella turcica.
AH and pregnancy AH shows an evident association with pregnancy15, 16. Caturegli and colleagues recently summarized the data reported in the literature dating from 1978 to 2004 totaling 210 women with AH6. Of these, 120 (57%) presented symptoms of AH during pregnancy or postpartum: symptoms of AH usually appear in the last month of pregnancy or in the first 2 months after delivery. AH during pregnancy is not associated with complications on the fetus or on the outcome of the pregnancy itself. Interestingly, in women with a previous history of AH, the risk of developing AH again during a second pregnancy, is not increased. The reason why the association between pregnancy and AH
subsists, has not yet been clarified. Pregnancy exerts various effects on autoimmune diseases; certain diseases (such as Graves disease17, rheumatoid arthritis18 and diabetes mellitus type 119) actually improve, while others (such as lupus erythmatosus20 and myasthenia21) may either improve or worsen. Conversely, some (like Wegener’s granulomatosis22) may even become aggravated during pregnancy.
The pituitary gland goes through significant anatomical modifications during pregnancy. It enlarges by about 33% of its initial weight mainly because of the estrogen-driven hypertrophy and hyperplasia of the lactotrophs23, 24. The percentage of lactotrophs, in fact, rises from 15-20% of total pituitary cells in nulliparous women to 50% at the end of gestation. Also, the blood supply to the gland changes during pregnancy so that increased blood flow is provided by systemic circulation, through newly formed vessels, and less from the hypothalamic-portal circulation25. It is not clear whether these anatomic modifications of the pituitary, occurring in concert with a modulation of the immune system during pregnancy, may support the susceptibility of the pituitary to an autoimmune attack.
Natural history of AH. The natural history of the fully developed state of AH is variable. The majority of patients improve after being given mass reducing treatment (surgery or glucocorticoid therapy26). These patients may require some form of hormone-replacement therapy, on the basis of the severity of the damage caused to the gland by mononuclear
infiltration. The minority of patients dies because of a misdiagnosed severe adrenal insufficiency (7%) and the remaining few improve spontaneously without treatment. Presently it is not clear whether AH has a silent preclinical phase. It would seem that glucocorticoid therapy, when started promptly, or the condition of spontaneous remission, can break the progression through the chronic phase.
Diagnosis of AH. At present, the diagnosis of certainty of AH can only be achieved by pathological examination of a pituitary biopsy which requires an invasive surgical intervention. The serological methods available, in fact, are non-antigen specific and lack accuracy in diagnosing AH (Table 6.1). Magnetic resonance imaging (MRI) of the pituitary does not always allow the differentiation of AH from other more common pituitary masses such as pituitary adenomas27. Typical, but not specific, MRI findings in AH include: pre-contrast homogeneous signal, homogeneous gadolinium enhancement of the pituitary, stalk thickening, suprasellar extension and intact sellar floor28, 29. MRI findings, albeit not specific for AH, need to be correlated with the clinical history and endocrine findings of the patient in order to provide useful information for the diagnosis.
2.2 PITUITARY ANTIBODIES IN ISOLATED HORMONE DEFECTS
Isolated pituitary hormone deficiencies are those conditions where only one of the pituitary hormones is lacking or is inactive. Most common are
the isolated defects of adrenocorticotropin (ACTH) and growth hormone (GH), whereas only a few cases have been reported with isolated gonadotropin, thyrotropin, or prolactin deficiencies. Four main causes have been recognized as isolated pituitary defects: genetic, iatrogenic, brain trauma or irradiation, and autoimmune. At times, however, their causes remain unknown (idiopathic forms).
Isolated ACTH deficiency. Its most common cause is the iatrogenic form (the one induced by glucocorticoid administration), but autoimmune forms are increasingly recognized. An autoimmune basis for isolated ACTH deficiency has been established using the following criteria: the pathological demonstration in rare cases of the typical lymphocytic infiltration in the anterior pituitary30, 31; the association with other, better characterized autoimmune diseases, such as Hashimoto thyroiditis, vitiligo, and premature ovarian failure32; the higher prevalence in the female sex, and the presence of pituitary antibodies. These antibodies have been measured by immunofluorescence (IF), immunoblotting (IB), and ELISA and were found to be positive in about 40% of patients (Table 6.1).
Isolated GH deficiency causes short stature in children, whereas it is usually asymptomatic in adults. In the majority of children no cause can be identified (idiopathic forms); in the remaining minority, isolated GH deficiency results from defects in the genes coding for GH1 or GH releasing hormone receptor. In adults, isolated GH deficiency is most
commonly caused by traumatic brain injury or external irradiation treatments, although several cases remain idiopathic. Autoimmunity has been proposed to explain some forms of idiopathic isolated GH deficiency based upon the presence of pituitary antibodies. Seven papers have tested pituitary antibodies in patients (mainly children) with isolated GH deficiency, showing a prevalence of about 15%33-37. This noted prevalence, although higher than that of healthy controls, is conversely low, overall. All indications therefore suggest the role of autoimmunity is unclear.
Isolated gonadotropin deficiency of autoimmune origin was first reported in 1985 by Barkan in two men with autoimmune polyglandular autoimmune type 238, and more recently by De Bellis and colleagues in a cohort of 21 cases39. These authors identified pituitary antibodies in 38% of the cases, as compared to 6% (3 out of 50) of healthy controls.
Isolated thyrotropin deficiency of autoimmune origin was first reported by Wong in 2004 in a biopsy proven case of lymphocytic hypophysitis40, and then by Hashimoto in a case using a series of 6 patients (one of whom was histologically proven)41. Pituitary antibodies against candidate pituitary antigens have been measured by Amino’s group in four additional patients, and found to be absent and low42-44.
2.3 PITUITARY ANTIBODIES IN NON-AUTOIMMUNE
Pituitary antibodies have been found in pituitary diseases not typically considered autoimmune in nature, but for which an autoimmune component has been proposed: most markedly in the empty sella syndrome and Sheehan syndrome.
