UNIVERSITA’ DEGLI STUDI DI PISA
FACOLTA’ DI MEDICINA E CHIRURGIA
Divisione di Ginecologia ed Ostetricia “P. Fioretti”
Direttore: Prof. G. Giusti
Tesi di Specializzazione
“Robot-assisted myomectomy: feasibility,
fertility outcome and short term results in
comparison to laparoscopy”
Relatore:
Chiar.mo Prof. A.Gadducci
Candidato:
Letizia Freschi
SUMMARY
INTRODUCTION: Laparoscopic myomectomy (LM) has recently gained a wide acceptance but this procedure remains technically highly demanding. Several concerns have been raised about the increased blood loss and an higher risk of postoperative uterine rupture of the pregnant uterus. The use of robotic technology provides instruments with a wrist function at the tip, movement downgrading, tremor elimination, a stable 3-Dimension view and a comfortable working position. Therefore this technology appear to enhance surgical dexterity and to improve fibroids enucleation and suture capability when compared with laparoscopy. OBJECTIVE: The aim of the present study is to evaluate the feasibility, short term results and fertility outcome of robot-assisted laparoscopic myomectomy (RALM). Moreover, our initial experience with robotic myomectomy has been compared with our experience with LM. METHODS: Data from 48 RALM performed in our department between the years 2007-2011 have been collected. Conception rate, abortion rate, incidence of feto-maternal morbidity or severe pregnancy and labour-related complications were reported; serum FSH and anti-mullerian hormone (AMH) assays and ultrasound valutation of antral follicle count (AFC) have been performed before and 6 months after operation. Number of cesarean sections and vaginal deliveries were described. Moreover, a retrospective chart review of 139 patients
undergoing RALM (n=70) or LM (n=69) has been made to compare the initial surgical outcome. RESULTS: The median age of the patients was 35 years (range 20-46 years) and median Body Mass Index (BMI) was 23 kg/m2 (range 18-35 kg/m2). Seven women (13%) became pregnant after RALM with 8 pregnancies. One pregnancy is actually on going; there were 5 deliveries with caesarian section and two spontaneous deliveries. No spontaneous abortions and no uterine rupture occorred. No significant modification of ovarian function was found after myomectomy. As far as comparison with LM, robotic seems to give advantages over laparoscopy in terms of bleeding, suturing and uterine wall reconstruction. CONCLUSION: RALM seems to be feasible with good short term results and to have a favorable impact on the endocrine and reproductive outcome of young patients.
II
NDEX
NDEX
1 INTRODUCTION ... 5
1.1 HYSTORICAL ESSAYS... 5
1.2 ROBOTIC IN GYNECOLOGY... 13
1.3 ROBOTIC APPLICATION IN BENIGN GYNECOLOGY... 15
1.4 ROBOTIC APPLICATION IN GYNECOLOGICAL ONCOLOGY... 18
1.5 ROBOTIC MYOMECTOMY... 21
2 MATERIAL AND METHODS... 24
2.1 PART 1 ... 24 2.2 PART 2 ... 25 2.3 SURGICAL PROCEDURES... 26 2.4 STATISTICAL ANALYSIS... 28 3 RESULTS... 29 4 DISCUSSION... 32 5 TABLES ... 39 REFERENCES... 45
11
II
NTRO DU
NTRO
DU CTION
CTION
1.1
1.1 HHYSTORICAL ESSAYSYSTORICAL ESSAYS
The era of robots in surgery commenced in 1994 when the first AESOP (voice controlled camera holder) prototype robot was used clinically and then marketed as the first surgical robot by the US FDA.
Since then, many robot prototypes like the Endoassist (Armstrong Healthcare Ltd., High Wycombe, Buck, UK), FIPS endoarm (Karlsruhe Research Center, Karlsruhe, Germany) have been developed to add to the functions of the robot and try to increase its utility.
In 1997, Intuitive Surgicals Inc. (Menlo Park, Calif) came out with their prototype robot called the da Vinci which was a master-slave manipulator with three arms, one for the camera and two for the operating instruments. This has been proved to be a breakthrough technology and stood the test of time since its inception.
The first machine was setup in Europe in 1997 and the first surgical procedure was reported by Himpens et al in March 1997 (1). Since its inception, the robot has been gradually upgraded from the first three-arm system to the current four arms, light weight and more versatile version called the Si-Type. The system basically has three components: the patient cart, the surgeon console and the endoscopic stack or column. The system has technical features which significantly improve the quality and control of
the visual field and thus enhance the dexterity of the surgeon and deliver a high quality three dimensional (3-D) vision to the surgeon manning the console. This technology allows intuitive tele-manipulation with tremor abolition, motion scaling and endo-wristed instruments. This is essentially what gives this technology an edge over the endoscopic technology which has been prevailing over the last two decades and overcomes some of the pitfalls of conventional laparoscopy which have probably limited the capabilities of the surgeon in the field of minimally invasive surgery (2). Patient cart
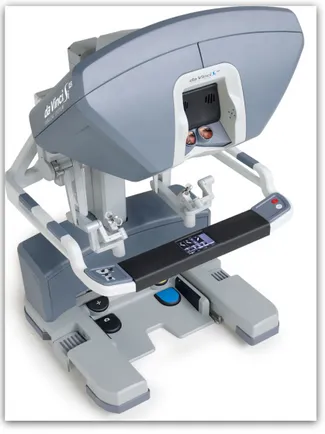
The patient cart of the Si-Type da Vinci weighs approximately 544 kg and is easily manoeuvrable on a wheel base. The cart is connected by color coded cables for the four arms to the console and the console in turn is connected to the main power circuits. The cart consists of four robotic arms and a monitor for the assistant surgeon at the patient side (Fig. 1).
Once in position, the cart is locked in place slightly away from the operating table. The system runs off the main power system and has an emergency five-minute internal power backup.
Each arm has a series of multiple positioning joints and a terminal pivot joint at the attachment with the port allowing easy positioning of the arms during setup and a full range of movement during the surgery (Fig. 2). Buttons provided at each joint allow manual adjustment by acting as a clutch, releasing the button locks the arm in place. The central, camera arm is compatible with a standard
12-mm port and the camera unit. The other three arms attach to specially designed 8-mm metal ports supplied with both blunt and sharp trocars. The arms are mechanically and electronically balanced for safety and ease of use and custom-fitted plastic drapes are available to drape the four arms to achieve sterility. The camera system (Insite vision system, Intuitive Surgical Inc.) has a dual lens system with two three-chip cameras and spatially separated within 12-mm casing. Hence, two complete optical systems are incorporated, representing the left and right eyes. The spatial separation of these images projected to the surgeon’s eyes in the binocular viewer allows true 3-D image perception at the console. The head end of the cart is fitted with a HD monitor for the benefit of the assistant surgeon and the scrub nurse. A wide variety of instruments available with the system are easily and rapidly changeable by the assistant surgeon or a trained scrub nurse at the patient side. Except for the ultrasonic dissector all the instruments are endowristed (Endo Wrist, Intuitive Surgical Inc.) and the instrument wrist is controlled by a cable system attached to four wheels on the instruments’ head that can be moved simultaneously by the robot to generate a single complex movement mimicking the motion of the human wrist. The human tremors are effectively abolished by position sensing, which occurs approximately 1500 times per second. There are six degrees of motion at the instrument tip and a seventh degree of freedom provided by the instrument itself (e.g. grasping or cutting). Each instrument has only 10 lives following which it needs to be discarded and replaced this is done by the system which counts down the ten sessions of use. The instruments can be sterilized. The instrument can be used any number of
times during one surgical procedure. However, a possibility of the instrument cable breaking off remains thus making the instrument unusable before 10 sessions.
Fig. 1 The Patient Cart
The surgical console
This consists of: master controllers, stereo viewers, left and right-side pods, touchpad and footswitch panel (Fig. 3).
