1. INTRODUCTION
1.1 Discovery of serotonin
It has been stateed that “the discovery of serotonin started out as an annoying artifact and ended as one of the most important discoveries in neuroscience” (Whitaker-Azmitia, 1999). Serotonin story begun in the early 1930’s, when Dr. Vittorio Erspamer, studying the smooth muscle constricting or contracting properties of various amines derived from animal skin and intestines, isolated from the enterochromaffin cells of the gut a substance capable of smooth muscle contraction, which he named enteramine (Erspamer and Vialli, 1937). Indipendently, Dr. Irvin Page, Maurice Rapport and Arda Green were trying to isolate vasoconstrictors from blood samples and needed to eliminate an interfering artifact produced when blood clotted. This substance, which increased vascular tone behaving as a “serum tonic factor”, was then isolated and named serotonin (Rapport et al., 1948). Just one year later, the same Maurice Rapport determined that the structure of serotonin was that of 5-hydroxytryptamine (5-HT, Fig 1.1, Rapport et al., 1949). In 1952 it was established that Erspamer’s enteramine and Page’s serotonin were the same chemical entity, which then took the final name serotonin. In 1953 Betta Mack Twarog, working in Page’s laboratory, finally found serotonin in mammalian brain and brought this newly discovered neurotransmitter into the field of neuroscience (Twarog and Page, 1953). 5-HT is a hydrophilic substance, therefore it does not pass the lipophilic blood– brain barrier readily. For this reason, its discovery in brain was exciting in that it indicated that 5-HT was being synthesized in brain.
In the 1960’s Professor Woolley described the comparable actions of serotonin and lysergic acid diethylamide (LSD), which was known to have psychedelic or psychotomimetic effects substantiating an important role of 5-HT in brain function and suggesting “the serotonin hypothesis about mental disease” (Woolley, 1962). This led to the foundations of the modern concept
of the role of serotonin in psychiatric disorders and opened a new field in neuroscience.
1.2 Synthesis and metabolism of serotonin
5-HT is biosynthesized in two main enzymatic steps from the essential dietary aminoacid L-tryptophan (Fig. 1.1):
1. Hydroxylation of L-tryptophan in 5-hydroxytryptophan (5-HTP) is catalyzed by tryptophan hydroxylase (Tph), which is the rate-limiting enzyme of the synthesis. This enzyme requires for its activity the presence of tetrahydrobiopterine (BH4), oxygen (O2), NADPH2 and iron (Fe2+).
2. Decarboxylation of 5-HTP in 5-HT is catalyzed by L-aromatic amino acid decarboxylase (AADC) with pyridoxal-phosphate as coenzyme.
In the pineal gland serotonin is also used as the precursor for the synthesis of melatonin, through other two enzymatic reaction performed by the 5-HT
N-acetyltransferase, generating the N-Acetyl serotonin intermediate, and the
5-hydroxyindole-O-methyltranferase.
Serotonin is degraded into 5-hydroxyindoleacetic acid (5-HIAA) by the mitochondrial associated enzyme, monoamine oxidase A (MAOA), whose enzymatic activity is not limited to serotonin, as it is also involved in the breakdown of norepinephrine and dopamine (Holschneider et al., 2001). In serotonergic neurons, 5-HT is packaged into vesicles by a transmembrane carrier called vesicular monoamine transporter 2 (VMAT2), also present in other monoaminergic neurons (Erickson et al.,1996). While VMAT2 is expressed primarily in the central nervous system (CNS), the other isoform of vesicular monoamine transporter, VMAT1, is present mainly in peripheral tissues and developing neurons (Peter et al., 1995). Serotonergic signalling also depends on 5-HT regulated transport, which primarily occurs via the serotonin transporter SLC6A4, formerly known as SERT or 5-HTT. SERT reuptakes serotonin from the synaptic cleft, determining the clearance of released 5-HT, and thus the control of the duration and magnitude of neurotransmission via 5-HT receptors (Zahniser and Doolen, 2001; Murphy et al., 2004). Thus, SERT has been the main target for a number of psychoactive drugs including antidepressants and psychostimulants: serotonin reuptake inhibitors (SSRIs) act primarily on SERT function to modulate serotonergic neurotransmission (Vaswani et al., 2003; Lesch and Gutknecht, 2005; Murphy and Lesch, 2008).
Tryptophan hydroxylase (Tph) is the rate-limiting enzyme for the biosynthesis of 5-HT. Tph belongs to a superfamily of aromatic amino acid hydroxylases, together with phenylalanine hydroxylase (Pah), responsible for phenylalanine catabolism, and tyrosine hydroxylases (Th), which catalyzes the first step in the biosynthesis of cathecolamines. They all form homotetramers, contain a mononuclear iron (Fe2+) and utilise dioxygen (O2)
(Fitzpatrick, 1999). The oxylase genes may have arisen from a common ancestor by gene duplication (Craig et al., 1986), and, unlike tyrosine hydroxylase and phenylalanine hydroxylase that are encoded by single genes, Tph activity is encoded by two distinct genes (Tph1 and Tph2) that are expressed in different organs and cells and whose activity is modulated post-translationally by phosphorylation (Hasegawa and Ichiyama, 1987; Makita et al., 1990; Ehret et al., 1991; Johansen et al., 1996, Walther et al., 2003; Zhang et al., 2004).
Tph1 was the first described Tph gene. It contains 11 exons and is
located on chromosome 11 in human and on chromosome 7 in mouse, near the th locus encoding for the tyrosine hydroxylase (Ledley et al., 1987; Stoll
et al., 1990; Stoll and Golman, 1991; Craig et al., 1991; Nielsen et al., 1992).
For more than a decade Tph1 has been thought to be the only Tph gene in vertebrates (Kim et al., 1991). However, several observations reported contradictions between the levels of expression of Tph1 mRNA and protein. Initial studies have shown that the Tph1 mRNA levels were 10- to 40-fold more abundant in the pineal gland than in the raphe neurons, whereas the protein levels as analyzed by anti-Tph immunoreactivity were similar. The discrepancy between the amounts of mRNA and protein was hypothesized to result from distinct Tph mRNA species with different translation efficiency or stability (Chamas and Sabban, 2002). Moreover, Tph from neoplastic mouse mast cells and Tph extracted from the rat brain were found to have different expression rate, regulation and molecular mass (Kuhn et al., 1980). The answer to these apparent contradictions came when Walther and colleagues functionally ablated the original Tph gene in mice by a gene targeting approach. Although Tph knockout mice had almost no detectable 5-HT in the duodenum and none in whole blood, they had close to normal levels of 5-HT in the brainstem (Walther et al., 2003). This led them to discover a second Tph gene, Tph2, which is located on chromosome 12 in human and on chromosome 10 in mice, near pah locus (encoding the phenylalanine hydroxylase) (Walther et al., 2003). The identification of Tph2
could explain the contradictions observed between the expression of Tph1 (formerly known as Tph) mRNA and protein (Walther et al., 2003). Indeed, the authors found that antibodies commonly used to identify Tph cross-reacted with both Tph1 and Tph2. Tph1 is responsible for peripheral 5-HT synthesis, which accounts for 80% of total 5-HT of the body and is taken up by blood platelets. Tph1 expression is detected in peripheral organs such as the enterochromaffin cells of the gut, the pancreas beta pancreatic cells and the pineal gland, which is considered to be a peripheral tissue since it is separated from the brain by the blood-brain barrier (Côté et al., 2003). Recently, Tph1 expression was found also in the placenta, where it is responsible for the synthesis of 5-HT in the earliest stages of embryonic development (Bonnin et al., 2011).
