6
Chapter 1. Separation anxiety disorder
1.1 Separation anxiety disorder
Separation anxiety has traditionally been defined as a childhood phenomenon. This disorder is conceptually rooted in both developmental research and attachment theory. Distress upon separation from one‟s attachment figure is the developmental norm during early childhood and is considered to be an evolutionarily adaptive mechanism designed to keep the defenseless child in close proximity to his adult caregiver (Ainsworth, 1973; Bowlby, 1982). Only when separation distress becomes prolonged, excessive, and developmentally inappropriate or impairing, is a psychiatric diagnosis typically made. The estimated prevalence of childhood separation anxiety disorder is 4% (American Psychiatric Association, 1994). Manicavasagar et al. (1996) proposed that the core symptoms of separation anxiety that is excessive and often disabling distress in the face of actual or perceived separation from major attachment figures may indeed persist or arise throughout adulthood.
1.2 Adult separation anxiety disorder
It has been a longstanding convention to regard separation anxiety disorder as a condition confined to childhood and adolescence (American Psychiatric Association, 2000). This age-restricted formulation contrasts with overall trends in the classification of other anxiety subtypes, in which it is increasingly acknowledged that several disorders can have their onset across a broad age range spanning childhood and adulthood (Kessler et al., 2005). The adult form of separation anxiety disorder (ASAD) has only recently been described in the psychiatric literature (Manicavasagar et al., 1997; 2000), this syndrome is listed in the DSM-IV (Dozier, 1999) and is frequently associated with mood disorders (Pini et al., 2005a; Shear et al., 2006). The National Comorbidity Study Replication was the first large-scale epidemiological study to include the diagnosis, revealing a lifetime prevalence of 6.6% (Shear et al., 2006). Apart from minor symptom differences associated with maturation, the adult pattern appears to parallel the established category of childhood separation anxiety disorder (Manicavasagar et al., 1997). Individuals with adult separation anxiety disorder report extreme anxiety about separations from major attachment figures (parents, partner, children), fear that harm would befall those close to them and need to maintain proximity to them.
7
Associated symptoms included a reluctance to leave places of safety, sleep difficulties (fear and avoidance of sleeping alone, and nightmares about separation). When faced with real or feared separations from family members, persons with ASAD are at risk of developing panic attacks (Manicavasagar et al., 1997). This condition may affect individual‟s behavior and lead to severe impairment in social relationships (Shear et al., 2006).
1.3 Biological Correlates of ASAD
Limited data are available concerning the possible etiologic factors underlying ASAD. A small study based on an anxiety clinic showed that children with separation anxiety disorder were much more likely to have one parent with ASAD (Manicavasagar et al., 2001). Familial concordance seemed particularly strong for mothers and daughters, a finding that is in accord with a twin study suggesting that females may have a stronger genetic loading for separation anxiety (Silove et al., 1995). However, little is known about the neurobiology of separation anxiety in humans. Several studies showed that translocator protein (TSPO) density values are associated with states of stress and anxiety and these study, also investigated whether TSPO density may be specifically associated with ASAD (Pini et al., 2005a, b; Chelli et al., 2008; Abelli et al., 2010). The first study of PD patients found that lower-density platelet TSPO was present only where there was comorbid ASAD. A relationship between TSPO density and severity of separation anxiety symptoms gave further weight to the findings. In a second study, TSPO platelet density was found to be lower in patients with major depression, but this effect was exclusively accounted for by the presence of comorbid ASAD; again, there was an inverse relationship between TSPO density values and the severity of separation symptoms. A third study found an association between lower platelet TSPO density and the presence and severity of ASAD in bipolar patients. These findings suggest that ASAD may be linked to a dysregulated neurobiological mechanism relevant to attachment and separation anxiety for which there are extensive animal models (Insell et al., 2001). TSPO participates in the binding and translocation of cholesterol into mitochondria (Jamin et al., 2005), an initial step in the production of steroids. The impact on neurosteroid biosynthesis in turn could influence cognitive functioning and behaviors associated with emotional states such as anxiety (Birzniece et al., 2006).
Others studies investigated oxytocin, a neurotransmitter or neuromodulator that influences attachment processes in animals and possibly humans (Insel, 1997; Donaldson et al., 2008). Oxytocin is involved in the regulation of social bonding and separation. Separation, the
8 disruption of social bonds, is a painful experience which might be buffered by oxytocin (Panksepp, 2003a). Accordingly Meinlschmidt et al., (2007) found stress system reactivity to be attenuated after application of oxytocin in subjects with early parental separation. There is good evidence that loneliness is the human equivalent of separation distress in animals (Panksepp, 2003b) and so Panksepp et al. (1997) hypothesized that an orally effective ligand for oxytocin receptors should prove to be a powerful alleviator of loneliness in humans. Although these biological inquiries are in their early phase, the preliminary results offer support for a link involving ASAD, and neurobiological markers thought to be relevant to the process of bond formation.
1.4 Pharmacological treatment of Anxiety Disorder
Anxiety disorders are highly prevalent disabling disorders (Kessler et al., 2005) that frequently turn into chronic clinical conditions (Nutt et al., 2002). Benzodiazepines such as diazepam are fastacting and effective antianxiety agents (Baldwin et al., 2005; Rudolph et al., 2006; Bandelow et al., 2008) and the most commonly prescribed anxiolytics. However, their side effects such as sedation and, following chronic administration, development of tolerance, consecutive abuse liability, and withdrawal symptoms render their use problematic in the long-term treatment of anxiety disorders. Currently, antidepressants such as selective serotonin reuptake inhibitors are first-line treatment for most anxiety disorders. However, their anxiolytic effects occur only after several weeks of treatment (Nutt et al., 2002; Baldwin et al., 2005; Bandelow et al., 2008). Thus, there is need for anxiolytic agents that retain the rapid anxiolytic potential of benzodiazepines but lack their unfavorable side effects. Neurosteroids are synthesized from cholesterol or steroidal precursors and modulate neurotransmitter receptors (Rupprecht et al., 1993; 1999; Balelli et al., 2005). The translocator protein ultimately promoting neurosteroid synthesis (Papadopoulos et al., 2006a; 2006b) and certain ligands of this protein have been shown to enhance neurosteroidogenesis in the brain (Romeo et al., 1993; Serra et al., 1999; Verleye et al., 2005) and to exert acute anxiolytic/anticonflict activity in rodent models (Okuyama et al., 1999; Serra et al., 1999; Kita et al., 2004, Verleye et al., 2005). Neurosteroids modulate GABAA receptors via an allosteric site different from that targeted by benzodiazepines (Belelli et al., 2005; Hosie et al., 2006; Rudolph et al., 2006). These distinct sites of action at the GABAA receptor might explain the lack of tolerance development and withdrawal symptoms after TSPO ligand-induced neurosteroidogenesis. Indeed, XBD173 binds to the TSPO with nanomolar affinity, enhances
9
GABAergic neurotransmission via induction of neurosteroidogenesis and shows anxiolytic efficacy in humans with a favorable side-effect profile, which suggests that the translocator protein represents a target for anxiolytic drug discovery (Rupprecht et al., 2009).
10
Chapter 2. Translocator protein: structure, functions and involvement in
pathology
2.1 Translocator protein
Translocator protein (18 kDa; TSPO), formerly known as the peripheral-type benzodiazepine receptor (PBR), was first identified in 1977 as an alternative binding site in the kidney for the benzodiazepine diazepam (Braestrup et al., 1977; Papadopoulos et al., 2006a). Because the benzodiazepine diazepam is a widely used drug ligand specific for the GABAA receptor in the central nervous system the subsequent identification of TSPO-specific ligands allowed for the pharmacological differentiation between TSPO and the GABAA receptor. This was accomplished by use of the isoquinoline carboxamide PK 11195, which binds with nanomolar affinity to TSPO but has no affinity for the GABAA receptor (Le FG et al., 1983). TSPO was then characterized by its ability to bind small molecule drugs, cholesterol, diazepam binding inhibitor (DBI), and porphyrins with diverse affinities (Papadopoulos et al., 2006a). In mammals, the biological significance of TSPO has been studied for decades and has been shown to be involved in a variety of cellular functions, including cholesterol transport and steroid hormone synthesis, mitochondrial respiration, mitochondrial permeability transition pore (MPTP) opening, apoptosis, and cell proliferation and differentiation (Fan et al., 2009). Although some cellular functions of TSPO are conserved, such as cholesterol-binding and transport, their biological significance seems to have adapted to serve specific functions critical for various tissues. Nevertheless, the conservation of TSPO throughout evolution highlights the significance of this protein for proper cellular function and development (Papadopoulos et al., 1997).