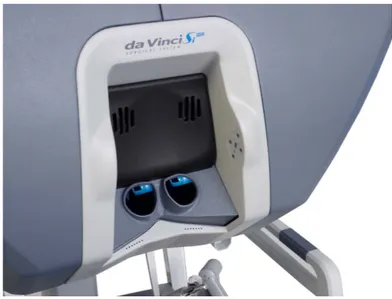
Fig. 3 The surgical console 1. Stereo viewer
The stereo viewer provides the video image for the Surgeon Console operator. The ergonomically designed view port provides head and neck support for added comfort during extended procedures. When the endoscope is activated, the stereo viewer's integrated left and right video channels provide the surgeon with continuous 3D video, extending the vision of the surgeon into the surgical field. The stereo viewer also displays messages and icons which convey status of the da Vinci Si system (Fig. 4).
Fig. 4 The stereo viewer
2. Master controllers
The master controllers provide the means for the surgeon to control the instruments and endoscope inside the patient. The master controllers are designed to allow natural range of motion and to provide ergonomic comfort, even during extended procedures.
To use the master controllers, the Surgeon Console operator grasps each controller with his/her index (or middle) finger and thumb. The operator activates and controls the EndoWrist instruments by bringing the finger and thumb together or apart; maneuvers the instruments and endoscope inside the patient by moving the hands and/or arms. These movements are precisely and seamlessly replicated at the Patient Cart, thereby virtually extending the operator's hands into the surgical field (Fig. 5). da Vinci® Si™ System Overview
2-5
DR
AFT/PRE-RELEASE/C
ONFIDENTIAL
2/2 7/0 9To use the master controllers, the Surgeon Console operator grasps each controller with his/her index (or middle) finger and thumb. The operator activates and controls the EndoWrist instruments by bringing the finger and thumb together or apart; maneuvers the instruments and endoscope inside the patient by moving the hands and/or arms. These movements are precisely and seamlessly replicated at the Patient Cart, thereby virtually extending the
operator's hands into the surgical field. (See 10.4 Surgical Controls on page 10-17 for details.)
Figure 2.6 Master Controllers
Stereo Viewer
The stereo viewer (Figure 2.7) provides the video image for the Surgeon Console operator. The
ergonomically designed view port provides head and neck support for added comfort during extended procedures.
When the endoscope is activated, the stereo viewer's integrated left and right video channels provide the surgeon with continuous 3D video, extending the vision of the surgeon into the surgical field. The stereo viewer also displays messages and icons which convey status of the
da Vinci Si system. (See Stereo Viewer Display on page 10-20 for details.)
11 Fig. 5 Master controllers
3. Touchpad
The touchpad is located in the middle of the surgeon console armrest and provides the means for selecting various system functions (Fig. 6).
Fig. 6 The touchpad da Vinci® Si™ System Overview
2-5
DR
AFT/PRE-RELEASE/C
ONFIDENTIAL
2/2 7/0 9To use the master controllers, the Surgeon Console operator grasps each controller with his/her index (or middle) finger and thumb. The operator activates and controls the EndoWrist instruments by bringing the finger and thumb together or apart; maneuvers the instruments and endoscope inside the patient by moving the hands and/or arms. These movements are precisely and seamlessly replicated at the Patient Cart, thereby virtually extending the
operator's hands into the surgical field. (See 10.4 Surgical Controls on page 10-17 for details.)
Figure 2.6 Master Controllers
Stereo Viewer
The stereo viewer (Figure 2.7) provides the video image for the Surgeon Console operator. The
ergonomically designed view port provides head and neck support for added comfort during extended procedures.
When the endoscope is activated, the stereo viewer's integrated left and right video channels provide the surgeon with continuous 3D video, extending the vision of the surgeon into the surgical field. The stereo viewer also displays messages and icons which convey status of the
da Vinci Si system. (See Stereo Viewer Display on page 10-20 for details.)
Figure 2.7 Stereo Viewer
System Overview
2-6
DR
AFT/PRE-RELEASE/C
ONFIDENTIAL
2/2 7/0 9 TouchpadThe touchpad (Figure 2.8) is located in the middle of the surgeon console armrest and
provides the means for selecting various system functions. (See 10.3 Touchpad Controls on
page 10-10 for details.)
Figure 2.8 Touchpad
Left-Side Pod and Right-Side Pod
The left-side and right-side pods (Figure 2.9) are located on either side of the surgeon console
armrest. The left-side pod provides ergonomic controls while the right-side pod is the location
for the Power button and Emergency Stop button. (See Ergonomic Setup on page 10-9 and
5.2 Powering On the System on page 5-2 for details.)
Figure 2.9 Left-Side and Right-Side Pods
Emergency Stop
4. The left-side and the right-side pods
The left-side and right-side pods are located on either side of the surgeon console armrest. The left-side pod provides ergonomic controls while the right-side pod is the location for the Power button and Emergency Stop button (Fig. 7).
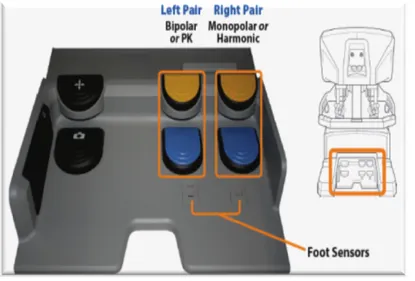
Fig. 7 Left-side and the right-side pods 5. Footswitch panel
The footswitch panel (Fig. 8) features two groups of footswitches. The three switches on the left control system functions are: master clutch, camera control and arm swap; the four switches on the right control energy activation for devices connected to the auxiliary connections on the core. The first on the left is the master clutch pedal pressing which both masters decouple from control of their instruments. This enables the surgeon to reposition the masters for ergonomic comfort and to reclaim space to maneuver the masters when they run out of working space while all instruments remain immobile. The second left pedal is for the camera control and focus: pressing it, it is possible to switch the masters from instrument control to camera (endoscope) control. In camera mode, the surgeon’s
System Overview da Vinci® Si™ 2-6
DR
AFT/PRE-RELEASE/C
ONFIDENTIAL
2/2 7/0 9 TouchpadThe touchpad (Figure 2.8) is located in the middle of the surgeon console armrest and
provides the means for selecting various system functions. (See 10.3 Touchpad Controls on
page 10-10 for details.)
Figure 2.8 Touchpad Left-Side Pod and Right-Side Pod
The left-side and right-side pods (Figure 2.9) are located on either side of the surgeon console
armrest. The left-side pod provides ergonomic controls while the right-side pod is the location
for the Power button and Emergency Stop button. (See Ergonomic Setup on page 10-9 and
5.2 Powering On the System on page 5-2 for details.)
Figure 2.9 Left-Side and Right-Side Pods
Emergency Stop Power System Overview da Vinci® Si™ 2-6
DR
AFT/PRE-RELEASE/C
ONFIDENTIAL
2/2 7/0 9 TouchpadThe touchpad (Figure 2.8) is located in the middle of the surgeon console armrest and
provides the means for selecting various system functions. (See 10.3 Touchpad Controls on
page 10-10 for details.)
Figure 2.8 Touchpad Left-Side Pod and Right-Side Pod
The left-side and right-side pods (Figure 2.9) are located on either side of the surgeon console
armrest. The left-side pod provides ergonomic controls while the right-side pod is the location
for the Power button and Emergency Stop button. (See Ergonomic Setup on page 10-9 and
5.2 Powering On the System on page 5-2 for details.)
Figure 2.9 Left-Side and Right-Side Pods
Emergency Stop
movement of both masters together, such as moving in or out, side to side, or rotating, translates into endoscope movements. When the surgeon releases the camera pedal, the masters resume control of the instruments. The last left-side pedal is the Arm Swap: it allows to swap control between two instrument arms associated with the same master. As far as the four switches on the right they correspond to cutting/coagulating with bipolar and monopolar energy .
