ContentslistsavailableatScienceDirect
Annals
of
Hepatology
j o ur na l ho me p ag e:w w w . e l s e v i e r . e s / a n n a l s o f h e p a t o l o g y
Concise
reviews
Obesity
and
liver
cancer
Carlo
Saitta
a,∗,
Teresa
Pollicino
a,b,
Giovanni
Raimondo
a,caDivisionofClinicalandMolecularHepatology,DepartmentofInternalMedicine,UniversityHospitalofMessina,Italy bDivisionofClinicalandMolecularHepatology,DepartmentofHumanPathology,UniversityofMessina,Italy
cDivisionofClinicalandMolecularHepatology,DepartmentofClinicalandExperimentalMedicine,UniversityofMessina,Italy
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received12July2019 Accepted12July2019 Availableonline20August2019
Keywords:
Hepatocellularcarcinoma Intrahepaticcholangiocarcinoma Non-alcoholicfattyliverdisease Non-alcoholicsteatohepatitis Obesity
a
b
s
t
r
a
c
t
Obesityprevalenceisrapidlyincreasingworldwide.Itisassociatedwithhugeeconomicandhealthcosts duetoitsclinicalconsequences,whichincludesincreasedincidenceoftype2diabetes,cardiovascular diseases,anddevelopmentofdifferentmalignancies.Inparticular,obesityisanindependentriskfactor forthedevelopmentofhepatocellularcarcinoma(HCC).Indeed,obesityishighlyprevalentinpatients withnon-alcoholicfattyliverdisease(NAFLD)thatisbecomingoneofthemostfrequentcausesofliver diseaseworldwide.NAFLD-relatedHCCisthemostrapidlygrowingindicationforlivertransplantation inmanycountries.ThehighermortalityratesfoundinobeseHCCpatientsmightberelatednotonly toaworseoutcomeafterHCCtreatments,butalsotoadelayeddiagnosisrelatedtoalowfrequency andapoorerqualityofabdominalultrasonographysurveillancethatisthetestuniversallyusedforHCC screening.Givenitsdiffusion,obesityisfrequentlypresentinpatientswithchronicliverdiseasesrelated todifferentetiologies,andinthesecasesitmayincreasetheHCCrisk,actingasanadditionalco-factor. Indeed,growingevidencedemonstratesthatahealthydietandregularphysicalactivitymayhavean impactinreducingtheoverallHCCrisk.Finally,animpactofobesityinthedevelopmentofintrahepatic cholangiocarcinomahasbeenpostulated,butmoreextensivestudiesareneededtodefinitivelyconfirm thisassociation.
©2019Fundaci ´onCl´ınicaM ´edicaSur,A.C.PublishedbyElsevierEspa ˜na,S.L.U.Thisisanopenaccess articleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Obesityisrecognizedasaglobalpandemic,consideringthat
fig-ureshavealmosttripledworldwidesince1975.TheWorldHealth
Organizationestimatedthatobesepeopleover18yearsofagewere morethan650millionin2016(about13%oftheworld’sadult pop-ulation[11%ofmenand15%ofwomen])[1].Furthermore,over340
millionchildrenandadolescentsaged5–19wereestimatedtobe
overweightorobesein2016,andthisimpliesthatthenumberof
obesesubjectswillincreaseinthenearfuture,giventhatchildhood obesityislinkedtoamuchhigherchanceofadultobesity[2],with allthenegativeeffectsintermsofhealthcareresourcesneededto dealwithitsconsequences.Indeed,thedevelopmentof cardiovas-culardiseasesandoftype2diabetesmellitus(T2DM)arethebest
knownobesity-relatedcomplications.However,obesityisalsoan
establishedriskfactorforthedevelopmentofseveralmalignancies,
suchasbreast,colorectal,endometrium,esophagus,gallbladder,
kidneyandpancreascancers,aswellasbone-marrow
malignan-cies(Fig.1)[3–6].Overall,obesityincreasesmortalityratesinall
∗ Correspondingauthor.
E-mailaddress:[email protected](C.Saitta).
cancers,asshowedinastudyfromtheAmericanCancerSocietyin
whichsubjectswithabodymassindex(BMI)greaterthan40had
deathrateshigherthanthoseinnormalweightindividuals(52%
higherinmenand62%higherinwomen)[7].Onthebasisofthe
relativerisksandassociationsobservedinthisstudy,itwas
esti-matedthat14%ofalldeathsfromcancerinmenand20%inwomen
wereattributabletobeingoverweightorobeseinU.S.A.[7]. Alargebodyofevidenceshowsaparticularlystrongassociation betweenobesityandhepatocellularcarcinoma(HCC)[6,8–14],and thisconcisereviewwillfocusontheepidemiologicalandclinical aspectsofthelivercancersinobesepeople.
2. Theburdenofobesity-associatedHCC
HCCisthefifthmostfrequentcancerandthesecondleading
causeofcancer-relatedmortalityworldwideinmen[15].A
con-stantlyincreasingtrendofHCCincidenceandmortalityhasbeen
observedinU.S.A. andmany Europeancountries. In U.S.A.,HCC
showsanincidenceincreasingby4.5%annually,anditisreported tobethemostrapidlygrowingcauseofcancer-relateddeaths[16]. Mostcasesof HCCariseinthecontextoflivercirrhosis,mainly duetochronichepatitisBvirus(HBV)andchronichepatitisCvirus (HCV)infectionsand/orheavyalcoholdrinking[17].ChronicHBV
https://doi.org/10.1016/j.aohep.2019.07.004
1665-2681/©2019Fundaci ´onCl´ınicaM ´edica Sur,A.C.PublishedbyElsevierEspa ˜na,S.L.U.Thisisan openaccessarticleundertheCCBY-NC-NDlicense(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Breast cancer
Kidney cancer
Endometrial cancer
Hematological malignancies
Esophageal cancer Gallbladder cancer Colorectal cancer Pancreatic cancer LIVER HCC ICC? OBESITY
Fig.1. Malignanciesassociatedwithobesity.Abbreviations:HCC,hepatocellular car-cinoma.ICC,intrahepaticcholangiocarcinoma.
