Supporting Information for
“Synthesis of Dichlorophosphinenickel(II) compounds and
Their Catalytic Activity in Suzuki Cross-Coupling Reactions: A
Simple Air-Free Experiment for Inorganic Chemistry
Laboratory”
Todsapon Thananatthanachon* and Michelle R. Lecklider
Department of Chemistry, University of Evansville, Evansville, IN 47722, United States
Table of Content
Page 1. Complete list of chemicals, equipment, and instruments S2
2. Student handout S3
3. Additional notes to instructors S7
4. Class data on % yield of the synthesized products, S11 the % conversion, and TON from the Suzuki cross-coupling
reactions
5. Photos for the setup of the air-free apparatus S12
6. Photos of the synthesized nickel products S14
7. UV-VIS spectra of the synthesized and standard nickel compounds S15 8. 19F{1H} NMR spectra from the Suzuki cross-coupling reactions S18
1. List of Equipment and Chemicals
Equipment and instrument
Nuclear Magnetic Resonance spectrometer capable of running 19F nucleus UV-VIS spectrometer
2-Neck round bottom flask Reflux condenser
Magnetic stir bar Sand bath or oil bath Tygon tubings 6-Inch test tube Plastic Y splitter
Disposable pasteur pipette Rubber septum
Glass adapter, inner joint Vacuume grease
Chemicals
Reagents and solvents
Name CAS
Nickel chloride hexahydrate (NiCl2⋅6H2O) 7791-20-0
Triphenylphosphine (PPh3) 603-35-0
Tricyclohexylphosphine (PCy3) 2622-14-2
1,2-Bis(diphenylphosphino)ethane (DPPE) 1663-45-2
Ethanol 64-17-5
Potassium phosphate (K3PO4) 7778-53-2
t-Amyl alcohol (or 2-methyl-2-butanol) 75-85-4
Phenylboronic acid 98-80-6 1-Bromo-4-trifluoromethylbenzene 402-43-7 Chloroform-D 865-49-6 Standards Name CAS 4-(trifluoromethyl)biphenyl 398-36-7 Bis(diphenylphosphine)nickel(II) chloride 14264-16-5 Bis(dicyclohexylphosphine)nickel(II) dichloride 115647-96-6 1,2-bis(diphenylphosphino)ethanenickel(II) chloride 146747-23-5
2. Student Handout
Synthesis and Catalytic Activity of Dichlorodiphosphinenickel(II) Compounds
Introduction
Catalytic processes are widely utilized in various chemical reactions, where more than 75% of all industrial processes require the use of catalysts. Catalysts enhance the rate of reaction by
reducing the activation energy, and are regenerated back at the end of the reaction. Since the catalysts are not consumed by the reaction, only a small amount (catalytic amount) of catalyst is required. However, the major drawback of current catalytic processes is the use of expensive precious metals such as Pd, Pt, Ru, Ir, and Rh, which results in a high operational cost. Recent research has focused on the utilization of an inexpensive and abundant first-row transition metal such as Ni, Fe, Cu, and Co. For example, an iron compound 1 [(iPrPNP)FeH(CO)Br)] has been demonstrated as an effective catalyst for the hydrogenation of ketones (Scheme 1).1
1
Scheme 1. The structure of [(iPrPNP)FeH(CO)Br)] catalyst 1 (left) and the catalytic
hydrogenation of acetophenone using catalyst 1 (right).
Another example of the use of first-row transition metal catalysts is the Suzuki cross-coupling reaction between aryl halide and arylboronic acid by various NiCl2(PR3)2 compounds (PR3 =
PPh3, PCy3, PMePh2, etc.) (Scheme 2).2
Scheme 2. Suzuki cross-coupling reaction using NiCl2(PR3)2 catalyst.
X = halide, R = alkyl, aryl, H
The mechanism of this Suzuki cross-couplings is presented in Scheme 3,3 which begins with a reduction of Ni(II) to Ni(0) by arylboronic acid. Ni(0) subsequently undergoes oxidative addition of aryl halide to form the intermediate A, which can undergo transmetallation, and reductive elimination to release the desired product. The Ni(0) is regenerated and is ready to accept another equivalent of aryl halide.
