Partial
Purification and Characterization
of
anEscherichia coli
Toxic
Factor
That Induces
Morphological Cell Alterations
A.CAPRIOLI,l* V. FALBO,' L.G. RODA,2 F. M.RUGGERI,' ANDC. ZONA3
Laboratorio di Ultrastrutture, Istituto Superiore diSanita,' andCattedra di Tecnica Fisiologica, Universita degli Studi di Roma,3 Rome; and Laboratorio di Farmacologia, Universita degli Studi diAncona, Ancona,2
Italy
Received 20 July 1982/Accepted14December 1982
Afactor produced by several strains ofEscherichia coli isolated from
enteritis-affected children has been shown to produce both a necrotizing effect onrabbit
skin and striking morphological alterations onCHO, Vero, and HeLa cells. The same strains were foundto have hemolytic activity on sheep erythrocytes. The toxic, cell-altering factor was demonstratedto bedifferent from both heat-labile and heat-stable enterotoxins and from Vero toxin.The main effect induced bythe
isolated factoroncultured cells wasthe formation of large multinucleated cells.
The partial purification achieved suggests that the same factor (most likely a
protein with amolecular weight of 70,000 to80,000) is responsible for toxicand
cell-altering activities, whereas a different molecular species is responsible for
hemolytic activity.
A number of Escherichia coli strains are known to produce heat-labile enterotoxin (LT) and heat-stable enterotoxin, whose properties
and role in diarrheal disease have been widely investigated (1-4, 9, 11, 18, 19). A cytotoxin active onVerocells has also been described by Konowalchuk et al. (13, 14), and its role in humaninfection byenteropathogenicE.coli has
subsequently been discussed (17). In addition,
several E. coli strains involved in sepsis or
urinarytractinfectionshavebeenreportedtobe hemolysin producers (12).
Inthis paper we report thefindingof a new E.
coli factor, designated CNF, obtained from strains of clinical relevance and showing an
activity which is cytotoxic for HeLa cells and
necrotizingonrabbit skin. Wealsodescribethe
mainbiological properties andchemical
charac-teristics of CNF.
MATERIALSANDMETHODS
Bacterial strains. Atotal of 213 E.ccli strainstested fortoxicactivitywereisolated from stoolspecimensof 110children (age, 2 years andunder) hospitalized for acutediarrheal disease. Strainswereidentifiedbythe
API20Etechnique (LaBalme, LesGrottes, France).
The followingE. coli strains were used as controls: E9638 (LT') and E1106/0 (positive for Vero toxin), kindlysupplied byB. Rowe(PublicHealthLaboratory Service,London,UnitedKingdom);andISS132
(posi-tivefor heat-stable enterotoxin)and K-12 J62carring
thehemolysinplasmid p212, fromourlaboratory
col-lection. E. coli K-12 J53 (pro met) was used as
negative controlin allexperiments.
Culture conditions. Cultures were maintained on
nutrientagarslantsat25°C. Erlenmeyer flasks(250ml)
containing20 mlofTrypticasesoybroth(BBL Micro-biology Systems, Cockeysville, Md.)wereinoculated
with 104 bacterial cells from exponentially growing
cultures ofcontrol and teststrains. After incubationin a rotary shaker at 37°C for 18 h, the cultures were
centrifugedat 10,000 x gfor30min at 4°C, and the supernatants werefilteredthrough 0.45-,umMF mem-branes(Millipore Corp., Bedford,Mass.). Ifrequired, mitomycin C was added (0.5 ,ug/ml) 3 h after the
inoculationofthe cultures. To obtain higheramounts
ofLT(2),cultures weresonicatedinan icebathat an amplitude of14 p.mpeaktopeak (three strokes,20 s
each) in an MSE apparatus (MSE Scientific Instru-ments, Crawley, United Kingdom). The cell extracts
thusobtainedwerecentrifugedat20,000xg at4°C for
20 min, and supernatants were filtered as described above.