Empty sella is the herniation of the subarachnoid space into the sella turcica. This herniation may occur because of anatomical variations of the diaphragma sellae (usually an incidental observation known as primary empty sella), or from a loss of intrasellar volume (secondary empty sella). The latter for example develops in pituitary adenomas that are surgically removed, treated with radiotherapy, or which undergo hemorrhagic infarction, or can be caused by Sheehan syndrome. AH may be the cause of secondary empty sella considering that the pituitary gland, following the initial increase in size, gradually becomes atrophic and fibrotic. Empty sella has been, in fact, demonstrated in 10% of AH patients who had at presentation a pituitary mass and later developed pituitary atrophy. An autoimmune basis for secondary empty sella was first proposed in 1986 by Okada, who reported a case of partial hypopituitarism with empty sella and pituitary antibodies after pregnancy45. Six subsequent papers have discussed the phenomena of pituitary autoimmunity in the context of an empty sella37, 46-50. In particular, Beressi and colleagues concluded their review by stating, “empty sella may in fact be the final term of initially undiagnosed lymphocytic hypophysitis”. Pituitary antibodies have been found in
patients with empty sella (Table 6.1) but with contrasting results. On the one hand, Komatsu detected through IF, antibodies recognizing mouse corticotroph cells (AtT-20) in 24 out of 32 patients with empty sella (75%); Mau found through IB, antibodies against human GH and ACTH in 2 out of 6 patients (33%); and Keda through ELISA, found them in 16 out of 38 patients (42%). Whereas, Bensing and colleagues found no evidence of autoimmunity in patients with empty sella: antibodies to 49 kDa alpha-enolase were present in 6 out of 30 patients (20%), similar to what had been in found in healthy controls (11 out of 50, 22%). It is certainly evident that the timing of pituitary antibodies measurement is important. Antibodies decrease over time when the antigenic load decreases, as when a gland is destroyed by the autoimmune attack and becomes atrophic. In 2003, Chiovato et al published that antibodies against the thyroperoxidase, thyroglobulin, and thyrotropin receptor progressively disappear after complete ablation of the thyroid (either by surgery or radioiodine), indicating that continued antibody production requires autoantigen persistence51. Thus, if empty sella is caused by AH, the pituitary gland at that stage is by definition atrophic and predicted to have a low antigenic load and, consequently, low or absent pituitary antibodies. In summary, AH could indeed be a possible cause of secondary empty sella, although the issue remains to be clarified.
Sheehan syndrome, first described in 1937, is an ischemic necrosis of the anterior pituitary caused by a severe peri-partum hemorrage.
Autoimmunity has been proposed as a mechanism for Sheehan syndrome, but the data are scanty. After the original report in 1965 by Engelberth & Jezkova, which described high titer of pituitary antibodies by complement consumption test in a woman with clinical signs of Sheehan syndrome 5 years after delivery, two case-control studies found pituitary antibodies in Sheehan patients at significantly higher prevalence than in healthy controls. Goswami and colleagues detected by IB antibodies to 49 kDa alpha enolase in 12 of 19 cases and in only 9 of 56 controls (p= 0.007)52. De Bellis and colleagues recently found by IF pituitary antibodies in 7 of 20 Sheehan cases and in none of 50 healthy controls (p= 0.001)53.
Pituitary adenoma. The prevalence of pituitary antibodies in pituitary adenoma, using different immunoassays in different series, ranges from 0% to 35%. . (Table 6.1) In the case of pituitary adenoma, a concomitant pituitary lymphocytic infiltration was described in x of 1440 cases (3%). In this large series the infiltrate was mainly perivascular, surrounding the lesion, not diffusing the entire gland, and the lymphocytic population was almost exclusively composed of T cells54.
Little data are reported on the association of pit autoimmunity with the pathological finding of lymphocytic infiltration55.
2.4 PITUITARY ANTIBODIES IN NON-PITUITARY
AUTOIMMUNE DISEASES
ranging in prevalence from a minimum of 5% in celiac disease to a maximum of 74% in eating disorders (Table 6.1). Autoimmune thyroiditis (represented by Graves disease and Hashimoto thyroiditis) is the most common condition where pituitary antibodies have been measured. The functional significance and clinical value of pituitary antibodies in these conditions remain to be established.
3. IN SEARCH FOR PITUITARY AUTOANTIGENS
Identification of “pathogenic” autoantigen(s) is a critical step to advance the field in any autoimmune disease. A pathogenic autoantigen causes disease when attacked by the patient’s own immune system (either during the initiation or the effector phase), and recreates the human disease when injected into experimental animals in an immunogenic context. Such identification has two clear benefits: 1) it allows the development of specific autoantibody assays that can be used to diagnose, monitor, or predict an autoimmune disease. 2) It allows reproduction in experimental animals of the human disease with a single protein (or even a single epitope), greatly facilitating understanding of the autoimmune response. For AH pathogenic autoantigens are yet to be discovered, although several candidates have been proposed during throughout the years: growth hormone, alpha-enolase, pituitary gland specific factors 1a and 2, and secretogranin II.
1 or growth hormone 2 was initially found to be recognized as a 22 kDa band by 11 of 15 (73%) patients with clinically suspected hypophysitis and 7 of 9 (78%) patients with isolated ACTH deficiency, but not by healthy controls, Hashimoto thyroiditis or Graves disease patients56. When Tanaka and colleagues expressed in vitro the full-length growth hormone 1, however, they reported a low recognition by hypophysitis (2 of 17, 12%), isolated ACTH deficiency (1 of 10, 10%), and other autoimmune diseases (2 of 31, 6%)42.
In 2002, a 13-mer sequence corresponding to the ubiquitous glycolytic enzyme alpha-enolase was detected by the serum of hypophysitis patients (who were previously shown to recognize a 49 kDa pituitary cytosolic band) but not by the normal serum57. Later, Tanaka and colleagues questioned the clinical utility of alpha-enolase antibodies. They expressed in vitro the full-length alpha-enolase and showed that it was recognized not only by hypophysitis patients (7 of 17, 41%), but also by pituitary adenomas (6 of 13, 46%), other autoimmune diseases (6 of 30, 20%), and healthy controls (2 of 46, 4%)43.
Pituitary gland specific factors 1a and 2 are of even more difficult interpretation. These two transcripts have been found by Amino’s laboratory during a gene expression profile of the human pituitary, but experimental support for the proteins is missing58. Factor 1a, now classified as chromosome 19 open reading frame 30, was expressed in vitro and reported to be recognized only minimally by hypophysitis sera
(1 of 17, 6%), isolated ACTH deficiency (2 of 10, 20%), and other autoimmune diseases (1 of 31, 3%). Factor 2 showed a similar low recognition in that study population42.
In 2007, Bensing and colleagues reported secretogranin II as the latest pituitary candidate autoantigens. They described a 70-yr-old man with clinically suspected hypophysitis, whose serum identified five clones in a human pituitary cDNA expression library all corresponding to secretogranin II. The protein is highly expressed in the pituitary but also in organs and tissues containing neuroendocrine cells59. This is an interesting candidate that needs to be tested in a larger number of cases and non-affected controls.
Overall, the above described candidate pituitary autoantigens are not useful as diagnostic markers. In addition, their pathogenicity has not been proven in experimental animals, leaving us with only circumstantial evidence of their role as real pituitary autoantigens.
4. PITUITARY ANTIBODIES DETECTION
In the natural history of organ specific autoimmune diseases, three distinct main phases can be identified: potential, sub-clinical and clinical, which are marked by the presence of specific autoantibodies. In these diseases, organ-specific autoantibodies can be detected in the serum of susceptible individuals months or even years before the clinical onset of the disease. Examples of such conditions, in which the detection of specific antibodies
preludes the clinical onset of the disease, are: type 1 diabetes mellitus, autoimmune thyroid diseases, autoimmune Addison disease, celiac disease and primary biliary cirrhosis.
This type of clear definition in early stages is not yet possible, in the case of AH, the main reason being that the auto-antigen/s target of the autoimmune attack is not known, consequently specific pituitary antibodies cannot be measured.