2.2 Molecular identity of TSPO
The TSPO gene is localized to the 22q13.31 chromosome in the human genome as a single copy and encodes 169 amino acids (Chang et al., 1992; Riond et al., 1991). Cloning and characterization of the TSPO gene in human and rat revealed that it is composed of four exons, with a large intron containing repetitive sequences separating the first and second exons. The first exon and parts of the second and fourth exons remain untranslated. Multiple
11 transcription start sites have been identified in the TSPO promoter in different species, including multiple internal transcription initiation sites (Giatzakis et al., 2004). Sequence analysis has shown that the human TSPO gene is regulated by a TATA-less, GC-rich promoter that contains five tandem putative binding sites for Sp factors. While this promoter architecture is commonly associated with housekeeping genes, a similar structure is found in the promoters of several growth factor receptor genes that have been implicated in the regulation of cell proliferation and carcinogenesis (Dimario, 2002). TSPO is expressed at the highest level in testis, and in Leydig, adrenal cortical, ovarian granulose, and luteal cells, as well as in placenta and brain glia cells, which are all know to form steroids de novo. Further studies have demonstrated that separate regions of the promoter drive TSPO transcription in steroidogenic and non-steroidogenic cells, suggesting that tissue specific transcriptional regulation accounts for differences in TSPO expression between these cell types (Giatzakis et al., 2007; Giatzakis et al., 2004).
2.3 Molecular structure of TSPO
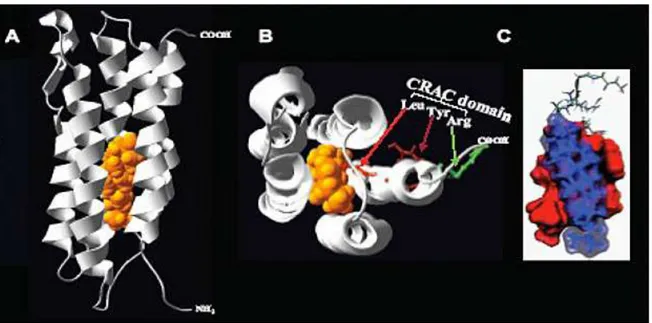
TSPO is heterotrimer composed of three protein components (Fig. 1): an isoquinoline binding protein (IBP; 18 kDa), a voltage-dependent anion channel (VDAC; 32 kDa); and an adenine nucleotide transporter (ANT; 30 kDa) (McEnery et al., 1992).
12 Fig. 1. The TSPO complex consists of several IBP molecules typically in conjugation with VDAC and ANT. Additional molecules, such as pk10, PRAX-1, and PAP7, can be linked to the TSPO. Furthermore, molecules of the Bcl-2 family and creatine kinase can be attached to VDAC and ANT. Endogenous ligands that bind to TSPO include the following: protoporphyrin IX, DBI and its metabolite triakontatetraneuropeptide (TTN), and phospholipase A2 (PLA2). At the functional end, TSPO and its ligands have been shown to be involved in mitochondrial permeability, apoptosis, steroidogenesis, cell proliferation, mitochondrial respiration, modulation of voltage-dependent calcium channels, ischemia, microglial activation, and immune response. (Reprinted from Veenman et al., 2006).
Intracellularly, TSPO is reported to be located primarily on mitochondrial membranes (Anholt et al., 1986; Antkiewicz-Michaluk et al., 1988; Mukherjee, 1989). Topographic analysis of TSPO distribution on the mitochondrial membrane has indicated that several IBP molecules can be associated with one VDAC molecule (Papadopoulos et al., 1994) and that this complex is located at the contact sites between the outer (OMM) and inner (IMM) mitochondrial membranes (Culty et al., 1999). In addition, it appears that the ratio of IBP to VDAC and ANT is tissue and treatment specific (Golani et al., 2001; Veenman et al., 2002). It has also been suggested that IBP may be present without interconnections with VDAC and ANT (Veenman et al., 2002). Structurally, VDAC forms the core of a protein complex, which in addition to IBP and ANT, may include several proteins, such as creatine kinase and proteins of the Bcl-2 family (Fig. 1) (Papadopoulos et al., 1999; Tsujimoto et al., 2000; Dolder et al., 2001). Other proteins which are found to interact with the TSPO complex are the following (Fig. 1): a protein of 10 kDa (pk10) (Blahos et al., 1995); PBR associated protein 1 (PRAX-1) (Galiegue et al., 1999); and PBR-associated protein 7 (PAP7) (Li et al., 2001a; Liu et al., 2003). It has been suggested that mitochondrial TSPO composed of IBP, VDAC, and ANT take part in apoptotic mechanisms (Veenman et al., 2004; Levin et al., 2005). VDAC and
13
ANT are the core components of the MPTP, of which extended opening may lead to mitochondrial swelling and subsequent release of mitochondrial cytochrome c, followed by the activation of a caspase cascade leading to apoptosis (Maaser et al., 2001; Verrier et al., 2003; Chelli et al., 2004; Kunduzova et al., 2004; Jorda et al., 2005). Mitochondrial IBP not conjugated with VDAC and ANT is considered to be sufficient for TSPO function in steroidogenesis (Lacapere et al., 2003). In particular, dimers of IBP are shown to possess enhanced binding capacity to cholesterol and PK 11195, which in turn leads to increased transport from cytosolic cholesterol into the mitochondria, determining the speed of formation of pregnenolone, the precursor for steroids (Lacapere et al., 2003).
2.4 Biosynthesis of neuroactive steroids
Steroid hormone receptors act as transcription factors in the regulation of gene expression (Evans, 1988; Truss et al., 1993; Rupprecht, 1997; Rupprecht et al., 1999). Meanwhile, there is increasing evidence that certain steroids may alter neuronal excitability via the cell surface through interaction with certain neurotransmitter receptors (Majewska et al., 1986; Paul et al., 1992; Lambert et al., 1995; Rupprecht, 1997; Rupprecht et al., 1999). The term „neuroactive steroids‟ has been coined for steroids with these particular properties (Paul et al., 1992). While the action of steroids at the genome requires a time period from minutes to hours that is limited by the rate of protein biosynthesis (McEwen, 1991), the modulatory effects of neuroactive steroids are fast occurring events requiring only milliseconds to seconds (McEwen, 1991). Thus, genomic and non-genomic steroid effects within the central nervous system provide the molecular basis for a broad spectrum of steroid action on neuronal function and plasticity. Indeed, 3α-reduced metabolites of progesterone and deoxycorticosterone such as 3α, 5α-tetrahydroprogesterone (3α, 5α-THP; 3α-hydroxy-pregnan-20-one; allopregnanolone) and 3α, tetrahydrodeoxycorticosterone (3α, 5α-THDOC; 3α, 21-dihydroxy-5α-pregnan-20-one; allotetrahydrodeoxycorticosterone), pregnenolone sulfate (PS) or dehydroepiandrosterone sulfate (DHEA-S) are allosteric modulators of specific neurotransmitter receptors such as γ-aminobutyric acid type A (GABAA) receptors (Evans, 1988; Paul et al., 1992). Due to their lipophilic nature steroids that are produced in various endocrine organs can easily cross the blood–brain barrier. However, a variety of neuroactive steroids may be synthesized in the brain itself without the aid of peripheral sources (Akwa et al., 1992; Baulieu, 1991; Baulieu, 1998). These steroids
14
that are formed within the brain from cholesterol (Fig.2) have been defined also as „neurosteroids‟ (Baulieu, 1998).
Fig.2 Biosynthesis of neuroactive steroids. (Reprinted from Rupprecht et al., 1999).
Progesterone may be formed from pregnenolone by the 3β-hydroxysteroid dehydrogenase/Δ5 -Δ4
-isomerase. The 5α-reductase catalyzes the reduction of progesterone and deoxycorticosterone into the pregnane steroids dihydroprogesterone (DHP) and 5α-dihydrodeoxycorticosterone (5α-DHDOC), respectively, the 5β-reductase reduces progesterone to 5β-dihydroprogesterone (5β-DHP). These are irreversible reactions in mammalian cells (Celotti et al., 1992). These pregnane steroids may be further reduced to the neuroactive steroids 3α, 5α-THP, 3α, 5β-tetrahydroprogesterone (3α, 5β-THP; 3α-hydroxy-5β-pregnan-20-one; pregnanolone) and 3α, 5α-THDOC by the 3α-hydroxysteroid oxidoreductase (Rupprecht, 1997).
