I
Phd Course
Biorobotics
2020/2021
Updating deep brain stimulation: novel
quantitative
methods to determine sweet spots and patients state
through electrophysiological activity
Author
Matteo Vissani
Supervisor
Dr. Alberto Mazzoni
Tutor
Dr. Alessandro Panarese
Keywords
Basal Ganglia
Deep Brain Stimulation
Parkinson’s Disease
Tourette Syndrome
Microelectrode Recordings
Local Field Potentials
Impulse Control Disorders
Deep Brain Stimulation (DBS) is a highly effective treatment to ameliorate pharmacologically intractable cardinal symptoms in movement disorders such as Parkinson’s Disease (PD), Tourette Syndrome (TS) and Dystonia. Promising results with DBS intervention are emerging for an expanding palette of brain diseases such as obsessive-compulsive disorder and depression. The objective of this work is tackling two current scientific DBS challenges. The first one hovers around the localization of neural targets that mediate optimal clinical outcomes for patients without emerging noxious DBS-induced side-effects – the so-called sweet spots. The second one deals with the computation of patient-state related biomarkers that could be potentially used to inform the pattern of the stimulation. Herein, I focused on developing quantitative tools to elucidate how different basal ganglia nuclei are functionally organized and to characterize the relationship between the neural activity with either the stimulation in situ or the state (e.g., clinical severity, motor behaviour) of the patient. These methods have been applied to a variegate repertoire of neural signals acquired either with explorative microelectrodes (intraoperatively) or DBS electrodes (postoperatively) from different DBS target nuclei in the basal ganglia (e.g., Subthalamic Nucleus and Globus Pallidus internus) in multiple cohorts of patients (e.g., PD, TS, and ICD). The results of this PhD thesis highlight the wealth of information contained in the neural activity of the basal ganglia and suggest new methods to exploit them for translational purposes. For instance, I found that discharge patterns of the STN neurons identify optimal sweet spots for stimulation in TS patients and that the temporal pattern of the STN oscillations in the beta range can characterize the encoding of dysfunctional motor behaviour in PD patients. This work also involved the use of computational models to grasp new insights into the pathological neuron activity associated with PD and the realization of an open-source platform where DBS imaging users can query the label of a specific region of the brain from different atlases. These results can contribute to the DBS field providing the necessary neuroscientific knowledge i) to build new algorithms to facilitate clinicians during the assessment of the optimal DBS electrode location, ii) to describe the relationship between neural oscillations and specific symptoms and/or compensatory activity and iii) to identify symptom-specific and task-related biomarkers to use as feedback signals for adaptive DBS paradigms.
It is always an extremely challenging quest to choose whom to thank when we attain such a fundamental and significant milestone as concluding the PhD in Biorobotics is for my intellectual and academic path. I am almost sure I fulfilled my duties, so that nobody will seemingly be held out from my claptrap.
First, from deep in down my heart, I want to thank Federica that provided me the love and the
emotional support I needed to not be swallowed by the darkest and toughest times in these last three years. She was always there to support me in the delicate task of keeping my shattering psyche in order. Second, I want to thank my parents for their continuous emotional and logistic support. They
allowed me to get this far and they are my biggest fans. Third, I want to thank all the good friends I was honored to entangle my experiences with. Among them, the sincerest thank must go to
Federico, Giorgio, Chiara, and Federico1, for the funny conversations and support, to Matteo for
being my favorite travelling companion during my Pisa-Macerata routes, and to Giuseppe, Cecilia, Sofia and Maria Letizia for the good vibes and moments during the Neuromatch Academy.
On top of that, I want to profoundly thank my supervisor Alberto Mazzoni, whose fundamental
contribution to my work could never be expressed in concise words. Just a hint: thank him for his extraordinary willingness to satiate my foolish ideas. I want to thank Alessandro Panarese for his
advices and Silvestro Micera for his wisdom, mentoring and his ability to inspire younger scientists.
I want to thank Silvia Ramat and Luigi Romito for their clinical expertise that immensely enriched
the quality of my work. I want to thank Andreas Horn for hosting me in his marvelous laboratory, for
the fruitful and inspiring discussions2 and for the Berlin good vibes. Thanks to all Horn’s laboratory
members for being my lighthouses in my Berlin’s experience.
Last but not least, I want to thank me for doing all this work and believing in me.
... I
Abstract ... IV
Acknowledgments ... V
Abbreviations ... IX
List of Figures ... XI
List of Tables ... XIII
Introduction ... 15
Motivations... 16
Summary of research products presented in this thesis ... 17
Outline ... 19
1.
Basal Ganglia and Deep Brain Stimulation: an overview ... 21
1.1
A System(at)ic View of Basal Ganglia ... 21
1.1.1 The Basal Ganglia circuit ... 23
1.1.2 The classical model of the Basal Ganglia: The Rate Model ... 24
1.1.3 Functional subdivision of the BG circuitry: the Tripartite Hypothesis ... 26
1.1.4 Parkinson’s Disease ... 29
1.1.5 Tourette Syndrome ... 31
1.1.6 Beyond the Rate Model ... 33
1.2
Deep Brain Stimulation Fundamentals ... 35
1.2.1 DBS Apparatus ... 35
1.2.2 Surgical Procedure ... 36
1.2.3 Suitable Targets for DBS ... 39
1.2.4 Therapeutic Mechanisms of DBS ... 39
1.2.5 An in-silico insight: what can we learn about DBS from models? ... 41
1.2.6 Seven open neural engineering challenges for a better DBS ... 44
2.
Toward well-defined “sweet spots” for Deep Brain Stimulation ... 45
2.1 The variance in the DBS outcome ... 45
2.2
Depth matters: millimetres are crucial ... 47
2.3
The wealth of intraoperative electrophysiology signals ... 48
2.3.3 “to MER” or not “to MER” ... 53
2.4
The contribution of imaging techniques ... 53
3.
Towards Adaptive Deep Brain Stimulation ... 56
3.1 The rationale of aDBS ... 56
3.2 Suitable feedback biomarkers for aDBS... 58
3.2.1 Neural feedback biomarkers for aDBS ... 58
3.2.2 Non-neural feedback biomarkers for aDBS ... 61
3.3 aDBS control strategies ... 63
3.3.1 Amplitude-responsive aDBS ... 63
3.3.2 Phase-responsive aDBS ... 64
3.3.3 Adaptive modulation of stimulation frequency and pulse width ... 64
3.4 The case of beta biomarker for aDBS in PD ... 65
3.5 Limitations and future directions for aDBS ... 67
4.
Quantitative characterization of intraoperative microelectrode recordings ... 70
Study 1. Spatio-temporal structure of single neuron subthalamic activity identifies
DBS target for anesthetized Tourette Syndrome patients ... 72
Introduction ... 72
Methods ... 73
Results ... 78
Discussion... 84
Study 1 Summary ... 88
Study 2. Impulsivity Markers in Parkinsonian Subthalamic Single-Unit Activity .... 90
Introduction ... 90
Methods ... 90
Results ... 95
Discussion... 97
Study 2 Summary ... 98
5.
Quantitative characterization of the basal ganglia involvement in task-related
activity in Parkinsonian patients ... 100
Study 3. Gait Initiation in Parkinson’s Disease: Impact of Dopamine Depletion and
Initial Stance Condition ... Errore. Il segnalibro non è definito.