On the other hand Tph2 mRNA expression is detected almost exclusively in the brain, with the exception of the myenteric plexus, and for this reason Tph2 is named also “neuronal-Tph” (Walther et al., 2003; Côté et
al., 2003; Côté et al., 2007). The two enzymes show 65% homology of the
amino acidic sequence, that reaches 83% in the catalytic domain, while the N-terminal region shows the highest divergence rate between the two isoforms, with Tph2 sequence containing 42 amino acids more than Tph1 (Walther et al., 2003).
Despite Tph1 expression in the periphery has been extensively described (Walther et al., 2003; Côté et al., 2003; Côté et al., 2007), the presence of Tph1 mRNA in the dorsal raphe has been controversially discussed: Nakamura and co-workers observed low Tph1 expression in mice dorsal raphe peaking at postnatal day (P) 21, although several authors described the exclusive expression of the second isoform, Tph2, in the raphe neurons, not observing any Tph1 expression (Walther et al., 2003; Zhang et
al., 2004; Malek et al., 2005; Gutknecht et al., 2008; Gutknecht et al., 2009;
unpublished observations).
1.3 Serotonin distribution and function
Serotonin has a multifunctional role in a variety of physiological systems, due to its ubiquitous distribution within and outside the CNS (Barnes and Sharp, 1999). Indeed, serotonin is synthesized within two main districts of the mammalian body: the gastrointestinal tract and the brain. Within the bowel, serotonin is produced by the enterochromaffin (EC) subtype of enteroendocrine cells and by the neurons of the myenteric plexus (Lovenberg et al., 1967; Legay et al., 1983; Côté et al., 2003; Gershon, 2004), via both Tph1 and Tph2 biosynthetic pathways, respectively. In these districts serotonin plays a critical role in enteric neurotrasmission, as well as in secretion and in peristalsis, thus a dysfunction in serotonergic signalling has been thought at the basis of gastroenteric diseases, such as the irritable bowel syndrome (Kim and Camilleri, 2000; Gershon, 2004; Sanger, 2008). 5-HT produced in the gastrointestinal tract is also released in the circulating blood where it is taken up and stored by SERT-expressing platelets that transport 5-HT to all vascularised tissues. Here serotonin contributes to blood coagulation, haemostasis and T cell-mediated immune responses (Sanger, 2008). Moreover, serotonin is also produced in the pineal gland, where it is the precursor for the synthesis of melatonin. Other peripheral sites in which 5-HT is present, even though in small amounts are the beta cells of the pancreas (Paulmann et al., 2009; Ohta et al., 2011), the parafollicular cells of the tyroid, the ovarian cumulus cells (Dube and Amireault, 2007), the dorsal root ganglia and the taste buds (Huang et al., 2005). Collectively, the peripheral source of serotonin produces more than 90% of the whole serotonin content. Apart its role in cardiovascular regulation (Thorin et al., 1990; Nebigil et al., 2000; Alenina et al., 2009) and gastrointestinal systems (Kato et al., 1999) described above, 5-HT is also involved in respiratory (Miyata et al., 2000; Erickson et al., 2007; Alenina et
al., 2009), pain sensitivity (reviewed in Hamel, 2007; Jann et al., 2007), and
Serotonin present within CNS, as the brain-blood barrier is impermeable to this neuromodulator, is synthesized in the serotonergic neurons of the raphe nuclei (Côté et al., 2003). In the brain, serotonin is involved in neural transmission (Turlejski, 1996), and has been implicated in the control of many physiological functions, such as the regulation of circadian rhythms (Morin, 1999), mood (Ruhé et al., 2007), learning (Chamberlaine et al., 2006; Lane et al., 2008) and memory (Meneses et al., 2007). Moreover, serotonin has a broad effect on the control of behaviours such as for example aggressiveness and social interaction (Olivier, 2005) or sexuality (Hull et al., 2004). More recently, several evidences have suggested that serotonin, before functioning as a neurotransmitter, is involved in the control of numerous developmental events.
During development, the necessary machinery for the biosynthesis of this monoamine appears at 11 days post coitum (dpc) - 11.5 dpc in the mouse hindbrain (Hendricks et al., 1999) and the expression of Tph1 in the enterochromaffin cells of gut, as well as the production of blood serotonin, begins as late as 15.5 dpc (Côté et al., 2007). Nevertheless, many serotonin receptors are expressed in different brain regions several days before the appearance of serotonergic innervation. Thus, exogenous sources must supply the embryo with serotonin at earlier stages of development, during a period of pronounced neurogenesis and circuitry formation (Lidov and Molliver, 1982). It has been demonstrated that the first and most important source of serotonin comes from the mother, as Tph1-/- dams, in which peripheral serotonin is abrogated, when mated to wild type males generate pups displaying abnormalities in the development of the brain and other tissues irrespective of their Tph1 genotype (Côté et al., 2007). More recently, the hypothesis of an exogenous extra-embryonic origin of serotonin at early stages of development to the foetus has been reinforced from the demonstration of the presence of a placental 5-HT synthetic pathway (Bonnin et al., 2011). Indeed, placental syncytiotrophoblasts express both
a significant decrease in 5-HT content in the forebrain. Thus, it has been proposed that there is a progressive switch from an early placental source of serotonin, which synthesizes 5-HT from maternal 5-HTP, to a later endogenous source (raphe neurons). Placental source would thus provide the embryo with the necessary serotonin during a period in which critical events in brain development require 5-HT (Gaspar et al., 2003).
In addition, specific cell types in several tissues, despite the lack of Tph or AADC enzymes, require the presence of 5-HT, that can be transiently uptaken thanks to the presence of SERT and VMAT2 (Gaspar et al., 2003). Such a mechanism, for instance, takes place during early stages of development, when maternal/placental serotonin is uptaken by non-neuronal tissues as heart, cranial mesenchyme and notochord (Lauder at al., 1988; Shuey et al., 1992). Similarly, at later stages, when 5-HT is synthesized by specific cell populations above described, its accumulation is observed in different neuronal population of thalamus, limbic cortex, and retina (Lebrand, 1996; Cases, 1998; Upton, 1999).
1.4 Serotonin signalling: 5-HT receptors
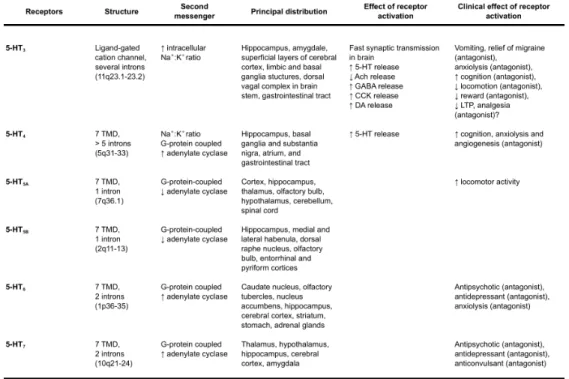
Serotonin exerts its action through multiple classes of receptors, which are expressed both pre-synaptically and post-synaptically in the central and periferic nervous system, and in non-neural tissues (Hoyer and Martin, 1997; Barnes and Sharp, 1999; Kroeze et al., 2002; Gershon, 2004) (Table 1).The first subdivision of 5-HT receptors was proposed in the 1950s, when Gaddum and Picarelli identified two receptor classes in the ileum of the guinea pig, 5-HT M and 5-HT D, based on their sensitivity to morphine (M) and dibenzyline (D), respectively. Almost 40 years later, molecular biology techniques allowed to classify serotonin receptors in 7 distinct families (5-HT1-7 receptors) based on amino acid sequence, signal transduction
mechanisms, pharmacology and functional criteria (Table 1, reviewed in Hannon and Hoyer, 2008). There are at least 15 different 5HT receptors, but this number is further increased of other 20 functional receptors by
post-transcriptional modifications, such as mRNA alternative splicing and, in the case of 5-HT2C receptor, mRNA editing (Burns et al., 1997). In the CNS, all
serotonin receptors are present and differentially expressed, covering almost all brain structures such as frontal cortex, hippocampus, amygdala, hypothalamus and striatum (Meltzer et al., 2003; Millan et al., 2006; Raymond et al., 2001; Bockaert, 2006).