This reaction may work both in the reductive and in the oxidative direction depending on the cofactors present in the environment (Rupprecht et al., 1993). Both the 5α-reductase and the 3α-hydroxysteroid oxidoreductase exist in various isoforms that are expressed in a tissue specific manner (Compagnone et al., 2000). Pregnenolone is also a precursor for dehydroepiandrosterone (DHEA). These two steroids exist also as conjugated sulfate esters, e.g. PS and DHEA-S, and fatty acid esters at concentrations frequently exceeding those of the free steroids (Baulieu, 1998). Both progesterone and DHEA are converted to androstenedione, which is a precusor of testosterone. Estradiol is formed by the aromatase either from testosterone or from androstenedione via estrone.
15
2.5 Modulation of γ-aminobutyric acid type A (GABAA) receptors by neuroactive steroids
The 3α-reduced metabolites of progesterone and deoxycorticosterone 3α, 5α-tetrahydroprogesterone (3α, 5α -THP; 3α-hydroxy-5α-pregnan-20-one; allopregnanolone) and 3α, 5α-tetrahydrodeoxycorticosterone (3α, 5α-THDOC; 3α, 21-dihydroxy-5 α -pregnan-20-one; allotetrahydrodeoxycorticosterone) were the first steroids that have been shown to modulate neuronal excitability via their interaction with γ-aminobutyric acid type A (GABAA) receptors (Majewska et al., 1986). GABAA receptors consist of various subunits that form ligand-gated ion channels with considerable homology to glycine, nicotinic acetylcholine and serotonin type 3 (5-HT3) receptors (Paul et al., 1992; Lambert et al., 1995; Wetzel et al., 1998) (Fig. 3). A variety of different classes of drugs act through GABAA receptors (Fig. 3): agonists for the GABA binding site, benzodiazepines, but also barbiturates, clomethiazol, neuroactive steroids, alcohols and anesthetics. While a specific and saturable binding site at GABAA receptors has been clearly identified for GABA and benzodiazepines, the occurrence for such a binding site has not yet been proven for the latter compounds. The assumption of a steroid binding site at this ligand-gated ion channel is based on pharmacological studies concerning the strong stereoselectivity and structure–activity relationship of the action of neuroactive steroids at this neurotransmitter receptor (Lambert et al., 1995).
The steroids 3α, 5α -THP and 3α, 5α-THDOC are potent positive allosteric modulators of GABAA receptors because they enhance the GABA evoked chloride current through increasing the frequency and/or duration of openings of the GABA-gated chloride channel (Majewska et al., 1986; Paul et al., 1992; Lambert et al., 1995). However, similar to barbiturates but in contrast to benzodiazepines, high concentrations in the micromolar range of these neuroactive steroids have been shown to exert a certain intrinsic agonistic activity in the absence of GABA (Puia et al., 1990).
2.6 TSPO and cholesterol
Experiments in multiple steroidogenic cell systems showed that pregnenolone production was stimulated upon exposure of the cells to TSPO ligands (Barnea et al., 1989; Mukhin et al., 1989). When these experiments were repeated in isolated mitochondria incubated with TSPO ligands a similar increase in pregnenolone was observed (Barnea et al., 1989; Papadopoulos et al.,1990). This increase was not seen in mitoplasts, mitochondria devoid of their OMM and
16 therefore deficient in TSPO. To determine the effect of TSPO ligands on the mitochondria, cholesterol content in the OMM and the IMM was measured both before and after TSPO ligand treatment (Krueger et al., 1990). This study revealed that ligand binding to TSPO induced the translocation of cholesterol from the OMM to the IMM and confirmed that TSPO participates in the binding and release of cholesterol at the OMM, an initial step in the production of steroids.
To identify the basis of the interaction of TSPO with cholesterol, molecular modeling and site-directed mutagenesis were used to identify potential binding sites. Previous studies had shown that TSPO spans the OMM in five alpha helices, composed of approximately 21 amino acids each. The 3-D models produced suggested the five alpha helixes come together to form a channel with a hydrophilic but uncharged interior surface (Lacapere et al., 2001) (Fig. 3). It was shown that the interior of the channel could bind a cholesterol molecule that had not been significantly modified, suggesting that TSPO could function as a transporter of cholesterol to the IMM. To identify the cholesterol-binding domain several deletion constructs were generated. A region on the C-terminus (Δ153–169) was identified as necessary for cholesterol binding by virtue of the mutant‟s reduced ability to take up cholesterol, although PK 11195 binding was unaltered (Li et al., 2001b). Further site-directed mutagenesis experiments identified the specific amino acids necessary for cholesterol binding, yielding a CRAC (cholesterol-recognition amino acid consenus) domain (Fig. 3).
Fig. 3 A) Molecular model of TSPO‟s five alpha helices in the presence of cholesterol, demonstrating a pore accommodating a cholesterol molecule. B) Ribbon diagram of mTSPO, showing the five helices as well as the CRAC domain, consisting of Leu/ Tyr/Arg residues, and cholesterol. C) Docking model of cholesterol to the TSPO CRAC domain. The accessible surface of the peptide and cholesterol molecules are represented in red and blue, respectively. (Modified from Jamin et al., 2005).
17 The CRAC domain showed the high nanomolar affinity for cholesterol that had been observed in other proteins interacting with cholesterol (Li et al., 2001b). These data suggest that the C-terminus of TSPO plays an important role in the uptake and translocation of cholesterol into the IMM. It should be mentioned that due to the important role of cholesterol in mammalian cells and the diverse localization of TSPO in many tissues, TSPO may play a more extensive role in the cell, participating in targeting cholesterol to mitochondria for membrane biogenesis and also cholesterol transport in the cell. This idea gained further support when treatment of both steroidogenic and non-steroidogenic cells with TSPO ligands resulted in a redistribution of cholesterol from the plasma membrane to lipid droplets (LD) (Falchi et al., 2007), suggesting a possible role for TSPO in the intracellular regulation and trafficking of cholesterol, independent of cell type.
2.7 TSPO distribution in healthy tissues
TSPO is found in most tissues, although its expression within each varies considerably (Casellas et al., 2002; Gavish et al., 1999; Papadopoulos et al., 2006a). Secretory and glandular tissues, such as adrenal glands, pineal gland, salivary glands, olfactory epithelium, ependyma, and gonads, are particularly rich in TSPO (Papadopoulos et al., 2006a). Intermediate levels of this protein are found in renal and myocardial tissues, and lower levels are present in the brain and liver (Giatzakis et al., 2004; Papadopoulos et al., 2006a). Subcellular localization studies demonstrated that TSPO is primarily an outer mitochondrial protein (Anholt et al., 1986; Basile et al., 1986; Ntkiewicz-Michaluk et al., 1988). In addition, it has been reported to be present in Golgi apparatus, lysosomes and peroxisomes (O‟Beirne et al., 1990), and on plasma membrane (Oke et al., 1992). Surprisingly, TSPO is present in mature human erythrocytes, which lack mitochondria and nuclei (Olson et al., 1988), and perinuclear and nuclear localization of TSPO has been observed in breast cancer cells (Hardwick et al., 1999).
2.8 TSPO expression in pathological specimens
TSPO expression levels have been correlated with certain disease states. It is highly expressed in steroidogenic cells such as testicular, adrenocortical, and brain glial tumor cells, whose proliferation can be regulated by TSPO drug ligands such as benzodiazepines (Garnier et al.,
18 1993). At the same time, TSPO levels are elevated in cancerous tissues of the breast, ovary, colon, prostate, and brain, compared to normal human tissues, suggesting a role for TSPO in carcinogenesis (Batarseh et al., 2010; Katz et al., 1988, 1990a,b). A positive correlation between TSPO levels and the metastatic potential of human breast and brain gliomas, as well as astrocytomas, has also beenshown (Batarseh et al., 2010; Benavides et al., 1988; Miettinen et al., 1995). The role of TSPO in tumor growth may be supported by the concerted effects of the receptor itself on cell proliferation and survival. Indeed, both proliferative and antiapoptotic properties have been ascribed to TSPO based on extensive data using TSPO drug ligands (Veenman et al., 2007). However, TSPO expression is upregulated in the brain at sites of injury and inflammation, as well as following a number of neuropathological conditions including stroke, herpes and HIV encephalitis, and neurodegenerative disorders such as Alzheimer‟s disease, multiple sclerosis, amyotrophic lateral sclerosis, Parkinson‟s disease, and Huntington‟s disease (Batarseh et al., 2010).
TSPO has been shown to be altered in anxiety and mood disorders as well. The concentration of TSPO determined through assays of radiolabeled PK 11195 binding to platelets showed a decrease in anxiety, panic, and post-traumatic stress disorders. These data suggest that TSPO may exert an anti-anxiety effect through the production of neurosteroids, if the same reduction in TSPO is seen in the CNS. In agreement with these findings it was shown that many drugs used in the treatment of psychiatric disorders act on specific enzymes of the neurosteroidogenic pathway, resulting in normal levels of the neurosteroid allopregnanolone (Guidotti et al., 2001). This was further confirmed when it was shown that DBI also has an effect on patients with schizophrenia (van Kammen et al., 1993) and suggests that TSPO ligands could play a role in the prevention and treatment of psychiatric disorders.
