Introduction
2.1 Histology of blood vessels
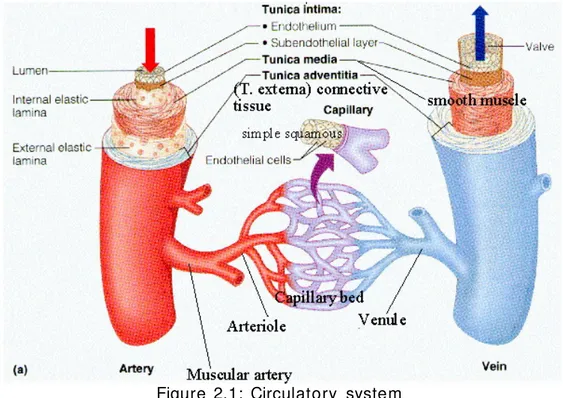
Blood vessels are part of the circulatory system and their function is to transport blood and nutrients throughout the body. They are divided into three types: arteries, veins and capillaries. Capillaries are microscopic vessels which enable the exchange of water and chemicals between blood and tissues, while arteries and veins carry blood from the heart to the capillaries or back towards the heart respectively (figure 2.1)[1].
Figure 2.1: Circulatory system
Arteries are generally divided into two types: elastic and muscular.
Elastic arteries have relatively large diameters and are located close to the heart (e.g. the aorta, the carotid and the iliac arteries). Muscular arteries, on the contrary, are more peripheral (e.g. femoral, celiac and cerebral arteries). However, some arteries exhibit morphol ogical structures of both types [2].
Blood vessels are composed of three layers, called from the luminal side outward, the tunica intima , the tunica media and the tunica adventitia (figure 2.2). The thickness of these three layers varies greatly depending on the size and type of vessel and on the age.
Figure 2.2: Blood vessel
•
The tunica intima is the innermost layer of an arte ry or vein . It ismade up of one layer of endoth eli al cells, which are in direct
contact with the blood, and is supported by an internal elastic lamina. It consists of:
1.
A layer of pavement endothe liu m , formed of polygonal,oval, or fusiform cells, with very distinct round or oval nuclei.
2.
A subendothelial layer, consisting of delicate connectivetissue with branched cells lying in the interspaces of the tissue .
3.
An elastic and fenestrated layer, which consists of amembrane containing a network of elastic fibers, having principally a longitudinal direction, and in which, under the
microscope, small elongated apertures or perforations may be seen, giving it a fenestrated appearance. In minute arteries the fenestrated membrane is a very thin layer, but in the larger arteries, and especially in the aorta , it has a very considerable thickness [3].
•
The tunica media is the middle layer of an artery or vein and It ismade up of smooth muscle cells and elastic tissue . It varies from smaller to larger arteries:
1.
In smaller arteries it principally consists of muscle fibersgrouped in fine bundles, arranged in lamellae and circularly disposed around the vessel. These lamellae vary in number according to the size of the vessel.
2.
In larger arteries (as the iliac , the femora l and the carotid ),elastic fibers unite to form lamellae which alternate with the layers of muscular fibers; these lamellae are united to one another by elastic fibers which pass between the muscular bundles, and are connected with the fenestrated membrane of the inner coat.
3.
In the largest arteries , as the aorta , the amount of elastictissue is considerably high; these vessels have a few bundles of white connective tissue in the middle coat. The muscle fiber cells are about 50 µm in length and contain well- marked, rod-shaped nuclei , which are often slightly curved.
As regards veins, the middle coat is composed of a thick layer of connective tissue with a transverse layer of muscular tissue. The white fibrous element is in considerable excess, and the elastic fibers are in much smaller proportion in the veins than in the arteries [4].
• The tunica adventitia is the outermost layer of a blood vessel and surrounds the tunica media . It is made up of collagen fibers, which anchor the blood vessel to the nearby organs, giving it stability. In larger vessels a network of small vessels (vasa vasorum) supply nutrients to the external tissue of the vessel wall [5].