Methods ... 103
Biomechanical Evaluation ... 104
Results ... 109
Discussion... 110
Study 4. Impaired reach-to-grasp kinematics in parkinsonian patients relates to
dopamine-dependent, subthalamic beta bursts ... 114
Introduction ... 114
Methods ... 115
Results ... 123
Discussion... 134
Study 4 Summary ... 137
Study 5. Correlated input to striatal population drives subthalamic nucleus
hyper-synchronization ... 139
Introduction ... 139 Methods ... 140 Results ... 142 Discussion... 144Study 5 Summary ... 145
Conclusions and Future Directions ... 147
Future Directions ... 149
Appendix A ... 152
Study 6. Disruption of layer-specific visual processing in the hyper-excitable cortex
... 153
Introduction ... 153 Methods ... 154 Results ... 158 Discussion... 164Study 6 Summary ... 168
Appendix B ... 170
References ... 174
BG Basal Ganglia
DBS Deep Brain Stimulation PD Parkinson’s Disease
FDA Food and Drugs Administration TS Tourette Syndrome
ET Essential Tremor
CBGT Cortico-basal ganglia-thalamocortical SNc Substantia Nigra pars compacta
SNr Substantia Nigra pars reticulata STN Subthalamic Nucleus
GPi Globus Pallidus internal GPe Globus Pallidus external MSN Medium Spiny Neuron FSI Fast Spiking Interneuron
VAdc densicellular ventral anterior motor thalamus VApc parvicellular ventral anterior motor thalamus VAmc magnocellular ventral anterior motor thalamus CM-pf centromedian-parafascicular nuclear complex RM Rate Model
DP Direct Pathway IP Indirect Pathway NHP Non-human primate ASM Action-Selection model HP Hyperdirect Pathway TH Tripartite Hypothesis
DaT SPECT Dopamine transporter Single-photon Computed Tomography UPDRS Unified Parkinson’s Disease Rating Scale
H&Y Hoehn and Yahr Stage Scale ICD Impulse Control Disorder
MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine 6-OHDA 6-hydroxydopamine
OCD obsessive-compulsive disorder
ADHD attention deficit hyperactivity disorder YGTSS Yale Global Tic Severity Scale LFP Local Field Potential
DBS Deep Brain Stimulation ET Essential Tremor
IPI Interpulse Interval CT Computed Tomography
MRI Magnetic Resonance Imaging AC Anterior Commissure
PC Posterior Commissure MER Microelectrode Recording ALIC anterior limb of internal capsule CR Coordination Reset
VIM ventral intermediate nucleus of thalamus Zi Zona Incerta
Y-BOCS Yale-Brown Obsessive Compulsive Scale
TWSTRS Toronto Western Spasmodic Torticollis Rating Scale GDS Global Dystonia Severity Rating Scale
Dys Dystonia
SUA Single-Unit Activity MUA Multi-Unit Activity
BUA Background Unit Activity ISI Interspike Interval
CV Coefficient of Variation PSD Power-Spectral Density
aDBS adaptive Deep Brain Stimulation ECOG Electrocorticography
EEG Electroencephalography EMG Electromyography
ESM Experience Sampling Method
cDBS Conventional Deep Brain Stimulation AM Amplitude Modulation
BIS Bispectral Index RS Rank Statistics DA Dopamine Agonist
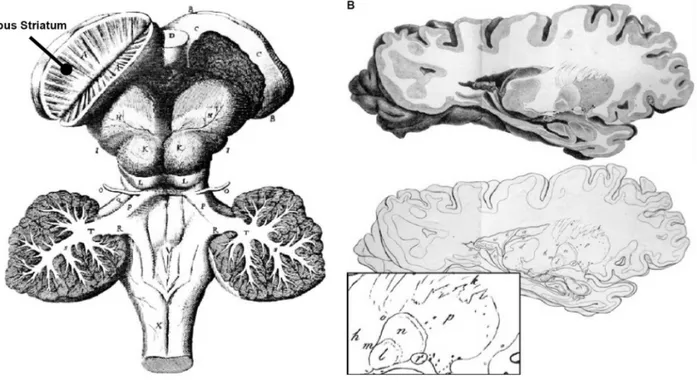
FIGURE 1.1HISTORICAL ANATOMICAL DEPICTIONS OF BASAL GANGLIA. ... 22
FIGURE 1.2STN-CENTRIC ACTION-SELECTION AND CANCELLATION MODEL PROPOSED BY MOSHER ET AL [58]. .... 27
FIGURE 1.3SCHEMATIC DIAGRAM OF THE THREE MAIN LOOPS IN THE CORTICO-BASAL GANGLIA-THALAMOCORTICAL NETWORK. ... 28
FIGURE 1.4THE CLASSICAL RATE MODEL OF THE BASAL GANGLIA UNDER HEALTHY CONDITION AND PD. ... 31
FIGURE 1.5THE CLASSICAL ACTION-SELECTION MODEL OF THE BASAL GANGLIA UNDER HEALTHY CONDITION AND TS. ... 33
FIGURE 1.6DBS APPARATUS AND LEAD DESIGNS USED FOR CLINICAL APPLICATIONS. ... 38
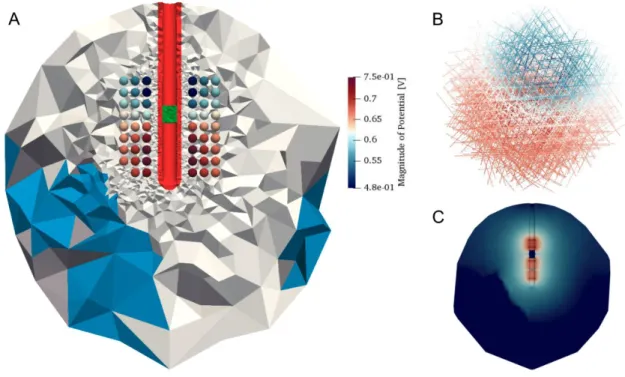
FIGURE 1.7EXAMPLE OF VOLTAGE-CONTROLLED STIMULATION (OSS-DBS) IN THE HUMAN BRAIN TISSUE. ... 43
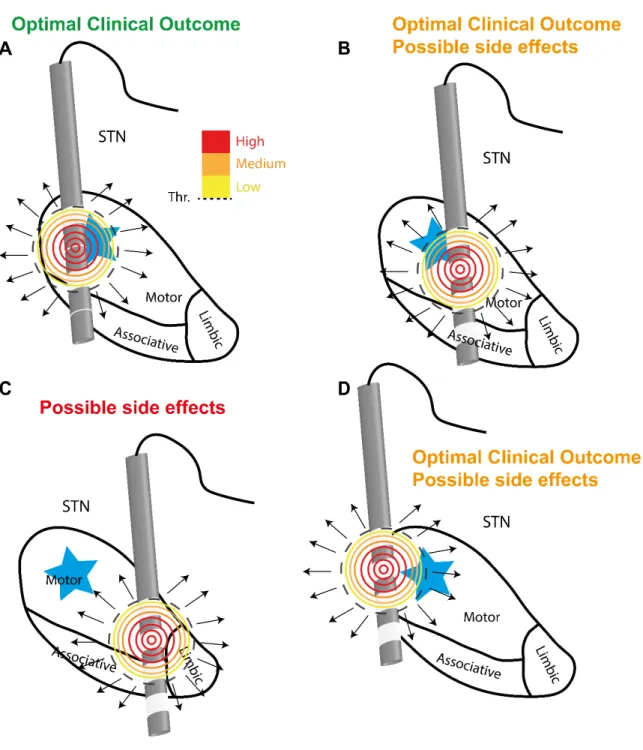
FIGURE 2.1QUALITATIVE EXPLANATION OF THE IMPORTANCE OF THE POSITION OF THE ACTIVE CONTACT IN DBSSTN FOR PD. ... 49
FIGURE 2.2 AN EXAMPLE OF MER DATA RECORDED DURING A DBS SURGERY ALONG THREE PARALLEL STN TRAJECTORIES. ... 51
FIGURE 2.3DIVERSITY IN THE MICROELECTRODE RECORDINGS SIGNAL. ... 52
FIGURE 3.1THE CLOSED-LOOP SCHEMA OF ADAPTIVE DEEP BRAIN STIMULATION (ADBS)... 57
FIGURE 3.2SCHEMATIC OF POSSIBLE ADBS TRIGGERS ... 65
FIGURE 3.3BETA OSCILLATION BREAKDOWN FOR ADBS IN PD ... 69
FIGURE 4.1SURGICAL TARGETING AND ELECTRODE PLACEMENT (RECONSTRUCTED USING LEAD-DBS V2.1.8). .... 79
FIGURE 4.2ONE-MONTH POST-OPERATIVE OUTCOME IN ABSOLUTE (LEFT) AND RELATIVE (RIGHT) TERMS. ... 81
FIGURE 4.3INTRAOPERATIVE MICROELECTRODE RECORDINGS OF PATIENT 1(LEFT HEMISPHERE) FOR DEPTHS CLOSE TO THE OPTIMAL CLINICAL DEPTH (INDICATED AS 0 MM). ... 82
FIGURE 4.4FIRING PATTERNS OF STN NEURONS. ... 83
FIGURE 4.5TOPOGRAPHICAL ANALYSIS OF DISCHARGE PATTERNS OF STN NEURONS ... 85
FIGURE 4.6SINGLE NEURON OSCILLATIONS AND FEATURE INFORMATION. ... 86
FIGURE 4.7GENERAL OVERVIEW OF METHODS AND RESULTS ... 97
FIGURE 5.1BIOMECHANICAL MEASUREMENTS. ... 106
FIGURE 5.2KINEMATICS MEASUREMENTS OF PARKINSONIAN PATIENTS (PD) AND HEALTHY CONTROLS (HC). ... 118
FIGURE 5.3VELOCITY PROFILES OF THE REACH-TO-GRASP TASK. ... 119
FIGURE 5.4CURVATURE ANALYSIS. ... 120
FIGURE 5.5COEFFICIENT C-SCORE COMPUTATION. ... 121
FIGURE 5.6ADDITIONAL KINEMATIC MEASUREMENTS. ... 125
FIGURE 5.7 SUBTHALAMIC NUCLEUS LOCAL FIELD POTENTIAL (LFP) SPECTRAL INFORMATION ABOUT REACH-TO -GRASP PHASES. ... 128
FIGURE 5.8FREQUENCY PEAK ANALYSIS. ... 129
FIGURE 5.9POWER ACROSS TASK PHASES FOR BETA RANGES AND CONVENTIONAL BANDS. ... 129
FIGURE 5.10BURST ANALYSIS FOR BETA RANGES SELECTED ACCORDING TO INFORMATION ANALYSIS. ... 130
FIGURE 5.11BURST ANALYSIS IN CONVENTIONAL BETA BANDS. ... 132
FIGURE 5.12BURST ANALYSIS FOR CONVENTIONAL THETA AND LOW GAMMA BAND. ... 132
FIGURE 5.16STRIATAL SYNCHRONICITY REVERBERATES IN DOWNSTREAM NUCLEI. ... 144
FIGURE 0.1LAMINAR RECORDINGS AND DATA PRE-PROCESSING ... 160
FIGURE 0.2DISRUPTION OF LAYER-SPECIFIC GAMMA MODULATION IN TENT ANIMALS ... 161