2.9 Psichiatric Disorder and steroids
Epidemiological studies suggest that the onset of psychiatric symptoms may be related to changes in the secretion of gonadal hormones (Hallonquist et al., 1993; Häfner et al., 1993). For example, the occurrence of clinical symptoms in schizophrenia have been shown to vary across the menstrual cycle (Hallonquist et al., 1993). Moreover, there is a difference between pre- and postmenopausal women with an increased vulnerability for the onset of schizophrenic episodes after the menopause (Häfner et al., 1993). Thus, it may be hypothesized that a sudden drop of steroid concentrations may contribute to the development of such disorders and a steroid replacement might be of therapeutic value. Positive allosteric
19 modulators of GABAA receptors, e.g. benzodiazepines, are effective anxiolytic substances. Thus, also 3α-reduced neuroactive steroids should exert anxiolytic effects. Indeed, such steroids were effective anxiolytics in different animal models, e.g. the elevated plus maze test (Crawley et al., 1986; Bitran et al., 1991; Wieland et al., 1991). Also progesterone as a precursor molecule for 3α-reduced neuroactive steroids may act as an anxiolytic via GABAA receptors (Bitran et al., 1995). Meanwhile, anxiolytic properties have also been demonstrated for synthetic analogues of 3α-reduced neuroactive steroids (Vanover et al., 2000). In women suffering from mixed anxiety–depressive disorder, significantly elevated plasma levels of PS have been reported (Bicikova et al., 2000). Studies in patients with panic disorder suggested that 3α-reduced neuroactive steroids may play a pivotal role in human anxiety in that they may serve as a counter regulatory mechanism against the occurrence of spontaneous panic attacks (Ströhle et al., 2002). Studies of neuroactive steroids during experimentally induced panic attacks in patients with panic disorder and healthy controls in our research group showed that there is a pronounced decrease in 3α, 5α-THP and 3α, 5β-THP together with an increase in 3β, 5α-THP following both cholecystokinin tetra peptide (CCK-4) and sodium lactate administration in patients with panic disorder. These changes in neuroactive steroid composition might result in a decreased GABAergic tone that may be related to the pathophysiology of panic attacks in patients with panic disorder. Although treatment with paroxetine did not further increase the concentrations of GABAergic neuroactive steroids, antidepressants such as SSRIs might be effective as antipanic agents also through stabilizing the equilibrium of endogenous neuroactive steroids (Ströhle et al., 2002). Neuroactive steroids may modulate neuronal function through their concurrent influence on neuronal excitability and gene expression. This intracellular cross-talk between genomic and non-genomic steroid effects provides the molecular basis for steroid action in the brain and the future development of such compounds in neuropsychopharmacology, both with regard to putative clinical effects and side effects. As an alternative to exogenous administration, also non-steroidal compounds that interfere with steroid synthesis, e.g. enzyme inhibitors or antidepressants, may be used to influence the equilibrium of endogenous neuroactive steroids. This may also provide a lead for the development of novel therapeutic strategies in the treatment of psychiatric diseases. In conclusion, endogenous or exogenous neuroactive steroids offer a considerable potential in the treatment of neuropsychiatric disorders (Rupprecht el al., 2003).
20
2.10 Anxiety Disorder and TSPO
It is well known that stress affects hormonal levels, and several studies have suggested that TSPO ligand binding density may be under hormonal control (Fares et al., 1987b, 1988, 1989; Bar-Ami et al., 1989; Weizman et al., 1992; Golani et al., 2001). Furthermore, it was shown that high dose steroid treatment of multiple sclerosis led to an increase in TSPO on blood mononuclear cells (Ferrero et al., 1992). Thus, it was considered possible that stress also affects TSPO levels. Indeed, assaying students immediately after an exam showed that increased TSPO density in platelets accompanies stress as compared to the controls (Karp et al., 1989). Furthermore, stress of war was also found to increase TSPO density in platelets (Weizman et al., 1994). It was suggested that TSPO density in platelets can be used as a promising biological marker of stressful conditions (Nakamura et al., 2002). A more recent study also showed that TSPO density in blood platelets was significantly increased following PhD examination (Droogleever Fortuyn et al., 2004). Not only the effects of acute stress on TSPO blood cell were studied, as discussed above, but also the effects of repeated chronic stress and posttraumatic stress disorder on blood cell TSPO levels. [3H]PK 11195 binding to platelet membranes was reduced immediately after actual repeated parachute jumps toward the end of parachute training of soldiers (Dar et al., 1991). Decreased platelet TSPO density was also observed in the posttraumatic stress disorder patients compared to controls (Gavish et al., 1996). Other studies suggested that TSPO levels in platelets of patients with generalized social phobia were decreased (Johnson et al., 1998). In obsessive–compulsive disorder patients, the relative content of TSPO mRNA in lymphocytes was significantly decreased only in chronic obsessive–compulsive disorder patients as compared to controls, whereas no significant changes were observed in episodic obsessive–compulsive disorder patients (Rocca et al., 2000). Also adolescent suicidal patients showed a significant decrease in platelet TSPO density compared to the non suicidal control groups (Soreni et al., 1999). Thus, platelet TSPO levels are typically reduced in patients suffering from various types of anxiety disorders, and also in suicidal patients. In peripheral blood mononuclear cells of generalized anxiety disorder patients, it was found that TSPO density was decreased by 45%, while TSPO mRNA levels were decreased by 70%, as compared to untreated healthy controls (Rocca et al., 1998). In addition, in a group of clinically anxious patients the average level of TSPO mRNA in lymphocytes was also found to be reduced compared to the control group (Nudmamud et al., 2000). Four weeks of diazepam treatment of anxious patients induced elevation of TSPO density of platelets (Weizman et al., 1987a). Peripheral blood mononuclear cells of generalized anxiety disorder patients treated with 2V-chloro-N-desmethyl-diazepam also
21 showed increases of TSPO density and TSPO mRNA levels back to the levels of the untreated healthy controls (Rocca et al., 1998). Furthermore, this suggests that the decrease of TSPO density in peripheral mononuclear cells of generalized anxiety disorder patients is due to a change at the transcriptional level (Rocca et al., 1998).
22
Chapter 3. Ala147Thr substitution in Translocator Protein (TSPO) is
associated with adult separation anxiety in patients with depression
3.1 Aim of the study
Anxiety disorders are known to be familial and heritable (Kendler et al., 1999), but the identification of susceptibility genes has been difficult to accomplish for their phenotypic complexity. Although the constellations of symptoms used as diagnostic criteria in the DSM-IV have been useful for clinical practice, it is unlikely that they are the optimal phenotype definitions for genetic analyses. Family, twin, and linkage studies suggest that genes confer susceptibility to anxiety proneness in a manner that cuts across clinical diagnostic labels (Smaller et al., 1998; Kaabi et al., 2006). New intermediate phenotypes of anxiety proneness (i.e., behavioural inhibition) and well-validated animal models have been demonstrated to provide crucial tools (Smaller et al., 2008). A well-validated anxiety animal model is represented by rats that have been subjected to a chronic stressor-daily maternal separation. In this anxiety animal model, separation distress has been shown to induce profound alterations in central mechanisms controlling hypothalamo-pituitary-adrenal (HPA) axis activity (Ladd et al., 1996; Plotsky et al., 2005). A growing body of evidence indicates that the neuroactive steroids, such as allopregnanolone, represent homeostatic molecules in the context of adaptation to stress by limiting the extent and duration of activation of HPA axis (Dubrosky et al., 2005). Studies on adult rats with heightened anxiety, developed after chronic maternal separation, evidenced that allopregnanolone, in response to a stressful experience, reduced the number of grooming, a well-documentated behavioural correlate of stress (Zimmerberg et al., 1999). A human condition that is likely to share some of those mechanisms described in animals is separation anxiety. In previous studies, were found alterations of TSPO expression levels in patients with ASAD (Pini et al., 2005a, b; Chelli et al., 2008). The rate-limiting step in the synthesis of neurosteroids is the transport of cholesterol into mithocondria, mediated by CRAC domain of TSPO (Jamin et al., 2005). The aim of this study was to test whether two allelic variants of TSPO gene, which cause the change of two aminoacids localized in CRAC domain, were associated with adult ASAD in a cohort of patients with a principal diagnosis of bipolar disorder or major depression.