The precise localization of each 5-HT receptor is still far from complete, and this is due also to the fact that many of these receptors show an early, as well as dynamic, expression profile (Lauder et al., 2000; Lein et
al., 2007; Bonnin et al., 2006). Moreover, since selective ligands for certain
receptor subtypes are not available, the anatomical distribution of these receptors (e.g. 5-HT1E, 5-HT2B, 5-HT5A receptors) is only based on their
respective mRNAs distribution, thus remaining elusive the subcellular (pre- post-synaptic) localization (Charnay and Leger, 2010). Within the brain, distribution and binding studies performed in rodents have shed light on the localization of many serotonin receptors (Table 1), and molecular biology techniques have contributed to unravel the signal cascade triggered by many of them (reviewed in Barnes and Sharp, 1999; Hannon and Hoyer, 2008). Like other neurotransmitters such as acetylcholine, glutamate, and γ-aminobutyric acid (GABA), serotonin acts primarily via two types of receptors: ionotropic and metabotropic receptors. Ionotropic receptors (channel receptors) have lower affinity for their neurotransmitter ligand but more rapid activation constant (few milliseconds). In contrast, metabotropic receptors (receptors acting through G-proteins activation and second messengers production) exhibit a high affinity for their ligand, but a slow activation constant (seconds or longer). With the exception of 5-HT3
receptors, which are ligand-gated ion channel receptors (Van Hooft and Yakel, 2003), the other 5-HT receptors are G-protein-coupled receptors with seven transmembrane domains. They can be further categorized into four groups according to their main second messenger system: the 5-HT1 receptors act through Gi/o proteins and are usually negatively coupled with
adenylate cyclase thus inhibiting cAMP production; the 5-HT2 receptors,
coupled to Gq proteins, activate phospholipase C (PLC) to increase the
hydrolysis of inositol phosphates and elevate intracellular Ca2+; the 5-HT 4,
5-HT6, and 5-HT7 receptors are positively coupled to Gs proteins; and the
Table 1 Serotonin receptors Adapted from Sodhi and Sanders-Bush, 2004 TMD,
transmembrane domain; ORF, open reading frame; VTA, ventral tegmental area; NE, norepinephrine/noradrenaline; GABA, γ-aminobutyric acid; CCK, cholecystokinin; DA, dopamine
Among the metabotropic serotonin receptors, the best-documented regional and cellular localizations have been reported for 5-HT1ARs, 5-HT1B/1DRs and
5-HT2CRs (Bockaert et al, 2006; Millan et al., 2008).
5-HT1A receptor (5-HT1AR) is among the most abundant and widely
distributed 5-HT receptor in the brain (Pedigo et al., 1981; Middlemiss and Fozard, 1983; Pompeiano et al., 1992; Pasqualetti et al, 1996). The highest density of 5-HT1ARs is found in the limbic areas, particularly in the
hippocampus and in the lateral septum, and also in cingulate and entorhinal cortex. Importantly, 5-HT1A receptor is localized in cell bodies and dendrites
of 5-HT neurons of the dorsal and median raphe, where it acts as autoreceptor, inhibiting cell firing and 5-HT release (Sotelo et al., 1990; Riad
induce the secretion of S100β, a growth factor expressed by astrocytes shown to have a positive role on serotonergic neuronal system development (Liu and Lauder, 1992 and Whitaker-Azmitia, 2001). Knockout mice for 5-HT1A showed increase anxiety and higher response to stress (Ramboz et al.,
1998; reviewed in Gross and Hen 2004). Interestingly, it has been demonstrated that the anxiety-related effects observed in the 5-HT1A
receptor null mice are developmentally related: conditional 5-HT1A mice in
which the gene inactivation is induced in adult life, behave normally, thus highlighting the importance of specific developmental periods in the acquisition of 5-HT-dependent behaviours.
5-HT1B and 5-HT1D are present on 5-HT terminals and on terminals of
other neurons, such as dopaminergic, GABAergic, and glutamatergic neurons, where they inhibit neurotransmitter release (Starke et al., 1989; Pauwels 1997; Sari et al., 1997; Riad et al., 2000). in situ hybridization studies have detected 5-HT1BRs mRNAs in rat basal ganglia (substantia
nigra, globus pallidus, vental pallium) and in many other brain regions (Bruinvels et al., 1994), while 5-HT1DRs mRNA is present in the nucleus
accumbens, caudate putamen and locus coeruleus (Hamblin et al., 1992; Bruinvels et al., 1994). 5-HT1BRs and 5-HT1DRs are also present in the
cortex (Bonnin et al., 2006), and in raphe nuclei of both human and rodent brain, likely acting as autoreceptors.
5-HT2CR expression has been detected in choroid plexus, cortex,
nucleus accumbens, amygdala, hippocampus, caudate nucleus and substantia nigra (Pazos et al., 1985; Mengod et al., 1990; Abramowski et al., 1995; Pasqualetti et al., 1999). All studies agree that the activation of 5-HT2CR inhibits dopaminergic neurons (Gobert et al., 2000; Ji et al., 2006),
although there is still disagreement on its localization, as some data suggest that 5-HT2CR is primarily localized on GABAergic neurons (Di Matteo et al.,
2001; Pasqualetti et al, 1999) while others suggest that 5-HT2CRs are
present on dopaminergic neurons (Ji et al., 2006). Serotonin 5-HT2C
where they mediate the serotonergic regulation of body weight and food intake (Tecott et al., 1995). Importantly, serotonergic signalling via 5-HT receptors is one of the main targets of the most common drugs used to treat neuropsychiatric disorders such as depression, schizophrenia and obsessive-compulsive behaviors. Thus, a better understanding of the transduction properties and of the regional and cellular distribution of each serotonin receptor will be crucial to clarify how a dysfunction in serotonergic signalling may lead to the onset of neuropsychiatric disorders.
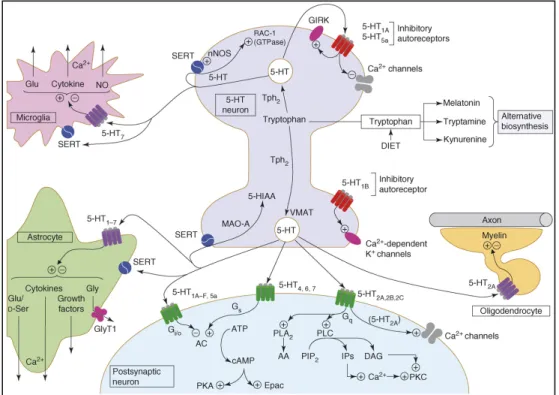
Figure 1.2 An integrated view of signalling at serotonergic neurons. 5-HT is derived
from dietary tryptophan by an action of tryptophan hydroxylase 2 (Tph2), and it is deactivated by monoamine oxidase (MAOA) after release and reuptake via 5-HT transporter (SERT). 5-HT is also converted into melatonin (in the pineal gland), tryptamine (in neurons) and kynurenine (in astrocytes). 5-HT receptors are localized both pre- and post-synaptically to serotonergic neurons: 5-HT1A and 5-HT1B inhibitory autoreceptors are localized on cell bodies and terminals, respectively, and 5-HT5a autoreceptors might also be present on the former. The major modes of transduction are
shown at the postsynaptic level and many receptors converge on specific signaling pathways. Abbreviations: 5-HIAA, 5-hydroxyindole amino acid; DAG, diacylglycerol; Gly, glycine; GlyT1, glycine transporter; IP, inositol phosphate; PIP2, phosphoinositol bisphosphate; D-Ser, D-serine; VMAT, vesicular monoamine transporter.