infectionis the leading causeof HCC worldwide and the main
riskfactorforHCCdevelopmentineasternAsiaandsub-Saharan
Africa,whilechronicHCVinfectionremainsanimportantrisk fac-torinU.S.A.andEurope.Indeed,thereisthehopethatthecure ofHCVbydirectantiviralagents(DAA)andtheefficacious treat-mentsavailableagainstHBVwillreducetheratesofHCCincidence
andmortality.However,despitetheseveryimportantadvancesin
thetreatmentofviralhepatitis,livercancerisstillaglobally rec-ognizedhealthcareissue.Possibly,theimpactontheglobalHCC
incidenceratesoftheHCVcurebyDAAwillbecomemoreevident
overtime[18].Nevertheless,thereisclearevidenceofaconstant riseofHCCincidencethatiscommonlyattributedtotheparallel
increaseofnon-alcoholic fattyliverdisease(NAFLD),which has
becometheleadingcauseof liverdiseaseinmany areasofthe
world[19],withaprevalencereaching30%ofthegeneral
popu-lation[20,21].Inabout20%ofNAFLDsubjects,liverhistologymay showfeaturesofhepatitis–non-alcoholicsteatohepatitis(NASH)
–characterizedbythepresenceofnecro-inflammationandoften
offibrosis,potentiallyevolvingtowardcirrhosisandHCC[22,23]. NASH-relatedHCCisthemostrapidlygrowingindicationforliver transplantationinU.S.A.[24].
NAFLDis stronglyassociatedwithfeaturesof metabolic
syn-drome(MS),andtheprobabilityofdevelopingNASHincreaseswith
thenumberofriskfactorsinvolved(obesity,T2DM,hypertension
anddyslipidaemia)[25,26].Inameta-analysisstudyofglobal
epi-demiology,obesityprevalenceamongpatientswithNAFLDwas
estimatedat51.3%,whereasamongpatientswithNASHitwas
cal-culatedtobe81.8%[27].Notsurprisingly,givingthecontinuous
riseofobesityprevalence,NAFLDhasbecomethemostcommon
etiologicfactorofliverdiseaseworldwide[21].Thevery strong
associationbetweenHCCandMShasbecomemoreevidentinthe
lasttwodecades.Ofnote,obesityinitselfhasbeenshowntobean independentriskfactorofHCC[8].
Inacohortofabout18,000London-basedgovernment
employ-ees,followedupforamedianof28years,thehazardratio(HR)
ofHCCdevelopmentinobeseindividualswas3.76[9].A
meta-analysisofcohortstudiesassessingtheassociationbetweenobesity
andlivercancershowedthatoverweight orobesesubjectshad
a17%and 89%increasedrisk ofHCC,respectively,comparedto
normalweightindividuals[10].Anothermeta-analysis,evaluating prospectiveobservationalstudiesassessingthestrengthof associ-ationsbetweenBMIanddifferentsitesofcancer,showedthatthe riskoflivercancerincreasesbyabout25%foreach5kg/m2increase ofBMI[11].Anadditionalmeta-analysisevaluated21prospective studies,showinga relativerisk ofHCCof1.39foreach5kg/m2
increaseinBMI,withthemostpronouncedriskincreaseamong
individualswithaBMI>32kg/m2[12].Asystematicreviewof10
cohortstudiesconductedin2010showedapositiveassociation
betweenobesityandrisk ofHCC inthemajority ofthestudies
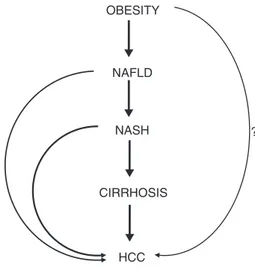
OBESITY NAFLD NASH CIRRHOSIS HCC ?
Fig.2.SchematicrepresentationofthephasesconnectingobesitytoHCC.The questionmark(?)highlightsthehypothesisofahepatocarcinogenicroleof obe-sityindependentofthenon-alcoholicfattyliverdisease.Abbreviations:NAFLD, non-alcoholicfattyliverdisease.NASH,non-alcoholicsteatohepatitis.HCC, hepa-tocellularcarcinoma.
analyzed[13].Thenecessitytotakeactioninordertoreducethe spreadofchildhoodobesityisfurtherhighlightedbyaDanishstudy, revealingthathigherBMIinchildhoodincreasestheriskofprimary
livercancerinadults,withaHRofHCCdevelopmentof1.36per
unitincreaseinBMI[14].Despitethefactthatsomeofthesestudies havelimitations(particularlyrelatedtotheabsenceofdataonthe presenceofco-factorsofliverdamage[i.e.,hepatitisvirus infec-tionsand/oralcoholintake]),thelinkbetweenobesityandHCCis verystrongandunanimouslyconsideredunquestionable(Fig.2).
