Functional biodiversity in green
manure crops: effects on
nitrogen dynamics and weed
suppression
Accademic Year
2017
/2018Phd Course Agrobiosciences
Functional biodiversity in green
manure crops: effects on nitrogen
dynamics and weed suppression
Author
Marzia Ranaldo
Supervisor
Prof. Paolo BàrberiTutor
Dr. Stefano Carlesi
D265ModTPhD/EN00
Scuola Superiore Sant’Anna
Institute of Life Science
Functional biodiversity in green manure
crops: effects on nitrogen dynamics and
weed suppression
PhD Candidate:
Marzia Ranaldo
Supervisor:
Professor Paolo Bàrberi
Tutor:
If a man does not keep pace with his companions, perhaps it is because he hears a different drummer. Let him step to the music which he hears, however measured or far away.
11
Declaration
I hereby declare that this PhD thesis comprise only my own research work except where indicated. Any external contribution regarding data collection or analysis as well as all the sources of information or any other material used have been fully acknowledged in accordance with the standard referencing rules. I certify that this thesis has not been previously submitted – neither partially nor totally – to any University or Institution for the award of any other degree.
13
Table of Contents
Acknowedgements ... 17 General Introduction ... 19 Structure of PhD thesis ... 28 References ... 301. Screening of cover crop species during early stages of development .. 37
1.1. Introduction ... 37
1.2. Materials and methods ... 38
1.2.1. Plant material ... 38
1.2.2. Germination test ... 43
1.2.3. Growth chamber trial ... 44
1.2.4. Data analysis ... 46
1.3. Results ... 47
1.3.1. Germination test ... 47
1.3.2. Growth chamber trial ... 47
1.4. Discussion ... 59
1.4.1. Germination test ... 59
1.4.2. Growth chamber trial ... 60
1.5. Conclusions ... 64
1.6. References ... 66
2. Functional diversity of cover crop mixtures increases stability of biomass yield and weed suppression ... 72
2.1. Introduction ... 72
2.2. Materials and methods ... 74
2.2.1. Data analysis ... 80
2.3. Results ... 83
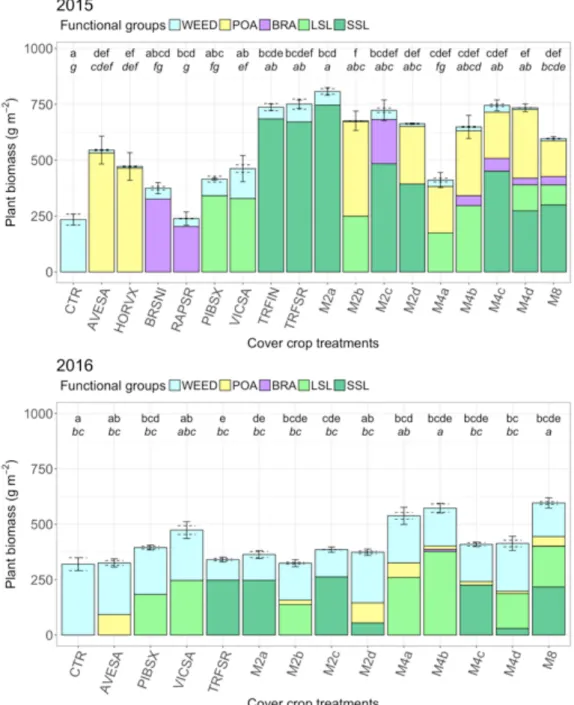
2.3.1. Cover crop biomass ... 83
2.3.2. Weed biomass ... 86
2.3.3. Weed suppression service provided by cover crops ... 87
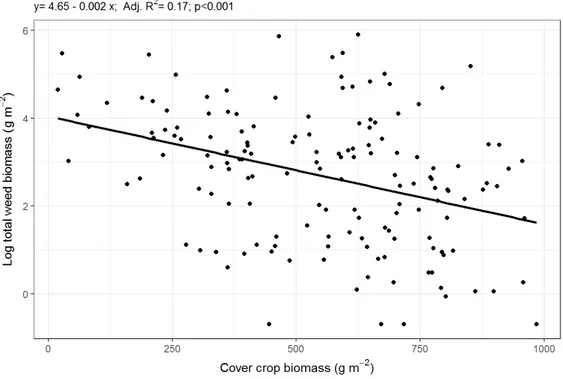
2.3.4. Relationship between cover crop biomass and weed biomass ... 87
2.3.5. Effect of diversity on cover crop and weed biomass ... 88
2.3.6. Effect of mixture functional composition on cover crop and weed biomass 90 2.3.7. Stability of cover crop performance and service provisioning ... 92
2.4. Discussion ... 93
2.4.1. References ... 98
3. Effect of cover crop functional diversity on nitrogen supply ... 106
3.1. Introduction ... 106
3.2. Materials and methods ... 108
14
3.3. Results ... 112
3.3.1. Nitrogen content in cover crops and weeds ... 112
3.3.2. Effect of cover crop nitrogen content on cover crop and weed biomass 113 3.3.3. Effect of species and functional diversity on cover crop nitrogen content 115 3.3.4. Stability of the nitrogen supply service ... 116
3.4. Discussion ... 117
3.5. Conclusions ... 119
3.6. References ... 121
4. Functional composition of cover crop mixtures can increase legumes biological fixation rate ... 126
4.1. Introduction ... 126
4.2. Materials and methods ... 128
4.2.1. Data analysis ... 133
4.3. Results ... 134
4.4. Discussion ... 140
4.5. Conclusions ... 144
4.6. References ... 146
5. Effect of cover crop mixtures on cash crop yield and nitrogen content 152 5.1. Introduction ... 152
5.2. Materials and methods ... 153
5.2.1. Data analysis ... 160
5.3. Results ... 161
5.3.1. Effect of cover crop treatments on aubergine yield ... 161
5.3.2. Fruit yield at the end of the cash crop cycle ... 162
5.3.3. Effect of cover crop diversity on aubergine yield ... 163
5.3.4. Effect of cover crop nitrogen and C/N ratio on aubergine yield ... 164
5.3.5. Effect of fertigation on aubergine yield ... 165
5.4. Discussion ... 167
5.5. Conclusions ... 169
5.6. References ... 170
6. Effect of cover crop dead mulch on weed abundance during aubergine cultivation ... 174
6.1. Introduction ... 174
6.2. Materials and methods ... 175
6.2.1. Data analysis ... 179
6.3. Results ... 180
6.4. Discussion ... 183
6.5. Conclusions ... 185
6.6. References ... 187
7. General discussion and conclusions ... 190
15
7.2. Implications for policy makers ... 201 7.3. Implication for farmers ... 202 7.4. References ... 204
17
Acknowedgements
This research was carried out within the frame of FERTILCROP project (http://www.fertilcrop.net/) funded via the ERA-net CORE Organic Plus (http://www.coreorganic.org). We would like to thank the Interdepartmental Centre for Agro-Environmental Research (CIRAA) ‘Enrico Avanzi’ of the University of Pisa for hosting the field trial and for the precious help during field operations. We would also like to thank Christian Frasconi and Daniele Antichi for their help and advice.
I would personally like to thank my supervisor, Prof. Paolo Bàrberi for his guidance and advice during my PhD, and for the time he dedicated helping me and answering my questions. I am grateful to Dr. Stefano Carlesi, my Tutor, for his essential help during field work, for his guidance throughout this PhD project realization, and his precious statistical advice. I am also grateful to Dr. Ambrogio Costanzo for his help and advice in the first phases of this research. I would like to thank Cian Blaix for his loving support and for English revision. I would also like to thank Giacomo, Roberta, Marco and Giovanni for their help during field operation. Their friendliness and humour made field work more enjoyable. Thanks to all the people that participated to field samplings and to all my colleagues for their cheerful company.
All my gratitude goes to my family, their love is making all my achievements possible.
19
General Introduction
At present, agriculture is facing many challenges. As human population is growing in number and wealth, crop global demand will increase by 100-110% from 2005 to 2050, especially due to increasing protein demand (Tilman et al., 2011). At the same time, as reported by FAO (2011), “roughly one-third of the edible parts of food produced for human consumption, is lost or wasted globally, which is about 1.3 billion ton per year”. It means that one third of the resources used for agricultural production (fuel, irrigation water, fertilizers, pesticides, antibiotics, etc.) is wasted as well, contributing to pollution and degradation of natural resources. Negative effects of the use of chemical inputs in farmlands on biodiversity (Donald et al., 2001) and natural resources (Blesh & Drinkwater, 2013) have been widely documented. Moreover, agriculture has been identified as one of the major contributors to greenhouse gases (GHG) emissions. The contribution of food waste is relevant also in this case: the FAO estimates that the impact of food waste in terms of GHG emission is higher than the whole India (FAO, 2013).