Current immunological tests, such as the measurement of pituitary antibodies, in fact, do not offer sufficient specificity and/or sensitivity in the diagnosis of AH. An ideal pituitary antibodies assay should identify not only the clinical but also the potential, and subclinical forms of autoimmune hypophysitis.
Pituitary antibodies have been measured both using non-antigen specific and antigen-specific methods. The methods, respectively, include mainly IF, IB, and ELISA60 and second, currently in vitro transcription/translation assays based on candidate pituitary autoantigens, which are summarized in Table 6.1.
Immunofluorescence for pituitary antibodies was first reported by Bottazzo in 1975, describing the recognition of prolactin-secreting cells (not prolactin itself) in patients with autoimmune endocrine diseases61. Immunofluorescence in general is known to be poorly sensitive as well as highly subjective in the interpretation of borderline results. Immunofluorescence of the pituitary, in particular, presents some
additional challenges. It was originally reported that only freshly obtained human pituitary glands are a suitable substrate, whereas post-mortem human pituitaries or rat and bovine pituitary yield low sensitivity or high background fluorescence. Subsequently, it was shown that adult human ACTH-producing cells express Fc receptors and therefore such pituitary sections bind aspecifically all immunoglobulins. Fetal ACTH cells do not express Fc receptors but are almost impossible nowadays to obtain61. Most recent studies of pituitary antibodies based on immunofluorescence utilize freshly obtained pituitary from young baboons. The importance of the species as the substrate for pituitary IF was emphasized by Gluck and Scherbaum in 199062. They tested a set of well-characterized sera (46 positive and 37 negative on human fetal pituitary) on pituitary glands from six other species (fetal Macaca
fascicularis, adult baboon, pig, beef, sheep, and rat) and reported very low
sensitivity or specificity. Therefore, different substrates may be a likely explanation for the variability observed in IF-based pituitary antibody tests (Table 6.1).
Immunoblotting is considered more sensitive than immunofluorescence, although no study has actually compared these two methods. Immunoblotting, first reported for pituitary autoimmunity by Patricia Crock in 1993, and is currently not used as a diagnostic tool in clinical laboratories33. It presupposes, in fact, to accurately know the molecular weight of pituitary autoantigen(s), a fact that still lacks substantial
evidence. Immunoblotting has been performed in research settings, using both whole pituitary cytosolic and membrane proteins as the antigenic substrate, and using glands from various species, making results difficult to compare across studies.
Antigen-specific methods, such as in vitro transcription/translation or more commonly, immunoassays, are the way of the future and will certainly further our understanding of AH once the pathogenic autoantigen(s) become identified.
5. ANIMAL MODELS OF AUTOIMMUNE HYPOPHYSITIS
Only 6 papers have been published on animal models of autoimmune hypophysitis, so far. In 1964 Beutner and Witesbky immunized 16 rabbits one-to-five times with rabbit anterior pituitary extracts, emulsified in complete Freund’s adjuvant63. They were able to induce specific antibody responses, but no pituitary pathology. In 1967 Levine reported the first successful model of experimental autoimmune hypophysitis, by immunizing rats with a single intracutaneous injection of rat pituitary tissue, emulsified in complete Freund’s adjuvant64. The adenohypophysis showed focal and diffuse lymphocytic infiltration in 6 out of the 14 rats (43%) 2 to 3 weeks after the immunization. Disease incidence could be increased to 75% by addition of a second immunologic adjuvant, pertussis toxin. The author subsequently showed that pituitary extracts from guinea pig were the most potent inducer of experimental autoimmune
hypophysitis (six of six rat recipients), whereas human and cow extracts were poorly effective, and dog and rabbit extracts not effective at all. In 1970 Beck and Melvin induced experimental autoimmune hypophysitis in one rhesus monkey by injecting her multiple times, over the course of three years, with human placental extracts and human chorionic somatommamotropin, both emulsified with Freund’s adjuvant. Histology showed lymphocytic infiltration of the adenohypophysis whereas the neurohypophysis was normal65.
In 1982 Klein induced lympho-plasmacytic infiltration of the anterior pituitary by injecting 12 rabbits with rabbit pituitary tissue emulsified in complete Freund’s adjuvant66. Eight to 16 weeks after the first injection, five of the seven experimental rabbits showed focal infiltration of the adenohypophysis with lymphocytes, some plasma cells, and a few eosinophils and fibrosis. None of the five controls showed histological abnormalities. In 1992, Yoon et al. immunized over 100 hamsters by injecting intra-dermally three times, at one-week interval, recombinant rubella virus E1 and E2 glycoproteins.67 Three weeks after the first injection, specific antibodies against the adenohypophysis were found in 95% of the hamsters. Eleven weeks after the first injection, a diffuse lymphocytic infiltration was found throughout the adenohypophysis. None of the hamsters that had received the control protein (nonglycosylated rubella nucleoprotein C) developed such lesions. The disease could be prevented by neonatal thymectomy, and could not be
produced by passive transfer of the autoantibodies, thus indicating that T cells are critical for disease induction and that antibodies are more important as markers of disease, rather than as a pathogenic player. Finally, Watanabe et al. in 2001 immunized 12 Lewis rats twice, at one-week interval, with rat pituitary extract emulsified in complete Freund’s adjuvant68. Three to six weeks after the first immunization, rats showed minimal lymphocytic infiltration in the adenohypophysis and developed antibodies directed against growth hormone, thyroid stimulating hormone and luteinizing hormone. It is unclear, however, whether these hormones represent the initiating autoantigens are rather the natural response of the immune system to the injection of hormone rich pituitary extracts.
6. AIM OF THE STUDY
This study was designed to further elucidate the physiopathology and the natural history of autoimmune hypophysitis, to assess the prevalence of pituitary autoimmunity in patients with AH and various endocrine diseases, and to finally identify the autoantigens target of the autoimmune attack.
In order to achieve these goals, the study was divided into two main parts: the “mouse study” and the “human study”.
MOUSE STUDY
7. DEVELOPMENT OF AN EXPERIMENTAL MOUSE MODEL OF AUTOIMMUNE HYPOPHYSITIS
Background and aim of the study
A mouse model that accurately replicates the pathology of human AH has not been developed.
Aim of the study was to develop a reliable mouse model of AH to further elucidate the phisiopathology of AH and to be used to finally identify the autoantigens of AH.
Materials and methods
Mice
The study was carried out in three stages and used a total of 514 mice: 462 immunized, 47 non-immunized, and 5 recipients of adoptively transferred splenocytes. Of the 462 immunized mice, 367 received proteins extracted from mouse pituitary glands emulsified in CFA, 49 proteins from mouse pituitary cell lines in CFA, 20 recombinant prohormone convertase 2 (PC2) in CFA, 15 control placental proteins in CFA, and 11 just CFA.
the mouse strain(s) susceptible to experimental autoimmune hypophysitis (EAH), in view of the known effects of the major histocompatibilty complex and sex on autoimmune diseases. Six inbred strains (Jackson Laboratory Bar Harbor, ME), used when 7-to-8 weeks old, were studied: A/J (H-2a, 15 females and 15 males), Balb/cJ (H-2d, 15 females and 10 males), C57BL/6J (H-2b, 15 females and 15 males), CBA/J (H-2k, 15 females and 10 males), FVB/NJ (H-2q,10 female), and SJL/J (H-2s, 17 females and 9 males).