2.2 Atherosclerosis
Atherosclerosis is an inflammatory vascular disease affecting arterial blood vessels and central in its developmental process (atherogenesis) stands the development of a an atheromatous plaque (figure 2.3). An atheromatous plaque results from accumulation of fatty substances between the tunica intima and the tunica media. Unlike functional high density lipoproteins, low density lipoproteins (plasma proteins that carry cholesterol and triglycerides ) fail to remove fats and cholesterol from the macrophages, thus leading to plaque formation [6] .
Figure 2.3: Atherosclerosis
2.2.1 Atheroge n es is
Atherogenesis is the developmental process of atheromatous plaques. It is characterized by a remodeling of arteries involving the concomitant accumulation of fatty substances called plaques. One recent theory suggests that, for unknown reasons, leukocytes , such as monocytes or basophils , begin to attack the endothelium of the artery lumen in cardiac muscle . The ensuing inflammation leads to the formation of atheromatous plaques in the arterial tunica intima . The bulk of these lesions is made of excess fat, collagen , and elastin . At first, as the plaques grow, only wall thickening occurs without narrowing of the lumen; stenosis is a late event, which may never occur and is often the result of repeated plaque rupture and healing responses.
2.2.2 Atheromatous plaque
The atheromatous plaque may be divided into three distinct components:
1. The atheroma , which is the nodular accumulation of a soft, flaky, yellowish material at the center of large plaques, composed of macrophages .
2. Underlying areas of cholesterol crystals.
3. Calcification at the outer base of older and more advanced lesions. Atherosclerosis causes two main problems. First, the atheromatous plaques , though long compensated for by artery enlargement, eventually lead to plaque ruptures and stenosis of the artery and, therefore, an insufficient blood supply to the organ it feeds. Second, if the compensating artery enlargement process is excessive, then a net aneurysm results. These complications are chronic, slowly progressing and cumulative. Most commonly, plaque suddenly ruptures, causing the formation of a thrombus that will rapidly slow or stop blood flow, leading to death of the tissues fed by the artery (infarction). One of the most common recognized scenarios is called coronary thrombosis of a coronary artery , causing myocardial infarction .
It is possible to distinguish two different plaque types:
1. The fibro- lipid plaque is characterized by an accumulation of lipid-laden cells underneath the intima of the arteries. It doesn’t involve the narrowing of the lumen due to compensatory expansion of the bounding muscular layer of the artery wall. Beneath the endothelium there is a "fibrous cap" covering the atheromatous "core" of the plaque. The core consists of lipid- laden cells (macrophages and smooth muscle cells) with elevated tissue cholesterol and cholesterol ester content, fibrin, proteoglycans, collagen and elastin.
These plaques usually produce the greatest damage to the individual when they rip.
2. The fibrous plaque is also localized under the intima, within the wall of the artery resulting in thickening and expansion of the wall and, someti mes, localized narrowing of the lumen with some atrophy of the muscular layer. The fibrous plaque contains collagen fibers (eosinophilic), precipitates of calcium (hematoxylinophilic) and, rarely, lipid- laden cells.
2.2.3 Risk incre asing physiological factors
There are several anatomic physiological and behavioral risk factors for atherosclerosis:
• Advanced age
• Diabetes or Impaired glucose tolerance (IGT) • Male sex
• Tobacco smoking • High blood pressure • Obesity
• A sedentary lifestyle
• Close relatives who have had some complication of atherosclerosis • Elevated serum levels of triglycerides
• Elevated serum levels of homocysteine • Elevated serum levels of uric acid
• Elevated serum fibrinogen concentrations • Elevated serum lipoprotein(a) concentrations • Elevated serum C-reactive protein concentrations
2.3 Coronary artery bypass
By means of coronary artery bypass surgery, blood flow can be “by passed“ by placing a healthy vessel of the patient (e.g. internal mammary artery) to deviate blood flow around the stenosis (figure 2.4). In case of coronary stenosis this procedure requires a sternotomy (an incision in the middle of the chest that separates the sternum to allow access to the heart) and the patient heart is connected to a heart- lung machine which is used to provide circulation and oxygenate the blood while the heart is stopped by the surgeons to work on it. Being an invasive surgical procedure, patients are usually admitted to an intensive care unit for one or two days and usually regain sufficient strength so that they can be discharged within 5 to 7 days after surgery [7].