It is evident that there is a strong need to redesign farming systems with the objective to meet growing population demands while reducing negative externalities. Agriculture, as it has been practiced from the mid 20th century to the present day, has been declared unsustainable widely by the scientific community and some alternative approaches have been proposed.
The need for a “sustainable” intensification of agriculture has emerged, but, as well explained by Tittonell (2014), “ecological intensification” or “sustainable intensification” should be addressed with caution and always contextualized. These terms do not always define agricultural systems in which input use is
20 reduced in favour of lower negative environmental impact (Rosset & Altieri, 1997). An increase in resource use efficiency can be reached in high-tech farms, but these systems are very vulnerable, due to their high dependency on external inputs and the cost of technological applications. Moreover, highly specialized farms, by their nature, do not take into account biodiversity loss. Biodiversity conservation is one of the major concerns at the global level, since “biodiversity contributes directly (through provisioning, regulating, and cultural ecosystem services) and indirectly (through supporting ecosystem services) many components of human well-being, including security, basic material for good life, health, good social relations, and freedom of choice and action” (Millennium Ecosystem Assessment, 2005a).
It has been proposed that long-term sustainability of agricultural systems can be effectively achieved through agroecology and related solutions, for two main reasons: (i) agroecology requires a paradigm shift from global industrialized agriculture, and consequently a re-design of food systems (Altieri et al., 1989); (ii) in agroecology, biodiversity (and not the use of external inputs) is the cornerstone of food production (Altieri, 1999; Duru et al., 2015). Farming systems designed upon agroecological principles do not allow for food and resource waste. Moreover, social issues related to food systems would be addressed since social justice and food sovereignty are two of the main points in the agroecological agenda (Gliessman & Tittonel, 2015).
Generally, in the European framework, agriculture is required also to facilitate biodiversity conservation. Several measures have been put in place by European policy makers to contain biodiversity loss and ensure a sufficient level of service provisioning (sensu Millennium Ecosystem Assessment, 2005b), but their effectiveness is questionable. This is because farmers do not completely understand the reason why they should perform some agricultural practices (e.g.
21 cover cropping) instead of others (e.g. monoculture). In the majority of cases, farmers merely try to comply with policy requirements to get an economic reward. Instead, in an agroecological perspective, biodiversity serves agriculture and not the other way around (Bàrberi, 2015). Instead of using external inputs, agroecosystem services are provided by (agricultural) biodiversity. This is achieved through the understanding agroecosystem functioning and the adaptation of ecological principles to farming systems (Moonen & Bàrberi, 2008; Doré et al., 2011 Malézieux, 2012; Garnier & Navas, 2012).
Agroecological practices and interactions among agroecosystem elements (crops, natural vegetation, pests, natural resources, humans, etc.) can be studied at different scales: at the field level, at the landscape level, and at the food system level (Wezel et al., 2009). This study focuses on agroecological practices carried out at the field scale. Several agronomic practices can be adopted in an agroecological framework. Some of them are already common practice in low input and organic farms (e.g. reduced tillage, organic fertilization, cultivar choice), while others need fine-tuning to be fully implemented (optimised crop rotations, intercropping and relay intercropping, agroforestry, allelopathic plants, direct seeding into living cover crops or mulch, integration and management of semi-natural elements, etc.) (Wezel et al., 2014). These less common agroecological practices imply a certain level of diversification and the management of agricultural biodiversity (agrobiodiversity). Research is needed to provide information on how agrobiodiversity should be managed to achieve the desired agroecosystem services.
The mechanisms upon which plant species diversity can improve ecosystem services provisioning have mainly been addressed in ecological studies (see e.g. Hooper et al., 2005; Petchey & Gaston, 2006; Dìaz et al., 2007), but there is still lack of information about the adaptation of these principles to agroecosystem
22 practices. A framework for the conceptualisation, evaluation and management of biodiversity in agroecosystems has been proposed by Moonen & Bàrberi (2008). When managing agrobiodiversity for the provision of target agroecosystem services, the functionality of agroecosystem elements should be taken into account. As formulated by Costanzo & Bàrberi (2014), functional agrobiodiversity may affect agroecosystem service provisioning through three possible mechanisms: (i) functional identity, based on the mass-ratio hypothesis (Grime, 1998), upon which the expression of a target service is given by the presence of a set of homogeneous functional traits carried by dominant species; (ii) functional composition, upon which the expression of a target service is given by the co-presence of complementary functional traits; and (iii) functional diversity s.s., based on the diversity hypothesis (Tilman et al. 1997), upon which the expression of a target service is given by the heterogeneity of traits of the species considered.
In our study, we focused on the agroecological practice of cover cropping. Cover crops are increasingly used by farmers (in either conventional, low input, or organic systems), who recognise their important role in providing benefits (i.e. services) to the agroecosystem (Wayman et al., 2016). Among others, cover crops can protect soil from erosion, improve water infiltration, increase soil organic matter content, and improve soil structure (Mazzoncini et al., 2011; Wortman et al., 2012). They can improve nutrient cycling (Kuo & Sainju, 1998), particularly nitrogen, thanks to the nitrogen-scavenging activity of non-legume species and the N2-fixing activity of legume species (Thorup-Kristensen et al.,
2001; Hauggaard-Nielsen et al., 2009; Mazzoncini et al., 2004). Together with nutrient cycling, supply of biologically fixed nitrogen is particularly important in organic and low input agroecosystems where reduction of external inputs is a clear priority. Nitrogen provided by legumes is a sustainable way of adding nitrogen to support agricultural production without using organic or chemical
23 fertilizers (Peoples et al., 1995; Peoples et al., 2009). In addition, cover crops proved to be an effective tool in weed management (Dyck & Liebman, 1995; Creamer et al., 1996; Altieri, 1999; Wortman et al., 2013), especially in low-input and organic farming.
Biomass productivity is a key agroecosystem service that cover crops should provide. It can be considered as a service per se and at the same time it is closely linked to the provision of other agroecosystem services (Foley, 1999; Tosti et al., 2014), such as weed suppression (Mirsky et al., 2013) and total nitrogen supply (Lawson et al., 2015). Biomass production of cover crops also affect effectiveness of weed control in reduced tillage systems (Wayman et al., 2015). Reduced tillage systems are part of the strategies contained within the framework of conservation agriculture (FAO, 2015). The application of conservation agriculture principles (minimal soil
disturbance, permanent soil cover and crop rotations) allow to reduce erosion, increase carbon storage in the soil, increase microbial activity, improve soil structure through higher macroporosity due to the presence of earthworms, reduce water run-off and leaching, and reduce fuel consumption (Peigné et al., 2007). Despite all the benefits, conservation tillage is not widely practiced in organic agriculture (Casagrande et al., 2015; Cooper et al., 2016) because of several disadvantages. Peigné et al. (2007) pointed out that: weed control can be a severe issue in conservation tillage, conservation tillage is not a valid alternative to ploughing in difficult soils and rainy areas, nitrogen degradation and availability can be lower in conservation tillage, and the choice of crops for this system is limited. The major obstacle hindering the adoption of conservation tillage in organic farming is the perception that yield may be reduced due to the above-mentioned drawbacks. Cooper et al. (2016) demonstrated that yield in organic agriculture are not always reduced in conservation tillage. Moreover,
24 higher weed presence in no-till systems can be overcome by improving crop rotations and by improving the management of cover crops and cover crop surface residues. With this study, we also aim at contributing to the growing need for practical solutions to the agronomic constraints linked to the adoption of no-till in organic systems.
Cover crop functionality can be improved by mixing cover crop species (Teasdale & Abdul-Baki 1998; Creamer, 1997; Schipansky et al., 2014; Finney & Kaye, 2017), but there is still a lack of information on the effect of functional diversity on cover crop service provisioning. In this PhD project, a functional approach (Moonen & Bàrberi, 2008; Costanzo & Bàrberi, 2014) was adopted for the design of cover crop mixtures. Our target services were: (i) cover crop biomass production, (ii) weed suppression, and (iii) nitrogen supply to the agroecosystem. The residual effect of cover crop mixtures on yield, weed abundance, and nitrogen uptake of a subsequent vegetable cash crop (aubergine, Solanum melongena L.) was analysed. A field experiment was carried out in a vegetable organic certified farm in central Italy under no-till conditions, aiming at contributing to the need of information about agroecological practices such as direct seeding/transplanting in cover crop mulches (Wezel et al., 2014).