The second stage used 344 SJL/J female mice and was designed to select the optimal immunogen dose, characterize the pituitary infiltrate by flow cytometry, demonstrate the transmissibility of disease upon splenocyte transfer, and define the evolution of EAH based on pituitary histopathology, antibodies, and function.
The third stage used 24 SJL/J female mice and tested recombinant PC2, a candidate pituitary autoantigens discovered in the second stage, for its ability to induce EAH.
All experiments were conducted in accordance with the standards established by the United States Animal Welfare Acts, set forth in NIH guidelines and the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee.
Immunogens
sexes made available by the Johns Hopkins animal care facility, and stored at -80 °C until use.
Glands were disrupted with a Polytron homogenizer in phosphate-buffered saline (PBS) pH 7.4, supplemented with protease inhibitors (Roche Applied Sciences, Indianapolis, IN) to prepare the pituitary whole extract. For each protein extraction, we used about 400 pituitary glands (approximately 780 mg total weight) in 4 ml of PBS that yielded around 100 mg of proteins. The pituitary whole extract was quantified by a bicinchoninic acid assay (Pierce), adjusted to 20 mg/ml, and used directly for immunization or further fractionated. In the latter case, the pituitary whole extract was centrifuged at low speed (1000g for 10 min at 4 °C) to pellet the nuclear fraction, and then at high speed (100,000g for 1 hr at 4 °C) to separate membranes from cytosol. The cytosolic fraction (corresponding to the supernatant) was adjusted to 2, 6, 8, or 10 mg/mL, and used for immunization. The membrane fraction (the ultracentrifugation pellet) was washed and resuspended in PBS/protease inhibitors at 6 or 8 mg/mL.
Mouse pituitary cell lines were αT1-1 (a precursor of gonadotrophs and thyrotrophs expressing the common α-subunit, N=5 mice), αT3-1 (an immature gonadotroph cell line, N= 5 mice), TαT-1 (a thyrotroph cell line, N= 5 mice), LαT2 (a gonadotroh cell line, N= 5 mice), GHFT5-1 (a precursor of somatotrophs and lactotrophs, N= 5 mice), and AtT-20 (a corticotroph cell line, N= 24 mice). Cells were cultured in the
recommended medium, harvested, and sonicated to prepare a total lysate. Cell line proteins were quantified and adjusted to 20 mg/ml.
Mouse recombinant PC2 produced in Chinese hamster ovary (CHO) cells (12) was used for the initial immunizations at a concentration of 1 mg/mL.
EAH induction protocols.
All proteins were emulsified 1-to-1 in CFA (which contained 5 mg/mL of heat-killed Mycobacterium tuberculosis, strain H37a, from BD Diagnostic Systems, Sparks, MD), and injected subcutaneously on day 0 in a volume of 100 µl (50 µL in the dorsal hind leg region and 50 µL in the controlateral inguinal region). Protein emulsions were injected again on day 7 in the opposite sites. Mice were sacrificed most commonly 28 days after the first immunization, but also on days 10, 14, 21, 28, 35, 56, 84, 112, 140, 252, or 370.
Adoptive transfer of T lymphocytes.
To prove that EAH is a cell-mediated autoimmune disease, single cell suspensions were prepared from the spleens of 4 SJL/J mice sacrificed 28 days after immunization with pituitary whole extract. Lymphocytes were cultured and expanded in 75 cm3 flasks in RPMI-10 medium, containing 200 mcg/mL of mouse pituitary proteins. After 7 days, the non-adherent cells (mainly T lymphocytes) were collected, washed, and resuspended in
sterile saline at a concentration of 2 x 108 cells/mL. T lymphocytes were injected into 5 naïve female SJL/J mice via the tail vein (of 2 x 107 cells per mouse). Two weeks after the adoptive transfer, recipients were sacrificed to collect pituitary, thyroid, and pancreas for histolopathology.
Pituitary histopathology.
To assess the presence and extent of mononuclear infiltration within the pituitary, glands from all 462 mice (the 514 total minus the 52 mice used for pituitary flow cytometry and thus without histopathology) were dissected, fixed overnight in Beckstead’s fixative, processed, and embedded in paraffin. At least five non-consecutive sections (1 every 10) were cut, stained by hematoxylin and eosin, and analyzed with a digital microscope as described69to quantify the mononuclear infiltration of the pituitary gland.
EAH was defined histopathologically as a mononuclear infiltration in the pituitary gland greater than 2% of the total anterior pituitary area; mice with such a degree of pituitary infiltration were considered the incidence cases. To assess the extent and evolution of pituitary fibrosis, a subset of 20 pituitaries (15 immunized and 5 CFA controls), representative of all post-immunization endpoints, were also colored by the Masson’s trichrome method, which stains collagen fibers blue, nuclei black, and cytoplasm red. To confirm the organ-specificity of the pituitary disease, thyroid gland and pancreas were also collected at sacrifice.
Pituitary immunohistochemistry.
To analyze the distribution and type of hematopoietic cells infiltrating the pituitary, immunohistochemistry was performed on deparaffinized and rehydrated sections as previously described70, using the following primary antibodies: B220, CD3, CD11c, CD45 (BD Biosciences, San Jose, VA), and F4/80 (AbD Serotec, Raleigh, NC). Sections were chosen from nine stage-2 mice (5 immunized and 4 controls) and from all stage-3 mice.
Pituitary flow cytometry.
To analyze the type, abundance, and activation state of hematopoietic cells infiltrating the pituitary, we studied 52 glands: 22 dissected from day-28 immunized mice, and 30 non-immunized controls. For each experiment, 5-to-7 pituitaries were pooled, minced into small pieces (~1 mm3), and digested for 30 minutes at 37 ºC in Dulbecco’s modified Eagle medium (Invitrogen) containing collagenase II (0.2% w/v, from Sigma). Pituitary cells from the digestion were passed through a 70-µm strainer (BD Biosciences), washed, and re-suspended in PBS, 1% BSA, 2 mM EDTA, and 0.02% sodium azide at a concentration of 107/mL. Cells were then processed as previously described69 and stained with fluorochrome-conjugated antibodies (all from BD Biosciences, except when indicated) recognizing the following markers: B220, CD3, CD4, CD8, CD11b, CD44, CD45, CD49b (a pan NK cell marker, clone DX5, from
eBiosciences, San Diego, CA), and CD69. Cells were then analyzed by FACScalibur cytometer using CellQuest software (BD Biosciences), gating first on the forward-side scatter to exclude aggregates and dead cells, and then on the CD45-side scatter to identify the hematopoeitic cells.