23 3.2 Experimental section
3.2.1 Subject
The study sample was recruited at the outpatients clinic of the Department of Psychiatry, University of Pisa. Overall, 182 patients with a DSM-IV diagnosis of bipolar disorder type I or II (N=90) or major depression (N=92) were included in the study. Patients with psychotic symptoms, schizophrenia and substance abuse were excluded from the study. Of the182 patients, 80 patients (18 males and 62 females, mean age 41.56±12.52 years) had separation anxiety and 102 patients (37 males and 65 females, mean age 43.07±11.88 years) did not. A group of 190 healthy control subjects (58 males and 132 females, age 40.40±12.1 years) was included in comparative analyses. They were recruited among students, teaching staff and technical personnel of University of Pisa and were thoroughly assessed by means of detailed psychiatric and medical interview. Volunteers a positive history or a current major psychiatric disorder were not included in the study. The group did not differ significantly on gender and age distribution from groups of patients with or without separation anxiety. The group of patients and controls had similar geographic origins (all individuals were Caucasians of Italian origin). The study design was approved by the University of Pisa Ethical Committee (study number 1968/2005). All subjects were informed of the nature of study procedures and provided written informed consent prior to participation.
3.2.2 Psichometric evaluation
All patients were evaluated by the SCID-I/P (First et al., 2002) for principal diagnosis and Axis I comorbidity. The presence of separation anxiety, was evaluated by the Structured Clinical Interview for Separation Anxiety Symptoms (SCI-SAS) which comprises a childhood section (SCI-SAS-C) and an adulthood section (SCISAS-A), respectively (Cyranowski et al., 2002). The eight separation anxiety disorder criteria were rated for both childhood and adulthood time frames, scored as 0 (not at all), 1 (sometimes), 2 (often) or ? (do not recall). In keeping with the DSM-IV guidelines, endorsement of three or more of the eight criterion symptoms (symptoms rated as „2‟ or „often‟) was used as a threshold to determine categorical (yes/no) diagnosis of separation anxiety disorder in childhood and adulthood. In addition, criterion B (symptoms lasting for at least 4 weeks) and criterion C (symptoms must cause
24 significant impairment) had to be fulfilled. When separation anxiety was assessed with reference to a specific relationship during childhood, the subject‟s relationship to his/her mother (or subject-identified primary caregiver) was utilized. Probes to assess adult separation anxiety were less relationship specific, assessing anxiety with reference to „separation from loved ones as an adult‟. The SCI-SAS showed excellent psychometric properties, including good internal consistency (Cronbach‟s alpha values were 0.79 for the child scale and 0.85 for the adult scale), a clear factor structure, and high levels of convergent and discriminate validity. The adult section of the SCI-SAS includes age-appropriate diagnostic criteria of separation anxiety disorder as experienced during adulthood. Details on psychometric properties of the SCI-SAS are reported in Cyranowski et al. (2002). Interviews were performed by experienced residents in psychiatry. Severity of depression and anxiety was evaluated by the Hamilton Rating Scale for Depression (HAM-D) (Hamilton, 1960). The relationship between specific TSPO genotypes and anxiety was assessed by using the „anxiety/somatization‟ HAM-D factor (Cleary et al., 1977).
3.2.3 DNA Genotyping
Genomic DNA from all subjects was isolated from saliva cells with a JETQUICK Blood and cell culture DNA spin kit (GENOMED, GmbH, Germany).
Genotyping for 439G>A (rs6971) and 485G>A (rs6972) SNPs of TSPO gene was performed using polymerase chain reaction-restriction fragment length polymorphism method, as reported previously (Kurumaji et al., 2000). The PCR reaction mixture contained a total volume of 30 µl consisting of 0.5 ng/µl genomic DNA, 200 µM of each dNTP, 100nM forward and reverse primers (forward: 5‟-TGGGACAGGCACTTGGGTGAAC-3‟; reverse: 5‟-AAGCGTGACGGCCACCACATCA-3‟), 1.25 mM MgCl2 and 1 U of AmpliTaq Gold
(Applied Biosystems) in an equipped buffer (1x). Thermal cycling was performed with an initial denaturation of 5 min at 95°C, followed by 30 cycles of 1 min at 94°C, 30 sec at 66°C, and 1 min at 72°C, followed by a terminal extension of 7 min at 72°C. The PCR products were digested with 5 U of Nru I or NlaIII according to manufacturer‟s recommendations and separated by electrophoresis on 2.5% Ultrapure agarose-TBE gels (Gibco BRL). For quality assurance, 5% of the samples were repeated blindly and each digestion run was carried out using extra samples with known genotypes.
25
3.2.4 Statistical analysis
Genotypes were tested for the fitting with Hardy–Weinberg equilibrium (HWE). Individual haplotypes were reconstructed using the software PHASE (Stephens et al., 2003). The linkage disequilibrium (LD) was measured by calculating the r2 and Lewontin‟s D‟ (Weiss et al., 2002). Genotype and haplotype distributions were compared using 2-test or Fisher‟s exact test. The associations between genotypes or haplotypes and diseases were analysed with the logistic regression model. Odds ratios (OR) and 95% confidence intervals (CI) were calculated using the common genotype or haplotype among controls as the reference group. For genotypes, analyses were performed both under a co-dominant and dominant model. In order to test the effects of genotype on the scores of SCI-SAS interview, one-way analysis of variance (ANOVA) and Tukey‟s post-hoc test were applied. Logistic regression was performed to predict the allelic variants of TSPO by adult separation anxiety controlling for the presence of comorbid anxiety disorders, namely panic disorder (PD), social anxiety disorder (SAD) and obsessive-compulsive disorder (OCD). A linear regression was used to test the association of genotype with adult separation anxiety disorder after controlling for severity of „free‟ anxiety as measured by the HAM-D „anxiety-somatization‟ factor score. Statistical analyses were performed using SPSS software (Version 16.0; SPSS Inc, Chicago, Ill).
26
3.3 Results and discussion
Demographic and clinical characteristics of the study sample are reported in table 1.
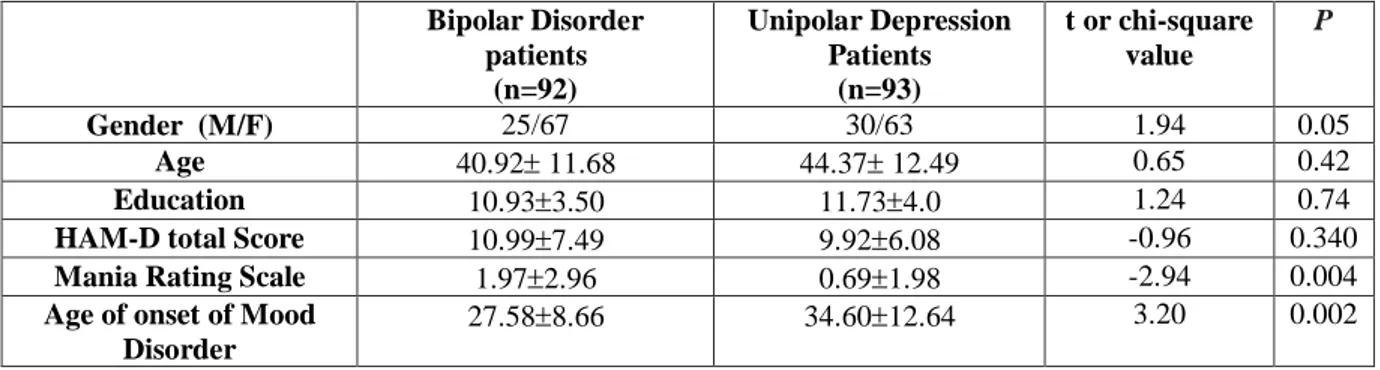
Table 1 Demographic and clinical characteristics of study sample.