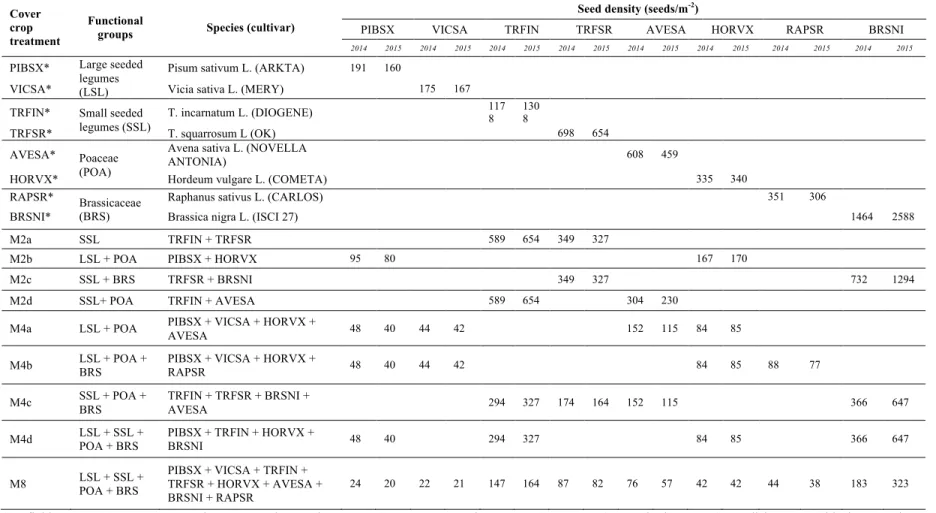
To select cover crop candidate species, a screening of cover crop species and varieties was performed in a growth chamber trial. Following this preliminary characterization, eight cover crop species belonging to four functional groups were selected. Among the species, the varieties that scored the highest for traits related to early establishment were selected, since plant development in the early growth stages is closely related to competitive ability against weeds (den Hollander et al., 2007).
Mixtures were designed with the aim of creating a gradient of diversity in terms of number of species and in terms of number of functional groups (Image 1). One
25 of the objectives of this project was to test the hypothesis that service provisioning by cover crop mixtures (cover crop biomass, weed suppression, and nitrogen supply, including the nitrogen fixed by legumes) can be enhanced by increasing the number of functional groups involved in mixtures. Several reasons can support the hypothesis that services provisioning can be enhanced in cover crop mixtures compared to monospecific stands. One of these is that monospecific stands are characterized by few functional traits linked to specific ecosystem services (e.g. legumes monocrop stands can provide high nitrogen quantity to the system, but they are not good competitors against weeds, while grasses do not fix nitrogen but are competitive against weeds, Ramirez-Garcia et al., 2015), while in cover crop mixtures, all functional traits needed for a target agroecosystem service (or multiple agroecosystem services) can be combined together. Another reason is that species diversity influences biomass productivity, and this can be achieved through the mechanism of complementarity of resource use (Loreau, 2000; Cardinale et al., 2007; Cardinale et al., 2011). Complementarity of resource use in cover crop mixtures can be achieved by mixing species characterized by complementary traits, e.g. species with shallow/deep root systems, fixing/non-fixing species prostrate/high-developing species. Diverse cover crop communities are expected to better exploit resource niches. For this reason, they are also expected to be more effective in suppressing weeds compare to monocrop stands since less resources are left available for weeds.
A second objective was to clarify which is the most important mechanism driving the provision of target agroecosystem services by cover crop mixtures among functional identity, functional composition and functional diversity s.s. We hypothesize that functional composition will be the most important mechanism driving cover crop service provisioning. This hypothesis is supported by the above-mentioned importance of complementarity of resource use in the
26 expression of a target agroecosystem service. Together with a better exploitation of environmental resources, positive synergies among cover crop species can be realized managing functional complementarity. An example is the increased yield and increased nitrogen fixation rate in legume-grasses mixtures compared to pure stands (Bedoussac et al., 2015, Tosti et al., 2014).
The last objective of this study was to test the hypothesis that the provision of target agroecosystem service is more stable in more diverse cover crop mixtures compared to monospecific stands. All crops can be vulnerable to adverse soil and environmental conditions, and all species respond differently to disturbance. The response to disturbance is driven by the set of functional traits that is present in the plant community (Hooper et al., 2005; Petchey et al., 2006; Díaz et al., 2007). If the set of trait in the cover crop is homogeneous (i.e. monospecific stand), the response to environmental changes will be homogeneous and, if negative, lead to the failure of the cover crop. If the functional trait pool is diversified (i.e. high diversity mixtures), there will be a diversified set of responses, of which, hopefully, some of them would be positive, maintaining a certain degree of service provisioning by the cover crop (i.e. the insurance hypothesis formulated by Bengtsson, 1998).
27
Image 1: Plots of cover crop mixtures at the experimental site in 2015. Image by Stefano
Carlesi.
Image 2: Cover crop four-species mixture (crimson clover, field pea, barley and black
28
Structure of PhD thesis
Figure 1: Scheme of the process followed in this study to assess agroecosystem services
provision of cover crop mixtures designed with a functional approach.
The first step in the adoption of a functional approach to the design of cover crop mixtures is to identify cover crop functional traits that are related to the target agroecosystem services. The choice of cover crop species must be based on the functionality of candidate cover crop species. The following step is to design cover crop mixtures using functional identity, functional complementarity or functional diversity of cover crop traits (Costanzo & Bàrberi, 2014) to improve the provision of target agroecosystem services: weed suppression, biomass production and nitrogen supply, including nitrogen derived by biological fixation. Cover crops can provide several agroecosystem services also to the subsequent cash crop. Cash crop yield can be affected by cover crop biomass; nitrogen supply to the cash crop can be driven by cover crop biomass and nitrogen content; weed control can be driven by cover crop biomass, especially in no-till
29 systems where cover crop biomass is left as dead mulch on the soil. The process described above is summarised in Figure 1.
Within this study, we address the issue of cover crop species selection in chapter one. In the second chapter, cover crop mixtures are described and the effect of functional diversity on weed control and biomass production is assessed. The effect of functional diversity of cover crop mixtures on nitrogen supply is discussed in chapter three, while the effect on biological fixation of legumes species in mixtures is analysed in chapter four. In the fifth chapter the effect of cover crop biomass and nitrogen content on cash crop yield and nitrogen uptake is described. In the last chapter, the effect of cover crop mixtures biomass on weed presence during cash crop cultivation is assessed (Figure 2).
30
References
Altieri, M. A. (1989). Agroecology: A new research and development paradigm for world agriculture. Agriculture, Ecosystems & Environment, 27(1), 37– 46
Altieri, M. A. (1999). The ecological role of biodiversity in agroecosystems. Agriculture, Ecosystems and Environment, 74(1–3), 19–31.
Bàrberi, P. (2015). Functional biodiversity in organic systems: the way forward? Sustainable Agriculture Research 4, 26-31.
Bedoussac, L., Journet, E. P., Hauggaard-Nielsen, H., Naudin, C., Corre-Hellou, G., Jensen, E. S., … Justes, E. (2015). Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agronomy for Sustainable Development, 35(3), 911–935.
Bengtsson, J. (1998). Which species? What kind of diversity? Which ecosystem function? Some problems in studies of relations between biodiversity and ecosystem function. Applied soil ecology, 10, 191–199.
Blesh, J., & Drinkwater, L. E. (2013). The impact of nitrogen source and crop rotation on nitrogen mass balances in the Mississippi River Basin. Ecological Applications, 23(5), 1017–1035.
Cardinale, B. J., Wright, J. P., Cadotte, M. W., Carroll, I. T., Hector, A., Srivastava, D. S., … Weis, J. J. (2007). Impacts of plant diversity on biomass production increase through time because of species complementarity. Proceedings of the National Academy of Sciences, 104(46), 18123–18128.
Cardinale, B. J., Matulich, K. L., Hooper, D. U., Byrnes, J. E., Duffy, E., Gamfeldt, L., … Gonzalez, A. (2011). The functional role of producer diversity in ecosystems. American Journal of Botany, 98(3), 572–592. Casagrande, M., Peigné, J., Payet, V., Mäder, P., Sans, F. X., Blanco-Moreno, J.
M., … David, C. (2016). Organic farmers’ motivations and challenges for adopting conservation agriculture in Europe. Organic Agriculture, 6(4), 281–295.
31 Cooper, J., Baranski, M., Stewart, G., Nobel-de Lange, M., Bàrberi, P., Fließbach, A., … Mäder, P. (2016). Shallow non-inversion tillage in organic farming maintains crop yields and increases soil C stocks: a meta-analysis. Agronomy for Sustainable Development, 36(1).