1.5 Serotonin and neuropsychiatric disorders
Starting from 1962, when Wooley proposed “the serotonin hypothesis about mental disease”, growing evidences have been strengthened the ideas that alterations in the normal central serotonergic signalling are involved in the pathophysiology of several neuropsychiatric disorders such as schizophrenia, depression, anxiety and autism (Mann et al., 1990; Lucki, 1998). Supporting this hypothesis is the fact that (SSRIs) are the most efficient antidepressants and are currently the first line treatment used not only for depression, but also for obsessive-compulsive disorders and anorexia nervosa.
Many lines of evidence suggest that serotonin may play a pivotal role in the etiology of behaviour and pathology relevant to autism spectrum disorders (ASD). The most consistent findings derive from neuroimaging, genetic and pharmacological intervention (Pardo and Eberhart, 2007; Moy and Nadler, 2008) showing that young autistic children lack the developmental peak in whole brain 5-HT synthesis capacity normally present in control infants (Chugani et al., 1999). Moreover genetic studies in autistic populations have identified abnormalities in several serotonin-related genes including Tph2 (Coon et al., 2005), SERT, MAOA and some serotonin receptors (5-HT2AR and 5-HT7R) (reviewed in Polleux and Lauder, 2004; Freitag, 2007). Further evidences supporting serotonin as a neurobiological factor in ASD comes from pharmacological interventions as selective serotonin reuptake inhibitor fluoxetine causes improvements in social behaviour while decreasing aggressive and stereotyped behaviours in children with autism (Hollander et al., 2003). On the contrary, tryptophan depletion results in decreased CNS serotonin and exacerbated symptoms in
patients with ASD, increasing various stereotyped autistic behaviours (McDougle et al., 1996).
There is growing evidence that chronic, endogenous 5-HT deficiency is implicated in depression vulnerability (reviewed in Levinson, 2006). The hypothesis originally derived from the clinical observation that drugs enhancing 5-HT neurotransmission by inhibiting SERT or MAOA displayed antidepressant activity (Coppen, 1967). Moreover, common functional polymorphisms of the SERT gene promoter have been associated to elevated trait anxiety, and increased vulnerability to affective disorders (reviewed in Levinson, 2006). In mice it has been demonstrated that alteration of serotonergic signalling through 5-HT1AR inactivation during early
postnatal development prompt to anxiety-like behaviour in the adult (Gross
et al., 2002). More recently, Jacobsen and collaborators have demonstrated
that the R439H mouse analogue of the R441H human polymorphism identified in the Tph2 gene, a mouse model of 5-HT deficiency, displayed depression-like alterations in serotonin biomarkers such as cerebrospinal fluid 5-HIAA and fenfluoramine-induced plasma prolactin (Jacobsen et al., 2011). All together these observations strongly support the hypothesis of the involvement of serotonergic neurotransmission deficiency in the origin of neurodevelopmental and affective disorders (reviewed in Ansorge et al., 2007).
The idea that serotonergic activity contributes to the etiology of schizophrenia evolved from the observation that lysergic-aciddiethylamide (LSD), a drug structurally similar to 5-HT, is hallucinogenic (Pieri et al., 1978) and it has been suggested to produce its psychotomimetic effects through the stimulation of 5-HT2Rs (Sanders-Bush et al., 1988). The LSD psychosis
has been found to be a close model for the reality distortion syndrome in schizophrenia (Slade et al., 1976). Moreover, changes in serotonin receptor and SERT gene expression, altered serotonin levels and behavioural studies support the hypothesis that dysfunctions in serotonergic system may alter synaptic plasticity that is sufficient to cause developmental changes leading
to schizophrenia (reviewed in Sodhi and Sanders-Bush, 2004). Impairment to the normal serotonin neurotransmission could be a contributory factor, if not a primary cause, also in some neuropsychiatric disorders leading to behavioural impairment, such as eating disorders, addiction and stress related disorders (Gaspar et al., 2003; Sodhi and Sanders-Bush, 2004). For example, antidepressants such as SSRI have been shown to promote neurogenesis in the adult hippocampus requiring several weeks to integrate into the neural circuits, which could account for the delay that is necessary before antidepressants start being effective (Santarelli et al., 2003).
Analysis performed to shed light on the biological functions of Tph2 gene has permitted the identification of genetic variants or different expression levels of this gene involved in the pathogenesis of various psychiatric disorders including depression, schizophrenia and aggression. Zhang and co-workers have also identified in the human Tph2 gene a functional SNP (G1463A), which replaces the highly conserved Arg441 with His (namely R441H, or Arg441His). This substitution results in 80% reduction in the enzymatic activity when expressed in cell culture system. Moreover, the authors found that the mutant 1463A allele was more abundant in subjects affected by unipolar major depression, suggesting that deficiency of brain 5-HT synthesis, due for example to a functional SNP mutation in Tph2 gene, may be an important risk factor for certain neuropsychiatric disorders, such as unipolar major depression (Zhang et al., 2005). To investigate the effects of R441H Tph2 mutation in vivo, Beaulieu and colleagues have generated a knockin mouse line in which the human R441H Tph2 allele is engineered at the equivalent R391H amino acid residue of the mouse Tph2 gene. The results showed that R439H mutation recapitulates the changes reported in the human genetic variant, leading to a 80% reduction in the 5-HT levels in the brain and induced the activation of GSK3β, a signalling molecule modulated by many psychiatric therapeutic agents (Beaulieu et al., 2008). Moreover, R439H Tph2 genetic variation is sufficient to induce behavioural abnormalities, which are alleviated by
pharmacological inactivation of GSK3β. These studies strongly support a role for serotonin in neuropsychiatric disorders, and identify GSK3β as a key factor in the pathway through which 5-HT deficiency may lead to abnormal behaviours, and a possible target for therapeutic strategies. In the last years, applying SNP haplotype and linkage disequilibrium studies, it has been reported an association between polymorphic variants of the human Tph2 gene and the pathogenesis of many behavioural disturbances: affective disorders (Harvey et al., 2004), aggressive behaviour (Kulikov et al., 2005), autism (Coon et al., 2005), attention-deficit/hyperactivity disorder (ADHD) (Walitza et al., 2005; McKinney et al., 2008), obsessive-compulsive disorders (ODC) (Mössner et al., 2006), major depression (Zill et al., 2004a) and suicide behaviour (Zill et al., 2004b).