Cell culture assays. Chinese hamsterovary(CHO), Vero, and HeLacells, obtained from the American Type Culture Collection (Rockville, Md.), were
pas-sagedby trypsinization and grown as monolayers at 36°C in Eagle minimal essential medium (GIBCO Laboratories, Grand Island, N.Y.) with Earle salts. The medium was supplemented with 5% fetal calf serumand 1%nonessentialaminoacids(GIBCO). LT assays were carried out in microtiter plates (Falcon Plastics, Oxnard, Calif.)as describedby Guerrant et
al. (10). Bacterial extracts were tested in Vero and HeLa cultures by the same procedure. To obtain
monolayers in eight-chamber tissue culture slides
(Lab-Tek 4808; Lab-Tek Products, Naperville,
Ill.)
eachchamberwasseeded with about104cellsin 0.36 ml of minimal essential mediumcontaining 1% fetal
calfserum and then was inoculated with 0.04 ml of
bacterialsample.Afterincubationat36°C, monolayers
were fixed in methanol and stained with May Grun-wald-Giemsa(E.Merck, Darmstaadt, Federal
CELL-ALTERING E. COLI TOXIC FACTOR -.I 4. * .4 . * -.4 -
'I
a., 4S.,IF
;,.o" .9 a.', "1! Sb .--Wl*v-
_ 14~I
*wA..l
1AF I; I}'." * I.&M .1 .. i'll lld x. : !i t.It
4, _s,-w
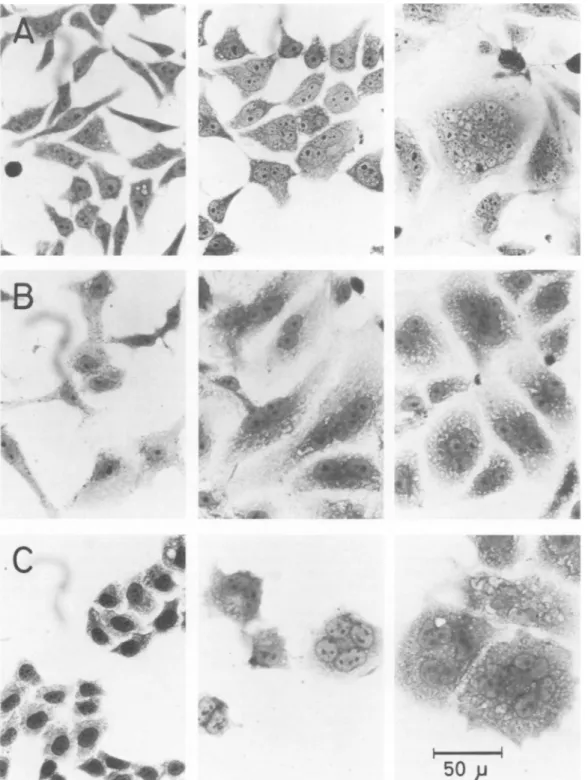
.' ; .AM a <¾./ ;.t C9Jr 4it.FIG. 1. ToxineffectonmonolayersofCHO(A), Vero (B). and HeLa(C)cells. From lefttoright,cells treated with: K-12J53 cell extract,18 h; ISS51 cell extract, 18 h: ISS51 cellextract, 3days.
lic ofGermany). To quantify toxin effects in HeLa
cultures,20microscopic fields(x400)foreach sample
wereexamined;viable cells (bytrypanblue exclusion) and cells containing one, two, or more nuclei were
counted. Data were expressed as cells per square
millimeter.Adenylate cyclase stimulationin CHO cell cultureswasevaluatedasdescribed by Guerrantetal.
(10); cAMP was determined by the protein binding
i_ . mkkI ..S6 . -.