Costanzo, A., & Bàrberi, P. (2014). Functional agrobiodiversity and agroecosystem services in sustainable wheat production. A review. Agronomy for Sustainable Development, 34(2), 327–348.
Creamer, N. G., Bennett, M. A., Stinner, B. R., Cardina, J., &Regnier, E. E. (1996). Mechanisms of Weed Suppression in Cover Crop-based Production Systems. HortScience, 31(3), 410–413.
Creamer, N. G., Bennett, M. A., &Stinner, B. R. (1997). Evaluation of cover crop mixtures for use in vegetable production systems. HortScience, 32(5), 866– 870.
den Hollander, N. G., Bastiaans, L., & Kropff, M. J. (2007). Clover as a cover crop for weed suppression in an intercropping design. European Journal of Agronomy, 26(2), 92–103.
Díaz, S., Lavorel, S., de Bello, F., Quétier, F., Grigulis, K., & Robson, T. M. (2007). Incorporating plant functional diversity effects in ecosystem service assessments. Pnas, 104(52), 20684–20689.
Donald, P. F., Green, R. E., & Heath, M. F. (2001). Agricultural intensification and the collapse of Europe’s farmland bird populations. Proceedings of the Royal Society B: Biological Sciences, 268(1462), 25–29.
Doré, T., Makowski, D., Malézieux, E., Munier-Jolain, N., Tchamitchian, M., &Tittonell, P. (2011). Facing up to the paradigm of ecological intensification in agronomy: Revisiting methods, concepts and knowledge. European Journal of Agronomy, 34(4), 197–210.
Duru, M., Therond, O., Martin, G., Martin-Clouaire, R., Magne, M. A., Justes, E., … Sarthou, J. P. (2015). How to implement biodiversity-based agriculture to enhance ecosystem services: a review. Agronomy for Sustainable Development, 35(4), 1259–1281.
Dyck, E., Liebman, M., & Erich, M. S. (1995). Crop-weed interference as influenced by a leguminous or synthetic fertilizer nitrogen source: I.
32 Doublecropping experiments with crimson clover, sweet corn, and lambsquarters. Agriculture, Ecosystems and Environment 56(2), 93–108. FAO. 2011. Global food losses and food waste – Extent, causes and prevention.
Rome
FAO. 2013. Food wastage footprint – Impacts on natural resources. Summary report. Rome
Finney, D. M., & Kaye, J. P. (2017). Functional diversity in cover crop polycultures increases multifunctionality of an agricultural system. Journal of Applied Ecology, 54(2), 509–517.
Finney, D. M., White, C. M., & Kaye, J. P. (2016). Biomass production and carbon/nitrogen ratio influence ecosystem services from cover crop mixtures. Agronomy Journal, 108(1), 39–52.
Foley, M. E. (1999). Genetic Approach to the Development of Cover Crops for Weed Management. Journal of Crop Production, 2(1), 77-93
Food and Agriculture Organization of the United Nations (2015) What is conservation agriculture? http://www.fao.org/ag/ca/1a.html
Garnier, E., & Navas, M. L. (2012). A trait-based approach to comparative functional plant ecology: Concepts, methods and applications for agroecology. A review. Agronomy for Sustainable Development, 32(2), 365-399.
Gliessman, S., &Tittonell, P. (2015). Agroecology for Food Security and Nutrition. Agroecology and Sustainable Food Systems, 39(2), 131–133. Grime JP (1998) Benefits of plant diversity to ecosystems: immediate, filter and
founder effects. Journal of Ecology, 86(6), 902–910.
Hauggaard-Nielsen, H., Gooding, M., Ambus, P., Corre-Hellou, G., Crozat, Y., Dahlmann, C., … Jensen, E. S. (2009). Pea–barley intercropping for efficient symbiotic N2-fixation, soil N acquisition and use of other nutrients in European organic cropping systems. Field Crops Research, 113(1), 64– 71.
33 Hooper, D. U., Chapin, F. S., Ewel, J. J., Hector, A., Inchausti, P., Lavorel, S., … Wardle, D. A. (2005). Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecological Monographs, 75(1), 3–35. Kuo, S., &Sainju, U. M. (1998). Nitrogen mineralization and availability of
mixed leguminous and non-leguminous cover crop residues in soil. Biology and Fertility of Soils, 26(4), 346–353.
Lawson, A., Cogger, C., Bary, A., & Fortuna, A. M. (2015). Influence of seeding ratio, planting date, and termination date on rye-hairy vetch cover crop mixture performance under organic management. PLoS ONE, 10(6), 1–19. Loreau, M. (2000). Biodiversity and ecosystem functioning: recent theoretical
advances. Oikos, 91(1), 3–17.
Malézieux, E. (2012). Designing cropping systems from nature. Agronomy for Sustainable Development, 32(1), 15–29.
Mazzoncini, M., Bàrberi, P., Cerrai, D., Rinaudo, V., Belloni, P., Università, A., … Avanzi, A. E. (2004). Effects of green manure on soil nitrogen availability and crop productivity in a Mediterranean organic farming system. Proceedings Eurosoil 2004, Freiburg (DE), 4-12 September, University, 1–9.
Millennium Ecosystem Assessment, 2005a. Ecosystems and Human Well-being: Biodiversity Synthesis. World Resources Institute, Washington, DC. Millennium Ecosystem Assessment, 2005b. Ecosystems and Human Well-being:
Synthesis. Island Press, Washington, DC.
Mirsky, S. B., Ryan, M. R., Teasdale, J. R., Curran, W. S., Reberg-Horton, C. S., Spargo, J. T., … Moyer, J. W. (2013). Overcoming Weed Management Challenges in Cover Crop–Based Organic Rotational No-Till Soybean Production in the Eastern United States. Weed Technology, 27(1), 193–203. Moonen, A. C., & Bàrberi, P. (2008). Functional biodiversity: An agroecosystem
approach. Agriculture, Ecosystems and Environment, 127(1–2), 7–21. Peigné, J., Ball, B. C., Roger-Estrade, J., & David, C. (2007). Is conservation
tillage suitable for organic farming? A review. Soil Use and Management, 23(2), 129–144.
34 Peoples, M. B., Brockwell, J., Herridge, D. F., Rochester, I. J., Alves, B. J. R., Urquiaga, S., … Jensen, E. S. (2009). The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis, 48(1– 3), 1–17.
Peoples, M. B., Herridge, D. F., &Ladha, J. K. (1995). Biological Nitrogen-Fixation - an Efficient Source of Nitrogen for Sustainable Agricultural Production. Plant and Soil, 174(1–2), 3–28.
Petchey, O. L., & Gaston, K. J. (2006). Functional diversity: Back to basics and looking forward. Ecology Letters, 9(6), 741–758.
Ramirez-Garcia, J., Gabriel, J. L., Alonso-Ayuso, M., & Quemada, M. (2015). Quantitative characterization of five cover crop species. The Journal of Agricultural Science, 153(7), 1174–1185.
Rosset, P. M., & Altieri, M. A. (1997). Agroecology versus input substitution: A fundamental contradiction of sustainable agriculture. Society & Natural Resources, 10(3), 283–295.
Schipanski, M. E., & Drinkwater, L. E. (2011). Nitrogen fixation of red clover interseeded with winter cereals across a management-induced fertility gradient. Nutrient Cycling in Agroecosystems, 90(1), 105–119.
Schipanski, M. E., Barbercheck, M., Douglas, M. R., Finney, D. M., Haider, K., Kaye, J. P., … White, C. (2014). A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agricultural Systems, 125, 12–22.
Schmid, B., Hector, A., Saha, P., & Loreau, M. (2008). Biodiversity effects and transgressive overyielding. Journal of Plant Ecology, 1(2), 95–102.
Teasdale, J. R., & Abdul-Baki, A. A. (1998). Comparison of mixtures vs. monocultures of cover crops for fresh-market tomato production with and without herbicide. HortScience, 33(7), 1163–1166.
Thorup-Kristensen, K., Magid, J., & Jensen, L. S. (2001). Catch crops and green manures as biological tools in nitrogen management in temperate zones. Advances in Agronomy, 79, 227–302.
35 Tilman, D., Balzer, C., Hill, J., &Befort, B. L. (2011). Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Sciences, 108(50), 20260–20264.
Tilman, D., Knops, J., Wedin, D., Recih, P., Ritchie, M., Siemann, E. (1997) The influence of functional diversity and composition on ecosystem pro-cesses. Science, 277, 1300–1302.
Tittonell, P. (2014). Ecological intensification of agriculture-sustainable by nature. Current Opinion in Environmental Sustainability, 8, 53–61.
