Chapter 4:
Angiotensin II (Ang II) induces the generation of procoagulant
MP by human mononuclear cells via AT2-mediated pathway
4.1 Introduction
4.1.1 Hypertension
Hypertension is a well know risk factor for thrombotic events and acute coronary syndrome. Individuals with high plasma renin, a protein that is a rate-limiting factor in the production of Ang II, have a fivefold increase in the rate of myocardial infarction compared with people with a low plasma-renin profile [72]. Moreover, captopril, an Ang II-converting enzyme inhibitor (ACEI), which decreases the level of circulating Ang II, has been shown to decrease the incidence of myocardial infarction [73]. Although the relationship between hypertension and the risk for atherosclerotic plaque rupture has been estabilished, the mechanism of this phenomenon is still poorly understood [74].
4.1.2 Renin Angiotensin System (RAS)
The activation of the RAS plays an important role in normal cardiovascular homoeostasis and its overactivity is involved in many cardiovascular pathologies, such as brain ischemia, renal failure, myocardial infarction, vascular injury and hypertension [75]. RAS is a bioenzymatic cascade that regulates the cardiovascular homeostasis by influencing vascular tone, fluid and electrolyte balance and the sympathetic nervous system [76]. The biological actions of the RAS are mediated primary by the active octapeptide Ang II. RAS is a system that was traditionally considered as a circulating endocrine system, whereby renin released by the juxtaglomerular cells of the kidney cleaves the liver-derived macroglobulin precursor angiotensinogen to produce the inactive decapeptide Angiotensin I, which it’s then converted to the active octapeptide Ang II by Angiotensin-Converting Enzyme (ACE) within the pulmonary circulation [77], [78]. In addition to the systemic circulating
RAS there are evidences that indicate that many tissues, like vasculature, heart, kidney and brain, are able to produce Ang II, which may thereby mediate autocrine, paracrine and intracrine effects. Numerous studies have also shown the presence of angiotensinogen, renin and ACE in such tissues [79]. Growing evidences showed the cytokine-like potential of locally-synthesized Ang II to promote endothelial dysfunction and vascular inflammation, two components of the atherogenic process.
4.1.3 Ang II
Ang II is the final affector of RAS and it can regulate blood pressure and electrolytic homeostasis (fig.4.1). The action of Ang II is mediated by two receptor subtypes, namely AT1 and AT2. Both receptors belong to the 7-transmembrane spanning receptor superfamily, but they only share 34% sequence homology [80]. All the well described actions of Ang II are mediated by the AT1 receptors and very little is still known about the AT2 receptor. AT1 receptor leads to a cascade of signaling pathways in several types of cells, that finally they leads to a decrease of NO and an increase of oxidized LDL, NF-kB, gene transcription factors and intracellular calcium. Depending on the local conditions, this leads to vasoconstriction, inflammation, proliferation, coagulation and increased atherogenicity.
Fig. 4.1. Bio-enzymatic cascade of the RAS [76].
4.1.4.Ang II type 1 receptors blockers (ARBs) and ACEIs
ARBs inhibit the RAS system by competitively and selectively binding the AT1 receptor and preventing its activation by Ang II. Compared to the well known class of ACEIs that can reduce directly the synthesis of the Ang II, ARBs seem to play an important role in the management of patients at increased cardiovascular risk. However, data from recent clinical trials [81], [82], shown that there are no substantive evidences to indicate that ARBs are able to reduce myocardial infarction (MI) and paradoxically, rates of MI seems to be increased after treatment with ARBs. ARBs bind AT1 receptor and consequently, increase the Ang II levels several-fold above the baseline and lead up to a negative-feedback loop. High concentrations of circulation Ang II result in a unopposed stimulation on the AT2 receptor, which is in addition up-regulated. AT2 receptor is mainly expressed in the fetal stage. In adults,
AT2 receptor is minimally expressed under normal circumstances and, although its effect seems to be opposite to those of the AT1 receptors, nowadays its intracellular pathway and its role in the adult cardiovascular system is still not well established. It has been assumed that stimulation of AT2 receptor leads to a decrease of MAP kinase and probably to an increase of cGMP, resulting in diminished cell growth, apoptosis and vasodilatation. Recent data suggested that this up-regulation may be harmful under certain circumstances through mediation of growth promotion, fibrosis and hypertrophy [83], [84], [85] as well as proatherogenic and proinflammatory effects [86], [87], [88].