FIGURE 0.3 LAYER-SPECIFIC CHANGES OF FIRING RATE IN PUTATIVE EXCITATORY AND FAST-SPIKING INHIBITORY NEURONS... 162
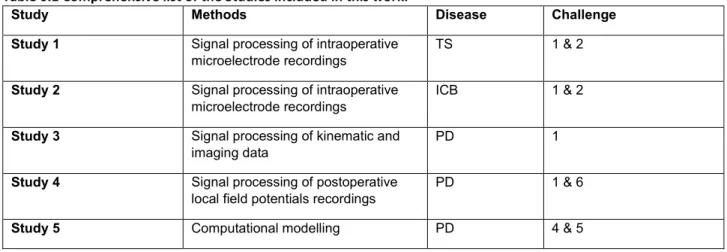
TABLE 0.1COMPREHENSIVE LIST OF THE STUDIES INCLUDED IN THIS WORK. ... 20
TABLE 1.1 OVERVIEW OF OSCILLATORY FEATURES RELATED TO PATHOLOGICAL SYMPTOMS IN PATIENTS WITH DIFFERENT DISORDERS. ... 34
TABLE 2.1DBS OUTCOMES FOR DIFFERENT DISEASES AND TARGETS IN THE LITERATURE. ... 46
TABLE 3.1COMPARISON OF SEVERAL POTENTIAL BIOMARKERS FOR ADBS. ... 63
TABLE 3.2SUMMARY OF BETA-BASED ADBS CLINICAL TRIALS FOR PD IN LITERATURE. ... 67
TABLE 4.1BASELINE CHARACTERISTICS OF 4 PURE TS PATIENTS. MEAN ±SD GIVEN IN THE LAST ROW WHERE APPROPRIATE. ... 75
TABLE 4.2STEREOTACTIC COORDINATES OF ACTIVE CONTACTS FROM THE POST-OPERATIVE CT-MRI FUSION (TS PATIENTS).BRAIN LOCALIZATION IS CLASSIFIED AS STN SENSORY-MOTOR (SM), ASSOCIATIVE (AS),FOREL’S FIELD H2(H2) OR MESENCEPHALIC RETICULAR FORMATION (MRF). ... 80
TABLE 4.3OSCILLATION FREQUENCIES OF STN CELLS ACROSS FREQUENCY BANDS (MEDIAN ± IQR/2). ... 86
TABLE 4.4DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF THE TWO GROUPS: THE BARRAT IMPULSIVENESS SCALE IS IN BOLD AS IT IS THE ONLY SCALE DISPLAYING SIGNIFICANT DIFFERENCE BETWEEN THE TWO GROUPS. ... 92
TABLE 4.5DISCRIMINATION PERFORMANCE OF SINGLE NEURAL FEATURES. ... 96
TABLE 5.1LIST OF THE BIOMECHANICAL PARAMETERS ANALYZED. ... 104
TABLE 5.2DEMOGRAPHIC, CLINICAL, AND BIOMECHANICAL DATA. ... 107
TABLE 5.3 STATISTICAL RESULTS OF THE COMPARISONS BETWEEN GROUPS AND THEIR CORRELATIONS WITH MOLECULAR IMAGING FINDINGS. ... 111
TABLE 5.4DEMOGRAPHIC AND CLINICAL CHARACTERISTICS. ... 115
TABLE 5.5MOLECULAR IMAGING FINDINGS. ... 124
TABLE 5.6MOST INFORMATIVE BETA LOW AND BETA HIGH FREQUENCY FOR EACH PATIENT. ... 128
TABLE 5.7CORRELATION BETWEEN KINEMATICS OF THE REACH-TO-GRASP TASK AND BETA HIGH-RANGE BURSTS FEATURES. ... 133
TABLE 5.8CORRELATION BETWEEN SPATIOTEMPORAL KINEMATICS OF THE REACHTO-GRASP TASK AND BETA HIGH -RANGE MODULATIONS. ... 133
TABLE 5.9CORRELATION BETWEEN STRIATAL DAT DENSITY AND KINEMATICS OF THE REACH-TO-GRASP TASK... 134
TABLE 5.10CORRELATION BETWEEN STRIATAL DENSITY AND BETA HIGH AND LOW RANGE BURSTS FEATURES. ... 134
TABLE 5.11NUMBER OF FIXED IN-DEGREE SYNAPSES INNERVATING EACH NEURON IN THE POSTSYNAPTIC TARGET. THE VALUE CORRESPONDING TO PRE-POST CONNECTIVITY REPRESENTS THE NUMBER OF PRESYNAPTIC PROJECTIONS ON EACH POSTSYNAPTIC CELL. ... 142
15
Introduction
For decades, researchers have studied the abnormalities in the human motor circuits of the Basal Ganglia (BG) suffering from different diseases (e.g., Parkinson’s Disease (PD) and Tourette Syndrome (TS)) to pin-point their causes. Through these endeavours, some pathophysiological findings have been associated with these diseases, e.g., dopamine depletion in PD. However, the precise aetiology of these diseases is yet to be discovered as such our knowledge on how different brain regions in the BG communicate and control behaviour in movement disorders is still elusive and coarse (Challenges 1 & 5 → Section 1.2.6). This lack of understanding may be the reason that
a cure does not exist for these pathologies. Current treatments are only symptomatic as they are designed to ameliorate the cardinal symptoms (e.g., tremors and rigidity in PD and tics in TS). The first line of treatment is the use of pharmacological drugs which are usually effective during the first– honeymoon – period. As the disease evolves and progresses, the dosage may be increased but only till the therapeutical results outweigh the adverse effects (e.g., levodopa-induced dyskinesia in PD). Once symptoms have become drug-refractory, the surgical treatment called Deep Brain Stimulation (DBS) is considered as a valid option for these patients. In DBS, electrical leads are implanted in specific regions of the brain to stimulate continuously them by a pulse generator implanted in the thoracic cavity. Over the years, certain brain structures have been considered as suitable targets for DBS (e.g., the Subthalamic Nucleus (STN) in PD). Moreover, promising results with DBS intervention are emerging for an expanding palette of brain diseases such as Impulse Control Behaviour (ICB), Obsessive Compulsive Disorder and Depression. Despite all the hype and the hope arising from the success of this technique, DBS is still associated with drawbacks, i.e., adverse effects and limited efficacy, and the knowledge of its mechanisms of action is still in its prime (Challenge 4 → Section 1.2.6): increasing consensus of its clinical utility was not paralleled by
comparable advancement in understanding its mechanism of action. The clinical efficacy of the DBS therapy strongly depends on the accuracy of the lead placement since suboptimal positioning can lead to inadequate or even detrimental DBS outcomes. However, the small size and the anatomical variability and complexity in the human BG and the terrific sensitivity of the DBS outcome to millimetres make the precise placement of the DBS lead a challenging task (Challenges 2 & 3 →
Section 1.2.6). DBS target localization procedure generally relies on preoperative stereotactic
imaging and in intraoperative electrophysiological recordings via microelectrodes. The time to take this critical decision is limited during the DBS surgery and the result strongly depends on the expertise of the neurosurgeons/neurologists. Thus, the development of quantitative methods that
delineate the functional organization of the target nucleus (Challenge 1 → Section 1.2.6) and
characterize the relationship between the neural activity and the stimulation in situ may support the clinical decision of the optimal stimulation spot (Challenge 2 → Section 1.2.6).