Tosti, G., Benincasa, P., Farneselli, M., Tei, F., & Guiducci, M. (2014). Barley– hairy vetch mixture as cover crop for green manuring and the mitigation of N leaching risk. European Journal of Agronomy, 54, 34–39.
Wayman, S., Cogger, C., Benedict, C., Collins, D., Burke, I., & Bary, A. (2015). Cover Crop Effects on Light, Nitrogen, and Weeds in Organic Reduced Tillage. Agroecology and Sustainable Food Systems, 39, 647–665.
Wayman, S., Kissing Kucek, L., Mirsky, S. B., Ackroyd, V., Cordeau, S., & Ryan, M. R. (2016). Organic and conventional farmers differ in their perspectives on cover crop use and breeding. Renewable Agriculture and Food Systems.
Wezel, A., Bellon, S., Doré, T., Francis, C., Vallod, D., & David, C. (2009). Agroecology as a science, a movement and a practice. Sustainable Agriculture, 2, 27–43.
Wezel, A., Casagrande, M., Celette, F., Vian, J. F., Ferrer, A., &Peigné, J. (2014). Agroecological practices for sustainable agriculture. A review. Agronomy for Sustainable Development, 34(1), 1–20.
Wortman, S. E., Francis, C. a., Bernards, M. a., Blankenship, E. E., & Lindquist, J. L. (2013). Mechanical Termination of Diverse Cover Crop Mixtures for Improved Weed Suppression in Organic Cropping Systems. Weed Science, 61(1), 162–170.
37
1. Screening of cover crop species during early
stages of development
1.1. Introduction
Cover crops are a very important tool in organic and low input systems. Cover crops can provide nitrogen to the agroecosystem, especially through N2 fixation
when legumes are involved, they can also reduce nutrient losses (Hauggaard-Nielsen et al., 2009; Campiglia et al., 2011), provide nutrients to subsequent cash crop (Benincasa et al., 2010; Tosti et al., 2012), and suppress weeds (Moonen & Bàrberi, 2004; Kruidhof et al., 2008; Price & Norsworthy, 2013). Weed suppression by cover crops is linked to cover crop stands functional traits (Garnier & Navas, 2012). Traits of cover crop species and varieties, such as plant height, root length, and biomass accumulation can be linked to weed suppressive ability of cover crop stands (Zare et al., 2012; Tardy et al., 2015; Damour et al., 2016; Jacob et al., 2016). It is important to select cover crop species and varieties carrying functional traits related to weed suppression to obtain the best cover crop stands for a specific environment or cropping system (Jannoyer et al., 2011). Selection of cover crop species and variety based on functional traits is particularly important in cover crop mixtures. The presence of specific multiple functional traits can drive agroecosystem services provisioning, and the composition of those traits must be carefully planned (Costanzo & Bàrberi, 2014). There is a wide variability of functional traits expression among cover crop species and varieties. Useful functional traits and genetic variability can also be found among plant species that could be potentially used as cover crops (Karlsson-Strese et al., 1996; Gebhard et al., 2013). However, the wide genetic variability of candidate cover crop species, especially in relation to their weed suppressive ability and suitability for inclusion in mixtures, has rarely been studied (Maul et al., 2011). Since little information on cover crop species and
38 varieties is available, we performed a screening of 34 cover crop species and varieties (including legumes, grasses, and brassicas) during early stages of development. Our trial aimed at highlighting differences in functional traits among cover crop varieties belonging to the same species and among species belonging to the same functional group.
1.2. Materials and methods
1.2.1. Plant material
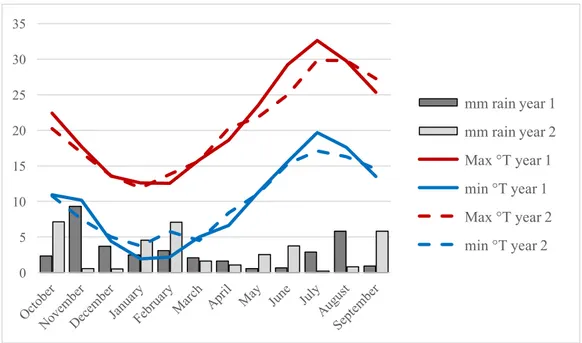
We chose to test different species that can be potentially used as cover crop in our target environment, the coastal plain of Tuscany (the average annual temperature is 14.6 °C and the average annual rainfall is 886 mm). To determine which species to include in the trial, we focused on their agroecological functionality.
We identified four functional groups that correspond to four botanical families of cover crop species, namely: Leguminosae, Graminaceae, Brassicaceae and Hydrophyllaceae.
As well known, Leguminosae species are characterized by the ability to fix atmospheric nitrogen. This function is performed thanks to the symbiosis with Rhizobium spp. bacteria. Because of the ability to introduce organic nitrogen in the agroecosystem they are usually sown as green manure crops (Giller & Cadish, 1995; Crews & Peoples, 2005; Cherr et al., 2006). Within this family, we chose seven annual species adapted to the Mediterranean environment: barseem clover (Trifolium alexandrinum L.), crimson clover (Trifolium incarnatum L.), squarrosum clover (Trifolium squarrosum L.), Persian clover (Trifolium resupinatum L.), bird’s-foot trefoil (Lotus corniculatus L.), common vetch (Vicia sativa L.), and field pea (Pisum sativum L.).
39 produce high biomass. As such, they are suited to be used as winter catch crops. Within this family we chose four species: barley (Hordeum vulgare L.), oats (Avena sativa L.), bristle oats (Avena strigosa Shreb.), and triticale (xTriticosecale Wittm.).
Brassicaceae species are also used as winter catch crops. Brassicaceae contain compound sugars (glucosinolates) that are able to develop allelochemical compounds through enzymatic degradation by myrosinase. The biologically active compounds that are formed are isothiocyanates (ITC), organic cyanides (CN), oxazolidinethiones (OZT), and ionic thiocyanate (SCN), depending on soil pH (Brown & Morra, 1996). They exert an allelopathic effect against weed seed germination and seedling growth (Vaughn et al., 2006; Alcántara et al., 2011; Kruidhof et al., 2014). Within this family, we selected six species: black mustard (Brassica nigra (L.) W.D.J. Koch), Ethiopian rape (Brassica carinata A. Braun), brown mustard (Brassica juncea (L.) Czern), oilseed rape (Brassica napus L.), radish (Raphanus sativus L.), and rocket (Eruca sativa Mill.).
In the Hydrophyllaceae family we have included one species: lacy phacelia (Phacelia tanacetifolia Benth.). This species is highly attractive for pollinators and is often seeded by beekeepers to feed honeybees (Williams & Christian, 1991; Barbir et al., 2014). It is also used as a viable catch crop (Bodner et al., 2010; Marinari et al., 2015).
We selected a higher number of legume species compared to the other families because they play a key role in cover cropping. Legumes N2 fixation provides
nitrogen to agroecosystems. The presence of at least one legume species in cover crops is essential when cover crops are used as green manure to maintain and increase soil nitrogen content and soil fertility. Due to legumes central role in the design of cover crops, we focused our efforts in characterising a higher number of legumes species compared to species belonging to other families.
40 2014, except for grasses, for which there was a higher availability of varieties. To select cultivars to be involved in the trial, we focused on traits that we assumed to be important for weed suppression, based on the information declared by breeders (Table 1.1). For leguminous species, we selected those cultivars for which an early development and a high resistance/tolerance to abiotic (frost) and biotic stresses were declared by breeders. The criteria adopted in choosing grass species cultivars were: (i) early development, (ii) resistance/tolerance to abiotic and biotic stresses, (iii) height.
Table 1.1: Description of cover crop species and varieties following the information given by
breeders.
Species Varieties Available information
Barseem clover (Trifolium
alexandrinum
L.)
Axi Adaptability to a wide range of soils and
environmental conditions; high number of leaves; early vegetative activity in spring; very good resistance to cold conditions and fungal diseases. Sacromonte No information available.
Bluegold No information available.
Nilodi Good performance in intercropping, very suitable
for cover cropping. Crimson clover
(Trifolium
incarnatum L.)
Diogene Can be utilized in mixtures; fast growth; good
adaptability to various soil conditions; highly resistant to cold and diseases.
Kardinal Quite rapid growth.
Clo Very resistant to cold conditions; suitable for cover
cropping. Squarrosum
clover (Trifolium
squarrosum L.)
Quadriga Very adaptable, resistant to low temperatures.
Bio No information available.
Ok No information available.
Persian clover (Trifolium
resupinatum L.)
Laser No information available.
Bird's-foot trefoil (Lotus
corniculatus L.)
Leo No information available.
Asca No information available.
Common vetch (Vicia sativa L.)
