GRAZIA RUTIGLIANO
Beyond classical thyroid hormone
3-iodothyronamine and
the trace amine-associated receptor 1
in the cross-talk between thyroid and brain
Sant’Anna School of Advanced Studies Institute of Life Sciences
Ph.D. Programme in Translational Medicine
Curriculum of Molecular Biomedicine, Experimental and Clinical Physiology and Pathophysiology
Tutor Prof Giorgio Iervasi Supervisor Prof Riccardo Zucchi
1
Summary
CHAPTER 1 INTRODUCTION ... 11
1.1 Thyroid hormone with focus on the central nervous system ... 11
1.2 Emerging role of 3-iodothyronamine ... 16
1.3 Trace amine-associated receptor 1 in the central nervous system ... 20
1.4 Unresolved issues in hypothyroidism ... 24
1.5 Objectives ... 29
1.5.1 Objective 1 - Human hypothyroidism ... 29
1.5.2 Objective 2 - Mouse model of hypothyroidism ... 30
1.5.3 Objective 3 - TAAR1 variants in patients with mental disorders ... 31
CHAPTER 2 MATERIALS AND METHODS ... 32
2.1 Human hypothyroidism... 32 2.1.1 Sample recruitment ... 32 2.1.2 Assessment ... 33 2.1.2.1 Neurocognitive tests ... 33 2.1.2.2 Psychometric scales ... 39 2.1.2.3 Biochemical parameters ... 42 2.1.3 Statistical analysis ... 43
2.2 Mouse model of hypothyroidism... 44
2.2.1 Animals ... 44
2.2.2 Treatments ... 44
2.2.3 Hormonal determinations ... 46
2
2.2.4.1 Elevated Plus Maze ... 51
2.2.4.2 Open Field Test... 53
2.2.4.3 Novel Object Recognition Test ... 54
2.2.4.4 Tail Suspension Test ... 55
2.2.4.5 Scoring of behavioural tests ... 58
2.2.5 Synthesis of [125I]-T1AM ... 58
2.2.6 Study of in vivo [125I]-T1AM biodistribution ... 60
2.2.7 Immunohistochemistry ... 60
2.2.8 Western blot analysis ... 61
2.2.9 Statistical analysis ... 63
2.3 TAAR1 variants in patients with mental disorders ... 64
2.3.1 Sample recruitment ... 64
2.3.2 Psychopathology assessment ... 65
2.3.3 Screening for TAAR1 variants ... 67
2.3.4 Structural human TAAR1 homology model ... 71
2.3.5 Functional characterization of TAAR1 variants ... 72
2.3.5.1 Cloning of plasmid constructs ... 73
2.3.5.2 Cell culture and transfection ... 77
2.3.5.3 Quantification of cell surface and total expression ... 77
2.3.5.4 Measurement of Gs/adenylyl cyclase activation ... 79
2.3.6 Statistical analysis ... 81
CHAPTER 3 RESULTS ... 83
3.1 Human hypothyroidism... 83
3.1.1 Socio-demographic and clinical characteristics ... 83
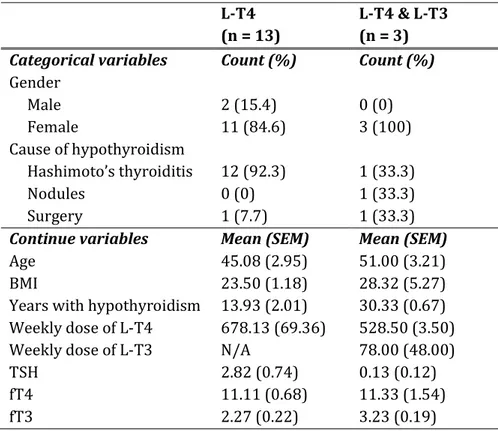
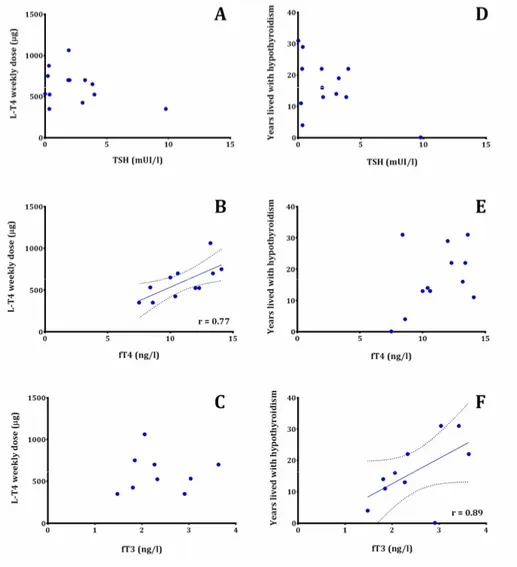
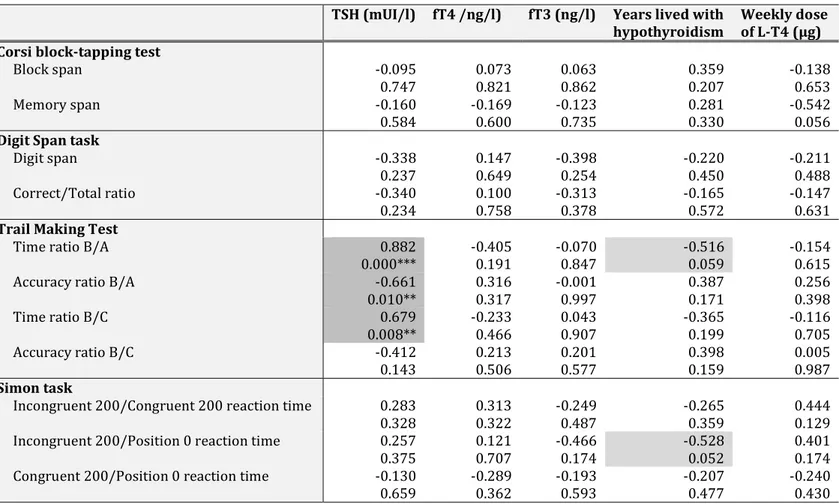
3.1.2 Relations between clinical and neurocognitive features ... 87
3.1.3 Relations between clinical and psychometric features ... 91
3.2 Mouse model of hypothyroidism... 95
3
3.2.2 Behavioural performances ... 99
3.2.2.1 Elevated Plus Maze ... 99
3.2.2.2 Open Field Test... 103
3.2.2.3 Novel Object Recognition Test ... 105
3.2.2.4 Tail Suspension Test ... 107
3.2.3 T1AM crosses the blood-brain barrier ... 108
3.2.4 TAAR1 is expressed in mouse hippocampus ... 110
3.2.5 TAAR1 expression in different organs ... 110
3.3 TAAR1 variants in patients with mental disorders ... 113
3.3.1 Socio-demographic and clinical characteristics ... 113
3.3.2 TAAR1 missense variants are enriched in patients ... 121
3.3.3 TAAR1 variants are in functional-structural key regions ... 126
3.3.4 R23C, Y131C and C263R decrease cell surface expression ... 128
3.3.5 R23C, Y131C and C263R dampen Gs signalling ... 131
3.3.6 RO5166017 could not rescue TAAR1-variant Gs signalling ... 136
CHAPTER 4 DISCUSSION ... 138
4.1 Human hypothyroidism... 138
4.2 Mouse model of hypothyroidism... 150
4.3 TAAR1 variants in patients with mental disorders ... 162
4.4 Conclusions ... 175
4
List of figures
FIGURE 1. OVERVIEW OF CLASSICAL AND NON-CLASSICAL TH WITH ASSOCIATED
INTERCONVERSION PROCESSES ... 19
FIGURE 2.GRAPHICAL REPRESENTATION OF THE NEUROCOGNITIVE TESTS... 38
FIGURE 3. GRAPHICAL REPRESENTATION OF THE APPARATUS USED FOR BEHAVIOURAL TESTS ... 57
FIGURE 4.CORRELATIONS BETWEEN BIOCHEMICAL PARAMETERS (TSH, FT4, FT3) AND: (A,B,C) WEEKLY DOSE OF L-T4 AND (D,E,F) YEARS LIVED WITH HYPOTHYROIDISM ... 86
FIGURE 5. CORRELATIONS SHOWING THE ASSOCIATION BETWEEN TSH LEVELS AND EXECUTIVE FUNCTIONS, ASSESSED WITH THE TRAIL MAKING TEST, IN L-T4- OR L-T4 &L-T3-TREATED HYPOTHYROID PATIENTS. ... 89
FIGURE 6.CORRELATIONS SHOWING THE ASSOCIATION BETWEEN CLINICAL PARAMETERS OF THYROID FUNCTION AND PSYCHOMETRIC SCALES ... 93
FIGURE 7.CHARACTERIZATION OF THE MOUSE MODEL OF HYPOTHYROIDISM ... 98
FIGURE 8.ELEVATED PLUS MAZE ... 102
FIGURE 9.OPEN FIELD TEST... 104
FIGURE 10.NOVEL OBJECT RECOGNITION TEST ... 106
FIGURE 11.TAIL SUSPENSION TEST... 107
FIGURE 12.[125I]-T1AM BIODISTRIBUTION TO DIFFERENT ORGANS IN VIVO ... 109
FIGURE 13.IMMUNOLOCALIZATION OF TAAR1 IN THE MOUSE HIPPOCAMPUS ... 111
FIGURE 14.TAAR1 IS EXPRESSED IN EVERY TESTED ORGAN EXCEPT FOR THE LIVER ... 112
FIGURE 15.PSYCHOPATHOLOGICAL CHARACTERISTICS OF OUR SAMPLE OF PATIENTS WITH MAJOR MENTAL DISORDERS ... 119
5
FIGURE 16.SEQUENCE ALIGNMENT OF HUMAN TAAR1 WITH ORTHOLOGUE GENES ACROSS SEVERAL SPECIES AND PARALOGUE GENES IN THE TAAR FAMILY ... 125
FIGURE 17.THE 3-D HOMOLOGY MODEL OF HUMAN TAAR1 BOUND WITH T1AM ... 127
FIGURE 18.QUANTIFICATION OF CELL SURFACE AND TOTAL EXPRESSION OF TAAR1 . 130
FIGURE 19.GS SIGNALLING PROPERTIES OF TAAR1-WT AND TAAR1-VARIANTS ... 133
FIGURE 20. ASSESSMENT OF TAAR1GS SIGNALING WITH THE GLOSENSORTM CAMP ASSAY ... 134
FIGURE 21.ASSESSMENT OF GS SIGNALING PROPERTIES OF TAAR1-WT AND -VARIANTS WITH THE ALPHASCREEN TECHNOLOGY ... 135 FIGURE 22.GS SIGNALING PROPERTIES OF TAAR1-WT/WT AND -WT/Y131C UPON CO
-STIMULATION WITH PEA AND RO5166017 ... 137
FIGURE 23.HOMEOSTATIC CONTROL OF THE HYPOTHALAMUS-PITUITARY-THYROID AXIS.