4.1.5 MP and hypertension
Plasma levels of MP are elevated in conditions where Ang II is implicated. Several studies showed that patients with hypertension, diabetes or hyperlipidemia exhibit elevated plasma platelet and EMP [89], [90]. Treatment with AT1 blockers as lipid-lowering agents, such as simvastatin, decreases plasma MP levels [89]. Several studies suggested that the up-regulation of TF in hypertensive patients is at least in part due to the activation of the RAS system, especially the local RAS contained within the monocytes/macrophages complex in atherosclerotic plaques. It’s well known the prothrombotic and procoagulant potential of Ang II, including platelet sensitization, inhibition of the fibrinolysis by PAI-1stimulation and activation of the TF expression [91]. Indeed, it has been demonstrated that the levels of circulation TF in patients with acute coronary syndromes are increased in comparison to the healthy controls and patients with stable angina, possibly reflecting the induction of TF synthesis in the coronary artery during the acute coronary syndromes. Furthermore, increased levels of MP bearing TF were found in patients with ST-elevation acute myocardial infarction compared to healthy controls [75].
4.2 Aim
In the present study we investigated whether Ang II induces the generation of procoagulant MP by human mononuclear cells and the possible mechanisms
involved in this phenomenon, thus contributing to the understanding of the thrombotic complications of systemic hypertension.
4.3 Material and Methods
4.3.1 Reagents and kits
RPMI 1640 medium, penicillin, streptomycin, L-glutamine, FBS, trypan blue, Ficoll-Hypaque, dextran, sodium citrate, calcium chloride, calcium A23187, Ang II, PD 123319 were obtained from Sigma (Milan, Italy). Losartan (LOS) and Olmesartan (OLM) were obtained from Guidotti, Pisa, Italy. Tromboplastin standard was obtained from Beckman Coulter (Milano, Italy). Human plasma was collected from a normal voluntary donor. Human anti-TF antibody was obtained from America Diagnostica inc. (Instrumentation Laboratory, Milano, Italy). The Zymuphen Mp-Activity kit was obtained from Hyphen BioMed (Neuville-sur-Oise, France). The Fluo-4NW Calcium Assay kit was obtained from Molecular Probes (Invitrogen, Milan, Italy). The Annexin V-FITC was obtained from Alexis (Vinci Biochemicals, Firenze, Italy). All other chemicals were obtained from the hospital pharmacy and were of the best grade available.
4.3.2 Mononuclear cells isolation and culture
Mononuclear cells were isolated either from fresh buffy coats obtained from the local blood bank or from the peripheral blood of normal volunteers as described [63]. Briefly, a fresh buffy coat was diluted 1:1 with sodium citrate 0.38% in normal saline solution, mixed gently with 0.5 volume of 2% Dextran T500, and left for 30 minutes for erythrocyte sedimentation. The leukocyte-rich supernatant was recovered and centrifuged for 10 minutes at 200 x g. The pellet was resuspended in 30 mL of sodium citrate solution, layered over 15 mL of Ficoll-Hypaque and centrifuged for 30 minutes at 350 x g at 4°C. The mononuclear cell-rich ring was recovered and washed twice in sodium citrate solution. Mononuclear cells were then resuspended in RPMI/10% FBS and allowed to adhere for 18 hours at 37°C on 24-well plates (2x106 cells/well) or on Petri dishes (4x106 cells/mL).
4.3.3 MP generation and purification
Mononuclear cells were washed three times with pre-warmed serum-free RPMI. For MP generation, Ang II (1 µM) and A23187 (12 µM) were added; after 15 min at 37°C the supernatants were recovered, cleared by centrifugation at 14,000 x g for 5 min at room temperature to remove dead cells and big cell fragments that might have detached during the stimulation, and immediately used for further experiments. To investigate the intracellular mechanism of Ang II on generation of procoagulant MP by mononuclear cells, LOS (1µM) and OLM (1µM), two Ang II AT1- receptor blockers, PD 123319 (1µM), an Ang II AT2-receptor blocker, were incubated with pre-washed mononuclear cells for 30 min before the addition of Ang II (1µM) or A23187 (1µM) for 15 min. The supernatants were collected and managed as described before.