N PiPr 2 PiPr 2 Fe H Br CO Ph O Me Ph OH Me 0.05 mol% 1 0.1 mol% KOtBu solvent, 4.1 atm H2 RT X (HO)2B NiCl2(PR3)2 K3PO4, dioxane + R R
Scheme 3. Proposed mechanism of the Suzuki cross-coupling
Hazards
Safety goggles must be worn at all time during the experiment. NiCl2⋅6H2O,
triphenylphosphine, and chloroform-D are harmful if swallowed or if inhaled, and can cause skin and eye irritation. Tricyclohexylphosphine, potassium phosphate, 4-(trifluoromethyl)biphenyl, bis(triphenylphosphine)nickel(II) chloride, and 1,2-bis(diphenylphosphino)ethanenickel(II) chloride are irritant, and can cause skin and eye irritation. Phenylboronic acid is toxic, and is harmful if swallowed. Ethanol, t-amyl alcohol, and 1-bromo-4-(trifluoromethyl)benzene are flammable liquids, and can cause skin and eye irritation. No hazardous classification is determined for 1,2-bis(diphenylphosphino)ethane and bis(tricyclohexylphosphine)nickel(II) chloride.
Experiment overview: In this experiment, your group will collaborate with two other groups to:
1. Synthesize three Ni catalysts containing three different phosphine ligands. The first two phosphines are monodentate PPh3 and PCy3. The third phosphine is bidentate
DPPE.
2. Determine the catalytic activity of the synthesized Ni compounds as well as the NiCl2⋅6H2O precursor in the Suzuki cross-coupling reaction between phenylboronic
acid and 1-bromo-4-(trifluoromethyl)benzene.
3. Determine the effect of the phosphine ligands on the synthesis and the catalytic activity of the nickel(II) products.
Ni0L n Cl R Ni Ln Cl R (HO)2B Ni Ln R R BCl(OH)2 Oxidative addition Transmetallation Reductive elimination (HO)2B b -b b A
General comments:
a) The solutions of NiCl2(PR3)2 compounds are somewhat air-sensitive, meaning that they
will gradually decompose in the presence of oxygen. Therefore, the syntheses of these complexes must be performed under an inert gas (N2) and in an oxygen-free environment.
The products, however, are quite stable in air once isolated in the solid form.
b) The reaction must be carried out in a closed system as presented in Figure 1, meaning that it cannot have any openings to air (or oxygen). Therefore, you will have to close all of the openings by using a glass stopper or rubber septum, except for one opening that will be connected to the N2 source. The glass stopper (or the rubber septum) is to be removed from the flask only when a reagent is being added. The reflux condenser is only required when the
solution is being heated to above the boiling point of the solvent.
Experimental
1. Synthesis of NiCl2(PR3)2 catalysts4
Assemble the apparatus as instructed and purge it with a medium flow of N2 for
approximately 5 minutes (check the N2 flow rate with the oil bubbler) to remove O2 and
H2O vapor before adding any reagents. Add NiCl2⋅6H2O (1.01 mmol) and ethanol (4 mL)
to a 2-neck round bottom flask. After stirring the reaction mixture until all of the nickel precursor is dissolved, add 2.10 mol equivalent of the monodentate phosphine to the flask. Question: what should be the mol equivalence if the bidentate DPPE is employed? Stir and heat the reaction mixture at reflux for 1 h. After cooling to RT and at 0 °C for 10 min, collect and wash the product with ethanol and ether. Characterize the synthesized nickel complex by UV-VIS spectroscopy in CH2Cl2.
2. Catalytic activity of NiCl2(PR3)2 catalysts in Suzuki cross-coupling reactions.2
Carry out the catalytic reaction under N2. Dissolve 10 mmol of K3PO4, 7.5 mmol of
phenylboronic acid, 5 mmol 1-bromo-4-(trifluoromethyl)benzene, and 10 mol% (with respect to the limiting reagent) of the prepared Ni catalyst in 25 mL of t-amyl alcohol. After stirring the reaction mixture at reflux overnight, prepare an NMR sample by adding
Figure 1. Apparatus setup To N2, and
oil bubbler
approximately 1 mL of the solution from the reaction mixture and 0.5–1 mL of CDCl3
into an NMR tube. Cap the tube and invert it several times to ensure thorough mixing. Obtain the percent conversion of the desired organic product and the turnover number of the catalyst by 19F{1H} NMR spectroscopy.