Pituitary antibodies by ELISA
To monitor the humoral immune response against pituitary proteins after immunization, sera were collected before (day 0) and several days after the first immunization (day 7, 10, 14, 21, 28, 35, 56, 84, 112, 140, 200, 252, and 370). Sera were serially diluted in PBS, and incubated overnight in Immunolon2 ELISA plates (Dynex Technologies, Chantilly, VA) precoated with mouse pituitary cytosolic proteins (625 ng/well). After washing, IgG and IgM recognizing pituitary antigens were detected using a secondary antibody, conjugated to alkakline phosphatase and directed against mouse G and M heavy chains (Jackson Immunoresearch Laboratories, West Groove, PA). Color development was measured at 405 nm using the Emax microplate reader (Molecular Devices, Sunnyvale, CA). Each plate included a homemade standard curve, made by diluting a pool of day 28 postimmunization sera 1:100, 1:400, 1:1600, 1:6400, and 1:25600 in PBS. A value of 5120 arbitrary units (AU) per microliter was assigned to the most concentrated standard (the 1:100 dilution), and corresponding values of 1280, 320, 80, and 20 to the other four standards.
This curve allowed us to compare results among plates, expressing results in AU/µl rather than in optical density. Pre-immune (day 0) sera were used to calculate the normal reference rangefor SJL/J female mice. Sera were tested in triplicates; sera with a coefficient of variation greater than 20% were excluded. The average coefficient of variation was 6.3%. Intra-assay variability was evaluated by measuring the same sera several times during the same assay procedure, and amounted to 4.9% ± 0.7%. Inter-assay variability was evaluated by measuring the same sera during different assay procedures, and amounted to 8.6% ± 1.2%.
Pituitary hormone function.
To study pituitary function after immunization, we initially attempted to measure anterior pituitary hormones but failed because the immunization protocol also induced antibodies against these hormones, thus markedly interfering with the assay performance. We therefore opted for measuring two key target hormones produced in response to pituitary control, using commercially available radioimmunoassays: total thyroxine (T4) (Diasorin Inc., Stillwater, MN), corticosterone (Diagnostic System Laboratories, Webster, TX), and insulin-like growth factor-1 (Diagnostic Systems Laboratories, Webster, TX). Assay standards and controls were run in duplicate, experimental samples in singlicate and undiluted. Pre-immune (day 0) sera were used to calculate the normal reference range for SJL/J female mice.
In vivo MRI of the mouse pituitary
MRI was performed using a horizontal 9.4 Tesla NMR spectrometer (Bruker Biospin, Billerica, MA, USA), and a 40 mm diameter birdcage coil as radio frequency transmitter and receiver. Scans were after gadolinium (Gd) injection (T1 post-contrast).
Mice were anesthetized using 1% isoflurane in a mixture of 75% air and 25% oxygen, and placed in a custom designed head holder with ear pins and nose cone to restrain head motion. Respiration was monitored using a small animal imaging system (SA instruments, Inc., Stony Brook, NY, USA), and kept at a rate of approximately 60-80 breaths per minute. Immunized mice were analyzed on day post-immunization 0 (N= 4), 12 (N= 5), 18 (N= 5), 25 (N= 3), 28 (N= 3), 32 (N= 4), 40 (N= 4), 70 (N= 6), 95 (N= 2), 100 (N= 1), 120 (N= 3), 145 (N= 3), 175 (N= 4), 225 (N= 8) or 300 (N= 3).
Mice were injected intra-peritoneally with Magnevist (gadopentetate dimeglumine, from Berlex Imaging, diluted 1:3 in saline solution) and received a dose of 0.65 mmol/kg in a total volume of 100 μl. Images were acquired using a 2D multiple slice spin echo sequence, with an echo time (TE) of 11 ms, a repetition time (TR) of 500 ms, and 10 signal averages. Each image contained 24 coronal slices (0.3 mm thick), covering the midbrain region. The in plane resolution was 0.1 mm x 0.1
mm. After the T1-weighted acquisition, co-registered T2-weighted images were acquired using a fast spin echo sequence, with TE/TR of 22/5000 ms, an echo train length of 4, and 8 signal averages. Images reconstruction was performed on the spectrometer console (Paravision, version 3.0.2, Bruker Biospin, Billerica, MA, USA).
Identification of candidate pituitary autoantigens by 2D-gel electrophoresis, immunoblotting, and mass spectrometry.
Pituitary proteins (cytosolic or membrane fractions) were precipitated with 10% trichloroacetic acid/acetone, and re-dissolved in 7 M urea, 2 M thiourea, 4 % CHAPS, 30 mM Tris-HCL (pH8.8). Proteins (250 µg) were initially loaded onto 7-cm long, “immobilized pH 3-10 gradient” strips (IPG strips, form GE Healthcare) to identify the most resolving isoelectric point for candidate autoantigens, which was later found to fall in between 5 and 7. Proteins were then loaded onto 24-cm long, pH 4-7 IPG strips, to further increase the resolution of candidate autoantigens in isoelectric focusing buffer (8 M urea, 4 % CHAPS, 0.2 % DTT, 1.5 % IPG buffer, 0.002 % bromophenol blue), [using the following programs: 10 hours at 20 volts, 30 min at 500 volts, 30 min at 1000 volts, 4.5 hours at 3000 volts]. Proteins were loaded onto a pair of identical pH 4-7 IPG strips in each experiment. After the first dimension, strips were cut into three fragments, each representing one pH unit. The pH 4-to-5 fragment was discarded because it did not contain pituitary autoantigens. The pH 5-to-6
and 6-to7 fragments were then run on 4-12% SDSPAGE for the second dimension. From the pair of identical strips, one gel set was stained by Coomassie blue and the other instead transferred to nitrocellulose membranes (GE Healthcare). These were blocked in PBS-3% BSA, and incubated overnight at 4 °C with diluted sera (1:200) from CFA-only mice. Membranes were then washed and incubated for 1 hour at room temperature with an anti-mouse IgG antibody, conjugated to horseradish peroxidase. Antibody binding was then detected using a chemoluminescent substrate (ECL, GE Healthcare). The membranes were then stripped, according to the manufacturer’s recommendation (GE Healthcare), and probed with the immune sera. Images produced by immune sera were compared to those from CFA-only sera to identify unique protein spots.
These spots were then matched to the gels stained by Coomassie blue, excised, in-gel digested with trypsin, and submitted for MALDI-TOF sequencing at the Proteomics Facility of the Johns Hopkins School of Medicine, as previously described71.
Statistical analysis
The study was mainly designed to analyze cross-sectionally the pituitary infiltration score at multiple time points after immunization.