3. MortalityinobesepatientswithHCC
A milestoneepidemiology paperpublishedby Calleet al.in
2003,whichevaluatedmorethan900,000U.S.adults,showedthat
therelativeriskoflivercancer-relatedmortalityinsubjectswitha BMIbetween30and34.9kg/m2andinthosewithaBMI>35kg/m2
was1.9 and4.5 timeshigher,respectively, thanthat ofnormal
weight individuals [7].Similarly, thepreviously reportedpaper
analyzingalargeEnglishpopulation,showedthatobese
individ-ualshadalivercancer-relatedmortality4timeshigherthanthat ofnormalweightsubjects[9].Recently,ameta-analysisincluding 9observationalstudiesforatotalnumberofmorethan1,500,000 individualsshowedthatobesesubjectshadatwo-foldincreaseof
HCC-relatedmortality.Suchassociationwasmoreevidentinmen
andWesternpopulations[28].AstudyconductedinUKshowed
that,between2000and2010,mortalityforlivercancerrose 1.8-fold,witha10-foldincreaseinHCCassociatedwithNAFLDandwith obesityandothermetabolicriskfactorsbeingpresentin66%ofthe cases,independentlyoftheunderlyingetiologyoftheliverdisease
[29].Obesitymayalsohaveanimpactontheoutcomeafterany
treatmentofthecancernodules.Inastudyanalyzingacohortof159 HCCpatientstreatedwithorthotopiclivertransplantation(OLT),an increasedincidenceoflife-threateningcomplicationswasfoundin
overweightandobesepatientscomparedtonormalweight
sub-jects,aswellasadoubledincidenceofHCCrecurrenceafterOLT (15%vs.7%)[30].InaU.S.retrospectivecohortof342HCCpatients
whounderwentOLTbetween1999and2010,aBMIhigherthan
30wasapredictorofHCCrecurrence,microvascularinvasionand
ofa pooroverallsurvival (OS),doublingthemortalityrisk after
transplantation [31].Anotherstudyperformedin Japanshowed
lowersurvivalratesinoverweightorobesepatientsundergoing
hepatectomyforrecurrentHCCcomparedtoindividualswith
negativelyinfluencestheoutcomeafterHCCtreatments,andthis maybeduetoahigherriskofpostoperativecomplications[33]. However,inthiscontextitisworthytobementionedthatseveral
studiesshowedthatHCCpatientswithhigherBMIhaveabetter
outcomeafterhepatectomythanthosewithlowerBMI[34–36],
whereasotherstudiesdidnotfindanydifferenceinoutcomesafter surgeryinpatientswithdifferentBMI[37–39].
4. HCCsurveillanceinobesepatients
Bydefinition,theaimofasurveillanceprogramistoachievea reductioninthemortalityratesofthetargetdiseasethroughan earlydiagnosis,anditshouldbecost-efficient.Theusefulnessand theapplicabilityofthediagnostictestsusedforsurveillancedepend onmanyfactors,suchastheincidenceofthediseaseinthetarget populationandtheavailabilityofthetestitself.Patientsathighrisk
ofdevelopingHCCshouldbeincludedinasurveillanceprogram
thatessentiallyconsistsofanupperabdominalultrasonography
performedeverysix months[40,41].Patients withcirrhosis are
atthehighestriskofHCC(infact,morethan80%ofHCCarisein thecontestofadvancedliverdisease)[42].However,grayareas
exist,andthere isthepossibilityof thedevelopmentofHCC in
non-cirrhoticlivers,inparticularincaseswithHBVinfectionand
incaseswithNAFLD.However,therealriskofHCCdevelopment
innon-cirrhoticNAFLDpatientsisstillunknown.Itisestimated
thatabout50%ofNASH-associatedHCCariseinthecontextofa
non-cirrhoticliver[43,44],butsuchincidenceof livercanceris
considerednot sufficienttopromoteanactiveultrasonographic
surveillance,consideringtheverylargeprevalenceof NAFLDin
thegeneralpopulation.Thus,timelydiagnosisofHCCarisingin
aNAFLDcontextisatruechallengeforthehepatologists,and
obe-sity–veryfrequentlypresentinNAFLDpatients–makesitmore
difficult,becauseofahigherchanceofapoor-qualityultrasound examinationinobesesubjects.
InaU.S.studyexamining“Surveillance,EpidemiologyandEnd
Results”(SEER)registriesbetween2004and2009,andincluding
almost5000HCCpatients,thosewithNAFLD-associatedliver
can-cerwereolder,hadshorterOSandhighertumor-relatedmortality thanpatients withHCC related tootheretiologies [45].This is
mainlyduetoadelayinthediagnosisduetouncertaintyinthe
surveillancebenefit.Theissuerelatedtothelackofsurveillance hasbeenassessedinastudyonU.S.veterans,showingthata
sig-nificantlyhigherproportionofpatientswithNAFLD-relatedHCC
(56.7%)didnotundergosurveillanceinthe3yearsprecedingHCC
diagnosis,compared withHCCpatientswithalcohol-(40.2%) or
HCV-relateddisease(13.3%)[46].Consequently,alowernumberof
patientswithNAFLD-relatedHCChadthechancetoreceive
tumor-specifictreatments[46].Aretrospectivecohortstudyconductedin theU.S.andincluding941patientsundergoingultrasound surveil-lanceforcirrhosisshowedthatobesepatientshad3–8-foldhigher
risk ofhaving aninadequate examination,with increasingBMI
leadingtoanincreasedriskoffailure.Infact,one-thirdofcirrhotics witha BMI>35 had a qualitativelyinadequate ultrasound [47]. Forthesereasons,thepossibilityofasurveillancewithcomputed
tomographyormagneticresonanceimaginghasbeenconsidered
forthesepatients,althoughitscost-effectivenesswouldbe imprac-tical.Indeed,thereisnoconsensusonwhatisthebestsurveillance
strategy– ifany– innon-cirrhoticobese patientswithNAFLD,
consideringthat theindividualriskof HCCdevelopmentis low,
particularlyifcomparedtotheriskincirrhoticsubjects[48,49].
Althoughgiventheactualincidenceratessurveillancecannotbe
recommendedinthissetting,itisundoubtedthatanefficacious
HCCriskstratificationinnon-cirrhoticobesesubjectswithNAFLD isanunmetneedatpresent.