Mery Easily adaptable to diverse environmental
condition; good performance in clay soils; very good resistance to cold and fungal diseases; low resistance to aphids.
Ereika Fast growth, good resistance to pests.
Senda No information available.
Field pea (Pisum
sativum L.)
Atos No information available.
Arkta No information available.
Lacy phacelia (Phacelia
tanacetifolia
Benth.)
Boratus No information available.
Mira No information available.
Barley (Hordeum
vulgare L.)
Cometa Medium growth, moderate resistance to cold
conditions; resistant to diseases; medium height.
Sphera Hybrid; early growth; medium height; very high
productivity.
Tattoo Hybrid; medium growth; medium height; very high
42 Oats (Avena
sativa L.)
Novella Antonia
Upright; fast early development.
Argentina Not upright; fast early development; shorter than NOVELLA ANTONIA.
Bristle oats (Avena strigosa Shreb.)
Pratex Rapid growth; competitive against weeds; high
tillering; good ground cover. Triticale
(xTriticosecale Wittm.)
Bienvenu Used as reference species for field trials; very fast early development.
Constant Medium height; adaptable and resistant to diseases. Black mustard
(Brassica nigra (L.) W.D.J. Koch)
ISCI 27 No information available.
Etihopian rape (Brassica carinata A. Braun) CT180 No information available. Brown mustard (Brassica juncea (L.) Czern)
ISCI 20 This variety is the most cultivated and the most tested in Italy. It has a long flowering period and it is very adaptable to different pedo-climatic
conditions. It has a strong bio-fumigant effect (13,3 µmol g-1 DM of glucosinolates compounds, of which sinigrin is the main one).
Oilseed rape (Brassica napus L.)
Fregat No information available.
Radish (Raphanus
sativus L.)
Carlos No information available.
Rocket (Eruca
sativa Mill.)
Nemat Can be a trap-crop against nematodes during
cultivation and has a bio-fumigant action at the moment of devitalization. Glucosinolates are concentrated in roots and above-ground biomass (12,9 µmol g-1 DM of glucosinolates compounds, of which erucin and raphanin are the most
1.2.2. Germination test
A germination test was carried out for all species and varieties, in a seed incubator (Mini Camera Termostatica MTC 200, Angelatoni Scientifica) under standard conditions. The germination test was performed for all species and varieties described in Table 1.1. All seeds were kept in a seed storage room at the Interdepartmental Centre for Agro-Environmental Research (CIRAA) “Enrico Avanzi” of the University of Pisa. Seed were stored for two consecutive years. Seed age at the moment of retrieval was not always declared by suppliers. Seed germinability was tested according to the guidelines of the Italian authority for seed certification (Centro di sperimentazione e certificazione delle sementi, CRA-SCS). Seed germinability is defined as the percentage of pure seeds (without extraneous material) that are able to generate normal seedlings, namely those whose essential structures have a balanced development, and are thus able to produce plants that have the capacity to accomplish their vegetative and reproductive cycle under favourable agronomic conditions (official methods indicated by the Italian Ministry of Agriculture and Forests, D.M. 22-12-1992; ISTA, 2013).
We randomly selected 100 pure seeds for each sample after eliminating seeds of extraneous species and debris. The trial was replicated four times. A filter paper substratum and Petri dishes (diameter of 12 cm) were used. Enough water to allow sufficient imbibition of seeds was applied. Because of their hard seed coat, Pisum sativum and Vicia sativa seeds were previously soaked in a volume of distilled water about three times their volume for 12 h. The germination test started immediately after soaking. In a preliminary test, we found that there was a high level of pathogen contamination on cereal seeds. To reduce the presence of pathogens, cereal seeds were washed in running water for 4 h before the
44 germination test. According to the official method, the paper was re-wetted when necessary during the trial. Petri dishes were wrapped with clear plastic film to retain evaporation (Baskin & Baskin, 1998).
Temperature of the growth chamber was set at 20°C throughout the duration of the experiment. This value was chosen because it falls into the range of mean temperatures required by all species involved in the trial. Seeds were exposed to a 12 h light-12 h dark daily cycle. A pre-refrigeration treatment was not necessary. The duration of the germination test was not fixed a priori. The trial was completed when the maximum germination of the sample was obtained.
After the first count of germinated seeds, carried out after three days, the number of subsequent counts was kept to a minimum to reduce the risk of damaging seedlings that were not yet sufficiently developed. Seedling and seeds were categorized into normal seedlings, abnormal seedlings, dead seeds, fresh ungerminated, and hard seeds, according to the International Seed Testing Association (ISTA, 2013) guidelines. Percent germination data, referring to normal seedlings, was expressed as:
Germination (%) = (Number seed germinated / number seeds on tray) * 100 The average of the four 100-seed replicates of the test represents the percentage germination.
1.2.3. Growth chamber trial
The experiment was carried out in a growth chamber (Armadio climatico 1400 FCU L-PT, Bertagnin Frigoriferi SnC) upon a randomized complete block design with five blocks and 34 treatments. The illumination and temperature cycle was of 12 h light at 17°C + 12 h dark at 13°C. These parameters were chosen to simulate environmental conditions in autumn in Mediterranean environment
45 (where the period of cover crop sowing is usually from mid-september to late October). Temperature inside the growth chamber was monitored and recorded during the entire experimental period with a WatchDog data logger (WatchDog 1000 Series Micro Stations – Temp/RH, Spectrum Technologies, Inc. Aurora. IL). The plant growth medium used derives from the decomposition of some Ericaceae (Erica gracilis J.C.Wendl, Erica scoparia L., Calluna vulgaris (L.) Hull), Graminaceae (Monilia caerulea (L.) Moench) and ferns in a sandy substratum (pH ~ 5; limited content of organic matter, macro and micro nutrients). This medium was chosen to avoid high vegetation growth due to high availability of nutrients.
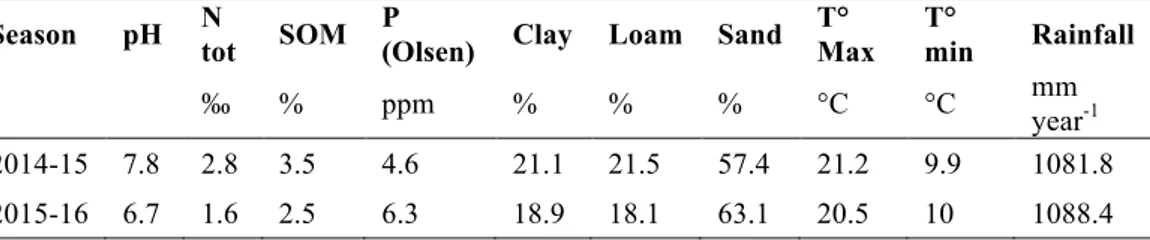
To inoculate Rhizobium spp., essential for leguminous species, each pot received ~ 100 g of soil taken from the organic field hosting the field experiment in the first year (chapter two), located in San Piero a Grado (43°40' lat. N, 10°20' long. E). The main characteristics of this soil are: clay 18.8%, silt 18.1%, sand 63.1%, pH 6.7, Total N 1.64 ‰, soil organic matter 2.53%, phosphorous (Olsen method) 6.3 ppm.
Five seeds per each treatment were sown in pots of 7 ´ 7 ´ 8 cm, which were initially watered until field capacity was reached, and re-watered when necessary. One week after their emergence, seedlings were thinned out to one seedling per pot.
Pots were arranged on five trays, placed on five shelves inside the growth chamber. Each tray represented one block. The distance between shelves (ca. 40 cm) was constant and enough to allow plant growth during the experiment. To reduce the experimental error, a re-randomization between pots within the trays and between trays inside the growth chamber shelves and compartments was performed weekly.
We recorded the developmental stages using the extended BBCH scale (Hess et al., 1997). Records were taken every two or three days for all treatments during
46 the entire duration of the trial, starting from early emergence of seedlings (08 BBCH stage) until the end of the trial (26 d in total).
At the end of the experiment, we measured shoot dry weight, root dry weight, height, root length, and number of roots for each sample. To isolate root systems, we soaked the pot in water and sieved the roots with a 0.2 mm sieve. Through gentle washing, particles of soil were removed from the roots. All plants were cut at the stem base and the stem was recorded separately from the roots. Shoot height, root length, and number of roots were recorded. For dicotyledonous species, we counted the number of roots developing from the main root; for grass species, we counted the number of secondary roots. Because of the limited root system development in samples of Brassicaceae, it was not possible to count the number of roots for those species. Samples of above-ground and below-ground biomass were oven-dried at 60°C until constant weight, and then weighted.