This score was expressed as percentage (continuous scale from 0 to 100) and calculated as follows: sum of the infiltrated areas in the anterior
pituitary divided by the total anterior pituitary area times 100. The posterior and intermediate lobes were not included in the measurements because their mononuclear infiltration was rare and scanty. The pituitary of non-immunized or CFA-only immunized mice contained only a few lymphocytes, representing about 0.5 ± 0.2% of the total area. Mice with an infiltration score greater than 2% were classified as EAH cases. Differences in the mean infiltration score were analyzed using a multiple linear regression model that included as covariates strain, sex, type of immunogen, dose of immunogen, and time post-immunization. Differences in EAH cumulative incidence among strains were assessed by calculating the relative risks. Secondary outcomes were serum pituitary antibodies, thyroxine and corticosterone, and the number of CD45 positive cells in the pituitary. Pituitary antibody values were first transformed to a logarithmic scale to approximate the normal distribution, and then analyzed by univariate regression using day post-immunization as covariate and fractional polynomial fit. Corticosterone and thyroxine were analyzed by univariate linear regression versus day post-immunization. The number of CD45 positive lymphocytes infiltrating the pituitary was compared among groups by Wilcoxon rank-sum test performed after the Kruskal-Wallis test.
Results
Considering the known influence of the MHC locus72 and sex73 on autoimmune diseases, we tested 146 mice from six inbred strains of both sexes for their susceptibility to EAH upon pituitary whole extract immunization. SJL/J developed EAH with significantly higher incidence (relative risk= 23, 95% confidence limits from 10 to 55, p<0.0001) and greater severity (p< 0.0001) than the other five strains (Figure 7.1A). Female SJL/J mice developed more severe EAH than male SJL/J mice (p<0.0001, Figure 7.1B), in keeping with the sex bias observed in patients with autoimmune hypophysitis6. Female FVB/N mice proved also susceptible to EAH (Figure 7.1A), although with lower incidence (5 of 10 mice) and milder severity (mean pituitary infiltration score = 6%, median = 3%). Based on these results, SJL/J females were used for all the other experiments presented in thesis.
2. The pituitary cytosolic fraction is enriched for autoantigen(s).
The mouse pituitary whole extract (filled squares in Figures 7.1C and 7.1D) induced EAH with the highest incidence and severity: incidence was >95% at doses ≥0.4 mg per injection (Figure 7.1C); severity increased in a dose-dependent manner from 0% at 0.2 mg, 3% at 0.4 mg, to 60% at 0.5 mg, plateauing thereafter (65% at 1 mg, Figure 7.1D). Pituitary cytosolic proteins (open circles in Figures 7.1C and 7.1D) were also reliable inducers of EAH: incidence and severity increased in a dose-dependent fashion, paralleling the trend seen with the whole extract. On
the contrary, pituitary membranes (filled triangles) induced only a very mild disease (Figure 7.1D) with low incidence (Figure 7.1C), and pituitary nuclei (open diamonds) were ineffective. These results indicate that the pathogenic autoantigens of EAH mainly reside in the cytosolic compartment of the pituitary gland.
The disease induced by the pituitary proteins was pituitary-specific. The mononuclear infiltration induced by pituitary immunization was seen only in the pituitary gland, and not in any of the thyroids and pancreases collected at the time of sacrifice, even when using the highest dose of pituitary extract. In addition, control placental proteins (filled circle in Figures 7.1C and 7.1D) were capable of inducing EAH, similar to what observed in non-immunized mice (open square) or CFA-only immunized mice (CFA controls, filled diamond).
Among the six mouse pituitary cell lines, the corticotroph AtT-20 line was the only one capable of inducing EAH, although with low incidence (1 of 24 mice, 4%, in Figure 7.1C, open triangle) and minimal severity (Figure 7.1D).
3. The pathology of murine EAH closely resembles the human disease
Following immunization with pituitary whole extract or cytosol, the pituitary gland developed a marked mononuclear cell infiltration of the anterior pituitary (Figure 7.2, compare the pituitary of an immunized mouse in panel B to that of a CFA control pituitary in panel A).
Infiltrating cells surrounded and destroyed the endocrine cells, ultimately effacing the normal acinar architecture (Figure 7.2, compare panel D to panel C). The intermediate and posterior lobes were not involved with this immunization protocol, perhaps because of insufficient antigen doses. The mononuclear cells were mainly composed of CD3+ T cells and B220+ B cells), which were present both diffusely throughout the gland and in focal collections. Other cell types like granulocytes (compare the G gate in panels C and D of Figure 7.3) and plasma cells (data not shown) also increased, although less abundantly, after immunization. Most of the pathological lesions were localized mainly in the anterior pituitary, whereas intermediate and posterior lobes only rarely contained scattered infiltrating lymphocytes.
4. T cells show an activated/memory phenotype and are the key mediators of EAH.
Flow cytometric analysis of the pituitary gland revealed that the percentage of CD45 positive hematopoietic cells increased from the normal 2% (Figure 7.3A) to 85% (Figure 7.3B) following immunization. Lymphocytes were the dominant population (gate L in Figure 7.3B), representing 70% of the total CD45 positive cells. CD3+ T cells were the most abundant lymphocytes followed by B220+ B cells, with average percentages of 78% and 10%, respectively (data not shown). CD4 T cells were about 3 fold more numerous than CD8 T cells (68% vs. 22%, Figure
7.3C), highlighting the key role for CD4+ T cells in disease pathogenesis. CD4 T cells, in fact, are the first cells to enter a gland targeted by autoimmunity, triggering the development of tertiary lymphoid follicles74 , and are the most abundant T cell subset in early disease phases75 . The majority of CD4+ cells showed an activated/memory phenotype, as indicated by homogeneously high levels of CD44 (Figure 7.3D). CD69, an early T-cell activation marker, was also expressed at high levels on about 50% of the CD4+ cells (Figure 7.3D). A similar activation profile was displayed by CD8+ cells (data not shown). The activated/memory phenotype of T cells is consistent with an ongoing inflammatory process within the pituitary gland. T cells were cPitAbsble of transferring EAH to naïve recipients upon adoptive transfer. All 5 recipients developed lymphocytic infiltration of the pituitary of mild severity (median infiltration score 5%, range 2 to 10%), whereas thyroids and pancreases were normal.
5. Evolution of murine EAH: from pituitary expansion to empty sella atrophy.
Lymphocytic infiltration first appeared in the pituitary gland on day 14 post-immunization and was characterized by small lymphoid infiltrates occupying about 5% of the gland (Figure 7.4A, arrow). These initial infiltrates were mainly present within the sinusoidal spaces and did not disrupt the normal acinar architecture (data not shown). The disease then
rapidly worsened by days 21 and 28, with infiltrating lymphocytes replacing about 50% of the anterior pituitary (Figure 7.4A), and remained severe after that suggesting that AH is a chronic disease where spontaneous resolutions are unlikely.