5. Obesityasariskco-factorofHCCdevelopmentin chronicliverdiseases
Becauseofitsdiffusion,obesityisfrequentlypresentinpatients withchronichepatitisBorCorwithalcoholicliverdisease,and itisconsideredanadditionalHCCriskfactorinthesesubjects.A
Taiwanesepopulation-basedstudy,conductedinabout24,000
sub-jects,revealedthatobesitywasassociatedwitha4-foldriskofHCC inanti-HCVpositivesubjects,a1.36-foldriskinHBV-infected,and a2-foldriskinsubjectswithoutviralinfections[50].Furthermore,
whenobesityanddiabeteswerepresenttogether,such
associa-tioncausedamorethan 100-foldincreasedriskofHCC inboth
HBVand HCVinfected subjects,suggesting apossible
synergis-ticeffectofmetabolicfactorsandviralhepatitis[50].AJapanese studywhichenrolledabout1500patientswithchronichepatitisC
showedthatoverweightandobesitywereindependentriskfactors
ofHCC,withaHRof1.86and3.1,respectively[51].A retrospec-tivestudyanalyzingliverbiopsiesfromHBVinfectedindividuals, showedthathistologicallydetectedliversteatosiswasan indepen-dentriskfactorforHCC(HR7.3)[52].Similarly,thepresenceof
radiologically-assessedNAFLDwasshown tobeariskfactorfor
HCCdevelopmentinchronichepatitis BpatientsinwhomHBV
wassuppressedbymeansofantiviraltherapy[53].Asynergistic effectofobesityandalcoholintakehasbeenidentifiedinastudy
prospectivelyevaluatingaTaiwanesepopulationwithchronic
hep-atitisB,wheretheriskofincidentHCCincreasedinbothoverweight (HR2.4)andobese(HR2.9)alcoholabusers[54].Alarge prospec-tivestudyfromUKhighlightedtheroleofobesityinpatientswith HCCarisinginthecontextofliverdiseasescausedbyother etiolo-gies,withmetabolicriskfactorsbeingpresentinuptotwo-thirds ofpatientswithHCC[29].Aretrospectiveanalysisconductedin
theU.S.onabout20,000explantedliversshowedthatobesitywas
anindependentpredictorof HCCinpatientswithalcoholic
cir-rhosis[55].Similarly,aFrenchretrospectivestudyanalyzing110
patientswithalcoholiccirrhosiswhounderwentOLTfoundthata
previoushistoryofbeingoverweightorobeseincreasedtherisk
ofHCC(oddsratio[OR]6.2),andthatthecontemporarypresence
ofT2DMincreasedtheriskwithanadditionaleffect(OR9.1)[56]. InacohortofFrenchpatientswithwellcompensatedalcoholicor
HCVrelatedcirrhosis,thecontemporarypresenceofobesityand
T2DMsignificantlyincreasedtheriskofHCCdevelopment(HR6)
[57].Altogether,thesedataconfirmthatobesityisanimportant additionalplayerinthedevelopmentofHCCinpatientswithliver diseaseduetodifferentcauses.
6. PossibleinterventionsforreducingHCCriskinobese patientswithNAFLD
GiventhestronglinkbetweenobesityandHCC,every
interven-tionaimedatreducingtheBMIatindividuallevelshouldreduce
theriskofHCCdevelopment.Theimpactofdietaryfactorsand
physicalactivityonHCChavebeenrecentlyreviewed[58].
Grow-ingevidencedemonstratesthatahealthydietmayhaveanimpact
inreducingtheriskofHCCdevelopment.Ithasbeenshownthat
a fruit-rich diet reduces HCC risk, while a low vegetable
con-sumptionincreasesthepossibilitiesof developingprimaryliver
cancer[59,60].Indeed,agoodadherencetoaMediterraneandiet appearstobeassociatedwitha50%reductionofHCCincidence[61].
Moreover,epidemiologic studieshave demonstratedthat
physi-calactivityisabletoreducetheriskofdifferentcancers[62–66]. Inalargeprospectively-followedTaiwanesecohort,acorrelation
betweenareducedriskofHCCandthedegreeofphysical
activ-itywasobserved[67].AnNIHstudyonabout500,000individuals
providedsimilarresults,showinga significantdecreased riskof
ofphysicalactivity[68].Concerningpharmacologicinterventions,
metforminaswellasstatinshavebeenassociatedwitha
signifi-cantlyreducedriskofHCCindiabeticsanddysplipidaemicpatients
withNAFLD[69–74].However,studiesspecificallyfocusedonthe
useoftheabovedrugsinobeseindividualsarelacking.Bariatric
surgeryisawellrecognizedtreatmentofmorbidobesity,andit
hasbeenshowntoinducedisappearanceofNASHinabout85%
ofpatientsafteroneyearoffollowuppost-surgery[75].Thus,one mayspeculatethat–inthelong-term–thissurgicalapproachcould
bebeneficialinreducingtheriskofHCCdevelopmentinmorbidly
obesepatients.However,thelackoflong-termfollowup
investi-gationsdonotallow–atpresent–toconfirmthishypothesis.
7. Istherealinkbetweenobesityandcholangiocarcinoma?
Cholangiocarcinoma(CC)is a malignanttumorof thebiliary
tract,thesecond mostcommonprimaryliver cancerafterHCC
[76].CCisclassifiedbasedonanatomiclocationsasintra-(ICC)or extrahepatic(ECC),whichareconsideredtwodistinctphenotypes, differingintheirpresentation,naturalhistory,management,and probablyalsointheirpathogenesis.ICCissimilartoHCCinits pre-sentation,andbothareoftenclassifiedasprimarylivercancersin
epidemiologicstudies.