C
4 VOL. 39, 1983 1301 -:.toi-041"
4w
.---& il. .IOr,
Al.
t IV' 1; I le Is .pl0
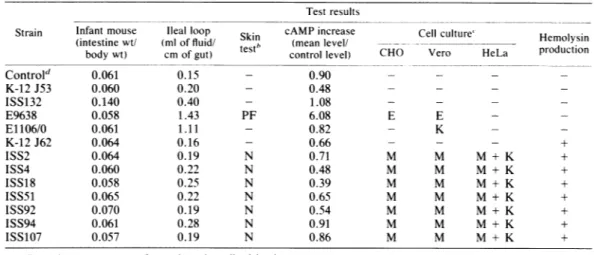
TABLE 1. Biologicaleffectsof cell extracts obtained from CNF-producing E. :oli strains"
Test results
Strain Infantmouse Ileal loop Skin cAMP increase Cell culture" Hemolysin
(intestinewt/ (mloffluid/
nb
(meanlevel/_odu
ctionbodywt) cmofgut) test control level) CHO Vero HeLa production
Control' 0.061 0.15 - 0.90 - - -K-12J53 0.060 0.20 - 0.48 - - -ISS132 0.140 0.40 - 1.08 - - -E9638 0.058 1.43 PF 6.08 E E -E1106/0 0.061 1.11 - 0.82 - K -K-12J62 0.064 0.16 - 0.66 - - - + ISS2 0.064 0.19 N 0.71 M M M + K + ISS4 0.060 0.22 N 0.48 M M M + K + ISS18 0.058 0.25 N 0.39 M M M + K + ISS51 0.065 0.22 N 0.65 M M M + K + ISS92 0.070 0.19 N 0.54 M M M + K + ISS94 0.061 0.28 N 0.91 M M M + K + ISS107 0.057 0.19 N 0.86 M M M + K +
Experimentswere performedasdescribed in the text. h - No effect;PF, vascular
response;
N.
necrosis.-.
Noeffect;
K,killing;
M. multinucleation; E,typical
morphologicalalterationsbyLT(elongation
inCHOcells,enlargementin Vero cells).
dUninoculatedTrypticase soy broth.
method with a reagent kit from the Radiochemical Centre(Amersham Corp.).
Serial dilutionsof eachsampleweretestedonHeLa cellculturestofollowevery stepofpurification. Data wereexpressedastoxic units permilliliter, determined fromthereciprocal ofthe lastdilution showing
detect-able morphological alteration.
Hemolysis test. Twodifferentprocedures were used.
(i)E. coli strains were tested on 5% sheep blood agar
plates, and afterovernightincubation (37°C), hemoly-sis halos were measured. (ii) Hemolytic activity on
fractionated samples was assayed by the following
procedure. Samples(100 ,ul each)of 5% washed sheep
erythrocytesandofthetestsample(adjustedto afinal concentration of0.9% NaCI)weremixedinglass test tubes (3 by 30 mm), incubated (37°C, 4 h), and
centrifuged. Hemoglobin release in the supernatant was evaluatedby absorbance at 540 nm.
Animal assays. To reveal LT, the ileal loop assay and skin test in rabbitswere carriedoutas described by Evansand co-workers (7, 8). The sucklingmouse
testfor heat-stable enterotoxin was performed bythe methodofDeanetal.(5). Necrotic reaction in rabbit skin wasevaluatedbyinspecting the ulceration inthe injection site.
Fractionationprocedure.High-pressuresteric exclu-sionchromatographywasperformedonacolumn (7.5
by 500 mm) of TSK G4000 SW (Toyo Soda Co., supplied byVarianAssociates, PaloAlto, Calif.)on a
Perkin Elmer series2/1 chromatographequippedwith
an LC 75 variable wavelength detector. The steric exclusion column was calibrated with the following proteins as standards: bovine serum albumin
(mono-mer and dimer), egg albumin, chymotrypsinogen, myoglobin (from horsehearts). Astandardcurve was
fitted withaleast-squares computerprogram.
Detection methods. Proteincontentwasfollowedby absorbance at 280 nm and by a microbiuret method (16). Amino acidanalysesofhydrolyzed samples(24 h in constant boiling 6 N HCI at 110°C underreduced
pressure) wereperformedon aCarlo Erbamodel3A29 amino acidanalyzer (CarloErbaStrumentazione, Cor-sico, Italy)equippedwith ninhydrin detection.