1.2.4. Data analysis
To highlight differences in varieties performance, we compared each variety against the other, using a non-parametric test (Wilcoxon rank sum test), while to determine differences in species performance among functional groups, we used an ANOVA followed by orthogonal contrasts and Tuckey’s HSD post-hoc test. A simple linear regression was performed to analyse the relationship between legumes total biomass and seed weight. All analyses were carried out using R (R Core Team, 2017) in RStudio (RStudio Team 2016), using agricolae package (de Mendiburu, 2016).
47
1.3. Results
1.3.1. Germination test
Seeds germinability was very variable depending on the species (Table 1.2). For clover species, a satisfying percentage of germinated seeds was found for berseem clover, while in the case of crimson and squarrosum clover the germinability was lower and there was more variability between varieties. Persian clover recorded the highest value of germinability within legumes (95.8%). In the small-seeded legumes group, the lowest germinability was recorded for bird’s-foot trefoil. High variability was found between Brassicaceae. Adequate germination rates were found only in the case of rocket (84.6%) and oilseed rape (95.4%). Low values of germinability were found also within the Poaceae species, ranging from 26 % in bristle oats (cv. Pratex) to 98 % in barley (cv. Cometa).
1.1.1. Growth chamber trial
1.1.1.1. Performances of cultivars within species
Data were recorded for all species but two: Ethiopian rape (cv. CT180) and brown mustard (ISCI 20). These two species in fact failed in the growth chamber trial because they did not germinate or their development was too poor and the seedlings eventually died. Nodules were present in all leguminous species, meaning that symbiosis with Rhizobium was actually established.
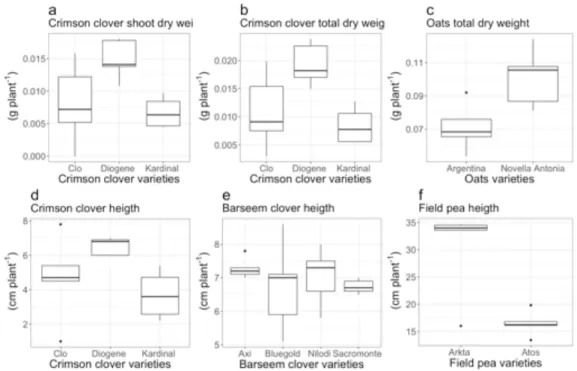
Significant differences in shoot dry weight were recorded only within the varieties of crimson clover; cv. Diogene scored 55 % higher shoot weight compared to cv. Kardinal (p<0.01; Figure 1.1a).
48
Tab 1.2: Average germination for cover crop species and varieties. Standard Deviation of the
mean is reported in brackets. Species have been coded upon EPPO Plant Protection Thesaurus (EPPT): http://eppt.eppo.org
Species Treatment code
Cultivar Germinability (%)
Barseem clover (Trifolium
alexandrinum L.)
TRFAL Axi 84.7 (4.7)
Sacromonte 88.7 (4.4)
Bluegold 88.0 (1.1)
Nilodi 86.1 (1.6)
Crimson clover (Trifolium
incarnatum L.)
TRFIN Diogene 78.3 (5.2)
Kardinal 66.7 (7.1)
Clo 82.7 (5.4)
Squarrosum clover (Trifolium squarrosum L.).
TRFSR Quadriga 82.0 (13)
Bio 79.3 (6.7)
Ok 76.9 (5.6)
Persian clover (Trifolium
resupinatum L.)
TRFRS Laser 95.8 (2.6)
Bird's-foot trefoil (Lotus
corniculatus L.)
LOTCO Leo 51.4 (13.5)
Asca 71.6 (5.2)
Common vetch (Vicia sativa L.) VICSA Mery 92.5 (3.5)
Ereika 79.2 (5.5)
Senda 93.7 (2.9)
Field pea (Pisum sativum L.) PIBSX Atos 94.7 (2.2)
Arkta 78.7 (27.6)
Lacy phacelia (Phacelia
tanacetifolia Benth.)
PHCTA Boratus 87.2 (4.5)
Mira 92.2 (7.5)
Barley (Hordeum vulgare L.) HORVX Cometa 93.8 (5.9)
Sphera 95.4 (1.2)
Tattoo 81.8 (15.6)
Oats (Avena sativa L.) AVESA Novella
Antonia
67.7 (23.8)
Argentina 54.8 (18.9)
Bristle oats (Avena strigosa Shreb.) AVESG Pratex 39.7 (17.4) Triticale (xTriticosecale Wittm.) TTLRI Bienvenu 80.4 (11.6) Constant 90.4 (2.8)
Black mustard (Brassica nigra (L.) W.D.J. Koch)
49 Etihopian rape (Brassica
carinata A. Braun)
BRSJU CT180 7.1 (3.7)
Brown mustard (Brassica
juncea (L.) Czern)
BRSCA ISCI 20 62.5 (5)
Oilseed rape (Brassica napus L.)
BRSNI Fregat 95.4 (1.3)
Radish (Raphanus sativus L.) ERUVE Carlos 62.0 (5.1)
Rocket (Eruca sativa Mill.) BRSNN Nemat 84.6 (4.3)
Differences in root dry weight were significant among varieties of oats, crimson clover and triticale. Among crimson clover varieties, root dry weight was significantly higher in cv. Diogene vs cv. Clo (+ 34 %, p < 0.05) and vs cv. Kardinal (+ 62 %, p < 0.01) (Figure 1.2c).
In the case of oats, cv. Novella Antonia had a 40 % higher root dry weight compared to cv. Argentina (p<0.05) (Figure 1.2d).
When analysing together the total dry weight, significant differences were found only for oats and crimson clover. In crimson clover, cv. Diogene scored the higher total biomass dry weight (0,02 g m-2, + 56% compared to cv. Kardinal, p<0.01; Figure 1.1b). For oats, cv. Novella Antonia total dry weight was significantly higher (+30 %) than cv. Argentina (p<0.05) (Figure 1.1c).
As for plant height, significant differences were found in crimson clover, barseem clover, and field pea. In crimson clover, cv. Diogene was the tallest variety (6.5 cm, 43 % taller than cv. Kardinal, p<0.05) (Figure 1.1d). We found significant differences among varieties of barseem clover only for plant height, where cv. Axi was taller than cv. Sacromonte (+ 7 %, p<0.05; Figure 1.1e) In the case of field pea, cv. Arkta was 46 % taller than cv. Atos (p<0.01) (Figure 1.1f).
As for root length, the only species that showed significant differences among varieties was crimson clover, where cv. Diogene had a 49 % higher value than cv. Kardinal (p<0.05) (Figure 1.2a).
Crimson clover varieties also showed significant differences in the number of roots (cv. Diogene 56 % higher than cv. Kardinal; Figure 1.2b.
50
Figure 1.1: Shoot parameters. Shoot dry weight in varieties of crimson clover (a); total dry
weight in varieties of crimson clover (b) and oats (c); height in varieties of crimson clover (d), barseem clover (e), and field pea (f).
Figure 1.2: Root parameters. Root length in varieties of crimson clover (a); number of lateral
roots in varieties of crimson clover (b); root dry weight in varieties of crimson clover (c), and oats (d).
1.1.1.2. Performances of species within functional groups
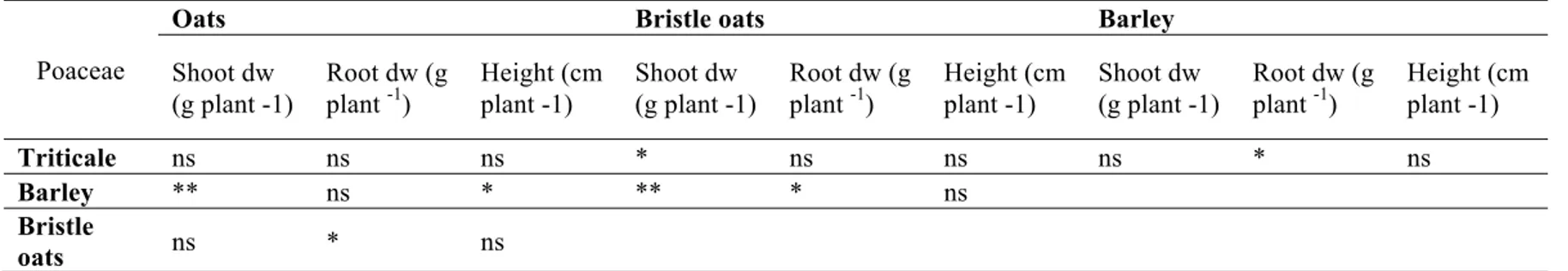
To complete our investigation, we analysed differences in performances of species within the same functional group. The performance of each species was based on the performances of all the varieties of that species taken together. Within the Poaceae family, significant differences in terms of shoot dry weight were found between triticale vs bristle oats, barley vs oats and barley vs bristle oats (Table 1.3; Figure 1.3a). Barley showed the maximum shoot dry weight, followed by triticale, oats and bristle oats. Moreover, significant differences in terms of root dry weight were found in Poaceae (Table 1.3; Figure 1.3b). Barley and oats had the highest root weight, and the difference between them was not significant, whereas bristle oats and triticale had the lowest value. Differences in height between Poaceae were not significant in all cases except for barley vs oats (Table 1.3; Figure 1.3c).