3. Catalytic activity of NiCl2⋅6H2O precursor in the Suzuki cross-coupling reaction.
Follow the same procedure as in step 2, but use NiCl2⋅6H2O as the catalyst instead of the
synthesized NiCl2(PR3)2 catalysts. The use of NiCl2⋅6H2O, will serve as a baseline for
the catalytic activities. Obtain the percent conversion of the desired organic product and the turnover number of the catalyst by 19F{1H} NMR spectroscopy.
Prelab questions
1. Provide an example of a catalyst and the reaction it catalyzes that you learned from organic chemistry.
2. Calculate the quantity in g or mL of every reagent used in this experiment.
3. Use Scheme 2 as an example to draw the structure of the desired product from the Suzuki cross-coupling reaction between phenylboronic acid and
1-bromo-4-(trifluoromethyl)benzene
4. Perform a SciFinder® search to obtain the chemical shift in the 19F{1H} spectrum of 1-bromo-4-(trifluoromethyl)benzene substrate and the desired product.
For lab report: your lab report must include the followings:
1. The percent yield of your synthesized nickel(II) product.
2. The percent conversion of the desired product and the TON of every nickel catalyst. 3. Discussion on the effects of the phosphine ligands on:
i. The rate of formation of the nickel compounds.
ii. The catalytic activity of the nickel compounds in the Suzuki cross-coupling reactions.
4. Discussion on the effect of the phosphine ligands on the structure and the magnetic properties of the nickel catalysts (it is known that NiCl2(PPh3)2 is tetrahedral while
NiCl2(PCy3)2 and NiCl2(DPPE) are square planar.5). References
1. Langer, R.; Leitus, G.; Ben-David, Y.; Milstein, D. Angew. Chem. Int. Ed. 2011, 50, 2120-2124.
2. Ramgren, S.D.; Hie, L.; Ye, Y.; Garg, N.K. Org. Lett. 2013, 15, 3950-3953.
3. Rosen, B.M.; Quasdorf, K.W.; Wilson, D.A.; Zhang, N.; Resmerita, A.M.; Garg, N.K.; Percec, V. Chem. Rev. 2011, 111, 1346-1416.
4. Standley, E.A.; Smith, S.J.; Muller, P.; Jamison, T.F. Organometallics 2014, 33, 2012-2018.
5. Christendat, D.; Butler, I.S.; Gilson, D.F.R.; Hynes, R.; Morin, F.G. Inorg. Chim. Acta
2004, 357, 3775-3779.
1. All chemicals and solvents were reagent grade and were used as received. No further purification of solvents such as degassing were performed.
2. The make and model of the instrument used in the experiment NMR spectrophotometer: Bruker ADVANCE III HD Nanobay
UV-VIS spectrophotometer: Agilent Cary 4000 Scanning UV-VIS Spectrophotometer 3. Representative calculation for the percent yield of the dichlorophosphinenickel(II)
compound NiCl2(PPh3)2
Mass of NiCl2(PPh3)2 obtained = 1.23 g
Mole of NiCl2(PPh3)2 obtained = (1.23 g NiCl2(PPh3)2)
1 mol NiCl2(PPh3)2 654.2 g NiCl2(PPh3)2 = 1.88 mmol
Mole of NiCl2⋅6H2O used = (0.96 g NiCl2⋅ 6H2O) 1 mol NiCl2
⋅ 6H2O 237.7 g NiCl2⋅ 6H2O = 4.04 mmol
= Theoretical yield of NiCl2(PPh3)2
Percent yield = 1.88 mmol 4.04 mmol×100 = 46.5 %
4. The experiment can be planned as follow:
During the regular lab period: the students synthesize and isolate the
dichlorophosphinenickel(II) compound. The students then obtain the UV-VIS spectrum of the synthesized product. Lastly, the students set up a catalytic reaction of the prepared nickel compound, and allow it to run overnight
The following day: the students turn off the reaction. They prepare an NMR sample and acquire a 19F{1H} NMR spectrum of the final solution mixture.