Electrophoresis. Electrophoresis was performed by
the method of Laemmli (15) on an LKB Uniphor
apparatus (LKBInstruments, Bromma, Sweden). RESULTS
Sonicated cell extracts of isolated E. coli strains were tested for LT in CHO cultures. Four of these proved to be LT producers,
whereas seven others (ISS2, 4, 18, 51, 92, 94, 107) induced unexpected morphological cell al-terations consisting of remarkable cell
enlarge-ment and the presenceof morethanonenucleus in each cell (Fig. 1). Similar alterations were
1000 / 800-E
,
E600- 400-,----'W ---200' 'N 20 40 60 80 100 time( hours)FIG. 2. Survival of HeLa cells with time. Cells treated with ISS51 cell extract (---), K-12 J53 cell
extract (- - -), and medium control ( ). Experi-ment wasperformedasdescribed in the text.
CELL-ALTERING E. COLI TOXIC FACTOR 1303
-60-
' U 40- i' 20-10 20 30 40 50 60 tim*(hours)FIG. 3. Relative frequencies of HeLa cells with
one(---),two(- - -), andmore( )nuclei,by
timeofexposurewithISS51 cellextract.Percentages ofmononucleated cells in controlmonolayerstreated
withK-12 J53cellextract wereconstantlyabout97%.
observedwhencultures of the samestrainswere treated withmitomycin C,thus inducingrelease
oftheinner cellcontents.Theoccurrenceof cell
lysing was confirmed by a 50% decrease in
turbidity, asdetermined by Klettreadings, and
bya drop of >90%in viability. Conversely, no
effectwasdetected whenCHOmonolayerswere
inoculated with the supernatants of untreated cultures ofthe toxic strains. All 7toxic strains werefoundtobepositive when tested for
hemo-lyticactivity,whereasonly 20hemolytic strains werefound among theremaining 206.
To characterizethe seven toxic strains, their cell extractswerecompared with extracts from
control strains in a set of biological assays
(Table 1). Cellextracts did notelicit fluid accu-mulation in rabbit ileal loops (the same result was obtained by inoculating about
1010
livingbacteriaperloop),nordidtheystimulate adenyl-ate cyclase in CHO cultures. In addition, anti-cholerahyperimmune serumfailed to neutralize thecytotoxic activity. These results as awhole suggest the presence of a specific factor other than LT. Furthermore, all seven E. coli strains werenegative in the infant mouse assay for heat-stableenterotoxin.
Cell extracts werealso able to produce mor-phological alterations in Vero and HeLa cell cultureswithin18 h, and the formation of giant multinucleated cells was observed after a few days (Fig. 1). HeLa cells died within 60 h of incubation unless the medium was changed dai-ly. CHO and Vero cells appeared to be more
resistant,their viability being unaffected over 5
days.Medium substitution 6hafterinoculation didnotinfluence the outcomeof morphological
changesin any of the cell lines tested.
Multinucleation wasobservedatlowcell
den-sities(lessthan 5,000cellspercm2)aswellasin
confluent monolayers.
Because theE. coli strains testedwere more
toxic forHeLacells, this cell line was used for the quantitative evaluation of the alterations induced by the cytotoxic factor. Monolayers
were treated withbacterialextracts andstained
at different times as described above. Determi-nations were made of cell viability (Fig. 2) and therelativefrequenciesof cellswith one, two,or more nuclei (Fig. 3).
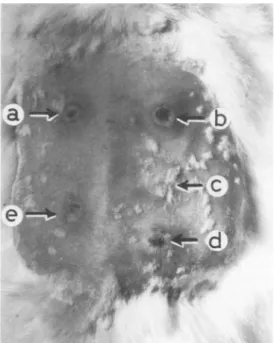
Intradermalinjection of cellextractsinrabbits caused anindurationareawithanecroticcenter up to a dilution of1:20 (Fig. 4). Subcutaneous inoculationin the abdomensofguineapigs
elicit-ed a large necrotic reaction; slightly more than 50%oftheanimals diedwithin 48 h andshowed
adiffusehemorrhagic patternduring autopsy.