6
List of tables
TABLE 1.HPLC PUMP CONDITIONS ... 49
TABLE 2.MASS SPECTROMETRY PARAMETERS ... 50
TABLE 3. BEHAVIOURAL INTERPRETATION OF THE VARIABLES RECORDED IN THE
ELEVATED PLUS MAZE TEST ... 53 TABLE 4. PRIMER PAIRS (THERMOFISHER SCIENTIFIC) USED TO PCR-AMPLIFY 3
PARTIALLY OVERLAPPING AMPLICONS SPANNING TAAR1 CODING REGION AND THE
5’- AND 3’-UNTRANSLATED REGION (UTR) ... 70
TABLE 5.PRIMER PAIRS (SIGMA-ALDRICH) USED FOR SITE-DIRECTED MUTAGENESIS ... 76
TABLE 6.SOCIO-DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF L-T4- AND L-T4&
L-T3-TREATED HYPOTHYROID PATIENTS ... 84
TABLE 7.CORRELATION MATRIX OF THE BIOCHEMICAL PARAMETERS ... 85 TABLE 8.CORRELATION MATRIX OF NEUROCOGNITIVE PERFORMANCES (ROW) VS CLINICAL CHARACTERISTICS (COLUMN) ... 90
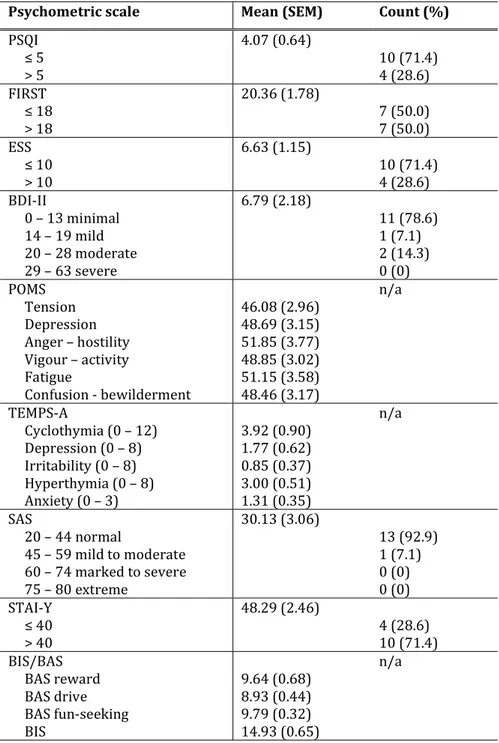
TABLE 9. PSYCHOLOGICAL MEASURES OF L-T4- AND L-T4 & L-T3-TREATED HYPOTHYROID PATIENTS ... 92
TABLE 10. CORRELATION MATRIX OF POMS SUBSCALES (ROW) VS CLINICAL CHARACTERISTICS (COLUMN) ... 94
TABLE 11.SERUM L-T4 CONCENTRATIONS IN THE 6 EXPERIMENTAL GROUPS ... 97
TABLE 12. MEASURES OF ANXIETY-RELATED BEHAVIOURS, SPONTANEOUS LOCOMOTOR ACTIVITY, DECISION-MAKING, RISK-ASSESSMENT, AND DEPRESSION-RELATED BEHAVIOURS IN 6-WEEK OLD C57BL/6J MICE FOLLOWING THE INDUCTION OF HYPOTHYROIDISM AND DIFFERENT REPLACEMENT TREATMENTS ... 101
TABLE 13.SOCIO-DEMOGRAPHIC AND CLINICAL CHARACTERISTICS ... 116
7
TABLE 15.CORRELATION MATRIX IN THE PSYCHOPATHOLOGY NETWORK ... 118 TABLE 16.TAAR1SNP/SNVS IN PATIENTS AND CONTROLS AND CORRESPONDING MINOR ALLELE FREQUENCIES ... 124
8
Abstract
Hypothyroidism is one of the most common endocrine disorders. Once the diagnosis is formulated, guidelines from all professional societies recommend levothyroxine (L-T4) monotherapy as the treatment of choice, under the assumption that peripheral conversion provides the exact amount of triiodothyronine (T3) to tissues. However, ~ 15% of hypothyroid patients do not achieve a satisfactory functional level, despite attainment of biochemical euthyroidism. Persistent symptoms include depression, anxiety, and cognitive impairments. It has been argued that combined T4 & L-T3 therapy might provide a more physiological treatment plan, although meta-analytical evidence remains inconclusive. Recently, it emerged that 3-iodothyronamine (T1AM), one of the active thyroid hormone metabolites, has pro-learning and anti-amnestic effects, and modulates pain threshold, sleep pattern, and food intake. Its reduced availability might result in some cognitive/neuropsychiatric symptoms traditionally attributed to thyroid hormone. T1AM binds to the trace amine-associated receptor 1 (TAAR1), a G-protein coupled receptor with a putative role in neurotransmission. The human gene for TAAR1 maps to locus 6q23, within a region associated with major mental disorders. Here, we performed a cross-sectional study of a sample of hypothyroid patients. We tested the correlation between clinical/biochemical variables and neurocognitive and psychometric variables. We found inverse relationships between: thyroid-stimulating hormone (TSH) levels and executive functions;
9
circulating levels of fT4 and fT3 and perception of sleepiness. Also, circulating levels of fT3 positively correlated with personal drive. Moreover, hypothyroid patients displayed higher anxiety levels than the general population.
We developed a pharmacological mouse model of hypothyroidism. Hypothyroid mice were administered various combinations of classical and non-classical thyroid hormones, i.e., L-T4, L-T4 & L-T3, L-T4 & T1AM, T1AM. Groups were compared with respect to hippocampus-dependent memory, locomotor activity, depression- and anxiety- related behaviours. Hypothyroid mice displayed significantly impaired hippocampus-dependent memory, which was almost fully normalized by L-T4 monotherapy. A larger improvement was observed upon L-T4 & T1AM replacement, while T1AM did not induce any effect per se. Hippocampus-dependent memory remained disrupted under L-T4 & L-T3, possibly due to thyrotoxicosis. No differences were found in anxiety, depression, and locomotion levels.
Our final aim was to identify and functionally characterize TAAR1 variants in patients suffering from major mental disorders. We detected 13 missense variants in TAAR1 coding region, with a significant enrichment in patients as compared to healthy controls (11 vs 1, 1 variant in both groups). Based on in silico predictions, we selected 3 variants - R23C, Y131C, C263R – for in vitro characterization. In cells co-transfected with wild type and mutated TAAR1 we observed a significant reduction of cell surface expression and dampening of ligand-activated Gs/adenylyl cyclase
10
signalling. R23C, Y131C, and C263R are rare in the general population and map to functionally important highly conserved positions in the protein.