5. The catalytic reaction can be scaled up or scaled down depending on the yield of the nickel(II) compounds obtained. However, the catalyst loading needs to be consistently maintained at the same mol% for every student (for example 10 mol% as recommended in the student handout).
6. The chemical shifts of the 1-bromo-4-(trifluoromethyl)benzene substrate and the desired 4-(trifluoromethyl)biphenyl are extremely close in the 19F{1H} NMR spectrum, which could be difficult to assign. The assignment of the signals can be accomplished by adding a small sample of 1-bromo-4-(trifluoromethyl)benzene substrate to a NMR sample. The signal that gains intensity will correspond to the substrate (See Figures S11 and S12). On the other hand, an addition of the standard 4-(trifluoromethyl)biphenyl product to a NMR sample. The signal that gains the intensity will correspond to the product (See Figures S8 and S13)
7. Representative calculation for the percent conversion of 4-(trifluoromethyl)biphenyl With NiCl2(DPPE) catalyst
Integration of the signal of the product = 1.000 Integration of the signal of the unreacted substrate = 0.1964
% conversion = 1.000
1.000 + 0.1964×100 = 83.6 %
8. Representative calculation for the turnover number of the catalyst With NiCl2(DPPE) catalyst
% conversion = 83.6 %
Mole of product formed = (83.6%)(5 mmol) 100 = 4.18 mmol
Mole of catalyst used (10 mol% of limiting substrate) = (10 mol%)(5 mmol) 100
= 0.5 mmol
TON = 4.18 mmol
0.5 mmol = 8.36
9. Amount of reagents needed Synthesis of nickel(II) compounds
NiCl2⋅6H2O 1.01 mmol 0.240 g
PPh3 2.12 mmol 0.556 g
PCy3 2.12 mmol 0.595 g
DPPE 1.06 mmol 0.422 g
Suzuki cross-coupling reactions
K3PO4 10 mmol 2.12 g
Phenylboronic acid 7.5 mmol 0.915 g
1-Bromo-4-(trifluoromethyl)benzene 5 mmol 0.70 mL
NiCl2(PPh3)2 0.5 mmol 0.327 g
NiCl2(PCy3)2 0.5 mmol 0.345 g
NiCl2(DPPE) 0.5 mmol 0.264 g
NiCl2⋅6H2O 0.5 mmol 0.119 g
10. More variables can also be altered for the second part (catalysis) of the experiment. The instructor could assign a student or a group of students to perform a reaction with
different variation, and pool the data at the end of the lab period. The possible variables are:
a. Exclusion of the base K3PO4
b. Changing the base: NaOH and Na2CO3
c. Changing the solvent: toluene and dioxane
d. Effect of free phosphine ligand. Additional phosphine ligand may help preventing a decomposition of the catalyst.
11. As stated in the Student Handout section, the solutions of the nickel phosphine
compounds are somewhat air-sensitive. However, since the rate of formation of these compounds are fast (instantly for NiCl2(PCy3)2 and NiCl2(DPPE), and 10 minutes for
NiCl2(PPh3)2), the inclusion of air do not have a significant effect on the synthesis of
these compounds. The instructor could omit the air-free apparatus set-up for this part of the experiment, if they desire to.
However, the second part of the reaction (catalysis) involves a reflux of the solution mixture overnight, therefore the presence of air will have a significant effect on the catalytic activity, and the air-free apparatus set-up is necessary.
12. The results of this experiment can be used to demonstrate not only the effect of the electron donating ability of phosphine ligands on the catalytic activity in the Suzuki coupling reaction, but also the effect to the structure and the magnetic properties of the nickel compounds, which can be explained by the crystal field theory. The PPh3 ligand is
less electron rich and therefore is a weaker field ligand than the PCy3 ligand. The
4-cooridnated transition metal compounds with a weak field ligand prefer a tetrahedral geometry, while the ones with a strong field ligand prefers a square planar geometry. From the crystal field theory, the d orbitals of the metal center split into e and t2 for the
tetrahedral structure, which make the complex with d8 electron configuration to be a paramagnetic. Four different energy levels for dxz and dyz, dz2, dxy, and dx2-y2 are resulted
for a square planar complex. Due to the absence of unpaired electrons in the d8 square planar complexes, these complexes exhibit paramagnetic properties.