Heating at75°C for 15 min or at 60°C for1 h
destroyedthetoxicactivity ofthesample,which wasfound tobestableatpHvaluesranging from
6 to 10.5. Since the above-mentioned observa-tions led us to suppose that toxic
activity
wascarried by proteinaceous material, further
ex-periments were carried out to obtain evidence
on thispoint.
Samples of bacterial culture (20 ml) were
centrifuged (20minat
4°C, 1,500
x g), suspend-ed in 5 ml of25 mMTris-hydrochloride
buffer(pH7.2), spun downagain, suspendedin 1.5 ml
FIG. 4. Local reactions in rabbit 40 h after intra-dermalinjection(0.1 ml)of cell extracts obtained from
ISS51 (a), ISS4(b), K-12J53(c), ISS18(d), and ISS2
(e).
VOL. 39, 1983
:.m r.7t
A'
A
C 1.5 -°0 .0 10 C'r-Q5 c-c)0 'Nj I.: 8 10 12 14 16 18 20 22 24 26 Elutionvolume (ml)
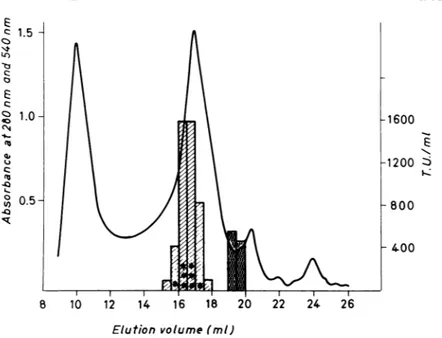
FIG. 5. Chromatogram fromTSK G4000SWcolumn(7.5 by 500 mm). The column, equilibrated in 25 mM
Tris-hydrochloride(pH7.2)and loaded with 175,uloffilteredsupernatant(45,000xg),waseluted at 1 ml min '.
Thesolidline represents absorbance at 280 nm; hatched bars representtoxicactivity onHeLa cells; dark bars represent hemolytic activity measured as absorbance at 540 nm; the number of asterisks is proportional to
necrotic activity in theskin testassay. TU,Toxic units.
of Trisbuffer, and sonicated as described above.
Sonicated cellswere finally centrifuged (20 min at40C, 45,000 x g),and the supernatant,filtered througha45-urmMillipore MFfilter, was imme-diately applied toaTSK G4000 SW column (7.5 by 500 mm). The column, equilibrated in the same buffer, was eluted at 1 ml/min, and the effluent was monitored at 280nm.
The chromatogram obtained from the G4000 column is shown inFig.5together with the toxic
activity determined by HeLa cell and skin test assays. Furthermore, the hemolytic activity of theTSK G4000fractionswasalsodeterminedas described above. Figure6shows thesame chro-matogram as Fig. 5 together with the carbohy-drate and protein contents of each fraction. From the results shown, it is evident that toxic activity is eluted by the G4000 column from15 to 17 ml, approximately 75% of the total toxic
activity being localized at 16 ml, whereas
ac-cordingtothe biuret assay,only 22% ofthetotal protein is localized in fraction 16. On the con-trary, hemolytic activity (Fig. 5, dark bars) is eluted at 19 ml. Figure 7 shows the electropho-retic pattern of the fractions from the TSK column.
From Fig. 6, it is also clear that the total
carbohydrate content of the original sample is
quite low, andno morethan 25
p.g
ofequivalenthexose isassociated with eachfraction, whereas theprotein contentis considerable. Indeed, the maximum protein concentration is associated
with thesecondmain peak in Fig. 5 and 6. Toxic
activity is also associated with the same peak.