Most DBS systems in current use are open-loop devices meaning that, once parameters are manually configured, they provide a continuous stimulation regardless of the changes of the state of the patient. The dynamic nature of some BG disorders (e.g., change of dominant symptom and severity) and other environmental factors, and mechanical factors (e.g., shift of the DBS lead position) may require a reprogramming of the DBS settings. However, DBS programming can be changed only during time-limited follow-up visits without controlling clinical fluctuations and rapid variations in symptoms. These issues fuelled the hypothesis that “always stimulating in the same manner” is not necessarily beneficial, but it may be more efficient to stimulate only as and when necessary in response to the online inferred state of activity in pathological brain circuits. This type of stimulation is called adaptive DBS (aDBS) which has the characteristics to cope with the aforementioned limitations. The whole point of aDBS is to implement control policies that can tailor the stimulation settings in real-time according to objective and quantifiable neural (e.g., local field potentials) and non-neural (e.g., kinematic and electromyographic signals) biomarkers. A primary beneficial aDBS effect is the mitigation of induced side-effects by sparing neural circuits from high-frequency stimulation when dysfunction is limited or by patterning stimulation so that it is far more selective for dysfunctional neural dynamics. A secondary beneficial aDBS effect is the reduction of power drains on the IPG. Potential aDBS biomarkers should embed symptom-specific information, be stationary over time, be easily recordable with a robust signal-to-noise ratio and discriminate between pathological and residual physiological (e.g., task-related activity) or compensatory activity (Challenges 1,6 & 7 → Section 1.2.6). The identification of suitable markers that can track the clinical
state considering the different demands per phenotype of the disease (Challenge 6 → Section 1.2.6)
and the implementation of control policies that include in their logic the behavioural state of the patients (i.e., what is doing the patient in that moment) (Challenge 7 → Section 1.2.6) are the two
major challenges that need to be achieved to thrive significantly the aDBS field.
Motivations
The poor understanding of the DBS therapeutic mechanisms and neural dynamics underlying the neurophysiopathology of symptoms and comorbidities in BG disorders are the main reasons that hinder the development of safer, more effective, and personalized DBS therapies. The objective of this work is two-fold:
• Objective 1: The localization of neural targets that mediate optimal clinical outcomes for patients without emerging noxious DBS-induced side-effects – the so-called sweet spots.
• Objective 2: The identification of robust and reliable biomarkers for intelligent adaptive DBS paradigms.
Following an analytical approach to tackle the complexity of these objectives, this work subdivided the Objectives 1 & 2 in seven open neural engineering Challenges (→ Section 1.2.6) that were
summarized in our recent review (Vissani et al 2020, Deep brain stimulation: a review of the open neural engineering challenges, Journal of Neural Engineering):
1. Assess the relationship between BG activity (e.g., dopamine neuromodulation, oscillations) and i) specific symptoms, ii) residual physiological and/or compensatory activity. (Objectives 1 & 2)
2. Online algorithms to assess optimal DBS electrode position and stimulation frequency during implant surgery (Objective 1).
3. Increase the spatial resolution of DBS electrodes (Objective 1).
4. Assess the biophysical mechanisms of clinical benefits associated to DBS (Objectives 1 & 2).
5. Develop in silico environment combining electrical field and spiking network models accounting for CBGT functional architecture to test DBS effects on pathological dynamics of the CBGT network (Objectives 1 & 2).
6. Identify symptom-specific (and task-related) biomarkers to use as control signals for adaptive DBS. (Objective 2).
7. Online algorithms to assess optimal stimulation pattern based on patient condition in adaptive DBS (Objective 2).
To achieve some of these challenges (→ Table 0.1), we developed quantitative tools to elucidate
how different basal ganglia nuclei are functionally organized and to characterize the relationship between the neural activity with either the stimulation in situ or the state (e.g., clinical severity, motor behaviour) of the patient. These methods have been applied to a variegate repertoire of neural signals acquired either with explorative microelectrodes (intraoperatively) or DBS electrodes (postoperatively) from different DBS target nuclei in the basal ganglia (e.g., Subthalamic Nucleus and Globus Pallidus internus) in multiple cohorts of patients (e.g., PD, TS, and ICB) (→ Table 0.1).
Data were acquired through a network of clinical collaborations (Besta Institute, Milan; Careggi Hospital, Würzburg University Hospital (Germany)) set up in the last three years. This work also involved the use of computational models to grasp new insights into the pathological neuron activity associated with PD.
The work reported in this thesis has resulted in the following publications and patents.
Journal Articles
[1] M. Vissani*, C. Palmisano*, J. Volkmann, G. Pezzoli, S Micera, A. Mazzoni and I.U. Isaias. Impaired reach-to-grasp kinematics in parkinsonian patients relates to dopamine-dependent, subthalamic beta bursts. (in press in NPJ Parkinson’s Disease)
[2] F. Micheli*, M. Vissani*, G. Pecchioli, F.Terenzi, S. Ramat and A. Mazzoni. “Impulsivity markers in parkinsonian subthalamic single-unit activity”, Movement Disorders, 2021
[3] A. Panarese, M. Vissani, N. Meneghetti. E. Vannini, M. Cracchiolo, S. Micera, M. Caleo, A. Mazzoni* and L. Restani*. “Disruption of layer specificity in hyper-excitable visual cortex” (under review)
[4] M. Vissani, I. Isaias, and A. Mazzoni. “Deep Brain Stimulation: a review of the open neural engineering challenges”, Journal of Neural Engineering, 2020
[5] M. Vissani, R. Cordella, S. Micera, R. Eleopra, L. Romito, and Mazzoni A. “Spatiotemporal structure of single neuron subthalamic activity identifies DBS target for anesthetized Tourette syndrome patients”, Journal of Neural Engineering, 2019.
[6] C. Palmisano, G. Brandt, M. Vissani, N.G. Pozzi, ..., and I. Isaias. “Gait Initiation in Parkinson’s Disease: Impact of Dopamine Depletion and Initial Stance Condition”, Front Bioeng Biotechnol, 2020.
Conference proceedings:
[1] J. Miehlbradt, C. Pierella, ..., M. Vissani, A. Mazzoni, and S. ... Micera. “Evolution ofCortical Asymmetry with Post-stroke Rehabilitation: A Pilot Study”. In: Conference of Neurorehabilitation (2018).
[2] F. Micheli*, M. Vissani*, G. Pecchioli, F. Terenzi, S. Ramat and A. Mazzoni. Biomarkers of Impulse Control Disorder in firing patterns of parkinsonian subthalamic nucleus, Proceedings of VII Congress of the National Group of Bioengineering (GNB), 2021.
[3] E. Manferlotti, M. Vissani, A. Kumar and A. Mazzoni. “Correlated inputs to the striatum generate beta over-synchronization in cortico-basal ganglia network”, 10th International IEEE EMBS
Conference on Neural Engineering, 2021 (under review)
Abstracts
[1] E. Manferlotti*, M. Vissani*, A. Kumar and A. Mazzoni. “Correlated inputs to the striatum generate beta over-synchronization in in-silico cortico-basal-ganglia network”, 29th Annual Computational Neuroscience Meeting, 2020.
[2] M. Vissani, R. Cordella, S. Micera, R. Eleopra, L. Romito, and A. Mazzoni. “Firing pattern of single neurons in the subthalamic nucleus of Tourette Syndrome patients identifies optimal deep brain stimulation target site: 1389”. In: MDS Conference 2019 (2019).
Title: “A system for monitoring and treating motor disorders with microrecordings and targeted electrical stimulations.”
Rif: 102020000026831 - Filed Nov 10th 2020
Inventors: Alberto Mazzoni, Matteo Vissani and Silvestro Micera.
Outline
This thesis consists of 6 chapters:
• Chapter 1 (→ Chapter 1) provides the background and associated literature on BG and DBS. It details the pathophysiology of BG with a focus on Parkinson’s Disease and Tourette Syndrome and the fundamental of DBS. The last section (→ Section 1.2.6) illustrates seven
open neural engineering challenges to optimize Deep Brain Stimulation and most of them have motivated the work in this thesis.
• Chapter 2 (→ Chapter 2) delves deeper into the conundrum of Deep Brain Stimulation,
illustrating the tremendous variance in the DBS outcome and the factors that may explain it. This Chapter strongly focuses on the importance of identifying meaningful and reliable sweet spots for stimulation (Challenges 1,2,3 and 4; → Section 1.2.6). Also, it illustrates how is
possible to achieve this objective using intraoperative microelectrode recordings and imaging techniques.