Our findings challenge the common view that thyroid replacement is easily accomplished in all hypothyroid patients. Response variability may relate to the complexity of the homeostatic control of thyroid status, operating at several levels of spatial and temporal organization. T1AM appears as a novel stakeholder in central effects of thyroid hormone, probably through TAAR1. Sub-functional TAAR1, either because of reduced availability of its endogenous ligand, or due to missense mutations, may represent a vulnerability factor for the development of mental disturbances.
11
Chapter 1
Introduction
1.1 Thyroid hormone with focus on the central
nervous system
Thyroid hormones (TH), namely thyroxine (T4) and 3,5,3’-triiodothyronine (T3), are crucial regulators of multiple processes and systems in vertebrates 1, 2. TH was the first
morphogen ever described. The pioneering demonstration that thyroidal extracts were able to transform the aquatic tadpoles into terrestrial frogs, and that such metamorphosis was arrested upon thyroid gland removal, unravelled the important role of TH as an ancient developmental regulator throughout phylogenesis
3. In particular, TH orchestrates many aspects of
neurodevelopment, including proliferation, survival, cell fate decision, migration, differentiation and maturation of neuronal and glial cells 4. For instance, TH initiates nervous system
remodelling in amphibians 5, fish 6, avian species 7, and even in
non-chordates, although the latter, not possessing a thyroid gland, rely on exogenous TH from food 8. In humans,
derangements of TH function in the foetus result in multi-organ complications. Epidemiological evidence in areas of iodine deficiency and data from children born to women with thyroid
12
disorders indicate that maternal TH deficiency dramatically alter neurodevelopment in the progeny, leading to physical and mental disturbances. These include cretinism, deafness, schizophrenia, attention deficit hyperactive disorder (ADHD) and autism 9-13. TH
continues to regulate neurogenesis also during adult life 4. In the
adult brain, TH signalling pathway represents a crucial endocrine signal for directing cell fate decision (i.e., neuron vs glia differentiation), cell proliferation, migration and differentiation of neural stem cells located in the neurogenic niches – active proliferative brain areas – of the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and the subventricular zone (SVZ) lining the lateral ventricle 4. TH-related disruption of adult
neurogenesis may result in cognitive deficits, neurodegeneration, and mood alterations 14-16. Furthermore, TH has a prominent role
in the control of metabolism and energy expenditure, in synergy with the sympathetic nervous system 17. As a matter of fact, most
TH actions occur in metabolically relevant tissues, such as brown adipose tissue, liver, skeletal muscle, heart and pancreatic islets
18. TH signalling is essential for adaptive thermogenesis 19, liver
lipogenesis 20, physical exercise-induced mitochondrial
adaptation 21, heart rate and cardiac output regulation 22, and
glucose-stimulated insulin secretion 23. Finally, TH is a
fundamental regulator of seasonal rhythms of physiological functions, such as metabolism, immune responses, reproduction and mood 24.
13
Attainment of the adequate amount of TH for the physiological requests of the body involves simultaneous activity of a complex set of evolutionary conserved plasma binding proteins, transporters, deiodinases, receptors and cofactors.
TH is produced and secreted by the thyrocytes, i.e., the follicular cells of the thyroid gland. According to the classical view, the thyroid gland contributes virtually the whole supply of T4, but only about 21% of daily T3 production, the remaining deriving from T4 to T3 deiodination by type 1 and 2 deiodinases (D1 and D2) 25. However, it has become apparent that the thyroid gland
can adjust circulating T3 levels to the body needs through de novo T3 formation 26, 27. The primary source of TH is thyroglobulin
(TG), a large homodimeric glycoprotein with a monomer molecular mass of 330 kDa and containing > 2745 residues (of which, ~70 are tyrosines, Y), that is synthesized by thyrocytes and secreted into the follicle colloid. TH synthesis also requires iodine, which is taken up as iodide (I-) across the basolateral membrane
of thyrocytes by the sodium/iodide (Na+/ I-) symporter, and then
moved across the apical membrane into the follicle colloid. Here, thyroperoxidase provides the oxidizing conditions required for: (i) iodination of TG tyrosine residues to form monoiodotyrosine (MIT) and diiodotyrosine (DIT); (ii) coupling of a MIT/DIT donor with a DIT acceptor to form L-T3 or L-T4, respectively 28, 29. The
basal synthesis and secretion of TH by the thyroid gland is relatively low 30, and is activated by pituitary thyroid-stimulating
14
hypothalamic thyrotropin-releasing hormone (TRH). Upon binding of TSH to its receptors (TSHR) in the basolateral plasma membrane of thyrocytes, cathepsins are released to the follicle colloid to initiate TG solubilization and TH liberation. Then, TG is re-internalized through endocytosed vesicles, which fuse with lysosomes, where TG is eventually degraded, and TH cleaved and liberated into the blood stream 31-34. Reciprocally, defensive
mechanisms are in place to avoid over-supply of TH to tissues/organs. First, TH feeds back information to the pituitary gland and the hypothalamus, to negatively regulate its own production, thereby setting an internal reference point 35. In
addition, TH availability can be controlled through binding to plasma proteins, activation of the pro-hormone T4 to the active form T3, transport across the cell membrane, and production of potential counter-regulatory derivatives, such as 3,3’,5’- triiodothyronine, or reverse T3 (rT3), and thyronamines 36-39.
Originally, because of its lipophilic structure, TH was believed to cross the plasma membrane by passive diffusion 40. Subsequent
investigations demonstrated the existence of saturable and stereospecific TH uptake, and then identified several families of TH transporters at the molecular level 41-43. The main carriers of
TH through the blood-brain barrier (BBB) are the monocarboxylate transporter 8 (MCT8) and the Na+ independent organic anion-transporting polypeptide 1C1 (OATP1C1) 44. Once
intracerebral, local availability of T3 is dependent on the conversion from L-T4 to L-T3, catalyzed by the astrocytic D2 45.
15
T3 is passed to neurons, and there metabolized into 3,5-diiodothyronine (T2) by type 3 deiodinase (D3) 46. Within the cell,
T3 is transported to the nucleus, where it binds to TH receptors (THR) to modulate the genomic regulation of hundreds of genes
47, 48. THR exist in different isoforms, TRα1 and -2, and TRβ1 and
-2, that are members of the nuclear receptor superfamily and bind to regulatory regions (thyroid response elements, TRE) of target genes as homo- or hetero-dimers with retinoid-X-receptors (RXRs), to either enhance or repress gene transcription. Unlike the other TRs, TRα2 does not bind TH, and its function is largely unclear 49, 50. The main TR isoform expressed in the brain is TRα1,
which accounts for 70-80% of TR transcripts 51. Studies in rodents
bearing mutations in TRα1 suggest that this receptor isoform might be responsible for many of the deleterious effects of hypothyroidism on brain development and function, namely delayed migration of cortical neurons, incorrect development of γ-aminobutyric acid (GABA)-ergic cells, defects in cerebellar development, and reduced adult neurogenesis 52. The detection of
TH-induced effects in a time frame of seconds or minutes, which cannot be altered by gene transcription inhibitors, suggested that TH have non-genomic effects, as well 53. The non-genomic effects
of TH are independent of nuclear uptake of T3 and intranuclear TRs. They appear to be initiated at receptors structurally related to TRs, such as truncated isoforms of TRα, at the level of the plasma membrane, the cytoplasm, or the mitochondria. Alternatively, structurally unrelated receptors have been
16
reported, like the plasma membrane integrin αvβ3 53. In the brain,
TH seems to promote the expansion of neural progenitor cells in the neocortex by binding to integrin αvβ3 54. Furthermore, T4 and
rT3 (but not T3) are able to initiate actin polymerization within minutes in cultured astrocytes, with no changes in actin mRNA expression or total actin content 55, 56. The same observation has
been replicated in vivo, in hypothyroid neonatal rodents, although the molecular target of this effect remains unknown 57.