This datum makes it very likely that the toxic
activity is due to material of a proteinaceous
nature. To confirm this hypothesis, amino acid analyses offractions 14 through 18 were run as
described above. It isclearfromthese analyses
that amino acids are associated with all of the
V) 50l 1'5 105 404-400 30 20 10 -300 ct-200 & -100 8 10 12 14 16 18 20 22 24 26 Elution volume (ml)
FIG. 6. Chromatogram from the TSK G4000 col-umn. Chromatographic conditions were the same as
for theexperimentsshown inFig.5.Symbols:
absorbance at 280 nm; - -- - protein as deter-minedbybiuret
assay:
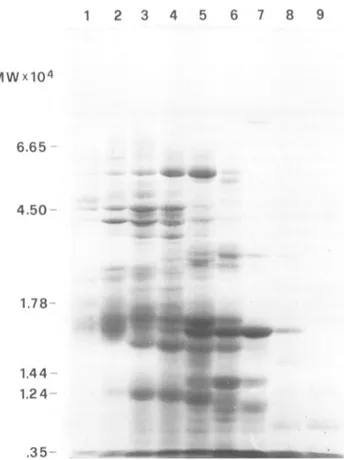
carbohydrate(as hex-ose) in micrograms per milliliter determined by the method of Duboisetal.(6).CELL-ALTERING E. COLI TOXIC FACTOR( 1 2 3 4 5 6 7 8 9 MWx104 6.65 4.50 1.78 1.44 1.24 -.35
-FIG. 7. Polyacrylamide gelelectrophoresis inthe presenceofsodiumdodecyl sulfate, performedon0.6-ml fractionsfrom the TSKG4000SWcolumn. Fractionscorrespondingtoeffluentat15to17 ml (wells1 through 4) arecytotoxicfor HeLacells: fractionscorrespondingto19 ml (wells 7 and8)arehemolytic. Inactive fractions. correspondingto18and 20ml,are in wells5. 6. and9.
assayed fractions, and 4.5 nM amino acid is present in fraction 16, confirming again the proteinaceous nature of the material to which
toxic activity is due.
The TSK G4000 column was calibrated as described above. Theresultingcalibrationcurve regression coefficient was 0.98, and in the cali-bration curve (data not shown) the elution vol-ume of the main toxic fraction (fraction 16)
corresponds to the elution volume ofaglobular
protein withamolecularweight of
approximate-ly 76,000.
DISCUSSION
The most relevantbiological properties of this E. coli toxin, termed CNF, can be summarized as follows. (i) It appears to be cytotoxic for HeLacells. (ii) Itsaction on CHO and Vero cells distinguishes it from Verotoxin and LT. (iii) It causes the formation of large multinucleated cells when tested in cell cultures; this effect is moreremarkablein HeLa andCHO cells than in Verocells. Vero and CHO cells remained viable
just as long as untreated controls did, whereas HeLacultures died within60 hafter inoculation. (iv)Thereis good evidence that the toxinis ofa proteinaceous nature.
The outcome of morphological alterations is notinfluencedby thedensity ofthetoxin-treated
monolayers; sucharesultmakes the occurrence ofacellfusion processunlikely, thussupporting
the hypothesis ofan inhibition of cell division. In vivo experiments show that CNF causes not only a local necrotic reaction but also sys-temic damage consisting of widespread hemor-rhage. No target organ was evidenced, and the observed alterations appear rather to be the result ofadiffuse vascular involvement.
The data also tend strongly to support the hypothesisthat toxicactivity is associated with material ofa proteinaceous nature, and specifi-cally with a protein with a molecular weight of about 70,000to80,000. Furthermore, hemolytic activity is consistently separated from toxic ac-tivity and is related to material with a much lower apparent molecular weight. The low fre-quency ofhemolyticstrains among E. coli
isolat-VOL. 39. 1983 1305 move ",14c. - "O..
40
* AqF. 4w.4w
40,ed from stool specimens (12), confirmed in the present study, makes it extremely difficult to hypothesize a casual association of the two activities in seven out of seven toxic strains examined. However, our findings very clearly show that the two activities are indeed due to different molecular species.
Inconclusion, our findings suggest that CNF plays the role of virulence factor. Although bacterial extracts and living bacteria themselves werenegative in the rabbit ileal loop test, it must be stressed that CNF-producing strains were all
derived from infants who had severe diarrhea and whose stool specimens proved negative for all otherbacterial or viral enteric pathogens.
Furtherinvestigations are in progress to char-acterize thebiochemical properties of CNF and its mode of action in vivo and in vitro.
ACKNOWLEDGMENTS
We are verygratefulto ValeriaDe Marco (University of Rome) for the aminoacidanalysesand to Ida Luzzi(Istituto Superiore diSanita, Rome) forherhelpinrabbit surgery.