Figure S1: Crystal field d orbital splitting diagram of a d8 tetrahedral compound (left, paramagnetic) and d8 square planar compound (right, diamagnetic)
4. Class data t2 e dxz dyz dz2 dxy dx2-y2
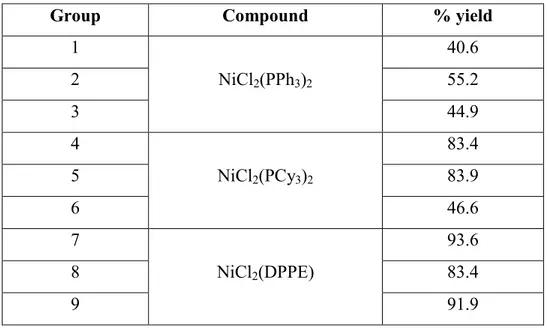
Table S1. % yield of the synthesized products
Group Compound % yield
1 NiCl2(PPh3)2 40.6 2 55.2 3 44.9 4 NiCl2(PCy3)2 83.4 5 83.9 6 46.6 7 NiCl2(DPPE) 93.6 8 83.4 9 91.9
Table S2. % Conversion and TON from the Suzuki cross-coupling reaction
Group Catalyst % conversion of
4-(trifluoromethyl)biphenyl TON of catalyst 1 NiCl2(PPh3)2 11.8 1.18 2 18.4 1.84 3 19.2 1.92 4 NiCl2(PCy3)2 27.3 2.73 5 20.7 2.07 6 28.4 2.84 7 NiCl2(DPPE) 83.6 8.36 8 94.9 9.49 9 66.2 6.62 Instructor NiCl2•6H2O 4.0 0.4
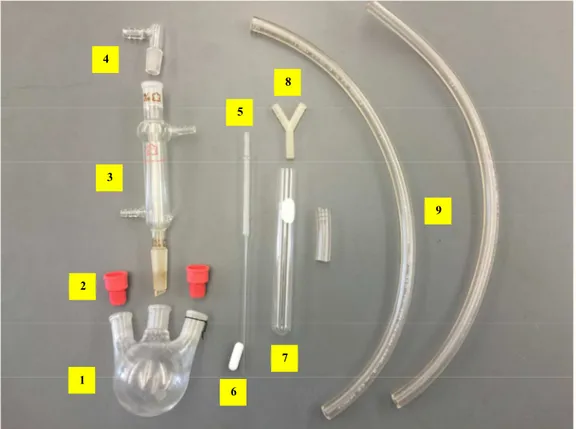
5. Photos for the setup of air-free apparatus
Figure S2. Glassware and equipment needed for the air-free apparatus
Number Item
1 2- or 3-neck round bottom flask 2 Rubber septum (or glass stopper)