1.2 Emerging role of 3-iodothyronamine
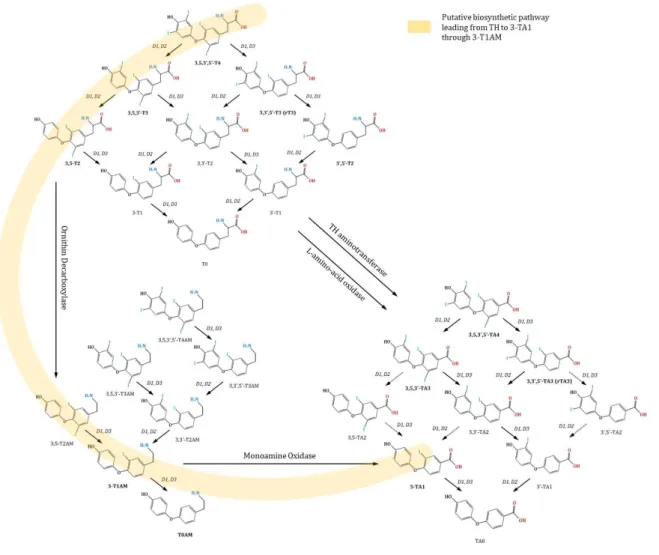
T4 and T3 undergo a complex metabolism in vivo, by several enzymes encompassing deiodinases, amine transferases, amine oxidases, decarboxylases, and several classes of conjugating enzymes, particularly sulfotransferases and UDP-glucuronosyl-transferases 58. The canonical paradigm, viewing T4 to T3
deiodination as the only activating reaction, and all the others as catabolic or inactivating reactions, turned out to be simplistic. It emerged, indeed, that some T4 or T3 metabolites can produce significant functional effects when administered to experimental preparations or intact animals via interaction either with TR, or with other receptors. Therefore, they are currently considered as chemical messengers further enriching TH signalling, and have become known as “novel thyroid hormones” or “active thyroid hormone metabolites”. These novel hormones include: T2; thyronamine, mostly 3-iodothyronamine (T1AM) and non-iodinated thyronamine (T0AM); thyroacetic acids, mostly
17
3,5,3’,5’-thyroacetic acid (TA4), 3,5,3’-thyroacetic acid (TA3), and 3-thyroacetic acid (TA1) (figure 1).
For the purposes of this dissertation, I will mainly focus on T1AM. Using liquid chromatography-mass spectrometry (LC-MS), T1AM has been detected in virtually every rodent tissue in concentrations on the order of a few pmol/g 59, 60. In human blood,
conflicting results have been reported, depending on the technique used for assessing T1AM 61-64. These discrepancies
might be explained with extensive binding to serum proteins, or with the formation of an adduct 65. More efforts are needed to
solve such technical pitfalls. T1AM biosynthetic pathway still awaits clarification. In a rat gut preparation, the exogenous administration of T4 or T3 was followed by deiodination to T2, then by decarboxylation to T2AM (catalyzed by ornithine decarboxylase), and further deiodination to T1AM (catalyzed by type III deiodinase) (figure 1) 66. However, it is unclear whether
this is the only biosynthetic pathway or whether T1AM synthesis from T3 may occur in other tissues. T1AM metabolism leads to a variety of derivatives, the major being the oxidative metabolite TA1 67. T1AM does not bind TRs but is a rather promiscuous
ligand for several molecular targets. It binds with nanomolar affinity to the trace amine-associated receptor 1 (TAAR1), other TAAR subtypes, alpha2 adrenergic receptors, transient receptor potential channels, and ApoB-100, a component of VLDL and LDL lipoproteins, the latter probably accounting for high affinity protein binding in serum 59, 67. The first functional effects of T1AM
18
were described in the heart, as a negative inotropic and chronotropic response, both in ex vivo working rat heart preparations, and in cardiomyocyte preparations 68-70. However,
these actions occurred at concentrations three order of magnitude higher than the endogenous levels, arguing against their pathophysiological plausibility. Instead, at lower concentrations (125 nM - 1.25 mM), T1AM elicited cardioprotective effects in an isolated rat heart model of ischemia-reperfusion injury, reducing infarct size in the absence of any hemodynamic effect 71. Subsequently, many other effects
have been reported, the most interesting being metabolic and neurological effects 59, 67, 72. T1AM modulates metabolic processes
by decreasing insulin and increasing glucagon secretion, increasing gluconeogenesis, and shifting to lipid catabolism. As for the neurological effects, intracerebral T1AM administration caused: a biphasic effect on feeding, sleep modulation with reduced non-REM sleep, increased locomotor activity, pro-learning and anti-amnestic effects, protection from toxic injury, increased autophagy, reduced pain threshold. Although the most interesting molecular target of T1AM is TAAR1, particularly at the level of the central nervous system (CNS), some of the elicited responses persisted in TAAR1 knock out animals 67. This suggests
the existence of a complex signaling system with multiple molecular targets and downstream transduction pathways.
19
Figure 1. Overview of classical and non-classical TH with associated interconversion processes
20
1.3 Trace amine-associated receptor 1 in the central
nervous system
A broad array of preclinical evidence points to a role of TAAR1 as a modulator of CNS functions, and to a potential pharmacological manipulation of its signaling pathway in the treatment of neuropsychiatric disorders. TAAR1 is a G protein-coupled receptor (GPCR), discovered in 2001 while searching for novel biogenic amines, which turned out to respond to some trace amines, instead 73, 74. The term trace amines refers to endogenous
amines, namely β-phenylethylamine, p-tyramine, tryptamine, octopamine, and synephrine. They derive from aromatic amino acids and are physiologically present in tissues at much lower concentrations (< 100 ng/g tissue) 75 than the classic biogenic
amines, such as dopamine, serotonin, norepinephrine, and histamine. While trace amines are major chemical messengers in invertebrates, in mammals they were originally believed to act as “false transmitters”, i.e. displacing classic biogenic amines from their storage and inhibiting their transporters 76. With the
discovery of TAARs, it became clear that trace amines may exert actions in their own respect 73, 77, 78. Through homology analysis,
it became clear that TAAR1 is the prototype of a class of aminergic receptors. In mammals, taar genes are highly homologous and cluster in a small region of a unique chromosome, with consistent transcriptional orientations across orthologs 79. All members of
21
transcripts, except for taar2, which contains two exons. Molecular evolutionary analyses identified an ancestral gene in the see lamprey 80. Then, several species-specific events of gene
duplications and pseudogenizations occurred, so that the number of taars is highly diverse among mammals, ranging from 0 in dolphins to 26 in the flying fox 80. The receptors are classified into
nine subfamilies (TAAR1 to TAAR9) 81. The oldest subfamily
includes TAAR1, which is the only TAAR that is not expressed in the olfactory epithelium and does not function as an olfactory receptor 80. Therefore, it appears that the divergence of younger
TAARs from TAAR1 was accompanied by a change in their expression pattern 80. From the functional point of view,
receptors in the TAAR1-4 cluster detect primary amines, while those in the TAAR5-9 cluster, which are specific to therian mammals, are predominantly stimulated by tertiary amines 82.
While most genomes contain a single well-conserved copy of the more anciently emerged taar subfamily genes (taar1-4), the latest subfamilies (taar5-9) underwent multiple species-specific duplications, with positive selection, e.g., in taar7 80. In primate
genomes, taar repertoires underwent accelerated pseudogenization 83. In particular, the human genome
encompasses six taars all present as single-copy genes, and mapping to a small genomic region of 108 kb located in chromosome 6q23 84. Functional TAAR3, TAAR4, and TAAR7 have
been lost 79, 80, before humans diverged from gorillas 85.taar gene
22
evolutionary factors. This hypothesis relates to the crucial role of many TAARs as olfactory receptors sensing important ethological signals, such as predator and prey odours, spoiled food, migratory cues, pheromones, and activating appropriate behaviours 86. For
instance, β-phenylethylamine, which is ligand for TAAR1 and TAAR4, is a carnivore odour from mountain lions, tigers and jaguars 86, 87. Therefore, taar might have been subject to positive
selection in those therian mammals that needed to discriminate among a wide number of volatile amines for increasing their survival fitness in ground-living. On the other hand, a much more relaxed selection – leading to taars deterioration - applied to those primates which, adapting to arboreality, relied less on olfaction for survival 83.
TAAR1 is widely express across the mammalian brain, mainly in limbic and monoaminergic areas involved in the regulation of emotion, cognition and reward 88. In particular, TAAR1 detection
has been reported in: hypothalamus and preoptic area, known to modulate sleep 89 and energy expenditure 90; ventral tegmental
area, a dopaminergic area critical for learning processes and motivated and addictive behaviours 91; amygdala, a complex
structure with a broad array of actions in emotional – especially fear – processing, reward learning and motivation, aggressive, maternal, sexual, and ingestive behaviours, and cognitive functions 92; dorsal raphe nucleus, a serotonergic region involved
in cognition, reward, pain sensitivity, and circadian rhythms 93;
23
autonomic, neuroendocrine and behavioural – defensive and reproductive – responses 94; parahippocampal region and
subiculum, which play a fundamental role in memory processes
95-97. In the CNS, TAAR1 appears to serve as a rheostat,
maintaining other neurotransmitters within a defined physiological range. As a matter of fact, TAAR1 engages in a cross talk with other monoaminergic systems, with interactions observed at the level of receptors and transporters that globally result in tonic inhibitory control of dopaminergic and serotonergic neurotransmission 88. Notably, TAAR1 knock out
(KO) mice display neurochemical and electrophysiological characteristics compatible with dopamine hyperactivity 96, 98-100.