Figure 2.4: Coronary artery bypass
2.4 Balloon Angioplasty
In order to reduce hospital stay, risks and costs associated with invasive surgery, stenosis can be treated with less invasive techniques.
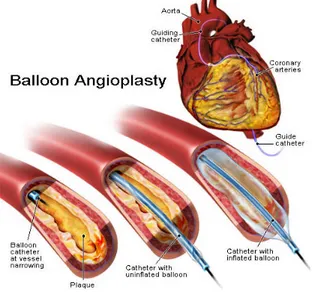
Angioplasty is used to dilate an area of stenotic artery by means of a catheter with an inflatable small sausage- shaped balloon on its tip. The balloon catheter is introduced through the skin of the groin, and someti mes of the arm (percutaneous = through the skin), and is placed inside a blood vessel (transluminal = in the lumen of a blood vessel). This technique can be applied in the coronary arteries treatmen t, so it is also called Percutaneous Transluminal Coronary Angioplasty (PTCA). Angioplasty physically opens the channel of diseased arterial segments (figure 2.5), relieves the recurrence of chest pain, increases the quality of life and decreases the risk of complications [8].
Figure 2.5: Balloon angioplasty
2.4.1 How PTCA is performed
A deflated balloon catheter is positioned in the narrowed segment of the target vessel. Subsequently, the balloon is inflated and, once the right luminal gain is achieved, the balloon is deflated and the catheter removed. Thanks to the limited invasive nature of this technique, patients recover faster compared to bypass surgery. However, two mechanisms limit the advantages of angioplasty over bypass surgery. On the short term, elastic recoil from the vessel is seen as soon as the balloon is removed, due to the energy released by the elastic fibers in the vessel wall after balloon deflation. On the long term, restenosis of the vessel is responsible for the renarrowing of the lumen. The major complications of balloon angioplasty mainly relate to arterial dissection and acute closure. Some dissections result from vigorous attempts at guidewire passage, however most are due to the injury induced by inflation of the balloon. To overcome these complications, st ent placement can be performed [7].
2.5 Endovascular Stent
Endovascular stents are medical devices inserted percutaneously into a stenotic artery to allow blood perfusion to the downstream tissue.
A metallic stent is typically a hollow cylindrical tube with a patterned slit structure and it is used as scaffold to maintain the perviety of a damged vessel after the PTCA surgery.
The first implanted stent was described by Dotter (1969) to treat arterial shrinkage, but clinical routine implantation began in the 1990s to improve the limitations of balloon angioplasty, such as restenosis and abrupt closure. If compared to angioplasty, the higher efficiency of stents is supported by randomized trials and clinical studies. Nevertheless,
problems and difficulties remain, such as migration, collapses, cloth formation or positioning difficulties.
Different typologies of stents are commercially available and it is important for the operator to know the different physical properties of the stent selected to treat a specific lesion [9].
There are two types of metallic stent:
• Self- expandable stent: made of a coil, mesh or zigzag design and
constrained inside a sheath (figure2.6). When the sheath is removed, the stent elastically springs open to a predetermined size that reflects the coronary artery diameter.
• Balloon expandable stent: pre- mounted on a balloon; when the
balloon is inflated, the stent plastically expands respecting the balloon diameter (figure 2.7).
Figure 2.6: Self- expandable stent
Figure 2.7: Balloon expandable stent
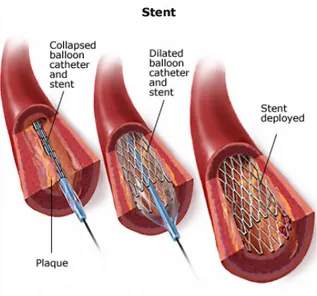
This technique consists in positioning a stent in the stenotic segment of the coronary artery (figure 2.8). This device is mounted on a balloon catheter and placed in the stenotic area.