1.6 Serotonin and development
The synthesis of 5-HT and the dynamic expression of its receptors during early embryonic development, together with the appearance of serotonergic innervation from the raphe nuclei in the embryonic telencencephalon by the time neuroepithelial cell proliferation, migration and differentiation take place, has led to the hypothesis that this neurotransmitter could behave as a growth regulator in specific developmental events (Lauder, 1993; Gaspar et
al., 2003). Moreover, recent discoveries of maternal and placental sources of
5-HT to the foetus have strengthened the idea that serotonin may have a major role in early embryogenesis (Côté et al., 2007; Bonnin et al, 2011). In order to address the role of 5-HT during CNS development, both pharmacological and genetic approaches have been used to perturb normal 5-HT levels and activity. Genetic models in mice demonstrated that excess of brain 5-HT levels obtained by knocking-out SERT or MAOA, genes involved in 5-HT re-uptake and degradation respectively, prevents the development of topographically organized whisker-barrel fields in the mouse somatosensory cortex (Cases et al., 1996; Persico et al., 2001). Recent findings showed that increased 5-HT transmission through the activation of
the 5-HT6 receptor could alter interneuron and pyramidal migration affecting normal cortical development (Riccio et al., 2009, 2012). Serotonergic manipulation via genetic inactivation of 5-HT receptors offered further evidence of an involvement of 5-HT in brain development. Targeted disruption of 5-HT1B receptor and combined 5-HT1B/1D showed that 5-HT signalling is required for appropriate hippocampal innervation and proper formation of the thalamocortical axon pathways, respectively (Ase et al, 2001; Bonnin et al, 2007; Daubert and Condron, 2010). Increased anxiety-like behaviour found in adult mice in which the 5-HT1A receptor was inactivated during the early postnatal period was associated to a likely altered development of hippocampal neural circuits (Gross et al., 2002). Prenatal depletion of 5-HT induced by exposure to p-chlorophenylalanine (PCPA) prompted to alteration in neuronal differentiation (Lauder and Krebs, 1978) and altered the postnatal expression of 5-HT receptors in the rat brain (Whitaker-Azmitia et al., 1987). Analysis of mouse models lacking most serotonergic neurons obtained inactivating genes involved in the specification of 5-HT identity, such as Lmx1b and Pet1, displayed breathing defects and behavior abnormalities but failed to show gross malformations on brain morphogenesis (Erickson et al, 2007; Hodges et al, 2008; Hendricks et al, 2003). Recently, four different groups and our lab have generated mouse models with depletion of brain serotonin obtained by means of targeted inactivation of Tph2 (Migliarini et al, 2008; Savelieva et al, 2008; Gutknecht et al, 2008; Alenina et al, 2009; Yadav et al, 2009). In good agreement among the different genetic models, serotonin withdrawal resulted in some physiological and behavioural disturbances (Savelieva et
al, 2008; Alenina et al, 2009; Migliarini et al, 2009; Yadav et al, 2009), even
though Tph2 knockout mice did not display any detectable cellular or morphological alteration in CNS development. How these results reconcile with the developmental role ascribed to serotonin? On one side, maternal/placental availability of serotonin during early foetal life may compensate the absence of endogenous 5-HT synthesis in the developing
brain, explaining the absence of overt CNS defects. On the other hand, it can be hypothesized that lack of serotonin may affect fine tunings of developmental events that take place late in foetal development or postnatally whose alterations require subtle analysis at the cellular level to be appreciated. Moreover, studies in invertebrate and vertebrate systems have shown that depletion of serotonin impinges on serotonergic fiber outgrowth and axon sprouting (Haydon et al., 1984; Budnik et al., 1989; Diefenbach et al., 1995; Sykes and Condron, 2008; reviewed in Daubert and Condron, 2010).
1.7 Anatomy of serotonergic system
Although brain serotonin was biochemically detected and its effects on behaviour and mood have been hypothesized already in the 1950s, it was only one decade later that serotonergic neurons could be directly visualized for the first time. In 1964 Dahlström and Fuxe by means of fluorescence histochemical detection reported that 5-HT producing neurons belong to a relatively small population of morphologically diverse neurons whose cell bodies are present largely within the brainstem raphe nuclei and particular regions of the reticular formation.
Serotonergic neurons are anatomically organized in the B1-B9 groups of the raphe nuclei and are born as two main groups in the embryonic hindbrain: a rostral cluster in the region anterior to the pontine flexure comprising the B4-B9 raphe nuclei) and a caudal cluster in the medulla which includes the B1-B3 nuclei (Dahlström and Fuxe, 1964; Lidov and Molliver, 1982) (Fig 1.3).
Raphe clusters of 5-HT neurons are found rostrally from the level of
the interpeduncular nucleus in the midbrain to the level of the pyramidal decussation in the medulla. Although there are only about 20,000 serotonergic neurons in the mouse and rat brain (around 300,000 in humans), they are able to densely innervate nearly all regions of the CNS through an extensive axonal projection system. The midline raphe nuclei
consist of the caudal linear nucleus (CLi, B8), the dorsal raphe nucleus (DR, B6, B7), the median raphe nucleus (MnR, B5, B8), raphe magnus nucleus (RMg, B3), raphe pallidus nucleus (RPa, B1), and the raphe obscurus nucleus (ROb, B2). Outside the raphe nuclei there are collections of 5-HT containing cell bodies in a region adjacent to the medial lemniscus called the B9 cell cluster, in the ventrolateral medulla called the B3 cluster, and in the central gray of the medulla oblongata (B4). The B3 group of serotonergic neuron clusters are thought to be lateral extensions of serotonergic neuron clusters in midline raphe.
Serotonergic neurons are generated along the rostro-caudal extent of the ventral rhombencephalon early during development, from 10 days post coitum (dpc) to 12 dpc in the mouse, and during the first month of gestation in primates (Levitt and Rakic, 1982; Briscoe et al., 1999). At this stage, when the embryonic hindbrain is transiently subdivided along its antero-posterior axis into 7 compartments, known as rhombomeres (Lumsden and Keynes, 1989), precursors of serotonergic neurons appear as two clusters in r1-r3 and in r5-r7. The lack of serotonergic differentiation in r4 which carries on in producing motorneurons separates two zones of serotonergic fate with partly distinct mechanisms of differentiation forming the basis for the two clusters of adult serotonergic neurons mentioned earlier (Pattyn et al., 2003) (Fig 1.4). Recently, Jensen and collaborators have used an intersectional and subtractive fate mapping approach to determine the link between the rhombomeric origin of serotonergic neurons and their final anatomical localization within the raphe nuclei (Jensen et al., 2008). They showed that the dorsal raphe nuclei (B6 and B7) are the first to be specified and derive entirely from rhombomere 1. The remaining rostral raphe nuclei appear to arise slightly later, from r1-r3, whereas, the caudal raphe nuclei arise from r5-r7 (Jensen et al, 2008).
Although the total number of serotonergic neurons is small compared to the total number of neurons in the CNS they provide, by way of an extensive and diffuse collateralization of their axons, a dense innervation to
all the brain areas and to the spinal cord. The rostral raphe nuclei produce mainly axonal projections ascending to the midbrain and forebrain, whereas the caudal raphe nuclei produce axons descending to the spinal cord (Wallace and Lauder 1983; Lidov and Molliver, 1982) (Fig 1.3).
Figure 1.3. CNS serotonin neurons and projections. The caudal nuclei (B1–
B3) in the medulla project axons to the spinal cord and the periphery, whereas the rostral raphe nuclei including dorsal raphe groups (B6 and B7) and the median raphe groups (B5, B8 and B9), project virtually to all rostral brain areas. Modified from Murphy et al., 2008.
The ascending projections arise mainly from the dorsal and median raphe nuclei and travel through the median forebrain bundle, reaching the diencephalon by 15 dpc in rats. From there they enter several other fiber systems to reach their target regions, such as the limbic system, hypothalamus, striatum and cerebral cortex (Lidov and Molliver, 1982). The features of serotonergic innervation to the forebrain are double: i) the fine varicose axon system (D fibers) arises from the dorsal raphe nucleus with fibers that branch profusely in their target areas. It is difficult to demonstrate the synaptic connections of these fibers and therefore the incidence of synapses on these fibers is controversial; ii) the basket axon system (M
fibers) arises from the median raphe nucleus with thick, non-varicose axons, giving rise to branches with characteristic axons that appear beaded, with round or oval varicosities. These large terminals make well-defined synapses with soma and dendrites of target cells. The two kind of projection probably coexist in all areas of the brain, but their ratio can vary considerably. The most common region of widespread coexistence is the cerebral cortex. The striatum, on the other hand, seems to receive an almost exclusive innervation by the fine varicose fiber system, while the granule and polymorph layers of the dentate gyrus receive a high concentration of basket fibers. Other well innervated regions of the forebrain have not yet been surveyed from this point of view (reviewed in Tork, 1990, Hensler, 2006).