In addition, TAAR1 has been documented in layer V pyramidal neurons in the PFC. In mice lacking TAAR1, the membrane resting potential of these neurons was more depolarized, and the amplitude and kinetics of NMDA-mediated excitatory post-synaptic currents (ePSCs) were decreased, as compared to wild-type (WT) neurons. Correspondingly, the composition of NMDA glutamatergic receptors was changed. From a behavioural point of view, the dysregulated dopaminergic and glutamatergic neurotransmissions in TAAR1-KO mice were associated with impairment in sensorimotor gating functions, perseverative and impulsive behaviours, and worse learning performances 99, 101-103, meaning with phenotypes of relevance for
modelling neuropsychiatric disorders. Also, TAAR1-KO mice presented enhanced sensitivity to the addictive effects of
24
amphetamine, methamphetamine, MDMA, and ethanol 96, 100, 102, 104. These experimental observations prompted the development
of TAAR1 agonists, to exploit for the treatment of neuropsychiatric disorders and substance abuse 105, 106. TAAR1
agonists proved able to control hyper-dopaminergia, classically considered the neurochemical underpinning of positive symptoms of schizophrenia. Furthermore, differently from commonly used antipsychotics, they also acted on hypo-glutamatergia, possibly related to negative and cognitive schizophrenia symptoms. Notably, common side effects of antipsychotics, such as extra-pyramidal side effects and olanzapine-induced weight gain, were modest and relatively rare. TAAR1 agonists demonstrated anti-craving effects for drugs that activate the dopaminergic reward pathway.
1.4 Unresolved issues in hypothyroidism
Hypothyroidism is a chronic disease resulting from deficient production of TH or inadequate action of TH on target tissues 107.
In 99% of cases, hypothyroidism arises as a primary defect of the thyroid gland to synthesize and release TH. The remaining cases are due to defects in the hypothalamic-pituitary-thyroid (HPT) axis, either in the production of TSH (secondary) or of TRH (tertiary). Peripheral (extra-thyroidal) hypothyroidism refers to consumptive hypothyroidism or tissue-specific hypothyroidism due to decreased sensitivity to TH 107. Primary hypothyroidism is
25
between 0.2 and 5.3% in Europe and the United States 108-110. In
iodine-replete areas, the main cause of hypothyroidism is autoimmune disease (~ 80% of cases), followed by surgery/radiation of the thyroid gland, post-partum, and iatrogenic causes 110, 111. Hypothyroidism represents an insidious
condition, as it presents with a variety of unspecific symptoms, including weight gain, tiredness, poor concentration, depression, diffuse muscle pain, menstrual irregularities, constipation, cold intolerance and dry skin 107. However, symptoms vary according
to the severity of biochemical hypothyroidism, hence they might become apparent even a long time after circulating free T4 (fT4) has started to decrease 112. It has been calculated that up to 5% of
the European population may have undiagnosed hypothyroidism
108. Moreover, ~ 30% of treated hypothyroid patients might be
receiving insufficient treatment 113. Undiagnosed, untreated or
undertreated hypothyroidism causes a significant burden on multiple organ systems, due to the negative effect on basal metabolic rate. Hypothyroidism is associated with coronary heart disease, infertility, neurosensory, musculoskeletal, and gastrointestinal symptoms. It might increase the risk of dementia. In general, it has a negative impact on quality of life, mostly due to weight gain, fatigue and mood susceptibility, and produces an increase in number of sick leave days and mortality 107, 114, 115.
The diagnosis of primary hypothyroidism is based on the laboratory findings of elevated TSH with low fT4. The term subclinical hypothyroidism refers to the finding of elevated TSH
26
with fT4 remaining within the reference range 114. Upon diagnosis
of hypothyroidism, guidelines from all professional societies indicate the initiation of levothyroxine (L-T4) monotherapy as the treatment of choice, with the goal to restore biochemical euthyroidism (normal serum TSH, fT4 and fT3 concentration), reduce symptoms and prevent long-term complications 112, 116, 117.
The first attempt to replace the function of the thyroid gland with exogenous TH dates back to 1890s, when an ovine thyroid gland was grafted into a patient with myxoedema 118. T4 was extracted
in 1914, and its chemical structure determined in 1926 115.
Despite the availability of synthetic T4 since the 1950s, desiccated animal thyroid gland – containing both T4 and T3 - remained the mainstay of therapy until the 1970s 115. Then, it was found out
that T4 was sufficient per se to successfully replace TH 119. Also, it
became clear that, out of the natural racemic mixture of levo (L)- and dextro (D)-T4, L-T4 was better absorbed and had greater physiological activity 120. Thus, the treatment strategy switched to
L-T4 monotherapy, and such has remained until today. In most cases, disease control is easily accomplished upon adequate L-T4 monotherapy 107. Its optimal efficacy-safety profile has made L-T4
one of the most prescribed drugs in the world 121, and an essential
medicine for basic health care, according to the World Health Organization 122.
While L-T4 treatment has been proven to be safe and well-manageable for many patients, there is a substantial portion (about 15%) of hypothyroid patients who do not get well on L-T4
27
monotherapy despite restoration of biochemical euthyroidism. Persistent complaints include difficulty to control body weight, fatigue, chronic malaise, and, above all, impaired psychological well-being, depression and anxiety 123. In 3 large
community-based studies, L-T4 monotherapy proved to be inadequate in treating neurocognitive and psychological disturbances, even after normalizing serum TSH concentrations 124-126. Such
variability in treatment response has challenged the “widely held belief that thyroid replacement is a simple uncomplicated process” 127. Excluding that the dosage of L-T4 is suboptimal (TSH
outside the normal range), many reasons can explain persistent symptoms in these patients. Some symptoms of dysphoria may be co-morbid to hypothyroidism and erroneously attributed to hypothyroidism, therefore they will not respond to treatment. Also, patients with psychological concerns could be more prone to seek medical advice and be offered a thyroid function screening. Alternatively, autoimmunity could affect psychological well-being independently from thyroid status 128. An intriguing
explanation is that serum TSH may not necessarily reflect the intracellular TH content in tissues, where membrane transport, intracellular T4 - T3 conversion, and T3 metabolism (inactivation?) might be subject to differential site-specific regulation 39, 123. Experimental evidence seems to support this
interpretation: in thyroidectomized rats, correction of tissue hypothyroidism could only be obtained combining L-T4 administration with L-T3 129, 130. Accordingly, in a different
28
experiment, only L-T4 & L-T3 treatment normalized tissue markers of T3-responsiveness, such as mitochondrial content, α-glycerophosphate dehydrogenase activity, and serum cholesterol levels 131. These observations have prompted several groups to
test whether the addition of synthetic L-T3 to standard L-T4 would provide any benefits in thyroid replacement. However, most of these clinical trials, summarised in 3 meta-analyses, failed to detect any difference between L-T4 monotherapy and combined L-T4 & L-T3 therapy, as concerns bodily pain, fatigue, body weight, serum lipids, psychiatric symptoms of depression and anxiety, and, broadly, psychological and physical well-being and quality of life 132-134. Recently, such negative results have
received further replication by 3 additional randomized clinical trials 135-137, except for some indication of the superiority of
combined L-T4 & L-T3 therapy over L-T4 monotherapy in ameliorating depressed mood 135. Nevertheless, somewhat
paradoxically, about half of the patients expressed a subjective preference for combined L-T4 & L-T3 therapy versus L-T4 monotherapy 135, 138-143, leaving the matter open to debate and
investigations. The latter should try to overcome the methodological drawbacks that affected previous studies, in detail: small sample size; heterogeneity in cause and severity of hypothyroidism; supra-physiological L-T4/L-T3 ratios; short duration of combination therapy with the possibility of carryover effects in trials with cross-over design 123. In the meanwhile, all
29
combination therapy, which might be only taken into account as an individual experimental approach in a small subset of patients with persisting symptoms despite optimal adherence to L-T4 treatment and serum TSH concentrations within the reference range 116, 117, 144, 145.
A further hypothesis speculates that tissue levels of TH derivatives, such as T1AM, might be decreased in hypothyroidism. In mice treated with methimazole, the development of hypothyroidism was associated with a corresponding decrease in T1AM tissue concentrations 146. Even
after L-T4-replacement, T1AM remained undetectable, despite an almost full recovery of fT4 and fT3, indicating that the intact function of the thyroid gland is needed for T1AM biosynthesis 146.
Based on these findings, it is tempting to speculate that reduced availability of T1AM might possibly result in some neuropsychiatric symptoms traditionally attributed to TH.