Fig. 8 The footswitch
1.2
1.2 ROBOTIC IN GYNECOLOG YROBOTIC IN GYNECOLOGY
Historically, surgery in gynecology has been accomplished via a vaginal or open abdominal approach. Although laparotomy may seem advantageous for the surgeon at first, with depth perception and tactile feedback from tissue, the large abdominal incision, prolonged hospitalization, increased postoperative analgesic requirements and higher morbidity are disadvantages for the patient. The days of a surgeon obtaining access to the
abdomen only at laparotomy have long passed. Laparoscopic surgery enables faster recovery with shorter hospitalization, improved cosmesis, de- creased blood loss, and less postoperative pain (3,4). Technical advancements have improved modern laparoscopy. These include high-intensity xenon and halogen light sources, improved hand instrumentation and energized devices. This technology has continued to grow rapidly in the area of minimally invasive gynecologic surgery. Despite these technologic advancements and proved benefits, more complex procedures such as management of advanced endometriosis and procedures that require extensive suturing such as myomectomy typically are still managed predominately by laparotomy.
One major obstacle to the more widespread acceptance and application of minimally invasive surgical techniques in gynecologic surgery has been the steep learning curve for surgeons that is associated with many of these advanced procedures. Other limitations of conventional laparoscopy include counterintuitive hand movement (fulcrum effect), an unsteady two-dimensional visual field, limited degrees of instrument motion within the body as well as ergonomic difficulty and tremor amplification (5). In an attempt to overcome these obstacles, robotics has been incorporated in the gynecologic resources as a possible solution. Overall, the integration of robotics in gynecology continues to be driven by the intent to improve the
surgical capabilities of the surgeon with consistent accuracy and precision in order to perform complex surgery at laparoscopy rather than at laparotomy. While a rationale for implementing robotics in gynecologic surgery is extremely important, it is equally important to note that it is not a replacement for minimally invasive procedures or approaches already done well. It is merely another laparoscopic tool that may provide a less invasive way to address gynecologic disease.
1.3
1.3 RROBOTIC APPLICATION I N BENIGN GYNECOLOOBOTIC APPLICATION IN BENIGN GYNECOLO GYGY
There are now reports on virtually every gynaecological procedure for benign disease to have been performed robotically.
Tubal re-anastomosis was one of the first gynaecological operations performed with the da Vinci surgical system. Robotic surgery for tubal anastomosis can be accomplished without significant differences in pregnancy and ectopic pregnancy rates and in similar operating time when compared to laparotomy. Return to normal activity is shorter with the robotic technique. Robotics seems to combine the advantages of open microsurgery and minimal invasive surgery without abdominal incision and compromised precision of conventional laparoscopy.
Management of fibroids laparoscopically is one of the more difficult tasks in minimally invasive surgery. To enucleate a leiomyoma and to close the defect with a multilayer-suture is challenging. Most conservative
surgery is therefore performed by laparotomy nowadays. With robotic assistance, a large percentage of myomectomies should be manageable by minimal invasive surgery as the da Vinci surgical system provides excellent suturing capabilities. Several studies have reported a decreased blood loss, complication rate and length of stay with the robotic minimal-invasive procedure when compared with an open myomectomy.
It has been estimated that one in nine women will have a hysterectomy in her lifetime, and up to 10% of these women will need surgical repair for vaginal prolapse. Laparoscopic colposacropexy has partially become a substitute for open surgery in the treatment of vaginal prolapse. However, technical difficulties and the potential significant increase in operative time have greatly limited its widespread use. Robotic surgery may overcome these limitations. In the largest reported series to date of 30 patients with post-hysterectomy vaginal vault prolapse, the conversion and complication rate after robotic-assisted laparoscopy was very low and almost all patients were discharged home on the first postoperative day. The satisfaction rate was very high and after a mean follow up of 24 months only one patient developed a recurrent prolapse. Long-term data are needed to confirm these results.
Hysterectomy is one of the most common gynaecological procedures performed in Australia. A definite trend towards laparoscopic hysterectomies has been seen since the 1990s. There has been a
transformation of hysterectomy techniques from both the abdominal and the vaginal approach to the laparoscopic-assisted vaginal hysterectomy (LAVH) and finally the total laparoscopic hysterectomy (TLH).
Several studies have evaluated the feasibility of robot-assisted hysterectomy. Large parts of the operation, such as securing the uterine vessels and cardinal ligaments, performing an accurate colpotomy and oversewing the vaginal cuff are performed with great ease, thus providing unique advantages as compared with conventional laparoscopy. Recently, Kho and colleagues from the Mayo Clinic published the largest series of robotic hysterectomies. They reviewed 91 consecutive patients who underwent a robotic-assisted hysterectomy with or without salpingo-oophorectomy. The mean total operating time (from incision to closure of the port sites, including cystoscopy) and console time (for surgeon to perform procedure at console, from beginning of robotic hysterectomy to closure vaginal cuff) were 128 and 73 min, respectively. Console time has not been significantly associated with body mass index (BMI). The mean estimated blood loss was 79 ml and no blood transfusions were required. The mean hospital stay was 1.3 days. The only intraoperative complication was an enterotomy which was repaired robotically (6). Prospective studies will now need to examine long-term clinical outcomes between conventional and robot-assisted laparoscopic hysterectomy.
The feasibility of complex endometriosis surgery with the da Vinci surgical system has been reported by various authors. The case numbers are small but the results are very promising. Robotic surgery seems to be particularly useful for complex cases with severe adhesions, scarring and difficult anatomical conditions in which fertility preservation would be extremely difficult with conventional laparoscopy or laparotomy.
The laparoscopic repair of vesico-vaginal fistulas is technically very challenging and has not gained popularity. Several small case series and case reports on successful repairs of vesico-vaginal fistulas with the da Vinci robotics system have now been published. Sundaram and colleagues reported a mean operative time of 233 min and an estimated blood loss of less than 70 ml. The mean length of hospital stay was five days. At six months follow up, the patients had a normal bladder function and there were no recurrent fistulas. These data suggest that robot-assisted vesico-vaginal fistula repair is feasible and results in low morbidity, a short hospital stay and a fast recovery (7).
1.4
1.4 RROBOTIC APPLICATION I N GYNECOLOGICAL ONCOOBOTIC APPLICATION IN GYNECOLOGICAL ONCO LOGY LOGY There has been a recent trend towards laparoscopic surgery in gynaecological oncology, especially for the management of endometrial cancer. Techniques include TLH and LAVH. Pre-requisite for the laparoscopic approach is that staging and extent of surgery is not compromised because of technical issues.
Studies published to date indicate no difference in prognosis whether the endometrial cancer is operated by laparoscopy or open surgery. The ‘Laparoscopic Approach to Carcinoma of the Endometrium (LACE)’ study reported that patients undergoing a Total Laparoscopic Hysterectomy (TLH) had a 74% decreased risk of a serious adverse event (AE) from their surgery when compared to patients treated with total abdominal hysterectomy (TAH). Moreover, patients undergoing TLH reported significantly greater postsurgical improvement of Quality of Life (QoL) compared to TAH. This improvement in QoL continued to favour the laparoscopic approach for up to 6 months post-surgery. These data suggest that laparoscopic surgical approach is feasible, safe and should become the standard treatment once survival data confirmed equivalence with respect to patterns of recurrence, disease-free and overall survival. Furthermore, two randomised clinical trials reported results on surgical Adverse events (AE) and the US Gynecologic Oncology Group (GOG) LAP2 showed that a laparoscopic surgical approach resulted in fewer post-operative moderate/severe surgical complications and shorter hospital stay than surgery through laparotomy.
Despite its advantages, minimal invasive surgery is not so widely used in gynaecological oncology. One reason is the inferiority of the laparoscopic approach in performing comprehensive radical dissections and achieving en bloc lymphadenectomies especially of the upper external iliac and para-aortic region when compared to the open approach.
hysterectomies for cervical cancer. There are issues with the length of the procedure, as type III laparoscopic hysterectomies take significantly longer than open cases. Furthermore, intraoperative complications of the urinary tract (for example as a result of thermal injuries) tend to be much more common in laparoscopic than in open cases.