The descending projections arise mainly from the raphe magnus and obscurus nuclei and also from the serotonergic cells located in the ventrolateral medulla. The projections of these nuclei are principally directed to the spinal cord (Tork, 1990, Hensler, 2006).
The establishment of the major axonal pathways within the serotonergic system can be subdivided into three main steps (Lidov and Molliver, 1982). During the first two phases serotonin axons grow out from serotonergic nuclei defining the primary pathway development and by the end of gestation selective pathways have developed reaching all the brain structures that will receive serotonergic innervation. An active sprouting of serotonin axon terminals exhibiting differential regional and temporal arborisation and proceeding within the first three weeks after birth characterizes the third phase of terminal field development (Lidov and Molliver, 1982).
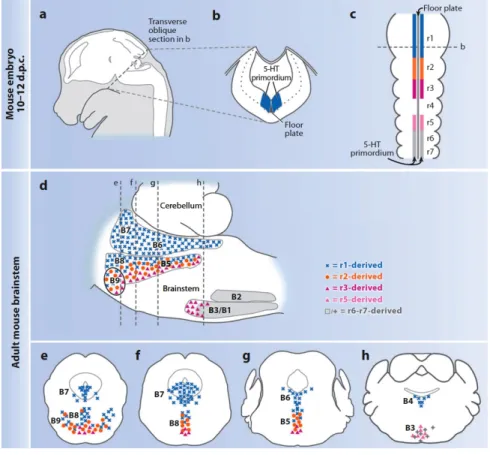
Figure 1.4 The organization of 5-HT neurons as defined by embryonic origin. (a–c) Schematic sagittal view of mouse embryo at 10–12 days post
coitum (d.p.c.) illustrating the whole brain (in white) (a), the transverse section (b), and the dorsal view of the hindbrain (c). The 5-HT primordium is situated on either side of the floor plate and spans almost the entire length of the hindbrain. (d) Sagittal scheme of adult brainstem compressed along the mediolateral axis. Blue represents the 5-HT progenitor cells in r1 (c) or r1-derived mature 5-HT neurons (d); orange, r2 5-HTprogenitors (c) or descendants (d); magenta, r3; pink, presumed r5; gray, r6–r7. The dashed lines represent coronal sections (e–h) presented rostral to caudal, left to right. r1-derived 5-HT neurons (blue) populate the B7, B6, and B4 groups in their entirety, as well as part of the B9, B8, and B5 nuclei. r2-derived 5-HT neurons (orange) populate the B9, B8, and B5 nuclei intermingled with both the r1-derived (blue) and the r3-derived (magenta) 5-HT neurons. Presumed r5-derived 5-HT neurons (pink) populate the B3 nucleus intermingled with more caudal (r6–r7) 5-HT neurons (gray). Figure modified from Kinney et al., 2009.
1.8 Development of the serotonergic system
The involvement of serotonin in so many complex behaviours and the fact that alterations in serotonergic signalling may contribute to the onset of neurodevelopmental disorders in adults has lead to an increasing interest in knowing how serotonergic neurons originate and are specified. Despite some aspects of serotonergic neuron development have been described already in the 1980’s (Lidov and Molliver, 1982; Wallace and Lauder, 1983), it is only with the advancements in molecular genetics that a more precise knowledge on the molecules and transcription factors involved in serotonergic phenotype specification has been achieved. Moreover, recent molecular studies have shed light on the heterogeneity among raphe neurons, in terms of their morphological, neurochemical and electrophysiological properties (Mamounas et al., 1991; Fu et al., 2010; Cordes et al., 2010; Calizo et al., 2011). Thus, not only a specific, common program for serotonergic differentiation is required, but also that each subset of serotonergic neurons necessitates precise transcriptional and environmental factors to finally determine its identity and the correct brain target of axonal projections (Wylie et al, 2010). The rostral and caudal raphe nuclei do not differ only for their anatomical localization within the brainstem, but also for their timing of appearance and for the specific factors required for their commitment and differentiation. In mice, serotonergic neurons are originated very early during foetal development, between 10 dpc and 11 dpc with the caudal group arising about one day later than the rostral (Levitt and Rakic, 1982; Briscoe et al., 1999). They originate as two wings lateral to the midline, in the ventral portion of the hindbrain.
During early brain development, different environmental signals confer a regional identity to neuronal precursors by virtue of the coordinates that the cells occupy along the anteroposterior and dorsoventral axes of the neural tube (Cordes, 2005). For serotonergic neurons, the mid-hindbrain boundary (MHB) expresses fibroblast growth factor 8 (Fgf8), whose importance in the patterning the neural tube has been demonstrated in
transgenic mice in which the MHB was displaced (Brodski et al., 2003). When the MHB was moved caudally, the serotonergic neurons were displaced caudally to the new boundary, and the rostral territories were converted into dopaminergic neurons. Conversely, when the MHB is shifted rostrally, the locus of production of the serotonergic neurons moved in the rostral direction (Brodski et al., 2003). Sonic Hedgehog (Shh), a morphogen produced by the notochord and floor plate, provides the dorsoventral positioning of precursors. The role of Shh was demonstrated using a transgenic mouse line in which one of its receptors, Smoothened, was constitutively active. In these mice, serotonergic precursors were dorsalized, and serotonergic neurons were misplaced in the cerebellum (Hynes et al., 2000). Interestingly, the rostral and caudal group of serotonergic neurons show a different requirement of these morphogen gradients. Indeed, the specification of the rostral group, which originates earlier and is in close proximity to the MHB, strongly depends on the expression of both SHH and Fgf8. Conversely, the caudal cluster is less dependent on Fgf8, whose expression is lower caudally, whereas it depends on the expression of Fgf4, which is secreted by the paraxial mesoderm, and thus its expression is strong in the caudal domain (Ye et al., 1998).
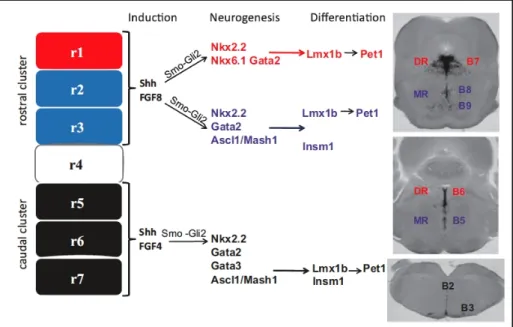
Over the last few years, analysis of mice mutants have identified a set of transcription factors that in some instances act sequentially and, other times, in parallel regulating the region-specific generation and differentiation of serotonergic neurons (Gaspar et al., 2003; Cordes, 2005; Scott et al., 2005; Flames and Hobert, 2011). Recent progress has been made in uncovering the molecular cascade that regulates the region-specific differentiation of 5-HT neurons, with particular emphasis on transcription factors that underlie their heterogeneity. Thus, there are two main known molecular pathways that are activated in the hindbrain: the first one involves, after Shh, Nkx2.2 (or Nkx6.1) and Foxa2, Gata2 and Gata3, and Lmx1b, while the other one includes Ascl/Mash1, Gata3, Lmx1b, Pet1 and Insm1 (Kiyasova and Gaspar, 2011) (Fig 1.5).