Bipolar Disorder patients (n=90) Major Depression Patients (n=92) t test/chi-square value p Gender (M/F) 25/65 30/62 0.50 0.48 Age 40.6 11.6 44.2 12.5 1.98 0.05 Education 10.933.50 11.734.0 1.24 0.74
HAM-D total Score 10.997.49 9.926.08 -0.96 0.340
Mania Rating Scale 1.972.96 0.691.98 -2.94 0.004
Age of onset of Mood Disorder 27.588.66 34.6012.64 3.20 0.002
Age of Onset of SAa 11.611.83 13.011.63 0.66 0.514
Subject (%) with ASAD 41 (45.6%) 39 (42.4%) -- --
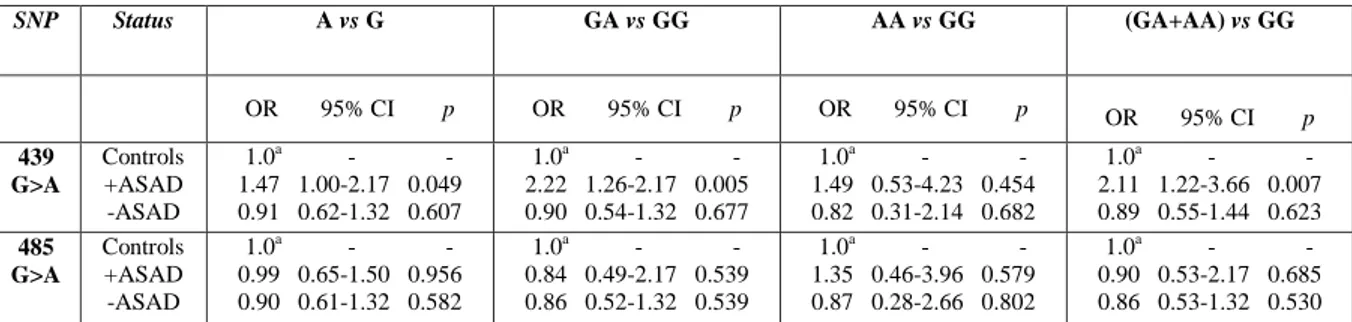
Genotype distributions were in HWE among controls and are reported in table 2 together with those of patients. The ORs values are reported in table 3.
Table 2 Raw data and statistical analyses for 439G>A and 485G>A SNPs within TSPO gene in patients with or without Adult Separation Anxiety and controls.
SNP Status Genotypes, n (%) HW Chi square (p) Alleles, n (%) Chi square (p)
GG GA AA G A 439 G>A Controls (n=190) + ASAD (n=80) - ASAD (n=102) 93 (48.9) 82 (43.2) 15 (7.9) 25 (31.2) 49 (61.3) 6 (7.5) 53 (51.9) 42 (41.2) 7 (6.9) YESa 7.84 (0.02) 0.28 (0.87) 268 (70.5) 112 (29.5) 99 (61.9) 61 (38.1) 148 (72.5) 56 (27.5) 3.87 (0.05) 0.265 (0.61) 485 G>A Controls (n=190) + ASAD (n=80) - ASAD (n=102) 97 (51.1) 83 (43.7) 10 (5.3) 43 (53.7) 31 (38.8) 6 (7.5) 56 (54.9) 41 (40.2) 5 (4.9) YESa 0.88 (0.64) 0.39 (0.82) 277 (72.9) 103 (27.1) 117 (73.1) 43 (26.9) 153 (75.0) 51 (25.0) 0.003 (0.96) 0.303 (0.58) +ASAD= patients with Adult Separation anxiety; -ASAD= patients without Adult Separation Anxiety; HW= Hardy-Weinberg equilibrium. a2=0.28, df=1, P=0.60; b2=2.11, df=1, P= 0.15.
Table 3 Raw data and statistical analyses for 439G>A and 485 G>A SNPs within TSPO gene in patients with or without Adult Separation Anxiety Disorder and controls. Common homozygotes GG represent the reference genotype.
+ASA= patients with Adult Separation anxiety; -ASA= patients without Adult Separation Anxiety; OR=Odd Ratios; a reference values for OR; CI=95% confidence interval of Odd Ratios.
SNP Status A vs G GA vs GG AA vs GG (GA+AA) vs GG OR 95% CI p OR 95% CI p OR 95% CI p OR 95% CI p 439 G>A Controls +ASAD -ASAD 1.0a - - 1.47 1.00-2.17 0.049 0.91 0.62-1.32 0.607 1.0a - - 2.22 1.26-2.17 0.005 0.90 0.54-1.32 0.677 1.0a - - 1.49 0.53-4.23 0.454 0.82 0.31-2.14 0.682 1.0a - - 2.11 1.22-3.66 0.007 0.89 0.55-1.44 0.623 485 G>A Controls +ASAD -ASAD 1.0a - - 0.99 0.65-1.50 0.956 0.90 0.61-1.32 0.582 1.0a - - 0.84 0.49-2.17 0.539 0.86 0.52-1.32 0.539 1.0a - - 1.35 0.46-3.96 0.579 0.87 0.28-2.66 0.802 1.0a - - 0.90 0.53-2.17 0.685 0.86 0.53-1.32 0.530
27 The analyses of 485G>A SNP did not reveal significant differences in the genotype distributions between depressed patients with or without adult separation anxiety and controls. With regard to 439G>A SNP, an excess of A439 carriers among the depressed patients with adult separation anxiety, compared to the control group was found. ANOVA and Tukey‟s test showed that 439G>A heterozygotes had a higher mean SCI-SAS total score than GG homozygotes (respectively, 7.413.40 vs 5.673.18, F= 3.69, P=0.028), suggesting an association of GA genotype with more severe symptoms. No significant differences were evidenced for 439G>A SNP genotype distributions in the group of patients without adult separation anxiety with respect to controls suggesting that this association was not accounted for by the principal diagnosis of bipolar disorder or major depression. No significant differences between genotypes of depressed patients were evidenced in the “anxiety” HAM-D factor scores (F=1.69, df=2, P=0.188). A logistic regression for adult separation anxiety, panic disorder, obsessive-compulsive disorder, social phobia, bipolar or unipolar depression diagnosis as predictive variables of 439G>A GA genotype was performed. The results showed that only adult separation anxiety was associated with the A allelic variant (adult separation anxiety: β=1.35, OR= 3.87, 95%CI 1.71-8.78, p=.001; PD: β=0.09, OR=1.09, 95%CI 0.49-2.41, p=.83; bipolar depression: β=-0.44, OR=0.64, 95% CI 0.30-1.38, p=.26; OCD: β=0.04, OR=1.04, 95%CI 0.36–3.03, p=.94; SAD: β=-0.76, OR=0.47, 95%CI 0.13-1.67, p=.24). At present, no other genetic association studies have been carried out in Caucasian population for these two SNPs. Two studies by Kurumaji et al., (2000; 2001) did not report an association of these allelic variants with schizophrenia or mood disorder in Japanese populations. Nakamura et al., (2006) have evidenced different allele distributions for 485G>A SNP in patients with panic disorder and in control subjects.
The measures of the LD were D‟= 0.91 and r2
= 0.137, suggesting that the two alleles are inherited together in chromosome 22 and this measurement is in agreement to previous analyses in Caucasians (HapMap project; www.hapmap.org ). The logistic regression analysis showed a trend for association of the haplotype A439-G485 with adult separation anxiety (OR=1.47; 95% CI 0.95-2.27).
The 439G>A SNP is likely to have a functional effect, because it codes for the aminoacidic change of Ala 147 in Thr. This aminoacidic position is in CRAC domain of TSPO, that is responsible for the uptake and translocation of cholesterol into mitochondria (Jamin et al., 2005). Substitution of a non-polar aminoacid with a polar aminoacid might determine an alteration of cholesterol translocation that is the first rate-limiting step in neurosteroid biosynthesis. However, we cannot exclude that the association could be due to other functional variants in LD with 439G>A. In the HapMap database, TSPO gene is included in a
28 large block of LD that could bear other important variants. One possible explanation for the negative finding for 485G>A SNP might be related to the type of aminoacidic substitution controlled by this polymorphism. In fact, the two aminoacids (Arg and His) encoded by the 485G>A SNP are basic and, therefore, this aminoacid substitution might not cause alterations of the cholesterol translocation function of TSPO.
This is the first report, to the best of our knowledge, of an association between A allele of TSPO 439G>A SNP and an intermediate anxiety phenotype, namely adult separation anxiety. Since the search for anxiety genes is in its early stages, identification of the TSPO gene for susceptibility to this specific anxiety phenotype would signal an advance in understanding of the pathophysiology of anxiety. However, these results should be conceived as a start point for addressing research to explore more in depth the role of TSPO in anxiety disorders.
29
Chapter 4. The spontaneous Ala147Thr aminoacid substitution within the
Translocator
Protein
influences
pregnenolone
production
in
lymphomonocytes of healthy individuals
4.1 Aim of the study
In cells that synthesize steroids de novo, steroidogenesis begins with the conversion of cholesterol to pregnenolone by the inner mitochondrial membrane cytochrome P450 side-chain cleavage (CYP11A1) enzyme. The rate of steroid formation depends on the rate of cholesterol transport from intracellular stores to the inner mitochondrial membrane (Stocco, 2000; Miller, 2007; Rone et al., 2009). A key element in the regulation of cholesterol transport into mitochondria is the TSPO and in particular, the CRAC sequence within the TSPO C-terminal domain (L144-S159) (Li et al., 1998; 2001b; Jamin et al., 2005; Papadopoulos et al., 2006b; Rone et al., 2009). The C-terminal domain is well conserved among species. However, two single nucleotide polymorphisms (SNPs) (rs6971, rs6972), which change the Alanine 147 into Threonine (Ala147Thr) and the Arginine 162 into Histidine (Arg162His), respectively, have been found in this TSPO region (http://www.ncbi.nlm.nih.gov/sites/entrez). These SNPs are associated with psychiatric disorders, as steroids and neurosteroids have an important role in the regulation of several CNS functions (Baulieu, 1998; Christenson et al., 2000; Melcangi et al., 2008). For example, positive associations have been found between the Arg162His SNP and panic disorder (Nakamura et al., 2006) and between Ala147Thr SNP and adult separation anxiety disorder
(chapter 3). In the present study, was investigated whether the Ala147Thr SNP could influence pregnenolone production in healthy volunteers. Due to the lack of assessable, typical steroidogenic tissues, the study was performed in a peripheral cell model, represented by circulating lymphomonocytes.