Several case series have now been published about robotic-assisted radical hysterectomies for endometrial and cervical cancer. Magrina and colleagues recently presented the first prospective study comparing the perioperative results of patients undergoing radical hysterectomy and lymph node dissection by robotics, laparoscopy and laparotomy for cervical and endometrial cancer. The mean operating time for a robotic, laparoscopic and laparotomic radical hysterectomy was 190, 220 and 167 min, respectively; the mean blood loss was 133, 208 and 444 ml respectively; the mean number of removed lymph nodes was 26, 26 and 28, respectively, and the mean hospital stay was 1.7, 2.4 and 3.6 days, respectively. There were no significant differences in intra- or post-operative complications among the three groups and no conversions in the robotics or laparoscopic groups. At a mean follow up of 31 months, none of the patients with cervical cancer had experienced a recurrence. The authors concluded that laparoscopy and robotics are preferable to laparotomy for patients requiring radical hysterectomy with advantages like significantly shorter operating times noted for robotics over laparoscopy. Their results are in accordance with other groups, although some reported the feasibility of a more radical lymph node
dissection with the robotic system when compared to conventional laparoscopy (8).
1.5
1.5 RROBOTIC MYOMECTOMYOBOTIC MYOMECTOMY
Endoscopic management of leiomyomas is one of the more challenging procedures in minimally invasive surgery and requires a skilled surgeon. Although 2 prospective trials have shown post-operative morbidity to be less and recovery faster with laparoscopic myomectomy, most procedures are still performed via laparotomy (9,10). The ability to enucleate leiomyomas and adequately perform a multilayered closure, the associated learning curve, concern about uterine rupture, and the risk of recurrence, which seems to be higher after laparoscopic myomectomy (LM) compared with laparotomy, may explain the reluctance to shift to a laparoscopic approach (11). The use of robot-assisted laparoscopy represents an attempt to overcome the difficulties encountered with the key steps of hysterotomy, enucleation, repair, and extraction during conventional laparoscopy (12). The earliest published series of robot-assisted laparoscopic myomectomy (RALM) was from Advincula et al. (13). In their series of 35 patients, mean (SD) myoma weight was 223.2 +/- 244.1 g (95% confidence interval [CI], 135.8–310.6). The mean number of myomas removed was 1.6 (range, 1–5), and the mean diameter was 7.9 +/- 3.5 cm (95% CI 6.6– 9.1). Mean estimated blood loss was 169 +/- 198.7 ml (95% CI 99.1-238.4). No blood transfusions were necessary. Mean operating time was 230.8 +/- 83 min (95% CI 201.6-260). Median length of stay for these patients was 1 day.
Total conversion rate was 8.6%, comparable to other published studies of conventional LM with ranges from 0% through 28.7%. Two of the reported conversions were secondary to an absence of tactile (haptic) feedback, which made enucleation of the leiomyomas difficult.
The largest comparative study to date of RALM compared with the criterion standard approach of laparotomy is also by Advincula et al. (14). Fifty-eight patients with symptomatic leiomyomas were studied in a retrospective case-matched analysis with 29 patients in each arm. As would be expected, there were no differences in the case-matched variables of age, body mass index (BMI), and fibroid weight. Noteworthy were the findings of decreased estimated blood loss (mean 195.69 +/- 228.55 ml [90% CR 50.00-700.00 ml] vs mean 364.66 +/- 473.28 ml [90% CR 75.00-1550.00 ml]) and length of stay (mean 1.48 +/- 0.95 days [90% CR, 1.00–3.00]) vs 3.62 +/-1.50 days [90% CR, 3.00–8.00]) compared with the laparotomy group. Both of these differences were statistically significant at p <.05. Complication rates were also lower in the robotic group. Operative time was longer in the robotic group (mean, 231.38 +/- 85.10 minutes [95% CI, 199.01–263.75]) vs 154.41 +/- 43.14 minutes [95% CI, 138.00–170.82 p < .05]).
In a smaller retrospective case-matched study, 15 patients who underwent RALM were compared with 35 patients who underwent LM (15). Mean surgical time was 234 minutes (range, 140–445 minutes) in the robotic group compared with 203 minutes (range, 95–330 minutes) in the conventional laparoscopy group. Estimated blood loss, length of stay and postoperative complications were not
significantly different between the 2 groups. The authors concluded that in the hands of a skilled surgeon, the robotic approach did not confer any major advantage over conventional laparoscopy.
In consideration of all these findings, the aim of our study is:
i) to evaluate the feasibility of RALM in infertile and/or symptomatic women and to analyze their fertility outcomes
ii) to compare our initial experience with RALM with our initial experience with LM in terms of immediate and post-operative surgical outcome.
22
M
M
ATERIAL AND METHODS
ATERIAL AND METHODS
2.1
2.1 PPART ART 11
Between 1 June 2007 and 31 March 2011, 48 regularly menstruating women younger than 46 years with median BMI 22.9 kg/m2 (range 17.2-35.3 kg/m2) underwent RALM in our Department. All the procedures were performed by the same surgeon. Patients had at least one subserous or intramural myoma measuring at least 3 cm of diameter (Tab. I). All women were recommended to avoid pregnancy within the first six months following surgery. A clinical follow-up regarding clinical outcome and subsequent pregnancies occurred after 1 and 6 months. Data regarding number, size and localization of myomas such as informations about the patient were registered prospectively. In case of pregnancy following the myomectomy the medical record has been required to register possible complication during the labour, the week of gestation at the time of delivery, the birth weight, the APGAR score, the type of delivery and in case of cesarian section the indication for the operation. Uterine rupture was defined as a complete separation of the wall of the pregnant uterus with or without expulsion of the fetus, endangering the life of the mother or fetus. The levels of serum follicle stimulating hormone (FSH) and anti mullerian hormone (AMH) as well as the ultrasound evaluation of antral follicle count
(AFC) were detected before operations on the third day of the menstrual cycle and 6 months after surgery (16).
2.2
2.2 PPART ART 22
A retrospective chart review included all patients with benign leiomyomata who underwent myomectomy by laparoscopic or robotic surgery. These patients were stratified into two groups according to the surgical route. Group I included 69 consecutive patients who underwent LM between March 1999 and April 2001. Group II included 70 consecutive patients who underwent RALM between January 2009 and February 2012. Robotic surgery was performed using the da Vinci or da Vinci Si system (Intuitive Surgical, Inc., Sunnyvale, CA). The same three surgeons performed all surgical procedures. Surgeons were at beginning of their LM experience between 1999 and 2001, although they had experience with laparoscopy since a couple of years. Between January 2009 and February 2012 the same surgeons began their experience with RALM, after performing at least five cases of other robotics gynecological procedures (simple hysterectomy or oophorectomy). Leiomyomata size, number, symptoms, or surgical history did not affect patient eligibility. We excluded all patients that received other concomitant surgical procedures in addition to myomectomy. The laparoscopic group included all eligible patients, and subjects were not matched with respect to specific characteristics of patients
in the robotic group. After January 2009 selection of patients for robotic surgery depended solely on the availability of the da Vinci system to the surgeon.
Patients’ data were retrieved from our electronic medical recording system as well as the patients’ paper charts. Demographic data including age, height, weight, BMI, parity were recorded. Pre-operative characteristics of the myomas including the number, location, depth of infiltration, the maximum diameter and the size were evaluated by pelvic ultrasounds. The weight of the myomas was evaluated postoperatively. Surgical outcome measures included surgical time “skin-to-skin” in minutes, estimated blood loss (calculated by subtracting irrigation volume from the total volume of fluid suctioned), number of uterine suture layers myoma, intraoperative and postoperative complications and the length of hospital stay. For robotics group, “skin-to-skin” time included both docking and console time. The investigation met the internal review board approval at Pisa University Hospital.