Atpresent, contrasting data are availableon a possiblelink
betweenobesityandCC[77–80].However,whenthestudieswere
limitedtotheICC,theresultsshowinganassociationwithobesity
appeartobemoreuniform.Indeed,thestartoftheobesity
pan-demicintheU.S.A.precededbyabout10yearstherapidincrease
ofICC incidenceobservedin the1980s in thatcountry[81]. In
addition,datafromtheSEERprogramshowedthatMSwas
sig-nificantlymorefrequentinpatientsdevelopingICC,andinthese
patientsobesitywasidentifiedasanindependentriskfactorofICC
[8].Alsoameta-analysisconfirmedthatobesityisanICCriskfactor
(OR1.6)[82].ArecentlypublishedpaperfromtheNational
Can-cerInstitutelinkedearlyadulthoodadipositytoICC.Inparticular,
itshowedthathigherBMIatage18 wasassociatedwitha34%
higherriskofsuccessiveICCdevelopment[83].Themostrecently publishedmeta-analysis,analyzingprospectivecohortsandnested case-controlstudies,revealedthatobesitywasassociatedwitha 49%increasedICCrisk[84].
8. Conclusions
Manyepidemiologicdatahaveidentifiedobesityasan
impor-tant risk factor for HCC development. Moreover, obesity is
associatedwithreducedsurvival inHCCpatients.Thismightbe
relatedtoalessefficacioussurveillancestrategyandaconsequent
delay in diagnosiswith more limited possibility of therapeutic
interventions,althoughthepossibilityofaworseoutcomeafter
curativetreatmentsinthesepatientscannotberuledout.Withthe growingepidemicofobesity,aparallelincreaseoftheprevalence ofNAFLDisforeseen,makingitthecandidatetobetheworldwide mostimportantriskfactorforHCCdevelopmentinthenearfuture.
Abbreviations
BMI bodymassindex
CC cholangiocarcinoma
DAA directantiviralagents
ECC extrahepaticcholangiocarcinoma
HR hazardratio
HBV hepatitisBvirus
HCV hepatitisCvirus
HCC hepatocellularcarcinoma
ICC intrahepaticcholangiocarcinoma
MS metabolicsyndrome
NAFLD non-alcoholicfattyliverdisease
NASH non-alcoholicsteatohepatitis
OR oddsratio
OLT orthotopiclivertransplantation
OS overallsurvival
SEER surveillanceepidemiologyandendresults
T2DM type2diabetesmellitus
Financialsupport
Thisresearchdidnotreceiveanyspecificgrantfromfunding
agenciesinthepublic,commercial,ornot-for-profitsectors.
Conflictsofinterest
Theauthorshavenoconflictsofinteresttodeclare.
References
[1]World Health Organization. Obesity and overweight. WHO; 2017, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
[2]SinghAS,MulderC,TwiskJW,vanMechelenW,ChinapawMJ.Trackingof childhoodoverweightintoadulthood:asystematicreviewoftheliterature. ObesRev2008;9:474–88.
[3]CalleEE,KaaksR.Overweight,obesityandcancer:epidemiologicalevidence andproposedmechanisms.NatRevCancer2004;4:579–91.
[4]Basen-EngquistK,ChangM.Obesityandcancerrisk:recentreviewand evi-dence.CurrOncolRep2011;13:71–6.
[5]BoeingH.Obesityandcancer–theupdate2013.BestPractResClinEndocrinol Metab2013;27:219–27.
[6]BhaskaranK,DouglasI,ForbesH,dos-Santos-SilvaI,LeonDA,SmeethL. Body-massindexandriskof22specificcancers:apopulation-basedcohortstudyof 5.24millionUKadults.Lancet2014;384:755–65.
[7]CalleEE,RodriguezC,Walker-ThurmondK,ThunMJ.Overweight,obesity,and mortalityfromcancerinaprospectivelystudiedcohortofU.S.adults.NEnglJ Med2003;348:1625–38.
[8]WelzelTM,GraubardBI, ZeuzemS,El-SeragHB,DavilaJA,McGlynnKA. MetabolicsyndromeincreasestheriskofprimarylivercancerintheUnited States:astudyintheSEER-Medicaredatabase.Hepatology2011;54:463–71.
[9]BattyGD,ShipleyMJ,JarrettRJ,BreezeE,MarmotMG,SmithGD.Obesityand overweightinrelationtoorgan-specificcancermortalityinLondon(UK): find-ingsfromtheoriginalWhitehallstudy.IntJObes2005;29:1267–74.
[10]LarssonSC,WolkA.Overweight,obesityandriskoflivercancer:ameta-analysis ofcohortstudies.BrJCancer2007;97:1005–8.
[11]RenehanAG,TysonM,EggerM,HellerRF,ZwahlenM.Body-massindexand incidenceofcancer:asystematicreviewandmeta-analysisofprospective observationalstudies.Lancet2008;371:569–78.
[12]WangY,WangB,ShenF,FanJ,CaoH.Bodymassindexandriskofprimaryliver cancer:ameta-analysisofprospectivestudies.Oncologist2012;17:1461–8.
[13]SaundersD,SeidelD,AllisonM,LyratzopoulosG. Systematicreview:the associationbetweenobesityandhepatocellularcarcinoma–epidemiological evidence.AlimentPharmacolTher2010;31:1051–63.
[14]BerentzenTL,GamborgM,HolstC,SorensenTI,BakerJL.Bodymassindexin childhoodandadultriskofprimarylivercancer.JHepatol2014;60:325–30.
[15]TorreLA,BrayF,SiegelRL,FerlayJ,Lortet-TieulentJ,JemalA.Globalcancer statistics,2012.CACancerJClin2015;65:87–108.
[16]WhiteDL,ThriftAP,KanwalF,DavilaJ,El-SeragHB.Incidenceofhepatocellular carcinomainall50UnitedStates,from2000through2012.Gastroenterology 2017;152:812–20.
[17]SingalAG,El-SeragHB.Hepatocellularcarcinomafromepidemiologyto pre-vention:translating knowledge into practice. ClinGastroenterol Hepatol 2015;13:2140–51.
[18]ChhatwalJ,KanwalF,RobertsMS,DunnMA.Cost-effectivenessandbudget impactofhepatitisCvirustreatmentwithsofosbuvirandledipasvirinthe UnitedStates.AnnInternMed2015;162:397–406.