Within the Brassicaceae family, the species with the highest root and shoot dry weight was radish. Black mustard had the lowest root dry weight, but the difference was significant only vs rocket and not significant vs oilseed rape (Table 1.4 and Figure 1.4a and Figure 1.4b)
Figure 1.3: Relevant parameters for Poaceae family: shoot dry weight (a), root dry weight (b)
Figure 1.4: Relevant parameters for Brassicaceae family: shoot dry weight (a), and root dry
weight (b).
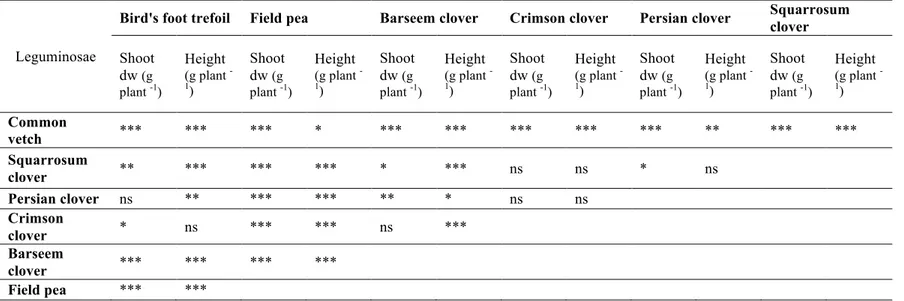
Within the Leguminosae family, we identified two sub-groups: one of legumes characterized by small seeds (crimson clover, squarrosum clover, barseem clover, Persian clover and bird’s-foot trefoil), hereafter referred to as Small Seeded Legumes (SSL) and the other comprising two species with large seeds (common vetch and field pea), hereafter referred to as Large Seeded Legumes (LSL). Differences for shoot dry weight between field pea and all the other species and between vetch and all the other species were highly significant (Table 1.5a; Figure 1.5a). Differences for shoot dry weight were significant also between field pea and common vetch. These two species had the higher shoot dry weight with field pea having the higher value above all species.
Within SSL, significant differences in shoot dry weight were found among them. Bird’s-foot trefoil was the species with the lowest development (Table 1.5a; Figure 1.5a).
The distinction of legumes into the two groups was also evident in the case of plant height: differences between LSL and SSL were always significant (Table 1.5a; Figure 1.5b).
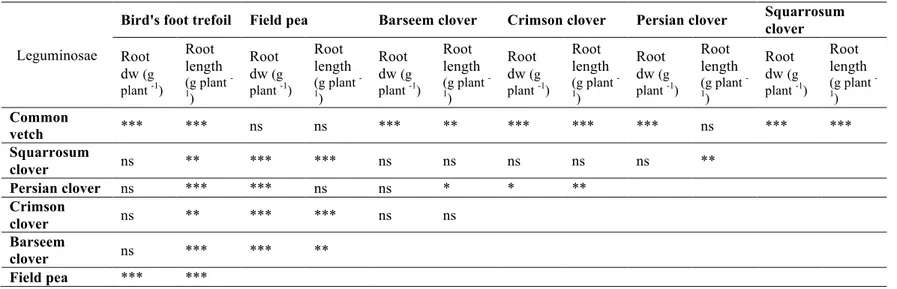
Differences in root dry weight were highly significant between LSL and SSL. Differences were not significant between common vetch and field pea. Within
54 the SSL group, significant differences were found only in the case of crimson clover vs Persian clover, with the latest showing a higher value (Table 1.5b; Figure 1.5c).
Significant differences in root length, were found only among legumes (Table 1.5b; Figure 1.5d). Persian clover root system was more developed than that of other clovers.
Figure 1.5: Relevant parameters for leguminosae family: shoot dry weight (a), height (b), root
dry weight (c), and root length (d).
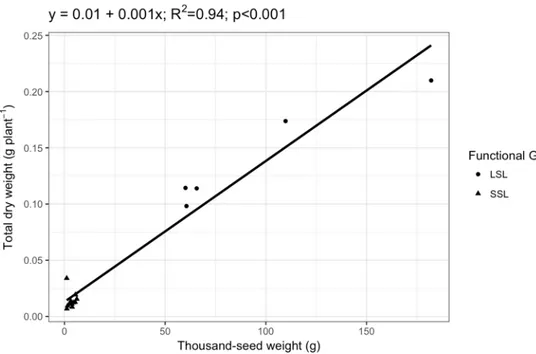
The distinction of legumes species in two functional groups is also supported by the analysis of the relationship between legumes total dry weight and seed weight (Fig. 1.6). A strong positive relationship was found between thousand-seed weight and total dry weight. Total dry weight was higher at higher seed weight. From Figure 1.6 it appears a clear distinction between two groups of legumes: legumes varieties characterized by low seed weight and low total plant dry weight
55 grouped at the bottom-left side of the graph, and legume varieties characterized by higher seed weight and total plant dry weight compared to the previous group on the upper part of the graph.
Figure 1.6: Relationship between total plant biomass dry weight and seed weight in legumes
varieties. Data are pooled by variety. Different shape indicates varieties belonging to a different functional group.
56
Table 1.3: Contrasts among Poaceae species for the parameters: shoot dry weight (dw), root dry weight (dw), and height. Significance codes: ***P
< 0.001; **P < 0.01; *P < 0.05.
Poaceae
Oats Bristle oats Barley
Shoot dw (g plant -1) Root dw (g plant -1) Height (cm plant -1) Shoot dw (g plant -1) Root dw (g plant -1) Height (cm plant -1) Shoot dw (g plant -1) Root dw (g plant -1) Height (cm plant -1) Triticale ns ns ns * ns ns ns * ns Barley ** ns * ** * ns Bristle oats ns * ns
Table 1.4: Contrasts among Brassicaceae species for the parameters: shoot dry weight (dw) and root dry weight (dw). Significance codes: ***P <
0.001; **P < 0.01; *P < 0.05.
Brassicaceae
Oilseed rape Black mustard Rocket
Shoot dw (g plant -1) Root dw (g plant -1) Shoot dw (g plant -1) Root dw (g plant -1) Shoot dw (g plant -1) Root dw (g plant -1) Radish ** * ns * ns * Rocket ns ns ns * Black mustard ns ns
57
Table 1.5a: Contrasts among Leguminosae species for shoot parameters: shoot dry weight (dw) and height. Significance codes: ***P < 0.001; **P <
0.01; *P < 0.05.
Leguminosae
Bird's foot trefoil Field pea Barseem clover Crimson clover Persian clover Squarrosum clover Shoot dw (g plant -1) Height (g plant -1) Shoot dw (g plant -1) Height (g plant -1) Shoot dw (g plant -1) Height (g plant -1) Shoot dw (g plant -1) Height (g plant -1) Shoot dw (g plant -1) Height (g plant -1) Shoot dw (g plant -1) Height (g plant -1) Common vetch *** *** *** * *** *** *** *** *** ** *** *** Squarrosum clover ** *** *** *** * *** ns ns * ns Persian clover ns ** *** *** ** * ns ns Crimson clover * ns *** *** ns *** Barseem clover *** *** *** *** Field pea *** ***
58
Table 1.5b: Contrasts among Leguminosae species for the root parameters: root dry weight (dw) and root length. Significance codes: ***P < 0.001;
**P < 0.01; *P < 0.05.
Leguminosae
Bird's foot trefoil Field pea Barseem clover Crimson clover Persian clover Squarrosum clover Root dw (g plant -1) Root length (g plant -1) Root dw (g plant -1) Root length (g plant -1) Root dw (g plant -1) Root length (g plant -1) Root dw (g plant -1) Root length (g plant -1) Root dw (g plant -1) Root length (g plant -1) Root dw (g plant -1) Root length (g plant -1) Common vetch *** *** ns ns *** ** *** *** *** ns *** *** Squarrosum clover ns ** *** *** ns ns ns ns ns ** Persian clover ns *** *** ns ns * * ** Crimson clover ns ** *** *** ns ns Barseem clover ns *** *** ** Field pea *** ***