• Chapter 3 (→ Chapter 3) introduces the motivations that hinged the development of the
adaptive Deep Brain Stimulation (aDBS). This Chapter furnishes a comprehensive literature on the rationale of aDBS with a focus on major developments and advances for this application. The last section (→ Section 3.5) highlights the current limitations of aDBS and
explains the importance of defining robust and reliable biomarkers for intelligent aDBS paradigms (Challenges 1,4,5 and 6; → Section 1.2.6).
• Chapter 4 (→ Chapter 4) illustrates 2 studies (→ Study 1 & → Study 2) regarding the development of computational methods to analyze retrospectively intraoperative microelectrode recordings acquired during DBS surgery to address Challenges 1 and 2 (→ Section 1.2.6).
• Chapter 5 (→ Chapter 5) illustrates 3 studies (→ Study 3 & → Study 4 & → Study 5 ) regarding the development of computational methods to investigate the role of BG in different motor states (e.g., gait and reach-to-grasp) in PD patients (→ Challenge 1,4,5 and 6 in
• Chapter 6 (→ Chapter 6) summarizes the main contributions of the work and provides future
directions in which the methods developed herein can be exploited towards the optimization of the DBS.
Finally, Appendix A reports one last Study (→ Study 6) not directly related to the aforementioned
studies.
Table 0.1 Comprehensive list of the Studies included in this work.
Study Methods Disease Challenge
Study 1 Signal processing of intraoperative
microelectrode recordings TS 1 & 2
Study 2 Signal processing of intraoperative
microelectrode recordings ICB 1 & 2
Study 3 Signal processing of kinematic and
imaging data PD 1
Study 4 Signal processing of postoperative
local field potentials recordings PD 1 & 6
21
C
HAPTER
1
1. Basal Ganglia and Deep Brain Stimulation: an
overview
Once considered a black box, the basal ganglia (BG) are becoming well growingly investigated and characterized through the application of research and clinical modalities. BG are strictly interconnected, both functionally and anatomically, with the cortex, the cerebellum, the thalamus, and other regions of the brain and they are thought to regulate a multitude of motor and cognitive behaviours in the daily life. More than thirty years ago, Albin, DeLong, and colleagues, came up with a box-and-arrow model of the BG pathways that have represented a starting point for the formulation of mechanistic hypotheses of BG physiology and dysfunction [1], [2]. From them on, preliminary investigation with electrical stimulation [3], [4] and ablation studies [5], [6] on single target BG nuclei prompted the neurosurgeon Alim Benabid to implant in the thalamus a lead to inject electricity through a Deep Brain Stimulation (DBS) apparatus to ameliorate symptoms in Parkinson’s Disease (PD) for the first time (in 1987) [7]. A particularly significant milestone was the approval of DBS as a therapy for PD in 1997 by the Food and Drug Administration (FDA). Basal ganglia neuromodulation is witnessing a remarkable surge in interest and it is going to herald a new era where basic neuroscience knowledge and neurotechnology are synergistically entwined with the ambitious goal to provide ever more personalized, precise, safe, and effective treatments for patients suffering from drugs-refractory BG disorders (e.g., PD, Tourette Syndrome (TS), Essential Tremor (ET), etc.) [8]. The next sections of this Chapter (→ Chapter 1) furnish a context for Basal Ganglia and Deep Brain
Stimulation, providing the basic knowledge to fully understand the core of this thesis. Parkinson’s Disease and Tourette Syndrome will be covered more in details as they are more related with the studies reported in this thesis.
1.1 A System(at)ic View of Basal Ganglia
The basal ganglia are a group of small subcortical nuclei which integrate information from widespread cortical areas and in turn project their outputs back to the cortex. The presence of structures at the basis of human brain is not a novel concept as it raised the attention of many scientist in the history. Prefatory anatomical representations of the BG appear in the works of classical anatomists such as Vesalius and the term Corpus Striatum to refer to subcortical
structures is attributed to Thomas Willis in his pioneering treatise Cerebri Anatome3 (1664). Most of
the modern nomenclature to identify the BG structures such as “internal capsule” and “external capsule” was introduced by Karl Friedrich Burdach in late 18th century. Finally, the term Basal
Ganglia has been proposed by Sir David Ferrier in his masterpiece The functions of the brain in 1887 where describes the basal ganglia as ganglionic masses, intercalated in the sweep of a projection system of fibres which connect the cortex with the crura cerebri and the periphery (→ Figure 1.1).
Figure 1.1 Historical anatomical depictions of basal ganglia.
(A) Thomas Willi’s view of the basal ganglia from Cerebri Anatome. There hemispheres have been removed
to illustrate the basal ganglia, and the corpus striatum (black line). The corpus striatum on the right side has been cut in half to show its characteristic striations. (B) Karl Friedrich Burdach’s representation of the basal
ganglia. The various components are defined clearly for the first time and their location is indicated by letters (e.g., d to thalamus; h to internal capsule; l-q to lenticular nucleus). The bottom panel displays the different components of the human lenticular nucleus (e.g., p to putamen, l to medial and n to lateral segments of globus pallidus). Modified from [9] under the CC BY 4.0 license https://creativecommons.org/licenses/by/4.0/.
After that, a wide corpus of research has been focused on the BG structure and anatomy in both healthy and disease conditions. The rise of neuroanatomical tracing [10], [11] and neuroimaging [12], [13] techniques, and post-mortem evaluations [14], [15], [16] in the last half of the 20th century
allowed the reconstruction of white matter anatomy of the human brain and the complete description of BG anatomy and connectivity in different animals.
1.1.1 The Basal Ganglia circuit
Four major nuclei are considered to form the Basal Ganglia: the Substantia Nigra (pars compacta (SNc) and pars reticulata (SNr), the Striatum (caudate nucleus, putamen and nucleus accumbes), the Subthalamic Nucleus (STN) and the Globus Pallidus (internal (GPi) and external (GPe)). BG nuclei can be broadly characterized in input nuclei, output nuclei and intrinsic nuclei. These small nuclei form the so-called Cortico-Basal Ganglia-Thalamocortical (CBGT) network with the thalamus and the cortex.
Input Nuclei
The Striatum is considered the major input structure of the BG, since it receives projections from the cerebral cortex, thalamus, and SNc [17], [18]. It is by far the largest subcortical brain structure in the human brain, with an estimated volume of ~10 cm3 [19]. It is composed by two different types of
neurons: projections or striatofugal neurons (~95%) and interneurons (~5%). The former is also called Medium Spiny Neurons (MSNs) for their cytoarchitecture, i.e., small to medium cellular somata (~15 µm of diameter) and the presence of postsynaptic specializations called dendritic spines. All striatal MSNs are GABAergic4 and their activity is modulated by the dopaminergic inputs (e.g.,
enhancing D1-type and depressing D2-type) coming from the SNc [20]. The MSNs containing D1-type receptors (MSND1) projects directly to the output nuclei GPi and SNr, whereas those presenting
D2-type receptors (MSND2) innervate the GPe. The latter are mainly represented by fast-spiking
GABAergic interneurons (FSI) that are influenced by both D1-type and D2-type receptors and strictly interconnected with both gap junctions and GABAergic synapses. Despite their small concentration, FSIs have a great role in regulating the striatal activity, i.e., a single FSI is able to block the action potentials in multiple MSNs [21], [22].
Output Nuclei
The GPi and the SNr are often grouped together as BG output nuclei because they share a broad palette of chemoarchitectural and cytoarchitectural characteristics and to some extent similar afferent and efferent systems [23]. The presence of a myriad of myelinated striatofugal fibers crossing the GPi and the lower cellular density gives the paler appearance to the GPi when compared to the Striatum. They both receive two different types of inputs: inhibitory contributions from the Striatum and GPe, and glutamatergic innervations from the STN (e.g., subthalamopallidal and subthalamonigral projections) [24]. They both exert a tonic inhibitory firing to the motor regions of the thalamus (e.g., ventral anterior motor thalamus (VAdc, VApc, and VAmc), and intralaminar nuclei such as the centromedian-parafascicular nuclear complex (CM-pf)), as well as brainstem
neurons (e.g., superior colliculus (SC) and pedunculopontine nucleus (PPN)) , which in turn regulate motor-related areas in the cerebral cortex influencing desired and unwanted behaviours [24], [25].
Intrinsic nuclei
The GPe – also known the lateral division of the globus pallidus – is really cytoarchitectural similar to the GPi. The GPi and the GPe are separated by a thin layer of myelinated fibers named the medial medullary lamina. The GPe receives mostly two types of afferents: GABAergic striatal innervations and glutamatergic subthalamic inputs. GPe neurons are reciprocally connected with the STN and the existence of such a GPe-STN closed-loop has led to consider the GPe as not simply as a relay station in the BG architecture, but more properly as an integrator hub [26]–[28]. The STN is a small nucleus situated between the thalamus and the Substantia Nigra that receives inhibitory inputs from the GPe [1], [2], [25] and glutamatergic projections the cortex [26], [29], the thalamus [23], [30], [31] , the brainstem, as well as dopaminergic projections from the SNc, as a part of the so-called nigro-extrastrial projection system [32]. The STN contains densely packed glutamatergic neuron’s projections that simultaneously innervate the GPi and SNr [31], [33], [34]. In addition, efferent STN neurons also project to specific thalamic targets (e.g., VAdc, VAmc, and CM-pf) [31], [35].