30
4.2 Experimental section
4.2.1 Subjects
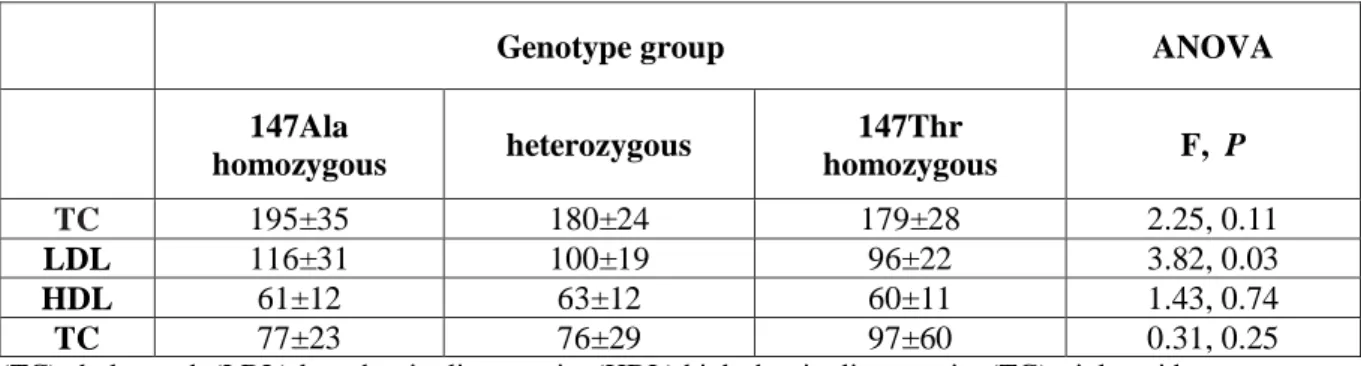
A total of 232 healthy volunteers have taken part in this study. All subjects (Caucasian of Italian origin) donated saliva for genomic DNA extraction and genotyping of the Ala147Thr SNP. Blood samples (taken between 9 and 10:30 AM after an overnight fast) were collected from 33 subjects for pregnenolone measurement and from 71 subjects for evaluation of the following circulating cholesterol parameters: total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL) and low density lipoprotein (LDL). Exclusion criteria for the study were: pregnancy, hypertension, obesity, diabetes, alcohol abuse and treatment of steroids or neuroactive drugs.
The study design was approved by the University of Pisa Ethical Committee (study number 1968/2005). All subjects were informed of the nature of the study procedures and provided written informed consent prior to participation.
4.2.2 DNA genotyping
Genomic DNA from all subjects was isolated from saliva cells with a JETQUICK Blood and cell culture DNA spin kit (GENOMED, GmbH, Germany). Genotyping of the Ala147Thr SNP (rs6971) was carried out using a modification of a previously described polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method, (paragraph 3.2.3) (Kurumaji et al., 2000). The PCR products were digested with 5 U of Nru I according to manufacturer‟s recommendations and separated by electrophoresis on 2.5% Ultrapure agarose-TBE gels (Gibco BRL). For quality assurance, 5% of the samples were repeated blindly and each digestion run was carried out using extra samples with known genotypes.
4.2.3 Pregnenolone measurement
Preparation of circulating cell samples: Blood samples were drawn into test tubes containing
Li-Heparin and then processed for mononuclear cell preparation, according to the method of Boyum (1968). The final cell pellet was resuspended in complete RPMI 1690 media
31 supplemented with 15% FBS, 2 mM L-glutamine, 100 units/ml penicillin and 100 mg/ml streptomycin. Cells were seeded in plate wells at a density of approximately 300,000 cells/well and maintained in a humidified atmosphere of 5% CO2 / 95% air at 37°C for 24 h.
The viability was greater than 90%, as assessed by the trypan blue exclusion method.
Characterization of pregnenolone synthetic enzyme expression: CYP450scc expression was estimated both at the mRNA and protein level using RT-PCR and Western-blot analyses, respectively, in random cell samples (n=7).
For RT-PCR analysis, 1 µg of total RNA was reverse transcribed using a quantiTect Transcription Kit in a total volume of 14 µl. Two microliters of the reverse transcription mixture were used for PCR. PCR amplification was performed with AmpliTaq Gold DNA Polymerase for 33 cycles at 95°C for 1 min, 57°C for 30 sec and 72°C for 1 min. PCR primers were as follows: sense, 5‟-GAGATGGCACGCAACCTGA-3‟; antisense, 5‟-CCCACATCGCTGAGGTGTT-3‟. Two microliters of the amplified product was subjected to semi-nested PCR using the conditions previously described for the first PCR. The semi-nested PCR primers were as follows: sense, CTCCTCAAAGCCAGCATCAA-3‟; antisense, 5‟-CCCACATCGCTGAGGTGTT-3‟. PCR products were separated by agarose gel electrophoresis on a 2% NuSieve-GTG agarose gel, visualized by ethidium bromide staining under ultraviolet light and subsequently purified using the QIAquick gel extraction system according to the manufacturer‟s instructions. Purified PCR products were sequenced by the Primm sequencing service (Primm srl, Milan, Italy) using an ABI 3730 automated DNA sequencer (Applied Biosystems, Foster City, California). Sequencing was carried out in both directions to confirm findings. Sequences were analyzed using the Geospiza‟s FinchTV software, version 1.4 (Goespiza Inc, Seattle) and aligned with the sequence of human CYP450scc mRNA, CYP11A1 (NCBI reference sequence of human CYP11A1 mRNA: NM_000781). As positive control, the steroidogenic glioma cell line ADF was used (Malorni et al., 1984). For Western-blot analysis, cell protein samples (50 µg) were run on a 12.5% SDS-Polyacrylamide gel and transferred onto a nitrocellulose membrane. The membranes were blocked for several hours in 5% blotting-grade blocker of nonfat dried milk containing 0.05% Tween 20 and incubated for 2 h at room temperature with a 1:1000 dilution of primary antibody (Millipore Corporation, developed against the 421-441 amino acid sequence of rat CYP11A1) in 5% blotting-grade blocker of nonfat dried milk. After three 10-min washes in Tris-buffered saline (TBS: 10 mM Tris-HCl, pH 8, and 150 mM NaCl) containing 0.05% Tween 20, the membranes were incubated for 1 h at room temperature with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:5000). The membranes were washed three times in TBS containing 0.05% Tween 20. The membranes were incubated for 1
32
min with ECL Plus Western Blotting agent (Amersham Pharmacia Biotech) and exposed to X-ray film (Amersham Pharmacia Biotech). Protein concentration was assayed using a Bio-Rad assay system. The steroidogenic glioma cell line ADF was used as positive control.
Pregnenolone assessment: lymphomonocytes from 33 subjects were incubated for 2 h with
serum-free salt medium (140 mM NaCl, 5 mM KCl, 10 mM glucose, 1.8 mM CaCl2, 1 mM
MgSO4, 10 mM Hepes, pH 7.4, and 0.1% BSA) containing the inhibitors trilostane (25 µM)
and SU 10603 (10 µM) at 37°C in a humidified atmosphere of 5% CO2 / 95% air. The
inhibitors were used to ensure that no further metabolism of pregnenolone would occur in the cells. The secreted pregnenolone in the cell medium was measured with an Enzyme Immunoassay (ELISA), as previously described (Da Settimo et al., 2008). Briefly, cell salt medium was retained and centrifuged at 1500 g for 10 min and used for quantitative determination of pregnenolone under the conditions recommended by the supplier (Pregnenolone ELISA, IBL Hamburg, Germany). ELISAs for each sample were performed in triplicate. Cross-reactivity with other steroids is typically below 1% and cross-reactivity with progesterone is 6%. The sensitivity of the assay was 0.05 ng/mL. Unknown samples were compared to concurrently run standards of pregnenolone using a one-site competition model.