In selected experiments, MP (12 mL) were further purified by ultracentrifugation (100,000 x g for 2 hours, 4°C); the pellet was resuspended in 250 μL of normal saline and used in a one-stage clotting assay to measure TF-dependent coagulation.
4.3.4 Measurement of MP
PS-positive MP in each sample were detected using the Zymuphen MP-activity kit (Hyphen BioMed, Neuville-sur-Oise, France) according to the manufacturer’s instructions and expressed as PS equivalents (nMPS).
4.3.5 Measurement of intracellular calcium concentration
Molecular Probes Fluo-4 NW Calcium Assay kit was used to measure the changes in [Ca2+]I of mononuclear cells. Briefly, pre-washed mononuclear cells on 96-multiwell
plate (1 x 106 cells/well) were loaded with 100 μl of the dye loading solution containing Fluo-4 NW dye and Probenecid according to the manufacturer’s instructions. The 96-well plate was incubated at 37 °C for 30-45 min in the dark and the different stimuli were added to the cells. The changes in Fluo-4 NW fluorescence were measured by the Wallac 1420 Victor 2 (PerkinElmer, Milan, Italy) at ʎex 494 nm
and ʎem 516 nm. The calcium mobilization was observed over the time (up to 80 sec)
and analyzed by the Wallac 1420 Software version 3 (PerkinElmer Life and Analitical Sciences, Wallac, Milan, Italy). The increase in [Ca2+]i fluorescence (RFU) was
calculated as the difference between the mean stimulation fluorescence value and the mean baseline fluorescence observed (Δ RFU).
4.3.6 Assessment of TF Activity Harbored by MP
TF activity was measured in MP generated in vitro from human mononuclear cells by one-stage clotting time assay according to standard protocol [65], except that the normal human plasma was made MP-poor by ultracentrifugation (100,000 x g for 2 hours, 4°C). The results were expressed in arbitrary units (UA) of procoagulant activity by comparison with a standard curve obtained using a human brain tromboplastin standard. This preparation was assigned a value of 1000 UA for clotting time of 30 sec. An anti-human TF Antibody (30 µg/mL) was used to assess the specificity of the test.
4.3.7 Data presentation and statistical analysis
Data are shown as mean ± SEM from n independent consecutive experiments. Comparison between two groups was performed by paired two-tailed Student’s t test. Comparison among groups was made by ANOVA for repeated measures followed by the correction of Bonferroni’s test. All analyses were performed by Graph Pad Prism Software version 4.00 for Windows (GraphPad Software, San Diego, Caliphornia, USA) and p values < 0.05 were considered statistically significant.
4.4 Results
4.4.1. Ang II induces the generation of procoagulant MP by mononuclear cells To investigate the effect of Ang II on MP generation, mononuclear cells were stimulated with Ang II (1 μM) for 15 min. As shown in figure 4.2, Ang II induces a statistically significant increase in MP (bas: 0.169 ± 0.026 vs. Ang II: 0.266 ± 0.040 nM PS; mean±SEM, **p<0.001).
Fig. 4.2 Exposure of mononuclear cells to Ang II for 15 min. MP were measured as equivalents of nM PS. Ang II treatment (1 µM) significantly increases total MP (n = 10, mean ±SEM,two-tailed paired t-test, **p<0.001).