1.1.2 The classical model of the Basal Ganglia: The Rate Model
The classical Rate Model (RM) of the Basal Ganglia originally proposed by Albin and DeLong [1], [2] in the 1980s and early 1990s has represented the cornerstone of our knowledge about subcortical structures. The RM sprang from various observations about deviations in BG structure (e.g., connectivity) and function (e.g., neurochemistry and physiology) in several diseases such as Parkinson’s Disease, Huntington’s Disease, and Hemiballismus5 [36]–[39]. The RM postulates that:
1. The BG implements a sequential and hierarchical physiological processing of the cortical input, relying on an interconnected network (i.e., CBGT) that can be subdivided in two major pathways: Direct Pathway (DR) and Indirect Pathway (IP).
2. The DP funnels the information directly from the Striatum (i.e., MSND1) to the GPi, whereas
the IP implies the multisynaptic activation of the Striatum (i.e., MSND2), GPe, STN and GPi.
3. The modulation of the firing rate of the output nuclei (e.g., GPi) regulates the behaviour and it potentially explains most of the deviations from the healthy condition. High/low GPi firing rate should suppress/promote specific actions.
4. The cortex may generate cortical commands, but their sustainment and execution heavily rely on the integrity of the BG as a positive feedback system.
5. Dopamine acts differentially on the DP and IP at the level of the Striatum. D1-type/D2-type receptors on the MSND1/MSND2 neurons facilitate the DP/IP, inhibiting/exciting the GPi, and
hence promoting/suppressing the cortex command.
6. The balance of the D1-type and D2-type dopamine acts a keystone role in the BG.
These key concepts, and in particular the idea that DP and IP act in opposite fashion to control the behaviour, led to a multitude of behavioural, physiological, and pathological predictions such as:
1. Dopamine has opposing physiological effects on the activity of MSND1 and MSND2 composing
the DP and IP, respectively.
2. The activity of the GPi should anticorrelate with the amplitude of the movement. 3. The activation of the DP should lead to increased or excessive movements.
4. The activation of the IP should lead to reduced movements or excessive bradykinesia. 5. The ablation or the inactivation of the DP/IP should lead to decreased/increased movements. For its intuitively simple mechanistic heuristics, the RM gained a pillar consideration and consensus. Over the years, many difficulties were encountered in trying to integrate new findings in the RM model, leading to puzzling and intriguing paradoxes [40]–[43] such as:
1. Pallidotomy (i.e., lesion of the GPi) should provoke a marked disinhibition of the thalamocortical projection, and hence a facilitation of movements. On the contrary, studies in parkinsonian MPTP-treated monkey models6 and in patients showed that pallidotomy does
not facilitate movements but it has opposite effects such as abolishing dyskinesias [44], [45]. 2. PD patients may present an increase of the GPi activity, but the latter is not necessary for
the former [46].
3. Single or combined lesion in the STN, GPi and motor thalamus is actually associated with marked improvement in PD patients [47].
Finally, neurons may encode information with both rate and timing coding, but the RM substantially have not yet explored the relation between neural discharge pattern and behaviour.
The Action selection Model: a natural extension of the Rate Model
The simplest formulation of the RM considers the DP and IP as ensembles of neurons with uniform responses. This hypothesis is not tenable as physiological recordings during motor and cognitive tasks from normal non-human primates (NHP) showed variegate responses of the neurons (even within the same anatomical nucleus). This heterogeneity of activity was included in the RM and advanced in a series of articles of Mink and colleagues, leading to the-so-called Action Selection Model (ASM) [48]–[51]. The ASM shares the key concepts of the RM but suggests that IP and DP may affect individual motor programmes, i.e., BG provides for action selection via focused disinhibition of the thalamus, and hence the cortex [52]. Indeed, an instantiation of a “go pathway”,
i.e., desired action, is the DP, which releases the motor thalamus from its state of constant inhibition. The slower multisynaptic IP is thought to play a role in terminating complete instantiated actions or inhibition unwanted motor commands that compete with the desired action [48].
A new role of STN: the Hyperdirect Pathway
The STN is known to be a node of the IP but its involvement in the BG activity remains elusive in the context of the RM. Indeed, in the RM realm, the STN is represented as a simple monolithic transmission relay that funnels the information to the final integrator hub, i.e., the GPi. In the last years, a third pathway was identified, the so-called Hyperdirect Pathway (HP), that directly connects glutamatergic cortical projections to the STN, bypassing the slower IP [53], [54]. The net effect of this pathway is inhibition through the combination of one excitatory and one inhibitory connection (STN-GPi and GPi-thalamus). Anatomical NHP tracing studies revealed the presence of prefrontal-subthalamic projections that terminate with a topography favouring the ventral regions of the STN [54]. Only recently, electrophysiological, electrical stimulation and tractography-based studies pointed towards the existence of the HP in humans [55]–[58]. The discovery of this fast, “direct” and negative monosynaptic cortico-subthalamic feedback has sparkled a breadth of new research positioning a broad role of the STN in switching [59], [60], selecting [61]–[63], and pausing actions [64], [65]. These observations led to the hypothesis that regions of the frontal cortex, involved in higher cognitive functions (e.g., right inferior frontal gyrus), would lead to a widespread and fast increase of a HP-driven inhibition to the thalamus, and hence to the cortex, to cancel and stop an action when the goal changes [58], [66], [67]. Considering the HP, the ASM can be extended as follows: to activate a desired motor pattern, the motor cortex wipes out competing and undesired motor outputs through the HP, funnels back the proper information to the thalamus and the cortex via the DP and suppresses excess and competing reinforcement through the IP. Moreover, in a recent study, Mosher and colleagues identified the presence of intermixed “go-pathway” and “stop-pathway” functions at single-neuron level in the STN. Go-pathway neurons fired just after the onset of the movement and they were found more dorsally. Since the STN is not an edge of the DP, the activity of these go-pathway neurons does not directly reflect the activation of the desired – “the centre” –motor program. Hence, they may potentially encode the inhibition of the competing motor programs – “the surround” –, facilitating the action of the DP. Stop-pathway neurons fired after the suppression of the movement and they were found more ventrally (i.e., where HP frontal-subthalamic projections likely innervate) in the STN. When these neurons fire, they may inhibit only the original intended motor program – “the centre” – during action cancellation and globally all the motor programs during action selection and cancellation (→ Figure 1.2 ).
1.1.3 Functional subdivision of the BG circuitry: the Tripartite Hypothesis
While several subdivisions of the BG have been proposed, the most influential model of the organization of the BG views them as components of both anatomically and functionally segregated
circuits that connect in a parallel manner distinct thalamic, cortical, and subcortical areas [24], [68], [69].
Figure 1.2 STN-centric action-selection and cancellation model proposed by Mosher et al [58].
The STN supports action selection by inhibiting competing motor programs (blue pathway in the surround) and action cancellation by inhibiting rapidly and globally all motor programs (red pathway in the centre and surround). Action cancellation is driven by the frontal-subthalamical HP projections that innervate ventrally the STN. For sake of simplicity, the scheme shows only the net effect of the thalamus bypassing the pallidal connections. Reproduced with permission from [58].