2.3
2.3 SSURGICAL PROCEDURESURGICAL PROCEDURES
In the laparoscopic group, the procedures were performed using a 10-mm scope with three ancillary ports. A longitudinal incision, using an unipolar hook scissor, was performed close to the midline. After cleavage plane identification, by choice of the operator, the leiomyoma was enucleated by
means of either adequate traction with a myoma drill, or a strong grasper and countertraction maneuvers with Manhes forceps, or scissors, or a hydrodissection technique. Coagulation of significant bleeding was performed using bipolar forceps and the myometrial edges were approximated, according to the depth of the uterine defect, with Vicryl CT 2-0 interrupted figure-eight sutures using intracorporeal or extracorporeal knots. In all cases leiomyomas were removed with a 12-mm automatic morcellator. As far as the robotic surgical technique, we used the four-arm da Vinci-Si robot and utilized a three-armed patient-side cart with 2 accessory ports placed on the patient’s left side and one sovrapubic. A 12 mm port is placed either at or above the umbilicus depending on the size of the uterus. This port accommodates the endoscope which provides the three-dimensional imaging. Pneumoperitoneum was obtained with a Veress needle technique followed by placement of the trocars. As a general rule, at least a hands-breadth distance or approximately 8–10 cm between the endoscope and top of an elevated uterus or leiomyoma during manipulation is necessary in order to allow for an adequate working distance between the endoscope and the uterine fundus. Two 8 mm ports that mount directly to the operating arms on the patient-side cart are placed in the left and the right lower quadrants, respectively. For larger uteri or leiomyomata, these ports are moved more cephalad. A fourth trocar serves as an accessory port and has been placed at the sovrapubic level. This last trocar is typically a 12
mm port in order to facilitate introduction of suture as well as instruments used for retraction, suction/irrigation, and specimen removal via a tissue morcellator. The uterine incision was made over the prominent bulge using monopolar scissor and subsequently myoma was removed using the tenaculum. Multilayered closure of the bed using interrupted figure-eight sutures using Vicryl CT 2-0 (Ethicon GmbH, Norderstedt, Germany) was performed with adequate hemostasis, continued along two or more layers in single, double or in multiple layers depending on the depth of the myoma. The myomas were retrieved by morcellator (Gynecare Morcellex®, Ethicon Inc, Somerville, NJ, USA).
2.4
2.4 SSTATISTATIS TICAL ANALYSISTICAL ANALYSIS
All data were analyzed with Prism software (GraphPad Software Inc., San Diego) and expressed as mean ± SD. The Mann Whitneytest was used to compute statistical significance, χ2 and Fisher’s exact tests were used to analyze differences between proportions and a paired Student t test to compare groups. P<.05 was considered statistically significant.
33
R
R
ESULTS
ESULTS
Among the 48 women, 41 (85.5%) had no pregnancy at the time of the investigation and 7 (14.5 %) had a total of 8 pregnancies of which 7 ended in live-term delivery and 1 actually on going. Eighty-nine myomas were removed with a median number of 1 (range 1-7) and a median size of 7 cm (range 1.5-9 cm). Mean time for surgery (including docking and undocking of the robot) was 121±46 minutes. Complications were few and mostly minor. Median length of post-operative hospital stay was two days (range 1-3 days). At the time of follow-up of the 9 women searching for a pregnancy 7 (77.8%) became pregnant with a total of 8 pregnancies achieved. The characteristics of these patients are shown in Tab. II.
The indication for myomectomy (in most of cases more than one myoma) for the 7 patients was primary or secondary infertility or recurrent spontaneous abortion associated with pain or pressure in 4 patients (66.7%). Six of the seven women (85.7%) conceived naturally. Median time from surgery to conception was 16 months. The pregnancy outcome of the 7 patients who delivered after robot-assisted laparoscopic myomectomy has shown in Tab. III.
One patient delivered two times two viable term neonates; all the other patients underwent elective cesarian section for previous uterine scar. No
ectopic pregnancy or uterine rupture was observed. As far as the valutation of the ovarian function we found that none patient experienced hot flushes after the procedures and pre-operative mean values of FSH, AMH and AFC of 8.1±2.8 mUI/mL, 5.2±0.9 ng/mL and 10.9±4.4 respectively with post-operative values at 6 months of follow-up of 7.8±3.5 mUI/mL, 4.9±1.0 ng/mL and 11.2±4.7 respectively, revealing no significant differences between levels before and after operation among all the patients.
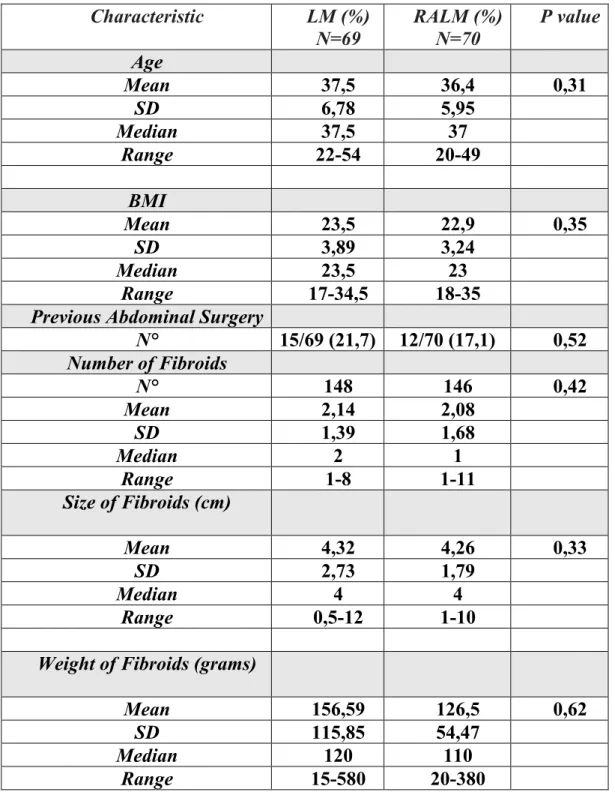
As far as the comparison between LM and RALM, one hundred thirty-nine patients were included in the study: 69 underwent LM and 70 underwent RALM. Patient and fibroid characteristics are shown in Table IV. No significant differences were noted between groups regarding the total number of myomas (148 for laparoscopy and 146 for robotic), the mean number of myoma for patient (2,14±1,39 for laparoscopy and 2,08±1,68 for robotics, p=ns) and the size (4,32±2,73 cm for laparoscopy and 4,27±1,79 for robotics, p=ns), as well as for patient characteristics. The weight of myomas resulted slightly higher in the laparoscopic groups, although not statistical significant. On the contrary location of myomas was different across groups. Patients underwent RALM showed more intramural myomas than women underwent LM and a large number of peduncolated myomas were treated by laparoscopy. No additional statistical significance were noted regarding the number of subsierosal or submucosal myomas, as well as the their uterine location (anterior or posterior wall and the fundus).
The intraoperative and immediate postoperative outcomes were compared across the two groups and are presented in Tab. V. The operative surgical time did not differ between LM and RALM. The operative surgical time is defined as the time from the incision to the closure of the skin. It reflects the absolute time of the surgical procedure. Overall, a significantly higher intraoperative blood loss was reported in the laparoscopic group compared to robotics (p=0.006). The number of suture layers of the uterus resulted different between LM and RALM: a higher percentage of patients underwent RALM in comparison with patients underwent LM, received at least a double closure of the uterine wall after myomas removing. Interestingly, 7 patients who underwent RALM received a three-layers suturing of the uterine defect, whereas all patients who underwent LM received only a single or a double-layers closures of the uterine incision (Tab. VI). Hospitalization was not different between LM and RALM. Four intraoperative uterine hemorrhagic complications were observed during laparoscopy and two of them required conversion to laparotomy, whereas no conversion to laparoscopy or laparotomy was recorded during robotics, although one patient underwent RALM showed intraoperative bleeding. No additional intraoperative or postoperative complications were observed in both groups.