4.2.4 Cholesterol parameters determination
Plasma concentrations of TC, TG and HDL were assessed from 71 subjects by standard enzymatic methods (Wako Chemicals GmbH, Germany). Low density lipoprotein was calculated using the Friedewald formula.
4.2.5 Statistical analysis
The extent to which genotypes deviated from Hardy-Weinberg equilibrium (HWE) was tested using Hardy-Weinberg Simulator software (HWSIM) (http://krunch.med.yale.edu/hwsim/) (Cubells et al., 1997). For a locus with two alleles, the locus is in HWE when the relationship between allele frequencies and genotype proportions follows the equation p2+2pq+q2=1, where p and q are the frequencies for major and minor alleles, respectively. Pearson‟s chi-squared test was performed to test for differences in gender frequency and the Student‟s t-test was conducted to compare the mean differences in age. Pregnenolone amount and circulating cholesterol parameters are expressed as means ± SEM (standard error of measurement) of the
33 raw data. One-way ANOVA followed by Bonferroni‟s test was applied to compare pregnenolone levels or circulating cholesterol parameters among genotype groups. P values less than 0.05 were considered statistically significant.
34
4.3 Results and discussion
4.3.1 Genotyping of the TSPO Ala147Thr SNP
The Ala147Thr SNP within the gene encoding TSPO, with the SNP identification number (RefSNP ID) rs6971, was studied in 232 Caucasian individuals (31.9% males, with a mean age of 36.513.6; 68.1 % females, with a mean age of 37.310.9). Genotype and allele distributions are summarized in Table 1. The Ala147Thr SNP genotype distribution conformed to Hardy-Weinberg equilibrium expectations. Testing for HWE can reveal occurrences of various phenomena, such as natural selection or random mating, within the population. Moreover, it provides a higher level of quality assurance by allowing the detection of systematic biases introduced by inappropriate genotyping protocols.
In Table 1, the Ala147Thr SNP genotype and allele distributions in the Caucasian population, reported in HapMap, are also shown. The Ala147Thr SNP genotype distribution in the study sample, stratified in females and males, was not significantly different between genders or mean ages (2=4.59, P=0.10; Student-t test t=0.48, P=0.629).
Table 1. Genotype and allele distributions for TSPO Ala147Thr SNP in the current study and in the HapMap.
Caucasian n Genotype n (%) HWEa 2 , P Allele n (%)
Current study Ala147b Ala147Thrc Thr147d Ala147 Thr147
total 232 113(48.7) 98(42.2) 21(9.1) 0.001, 0.97 324(69.8) 140(30.2) females 158 74(46.8) 73(46.2) 11(7.0) 221(69.9) 92(30.1) males 74 39(52.7) 25(33.8) 10(13.5) 103(69.6) 45(30.4) HapMap-CEU 60 28(46.7) 23(38.3) 9(15.0) 79(65.8) 41(34.2) CEU_GENO_PANEL 59 28(47.5) 22(37.3) 9(15.3) 78(66.1) 40(33.9) a
Hardy-Weinberg equilibrium ; aAla147 homozygous genotype, bAla147Thr heterozygous genotype, c147Thr homozygous genotype.
The TSPO SNP showed a frequency of 30.2% for the minor allele, encoding the amino acid Threonine 147. This and the previous results (paragraph 3.3) contribute to better estimate of the Ala147Thr SNP frequency in the Caucasian population, since the genotype distribution was obtained in a larger sample compared to those reported in the HapMap database . To the best of our knowledge, evaluation of genotype distribution of this SNP has only been performed in large samples of Japanese individuals (Kurumaji et al., 2000; 2001; Nakamura et al., 2006).
35
4.3.2 Characterization of pregnenolone synthetic enzyme expression
In order to investigate CYP11A1 expression in lymphomonocytes, RT-PCR and Western-blot analyses were performed (n=7). As a positive control, the human steroidogenic glial cell line, ADF, was used (Malorni et al., 1994). The presence of CYP11A1 mRNA was identified in total RNA using specific primers designed to amplify the 6th and 9th exons of the human CYP11A1 gene. Fragments of the expected size (355 bp) were consistently detected on agarose gels, and sequencing showed complete identity with the corresponding sequence deposited in GenBank (NM_000781) (Fig. 1).
36
Fig. 1. RT-PCR and nucleotide sequencing of the amplified products. At the top of figure, the RT-PCR analysis showed the expected-size fragment (355 bp) in human lymphomonocytes (lane 3) and the positive control (ADF steroidogenic cell line, lane 1). Samples without reverse transcriptase (lanes 2 and 4). The expected-size fragments (355 bp) were purified and sequenced to check the specificity of cDNA nucleotide sequences obtained for lymphomonocytes (LM) and ADF cells. To this aim cDNA sequences were compared with the human CYP11A1 mRNA sequence reported in the GenBank database (GenBank accession n°. NM_000781). At the bottom of figure, the comparison of nucleotide sequences obtained for both lymphomonocytes (LM) and ADF cells showed complete identity with the published human CYP11A1 mRNA sequence.
The presence of CYP11A1 protein was verified using a commercial polyclonal antibody developed against the 421-441 amino acid sequence of rat CYP11A1. As shown in Fig. 2, the anti-CYP11A1 antibody recognized the 56.1 KDa human CYP11A1 protein (GenBank accession number: NP_000772) in lymphomonocytes and in the positive control ADF cell line. The smaller human CYP11A1 isoform (42.0 KDa; GeneBank accession number: NP_001093243) was also present in the lymphomonocytes. The expression of isoform a had been also identified in tissues that do not primarly function to synthesize steroid hormones (Morales et al., 1999; Slominski et al., 2000; Shibuya et al., 2003, Oka et al., 20007; Teplyuk et al., 2009). Less data are available about isoform b (http://www.ncbi.nlm.nih.gov/protein).
37
Fig. 2. Western blot analysis of human CYP11A1 enzyme. Anti-CYP11A1 antibody recognizes the 56.1-kDa human CYP11A1 (GenBank accession n°. NP_000772) in lymphomonocytes (LM) or in the positive control ADF cell line. The smaller human CYP11A1 isoform (42.0 kDa; GenBank accession n°. NP_001093243) was also evidenced in lymphomonocytes. Anti-GAPDH antibody recognizes the 36.0-kDa human GAPDH (GenBank accession n°. NP_002037) in lymphomonocytes or the ADF cell line.
4.3.3 Pregnenolone assessment
The functional activity of CYP11A1 was determined in lymphomonocytes of 33 healthy individuals (36.4% male, mean age 30.67.9; 63.6% female, mean age 30.09.7) by measuring the pregnenolone released by cells and accumulated in salt medium. Approximately 3x105 cells produced 0.1165±0.04375 ng/ml of pregnenolone during a period oh 2h. In order to establish that pregnenolone came from the catalytic activity of CYP11A1, the samples were treated with the specific CYP11A1 inhibitor aminoglutethimide (20 µM). Pregnenolone levels were not detectable after the aminoglutethimide treatment. This result is in agreement with other reports that have previously demonstrated the presence and/or activity of the CYP11A1 enzyme in immune cell populations of other species (Lachner et al., 2001; Oka et al., 2007). As expected, this value is much lower than that in cells that primarily function to synthesize steroid hormones (Campiani et al., 1996; Nishi et al., 2001; Rommerts et al., 2001; Johansson et al., 2002) (i.e. 4.9±0.06 ng and 21±0.9 ng of pregnenolone production in MA-10 Leydig and the Y-1 adrenocortical cell lines (~1x105 cells), respectively).
4.3.4 Comparison of pregnenolone production in genotype groups
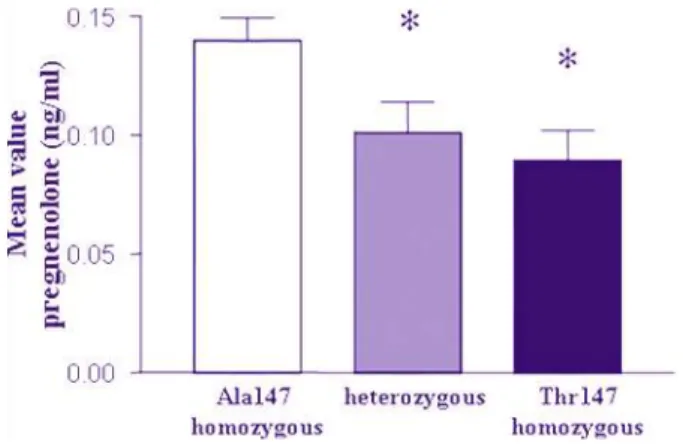
Mean values of pregnenolone levels produced by human lymphomonocytes were stratified into the three genotype groups of the TSPO Ala147Thr SNP: Ala147 homozygous individuals