The stent positioning is simplified by the employment of small radiopaque markers on the catheter and often on the stent too. These markers are angiographically visible and they help the operator to put the stent in optimal position.
When the stent is correctly positioned in the site of the lesion, the balloon is inflated so that the stent expands and it comes into contact with the artery wall. The pressure used to expand the stent varies from 6 atm to 12 atm.
Then, the balloon is deflated while the expanded stent remains in contact with the artery wall. With time, the artery wall cells grow around the strut of the device, thus further fixing it in its position. The success percentage of this technique is generally high, however a few complications may set in, such as restenosis or elastic recoil, which will cause the failure of the device [10].
Figure 2.8: Stent implantation
2.6. Stent project features
The ideal stent is inexpensive to manufac ture, easy to deliver and expand, sufficiently rigid to provide support, able to deliver therapeutic agents and biocompatible.
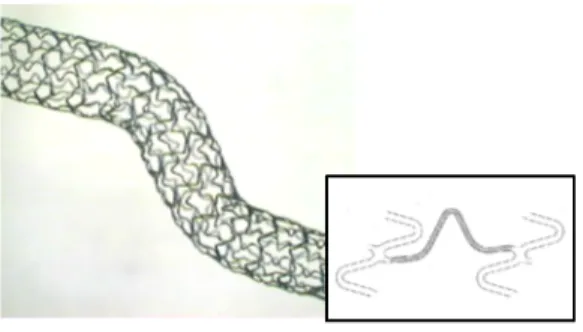
• Good flexibility in order to advance through the vascular tree, which
can present narrow and tortuous passage, and reach the target site (figure 2.9).
Figure 2.9: Stent flexibility
• Uniformity in order to avoid acute vascular injuries due to a
non-uniform expansion (dogboning) (figure 2.10) and foreshortening. Foreshortening affects the precision of stent positioning, while friction between the stent and the arterial wall can injure the endothelium.
Figure 2.10: Dogboning
• High radial strength in order to support the vessel lumen by minimizing post- stenting vessel recoil
• Low elastic recoil in order to achieve a final lumen diameter consistent with the target vessel diameter. In addition, undesirable shearing along the arterial walls may be caused by longitudinal recoil after the balloon is deflated
• Optimal scaffolding in order to avoid the prolapsed of the vessel tissue within the stent mesh and to obtain a uniform drug release. However, a low artery- stent contact surface area should be maintained, because the body- foreign material of the stent can initiate an aggressive thrombotic response.
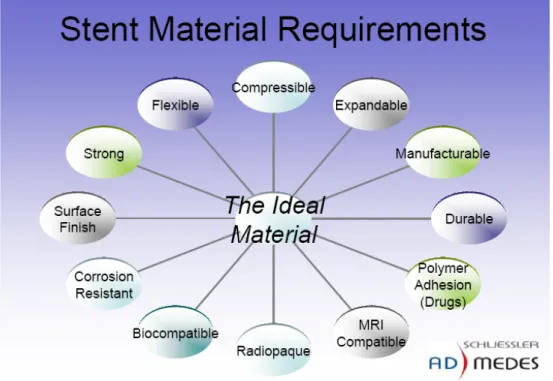
Metallic characteristics, bulk and surface properties, design and chemistry are all important factors to consider when conceiving an optimal stent. Materials to be used as stent backbone must fulfil stringent physical, mechanical and chemical properties (figure 2.11). The metal of an expandable stent must have enough plasticity to remain at the required size when expanded.
Self- expanding stents, moreover, have to be composed of sufficiently elastic metals so that they can be compressed and then oxide layer on many metals and corrosion particles may migrate from the metallic implants to other parts of the body.