44
D
D
ISCUSSION
ISCUSSION
In our series we found an overall pregnancy rate of 78% in the 9 patients
searching for pregnancy at the time of surgery, following RALM. We must take into consideration that operated women were counselled not to become pregnant within six months of surgery and that the postoperative observational time has been relatively short. Thus, our fertility rate can be considered high and similar to that published by Lönnerfors et al. suggesting no different fertility outcome and pregnancy rates following RALM for deep intramural mioma with possible impact on fertility, equaling results reported in literarure after laparoscopic or laparotomic myomectomy (17). No uterine rupture has been registered after surgery also following spontaneous vaginal delivery and the blood lost during the surgery was minimal as well as the post-operative hospital stay. As far as the
potential impact of myomectomy on ovarian function, to date it is unknown
whether there is a subclinical ovarian impact on younger women; furthermore most of the studies published to date have measured only serum FSH which is a relatively insensitive measure of ovarian reserve in younger women. Recent studies, infact, suggested that other ovarian markers, such as AMH, may be more sensitive and accurate in predicting ovarian reserve compared with FSH. Our
results, demonstrating no statistically significant differences between mean
pre-operative FSH, AMH and AFC values and that detected at 6 months of follow-up, suggest that RALM doesn’t adversely affect ovarian function (18). Laparotomy has long been the standard surgical approach to myomectomy because it allows
easy access to the uterus for the removal of large fibroids. However, it usually requires a large incision and, compared with minimally invasive surgery, is associated with longer hospitalization, higher levels of postoperative analgesia and increate morbidity (19).
Currently available instruments make LM feasible, and several comparative trials have been published to evaluate the value of laparoscopic access as an alternative to laparotomy: laparoscopic approach is associated with faster postoperative recovery, less postoperative morbidity and shorter hospital stay compared with abdominal surgery with no significance found in terms of major complications, pregnancy and recurrence (20,21). As regards the uterine rupture during pregnancy, after abdominal myomectomy it seems to be a rare event, based on reviews of delivery records of large numbers of women. Two studies comprising 236.454 deliveries reported 209 instances of uterine rupture of which only 4 were attributable to previous myomectomy (22,23).
The incidence of uterine rupture after LM is unknown however was estimated to be approximately 1% by Dubuisson et al. (24) in his series (100 cases). Nine other series (a total of 156 cases) reported none uterine ruptures (25). It is not clear whether the laparoscopic procedure is associated with higher risk of subsequent rupture or whether these cases are being more systematically reported but when the procedure is performed via laparoscopy, because of the limitations of fixed port placement, the
repair is more likely to be completed using the bulk closure technique (26). So, failure to adequately suture myometrial defects, lack of hemostasis with subsequent hematoma formation, or excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium have all been postulated to interfere with myometrial wound healing and increase the risk of rupture (27,28). For example, the lack of suture or suturing only the superficial layers of the myometrium, resulted in thin or dehiscent scars (29,30,31) and inappropriate use of electrocautery may sometimes have induced in-depth necrosis of the myometrium with an adverse effect on healing (32). Several cases of uterine rupture reported after myolysis confirm this negative effect of electrocoagulation. So, the correct repair of the uterine incision is fundamental: the suture must take up the whole depth of the edges of the hysterotomy in order to ensure that the whole of the myomectomy bed is brought into contact, so that secondary formation of a haematoma in the myometrium is avoided (33) and when the myomectomy bed is deep or the uterine cavity opened, two layers may be necessary to close the myometrium (34,35).
Robotic technology, offering a translation of open surgical skills to
laparoscopy, may improve efficiency, accuracy, ease and comfort
associated with the performance of laparoscopic operations because the da Vinci three-dimensional vision offers superior visualization and definition
of the operative anatomy. In addition to helping close dead space and prevent hematoma formation the robotic technology, moreover, enhances the precision of the myomectomy and decreases risk of perforation of the uterine cavity which may predispose the patient to synechiae or a weak scar.
By the comparison of our first RALM with our first LM appears that
RALM is a feasible and safe procedure, although all surgeons were at their initial experience, confirming similar findings from different surgical series (36). In particular, our data showed that RALM might have some advantages over traditional laparoscopy in two critical surgical steps of myomectomy: 1) bleeding and 2) suturing of uterine defect following myoma enucleation. These finding were demonstrated, at least, when surgeons were at their initial experience performing both RALM and LM.
Previous studies have demonstrated the advantages of robotic technologies to reduce bleeding during uterine surgery in comparison with laparotomic myomectomy. Our series supported also the evidence that bleeding during myomectomy was reduced when surgeons used robotics instead of traditional laparoscopy (37). Similarly, Falcone et al. demonstrated that the hemoglobin drop following myomectomy was significantly lower in the robotic group compared with laparoscopy and laparotomy. On the contrary, the other two available series reported
divergent findings: Nezhat et al. and Bedient et al. reported no differences in the estimated blood loss between robotic and laparoscopic cases (38).
Interestingly, our series provide evidence that the use of robotics might enhance suturing performance during myomectomy. Indeed, the number of patients that receive at least a double-layers suturing of the uterine defect was higher during RALM than using laparoscopy. Similarly, surgeons decided to perform a three-layers suturing only when they used robotics. However, the increased number of suture during RALM did not induce an increase of operating time that resulted not different between groups. This finding supports the concept that technical advantages of robotics might improve the quality of uterine surgery during myomectomy. It has been previously demonstrated that RALM is a feasible technique for removal and suturing of deep intramural myomas unfavorably localized for traditional laparoscopy. Similarly, Bedient et al. believe that robotic surgery improved fibroid enucleation and layered closure of the uterine incisions when compared with laparoscopy and it provided an unsurpassed level of surgeon comfort. However, the available surgical series evidenced the laparoscopic myomectomies performed with the assistance of the robot might be longer compared with standard LM. This additional surgical time has been mainly attributed to assembly and disassembly of the robot and substituting the instrumentation for the robotic arms during the procedure. Our series
showed that docking time did not increased total robotic operating time in comparison with LM, when surgeons’ experience with two new technical instrumentations was equally comparable. This finding could be related, at least in part, to the reduced surgeons’ dexterity during suturing at their beginning of laparoscopic experience. Similarly, the only conversions that we analyzed in our series were caused by uterine bleeding and relative difficulty to perform hemostasis suturing by laparoscopy. Multilayered closure of myometrium together with limited use of electrosurgery, is a recommendation to reduce the risk of uterine rupture following LM (39). The use of robotics could improve uterine wound healing and subsequent risk of rupture in case of deep intramural myomas or multiple uterine incisions. At the same time, robotics could allow a less skilled or experienced laparoscopist to perform safe suturing in a shorter time period. Robotics is the latest technological innovation. Learning curve and surgeon experience affect surgical results and the need to study outcomes of patients treated by surgeons who are equally competent in LM and RALM may be now difficult to accomplish. Comparing the initial surgeon experience using two difference technologies could reduce confounding factors, evidencing the proper role of technological innovation.
In conclusion, RALM is a promising new technique that may overcome many of the surgical limitations of conventional laparoscopy and although
the absence of haptic or tactile feedback may be viewed as a limitation, both the improved visualization and instrument dexterity in this approach overcome any difficulties that may be encountered with the dissection (40). Our data confirm that RALM may have some additional advantages compared to LM in term of bleeding and uterine suturing without compromise operation length, at least when surgeons were at the beginning of their experience for endoscopic treatment of symptomatic uterine
myomas. Moreover, our results demontrate a good fertility and pregnancy
outcomes after RALM also in comparison to that reported in literature after laparoscopy or laparotomy suggesting that RALM allows transposition of the classic abdominal myomectomy technique to the laparoscopic arena. It is safe and reproducible and, with the expected advancements of surgical robotics, has the potential to be adopted by more infertility specialists as part of their armamentarium for comprehensive reproductive care. However, additional randomized trials are needed to compare LM and RALM in term of fertility and obstetric outcome and to individualize possible specific indications for the different tecniques.