1.5 Objectives
1.5.1 Objective 1 - Human hypothyroidism
In a cross-sectional, retrospective study of a sample of patients affected with hypothyroidism – both overt and sub-clinical – undergoing replacement treatment with T4 monotherapy or L-T4 & L-T3 combination therapy, we tested the correlations: (1) between clinical/biochemical variables;
30
(2) between clinical/biochemical and neurocognitive variables;
(3) between clinical/biochemical and psychometric variables. 1.5.2 Objective 2 - Mouse model of hypothyroidism
As primary aim, we developed and characterized a pharmacological (methimazole & potassium perchlorate-induced) mouse model of hypothyroidism.
As secondary aim, we compared: (1) Hypothyroid mice;
(2) L-T4-treated, hypothyroid mice;
(3) L-T4 & L-T3-treated, hypothyroid mice; (4) L-T4 & T1AM-treated, hypothyroid mice; (5) T1AM-treated, hypothyroid mice;
(6) Euthyroid mice
with respect to behavioural tests assessing hippocampus-dependent memory, locomotor activity, depression- and anxiety- related behaviours.
As tertiary aim, we tested whether T1AM crosses the BBB, and whether its receptor TAAR1 is expressed in mouse hippocampus. Also, we assessed TAAR1 expression in different tissues/organs.
31
1.5.3 Objective 3 - TAAR1 variants in patients with mental disorders
Our main aim was to demonstrate an association between disruption of TAAR1 functions and mental disorders. This was articulated into 2 sub-aims:
(1) to screen a cohort of patients suffering from major mental disorders for TAAR1 variants;
(2) to perform functional in vitro characterization for a subset of the identified variants, in terms of cell surface expression and Gs signalling.
32
Chapter 2
Materials and Methods
2.1 Human hypothyroidism
2.1.1 Sample recruitment
A cross-sectional, retrospective, single-centre, no profit study was conducted at Fondazione G. Monasterio, between January 2017 and July 2019. We recruited patients aged 18 to 65 years, affected by hypothyroidism – both overt and sub-clinical – undergoing replacement treatment with L-T4 monotherapy or L-T4 & L-T3 combination therapy. Subjects were excluded if there was a history of severe medical or neurological disorders; estimated IQ < 60; acute intoxication; pregnancy; incapacity to give valid informed consent. In addition, current or past history of any mental disorder applied as an exclusion criterion. Potential participants were identified by the Endocrinologists operating at Fondazione G. Monasterio among their patients. Those willing to take part in research were contacted by phone by the experimenters (Grazia Rutigliano or Francesca Scintu), and invited to attend to two visits. During the first visit: (i) written informed consent was obtained from all the participants to use data about clinical, psychometric, and neurocognitive measures
33
and treatment; (ii) diagnosis of mental disorders was excluded according to the Structural Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5),
Research Version (SCID-5-RV) 147; (iii) socio-demographic and
clinical characteristics were collected, namely: gender, age, body mass index (BMI), cause of hypothyroidism, years lived with hypothyroidism, type of replacement treatment (L-T4 monotherapy or L-T4 & L-T3 combination therapy) and weekly dose of L-T4 or L-T4 & L-T3; (iv) neurocognitive tests were performed; (v) participants were delivered a battery of self-administered psychometric scales to rate at home by the date of the second visit. During the second visit, participants returned the rated questionnaires, having the possibility to discuss doubts with the experimenters. Then, high-density - 128-channel – electroencephalogram (EEG) was recorded in resting state and during an oddball task consisting of passive viewing of emotional pictures. The latter will not be discussed further, as the data are still under elaboration at the moment of writing.
2.1.2 Assessment
2.1.2.1 Neurocognitive tests
Neurocognitive tests were run using the Psychology Experiment Building Language (PEBL), version 2.0, a free, open-source software system licenced under the GNU Public License 2.0, available at http://pebl.sourceforge.net 148. A unique computer
34
was used for all participants. The following measures were collected:
1. Short-term memory for visual sequences (forward), with the Corsi block-tapping test (directory name: corsi) 149-151. The
Corsi block-tapping test is a span task broadly used for assessing visuospatial short-term memory. The test consists of nine blocks, which are lit up on the screen in a given sequential order, which the participant must subsequently reproduce, by clicking on the blocks with the mouse in the same order they are lit up. The test starts with 2 sequences of 2 blocks. If at least 1 of these is reproduced correctly, in the next 2 trials a sequence of increased length is administered. The test is terminated when the participant fails to reproduce 2 sequences of equal length (Figure 2.A). As output variables, we recorded: (a) block span, computed as the longest length at which at least one sequence was correctly recalled; (b) memory span, which is computed adding to the minimum list length, i.e., 2, the total number correct, and dividing by the number of trials at each length, i.e., 2.
2. Simple digit span task (forward), with the Digit Span (directory name: dspan) 152. The Digit Span task is a standard
span task, used for assessing verbal short-term memory. The test consists in the visual presentation on the screen of number strings, which the participant must subsequently reproduce, by typing the numbers in the same order on the keyboard. The test starts with a 3-number string. Two trials
35
are given per number string of the same length. If at least 1 of these is reproduced correctly, in the next 2 trials a string of an increased length is administered. The test is terminated when the participant fails to reproduce 2 strings of equal length (Figure 2.B). As output variables, we recorded: (a) digit span, computed as the longest length at which at least one string was correctly recalled; (b) ratio between the total number of correct reproductions and the total number presented strings. 3. Connect-the-dots task requiring executive switching, with the
Trail-making test, TMT (directory name: ptrails) 153.
Classically, the TMT measures how long participants take to connect dots that are either numbered (Trails part A: 1-2-3) or alternated between numbers and letters (Trails part B: 1-A-2-B-3-C). As such, the TMT is used for assessing visual attention (Trails A) and cognitive flexibility (Trails B). The computerized version administered was slightly modified, in that it contained a third type of problem, with dots only marked with letters (Trail part C: A-B-C). The TMT consisted of 12 trails and alternated 4 trials with only numbers (Part A), 4 trials with only letters (Part C) and 4 trials with alternating numbers and letters (Part B). Each Part A and Part C trial has a corresponding Part B trial, an isomorphic problem, mirrored along the vertical axis, with an equal distance to connect all the dots. The participant is instructed to click on each dot in sequence as quickly as possible. The test continues until the participant has successfully clicked on all the dots in the
36
correct order (Figure 2.C). As output variables, we recorded: (a) B/A and B/C time ratio, computed as the ratio between the total time needed to complete Part B and Part A or C, respectively; (b) B/A and B/C accuracy ratio, being the accuracy computed as the minimum number of clicks necessary to complete each trial divided by the actual number of clicks.
4. Stimulus-response compatibility and interference test, with the Simon task (directory name: simon) 154. The Simon task is
used for assessing executive function, response suppression, and interference suppression. The participant is instructed to respond to the colour of the stimulus, by clicking on the left vs right shift key in response to a blue vs red stimulus, respectively. While responding, the participant must ignore the position of the stimulus presentation along the horizontal axis, which might be neutral (i.e., position 0 in the middle of the screen), congruent (i.e., blue-left, red-right) or incongruent (i.e., blue-right, red-left) with respect to the instructed response. In the computerized version administered, the distance from position 0 was modulated over 3 increasing horizontal steps (50, 100, 200), under the assumption that the more offset the stimulus, the larger the interference (Simon effect) 154. By aggregating over left versus
right positions (e.g., averaging together far right congruent and far left congruent, far right incongruent and far left incongruent, etc.), we generated a simple interference score
37
with 7 levels (incongruent 200, 100, 50, position 0, congruent 50, 100, 200, Figure 2.D). As output variables, we recorded: (a) ratio between the reaction time at position incongruent 200 vs congruent 200; (b) ratio between the reaction time at position incongruent 200 vs position 0; (c) ratio between the reaction time at position congruent 200 vs position 0.
38
Figure 2. Graphical representation of the neurocognitive tests: (A) Corsi block-tapping test; (B) Digit Span test; (C) Trail-making test; (D) Simon task
39
2.1.2.2 Psychometric scales
Participants were administered a comprehensive battery of psychometric scales to investigate sleep, mood, anxiety, stress sensitivity, and motivational system, including:
1. Pittsburgh Sleep Quality Index (PSQI) 155, a 19-item self-report
questionnaire which measures sleep quality over the previous month. The PSQI provides information about three factors: (i) sleep efficiency, including sleep duration and habitual sleep efficiency; (ii) perceived sleep quality, including subjective sleep quality, sleep latency and use of sleep medication; (iii) daily disturbances, including sleep disturbances and daytime dysfunction. These domains are globally scored as a single factor of sleep quality, which indicates substantial sleep disturbances when higher than 5.
2. Ford Insomnia Response to Stress Test (FIRST) 156, a 9-item
questionnaire whose total score captures the constructs of vulnerability to sleep disturbances induced by stress and physiological hyperarousal. The FIRST has a Likert-scale format, with 4 response options: 1 = not likely; 2 = somewhat likely; 3 = moderately likely; 4 = very likely. A cut-off of 18 appears to predict incident insomnia at 1-year follow-up with 62%-sensitivity and 67%-specificity 157.