[19]StepanovaM,DeAvilaL,AfendyM,YounossiI,PhamH,CableR,etal.Direct andindirecteconomicburdenofchronicliverdiseaseintheUnitedStates.Clin GastroenterolHepatol2017;15:759–66.
[20]WilliamsCD,StengelJ, AsikeMI,TorresDM,ShawJ, ContrerasM,etal. Prevalenceofnonalcoholicfattyliverdiseaseandnonalcoholicsteatohepatitis amongalargelymiddle-agedpopulationutilizingultrasoundandliverbiopsy: aprospectivestudy.Gastroenterology2011;140:124–31.
[21]YounossiZ,AnsteeQM,MariettiM,HardyT,HenryL,EslamM,etal.Global burdenofNAFLDandNASH:trends,predictions,riskfactorsandprevention. NatRevGastroenterolHepatol2018;15:11–20.
[22]EuropeanAssociationfortheStudyoftheLiver(EASL),EuropeanAssociationfor theStudyofDiabetes(EASD)&EuropeanAssociationfortheStudyofObesity (EASO).EASL-EASD-EASOClinicalPracticeGuidelinesforthemanagementof non-alcoholicfattyliverdisease.JHepatol2016;64:1388–402.
[23]EstesC,RazaviH,LoombaR,YounossiZ,SanyalAJ.Modelingtheepidemic ofnonalcoholicfattyliverdiseasedemonstratesanexponentialincreasein burdenofdisease.Hepatology2018;67:123–33.
[24]WongRJ,CheungR,AhmedA.Nonalcoholicsteatohepatitisisthemostrapidly growingindicationforlivertransplantationinpatientswithhepatocellular carcinomaintheU.S.Hepatology2014;59:2188–95.
[25]DixonJB,BhathalPS,O’BrienPE.Nonalcoholicfattyliverdisease:predictorsof nonalcoholicsteatohepatitisandliverfibrosisintheseverelyobese. Gastroen-terology2001;121:91–100.
[26]Sepulveda-VillegasM,RomanS,Rivera-I ˜niguezI,Ojeda-GranadosC, Gonzalez-AldacoK,Torres-ReyesLA,etal.Highprevalenceofnonalcoholicsteatohepatitis andabnormalliverstiffnessinayoungandobeseMexicanpopulation.PLoSOne 2019;14:e0208926.
[27]YounossiZM,KoenigAB,AbdelatifD,FazelY,HenryL,WymerM.Global epi-demiologyofnonalcoholicfattyliverdisease–meta-analyticassessmentof prevalence,incidence,andoutcomes.Hepatology2016;64:73–84.
[28]GuptaA,DasA,MajumderK,AroraN,MayoHG,SinghPP,etal.Obesity is independentlyassociated withincreasedrisk ofhepatocellular cancer-relatedmortality:asystematicreviewandmeta-analysis.AmJClinOncol 2018;41:874–81.
[29]DysonJ,JaquesB,ChattopadyhayD,LochanR,GrahamJ,DasD,etal. Hepato-cellularcancer:theimpactofobesity,type2diabetesandamultidisciplinary team.JHepatol2014;60:110–7.
[30]MathurA,FrancoES,LeoneJP,Osman-MohamedH,RojasH,KemmerN,etal. Obesityportendsincreasedmorbidityandearlierrecurrencefollowingliver transplantationforhepatocellularcarcinoma.HPB2013;15:504–10.
[31]SiegelAB,LimEA,WangS,BrubakerW,RodriguezRD,GoyalA,etal. Dia-betes,bodymassindex,andoutcomesinhepatocellularcarcinomapatients undergoinglivertransplantation.Transplantation2012;94:539–43.
[32]UtsunomiyaT,OkamotoM,KameyamaT,MatsuyamaA,YamamotoM,Fujiwara M,etal.Impactofobesityonthesurgicaloutcomefollowingrepeathepatic resectioninJapanesepatientswithrecurrenthepatocellularcarcinoma.World JGastroenterol2008;14:1553–8.
[33]RongX,WeiF,GengQ,RuanJ,ShenH,LiA,etal.Theassociationbetweenbody massindexandtheprognosisandpostoperativecomplicationsof hepatocel-lularcarcinoma:ameta-analysis.Medicine2015;94:e1269.
[34]MathurAK,GhaferiAA,SellK,SonnendayCJ,EnglesbeMJ,WellingTH. Influ-enceofbodymassindexoncomplicationsandoncologicoutcomesfollowing hepatectomyformalignancy.JGastrointestSurg2010;14:849–57.
[35]ItohS,IkedaY,KawanakaH,OkuyamaT,KawasakiK,EguchiD,etal.Theeffectof overweightstatusontheshort-termand20-youtcomesafterhepaticresection inpatientswithhepatocellularcarcinoma.JSurgRes2012;178:640–5.
[36]OkamuraY,MaedaA,MatsunagaK,KanemotoH,UesakaK.Negativeimpactof lowbodymassindexonsurgicaloutcomesafterhepatectomyfor hepatocellu-larcarcinoma.JHepatobiliaryPancreatSci2012;19:449–57.
[37]NishikawaH,ArimotoA,WakasaT,KitaR,KimuraT,OsakiY.Therelation betweenobesityandsurvivalaftersurgicalresectionofhepatitisCvirus-related hepatocellularcarcinoma.GastroenterolResPract2013;2013:430438.
[38]ItohS,ShirabeK,MatsumotoY,YoshiyaS,MutoJ,HarimotoN,etal.Effect ofbodycompositiononoutcomesafterhepaticresectionforhepatocellular carcinoma.AnnSurgOncol2014;21:3063–8.
[39]LiuXY,XuJF.Liverresectionforyoungpatientswithlargehepatocellular carci-noma:asinglecenterexperiencefromChina.WorldJSurgOncol2014;12:175.