The Nkx2.2 homeodomain transcription factor acts downstream of Shh signalling (Briscoe et al., 1999) and it appears to be sufficient to induce the differentiation of both ventral motor neurons (Pattyn et al., 2003) and a significant proportion of serotonergic neurons (Craven et al., 2004). In
Nkx2.2 –/– mice, most of serotonergic neurons are lost, with the exception of
some r1-derived neurons. The specification of r1 serotonergic neurons is driven by the Nkx2.2-related homeodomain transcription factor Nkx6.1 (Vallstedt et al., 2001) and Foxa2 (Jacob et al., 2007), whose role is to repress ventral motor neuron generation in favour of a serotonergic phenotype. Nkx(s) factors drive the expression of Gata2 and Gata3 zinc finger transcription factors, which are required for all serotonergic neurons and in a serotonergic cluster-specific manner, respectively (Craven et al., 2003). Gata2 –/– embryos, which die between 9.5 dpc and 10.5 dpc for hematopoietic defects (Tsai et al., 2004), showed a complete absence of serotonergic neurons, even though Gata3 expression was maintained in rhombomere 1. Conversely, Gata3 has a cluster-specific role (van Doornick
et al., 1999). Gata3 is actually expressed in both the rostral and caudal
clusters of serotonergic neurons starting at E10.5–E11.5, but it is required for the development of serotonergic neurons in the caudal raphe nuclei only (Pattyn et al., 2004; van Doorninck et al., 1999). In Gata3 –/– embryos, the rostral group of serotonergic neurons differentiated normally, but the caudal 5-HT neurons were strongly affected by the mutation (Pattyn et al., 2004). Lmx1b activation follows that of Gata genes, in both the rostral and caudal domains of the raphe. Lmx1b knock-out resulted in a complete loss of serotonergic neurons, and since the expression of upstream factors such as Shh and Nkx2.2 was mantained, serotonergic neurons were born, but do not acquire their neurochemical phenotype (Ding et al., 2003). Moreover, Cheng and collaborators have demonstrated that the co-expression of Nkx2.2,
Lmx1b and Pet1 is sufficient to induce ectopic serotonergic neurons in the
The parallel pathway starts with the Ascl/Mash1 transcription factor. Ascl/Mash1 is a basic-helix-loop-helix transcription factor, which is detected in r1-r7 during motor neurons generation and is essential for serotonergic neuron development in this zone. Pattyn and collaborators have demonstrated that Ascl/Mash1 mutant embryos were totally devoid of serotonergic neurons already at 11.5 dpc, thus its action is important in the differentiation of serotonergic neurons, even thouh Ascl/Mash1 requires
Gata3 as a downstream factor (Pattyn et al., 2004).
More recently, the iroquois homeodomain factors Irx1 and Insm1 have been identified in zebrafish and mice, respectively (Cheng et al., 2007; Jacob et al., 2009). Insm1 mutant mice showed a marked reduction in serotonergic neurons, with no changes in the progenitor number, but with an abnormal expression of postmitotic serotonergic markers such as Lmx1b and Pet1 (Jacob et al., 2009). Thus, Ascl/Mash1 and Ismn1 are part of the regulatory mechanism that specifies serotonergic neurons.
Figure 1.5. Induction, neurogenesis and differentiation of raphe 5-HT neurons.
Gli2 Nkx2.2, Nkx6.1, Gata2, Ascl1⁄Mash1 in rhombomere 1 (in red) and Nkx2.2, Gata2 and Ascl1⁄Mash1 in rhombomeres 2 and 3 (in blue). Terminal differentiation for the dorsal raphe (DR) 5-HT neurons is controlled by Lmx1b and Pet1 (in red), whereas that in the median raphe (MR) is controlled by Lmx1b, Pet1 (in blue). Transcriptional regulation of the 5-HT neuron development in the caudal cluster is regulated by Shh and Fgf4 during induction, Nkx2.2, Gata3 and Ascl1⁄Mash1 during neurogenesis and Lmx1b, Pet1 and Insm1 for the terminal differentiation. Tph2 expression (in situ hybridization) on coronal sections shows the raphe nuclei that arises from each hindbrain cluster. r1–r7, rhombomere 1–7. Modified from Kiyasova
et al., 2011.
In vertebrates, the timing of development and establishment of ascending serotonergic innervation proceed with a sequential pattern that can be subdivided into three phases (Lidov and Molliver, 1982). During the first two phases serotonin axons grow out from serotonergic nuclei defining the primary pathway development and by the end of gestation selective pathways have developed reaching all the brain structures that will receive serotonergic innervation. An active sprouting of serotonin axon terminals exhibiting differential regional and temporal arborisation and proceeding within the first three weeks after birth characterizes the third phase of terminal field development (Lidov and Molliver, 1982). Thanks to genetic studies and analyses of mutant animals, the molecular mechanisms that control each developmental step of the formation of serotonergic circuitry are beginning to be elucidated. Some molecules have been implicated in the development of the serotonergic innervation for the proper outgrowth of serotonergic axons and for their correct sprouting within target brain regions (reviewed in Kiyasova and Gaspar, 2011). Wnt/planar cell polarity signalling has been extensively shown to play a pivotal role in establishing the antero-posterior axonal and cellular organization of monoaminergic systems. Some of these core components are expressed in the raphe 5-HT neurons, such as Frizzled3 and Vangl2 (Fenstermaker et al., 2010). Recent analysis of
Frizzled3 -/- and Vangl2 -/- mice showed that, at 12.5 dpc, ascending and
Once properly oriented, serotonergic fibers are guided along the midline to the forebrain thanks to the interaction between Slit proteins and their receptors. It has been demonstrated that in both Slit2 -/- and double
Slit1::Slit2 -/- serotonergic fibers showed an abnormal trajectory through the
medial forebrain boundle, demonstrating that these proteins play an essential role in the guidance of 5-HT axons (Bagri et al., 2002).
On reaching the forebrain, specific molecular cues guide 5-HT axons towards different structures. Mutant animals for the growth-associated
protein 43 (GAP-43) show already at early stages of brain development an
abnormal pathfinding of 5-HT axons that fail to innervate the cortex and hippocampus (Donovan et al., 2002). Axon targeting defects have been also observed in mice lacking protocadherin-α (Pcdha), in which serotonergic fibers developed with a normal distribution pattern and reach their target districts. However, by three weeks after birth, the density of serotonergic axons in Pcdha mutants was abnormal suggesting that Pcdha might control terminal field development of 5-HT fibers (Katori et al., 2009). Another candidate factor exhibiting specific effect on serotonergic axonal outgrowth is brain-derived neurotrophic factor (BDNF), which promotes sprouting of serotonergic axons when infused in the brain of adult animals (Mamounas et
al, 1995, 2000), but produce a progressive serotonergic hypoinnervation in
BDNF heterozygous mice (Lyons et al, 1999). S100β is a member of a large family of calcium-binding proteins. Stimulation of 5-HT1A receptor may induce
S100β release from the astroglial cells (Whitaker-Azmitia and Azmitia, 1994; Ahlmeyer et al., 2000). S100β has a significant involvement on the terminal growth of serotonergic neurons, as it is transiently expressed in high amounts in the fetal rat brain when serotonin neurons are developing (Van Hartesveldtand et al., 1989; Lui and Lauder, 1992). In adult animals, transplantation of astroglial cells containing S100β can cause sprouting of serotonin terminals.