The most recent stents are manufactured in 316L (low carbon content). This alloy is predominantly iron (60% to 65%) mixed with chromium (17% to 18%) and nickel (12% to 14%).
The chromium content is used to give a good corrosion protection behaviour (passivation) and contributes to strength and hardness. Thus, 316L stainless steel provides good resistance to corrosion, and has excellent mechanical properties but biocompatibility remains limited by the thrombosis issue.
Other materials can be used, such as a Nitinol alloy, which has a good biocompatibility, or tantalum, which has several advantages in terms of radiopacity, biocompatibility, mechanical properties and lack of ferromagnetism. After implantation, the tantalum stent wire undergoes oxidation, resulting in an oxide that is very stable and extremely resistant to degradation. In the circulation, the thin layer of inert tantalum peroxide creates an electrically negative charge that could reduce adhesion of negatively charged platelets.
Surface treat ment too, has been shown to modify the performance of stents. In fact mechanical polishing can decrease surface irregularities and thus reduce thrombosis and neointimal growth [11].
A method to obtain a smooth surface, is treating the stent with acid-pickling and then electrochemical polishing.
expanded and retain sufficient radial hoop strength to prevent vessel recoil or closure once in place.
Among chemical characteristics, corrosion properties are paramount. Formation of a surface metal oxide- film delays corrosion, and for some metals, such as chromium and titanium, this passivation is highly effective. However, saline liquids (such as blood) could destabilize the oxide layer on many metals. The corrosion particles, which are likely to form, may migrate from metallic implants to other parts of the body.
Figure 2.11: The ideal material
The stent design, structure, geometry and dimension s play a crucial role in the delivery process, the stent performance and the procedural success.
Two types of design are currently used [12] (figure 2.12):
• Coil stent , which may be obtained from a continuous wire or a series
of flat sheet coils.
• Tube stent , which are cut from a steel tube (e.g. Palmaz- Schatz
stent) or, occasionally, from a flat sheet of metal which is then rolled in a tube- shape and welded.
In coil stents there are fewer or no connections between struts. Therefore, coil stents show great flexibility, but poor radial strength and allow tissue prolapse within the widely- spaced wire elements.
As regards tube stent , two types of design can be distinguished:
• Slotted tube design • Modular design
Both are laser- cut from a metallic tube, although some modular designs are composed of wires which are welded together. The distinction between slotted tube and modular is less clear with modern stent designs, because tubes can be cut with few links and open cell design to produce a quasi-modular structure. Some designs of slotted tubes are manufactured with two versions, with more or less connecting elements, explicitly to create closed or open cell designs; the former provides extra hoop strength for lesions prone to recoil, and the latter provides increased flexibility to access tortuous vessels.
The dimensions of the stent, such as length and width can influence its deliverability, visibility and flexibility. A work from Thoraxcentre has clearly demonstrated that restenosis rates are predictable from the stent vessel dimensions. In their clinical studies, results clearly showed that a short stent has lower cases of restenosis than a long stent [13]. A similar study of the stent width suggested that a wide diameter stent is more favourable than a narrow one.
Stent geometry is another important aspect in the design. The result of studies on rabbit iliac arteries, indicated that fewer struts induce greater chance of restenosis compared to more struts [14]. This is probably due to the greater injury caused by a stent with fewer struts, presumably because the cross-sectional polygon produced by the stent with fewer struts was more angulated and liable to produce deep arterial injury. Moreover, a stent with few struts gives less radial support in the case of calcified plaques.
Another important aspect of the strut design is the thickness; thinner struts may have two differing effects upon the arterial wall. On the one hand, thin struts may damage tissue more easily and therefore more deeply than thick struts. On the other hand, they may cause less angulations and stretching of the arterial wall than wide struts. Moreover, in vitro studies of endothelial cell migration and attachment in conditions of flow, suggest that thicker struts may be slower to allow the development of a layer of endothelial cells [15].
Finally, another important aspect of the geometry is the shape of the strut cross- section (figure 2.13). A round cross- section area is ideal for smoothness, while a square design is not recommended because it
interferes with the blood flow due to their sharp edge which can slice blood cells.