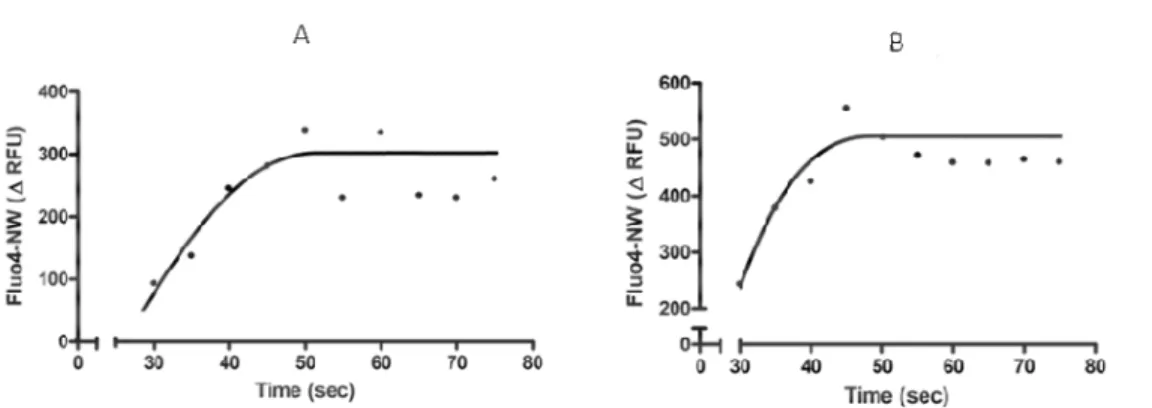
4.4.2 Ang II induces the mobilization of [Ca]i2+ in mononuclear cells
Ang II can induce an increase in [Ca]i2+ in many different systems [92], [93], [94].
Because calcium mobilization is involved in MP generation [5] we investigated whether Ang II increases [Ca]i2+ in our conditions. Figure 4.3 shows that Ang II
induces a rapid and significant increase in [Ca]i2+. A23187 was used as a positive
control.
Fig. 4.3 Evaluation of intracellular calcium mobilization in mononuclear cells treated with Ang II (1 µM) (A) or A23187 (12 µM) as positive control (B). One curve is representative of four independent experiments.
4.4.3 AngII-induced MP express TF on their surface
The procoagulant activity of MP due to the exposure of PS on their surface can also be enhanced by the presence of functional TF [45]. To evaluate whether Ang II can induce the generation of TF+-MP by mononuclear cells, the procoagulant activity of purified MP released by treated and untreated cells was analyzed by a one-stage clotting assay. As shown in figure 4.4, Ang II treatment induces a 3.6-fold increase in TF activity of MP. Identity of the procoagulant activity shown by the one stage assay with TF was confirmed by an anti-human TF antibody (data not shown).
Fig. 4.4 Effect of Ang II on generation of TF-bearing MP by mononuclear cells. Ang (1 µM, 15 min) induces a significant increase in TF-MP+ (n= 10, mean ±SEM, two-tailed paired t-test, *p<0.05). The procoagulant activity was measured by a one stage clotting test.
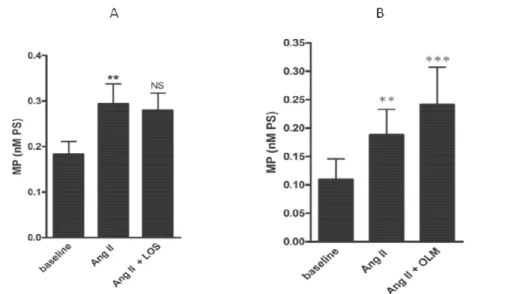
4.4.4 AngII-induced MP generation is mediated by AT2 but not AT1 receptor
Because AngII acts through the interactions with at least two distinct receptors, AT1 and AT2, we investigated which receptor was involved in AngII-induced MP generation. To this end, mononuclear cells were pre-treated with two different selective AT1 antagonists, LOS and OLM, or with a selective AT2 antagonist, PD123319. Exposure of mononuclear cells to the AT1 selective antagonists had no inhibitory effect on MP generation (fig. 4.5a) which was in fact increased by OLM (bas: 0.010 ± 0.036; Ang II: 0.189 ± 0.045; OLM + Ang II: 0.241 ± 0.067 nM PS; mean±SEM, **p<0.001, ***p<0.0001 (fig. 4.5b). On the contrary, cell treatment with the selective AT2 antagonist, PD123319, completely inhibited MP production (bas: 0.194 ± 0.036; Ang II: 0.289 ±0.055; PD123319+Ang II: 0.202 ± 0.043 nM PS; mean±SEM, **p<0.001, *p<0.05), (fig. 4.6a). To confirm the specificity of this
inhibitory effect, mononuclear cells were treated with A23187. AT2 selective antagonist PD123319 had no effect on A23187-induced generation of MP (fig.4.6b).