55
T
T
ABLES
ABLES
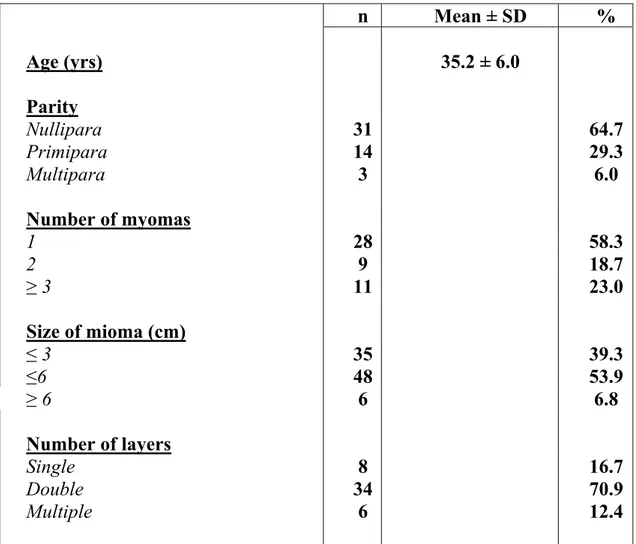
n Mean ± SD % Age (yrs) 35.2 ± 6.0 Parity Nullipara 31 64.7 Primipara 14 29.3 Multipara 3 6.0 Number of myomas 1 28 58.3 2 9 18.7 ≥ 3 11 23.0 Size of mioma (cm) ≤ 3 35 39.3 ≤6 48 53.9 ≥ 6 6 6.8 Number of layers Single 8 16.7 Double 34 70.9 Multiple 6 12.4
Tab. I Main characteristics of the 48 patients underwent robot-assisted laparoscopic myomectomy
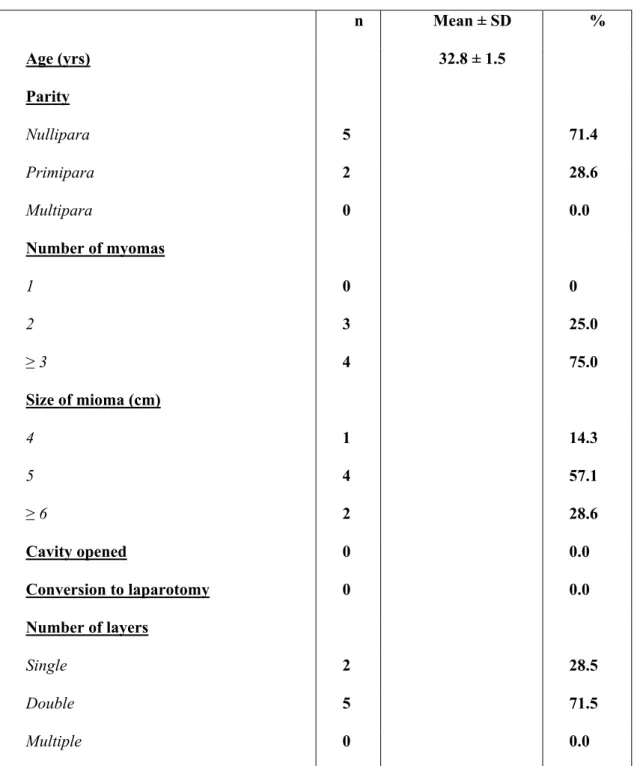
Tab. II Main characteristics of the 7 patients who became pregnants after
robot-assisted laparoscopic myomectomy
n Mean ± SD % Age (yrs) 32.8 ± 1.5 Parity Nullipara 5 71.4 Primipara 2 28.6 Multipara 0 0.0 Number of myomas 1 0 0 2 3 25.0 ≥ 3 4 75.0 Size of mioma (cm) 4 1 14.3 5 4 57.1 ≥ 6 2 28.6 Cavity opened 0 0.0 Conversion to laparotomy 0 0.0 Number of layers Single 2 28.5 Double 5 71.5 Multiple 0 0.0
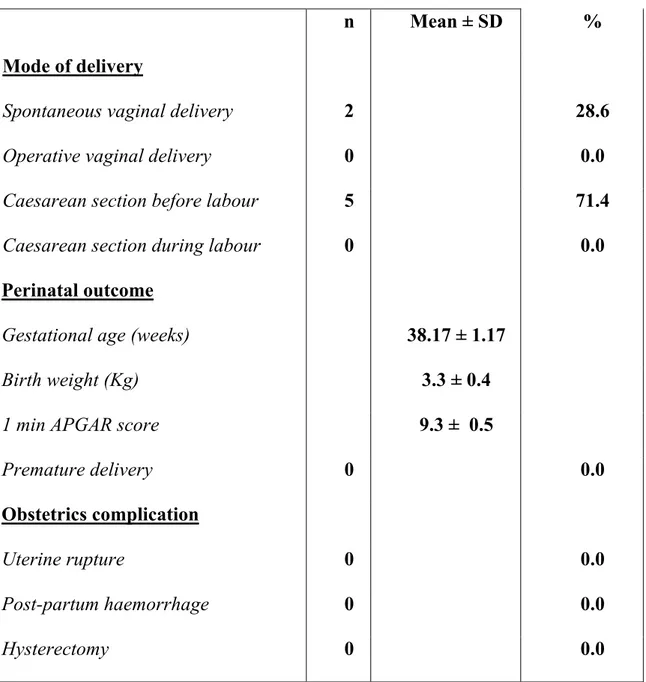
n Mean ± SD % Mode of delivery
Spontaneous vaginal delivery 2 28.6
Operative vaginal delivery 0 0.0
Caesarean section before labour 5 71.4
Caesarean section during labour 0 0.0
Perinatal outcome
Gestational age (weeks) 38.17 ± 1.17
Birth weight (Kg) 3.3 ± 0.4
1 min APGAR score 9.3 ± 0.5
Premature delivery 0 0.0
Obstetrics complication
Uterine rupture 0 0.0
Post-partum haemorrhage 0 0.0
Hysterectomy 0 0.0
Tab. III Delivery characteristics, maternal, and perinatal morbidity for the 7
at term deliveries after robotic-assisted laparoscopic myomectomy (RALM)
Characteristic LM (%) N=69 RALM (%) N=70 P value Age Mean 37,5 36,4 0,31 SD 6,78 5,95 Median 37,5 37 Range 22-54 20-49 BMI Mean 23,5 22,9 0,35 SD 3,89 3,24 Median 23,5 23 Range 17-34,5 18-35
Previous Abdominal Surgery
N° 15/69 (21,7) 12/70 (17,1) 0,52 Number of Fibroids N° 148 146 0,42 Mean 2,14 2,08 SD 1,39 1,68 Median 2 1 Range 1-8 1-11 Size of Fibroids (cm) Mean 4,32 4,26 0,33 SD 2,73 1,79 Median 4 4 Range 0,5-12 1-10
Weight of Fibroids (grams)
Mean 156,59 126,5 0,62
SD 115,85 54,47
Median 120 110
Range 15-580 20-380
Tab. IV Comparison between RALM and LM: Patients and fibroids
Laparoscopic
(%) Robotic (%) valueP
Operation Time (min) Mean 120,98 118,52 0,57 SD 45,49 43,59 Median 120 120 Range 30-210 60-275 Blood Loss (mL) Mean 232,74 138,42 0,006 SD 183,14 67,66 Median 190 130 Range 10-800 30-550 Hospital Stay (d) Mean 1,94 2,03 0,72 SD 0,376317561 1,068580872 Median 2 2 Range 1-3 1-7 Major Complications Bleeding N° 4/69 (5,8) 2/70 (2,8) 0,44 Conversions to Laparotomy N° 2/69 (2,9) 0/70 (0) 0,24
Tab. V Comparison between RALM and LM: Intraoperative and immediate
Myoma location Laparoscopic (%) Robotic (%) P value Subserosal 83/148 (56,1) 80/146 (58,2) 0,92 Intramural 41/148 (27,8) 58/146 (41,1) 0,04 Submucosal 1/148 (0,7) 1/146 (1,3) 1,00 Broad ligament 8/148 (5,4) 4/146 (2,8) 0,37 Peduncolated 15/148 (10,1) 3/146 (3,4) 0,005 Anterior 48/148 (32,4) 42/146 (28,7) 0,52 Posterior 55/148 (37,2) 66/146 (45,2) 0,19 Fundal 45/148 (30,4) 38/146 (26,1) 0,63 Suture layers One Layer 59/69 (85,5) 9/70 (12,8) <0.0001 Two Layer 10/69 (25,5) 54/70 (77,2) <0.0001 Three Layer 0/69 (0) 7/70 (10) 0.006
Tab. VI Comparison between RALM and LM: Myoma location and suture
R
R
EFERENCES
EFERENCES
1. Zmora O, Gervaz P, Wexner SD. Trocar site recurrence in laparoscopic surgery for colorectal cancer. Surg Endosc 2001;15:788-93
2. Himpens J, Lemans G, Cadiere GB. Telesurgical laparoscopic cholecystectomy. Surg Endosc 1998;12:1091
3. Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A random- ized prospective study of laparoscopy and laparotomy in the manage- ment of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109–14
4. Lo L, Pun TC, Chan S. Tubal ectopic pregnancy: an evaluation of laparoscopic surgery versus laparotomy in 614 patients. Aust N Z J Obstet Gynecol. 1999;39:185–7
5. Stylopoulos N, Rattner D. Robotics and ergonomics. Surg Clin North Am. 2003;83:1–12
6. Kho RM, Hilger WS, Hentz JG, Magtibay PM, Magrina JF. Robotic hysterectomy: technique and initial outcomes. Am J Obstet Gynecol. 2007 Jul;197(1):113.e1-4. Erratum in: Am J Obstet Gynecol. 2007 Sep;197(3):332
7. Sundaram BM, Kalidasan G, Hemal AK. Robotic repair of vesicovaginal fistula: case series of five patients. Urology. 2006 May;67(5):970-3
8. Magrina JF, Kho RM, Weaver AL, Montero RP, Magtibay PM. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008 Apr;109(1):86-91. Epub 2008 Feb 14.
9. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized com- parison with abdominal myomectomy. Hum Reprod. 2000;15:2663–2668
10. Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654–658
11. Doridot V, Dubuisson JB, Chapron C, et al. Recurrence of leiomyomata after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2001;8:495–500
12. Senapati S, Advincula AP. Surgical techniques: robot-assisted laparoscopic myomectomy with the daVinci surgical system. J Robotic Surg. 2007;1:69–74
13. Advincula AP, Song A, Burke W, Reynolds RK. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 2004;11:511–18
14. Advincula AP, Xu X, Goudeau S, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and costs. J Minim Invasive Gynecol. 2007;14:698–705
15. Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-as- sisted laparoscopic myomectomy compared with standard laparoscopic myomectomy: a retrospective matched control study [published online ahead of print March 31, 2008]. Fertil Steril. 2009;91:556–9
16. Farmer RM, Kirschbaum T, Potter D, Strong TH, Medearis AL. Uterine rupture during trial of labor after previous cesarean section, Am J Obstet Gynecol 1991 Oct;165 (4 Pt 1):996-1001
17. Lönnerfors C and Persson J. Pregnancy following robot-assisted laparoscopic myomectomy in women with deep intramural myomas. J.Acta Obstet Gynecol Scand 2011 Sep;90 (9):972-7. doi: 10.1111/j.1600-0412.2011.01207.x. Epub 2011 Jul 20
18. Hovsepian DM, Ratts VS, Rodriguez M, Huang JS, Aubuchon MG, Pilgram TK. A prospective comparison of the impact of uterine artery embolization, myomectomy and hysterectomy on ovarian function. J Vasc Interv Radiol. 2006 Jul;17(7):1111-5
19. Ascher-Walsh CJ and Capes TL. Robot assisted laparoscopic myomectomy in women with a limited number of mioma. J Minim Invasive Gynecol 2010 May-Jun;17(3):306-10. Epub 2010 Mar 19
20. Falcone T and Bedaiwy MA. Minimally invasive management of uterine fibroids. Curr Opin Obstet Gynecol. 2002 Aug;14(4):401-7 21. Jin C, Hu Y, Chen XC, Zheng FY, Lin F, Zhou K, Chen FD, Gu HZ.
Laparoscopic versus open myomectomy-a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009 Jul;145(1):14-21. Epub 2009 Apr 23
22. Garnet JD. Uterine rupture during pregnancy: an analysis of 133 patients. Obstet Gynecol. 1964;23:898–905
23. Palerme GR and Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94:571–6
24. Dubuisson JB, Fauconnier A, Deffarges JV, Norgaard C, Kreiker G, Chapron C. Pregnancy outcome and deliveries following laparoscopic myomectomy. Hum Reprod 2000; 15:869- 73
25. Seinera P, Farina C, Todros T. Laparoscopic myomectomy and subsequent pregnancy: results in 54 patients. Hum Reprod 2000; 15:1993-6
26. Ascher-Walsh CJ, Capes TL. Robot-assisted Laparoscopic Myomectomy Is an Improvement Over Laparotomy in Women with a Limited Number of Myomas. J Minim Invasive Gynecol. 2010 May-Jun;17(3):306-10. Epub 2010 Mar 19
27. Quaas AM, Einarsson JI, Srouji S, Gargiulo AR. Robotic myomectomy: a review of indications and tecniques. Rev Obstet Gynecol. 2010 Fall;3(4):185-91
28. Gargiulo AR and Nezhat C. Robot-assisted laparoscopy, natural orifice transluminal endoscopy, and single-site laparoscopy in reproductive surgery. Semin Reprod Med. 2011 Mar;29(2):155-68. Epub 2011 Mar 24
29. Nezhat C, Nezhat F, Silfen SL, Schaffer N, Evans D. Laparoscopic myomectomy. Int J Fertil 1991 Sep-Oct;36(5):275-80
30. Hasson HM The role of laparoscopy in myomectomy. Int J Fertil Menopausal Stud. 1996 May-Jun;41(3):276-9
31. Harris WJ. Uterine dehiscence following laparoscopic myomectomy. Obstet Gynecol 1992 Sep;80(3 Pt 2):545-6
32. Dubuisson JB, Chavet X, Chapron C, Gregorakis SS, Morice P. Uterine rupture during pregnancy after laparoscopic mypmectomy. Hum Reprod. 1995 Jun;10(6):1475-7
33. Miller CE, Johnston M, Rundell M. Laparoscopic myomectomy in the infertile woman. J Am Assoc Gynecol Laparosc 1996 Aug;3(4):525-32
34. Dubuisson JB, Chapron C. Uterine fibroids: place and modalities of laparoscopic treatment. Eur J Obstet Gynecol Reprod Biol. 1996 Mar;65(1):91-4. Review
35. Ostrzenski A. A new laparoscopic myomectomy technique for intramural fibroids penetrating the uterine cavity. Eur J Obstet Gynecol Reprod Biol. 1997 Aug;74(2):189-93
36. Quaas AM, Einarsson JI, Srouji S, Gargiulo AR. Robotic myomectomy: a review of indications and techniques. Rev Obstet Gynecol. 2010 Fall;3(4):185-91
37. Barakat EE, Bedaiiwy Ma, Zimberg S, Nutter B, Nosseir M, falcone T. Robot-assisted laparoscopic and abdominal myomectomy: a comparison of surgical outcomes. Obstet Gynecol. 2011 Feb;117 (2 Pt 1):256-65
38. Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009 Dec;201(6):566.e1-5
39. Risk factors for uterine rupture after laparoscopic myomectomy. Parker WH, Einarsson J, Istre O, Dubuisson JB. J Minim Invasive Gynecol. 2010 Sep-Oct;17(5):551-4
40. Mao SP, Lai HC, Chang FW, Yu MH, Chang CC. Laparoscopy-assisted robotic myomectomy using the da Vinci system. Taiwan J Obstet Gynecol. 2007 Jun;46(2):174-6.