Further analysis of individual pituitary scores (Figure 7.4B), rather than means as in Figure 7.4A, revealed the existence of two modes of EAH early development. In the majority of female SJL/J mice, EAH developed very rapidly and severely (Figure 7.4B, open circles). In the remainder, EAH was milder and progressed more slowly but still with an upward trend (Figure 7.4B, closed diamonds). The pituitary score differed on average by 27% (95% confidence limits from 15% to 40%, p<0.0001) between the fast and slow responders. The pituitary gland on day 28 post-immunization was markedly enlarged, inflamed, and firmly adherent to the surrounding meningeal structures (Figure 7.5B, compare it to the normal pituitary of a CFA-only immunized control in Figure 7.5A). With time, the pituitary gland markedly shrank, resulting in an atrophic tissue (Figure 7.5C) that contained only minimal adenohypophysis by day ≥250 post-immunization. The disease remained mainly lymphoid in nature throughout the entire course; by day ≥140 post-immunization, lymphocytes could be seen in large aggregates (Figure 7.5D). In addition, other pathological features appeared at later time points. Multinucleated giant cells (Figure 7.5E) could be seen between days 35 and 84, which
directly related to disease severity and resembled some of the lesions observed in patients with granulomatous hypophysitis. Fibrotic changes appeared as early as day 35 or 56, and became more prominent at later time points (Figure 7.5F).
6. MRI follow-up: pituitary volume initially increases then gradually decreases after immunization
The MRI images, obtained after Gd injection at different day (from 0 to 300) post-immunization, confirmed that the pituitary gland increases in size during the first phases of the disease, and then begins to gradually decrease. At later stages, when atrophic changes such as fibrosis are demonstrated by the pathology, the pituitary volume shrinks. (Fig. 7.6). In particular, the immunization with pituitary proteins induced marked changes in pituitary volume, which could be schematically classified into 3 phases: initiation (from day 18 to 40), florid (from day 40 to 120), and atrophy (from day 120 onward).
Initiation phase. Pituitary volume began to increase on day 18 post-immunization, although still remaining within the normal range, and became significantly greater than baseline on day 25.
Florid phase. Peak volume was reached approximately 1 month after immunization: 3.95 ± 1.6 on day 28, and 4.69 ± 0.74 mm on day 32, Figure 6). Volume remained significantly greater than baseline on days
40, 70, 95, and 120 post-immunization (Figure 7.6), and then progressively decreased.
Atrophic phase. At about 7 months (day 225) post-immunization, volume became significantly smaller than baseline (1.99 ± 0.67), reaching atrophic values at 10 months (day 300, 1.08± 0.38, Figure 7.6).
Pituitary volume strongly correlated with pituitary weight [Pearson correlation coefficient (r) of 0.857], so that volume could be used to predict actual weight in the univariate linear regression analysis (beta coefficient of 0.8576, p< 0.0001, r2 0.735) (data not shown).
7. Pituitary antibodies predict hypophysitis severity in the florid phase of EAH. Pituitary antibodies increased rapidly after immunization, with a
steep slope between days 0 and 21 (Figure 7.7A), a period called the “initiation phase”. Pituitary antibodies did not correlate with disease severity at this time (Figure 7.7B, filled diamonds), because pituitary disease is minimal during the initiation phase. Between day 21 and 140 post-immunization, pituitary antibodies remained elevated and did not change significantly in titer (Figure 7.7A). During this period, the “florid phase”, antibody levels directly correlated with disease severity (Figure 7.7B, open squares): the pituitary infiltration score increased on average 17% for every unit increase in the logarithm of the antibody AU (95% CI between 8% and 27%, p=0.001). Beyond day 140, the atrophic phase, the
pituitary antibody titers gradually declined, although remaining significantly higher than at baseline even 370 days post-immunization (Figure 7.7A), and did not correlate with EAH severity (Figure 7.7B, filled triangles).
8. Hypopituitarism is the common end-point of EAH
We studied the function of the pituitary gland throughout the course of EAH by measuring the serum levels of corticosterone, and thyroxine and insulin-like growth factor-1. Overall, all hormones decreased with time (Figures 8.8A, 8.8B), indicating that hypopituitarism is the final outcome of the lymphocytic infiltration of the pituitary gland. Corticosterone levels, however, were more informative. They dropped below the normal range sooner than thyroxine levels (day 56 in Figure 7.8A versus day 112 in Figure 8.8B), suggesting that the ACTH-producing cells are an earlier or preferential target of the autoimmune response in the pituitary. They also showed in some of the mice a marked increase at day 28, possibly reflecting the host’s attempt to control the autoimmune attack on the pituitary by increasing the output of ACTH and glucocorticoids. Indeed, mice with the highest corticosterone levels were generally the ones with the milder pathology (Figure 8.8C, open squares in the lower right region). By contrast, mice with the most severe EAH often had the lowest corticosterone levels (Figure 8.8C, open squares in the upper left region). All mice with atrophic pituitaries developed hypocortisolism (Figure
8.8C, filled triangles). Thyroxine levels also showed an inverse correlation with pituitary pathology, with the lowest levels observed in mice with the most severe disease (Figure 8.8D, upper left region).
9. Novel autoantigens in EAH.
The above described mouse model of AH was also developed with the goal of obtaining a tool that could aid in the discovery of pituitary autoantigen(s), which have thus far escaped identification. We used a proteomic approach based on 2D-gel electrophoresis, immunoblotting comparison of immune versus control sera, and protein sequencing by MALDI-TOF mass spectrometry of the protein spots recognized by the immune sera. Immune sera recognized 8 unique spots from the pituitary cytosolic fraction, and 7 from the pituitary membrane fraction (data not shown). Sequencing yielded 62 (Table 7.1) and 56 (Table 7.2) candidate autoantigens, respectively. These 118 candidates need to be tested systematically for their ability to induce EAH in mice, and the results then validated in humans. Some candidates, however, like sorting nexin 1 and PC2 in the cytosolic fraction and proopiomelanocortin and aminopeptidase B in the membrane fraction, are already attractive because of the high pituitary expression76, 77, specific subcellular location78, 79, and clinical notion that human corticotroph cells are the most frequently damaged cells in AH patients6. We arbitrarily selected PC2 from the list of 118 candidate autoantigens as a tester to establish the system for
mammalian expression and purification. Upon immunization, PC2 induced a hematopoietic infiltration of the pituitary gland that was significantly greater than that seen in CFA-only immunized mice (p= 0.0028 by Wilcoxon rank-sum test, Supplemental Figure 4A). EAH incidence and severity, however, were low: 7 of 20 mice (35%) developed an infiltration greater than 2%, and in only one mouse was the infiltration moderately severe.
Nevertheless, the infiltration was pituitary specific and not seen in thyroids or pancreases (data not shown). Overall these results suggest that PC2 is a minor pituitary autoantigen and that additional players contribute to EAH initiation and progression.
HUMAN STUDY
8. PREVALENCE AND FUNCTIONAL SIGNIFICANCE OF
PITUITARY AUTOIMMUNITY IN PATIENTS WITH
AUTOIMMUNE THYROID DISEASES, PITUITARY ADENOMA AND IN NORMAL SUBJECTS
Background and aim of the study
The prevalence and the clinical consequences of pituitary autoimmunity are not clear.
Aim of this study was to evaluate the prevalence of pituitary antibodies in a large cohort of patients with autoimmune and non-autoimmune endocrine diseases using immunofluorescence. In a subgroup of patients resulted positive for pituitary antibodies we also performed a functional testing of pituitary hormone secretion.