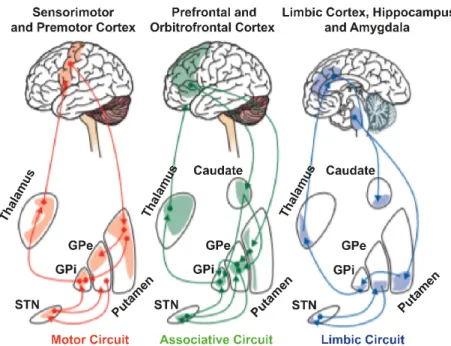
The classical Tripartite Hypothesis (TH) proposed by Alexander and colleagues in 1986 posits that the BG circuitry can be subdivided into three circuits – motor circuit, associative circuit, and limbic circuit [68] – based on their relationship with relevant cortical projection areas and the engagement of these regions (→ Figure 1.3). For example, projections from different cortical areas (motor,
prefrontal, limbic, etc.) terminate in different regions of the Striatum. These striatal regions project to different regions of the other BG nuclei, segregating them in homological maps (motor, associative and limbic). Moreover, somatotopy is well defined in each motor territory of the BG nuclei as motor information coming from different body parts is mapped in distinct regions. Thus, in addition to the well-known role of the BG in the motor domain (i.e., motor control, action selection, motor planning, and motor execution) [25], [69], [70], there is now a huge consideration of the BG as pillar structures for other functions such as reward-related behaviours (i.e., reinforcement learning), implicit learning and habit formation (i.e., automatic responses that can be performed by the motor circuit), emotions, attention, time estimation, etc [51], [71], [72]. The TH was applied to the STN subdividing it into three functional regions (motor, associative and limbic) based on the connections which arise from the different brain regions conjoining with the functional circuits [68]. Moreover, functional maps obtained by electrophysiological recordings of the STN showed a further segregation of the STN motor region in a somatotopic fashion, i.e., dorsolateroposterior area of the STN tends to be arm-responsive, whereas leg-responsive regions tend to be more ventromedioanteriorly. Recent studies have
questioned the TH claiming that these parallel pathways may be more than three and not be as segregated as once thought [73]–[75]. Indeed, evidence seems converging toward a topographical organization of the STN without defined anatomical borders, with at least partial overlap between functional subregions within the structure (i.e., presence of cross-talks in terms of converging axonal afferents on large dendritic fields of neuronal populations) [28], [54], [76]–[78]. The extent of this functional overlap is still unclear and represents a point of further investigation. Some experts have shifted this concept even more, viewing these pathways as a continuum gradient rather than entities with well-defined borders [79], [80]. Since BG are involved in many aspects of the human behaviour, an alteration or degradation of the normal physiological BG activity can potentially lead to a variegate spectrum of neurological disorders comprising Parkinson’s Disease, Tourette Syndrome, Dystonia, Impulse Control Disorder, Essential Tremor, etc.
Figure 1.3 Schematic diagram of the three main loops in the Cortico-basal ganglia-thalamocortical network.
The motor circuit (red) comprises mainly the primary, supplementary and pre-motor cortex; the dorsolateral part of the caudal putamen and of the head of the caudate nucleus; the ventrolateral two thirds of the pallidal complex and part of the lateral substantia nigra; the ventrolateral, vental anterior and centromedian nucleus of the thalamus. The associative circuit (green) comprises mainly the dorsolateral and ventrolateral prefrontal cortex; the rostral regions of the striatum; the dorsomedial regions of the pallidal complex and most of the substantia nigra; the parvocellular part of the dorsomedial nucleus of the thalamus. The limbic circuit (blue) comprises the orbitofrontal cortical regions and the anterior cingulate; the nucleus accumbens and the ventral pallidum; the rostral GPe and the rostral ventromedial GPi; the medial regions of the substantia nigra and the ventral tegmental area of the mesencephalon; the parvocellular part of the ventral anterior nucleus and the magnocellular part of the dorsomedial nucleus of the thalamus. The same subdivision can be applied to the STN. The STN is subdivided in two rostral thirds and a caudal third. Furthermore, the two rostral thirds are subdivided into medial (medial third) and lateral portions (lateral two-thirds). The medial portion of the rostral two-thirds is thought to comprise the limbic and part of the associative territories. The ventral aspect of the lateral portion of the rostral two-thirds composes the other portion of the associative territory. The dorsal aspect of the lateral portion of the rostral two-thirds and the caudal third of the nucleus are related to motor circuits. Modified from [81] under the CC BY 4.0 license https://creativecommons.org/licenses/by/4.0/.
1.1.4 Parkinson’s Disease
Neurological conditions are the leading source of disability worldwide, and the prevalence of Parkinson Disease (PD) is increasingly more rapidly that other neurological disorders (~7 milions individual with PD in 2016) [82]. PD is the most common type of parkinsonism, a term reflecting a group of neurological disorders with PD-like movement alterations such as tremor, rigidity, and bradykinesia, characterized by death of dopaminergic neurons in the SNc. PD is more common in men (1.4 male-to-female ratio), with a peak of prevalence between 85-90 years [83]. The median age onset of the disease is 60 years and the mean duration7 of the disease is 15 years [84]. About
5-10% of PD cases are diagnosed between 20-50 years and classified as young onset PD [85]. Different studies estimated that the annual incidence is around 10-15 per 100000 persons, meaning that PD-related socioeconomical costs are expected to increase in the future [86]. In most PD patients, there is not an external identifiable cause of the disease and such PD is termed as idiopathic or primary. Studies have identified some risk factors like dairy products, alcohol, and pesticides, etc., and some protective habits such as exercise associated with PD [87]. Only few PD cases termed familial or hereditary have been associated to genetic factors.
Diagnosis and symptoms
The diagnosis of the PD is primarily based on history and physical examination [88]. The most commonly diagnostic criteria are UK Brain Bank [89] (last revised by Movement Disorder’s Society in 2016 [90]). When the presence of parkinsonism is uncertain, Dopamine transporter single-photon emission computed tomography (DaT SPECT) can be used to detect the nigrostriatal cell loss with an exceptional specificity and sensitivity (98%-100%) [91]. Bradykinesia, rigidity, rest tremor and loss of postural reflexes are generally considered the cardinal signs of PD. PD patients can also present other motor symptoms (e.g., gait disturbances (i.e., periods of freezing of gait), dysarthria, hypomimia, dysphagia) as well as non-motor symptoms (e.g., sleep disturbances, sensory abnormalities, autonomic dysfunction, and cognitive decline) [92]. PD is an extremely heterogeneous disease, but recent data-driven clustering approaches suggested that it is possible to define three PD subtypes (e.g., mild motor predominant8 (49%-53% of cases), intermediate9 (35%-39% of cases)
and diffusive malignant10 (9%-16% of cases)) with different implications for diagnosis, prognosis,
and expected treatment response [93]–[96]. PD is also a neurodegenerative disease as symptoms of the patient worsen with time due to the progressive death of neurons in different parts of the brain. To study the evolution of the disease in each patient individually, rating scales that describe with numbers the severity of the symptoms are needed. Currently, there are two rating scales that are widely accepted in the clinical practice: Unified Parkinson’s Disease Rating Scale (UPDRS) and
7 Duration of the disease: time span from the diagnosis to the death.
8 Mild motor predominant: younger age at onset, mild symptoms and good medication response. 9 Intermediate: intermediate age at onset and symptomatology, moderate response to medication.
Hoehn and Yahr Stage Scale (H&Y) [97]. The UPDRS was proposed in 1995 and it consists of 4 parts: Part I Mentation, Behavior and Mood (i.e., 4 items to evaluate mental and mood dysfunction); Part II Activities of Daily Living (i.e., 13 items related to the difficulties that the patient faces for daily living activities); Part III Motor (i.e., 14 items to assess the severity of the motor symptoms) and Part IV Complications (i.e., to assess complications potentially related to the medication). Each item spans between 0 (normal) and 4 (severe). The final score is obtained summing the ratings for each part. The H&Y was proposed in 1967 by Melvin Yahr and Margaret Hoehn, and it was designed as a five-point scale (0-5): Stage 1 (i.e., unilateral symptoms), Stage 2 (i.e., bilateral symptoms), Stage 3 (i.e., presence of postural instability), Stage 4 (i.e., loss of independence) and Stage 5 (i.e., being bed-bound).
Treatments
A cure for PD does not exist. Current treatments of PD focus on ameliorating the symptoms to extend the independence and increase the quality of the life of the patient as long as possible. These symptomatic treatments must be individualized, mainly evaluating the spectrum and the severity of the symptoms, and the patient’s response to medication. Since dopamine cannot be directly used to compensate the dopamine depletion (i.e., it cannot cross the blood brain barrier),its precursor Levodopa is used [98]. Levodopa is currently the most effective oral treatment for PD symptoms, but its posology must be carefully calibrated by the clinicians. Hence, different motor and non-motor side-effects, such as dyskinesias (in up to 80% of patients after few years of chronic treatment) [99]– [101] and behavioural addictions or impulse control disorders (ICD) (e.g., gambling disorders, binge eating disorders, compulsive sexual behaviour and compulsive shopping in more than 25% of PD patients) [102]–[104] are associated with dopaminergic medications. Before the chemical era of the Levodopa, surgical ablation of some portions of the thalamus (thalamotomy), or STN (sub-thalamotomy) or the pallidum (pallidotomy) was considered to ameliorate PD symptoms. With the worsening of symptoms, the therapeutic benefits of higher doses of levodopa are surpassed by the adverse effects. For this reason, patients with advanced PD are usually treated with complementary treatments in such a way that it reduces the dosage of levodopa without altering the therapeutic effects. When symptoms become harshly drug-refractory, Deep Brain Stimulation becomes a valid option (→ Section 1.2).