This workwaspartially supportedby the Progretto Finaliz-zatoChimica FineeSecondaria oftheItalian Nation Council forScientific Research.
LITERATURECITED
1. Alderete, J.F., and D. C. Robertson. 1978. Purification and chemical characterization ofthe heat-stable entero-toxinproduced byporcinestrain ofenterotoxigenic Esch-erichia c oli. Infect. Immun. 19:1021-1030.
2. Clements,J.D., and R. A. Finkelstein. 1979. Isolation and characterization ofhomogeneous heat-labile enterotoxins withhighspecific activity from Escherichia colicultures. Infect. Immun. 24:760-769.
3. Clements, J. D., R.J. Yancey, and R. A. Finkelstein. 1980.Properties ofhomogeneous heat-labile enterotoxin from Escherichiaco/i.Infect.Immun. 29:91-97. 4. Dallas, W. S., and S. Falkow. 1980. Aminoacidsequence
homology between cholera toxin and Escherichia co/i
heat-labiletoxin. Nature(London)288:499-501. 5. Dean, A.G.,Y.Ching,R.G.Williams,andL. B. Harden.
1972. TestforEscherichia colienterotoxin usinginfant mice: application in a study of diarrhoea inchildren in Honolulu. J. Infect. Dis. 125:407-411.
6. Dubois, M., K. A.Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith.1956. Colorimetric methodforthe determi-nation of sugar and related substances. Anal. Chem. 28:350-356.
7. Evans, D. G., D. J. Evans, Jr., and N. F. Pierce. 1973. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli.Infect.Immun.7:873-880.
8. Evans, D. J., Jr., D. G. Evans, and S. L. Gorbach. 1973. Production of vascularpermeabilityfactor by enterotox-igenic Escherichia coliisolatedfrom man. Infect.Immun. 8:725-730.
9. Gill, D.M., J. D.Clements,D. C. Robertson, and R. A. Finkelstein. 1981. Subunit number and arrangement in Escherichia coliheat-labile enterotoxin. Infect. Immun. 33:677-682.
10. Guerrant, R. L., L. L. Brunton, T. C. Schnaitman, L.I. Rebhun, and A. G. Gilman. 1974.Cyclicadenosine mono-phosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Esc/herichia coli. Infect.Immun. 10:320-327.
11. Guerrant, R. L., J. M. Hughes, B. Chang, D. C. Robert. son, and F. Murad. 1980.Activationof intestinal guanyl-atecyclase byheat-stable enterotoxin of Escherichiaco/i: studies of tissuespecificity, potentialreceptors and inter-mediate.J. Infect. Dis. 142:220-228.
12. Hughes, C., D. Muller, J. Hacher,and W. Goebel.1982. Genetics andpathogenicrole of Escherichia c oli haemoly-sin. Toxicon20:247-252.
13. Konowalchuk, J., N. Dickie,S.Stavric, and J. I. Speirs. 1978.Properties ofanEscherichia colicytotoxin.Infect. Immun. 20:575-577.
14. Konowalchuk, J.,J.I. Speirs,andS. Stavric. 1977. Vero response to a cytotoxinof Escherichia (oli. Infect. Im-mun. 18:775-779.
15. Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature(London)227:680-685.
16. Munkres, K. D., and F. M. Richards. 1965. The purifica-tionandproperties ofneurospora malatedehydrogenase. Arch. Biochem.Biophys. 109:466-479.
17. Scotland, S. M., N. P. Day, G. A.Willshaw,and B. Rowe. 1980.CytotoxicenteropathogenicEscherichia coli. Lan-ceti:90.
18. Smith,H. W., and C. L.Gyles. 1971. The effect of cell free fluids prepared fromculturesofhumanand animal enteropathogenic strains of Escherichia coli on ligated intestinal segments ofrabbitsandpigs. J. Med.Microbiol. 3:403-409.
19. W.H.O. ScientificWorking Group.1980.Escherichiacoli diarrhoea.Bull.W.H.O.58:23-36.