[40]EuropeanAssociationfortheStudyoftheLiver.EASLclinicalpractice guide-lines:managementofhepatocellularcarcinoma.JHepatol2018;69:182–236.
[41]MarreroJA,KulikLM,SirlinCB,ZhuAX,FinnRS,AbecassisMM,etal.Diagnosis, staging,andmanagementofhepatocellularcarcinoma:2018practice guid-ancebytheAmericanAssociationfortheStudyofLiverDiseases.Hepatology 2018;68:723–50.
[42]FornerA,ReigM,BruixJ.Hepatocellularcarcinoma.Lancet2018;391:1301–14.
[43]PiscagliaF,Svegliati-BaroniG,BarchettiA,PecorelliA,MarinelliS,TiribelliC, etal.Clinicalpatternsofhepatocellularcarcinomainnonalcoholicfattyliver disease:amulticenterprospectivestudy.Hepatology2016;63:827–38.
[44]ParadisV,ZalinskiS,ChelbiE,GuedjN,DegosF,VilgrainV,etal. Hepatocel-lularcarcinomasinpatientswithmetabolicsyndromeoftendevelopwithout significantliverfibrosis:apathologicalanalysis.Hepatology2009;49:851–9.
[45]YounossiZM, OtgonsurenM,HenryL,VenkatesanC,MishraA,ErarioM, etal.Associationofnonalcoholicfattyliverdisease(NAFLD)with hepatocel-lularcarcinoma(HCC)intheUnitedStatesfrom2004to2009.Hepatology 2015;62:1723–30.
[46]MittalS,SadaYH,El-SeragHB,KanwalF,DuanZ,TempleS,etal.Temporal trendsofnonalcoholicfattyliverdisease-relatedhepatocellularcarcinomain theveteranaffairspopulation.ClinGastroenterolHepatol2015;13:594–601.
[47]SimmonsO,FetzerDT,YokooT,MarreroJA,YoppA,KonoY,etal.Predictors ofadequateultrasoundqualityforhepatocellularcarcinomasurveillancein patientswithcirrhosis.AlimentPharmacolTher2017;45:169–477.
[48]Kawamura Y, Arase Y, Ikeda K,SekoY, Imai N,Hosaka T,et al. Large-scalelong-termfollow-upstudyofJapanesepatientswithnon-alcoholicfatty liverdiseasefortheonsetofhepatocellularcarcinoma.AmJGastroenterol 2012;107:253–61.
[49]AraseY,KobayashiM,SuzukiF,SuzukiY,KawamuraY,AkutaN,etal.Difference inmalignanciesofchronicliverdiseaseduetonon-alcoholicfattyliverdisease orhepatitisCinJapaneseelderlypatients.HepatolRes2012;42:264–72.
[50]ChenCL,YangHI,YangWS,LiuCJ,ChenPJ,YouSL,etal.Metabolicfactorsand riskofhepatocellularcarcinomabychronichepatitisB/Cinfection:afollow-up studyinTaiwan.Gastroenterology2008;135:111–21.
[51]OhkiT,TateishiR,SatoT,MasuzakiR,ImamuraJ,GotoT,etal.Obesityisan independentriskfactorforhepatocellularcarcinomadevelopmentinchronic hepatitisCpatients.ClinGastroenterolHepatol2008;6:459–64.
[52]ChanAW,WongGL,ChanHY,TongJH,YuYH,ChoiPC,etal.Concurrentfatty liverincreasesriskofhepatocellularcarcinomaamongpatientswithchronic hepatitisB.JGastroenterolHepatol2017;32:667–76.
[53]ChoH,ChangY,LeeJH,ChoYY,NamJY,LeeYB,etal.Radiologicnonalcoholic fattyliverdiseaseincreasestheriskofhepatocellularcarcinomainpatients withsuppressedchronichepatitisB.JClinGastroenterol2019,inpress.
[54]LoombaR,YangH,SuJ,BrennerD,IloejeU,ChenCJ.Obesityandalcohol syn-ergizetoincreasetheriskofincidenthepatocellularcarcinomainmen.Clin GastroenterolHepatol2010;8:891–8.
[55]NairS,MasonA,EasonJ,LossG,PerrilloRP.Isobesityanindependentriskfactor forhepatocellularcarcinomaincirrhosis?Hepatology2002;36:150–5.
[56]PaisR,LebrayP,RousseauG,CharlotteF,EsselmaG,SavierE,etal.Nonalcoholic fattyliverdiseaseincreasestheriskofhepatocellularcarcinomainpatients withalcohol-associatedcirrhosisawaitinglivertransplants.ClinGastroenterol Hepatol2015;13:992–9.
[57]N’KontchouG,PariesJ,HtarMT,Ganne-CarrieN,CostentinL,Grando-Lemaire V,etal.Riskfactorsforhepatocellularcarcinomainpatientswithalcoholicor viralCcirrhosis.ClinGastroenterolHepatol2006;4:1062–8.
[58]SaranU,HumarB,KollyP,DufourJF.Hepatocellularcarcinomaandlifestyles. JHepatol2016;64:203–14.
[59]MandairDS,RossiRE,PericleousM,WhyandT,CaplinM.Theimpactofdiet andnutritioninthepreventionandprogressionofhepatocellularcarcinoma. ExpertRevGastroenterolHepatol2014;8:369–82.
[60]Yu MW, HsiehHH, Pan WH,Yang CS,Chen CJ. Vegetableconsumption, serumretinollevel,andriskofhepatocellularcarcinoma.CancerRes1995;55: 1301–5.
[61]TuratiF,TrichopoulosD,PoleselJ,BraviF,RossiM,TalaminiR,etal. Mediter-raneandietandhepatocellularcarcinoma.JHepatol2014;60:606–11.
[62]WuY,ZhangD,KangS.Physicalactivityandriskofbreastcancer:a meta-analysisofprospectivestudies.BreastCancerResTreat2013;137:869–82.