3 Reflux condenser
4 Glass adapter, inner joint 5 Disposable Pasteur pipette
6 Magnetic stir bar
7 6-inch test tube
8 Y splitter 9 Rubber tubings 1 2 3 4 5 6 7 8 9
Figure S3. Assembled air-free apparatus
6. Photo of the synthesized nickel products N2 in N2 out
Oil bubbler With vegetable oil
Figure S4. Synthesized nickel products
Left: NiCl2(PPh3)2 = green
Center: NiCl2(PCy3)2 = pink
Right: NiCl2(DPPE) = orange
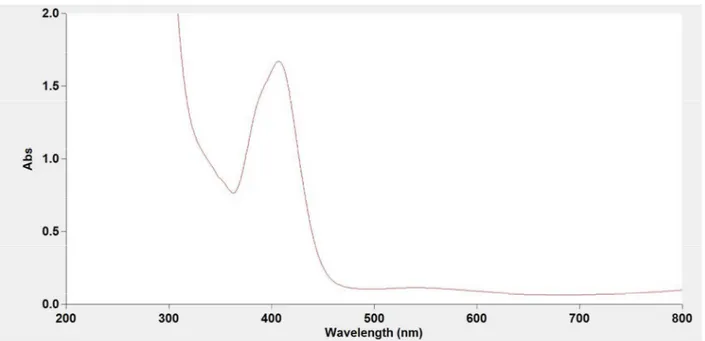
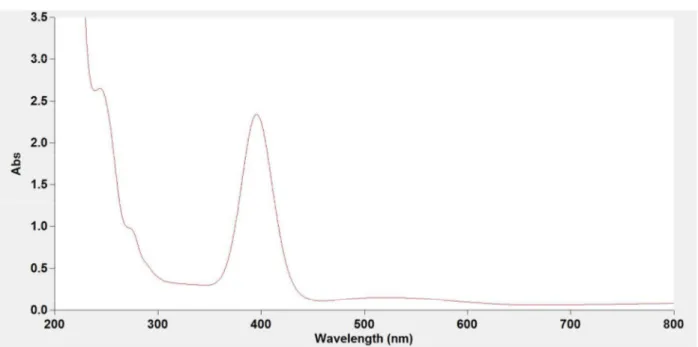
Figure S5. UV-VIS spectra of the synthesized (top) and the standard (bottom) NiCl2(PPh3)2 in
Figure S6. UV-VIS spectra of the synthesized (top) and the standard (bottom) NiCl2(PCy3)2 in
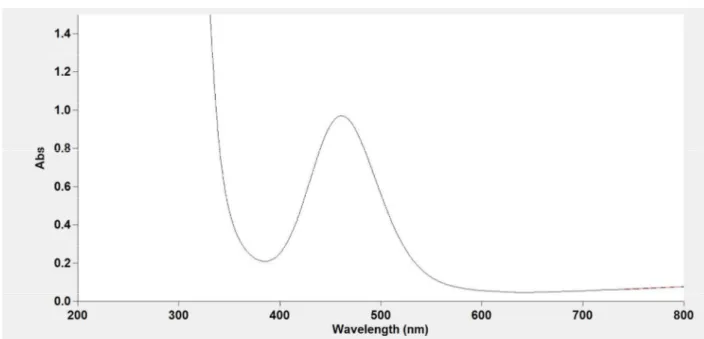
Figure S7. UV-VIS spectra of the synthesized (top) and the standard (bottom) NiCl2(DPPE) in
CH2Cl2. The λmax = 461 nm was observed.
Figure S8. 19F{1H} NMR spectrum of the Suzuki cross coupling reaction with NiCl2(PPh3)2
