Macrosomia, transient neonatal hypoglycemia and monogenic diabetes in a family with heterozygous mutation R154X of HNF4A gene.
Testo completo
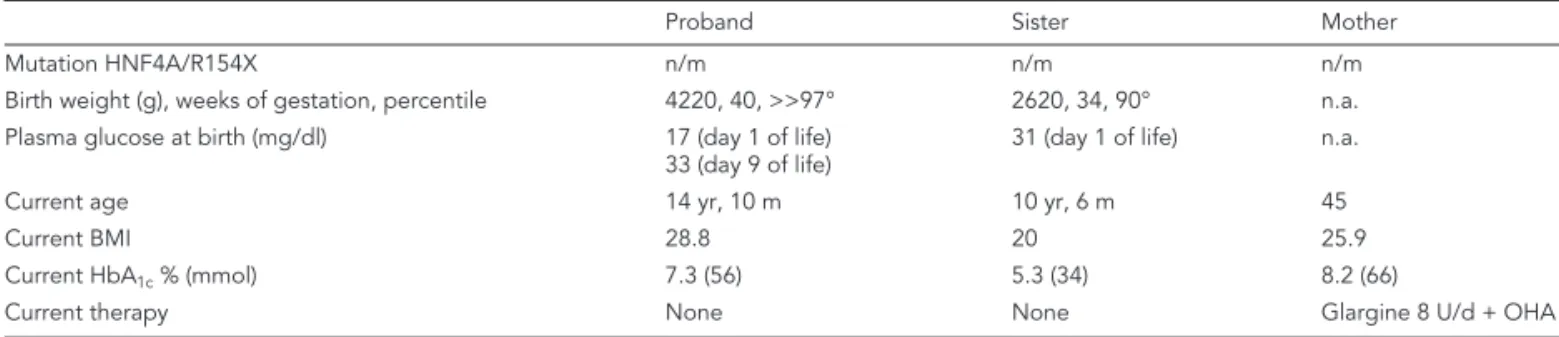
(2) JEI_11_008_Barbetti.qxp:.. 26-04-2011. 10:42. Pagina 253. HNF4 mutation and neonatal hypoglycemia. REFERENCES 1.. 2.. 3.. 4.. Pearson ER, Boj SF, Steele AM, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4a gene. PLoS Med 2007, 4: e118. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32: 1327-34. Fajans SS, Bell GI. Macrosomia and neonatal hypoglycemia in RW pedigree subjects with a mutation (Q268X) in the gene encoding hepatocyte nuclear factor 4a (HNF4a). Diabetologia 2007, 50: 2600-1. Conn JJ, Simm PJ, Oats JJN, et al. Neonatal hyperinsulinaemic hypoglycaemia and monogenic diabetes due to a heterozygous mutation of the HNF4a gene. Aust N Z J Obstet Gynaecol 2009, 49: 328-30.. 5.. Kapoor RR, Locke J, Colclough K, et al. Persistent hyperinsulinemic hypoglicemia and maturity-onset diabetes of the young due to heterozygous HNF4a mutations. Diabetes 2008, 57: 1659-63.. 6.. Flanagan SE, Kapoor RR, Mali G, et al. Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations. Eur J Endocrinol 2010, 162: 987-92.. 7.. Lindner T, Gragnoli C, Furuta H, et al. Hepatic function in a family with a nonsense mutation (R154X) in the hepatocyte nuclear factor4alpha/MODY1 gene. J Clin Invest 1997, 100: 1400-5.. 8.. Pinney SE, MacMullen C, Becker S, et al. Clinical characteristics and biochemical mechanism of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest 2008, 118: 2877-86.. r t i d. K e ic. E E S U , L 1 A 1 N O 0 S ©2 PER FOR. 253. s i t ur. LY N O.
(3)
Figura
Documenti correlati
Le ragioni sono associate con l’aumento di incidenza epidemiologica di tali epatopatie (120) e al fatto che la biopsia in questo caso può essere utile non solo nella
leggi speciali che contemplano per particolari enti la possibilità di emettere obbligazioni, si desumerebbe la connessione della facoltà di creare titoli di massa
In the proposed approach all the trajectories are planned in the relative stitch frame Σ s (see Fig. To this purpose, an interactive stitch selection strategy has been
gambling, our aim was to develop a new brief scale to assess pathological gambling among adolescents (Gambling Behavior Scale for Adolescents, GBSA) referring to
Sulla base di 119 imprese industriali con disponibilità di dati di bilancio nella banca dati Compustat durante il periodo 1962 fino a 1980 (compresi) ed un set iniziale di
Use of all other works requires consent of the right holder (author or publisher) if not exempted from copyright protection by the
perniciosus infectivity expressed as promastigote multiplication and foregut migration at 96 h from biting (potential transmissibility) was clearly related to the same clinical
Percentage increase in von Mises stress obtained combining the average value of the three most important anatomical parameters (average case: BMA, CoFRinf and PDIs), their