[63]Robsahm TE,AagnesB,HjartakerA,LangsethH,BrayFI, LarsenIK.Body massindex,physicalactivity,andcolorectalcancerbyanatomicalsubsites: asystematicreviewandmeta-analysisofcohortstudies.EurJCancerPrev 2013;22:492–505.
[64]SunJY,ShiL,GaoXD,XuSF.Physicalactivityandriskoflungcancer:a meta-analysisofprospectivecohortstudies.AsianPacJCancerPrev2012;13:3143–7.
[65]BuffartLM,SinghAS,vanLoonEC,VermeulenHI,BrugJ,ChinapawMJ.Physical activityandtheriskofdevelopinglungcanceramongsmokers:ameta-analysis. JSciMedSport2014;17:67–71.
[66]PijpeA,MandersP,BrohetRM,ColleeJM,VerhoefS,VasenHF,etal. Physi-calactivityandtheriskofbreastcancerinBRCA1/2mutationcarriers.Breast CancerResTreat2010;120:235–44.
[67]WenCP,LinJ,YangYC,TsaiMK,TsaoCK,EtzelC,etal.Hepatocellular carci-nomariskpredictionmodelforthegeneralpopulation:thepredictivepower oftransaminases.JNatlCancerInst2012;104:1599–611.
[68]BehrensG,MatthewsCE,MooreSC,FreedmanND,McGlynnKA,EverhartJE, etal.Theassociationbetweenfrequencyofvigorousphysicalactivityand hep-atobiliarycancersintheNIH-AARPDietandHealthStudy.EurJEpidemiol 2013;28:55–66.
[69]LeeMS,HsuCC,WahlqvistM,TsaiHN,ChangYH,HuangYC.Type2diabetes increasesandmetforminreducestotal,colorectal,liverandpancreaticcancer incidencesinTaiwanese:arepresentativepopulationprospectivecohortstudy of800,000individuals.BMCCancer2011;11:20.
[70]LaiSW,ChenPC,LiaoKF,MuoCH,LinCC,SungFC.Riskofhepatocellular car-cinomaindiabeticpatientsandriskreductionassociatedwithanti-diabetic therapy:apopulation-basedcohortstudy.AmJGastroenterol2012;107:46–52.
[71]ChenHP,ShiehJJ,ChangCC,ChenTT,LinJT,WuMS,etal.Metformindecreases hepatocellularcarcinomariskinadose-dependentmanner:population-based andinvitrostudies.Gut2013;62:606–15.
[72]SinghS,SinghPP,SinghAG,MuradMH,SanchezW.Anti-diabeticmedications andtheriskofhepatocellularcancer:asystematicreviewandmeta-analysis. AmJGastroenterol2013;108:881–91.
[73]El-SeragHB,JohnsonML,HachemC,MorganaRO.Statinsareassociatedwith areducedriskofhepatocellularcarcinomainalargecohortofpatientswith diabetes.Gastroenterology2009;136:1601–8.
[74]DongiovanniP,PettaS,MannistoV,MancinaRM,PipitoneR,KarjaV,etal. Statinuseandnon-alcoholicsteatohepatitisinatriskindividuals.JHepatol 2015;63:705–12.
[75]LassaillyG,CaiazzoR,BuobD,PigeyreM,VerkindtH,LabreucheJ,etal.Bariatric surgeryreducesfeaturesofnonalcoholicsteatohepatitisinmorbidlyobese patients.Gastroenterology2015;149:379–88.
[76]CarriagaMT,HensonDE.Liver,gallbladder,extrahepaticbileducts,and pan-creas.Cancer1995;75:171–90.
[77]WelzelTM,GraubardBI,El-SeragHB,ShaibYH,HsingAW,DavilaJA,etal.Risk factorsforintrahepaticandextrahepaticcholangiocarcinomaintheUnited States:a population-basedcase–controlstudy.ClinGastroenterolHepatol 2007;5:1221–8.
[78]GraingeMJ,WestJ,Solaymani-DodaranM,AithalGP,CardTR.Theantecedents ofbiliarycancer:aprimarycarecase–controlstudyintheUnitedKingdom.Br JCancer2009;100:178–80.
[79]WelzelTM,MellemkjaerL,GloriaG,SakodaLC,HsingAW,ElGhormliL,etal. Riskfactorsforintrahepaticcholangiocarcinomainalow-riskpopulation:a nationwidecase–controlstudy.IntJCancer2007;120:638–41.
[80]ChaiteerakijR,YangJD,HarmsenWS,SlettedahlSW,MettlerTA,Fredericksen ZS,etal.Riskfactorsforintrahepaticcholangiocarcinoma:associationbetween metforminuseandreducedcancerrisk.Hepatology2013;57:648–55.
[81]SahaSK,ZhuAX,FuchsCS,BrooksGA.Forty-yeartrendsin cholangiocar-cinomaincidenceintheU.S.:intrahepaticdiseaseontherise.Oncologist 2016;21:594–9.
[82]PalmerWC,PatelT.Arecommonriskfactorsinvolvedinthepathogenesisof primarylivercancers?Ameta-analysisofriskfactorsforintrahepatic cholan-giocarcinoma.JHepatol2012;57:69–76.
[83]YangB,PetrickJL,KellySP,GraubardBI,FreedmanND,McGlynnKA.Adiposity acrosstheadultlifecourseandincidenceofprimarylivercancer:theNIH–AARP cohort.IntJCancer2017;141:271–8.
[84]PetrickJL,ThistleJE,Zeleniuch-JacquotteA,ZhangX,Wactawski-WendeJ,Van DykeAL,etal.BodyMassIndexdiabetesandintrahepaticcholangiocarcinoma risk:theLiverCancerPoolingProjectandMeta-analysis.AmJGastroenterol 2018;113:1494–505.