Pathophysiology
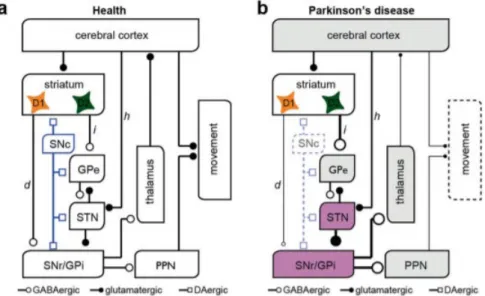
Earliest models of the pathophysiology of PD were based on the RM, suggesting that the depletion of dopamine has two-fold effect on BG: reduce activation of the DP and disinhibition of the IP. This would lead to a net increase of the GPi firing rate, and hence to a higher opposition to movements (→ Figure 1.4 ). This model availed of a huge appeal and consensus for its simplicity and it was
dopaminergic-induced imbalance of the DP and IP potentially explains the PD hypokinetic symptoms such as bradykinesia and rigidity, but it provides no explanation for the hyperkinetic aspects of PD, such as tremor, and the whole realm of non-motor symptoms.
Figure 1.4 The classical Rate Model of the Basal Ganglia under healthy condition and PD.
Cortical excitatory inputs are process through the direct (d), indirect (h) and hyperdirect (h) pathways to influence the neuronal activity of the Basal Ganglia output nuclei, which, in turn, regulate the activity of downstream cortical activity. In the Parkinsonian State the degeneration of dopamine neurons in SNc results in the hyperactivity of the indirect pathway (i) and hypoactivity of the direct pathway (d), leading to an enhanced inhibition of the motor areas of the thalamus and cortex. Hyperactivated regions (purple) and hypoactivated regions (gray) respect the healthy state are reported. Reproduced with permission from [112]. Abbreviation: Pedunculopontine nucleus (PPN).
1.1.5 Tourette Syndrome
In his 1885 paper, Georges Albert Gilles de la Tourette, one of the pupils of the widely considered founder of the modern clinical neurology Jean-Martin Charcot, provided a clear description of nine patients suffering from a “malady of tics”, after renamed Tourette Syndrome (TS) [113]. TS is a complex, childhood-onset, neurodevelopmental disorders characterized by motor and phonic tics and a variety of behavioural comorbidities, such as obsessive-compulsive disorders (OCD) and attention decifit hyper hyperactivity disorders (ADHD) [114], [115]. Although the pathogenesis of TS is still not fully understood, it is now widely accepted that TS has a relevant hereditary component and it likely results from a mix of genetic and environmental factors [116], [117]. TS usually begins in childhood (average onset age 4-6 years) with a prevalence of 3-9/1000 children and a male-to-female ratio of 3-4:1. Studies suggest that motor tics precede phonic tics, and they reach the maximum of severity at around 10-12 years. OCD and behavioural comorbidities have their peak during the adolescence [115], [118]–[120]. Most patients (80-90%) have a complete (or nearly complete) remission of the diseases after 21 years of age, but behavioural comorbidities usually persist until the adulthood. In the remaining 10-20% of TS patients, symptoms fluctuate, persist, or even worsen [115].
Diagnosis and symptoms
TS can generally be diagnosed based on targeted neurological and family history examinations. Diagnostic TS criteria were specified in the 2012 American Pychiatrics Association Diagnostic and Statistical Manual of Mental Disorders (DSM-V) [114]. Motor and phonic tics (i.e., quick, rapid, recurrent, non-rhythmic, brief movements or vocalizations) are the characteristic signs of TS. They usually have a waxing and waning course with an inverse power law of temporal scaling [121], [122]. Many people with TS report that their tics are a response to relieve uncomfortable premonitory sensations or an involuntary urge. Although tics are commonly considered as involuntary acts, often patients can exercise a degree of volitional inhibition over the release of their tics as a coping strategy in social settings. Suppression is not always successful, it requires a huge burden, and usually only temporarily prevents tic release [123]. For setting a careful diagnosis, Yale Global Tic Severity scale (YGTSS) can be used to evaluate the tics in terms of number, severity, frequency, intensity. complexity and interference with the quality of the life. The result of the scale is the Total YHTSS score (0-100) [124].
Treatments
The first-line therapy should be the neurophysiological intervention to increase the self-esteem, to avoid depressing feelings and to increase the awareness of the tics in such a way as it implements specific behavioural strategies that reduce them [125]. The second-line therapy is the neuropharmacological treatment. Neuropharmacology intervention is suggested when tics cause severe emotional problems (e.g., depression), social problems (e.g., social isolation), discomfort (e.g., pain) and functional interference with the daily living activity. The most effective drugs seem to be antipsychotics haloperidol, pimozide, and risperidone [126]. The last treatment option is neurosurgical treatment, especially DBS (→ Section 1.2). This option should be reserved for
patients who do not respond to neurocognitive interventions or pharmacotherapy, or those patients who respond to treatment, but have very serious side effects [127].
Pathophysiology
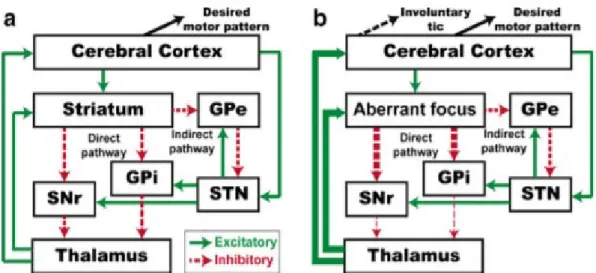
TS pathophysiological mechanisms have still not been understood. An interesting hypothesis proposed by Mink and colleagues, based on the ASM, posits that an aberrant focus of striatal neurons inhibiting GPi and SNr could foster the disinhibition of competing unwanted motor patterns such as tics (brake removal action, → Figure 1.5) [128]. Multiple tics would result from abnormal
excessive activity of multiple discrete sets of striatal neurons. Detailed knowledge of the nature of this striatal perturbation is still at an early stage of understanding, but some mechanisms are hypothesized: i) the disruption of the striatal GABAergic transmission due to a loss of striatal neurons [129], [130], ii) the striatal dopaminergic hyperinnervation [131]and iii) the failure of cortical internal inhibition in some cortical areas [132], [133].
Figure 1.5 The classical Action-Selection Model of the Basal Ganglia under healthy condition and TS.
Under normal conditions (a) desired motor patterns are disinhibited by the direct pathway, and competing motor programmes are suppressed by the indirect pathway. In TS (b), aberrant striatal neurons release the brake action of the GPi on unwanted motor programs, leading to tics. Excitatory connections (red) and inhibitory connections (green) are reported. The thickness represents the related activity of the projection. Reproduced with permission from [120].
1.1.6 Beyond the Rate Model
As extensively discussed above, for several years, the most appealing theory underlying movement disorders phenomenology was the RM [134]. This theory posited that an over-activation and under-activation of the GPi are a causal mechanism of hypokinetic disorders (e.g., bradykinesia in PD) and hyperkinetic ones (e.g., tics in TS), respectively. Despite the intuitiveness of this hypothesis, there are many contravening observations that make this theory no longer tenable (refer to [135] for a review). New theories on the BG genesis of movement disorders have been developing thanks to the paradigm shift of analyzing BG activity not only through the rate of single neurons but considering the discharge pattern and the local field potentials (LFP). LFP is a population level measure of the effects of synaptic input currents in the extracellular medium [136]. Most of the LFP studies in literature were performed through LFP recordings from externalized DBS electrodes few days after the DBS surgery (→ Section 1.2.1 and Chapter 3). New devices such as the PC+S and PerceptTM
(Medtronic Inc) or AlphaDBS (Newronika Srl) allow LFP recordings also directly from chronically implanted impulse generator [137]. Accumulating evidence suggested that aberrant firing patterns (i.e., bursting activity), synchronized firing at the single neuron level and abnormal oscillations at the population level (i.e., LFP) may result in a failure of the normal functioning of the CBGT network, and potentially hinge the surge of disorders such as PD and TS [138]–[145]. Increased bursting in the Striatum, GPe, GPi, STN and thalamus [27], and increased synchrony [146] in all BG nuclei were found in PD animal models and patients [147]–[152]. Tremor-related and akinetic-related single neurons oscillating at 4-7 Hz (theta frequency range) and 13-30 Hz (beta frequency range) were found in the STN in PD patients [153], [154]. At the LFP level, subjects with PD display an abnormal
![Figure 1.2 STN-centric action-selection and cancellation model proposed by Mosher et al [58]](https://thumb-eu.123doks.com/thumbv2/123dokorg/2926205.18610/27.892.217.652.180.498/figure-centric-action-selection-cancellation-model-proposed-mosher.webp)