1.9 Pet1 and serotonergic neuron heterogeneity
The transcriptional pathways of serotonergic neuron differentiation described above converge on the activation of the ETS transcription factor Pet1 (plasmacytoma expressed transcription factor 1) (Hendricks et al, 1999; Kiyasova and Gaspar, 2011). Pet1 orthologue in humans, FEV (Fifth Ewing Variant), has been shown to contribute to a subset of Ewing’s sarcoma tumours after a chromosomal translocation in the EWS locus. The onset of Pet1 expression in the mouse brain has been described to occur at approximately 11 dpc in post-mitotic precursors within the mantle layer (Pfaar et al., 2002; Pattyn et al., 2003), and its expression is confined to two bilateral wings in the ventral hindbrain at the level of rhombomeres r1-r3 and r5-r8. Pet1 expression is first seen in the former (rostral) domain, followed approximately one day later, by the expression in the latter (caudal) domain (Hendricks et al., 1999). In both domains Pet1 expression precedes the expression of markers of serotonergic terminal differentiation, such as Tph2 and SERT, and 5-HT production of 12 hours (Hendricks et al., 2003; Liu et
al., 2010). Thus, to date, Pet1 is known to be the only gene selectively
expressed in precursors as well as in developing and adult serotonergic neurons in the brain (Hendricks et al., 1999; Hendricks et al., 2003; Pattyn et
al., 2003; Craven et al., 2004). Additionally, Pet1 is also expressed in a small
number of peripheral tissues as the entherochromaffin cells of the gut, the adrenal medulla and beta cells of the pancreas (Fyodorov et al., 1997; Ohta
et al., 2011).
Mice lacking Pet1 displayed a 70% loss of serotonergic neurons (Hendricks et al., 2003), as well as an overall reduction of the expression of genes required for 5-HT synthesis (Tph2 and AADC), uptake (SERT), and storage (Vmat2). This differentiation defects resulted in a reduction of 5-HT in the adult brain, that is associated with an increased anxiety and aggressive behaviour in Pet1 -/- mice (Hendricks et al., 2003). However, the fact that approximately the 30% of serotonergic neurons differentiated in all of B1-B9 raphe nuclei has led to the hypothesis that serotonergic neurons
may be heterogeneous. This view was supported by studies in zebrafish, which have shown that serotonergic neurons can be grouped at least in two populations, one dependent upon and the other not dependent upon Pet1 gene (Lillesaar et al., 2007). Moreover, analysis of transgenic mice in which
LacZ expression was driven by serotonergic neuron-specific regulatory
regions of Pet1 has revealed that Pet1 autoregulation is required for a subset, but not all, of serotonergic neurons (Scott et al., 2005). Furthermore, in Pet1 knockout brains, 5-HT- and SERT-labelled serotonergic projections were concentrated in specific brain districts, where they showed a comparable density to controls (Kiyasova et al., 2011). In particular, innervation in the amygdala and in the hypothalamic paraventricular nucleus, two structures involved in stress responses, was preserved. Moreover, in
Pet1 -/- animals 5-HT release occurs normally in those brain areas that were
still innervated. Thus, Pet1 knockout indicates a functional subset of serotonergic neurons whose differentiation occurs in a Pet1-independent manner (Kiyasova et al., 2011). These results strengthen the hypothesis that serotonergic neurons are intrinsically heterogeneous, and open the question of how Pet1-independent serotonergic neurons can differentiate. Obvious explanations include redundancy with another unknown ETS transcription factor or a possible later final wave of serotonergic neuron generation that is
Pet1-independent. Alternatively, heterogeneous levels or types of
transcriptional determinants may be expressed within individual serotonergic neurons, or local signals from other cell types within raphe nuclei can act to establish and/or maintain serotonergic fate in a Pet1- independent manner (Kiyasova et al., 2011).
1.10 Tph2 and serotonergic system development
During the last few years, Tph2 has been genetically targeted by four different groups, and by our lab (Savelieva et al, 2008; Gutknecht et al, 2008; Alenina et al, 2009; Yadav et al, 2009; Migliarini et al, 2008, submitted). In all the animal models 5-HT levels in raphe nuclei were
dramatically reduced, whereas peripheral 5-HT content was unchanged (Savelieva, 2008; Alenina et al., 2009). Strikingly, beside some relatively mild alterations, animals lacking Tph2-derived serotonin are viable, morphologically normal and fertile (Gutknecht et al., 2008; Savelieva et al., 2008, Alenina et al., 2009; Yadav et al., 2009, Migliarini et al., submitted). However the lack of serotonin in these mice leads in some cases to increased lethality during the first four weeks after birth (Alenina et al., 2009; Migliarini et al., submitted). Also, Tph2 -/- mice showed severe postnatal growth impairment (Savelieva et al., 2008; Alenina et al., 2009, Migliarini et
al., submitted), as well as altered autonomic control of sleep, breathing,
thermoregulation, heart rate and blood pressure (Alenina et al., 2009). Moreover, impairment to the normal bone mass and fat pad associated to an altered appetite and energy expenditure in the Tph2 null mice was linked to the leptin-pathway, demonstrating a serotonin-dependent mechanism regulating homeostatic functions (Yadav et al., 2009). Furthermore, the Tph2 null mutation promoted aggressive behaviour (Savelieva et al., 2008; Alenina et al., 2009) and maternal neglect (Alenina et al., 2009), thus demonstrating that Tph2-derived 5-HT is surprisingly not essential for the adult life, but it is involved in the regulation of behaviour and autonomic pathways (Alenina et al., 2009). Interestingly, combined deletion of Tph1 and
Tph2 resulted in a near total loss of 5-HT in both the brain and the periphery,
worsening the behavioural phenotype observed in the Tph2 knockout mice (Savelieva et al., 2008; Yadav et al., 2009).
1.11 Aim of the thesis and experimental approach
I have focused my three years research doctorate activity on the study of the development of the central nervous system, using mouse molecular genetics as approach. In particular, I studied of the role of the serotonin during CNS development, following the hypothesis that, before acting as neurotransmitter, serotonin behave as a growth regulator during specific developmental events.
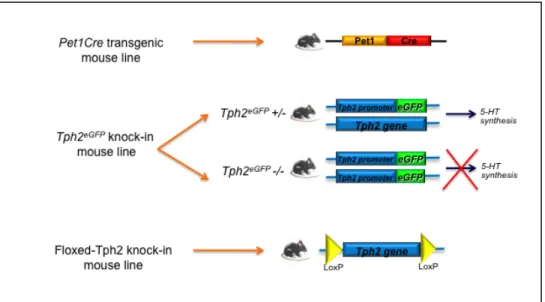
In order to address the role of 5-HT during CNS development it is crucial to use appropriate genetic models, so I took advantage of the previously generated Pet1Cre transgenic mouse line and Tph2::eGFP knockin mouse allele to get insights into serotonergic system development. The Pet1Cre mouse line, thanks to the Cre/loxP system, allows the genetic fate mapping of Pet1 positive cells, by expressing Cre recombinase under the transcriptional control of a 210 Kb genomic region surrounding the Pet1 transcription factor gene locus. The Tph2::eGFP knock-in mouse line, in which the eGFP replaces the Tph2 coding region, allows to generate animals depleted of brain serotonin and to study the effects of lack of central serotonin on development. Moreover, thanks to the presence of eGFP, Tph2::eGFP mouse line permits to trace serotonergic neurons and their projections, in both heterozygous and 5-HT-depleted mice. Finally, I generated a floxed-Tph2 knock-in mouse line, in order to allow a conditional, time and space-controlled, inactivation of serotonin synthesis within the brain (Fig.1.6).
Figure 1.6 Diagrams showing different genetic tools in mouse used and/or generated for the analyses described in the present PhD thesis