Figure 2.13: Strut cross- section
2.7 Drug eluting stent s
The goal to eliminate restenosis, or at least to reduce its rate, has stimulated researchers to explore new technologies and adjunct therapy modalities to restore and preserve the acute and long- term outcomes following stenting [16]. The three major components of restenosis following a percutaneous coronary intervention that should be target are:
• An exuberant cellular proliferation and matrix synthesis (intima
hyperplasia) triggered by injury to the vessel wall [17];
• An acute elastic recoil immediately following balloon deflation ; • A late vascular contraction (remodelling) resulting in a decrease in
total vessel diameter [18].
While coronary stenting eliminates elastic recoil and vessel contracture by acting as a mechanical scaffold within the vessel, it is unable to inhibit excessive neointi mal formation. Stents were associated with an increase of neointimal formation compared to balloon angioplasty as a result of excessive injury to the vessel wall and the inflammatory process resulted from the interaction of the metal with the vessel wall. Furthermore, platelet activation, cytokines release and matrix production contribute to the process of restenosis following stenting [19].
restenosis than the bare metal stents; they inhibit restenosis and thrombosis still maintaining their metallic properties.
There are two types of DES:
• Special antithrombogenetic substances are directly fixed on the
stent surface.
• The stent is coated with a drug loaded polymer which acts as a
barrier between metal and blood and release the necessary drugs to prevent thrombosis and restenosis.
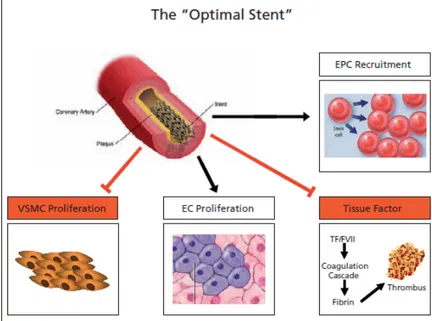
Therefore, the optimal DES will be coated with a simple- structure neutral polymer which does not elicit local inflammatory responses and does not favour platelet adherence (figure 2.14). The optimal stent will have the “right drug for the right condition ” approach and will release a multitude of agents able to maintain intact local homeostasis [20].
Figure 2.14 : The “opti mal stent”
2.7.1 Drug delivery mechanisms
The drug delivery mechanisms can be divided into physical and chemical mechanisms. The physical mechanisms include diffusion of drug molecules through a polymer layer, dissolution or degradation of polymer matrix controlling the drug release rate, osmotic pressure for drug release and use of ion exchange for ionic drug. One of the main advantages of physical mechanisms is the control of the drug release kinetics by the drug delivery system itself. Each system has predetermined drug release kinetics that can be adjusted by varying simple parameters, e.g. thickness of the polymer membrane, type of the polymer use and surface area. The chemical mechanisms are base on breaking of covalent bonds that connect drug molecules to a delivery vehicle, such as polymer chains. The main disadvantage of using chemical mechanisms is that drug molecules
undergo to chemical modified for grafting to delivery vehicle, and these result in new chemical entities called prodrugs.
Diffusion controlled drug release can occur in two different ways:
• The drug reservoir is covered with a thin polymer layer, which
functions as a rate- controlling membrane. At the steady state, the drug release remains constant, resulting in a zero order release.
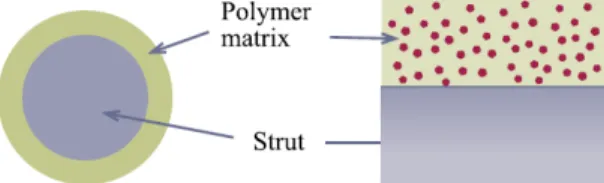
• Matrix devices disperse a drug inside the polymer matrix, and the
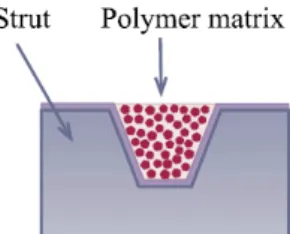
drug is released into the environment without any rate- controlling barrier layer (figure 2.15).