3. Epworth Sleepiness Scale (ESS) 158, a short questionnaire that
measures perception of sleepiness in ordinary life situations. It consists of rating the chance of sleeping in 8 situations (e.g., sitting and reading, watching TV, etc) on a 4-point scale: 0 =
40
would never doze; 1 = slight chance of dozing; 2 = moderate chance of dozing; 3 = high chance of dozing. A total score is calculated, which normally ranges between 0 and 10, while scores higher than 10 require further medical assessment. 4. Beck Depression Inventory-II (BDI-II) 159, a self-report
inventory widely used to assess the severity of depression in the last 2 weeks, according to the DSM diagnostic criteria for major depressive disorder. The BDI-II encompasses 21 items - related to sadness, irritability, hopelessness, cognitions of guilt and feelings of punishment, physical symptoms such as fatigue, weight loss, and lack of interest in sex – scored on a scale from 0 to 3. Total scores 0-13 indicate minimal depression, 14-19 mild depression, 20-28 moderate depression, 29-63 severe depression.
5. Profile of Mood States (POMS) 160, a self-report measure of
mood during the last week, broadly used in the context of clinical psychology and medicine. The POMS includes 58 items, which can be answered on a 5-level scale ranging from not at all to very strong. The items load on 6 subscales: tension-anxiety, 9 items, score range: 0 – 36; depression, 15 items, score range: 0 – 60; anger - hostility, 12 items, score range: 0 -48; vigour – activity, 8 items, score range: 0 – 32; fatigue, 7 items, score range: 0 -28; and confusion – bewilderment, 7 items, score range: 0 - 28. These scores are then converted automatically into standard T-scores.
41
6. Temperament Evaluation of the Memphis, Pisa, Paris and San Diego – Autoquestionnaire (TEMPS-A) 161, a 39-item
self-report measure of the affective temperaments with depressive (D, score range: 0 - 8), cyclothymic (C, score range: 0 - 12), hyperthymic (H, score range: 0 - 8), irritable (I, score range: 0 - 8), and anxious (A, score range: 0 -3) subscales. The TEMPS-A allows the assessment of the full affectivity spectrum, including sub-clinical manifestations and pre-morbid traits.
7. Zung Self-Rating Anxiety State (SAS) 162, a self-report measure
of anxiety levels focusing on the most common general anxiety disorders (e.g., I feel afraid for no reason at all, I can feel my heart beating fast, etc.). The SAS consists of 20 items - of which 15 assessing increased anxiety level and 5 representing positive/non-anxiety statements - with 4 response options: 1 = a little of the time; 2 = some of the time; 3 = good part of the time; 4 = most of the time. Scores are interpreted as follows: 20-44, normal range; 45-59, mild to moderate anxiety levels; 60-74, marked to severe anxiety levels; 75-80, extreme anxiety levels.
8. State-Trait Anxiety Inventory, Form Y (STAI-Y) 163, a
self-report of trait anxiety, that is of the tendency to perceive stressful situations as dangerous or threatening and to respond to such situations with elevations in the intensity of anxiety reactions. The STAI-Y is made up of 20 items (e.g., I feel pleasant, I feel nervous and restless, etc), that are scored on a
42
4-point scale: 1 = almost never; 2 = sometimes; 3 = often; 4 = almost always. Scoring needs to be reversed for anxiety-absent items. Total scores range from 20 to 80, with a cut-off of 39-40 indicating clinically significant anxiety symptoms 164.
9. Behavioural Inhibition/ Behavioural Activation Scales (BIS/BAS) 165, which provides a measure of the two
motivational systems of: (a) aversive motivation, punishment and non-reward, BIS; (b) appetitive motivation, reward and non-punishment, BAS. The BIS scale is measured on one subscale and includes items such as I worry about making mistakes, and Criticism or scolding hurts me quite a bit. The BAS scale is a composite scale encompassing 3 subscales: (i) reward responsiveness (e.g., It would excite me to win a contest); (ii) drive (e.g., I go out of my way to get things I want); (iii) fun-seeking (e.g., I crave excitement and new sensations). The BIS/BAS scales are made up of 24 items, scored on a 4-point scale ranging from 1 = very true for me to 4 = very false for me.
2.1.2.3 Biochemical parameters
Participants provided the laboratory values of thyroid function tests – TSH (normal range: 0.7 – 7 mUI/l), free thyroxine (fT4; normal range: 7.8 – 19.4 ng/l), and free triiodothyronine (fT3; normal range: 2.0 – 5.2 ng/l) – closest to the date of recruitment.
43
2.1.3 Statistical analysis
Socio-demographic, clinical, neurocognitive, and psychometric characteristics were described using absolute and relative frequencies for categorical variables and mean and standard error of the mean (SEM) for continuous variables. Spearman’s rank-order partial correlations were run to assess the relationship: (i) between clinical variables; and to test the association between clinical variables and (ii) neurocognitive, and (iii) psychometric variables, adjusting for the effect of age. Statistical significance was set at ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001 and ∗∗∗∗p ≤ 0.0001. Statistical analyses were conducted using SPSS statistical package version 22 (SPSS Inc, Chicago, IL) and visualised using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA).
44
2.2 Mouse model of hypothyroidism
2.2.1 AnimalsFour to five-week old C57BL/6J male mice were maintained in an air-conditioned animal room with a 12-h light/dark cycle. Mice were housed in plastic cages with five to six animals per cage and were provided with a pelleted basal diet and tap water ad libitum. All experiments were conducted in accordance with the principles of animal care and experimentation in the guidelines of the Italian Ministry of Health (Legislative Decree n. 116/92) and the European Community (European Directive 86/609/EEC). The Italian Ministry of Health approved the use of animals in this protocol (65E5B.10, n.734/2017-PR, 10/10/2017). The experiments were conducted at the Institute of Neuroscience, National Research Council, Pisa, by Grazia Rutigliano, with the valuable technical assistance of Sabina Frascarelli.
2.2.2 Treatments
Six experimental groups were analysed: (1) Hypothyroid mice (H);
(2) L-T4-treated, hypothyroid mice;
(3) L-T4 & L-T3-treated, hypothyroid mice; (4) L-T4 & T1AM-treated, hypothyroid mice; (5) T1AM-treated, hypothyroid mice;
45
Hypothyroidism was induced by administration of Methimazole (0.20 mg/g/die, Sigma-Aldrich) and Potassium Perchlorate (0.30 mg/g/die, Sigma-Aldrich) in drinking water for 21 days, starting from postnatal day 42. Euthyroid mice received simply tap water. Body weight (BW), water intake, and food consumption were monitored once per week throughout the hypothyroidism induction period.
At the end of the 21-day treatment, mice were implanted with subcutaneous ALZET® osmotic pumps, continuously delivering the different replacement compounds. Indeed, given the short half-life of L-T3 (< 24 h 166, 167) and T1AM (~ 40 min 168), we opted
for ALZET® osmotic pumps to ensure constant compound levels in plasma, at the same time avoiding extensive animal handling, thereby minimizing the potentially disrupting effect of animal distress on behavioural performances. ALZET® osmotic pumps with reservoir volume of 100 μl and delivery rate of 0.11 μl/h were used. The filling solution was prepared in sterile 1% bovine serum albumin (BSA), 0.9% saline, NaOH 0.1 M. The hormonal concentration in the solution was calculated to provide:
(1) H, no hormones provided; (2) 0.04 μg L-T4/g BW/die 169;
(3) 0.03 μg L-T4 & 0.007 μg L-T3/g BW/die (according to the physiological T4:T3 secretion ratio of 6:1 from the mouse thyroid 170);
(4) 0.04 μg L-T4 & 0.004 μg T1AM/g BW/die; (5) 0.004 μg T1AM/g BW/die;
46
(6) E, no hormones provided.
In absence of previous data on chronic T1AM administration, the dose for the present experiment was calculated multiplying by 28 (length of replacement treatment, in days) the minimum acute dose of T1AM which was reported to improve cognition in mice, namely 4 μg/kg BW 171.
For subcutaneous pump implantation, mice were anesthetised with 1.5% isoflurane in 100% oxygen in an anesthetic chamber. The skin was disinfected over the implantation site, on the back, slightly posterior to the scapulae. Then, a 1-cm incision was made perpendicular to the spine, and a pocket was created for the insertion of the pump. The wound was closed with 2-3 sutures. After the surgery, wound healing was monitored daily for 72 h. L-T4, L-T3 and T1AM used in the replacement treatments were purchased from Sigma-Aldrich.
2.2.3 Hormonal determinations
At time 0 and at the end of the replacement treatment with ALZET® osmotic pumps, blood was collected through the tail vein, and centrifuged (3˙500 rpm for 10 min at 4°C). Serum was aspirated and stored for the determination of L-T4.