Subjects and methods
Subjects
In the first section of the “human study” we conducted a health survey in which we enrolled a total of 1718 consecutive subjects to detect pituitary autoimmunity using immunofluorescence. The study group included 961 patients with autoimmune thyroid diseases (117 men and 844 women, age 43±14 years): 707 with Hashimoto thyroiditis, 254 with Graves disease,
293 patients with pituitary adenoma, (87 men (age 53±14 years) and 185 women (age 46±16) years): 30 ACTH-, 69 GH-, 71 PRL-, 13 TSH- and 110 non-secreting pituitary adenoma, and 464 healthy subjects (98 men and 367 women, age 38±15 years).
The study was approved by the institutional ethic committee, previous obtainment of an informed consent.
Study protocol
This study was a health survey in which we analyzed the prevalence of PitAbs in all consecutive unselected patients that were examined at the Endocrinology Department in Pisa in the time frame 2004-2006. Subsequently, in order to detect functional impairment of the pituitary function related to PitAbs positivity, pituitary function was evaluated, by basal and stimulated pituitary hormone measurement, in a selected group of the original population of patients with AITD and PitAbs. MRI of the hypothalamic-pituitary region was performed when evidence of pituitary functional defects was found.
Hormones and organ-specific antibodies measurement and thyroid ultrasound
Serum free T4 (FT4) and free T3 (FT3), TSH, anti-thyroglobulin (TgAb) and anti-thyroperoxydase (TPOAb) antibodies, anti-TSH receptor antibody, gastric anti-parietal cell (PCAb) and anti-transglutaminase
(tTGAb) antibodies, anti-21OH (21-OHAb) and anti-glutamic-acid decarboxylase (GADAb) antibodies were assayed by commercial kits in all patients and controls.
Thyroid ultrasound was performed in all subjects with a real-time instrument with 7.5 MHz linear transducer.
Dynamic tests of pituitary function
PitAbs-positive patients underwent dynamic testing for pituitary function assessment. Eleven patients (9.7%) refused further examinations. Dynamic tests were performed as previously reported80. Pituitary-adrenal axis was investigated by testing the response of ACTH and cortisol to CRH (100 µg as an iv bolus). Pituitary-gonadal axis was investigated with the measurement of basal serum 17-β estradiol (E2) or total testosterone (T) and by testing the response of LH and FSH to GnRH (100 µg as an iv bolus) in pre-menopausal women. GH secretion was investigated by assessing its response to GHRH (1 µg/Kg bw as an iv bolus) plus arginine (diluted 30%, 0.5 mg/Kg bw), and by measuring baseline serum IGF-I. Serum PRL was also measured at baseline. Normal cortisol response to CRH was above 210 µg/. Normal gonadotropin responses to GnRH were: for women (follicular phase) LH increase of 12-23 IU/l (17±3 IU/l) and FSH increase of 3±1 IU/l; for men LH increase of 32±6 IU/l and FSH increase of 3±1 IU/l. In normal weight subjects, diagnosis of mild GH
deficiency (GHD) was defined as a peak GH response to GHRH + arginineless than 16.0 µg/l, while GHD was severe if peak GH response was less than 9.0 µg/l81. In overweight and obese patients appropriate cut-off values were applied82.
Pituitary MRI
PitAbs-positive patients with abnormal pituitary dynamic tests were submitted to MRI of the hypothalamus-pituitary region with a 1.5 T system (General Electric, Signa Infinity Twinspeed-Milwaukee, WI, USA). MRI protocol included Spin Echo T1 weighted sequences (TR 500 msec, TE 15 msec, Field of view 16 cm, matrix 256x256, slice Tk 3 mm) in the sagittal and coronal planes before and after the administration of contrast media (gadolinium). Evaluation of MRI scans was performed by a single operator.
Immunofluorescence to detect pituitary antibodies
Pituitary antibodies were measured by immunofluorescence on a substrate of primate pituitary in all the subjects of the study group (N=1718) using the commercially available kit (Euroimmun Medizinsche Labordiagnostika AG, Lubeck, Germany), and read on a Zeiss fluorescence microscope. Pituitary antibodies test was considered positive starting at dilution 1/10. All sera were read blindly by two investigators. Samples were considered positive when a diffuse immunofluorescence
pattern showing an intracytoplasmatic staining was observed in the majority of the fields. To quantify the positivity, all positive samples were further diluted at 1/30 and 1/90.
Results
1. Pituitary antibodies are detected in patients with autoimmune thyroid diseases and in patients with pituitary adenoma with greater frequency than in controls.
Pituitary antibodies were found in 92 out of 707 Hashimoto thyroiditis patients (13%), 18 out of 254 Graves disease patients (7%), 15 out of 293 adenoma patients, 3 out of 469 healthy subjects. Pituitary antibodies were more frequent in Hashimoto thyroiditis (p<0.0001), Graves disease (p=0.002) and in adenoma patients (p=0.002) than in healthy subjects. When we compared the autoimmune thyroid diseases groups [(Hashimoto thyroiditis and Graves disease) AITD] with the pituitary adenoma group, PitAbs prevalence was higher, but not significantly higher, in the AITD group (p=0.051, Fig. 8.1)
In the group of pituitary adenoma, pituitary antibodies were found in 2 out of 30 patients with ACTH-secreting adenoma (6.6%), in 3 out of 69 patients with GH-secreting adenoma (4.3%), in 6 out of 71 PRL-secreting adenoma (8.4%), in 4 out of 110 non-secreting pituitary adenoma (3.6%), and in none of the TSH-secreting adenoma patients. No overall difference
was observed when we compared PitAbs prevalence among the 5 pituitary adenoma types (p=0.55 Figure 8.1).
In the AITD group, pairwise comparison showed that pituitary antibodies were more frequently positive in Hashimoto thyroiditis patients than in Graves disease patients (p=0.011). Thyroid status did not influence pituitary antibodies presence in either Hashimoto thyroiditis and Graves disease patients.
The titer of PitAbs differed among the disease categories: 66 Hashimoto thyroiditis and 14 Graves disease patients and 10 adenoma patients had 1/10 titer, 18 Hashimoto thyroiditis, 3 Graves disease and 5 adenoma patients had 1/30 titer, and 8 Hashimoto thyroiditis and 1 Graves disease had 1/90 titer.
2. Pititary autoimmunity in patients with AITD is frequently associated to other organ specific antibodies.
Before PitAbs determination, AITD was associated with other autoimmune disorders in 100 patients (10.4%); these disorders included atrophic gastritis (n=43), Addison’s disease (n=26), chronic hypoparathyroidism (n=3), diabetes mellitus type 1 (n=13), celiac disease (n=13), vitiligo (n=16), premature ovarian failure (n=10), Sjogren’s sindrome (n=2), in the context of APS type 1 (3 patients), 2 (28 patients), or 3 (99 patients). Conversely, AITD was a priori isolated in 861 of 961 patients (89.6%). When we evaluated the 110