In the early days of developing controlled drug delivery technologies it was thought that the zero- order release would be more desirable than other forms of drug release profiles. The drug delivery system, however can be equally effective regardless of their release kinetics, as long as, the drug concentration in the blood is maintained between the maximum safe concentration (Cmax) and the minimum effective concentration (Cmin) [21].
Figure 2.15: Polymer matrix
2.7.2 Drugs used in controlling restenosis
Ideally, the drug should inhibit the formation of neointi mal hyperplasia by suppression of one or more of the processes of platelet activation, acute inflammat ion, smo oth muscle cell migration, smooth muscle cell proliferation, extracellular matrix production, angiogensis and vascular remodelling; it should also preserve vascular healing and allow re-endothelialization of the injured vessel wall [22]. Since the inflammatory and proliferative responses are results of the complex cascade of events, various cells and tissue components involved in the vascular reparative process are all potential targets of therapeutic approaches [23]. For this reason, various drugs with widely different properties can be used for the same purpose of reducing neointimal proliferation [24]. A detailed understanding of the biological events following the stent implantation allows a proper selection of drugs for DESs based on physicochemical properties, such as molecular weight, water solubility, and portioning coefficient. It is the physiochemical properties of a drug that largely determines the drug delivery technologies. The physiochemical properties of a drug are also important in drug absorption into the surrounding tissue [25]. The drug, the polymer, and their interactions, significantly affect the clinical outcome. [26]
• Immuno- suppresive agent s: sirolimus, tacrolimus and everolimus
• Cellular proliferation inhibitors: paclitaxel and actinomycin D
• Anti- inflammatory agents: dexamethasone
• Prohealing agents
2.7.3 Coating strategies
Vascular smooth muscle cell start proliferation only a day after the injury resulting from stet deployment for about two weeks [21]. Thus, it is believed that antirestenotic drugs need to be delivered for at least three weeks after stent deployment to prevent smooth muscle cell migration and proliferation [27]. It is important to have the precise control of the drug release mechanism, in order to tailor the drug release kinetic to the pathophysiologic phases of restenosis, depending on the specific action of the drug. Drug delivery for long periods from the stents requires a controlling system, which is usually a polymeric system [28]. The rate-controlling system ensures drug retention during stent deployment and modulates drug- elution kinetics. The controlled drug delivery technology also is able to target distinct phases of the restenotic process by altering the release kinetics of the same drugs or different drugs.
Many different materials can be used to coat the stent surface; The most commonly employed are: polyurethane, silicon, acrylic polymers, polyethylene and polyvinylpirrolidone.
There are also different coating techniques, such as dip coating , spray coating , plating , sputtering , and surface induced mineralization .
Instead of coating the drug–polymer layers throughout the entire strut surfaces, some approaches utilized filling the reservoir spaces in the form of grooves and holes present on the struts using microdispensers (figure 2.16). The grooves and holes vary in size and shape. In these approaches, the strut should have dimension large enough to have the reservoir spaces.
2.7. 4 Multilayer systems
A really promising m ethod for DES is a polymer layered coating (figure 2.17). It consists in obtaining a first layer (primer) on the bare stent surface and then a second layer (coating), upon the primer, containing the drug. The primer is selected to have a good adhesion on the metallic support, while the coating must be able to homogeneously disperse the drug. A third layer, the top coating, could be added to control the drug diffusion, working as additional barrier, in order to prolong its efficacy as much as possible. It should prevent the “burst effect”, which appears when the drug is quickly released by the surface of the polymer when this is placed into a physiological environment.
The advantages of these multilayer system s are: • Great adhesion capability.
• The multilayer coating ensure the release of active agents which inhibit the smooth muscular cell proliferation.
• It allows the release of a wide range of drugs in a relatively long
period of time.
• The stent is able to inhibit restenosis and thrombosis.