Hormonal quantification was performed by Marco Borsò according to a method developed and previously described by Prof Alessandro Saba in our laboratory 172.
Briefly, 100 µL of plasma were placed in a 2mL Eppendorf tube with an appropriate amount (2.8 pmol T3 , 2.4 pmol
13C6-47
T4) of stable isotope labelled internal standards (IS). Samples were vortexed, kept 30 min at room temperature and, then, deproteinized with 300µL of cold-acetone for 30 min at 4°C. After centrifugation at 22780 x g for 10 min, the supernatants were dried at 40 °C under a gentle stream of nitrogen. Samples were reconstituted with 500 µL of 0,1 M potassium buffer (PH=4) prior to loading onto Agilent (Santa Clara, CA, USA) Bond-Elut Certify 130 mg SPE cartridges, pre-conditioned by consecutive wetting with 2 mL of dichloromethane/isopropanol (75/25 by volume), 2 mL of methanol and 2 mL of 0,1 M potassium buffer (PH = 4). Each cartridge was washed with 3.5 mL of water, 2 mL of 0.1 M hydrochloric acid, 7 mL of methanol and 3.5 mL of dichloromethane/isopropanol (75/25 by volume). After complete dryness, samples were eluted with 2 mL of dichloromethane/isopropanol/ammonium hydroxide (70/26.5/3.5 by volume), dried under nitrogen and derivatized adding 200 µL of 3.0 N hydrochloric acid in n-butanol and incubating for 45 min at 60 °C. This derivatization step allows the formation of the corresponding butyl esters of TH. Afterwards, samples were dried again as mentioned above, reconstituted with 100 µL of acetonitrile/0.1 M hydrochloric acid (50/50 by volume) and injected into the HPLC-MS-MS system.
Stock solutions of T3 and T4 were separately prepared at 1µg/mL concentration in methanol. Calibration curves were daily prepared by serial dilution with methanol at a concentration ranging from 0.1 to 100 ng/mL.
48
Instrument layout was made up of an Agilent (Santa Clara, CA, USA) 1290 UHPLC system, comprehending a binary pump, a column oven set to 20°C and a thermostated autosampler, coupled to an AB-Sciex (Concord, Ontario, Canada) QTRAP 6500+ triple quadrupole mass spectrometer, equipped with an IonDrive™ Turbo V source. The integrated switching valve was used to discard both head and tail of the HPLC runs. Chromatographic separations were carried out using a 110 Å, 2x50 mm, 3µm particle size, Gemini C18 column (Phenomenex, Torrance, CA), protected by a C18 Security Guard Cartridge. System control, data acquisition and analyses were performed using an ABSciex Analyst® version 1.7 software.
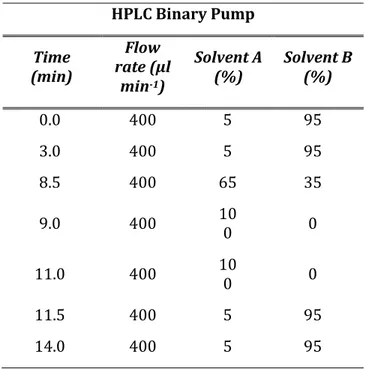
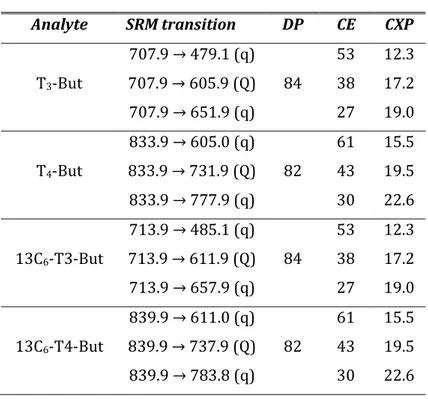
1μL of each sample were injected to the UHPLC system and the chromatographic separations were carried out with a flow rate of 400 µl min-1 using methanol/acetonitrile (20/80 by volume) added with 0.1% formic acid as solvent A and water containing 0.1% formic acid as solvent B. Mobile phases gradient conditions are shown in Table 1. Mass spectrometry selected reaction monitoring (SRM) method operated in positive electrospray ionization (ESI(+)) mode. For each compound, after the optimization of declustering potential (DP), collision energy (CE) and collision exit potential (CxP), three transitions were considered in the analysis. Based on the highest signal/noise ratios, one of them was used as quantifier (Q) and the other two as qualifiers (q). The SRM transitions and related parameters are shown in Table 2. Additional operative parameters were set as
49
follow: Collision gas (CAD), N2; operative pression with CAD gas, 3.3 mPa; Curtain gas (CUR), 20 arbitrary units; Gas source 1 (GS1), 60 arbitrary units; Gas Source 2 (GS2), 45 arbitrary units; ion spray voltage (IS), 5.50 kV; Source temperature (TEM), 650 °C; Entrance potential (EP), 10 V.
Table 1. HPLC pump conditions
HPLC Binary Pump Time (min) Flow rate (µl min-1) Solvent A (%) Solvent B (%) 0.0 400 5 95 3.0 400 5 95 8.5 400 65 35 9.0 400 100 0 11.0 400 100 0 11.5 400 5 95 14.0 400 5 95
50
Table 2. Mass spectrometry parameters
Operative Parameters Analyte SRM transition DP CE CXP 707.9 → 479.1 (q) 53 12.3 T3-But 707.9 → 605.9 (Q) 84 38 17.2 707.9 → 651.9 (q) 27 19.0 833.9 → 605.0 (q) 61 15.5 T4-But 833.9 → 731.9 (Q) 82 43 19.5 833.9 → 777.9 (q) 30 22.6 713.9 → 485.1 (q) 53 12.3 13C6-T3-But 713.9 → 611.9 (Q) 84 38 17.2 713.9 → 657.9 (q) 27 19.0 839.9 → 611.0 (q) 61 15.5 13C6-T4-But 839.9 → 737.9 (Q) 82 43 19.5 839.9 → 783.8 (q) 30 22.6 2.2.4 Behavioural testing
Over the 2 weeks preceding behavioural testing, mice underwent daily 10-min sessions during which they were extensively habituated to experimental handling. Over the last 3 consecutive days before the start of the experiment, mice were individually habituated for one hour to the behaviour testing room, a soundproof environment with 100 lux illumination level. Behavioural testing was performed at the end of the replacement treatment with ALZET® osmotic pumps, and included, in the
51
following order: (1) Elevated Plus Maze (EPM), to assess anxiety-related behaviours 173; (2) Open Field Test (OF), to assess
locomotion and anxiety-related behaviours 174; (3) Novel Object
Recognition Test (ORT), to assess hippocampus-dependent memory 175; (4) Tail Suspension Test (TST), to assess
depression-related behaviours 176.
2.2.4.1 Elevated Plus Maze
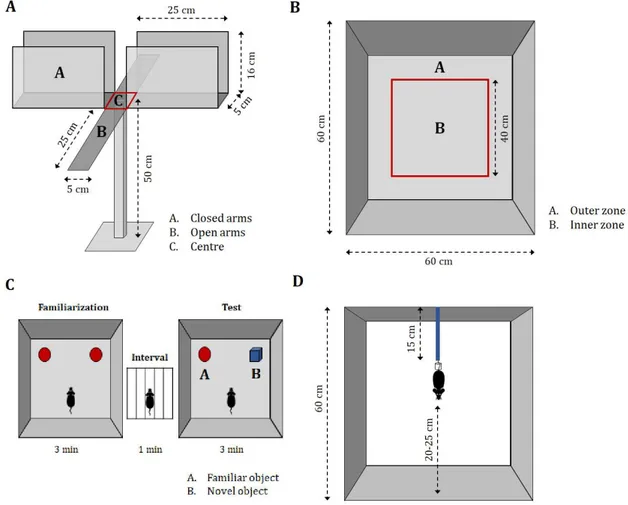
The EPM is broadly used to assess anxiety-related behaviours in rodents. It derives from the ethological preference of rodents for sheltered, enclosed, and dark spaces over open, heightened, and unprotected spaces. The EPM measures the conflict between the two opposite natural instincts of curiosity, which results in exploration of the open, unprotected spaces, and fear/avoidance, which results in the tendency to preferentially approach dark, enclosed spaces. The general principle is that the higher the “anxiety” levels, the lower the proportion of explorations in the open spaces in favour of the dark spaces.
The EPM apparatus was made of black Plexiglas and consisted of 4 arms (25 cm [length] x 5 cm [width]) forming a plus sign, elevated 50 cm above the floor. Two opposite facing arms have walls (16 cm [height]) and open roof (closed arms), while the other two opposite facing arms have no walls (open arms) (Figure 3.A). The entire apparatus was placed in a squared arena (60 x 60 cm, normally used for the ORT) to protect the mice that fall or attempt to escape during the test.