Figure S9. 19F{1H} NMR spectrum of the Suzuki cross coupling reaction with NiCl2(PCy3)2
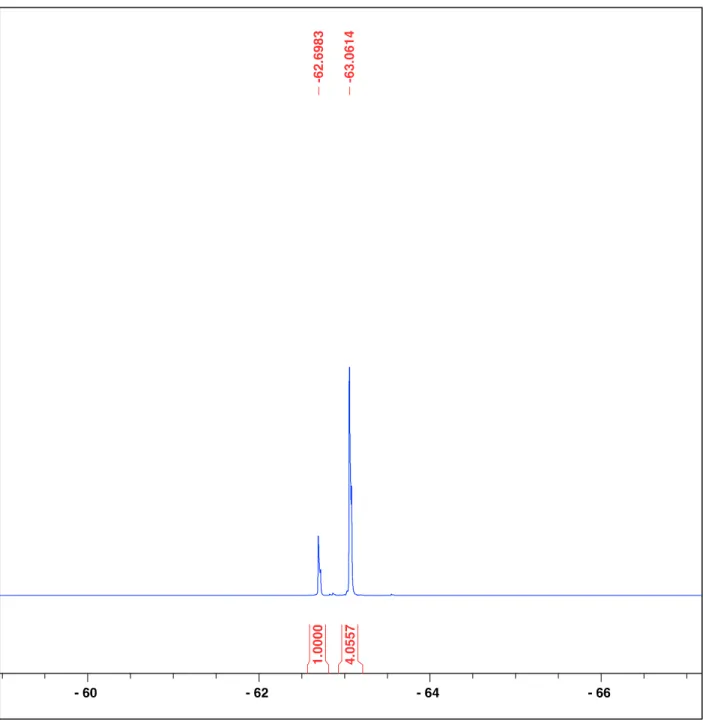
Figure S10. 19F{1H} NMR spectrum of the Suzuki cross coupling reaction with NiCl2(DPPE)
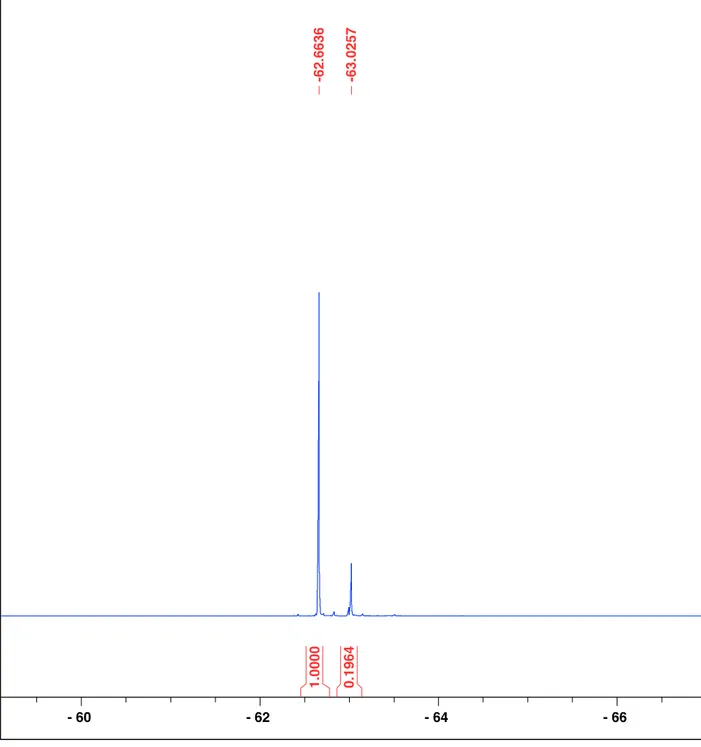
Figure S11. 19F{1H} NMR spectrum of the Suzuki cross coupling reaction with NiCl2⋅6H2O
Figure S12. 19F{1H} NMR spectrum of the Suzuki cross coupling reaction with NiCl2⋅6H2O
catalyst (same sample in Figure S11) with 1-bromo-4-(trifluoromethyl)benzene added. The upfield signal at δ = -63.2 ppm increases in intensity, therefore it corresponds to the unreacted
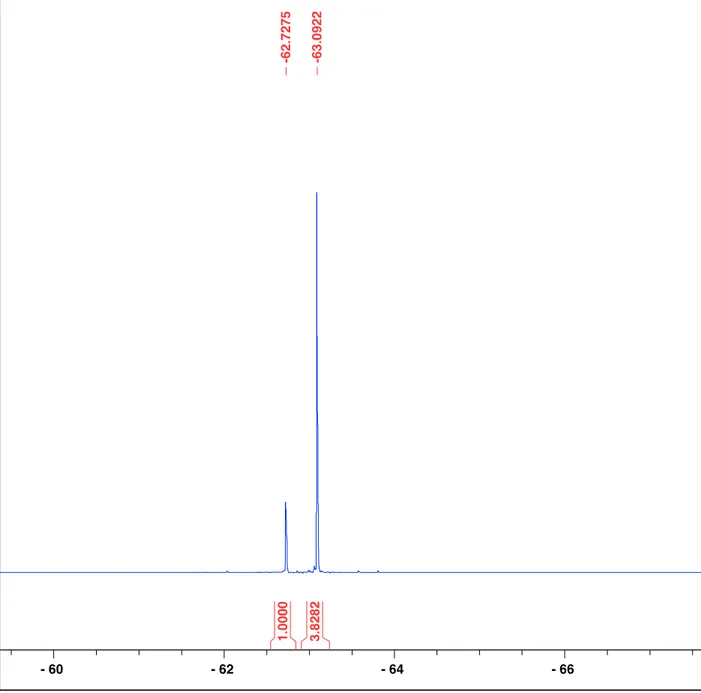
Figure S13. 19F{1H} NMR spectrum of the Suzuki cross coupling reaction with NiCl2(PPh3)2
catalyst (same sample in Figure S8) with standard 4-(trifluoromethyl)biphenyl added. The downfield signal at δ = -62.7 ppm increases in intensity, therefore it corresponds to the desired