Fig. 4.5 Effect of two AT1 selective antagonists on MP generation by mononuclear cells. Either LOS (A) or OLM (B) had no effect on the MP release. The cells were pre-treated with LOS (1 µM) or OLM (1 µM) for 30 min before the stimulation with Ang II (n= 6, mean ±SEM, Anova analysis, **p<0.001,***p<0.0001).
Fig. 4.6 Effect of an AT2 selective antagonist on MP generation by mononuclear cells. Mononuclear cells were pre-treated with PD 123319 (1 µM) for 30 min before the stimulation with Ang II (1 µM) (A) or A23187 (1 µM) (B). PD 123319 reduces completely the Ang II-induced MP release by the cells but it had not effect on the A23187-induced MP generation. (n= 6, mean ±SEM, Anova analysis, **p<0.001, *p<0.05).
4.5 Discussion
Systemic hypertension is a well established risk factor for acute ischemic events even though the mechanisms involved are not fully understood. Experimental evidences have shown that the TF expressed by mononuclear cells and endothelial cells may contribute to the pathogenesis of acute ischemic syndromes at the coronary level. The activation of RAS system up-regulates TF synthesis at least in part through Ang II/AT1R-mediated activation of the transcription factor NF-kB. Ang II is the main effector of the RAS system and can itself modulate the expression of TF by mononuclear cells and endothelial cells [75]. Burger and coworkers demonstrated that Ang II has a direct stimulatory effect on EMP formation, involving a novel AT1 receptor/NADPH oxidase/Rho kinase pathway targeted to lipid rafts/caveolae. In turn, EMP can stimulate endothelial ROS formation and a pro-inflammatory response, suggesting a system wherein Ang II promotes endothelial injury through its own endothelial-derived MP [95]. Monocytes/macrophages play an important role in the development of atherosclerosis and formation of vulnerable plaques. Upon endothelial dysfunction, possibly caused by deposition of LDL and cholesterol, monocytes can infiltrate into the vascular wall in response to the secretion of TNF-α produced by other monocytes and to GM-CSF produced by the endothelial cells. Kim et al. demonstrated that Ang II, through AT2 receptor and ciclooxygenases, plays a crucial role in production of metalloproteinase-1 (MMP-1) by monocytes stimulated with TNF-α and GM-CSF, which may lead to atherosclerotic plaque rupture. This data correlate with an increased level of circulating Ang II in hypertension patients. High concentrations of Ang II, therefore, can facilitate the migration of monocytes towards the atherosclerotic plaque and induce them to release MMP-1, which has the ability to degrade the collagen in the fibrous cap of an atheroma, resulting in the rupture of the plaque. Blocking the AT2 receptor with the PD123319 antagonist can
block the MMP-1 production, suggesting an important role of AT2 receptor in promoting atherosclerosis.
ACEi have been widely used for the treatment of hypertension. It has been well established that ACEi improve cardiac function and remodeling and prolong survival in patients with heart failure. Recently, ARBs constitute a new important class of antihypertensive drugs. They block the AT1 receptor preventing the binding with Ang II and leading to an increased plasmatic concentration of renin and Ang II by a negative feedback loop. The increased circulating Ang II can act only on the AT2 receptor, that it’s up-regulated and overexpressed. Nowadays it’s not clear if ARBs are really more advantageous than ACEi. Some clinical trials have demonstrated that ARBs not only don’t give a real advantage in therapy against hypertension and heart failure but that the up-regulation of AT2 may be also harmful under certain circumstances through mediation of growth promotion, fibrosis and hypertrophy. In conclusion, our data demonstrated that Ang II can stimulate mononuclear cells to release MP associated to a TF dependent procoagulant activity via AT2 mediated pathway, underling the main role of TF-MP+ in thrombotic events and suggesting a possible explanation of the deleterious effect of ARBs in some hypertensive patients [82]. Although at present there is insufficient knowledge of the effects of stimulation of the AT2 receptor, further understanding of AT2 receptor and the role of TF-MP+ may contribute to new therapeutic strategies for cardiovascular diseases and hypertension.
![Fig. 4.1. Bio-enzymatic cascade of the RAS [76].](https://thumb-eu.123doks.com/thumbv2/123dokorg/7560004.110381/3.918.190.783.100.589/fig-bio-enzymatic-cascade-ras.webp)